Abstract
Young adults typically deactivate specific brain regions during active task performance. Deactivated regions overlap with those that show reduced resting metabolic activity in aging and dementia, raising the possibility of a relation. Here, the magnitude and dynamic temporal properties of these typically deactivated regions were explored in aging by using functional MRI in 82 participants. Young adults (n = 32), older adults without dementia (n = 27), and older adults with early-stage dementia of the Alzheimer type (DAT) (n = 23) were imaged while alternating between blocks of an active semantic classification task and a passive fixation baseline. Deactivation in lateral parietal regions was equivalent across groups; in medial frontal regions, it was reduced by aging but was not reduced further by DAT. Of greatest interest, a medial parietal/ posterior cingulate region showed differences between young adults and older adults without dementia and an even more marked difference with DAT. The temporal profile of the medial parietal/posterior cingulate response suggested that it was initially activated by all three groups, but the response in young adults quickly reversed sign, whereas DAT individuals maintained activation throughout the task block. Exploratory whole-brain analyses confirmed the importance of medial parietal/posterior cingulate cortex differences in aging and DAT. These results introduce important opportunities to explore the functional properties of regions showing deactivations, how their dynamic functional properties relate to their baseline metabolic rates, and how they change with age and dementia.
Functional neuroimaging studies of aging and dementia of the Alzheimer type (DAT) typically focus on brain regions that show positive activations, defined as increases in signal during an active task compared with a more passive baseline (1-3). Here we report a systematic exploration of aging- and DAT-related differences in negative activations, or deactivations, defined as decreases in signal during the task as compared with baseline.
In young adults, a specific set of regions consistently shows deactivation that generalizes across a wide range of tasks and stimulus modalities, and has been detected by using both positron emission tomography and functional MRI (4-7). These commonly deactivated regions include large sections of the lateral parietal cortex, the medial parietal cortex (including posterior cingulate and precuneus), and the medial frontal cortex (6, 7). The functional significance of these deactivations remains unclear. One current hypothesis is that the regions involved constitute a ”default network” supporting processes more active during passive-than active-task conditions (8). Suggested default activities include monitoring the environment, monitoring one's internal state and emotions, and various forms of undirected thought. Engaging in a goal-directed, active task may redirect processing resources from default activities to task-specific processes and regions.
Several lines of evidence support the idea of a default network. First, both the medial frontal and the medial parietal/posterior cingulate cortex show high resting metabolism as measured by using fluorodeoxyglucose positron emission tomography (8, 9). Second, a recent study found high temporal correlations in blood oxygenation level-dependent signal fluctuations during passive conditions across the medial frontal, lateral parietal, and medial parietal/posterior cingulate cortex, implying functional connectivity (10). Third, deactivation magnitudes increase with task difficulty, suggesting increased diversion of attention from default processing to task-related processing (7). Finally, active tasks designed to target processes thought to be ongoing in the default state can produce positive activations in these regions of the cortex. For example, dorsal medial frontal aspects of the network can be activated in tasks requiring reference to one's internal state or emotions (11, 12).
Relevant to aging research, regions corresponding to those that show functional deactivations in young adults show reduced resting metabolism (measured by using fluorodeoxyglucose positron emission tomography) with aging, and this hypometabolism is even more pronounced in DAT (13-17). For example, a large multicenter study (n = 505) found inverse correlations between age and resting metabolism in the medial frontal cortex for adults without dementia (15). Hypometabolism in the lateral parietal cortex and posterior cingulate was associated with dementia severity in DAT participants, but not with aging. DAT-associated resting hypometabolism in posterior cortical regions is found so consistently that it has been proposed as an early diagnostic marker of the disease (16, 17).
The medial parietal/posterior cingulate cortex, near the retrosplenial cortex, has dense reciprocal connections with medial temporal regions (18) that are involved in memory function and that are the earliest and most prominent sites of pathology in Alzheimer's disease (19). Studies in both animals and humans show that lesions including the entorhinal and perirhinal cortex lead to reduced posterior cingulate resting metabolism similar to that seen in DAT (20-24). It has been suggested that posterior cortical regions, in particular, the posterior cingulate and precuneus, may be involved in memory function, and that DAT-related hypometabolism may reflect memory impairment (25-29).
Despite extensive findings that these regions show functional deactivations in young adults, and reduced resting metabolism in old age and DAT, little is known about their dynamic activity profiles, or how their functional characteristics may differ in old age and DAT. In the present study, young adults, older adults without dementia, and older adults with early-stage DAT were imaged while they alternated between a passive fixation baseline condition and an active semantic classification task shown to promote memory for words (30). Semantic classification tasks are often associated with greater positive activation responses in nondemented aging and DAT relative to young adults, possibly reflecting compensation for increased task difficulty (1-3, 31). Here, the focus is on the magnitude and temporal profile of activity in frontal and parietal regions where young adults typically show deactivations.
