Abstract
OBJECTIVE
To elucidate the mechanism of the unique beneficial effect of intravitreal steroid therapy on diabetic macular edema, we investigated the effect of locally administered triamcinolone acetonide (TA) on the expression of vascular endothelial growth factor (VEGF)-A and its receptors in retinas of rats with streptozotocin (STZ)-induced diabetes. We then correlated the expression of these proteins with breakdown of the blood-retinal barrier (BRB).
RESEARCH DESIGN AND METHODS
Thirty-two eyes of 16 diabetic and nondiabetic rats were divided into four groups. TA was injected into the vitreous of the right eye, and saline was injected into the left eye (control) 3.5 weeks after induction of diabetes. Retinas were harvested 48 h following treatment. mRNA and protein expression of VEGF-A, VEGF-A receptor 1 (fms-like tyrosine kinase [FLT]-1), and VEGF-A receptor 2 (fetal liver kinase [FLK]-1) were determined by real-time RT-PCR and immunohistochemistry. BRB permeability was quantitated by measuring extravasated endogenous albumin and retinal thickness.
RESULTS
Diabetes-induced retinal thickness and albumin extravasation were significantly reduced in TA-treated diabetic retinas to a level similar to that in sham-treated nondiabetic eyes. A close correlation between albumin leakage and increased expression of both Vegf-a and Flk-1 was noted in the diabetic retinas. TA downregulated the expression of Vegf-a and Flk-1 but upregulated the expression of Flt-1. TA did not alter the expression of these genes in nondiabetic retinas.
CONCLUSIONS
Intravitreal injection of TA stabilizes the BRB in association with regulation of Vegf-a, Flk-1, and Flt-1 expression in retinas in the early stages of diabetes.
As the prevalence of diabetes progressively rises throughout the world, improving the treatment of macular edema, a common cause of loss of vision from diabetic retinopathy (DR), has become a major goal of ophthalmic research (1). Laser treatment has been proven to reduce the risk of further loss of vision in patients with clinically significant macular edema but is less effective in restoring vision once it already has deteriorated. Moreover, laser treatment is an intrinsically destructive procedure that itself can lead to loss of vision through progressive enlargement of laser scars with time (2).
Breakdown of the blood-retinal barrier (BRB) is an early event in the pathogenesis of DR (3). When local compensatory mechanisms are overcome, frank vasogenic macular edema develops. The anatomical correlate responsible for the BRB is the tight junction-associated proteins between retinal vascular endothelial cells (inner BRB) and retinal pigment epithelial cells (outer BRB). It is strongly suggested that the inner BRB (rather than the outer BRB) is the primary site of the vascular leak that results in diabetic macular edema in both human and animal studies (4,5). In the retinas of rodents, the inner BRB has been shown to become permeable to high–molecular weight molecules in as little as 2 weeks after induction of diabetes (6).
Vascular endothelial growth factor (VEGF)-A/vascular permeability factor is believed to mediate increased vascular permeability and colocalize with extravasated albumin in major retinal diseases such as DR (7,8) and age-related macular degeneration (AMD) (9). VEGF-A activates two tyrosine-kinase receptors, fms-like tyrosine kinase-1 (FLT-1, vascular endothelial growth factor receptor-1), and fetal liver kinase-1 (FLK-1, vascular endothelial growth factor receptor-2) (10), which autophosphorylate to initiate a signaling cascade that results in increased microvascular permeability and angiogenesis (11). Inhibition of the VEGF-A overexpression is thus a potential treatment for DR and AMD (12,13).
