biochemistry, medical sciences. For the article ”Structure of the topoisomerase II ATPase region and its mechanism of inhibition by the chemotherapeutic agent ICRF-187,” by Scott Classen, Stephane Olland, and James M. Berger, which appeared in issue 19, September 16, 2003, of Proc. Natl. Acad. Sci. USA (100, 10629-10634; first published September 8, 2003; 10.1073/pnas.1832879100), the authors note that the recently published structure of the Saccharomyces cerevisiae topoisomerase II ATPase region bound to adenosine 5′-[β,γ-imino]triphosphate (ADPNP) and ICRF-187 contains an error. The drugs ICRF-187 and ICRF-186 are the respective (S)- and (R)-specific enantiomers of the racemic compound ICRF-159 (1, 2). During model building of our drug-inhibited complex, we relied on coordinates downloaded from the National Cancer Institute database for ICRF-187. We now have discovered that this file actually contains the ICRF-186 stereoisomer, and that the structure of the drug bound to topoisomerase II was correspondingly modeled incorrectly. Because the electron density for the ethanediyl linker region of the drug that contains the chiral center is poorly defined, the mistake was not immediately evident.
We have now rebuilt and refined the correct (S)-enantiomeric ICRF-187 compound into the ATPase region (see corrected Fig. 5 and legend below). Compared with the original model containing (R)-ICRF-186, the new model with (S)-ICRF-187 alters the angle at which the single methyl group extends from the chiral center of the linker. Despite this change, the well defined electron density of the two dioxopiperazine rings constrains the ethanediyl linker of ICRF-187 to adopt a slightly different twist than observed previously, shifting the coordinate position of the methyl substituent by only ≈0.5 Å. As a consequence, the methyl-pi interaction postulated to exist between the ICRF-187 ethanediyl linker and Tyr28 is maintained. Other interactions observed between the drug and topoisomerase II are similarly unaffected, and all discussions and conclusions of the paper still stand. Indeed, both ICRF-186 and ICRF-187 inhibit topoisomerase II with virtually the same Ki values (2-4), an observation that can be explained by the absence of stereospecific interactions between these drugs and the enzyme. The new coordinates have been deposited in the Protein Data Bank, www.rcsb.org (PDB ID code 1QZR).
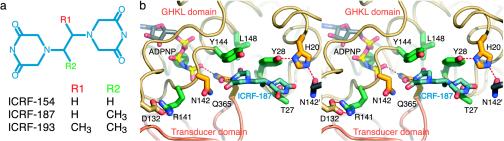
Fig. 5.
Details of bisdioxopiperazine compounds and the ICRF-187-binding site. (a) Schematic of different bisdioxopiperazine compounds. (b) Stereo figure of drug-binding site (compare with Fig. 5 of the original paper). One protomer has been removed for clarity. ADPNP, ICRF-187, and residues within 5 Å of the drug are shown in stick representation and colored as in the original paper.
References
- 1.Hempel, A., Camerman, N. & Camerman, A. (1982) J. Am. Chem. Soc. 104, 3453-3456. [Google Scholar]
- 2.Hasinoff, B. B., Kuschak, T. I., Yalowich, J. C. & Creighton, A. M. (1995) Biochem. Pharmacol. 50, 953-958. [DOI] [PubMed] [Google Scholar]
- 3.Hasinoff, B. B., Kuschak, T. I., Creighton, A. M., Fattman, C. L., Allan, W. P., Thampatty, P. & Yalowich, J. C. (1997) Biochem. Pharmacol. 53, 1843-1853. [DOI] [PubMed] [Google Scholar]
- 4.Snapka, R. M., Woo, S. H., Blokhin, A. V. & Witiak, D. T. (1996) Biochem. Pharmacol. 52, 543-549. [DOI] [PubMed] [Google Scholar]