Methods
Subjects. Eighty-nine subjects participated in the study. Data from seven subjects (two young adults, four older adults without dementia, and one DAT participant) were excluded because their data fell more than 3 SDs away from the mean for their group. Clinical and demographic data for the included subjects are presented in Table 1. Young adults were Washington University students or staff. Older adults were recruited from the Washington University Alzheimer's Disease Research Center and classified by using the Clinical Dementia Rating (CDR) scale (32) as nondemented (CDR = 0) or in the early stages of DAT (19 participants with CDR = 0.5, four participants with CDR = 1). Although several of our DAT participants had cognitive test scores that might qualify for classification as mild cognitive impairment, a CDR score of 0.5 or greater in this sample is highly predictive of Alzheimer's disease, both in clinical progression and neuropathologic diagnosis at autopsy (33). All testing was conducted within guidelines established by the Washington University Human Studies Committee.
Table 1. Demographic and performance variables.
| Young (n = 32) | Old (n = 27) | DAT (n = 23) | |
|---|---|---|---|
| Female/male | 15/17 | 17/10 | 8/15 |
| Age* | 22.0 (0.6) | 76.1 (1.7) | 77.0 (1.3) |
| Education, yr | 15.4 (0.6) | 14.2 (0.6) | |
| MMSE† | 29.0 (0.2) | 26.0 (0.7) | |
| WAIS information† | 21.0 (0.9) | 17.6 (1.1) | |
| WAIS block design† | 32.3 (1.8) | 25.6 (2.1) | |
| WAIS digit symbol† | 47.3 (2.1) | 36.3 (2.6) | |
| Boston naming† | 56.1 (0.7) | 50.7 (1.4) | |
| Word fluency (S + P)† | 32.9 (2.0) | 25.8 (2.0) | |
| WMS mental control† | 7.6 (0.3) | 6.5 (0.4) | |
| WMS digit span (backward)† | 5.0 (0.2) | 4.1 (0.2) | |
| WMS logical memory† | 9.8 (0.9) | 6.4 (0.6) | |
| Reaction time, ms* | 934.5 (20.6) | 1,081.9 (32.4) | 1,159.0 (33.4) |
| Classification accuracy, %, *† | 90.0 (0.5) | 85.4 (1.6) | 80.3 (2.0) |
| Corrected recognition, %, *† | 78.0 (2.2) | 62.5 (3.6) | 31.2 (5.2) |
Means and standard errors (in parentheses) for demographic and performance variables. MMSE, Mini Mental State Evaluation; WAIS, Wechsler Adult Intelligence Scale; WMS, Wechsler Memory Scale. Variables with values for all three groups were analyzed by using one-way ANOVA followed by post hoc t tests. Variables with values only for the older adult groups were analyzed by using independent sample t tests. *, significant difference between young and old without dementia; †, significant difference between old without dementia and old with DAT, P < 0.05.
Imaging Methods. Images were collected by using a 1.5T Siemens Vision scanner (Siemens, Erlangen, Germany). Cushions, a plastic face mask, and headphones were used to reduce movement, dampen scanner noise, and communicate with the participant. Stimuli were projected (AmPro model LCD-150, Melbourne, FL) onto a screen at the head of the bore and viewed by a mirror attached to the head coil. Participants were fitted for MR1-compatible lenses based on autorefractor readings (Marko Technologies, model 760A, Jacksonville, FL) and subjective report of improved acuity. Participants not in need of vision correction wore plain lenses. Behavioral responses were collected by using a fiber-optic button-press device held in both hands.
Each scanning session began with a scout image to center the field of view on the brain, followed by a high-resolution (1 × 1 × 1.25 mm) structural T1-weighted magnetization-prepared rapid gradient echo sequence (MPRAGE) [repetition time (TR) = 9.7 ms; echo time (TE) = 4 s; flip angle = 10; inversion time (TI) = 20 ms; delay time (TD) = 200 ms]. Two functional runs followed using an asymmetric spin-echo sequence sensitive to blood oxygenation level-dependent contrast (TR = 2.5 s; T2* evolution time = 50 ms). Each functional run acquired 76 sequential whole-brain image acquisitions (16 8-mm-thick contiguous slices, 3.75 × 3.75 mm in-plane resolution, aligned with the anterior-posterior commissure plane).