Triamcinolone acetonide (TA) is an intermediate acting corticosteroid suspension. Intravitreal injections of TA (IVTA) have shown remarkable efficacy for the treatment of advanced DR, in particular diabetic macular edema (14,15). IVTA has been shown to inhibit VEGF-induced breakdown of the BRB in rabbit and leukostasis in rat models of DR (16,17). In a 2-year, double-masked, placebo-controlled, randomized clinical trial, eyes with recalcitrant diabetic macular edema involving the fovea that were treated with IVTA had twice the chance of improving vision and half the risk of further loss. However, a high incidence of cataract and elevated intraocular pressure was found in IVTA-treated eyes (18). If the unique beneficial effect of intravitreal steroid therapy on macular edema is to be distinguished from its high risk of local adverse events (glaucoma and cataract), the precise mechanism of action of TA needs to be elucidated. In this study, we determined the effect of IVTA on the expression of VEGF-A and its receptors in a rodent model of early DR.
RESEARCH DESIGN AND METHODS
Animals.
Male Sprague-Dawley (SD) rats (aged 8–10 weeks, ARC Laboratories, Perth, Australia), weighing 240–250 g, were used in this study. The animals were cared for in accordance with The Association for Research in Vision and Ophthalmology statement for the use of animals in ophthalmic and vision research. All animals were housed with environmental enrichment with free access to food and water. Experimental procedures were conducted under aseptic conditions and were approved by the Animal Ethics Committee of the University of Sydney.
Induction of diabetes.
Diabetes was induced with streptozotocin (STZ) (Sigma) in SD rats according to the published protocol with slight modifications (19,20). Rats were given a single dose of 65 mg/kg STZ i.p. in 100 mmol/l citrate buffer (pH 4.5); weight- and age-matched nondiabetic control rats received vehicle only. Eight animals were used per group for each set of experiments. After STZ injection, body weights were recorded daily, and diabetic status was confirmed by tail vein blood glucose estimation using an automated Accu-Chek glucometer (Roche Diagnostics, Indianapolis, IN) after 48 h. Rats with nonfasting blood glucose levels >13.8 mmol/ml were deemed diabetic; those with lower values were excluded from this study. Maintenance of a diabetic state was confirmed by weekly tail vein blood glucose measurements.
Treatment.
Intravitreal treatment with TA or saline was performed 3.5 weeks after induction of diabetes. Rats were divided into four experimental treatment groups:
Diabetic eyes with IVTA treatment (IVTA-treated diabetic retinas, n = 8, right eyes)
Diabetic eyes with sham treatment (sham-treated diabetic retinas, n = 8, left eyes)
Nondiabetic eyes with IVTA treatment (IVTA-treated nondiabetic retinas, n = 8, right eyes)
Nondiabetic eyes with sham treatment (sham-treated nondiabetic retinas, n = 8, left eyes).
Intravitreal injection of TA.
STZ-induced diabetic or nondiabetic animals were anesthetized by isoflurane inhalation and a 4:1 mixture of ketamine hydrochloride (80 mg/kg) and xylazine hydrochloride (4 mg/kg) i.m. Pupils were dilated with one drop each of 0.5% tropicamide and 2.5% phenylephrine hydrochloride. For additional topical anesthesia, 0.4% benoxinate-HCl of eye drop was applied followed by 0.5% of levofloxacin ophthalmic solution to prevent infection. A single dose of 56 μg TA (Kenacort-A 40, Victoria, Australia) in a volume of 5.6 μl (10 mg/ml) was injected into the vitreous of the right eye with a 32-gauge microinjector (Hamilton, Reno, NV) under a dissecting microscope (17). The contralateral eye, which received an equal volume of saline, served as a sham-treated control. A 30-gauge needle was used to make a punch incision 1 mm posterior to the temporal limbus, and a microinjector needle was then inserted through the incision, ∼1.5 mm deep, angled toward the optic nerve.
Preparation of retinas for histopathology/immunohistochemistry and real-time RT-PCR.