The first four image acquisitions of each run were excluded from analysis. The remaining 72 image acquisitions (hereafter referred to as time points) alternated between blocks of 9 passive baseline trials and 12 active task trials. During baseline blocks, subjects fixated a continuous, centrally presented crosshair. During task blocks, subjects made semantic classification judgments on visually presented words (2,000-ms duration; 500-ms intertrial interval), pressing a right-hand button if a word represented a living thing or a left-hand button if it represented something nonliving. They were not instructed to try to remember the words presented during this task, but it nonetheless promotes effective incidental encoding (30). Immediately after the two task runs, participants completed an old/new recognition test in which previously presented words were randomly intermixed with an equal number of new words. Recognition data were lost for one young adult.
Functional MRI Analysis. Functional data were first preprocessed to correct for slice timing, odd/even slice intensity differences, head movement, and differences in whole-brain intensity by using procedures similar to Logan et al. (31). Individual subject data were transformed into stereotaxic atlas space (34, 35) and resampled to 2-mm3 isotropic voxels. The atlas-representative target image was composed of a merged young-adult/older-adult reference. This target-image strategy minimizes systematic age differences in the atlas location of cortical structures.** The atlas-transformed image for each subject was checked against a reference average to ensure appropriate registration.
After preprocessing, magnitude estimates were computed for each person based on an implementation of the general linear model (see ref. 36). Task-related activity was modeled as an extended gamma function (37) with a mean and slope coded for each run. The model was computed for each voxel separately, resulting in magnitude estimates for each subject for task versus baseline. Magnitude estimates were scaled to percent signal change and entered into a priori regional analyses and also map-wise exploratory analyses that used a mixed-effects model.
Four a priori regions were defined on a separate large sample of young adults pooled across several studies of intentional memory encoding (ref. 38; see also ref. 31). Peak locations of activation or deactivation served as region seed points, and regions were defined to include all significant voxels with the same sign within 12 mm. Regions are labeled by their approximate Brodmann area (BA; based on ref. 34). The left frontal region (BA 44/6; x, y, and z peak location = -43, 3, and 32) is activated during controlled processing tasks using verbal materials. It was selected to test whether the current data replicate typical findings of preserved or increased positive activation for older adults without dementia and with DAT in semantic classification tasks (1-3, 31). The other regions were selected because they show deactivation across many cognitive tasks (4-7). These regions included a lateral parietotemporal region (BA 40; peak 45, -73, 38), a medial frontal region (BA 10; peak 1, 37, 6), and a medial parietal/posterior cingulate region (BA 31; values averaged around left- and right-hemisphere peaks, -11, -31, 39; 11, -35, 38). Magnitude estimates for these regions were computed for each subject in the current study and served as the dependent measure for ANOVA.
The temporal profile of activity within each region was plotted without temporal smoothing or other secondary modeling (38). Values for each task block and its subsequent baseline block were averaged first within each subject and then across subjects within a group to produce the time courses shown in Fig. 1. Normalization to correct for individual differences in signal intensity was achieved by setting the mean of the first four points of the initial baseline block (not included in the averages) to 1,000.
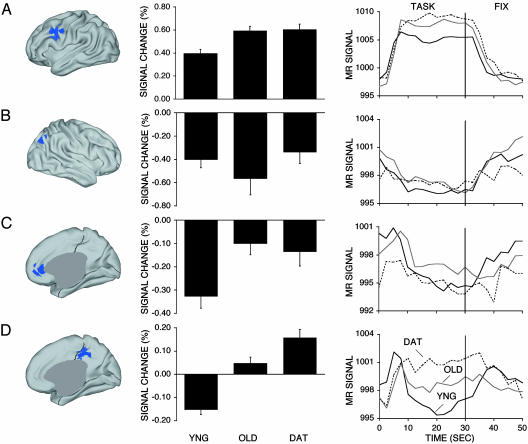
Fig. 1.
Regional analyses showing effects of age and dementia. (Left) Regions are projected onto an inflated representation of the cortex (40) and include left frontal cortex BA 44/6 (A), right lateral parietal cortex BA 40 (B), medial frontal cortex BA 10 (C), and medial parietal/posterior cingulate cortex BA 31 (D). References to specific BAs are tentative and used as a heuristic based on ref. 34. Mean magnitude estimates with standard error bars for activity in each region for each group are shown in Center, followed by the mean time courses of blood oxygenation level-dependent signal across active task and passive fixation baseline conditions for each group in Right. Note reversed sign of net response and altered time course in the posterior cingulate region (D).
Results
Behavioral data are shown in Table 1. The groups differed in response time for the semantic classification task [F(2, 79) = 16.68, P < 0.0001]. The older adults without dementia were slower than the young adults [t(57) = 3.96, P < 0.0005], as were the DAT participants [t(53) = 6.03, P < 0.0001], but the two older adult groups did not differ significantly [t(48) = 1.65, P = 0.11]. Group differences also occurred in classification accuracy [F(2, 79) = 12.35, P < 0.0001]. The older adults without dementia were less accurate than were the young adults [t(57) = 2.98, P < 0.005], and DAT participants were less accurate than the older adults without dementia [t(48) = 2.03, P < 0.05]. However, all groups achieved at least 80% accuracy overall. This contrasts with the results for the recognition memory test, where the DAT participants performed very poorly. Large group differences occurred in recognition memory [F(2, 78) = 46.57, P < 0.0001]. Older adults without dementia had worse memory performance than young adults [t(56) = 3.77, P < 0.0005], and DAT participants were additionally worse than older adults without dementia [t(48) = 5.39, P < 0.0001].
Examination of functional activation patterns suggested differences associated with aging and DAT. Fig. 1 illustrates the a priori regions, along with the mean estimates of signal change and the temporal profile of signal change within them. The left frontal region (BA 44/6) was chosen because it typically shows positive activation in tasks involving controlled verbal processing and has been associated with preserved or even greater activation in aging and DAT (1-3, 31). We found positive activations in this region that differed between the groups [F(2, 79) = 9.21, P < 0.001]. The group differences were largely due to age. The older adults without dementia showed greater activation than did the young adults [t(57) = 3.66, P < 0.001], as did the DAT [t(53) = 3.70, P < 0.001], but the two older adult groups did not differ (t < 1).
The three remaining regions (BA 40, BA 10, and BA 31) were chosen because they correspond to regions typically showing functional deactivation in young adults and reduced resting metabolism in aging and DAT (4-10, 13-17). They were first entered into an omnibus group X region ANOVA, which found a significant interaction [F(4, 158) = 3.10, P < 0.05]. The significant group X region interaction suggests that various group-dependent effects exist within these regions that are not likely to be artifacts of systematic, main-effect group differences in hemodynamic response properties (39). One-way ANOVAs followed by specific t tests were next used to compare group values for each region.
There were no group differences in activation for BA 40 (P = 0.30). BA 10 showed an effect of group [F(2, 79) = 5.666, P < 0.001], which was largely driven by age. The older adults without dementia showed less deactivation in this region than did the young adults [t(57) = 3.19, P < 0.01], as did the DAT participants [t(53) = 2.40, P < 0.05], but the two older adult groups did not differ (t < 1).
The most intriguing results were found for the medial parietal/ posterior cingulate region (BA 31), which showed a significant effect of group [F(2, 79) = 33.98, P < 0.0001]. Marked quantitative and qualitative differences were observed between the groups. Specifically, young adults showed deactivations, a pattern notably different from either the older adults without dementia [t(57) = 5.93, P < 0.0001], or DAT participants [t(53) = 7.88, P < 0.0001]. The group without dementia showed a trend toward positive activation that was only marginally different from zero (P = 0.09). The DAT group had positive activations that were significantly greater than those shown by the group without dementia [t(48) = 2.51, P < 0.05].
Correlational analyses within the groups did not reveal a relation between behavioral measures and deactivation magnitudes for any of the regions. However, BA 10, which showed an effect of age in the group comparisons, also showed a significant correlation with age within the older adult group without dementia, such that increasing age associated with a trend away from deactivations toward positive activations (r = .58, P < 0.005). The majority (8 of 11) of participants without dementia who were older than 80 years of age had magnitude estimates with a positive sign.
To consider whether our results were influenced by gender, we replicated the ANOVAs on subsets matched for gender (eight females and eight males per group). The two older adult subgroups were matched as closely as possible for age, although the DAT (78.0 ± 1.4 years) were slightly older than those without dementia (74.6 ± 1.5 years). All patterns remained the same but, in some cases, failed to reach statistical significance because of the reduced power associated with smaller groups.
The temporal profile of activity within each region is plotted in Fig. 1 Right. Of most interest is the difference between the time courses for BA 31 and BA 40. BA 40 showed an initial decrease and sustained deactivation relative to baseline throughout the task block for all groups. In contrast, BA 31 showed an initial increase at the beginning of the task block. For young adults, the time course quickly reversed sign and was lower than baseline for the remainder of the task block, replicating the pattern shown by Konishi et al. (38). For older adults without dementia, the subsequent decrease was roughly equivalent to baseline levels. Strikingly, the DAT maintained the increase and thus had a net positive activation throughout the task block.