To detect VEGF-A, FLT-1, or FLK-1 antigen, the chest cavity was opened following anesthesia as described above. A 20-gauge perfusion cannula was introduced into the aorta via the left ventricle. Each rat was perfused with 60 ml PBS at 37°C to clear the retinal vessels then perfused with 40 ml 2% paraformaldehyde (PFA) in 0.1 mol/l phosphate buffer (PBS), pH 7.4, after achieving drainage from the right atrium. Eyes were collected and fixed in 2% PFA overnight at 4°C. The fixed eyes were processed routinely and embedded in paraffin wax. To quantitate BRB breakdown (immunochemical analysis of albumin leakage), rats were killed without perfusion.
For real-time RT-PCR studies, each retina was dissected and frozen immediately in liquid nitrogen after perfusion with 100 ml PBS. Retinas were stored at −80°C until extraction of RNA.
RNA isolation and characterization.
Total RNA was isolated from individual retinas using TRI reagent (Sigma) and purified with a RNeasy Mini kit (Qiagen, Victoria, Australia) according to the manufacturer's instructions. RNA concentrations and A260/A280 and A260/A230 ratios were measured using a NanoDrop spectrophotometer. RNA integrity was assessed with an Agilent 2100 Bioanalyzer using an Agilent RNA 6000 Nano LabChip kit (Agilent Technologies, Palo Alto, CA).
Two-step real-time RT-PCR.
Reverse transcription (RT) was carried out using a SuperScript III First-Stand Synthesis System for RT-PCR kit (Invitrogen, Australia). RNA (1 μg) was reverse transcribed using 2.5 μmol/l Oligo(dT) primer after DNase I treatment according to the manufacturer's instructions. A non-RT control (minus RT) was performed for each RNA sample to test genomic DNA contamination. The RT reaction was performed at 50°C for 50 min after denaturing at 65°C for 5 min. The RT reactions were terminated by incubation at 85°C for 5 min.
Real-time PCR was performed using a Platinum SYBR Green qPCR SuperMix-UDG kit (Invitrogen, Australia) on a Rotor-Gene 3000 real-time thermal cycler according to the manufacturer's instruction. The same real-time PCR cycling condition was used for all target genes: 50°C for 2 min (UDG reaction), 95°C for 2 min, 45 cycles of 95°C, 15 s; 60°C 20 s; 72°C 20 s. Melting curve analysis and agarose gel electrophoresis were performed to confirm the purity of the qPCR products. All samples were performed in duplicate, and replicate data were collected for further comparative analysis. The rat gene specific primer pairs used for PCR are listed in Table 1.
TABLE 1.
Primers and product size for real-time RT-PCR
| Target genes | GenBank accession no. | Forward primer | Reverse primer | Product size (bp) |
|---|---|---|---|---|
| Gapdh | NM_017008.3 | 5′- GAGCTGAATGGGAAGCTCAC-3′ | 5′-AAAGGTGGAGGAGTGGGAGT-3′ | 216 |
| β-actin | NM_031144.2 | 5′-AGCCATGTACGTAGCCATCC-3′ | 5′- CTCTCAGCTGTGGTGGTGAA−3′ | 228 |
| Vegf-a | NM_031836.1 | 5′-AGAAACCCAATGAAGTGGTG-3′ | 5′- ACTCCAGGGCTTCATCATTG-3′ | 177 |
| Flt-1 | NM_019306.1 | 5′- TCCCTCAGCCTACCATCAAG-3′ | 5′- GAGAGTCAGCCACCACCAAT-3′ | 207 |
| Flk-1 | NM_013062.1 | 5′-ACAGCATCACCAGCAGTCAG-3′ | 5′- CCAAGAACTCCATGCCCTTA-3′ | 168 |
The pairs of primers were designed according to rat gene sequences published in GenBank.
Relative quantification and statistical analysis.
The BestKeeper program (http://www.gene-quantification.info) was used to determine the better reference gene between two widely used housekeeping genes (Gapdh and β-actin) for our study.
The relative quantification analysis was performed using the Relative Expression Software Tool (REST-MCS). The statistical significance of variations in gene expression was analyzed by the pairwise fixed reallocation randomization test provided by the REST software. P values <0.05 were considered significant. Data are shown as means ± SEM.