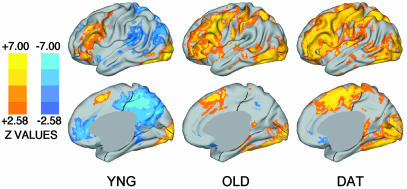
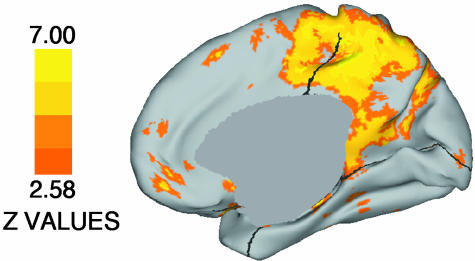
Exploratory whole-brain analyses were next performed to confirm and extend the a priori regional analyses. Fig. 2 presents statistical activation maps projected onto lateral and medial surfaces (41). Both older adult groups show robust positive activations, but only sparse deactivations. An ANOVA comparing the three groups (Fig. 3) revealed a significant and extensive difference in the medial parietal/posterior cingulate cortex, overlapping with the region showing notable aging and DAT effects in the a priori analyses. The medial parietal/posterior cingulate cortex was the site of some of the largest group differences found throughout the brain. Pairwise comparisons (not shown) revealed that it was the site of large group differences between the young adult group and either older adult group and between the older adults without dementia and with DAT.
Fig. 2.
Statistical activation maps for young, old, and DAT groups. Warm colors (red, yellow) show positive activations; cool colors (blues) show deactivations. Brighter colors indicate areas of greater statistical significance. (Upper) The left lateral cortical surface for each group. (Lower) The right medial surface. These maps complement the a priori statistical analyses shown in Fig. 1. Because different numbers of subjects contribute to each of the maps in the current figure, apparent differences in z scores across the groups should not be quantitatively interpreted.
Fig. 3.
Map of statistically significant group differences across the brain. Brighter colors indicate areas of greater statistical significance. Note that the medial parietal/posterior cingulate cortex showing large group differences here overlaps heavily with the BA 31 region showing robust group differences in the a priori analyses (compare with Fig. 1D).
Inspection of the group statistical activation maps in Fig. 2 raised the question of whether the DAT participants showed significant positive activations in medial parietal/posterior cingulate regions or simply a failure of deactivation. To address this question, we generated two additional, post hoc bihemispheric regions of interest based on peak locations of group differences identified in the group ANOVA shown in Fig. 3. The first (peaks -9,-35, 40 and 7, -29, 40) was located somewhat more specifically in posterior cingulate gyrus than our a priori region, and was chosen because the posterior cingulate is a frequent focus in fluorodeoxyglucose positron emission tomography studies of aging and DAT (13-17). A second, more dorsal and posterior region located primarily in precuneus (peaks -7, -49, 52 and 9, -43, 44) was included because it contained the medial cortex voxels of highest significance in the between-group comparisons.
Magnitude estimates obtained for these post hoc regions revealed similar results as those found for the a priori medial parietal/posterior cingulate region. Young adults showed deactivations, older adults had slightly positive magnitude estimates with values near zero, and DAT participants showed positive activations with absolute magnitudes approximately as large as the deactivations shown by young adults. Importantly, for both the posterior cingulate and precuneus regions, the DAT participants' activation magnitudes were significantly above zero (the P value was <0.005 for both). Thus, while they await replication, the current results suggest that the medial parietal/posterior cingulate cortex, consistently associated with functional deactivation in young adults, is associated with small but significant positive functional activation in older adults in the beginning stages of DAT.
Discussion
The present study found marked age- and DAT-related differences in brain regions that are associated with functional deactivation in young adults (4-10). In particular, the medial parietal/posterior cingulate cortex showed DAT-specific differences that were linked to positive, sustained activation. The details of these findings, how they relate to our understanding of default states, and their implications for characterizing and detecting DAT are discussed below.
Although medial frontal, lateral parietal, and medial parietal/posterior cingulate regions are often described as a network mediating resting state or ”default” cognitive processing in passive conditions (4-10), several dissociations among them were noted. Even within the young adult group, these regions had distinct time courses: The lateral parietal region showed a time course inverse of that often found for positive activations, with a decrease at the onset of the active task that was maintained through its duration. In contrast, the medial parietal/posterior cingulate region showed an initial increase in activation that in young adults quickly reversed to below-baseline levels. More obvious were the group-based differences. The lateral parietal cortex was similarly deactivated for all groups, medial frontal cortex deactivations were reduced in old age but did not show further reduction in DAT, and the medial parietal/posterior cingulate showed activity in opposite directions for young adults versus the DAT participants. The reduced deactivations in the older adult groups are especially surprising, given that the performance data suggested that they found the task more difficult than did the young adults. In young adults, greater task difficulty is usually associated with greater deactivation (7).
The medial parietal/posterior cingulate cortex was the site of the largest and most intriguing differences between the groups. Compared with the young adults, DAT participants showed activity that was equivalent in magnitude but opposite in sign. Furthermore, group differences were also easily observed in the time courses. Whereas young adults showed decreases in this region after a transient initial activation, DAT participants maintained an above-baseline activation throughout the task period. The magnitude of the DAT participants' medial parietal/posterior cingulate activation is not as large as for the left frontal region, which is also associated with positive activations in young adults; future investigations using DAT participants should inspect medial parietal/posterior cingulate regions carefully to determine the specific quantitative properties of the DAT pattern.
Functional activation of the medial parietal/posterior cingulate in DAT may at first seem counterintuitive, given its strong association with DAT-related reductions in resting metabolism (13-17). However, in the schizophrenia literature there are also reports of patients showing functional activations in regions where control subjects show deactivations and that correspond to regions where patients have reduced resting metabolism (41-43). Taken together, these patterns suggest that the relation between functional activity patterns and resting metabolism needs further investigation.
We suspect that DAT-related changes in the medial parietal/ posterior cingulate cortex are related to damage of the entorhinal/perirhinal cortex (19). As described, medial temporal regions are a major source of projections to the medial parietal/ posterior cingulate, and their disruption leads to medial parietal/posterior cingulate hypometabolism in both humans and animals (18, 20-24). The DAT-related activation in the current study might thus reflect a loss of regulation or functional inhibition. Alternatively, it may reflect compensation for damage to the medial temporal regions, perhaps by recruitment of alternative posterior attention networks (44). Regarding to the possibility of compensation, a recent study of a patient with amnesia suggests that connections from this region to the medial temporal and frontal lobe regions may play an increased role in memory processing after medial temporal lobe damage (45).
The age- and DAT-related differences in functional deactivation reported here represent a new class of ”senior-specific” functional activity patterns complementary to age- and DAT-related differences in positive activation (1-3). These findings make connections between the existing literature on age- and DAT-related differences in resting-brain metabolism (13-17), and the rapidly growing literature on task-related deactivations in young adults (4-12). DAT-related changes in medial parietal/ posterior cingulate cortex activation provide a candidate marker that may aid detection and monitor progression of Alzheimer's disease. It will be of particular interest to determine whether such changes can serve as a preclinical marker for Alzheimer's disease, and whether those individuals without dementia who show atypical activation patterns are more likely to convert to dementia. Changes in deactivation patterns have also been reported in other populations whose brain function differs from that of healthy young adults, including patients with amnesia, schizophrenia, or fragile X syndrome (42, 45-47). In conclusion, these results introduce important opportunities to explore the functional properties of regions showing deactivations, how their dynamic functional properties relate to their baseline metabolic rates, and how they change with age and dementia.
Acknowledgments
We thank the Washington University Alzheimer's Disease Research Center for clinical assistance and participant recruitment, Glenn Foster, Rich Nagel, and Linda Hood for assistance with MRI data collection, and Martha Storandt and Betsy Grant for assistance with psychometric data. David Van Essen generously provided the use of caret software. This study was supported by National Institutes of Health Grants AG00030, AG05681, AG03991, MH57506, NS06833, and P50 AG05681, the Alzheimer's Association, the James S. McDonnell Foundation, and the Howard Hughes Medical Institute. C.L. was supported by a Richard and Mildred Poletsky Education Award.
Abbreviations: CDR, Clinical Dementia Rating; DAT, dementia of the Alzheimer type; BA, Brodmann area.
Footnotes
Snyder, A. Z., Morris, J. C., Williams, L. & Buckner, R. L. (2002) Neurobiol. Aging 23, 1337 (abstr.).