Morphological analysis.
Retinal thickness was evaluated by using Image-Pro Plus (IPP) 4.5 (Media Cybernetics, Silver Spring, MD) on 5 μm hematoxylin and eosin–stained sections. The eyes were cut vertically through the center of the cornea and optic nerve, and both halves were embedded face down. Care was taken to keep this cutting angle consistent for all eyes measured for retinal thickness. Images of retinal sections were obtained, and the thickness of both the retinas and sclera was measured using IPP4.5 with a reference micrometer (Olympus, Australia) under the same conditions. Twenty lines were manually drawn by an observer masked to the status of the rat from which the eye was obtained, from the inner limiting membrane to the tips of the photoreceptor outer segments for each of 20 sequential fields. The mean scleral width was also calculated and found to be similar among the four groups, indicating that differences in retinal thickness were not due to systematic sampling error (not shown).
Immunohistochemical detection of retinal VEGF-A, FLK-1, FLT-1, and albumin.
Standard indirect immunohistochemical methods were used to detect leakage of albumin and the expression of VEGF-A, FLK-1, and FlT-1 (21). The paraffin-embedded retinal sections (5 μm) were dewaxed and dehydrated. The sections were then quenched with H2O2 blocker (Dako, Australia) for 10 min at room temperature and washed three times in Tris-buffered saline (TBS), pH 7.6.
For detection of albumin leakage in the retinas, the sections were incubated with sheep anti-human albumin polyclonal antibody (Abcam, Cambridge, U.K.) at a 1:500 dilution for 1 h at room temperature. The sections were further incubated with rabbit anti-sheep HRP-Streptavidin immunoglobulin (Abcam) for 30 min at room temperature after three washes with TBS. The sections were developed with 3-amino-9-ethylcarbazole chromogen (AEC; Dako) for 3 min and counterstained with hematoxylin.
For detection of VEGF-A, FLK-1, and FLT-1, the sections were treated with antigen retrieval solution (Dako) at 90°C for 10 min, subsequently cooled to room temperature, and then quenched with H2O2 blocker. Sections were incubated with rabbit anti-human polyclonal primary antibody [VEGF-A (1:100); FLK-1 (1:200) or FLT-1 (1:50) antibody] (Abcam) for 1 h at room temperature. The sections were further incubated with goat anti-rabbit HRP-Streptavidin immunoglobulin (Abcam) for 30 min at room temperature. Slides were developed with diaminobenzidine (DAB; Dako) for 5 min and counterstained with hematoxylin.
An isotype-matched negative control was used for all the antibodies (data not shown).
Quantification and statistical analysis of immunohistochemical staining.
The peroxidase activity was visualized using DAB. The numbers of VEGF-A–, FLT-1 –, and FLK-1–positive stained cells were counted per high- power field (400×) by using IPP4.5 as described previously (22,23). Briefly, the threshold of positive signal was defined for each antibody for all the sections following different treatments. Color signal above the threshold for each antibody defined was deemed to be positive, whereas any signal below the threshold was regarded as negative. Analyses consisted of 20 fields per retina. The entire retina was measured with reference to a micrometer (Olympus) under the same condition sequentially. The number of positive signals versus total cell numbers within each field of the retina was determined with IPP 4.5 and was expressed as positive cell numbers per millimeter, as described previously (24).
Extravasated albumin was visualized with AEC Chromogen. The threshold of positive staining of albumin was defined for all sections before automate counting. The intravascular space was excluded by the analysis. The ratio of the area of positive signal versus the total retinal area in each field was determined with IPP 4.5 and expressed as image arbitrary units (IAUs). All images analyzed with IPP 4.5 were evaluated by an experienced masked histopathologist.
Statistics for immunostaining.
All data are presented as means ± SEM. One-way ANOVA was performed for statistical analysis (GraphPad Prism Version 4.0, San Diego, CA). Differences between group means with P values of <0.05 were regarded as being statistically different.