References
- 1.Reuter-Lorenz, P. A. (2002) Trends Cognit. Neurosci. 6, 394-400. [DOI] [PubMed] [Google Scholar]
- 2.Grady, C. L., McIntosh, A. R., Beig, S., Keightley, M. L., Burian, H. & Black, S. E. (2003) J. Neurosci. 23, 986-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabeza, R. (2002) Psychol. Aging 17, 85-100. [DOI] [PubMed] [Google Scholar]
- 4.Gusnard, D. A. & Raichle, M. E. (2001) Nat. Rev. Neurosci. 2, 685-694. [DOI] [PubMed] [Google Scholar]
- 5.Mazoyer, B., Zago, L., Mellet, E., Bricogne, S., Etard, O., Houde, O., Crivello, F., Joliot, M., Petit, L. & Tzourio-Mazoyer, N. (2001) Brain Res. Bull. 54, 287-298. [DOI] [PubMed] [Google Scholar]
- 6.Shulman, G. L., Fiez, J. A., Corbetta, M., Buckner, R. L., Miezin, F. M., Raichle, M. E. & Petersen, S. E. (1997) J. Cognit. Neurosci. 9, 648-663. [DOI] [PubMed] [Google Scholar]
- 7.McKiernan, K. A., Kaufman, J. N., Kucera-Thompson, J. & Binder, J. R. (2003) J. Cognit. Neurosci. 15, 394-408. [DOI] [PubMed] [Google Scholar]
- 8.Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A. & Shulman, G. L. (2001) Proc. Natl. Acad. Sci. USA 98, 676-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phelps, M. E., Mazziotta, J. C., Kuhl, D. E., Nuwer, M., Packwood, J., Metter, J. & Engel, J., Jr. (1981) Neurology 31, 517-529. [DOI] [PubMed] [Google Scholar]
- 10.Greicius, M. D., Krasnow, B., Reiss, A. L. & Menon, V. (2003) Proc. Natl. Acad. Sci. USA 100, 253-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gusnard, D. A., Akbudak, E., Shulman, G. L. & Raichle, M. E. (2001) Proc. Natl. Acad. Sci. USA 98, 4259-4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kjaer, T. L., Nowak, M. & Lou, H. C. (2002) NeuroImage 17, 1080-1086. [PubMed] [Google Scholar]
- 13.Benson, D. F., Kuhl, D. E., Hawkins, R. A., Phelps, M. E., Cummings, J. L. & Tsai, S. Y. (1983) Arch. Neurol. 40, 711-714. [DOI] [PubMed] [Google Scholar]
- 14.Loessner, A., Alavi, A., Lewandrowski, K. U., Mozley, D., Souder, E. & Gur, R. E. (1995) J. Nucl. Med. 36, 1141-1149. [PubMed] [Google Scholar]
- 15.Herholz, K., Salmon, E., Perani, D., Baron, J. C., Holthoff, V., Frolich, L., Schonknecht, P., Ito, K., Mielke, R., Kalbe, E., et al. (2002) NeuroImage 17, 302-316. [DOI] [PubMed] [Google Scholar]
- 16.Leon, M. J., Convit, A., Wolf, O. T., Tarshish, C. Y., DeSanti, S., Rusinek, H., Tsui, W., Kandil, E., Scherer, A. J., Roche, A., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 10966-10971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silverman, D. H., Small, G. W., Chang, C. Y., Lu, C. S., de Aburto, M. A. K., Chen, W., Czernin, J., Rappoport, S. I., Pietrini, P., Alexander, G. E., et al. (2001) J. Am. Med. Assoc. 286, 2120-2127. [DOI] [PubMed] [Google Scholar]
- 18.Vogt, B. A., Finch, D. M. & Olson, C. R. (1992) Cereb. Cortex 2, 435-443. [DOI] [PubMed] [Google Scholar]
- 19.Braak, H. & Braak, E. (1991) Acta Neuropathol. 82, 239-259. [DOI] [PubMed] [Google Scholar]
- 20.Perani, D., Bressi, S., Cappa, S. F., Vallar, G., Alberoni, M., Grassi, F., Caltagirone, C., Cipolotti, L., Franceschi, M., Lenzi, G. L. & Fazio, F. (1993) Brain 116, 903-919. [DOI] [PubMed] [Google Scholar]
- 21.Reed, L. J., Marsden, P., Lasserson, D., Sheldon, N., Lewis, P., Stanhope, N., Guinan, E. & Kopelman, M. D. (1999) Memory 7, 599-612. [DOI] [PubMed] [Google Scholar]
- 22.Aupee, A. M., Desgranges, B., Eustache, F., Lalevee, C., de la Sayette, V., Viader, F. & Baron, J. C. (2001) NeuroImage 13, 1164-1173. [DOI] [PubMed] [Google Scholar]