RESULTS
Retinal gene transcription of Vegf-a, Flk-1, and Flt-1.
Total RNA yield and quality.
The average yields of total RNA extracted from retinas in different groups were comparable. The ratios of A260/A280 and A260/A230 for all RNA samples were above 2 (2.10 ± 0.005 and 2.03 ± 0.002, respectively), indicating limited protein and organic contaminants. RNA integrity number (RIN), obtained automatically by the Agilent 2100 Expert software, was similar between the diabetic and nondiabetic groups (8.7 ± 0.2 vs. 8.4 ± 0.01, P = 0.88).
A single PCR fragment was obtained for each gene-specific primer pair with the expected length as determined by 1.5% agarose gel electrophoresis. This was confirmed with a unique peak in the melting curve analysis for each real-time PCR reaction (data not shown).
β-actin was selected as a reference gene for this study based on the results obtained from BestKeeper software (Tables 2 and 3).
TABLE 2.
Results from BestKeeper descriptive statistical analysis showing variation in the capability potential (CP) values
| Factor | n | GM (CP) | AM (CP) | Min (CP) | Max (CP) | SD (±CP) | CV (%CP) |
|---|---|---|---|---|---|---|---|
| Gapdh | 32 | 19.75 | 19.88 | 16.06 | 16.50 | 1.66 | 8.35 |
| β-actin | 32 | 14.01 | 14.03 | 13.00 | 15.80 | 0.59 | 4.17 |
Housekeeping gene expressions were assessed on total RNA templates isolated from retinas. GM (CP), the geometric mean of CP; AM (CP), the arithmetic mean of CP; Min (CP) and Max (CP), the extreme values of CP; SD (±CP), the SD of the CP; CV (%CP), the coefficient of variance expressed as a percentage of the CP level.
TABLE 3.
Results from BestKeeper correlation analysis
| BestKeeper vs. | GAPDH | β-actin |
|---|---|---|
| r | 0.909 | 0.815 |
| P | 0.001 | 0.003 |
Measures of correlation between the two candidate gene expressions and the Bestkeeper in an index computed from the best candidate genes.
Relative changes in Vegf-a, Flk-1, and Flt-1 mRNA expression.
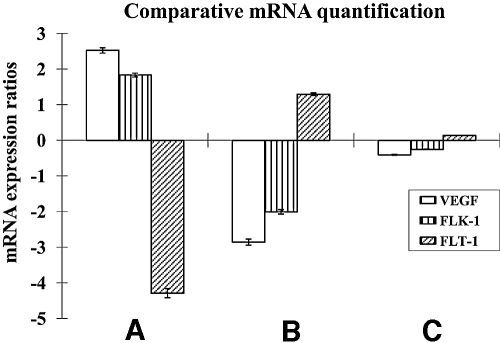
Diabetes and TA treatment differentially regulated the transcription of Vegf-a and its receptors. Transcription of Vegf-a and Flk-1 was significantly increased by 2.5- and 1.8-fold (PVegf-a = 0.001 and PFlk-1 = 0.031), respectively, but Flt-1 was downregulated 4.3-fold (PFlt-1 = 0.005) in sham-treated diabetic retinas compared with sham-treated nondiabetic retinas (Fig. 1A). Vegf-a and Flk-1 transcription was significantly downregulated 2.9- and 2.0-fold, respectively (PVegf-a = 0.030 and PFlk-1 = 0.028); however, Flt-1 was significantly upregulated 1.3-fold (PFlt-1 = 0.002) with IVTA treatment (Fig. 1B). There was no statistically significant difference in the transcription of Vegf-a and Flk-1 or Flt-1 between sham-treated and IVTA-treated nondiabetic groups (PVegf-a = 0.519, PFlk-1 = 0.679, and PFlt-1 = 0.909) (Fig. 1C).