- 23.Meguro, K., Blazoit, X., Kondoh, Y., Le Mestric, C., Baron, J. C. & Chavoix, C. (1999) Brain 122, 1519-1533. [DOI] [PubMed] [Google Scholar]
- 24.Millien, I., Blaizot, X., Giffard, C., Mezenge, F., Insausti, R., Baron, J. C. & Chavoix, C. (2002) J. Cereb. Blood Flow Metab. 22, 1248-1261. [DOI] [PubMed] [Google Scholar]
- 25.Andreasen, N. C., O'Leary, D. S., Arnt, S., Rezai, K., Watkins, G. L., Ponto, L. L. B. & Hichwa, R. D. (1995) NeuroImage 2, 296-305. [DOI] [PubMed] [Google Scholar]
- 26.Cabeza, R. & Nyberg, L. (2000) J. Cognit. Neurosci. 12, 1-47. [DOI] [PubMed] [Google Scholar]
- 27.Desgranges, B., Baron, J. C., Lalevee, C., Giffard, B., Viader, F., de la Sayette, V. & Eustache, F. (2002) Brain 125, 1116-1124. [DOI] [PubMed] [Google Scholar]
- 28.Maddock, R. J. (1999) Trends Neurosci. 22, 310-316. [DOI] [PubMed] [Google Scholar]
- 29.Valenstein, E., Bowers, D., Verfaellie, M., Heilman, K. M., Day, A. & Watson, R. T. (1987) Brain 110, 1631-1646. [DOI] [PubMed] [Google Scholar]
- 30.Kapur, S., Craik, F. I. M., Tulving, E., Wilson, A. A., Houle, S. & Brown, G. M. (1994) Proc. Natl. Acad. Sci. USA 91, 2008-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Logan, J. M., Sanders, A. L., Snyder, A. Z., Morris, J. C. & Buckner, R. L. (2002) Neuron 33, 827-840. [DOI] [PubMed] [Google Scholar]
- 32.Morris, J. C. (1993) Neurology 43, 2412-2414. [DOI] [PubMed] [Google Scholar]
- 33.Morris, J. C., Storandt, M., Miller, J. P., McKeel, D. W., Price, J. L., Rubin, E. H. & Berg, L. (2001) Arch. Neurol. 58, 397-405. [DOI] [PubMed] [Google Scholar]
- 34.Talaraich, J. & Tournoux, P. (1988) Co-Planar Stereotaxic Atlas of the Human Brain (Thieme, New York).
- 35.Maccotta, L., Zacks, J. M. & Buckner, R. L. (2001) NeuroImage 14, 1105-1121. [DOI] [PubMed] [Google Scholar]
- 36.McDermott, K. B., Buckner, R. L., Petersen, S. E., Kelley, W. M. & Sanders, A. L. (1999) J. Cognit. Neurosci. 11, 631-640. [DOI] [PubMed] [Google Scholar]
- 37.Boynton, G. M., Engel, S. A., Glover, G. H. & Heeger, D. J. (1996) J. Neurosci. 16, 4207-4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Konishi, S., Donaldson, D. I. & Buckner, R. L. (2001) NeuroImage 13, 364-374. [DOI] [PubMed] [Google Scholar]
- 39.Buckner, R. L., Snyder, A. Z., Sanders, A. L., Raichle, M. E. & Morris, J. C. (2000) J. Cognit. Neurosci. 12, 24-34. [DOI] [PubMed] [Google Scholar]
- 40.Van Essen, D. C. & Drury, H. A. (1997) J. Neurosci. 17, 7079-7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cleghorn, J. M., Szechtman, H., Garnett, E. S., Nahmias, C., Brown, G. M., Kaplan, R. D., Szechtman, B. & Franco, S. (1991) Psychiatry Res. 40, 135-153. [DOI] [PubMed] [Google Scholar]
- 42.Fletcher, P. C., McKenna, P. J., Frith, C. D., Grasby, P. M., Friston, K. J. & Dolan, R. J. (1998) Arch. Gen. Psychiatry 55, 1001-1008. [DOI] [PubMed] [Google Scholar]
- 43.Turetsky, B. I., Moberg, P. J., Mozley, L. H., Moelter, S. T., Agrin, R. N., Gur, R. C. & Gur, R. E. (2002) Neuropsychology 16, 481-490. [PubMed] [Google Scholar]
- 44.Posner, M. I. & Petersen, S. E. (1990) Annu. Rev. Neurosci. 13, 25-42. [DOI] [PubMed] [Google Scholar]
- 45.Maguire, E. A., Vargha-Khadem, F. & Mishkin, M. (2001) Brain 124, 1156-1170. [DOI] [PubMed] [Google Scholar]
- 46.Spence, S. A., Liddle, P. F., Stefan, M. D., Hellewell, J. S. E., Sharma, T., Friston, K. J., Hirsch, S. R., Frith, C. D., Murray, R. M., Deakin, J. F. W. & Grasby, P. M. (2000) Br. J. Psychiatry 176, 52-60. [DOI] [PubMed] [Google Scholar]
- 47.Tamm, L., Menon, V., Johnston, C. K., Hessl, D. R. & Reiss, A. L. (2002) J. Cognit. Neurosci. 14, 160-171. [DOI] [PubMed] [Google Scholar]