FIG. 1.
mRNA expression ratios for Vegf-a, Flk-1 and Flt-1. A: Sham-treated diabetic retina/sham-treated nondiabetic retina. B: IVTA-treated diabetic retina/sham-treated diabetic retina. C: IVTA-treated nondiabetic retina/sham-treated nondiabetic retina.
Retinal protein expression of VEGF-A, FLK-1, and FLT-1.
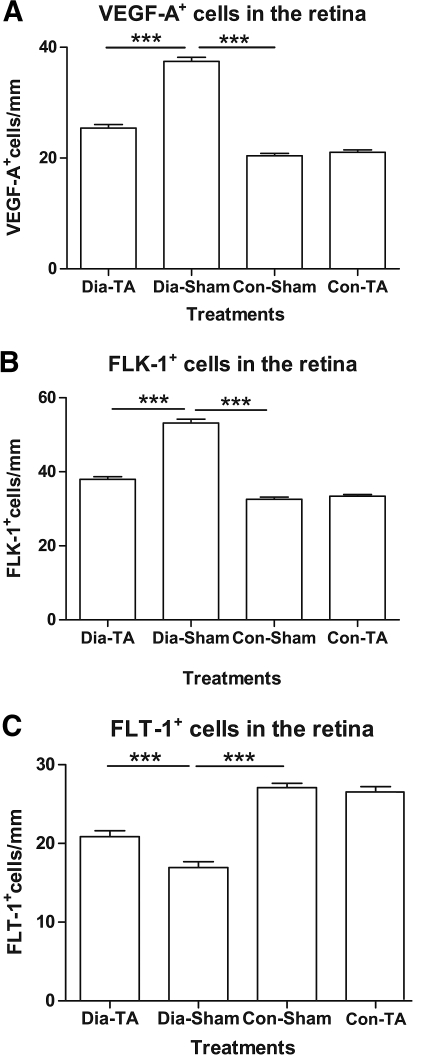
The expression of VEGF-A and its two receptors (FLK-1 and FLT-1) was found predominantly in the ganglion cell layer, inner nuclear layer, and outer plexiform layer of all four treatment groups. In nondiabetic retinas, immunoreactivity against VEGF and FLK-1 was weak in these layers in contrast to that in the diabetic retinas. The expression of VEGF-A was increased by 1.8-fold in the sham-treated diabetic retinas (37.44 ± 0.74 vs. 20.40 ± 0.44 positive cells/mm, P < 0.0001) compared with sham-treated nondiabetic retinas. The number of VEGF-A–positive cells was reduced by 32.1% in the IVTA-treated diabetic retinas in contrast to the sham-treated diabetic retinas (25.41 ± 0.62 vs. 37.44 ± 0.74 positive cells/mm, P < 0.001) (Fig. 2A). Similarly, FLK-1 expression was increased by 1.6-fold in the sham-treated diabetic retinas compared with that in sham-treated nondiabetic retinas (53.17 ± 1.08 vs. 32.95 ± 0.47 positive cells/mm, P < 0.0001). The number of FLK-1–positive cells was significantly decreased by 28.5% in the diabetic retinas with IVTA treatment compared with that in sham-treated diabetic retinas (37.99 ± 0.71 vs. 53.17 ± 1.08 positive cells/mm, P < 0.0001) (Fig. 2B). In contrast to the elevated levels of VEGF and FLK-1, FLT-1 expression was reduced by 37.5% in diabetic retinas (16.94 ± 0.76 vs. 27.09 ± 0.56 positive cells/mm, P < 0.0001) (Fig. 2C). The number of FLT-1–positive cells in IVTA-treated diabetic retinas increased by 1.2-fold compared with that in the sham-treated diabetic retinas (20.87 ± 0.73 vs. 16.94 ± 0.76 positive cells/mm, P = 0.0002) (Fig. 2C) but was still significantly less than both TA-treated or sham-treated nondiabetic retinas (20.87 ± 0.73 vs. 26.53 ± 0.67 positive cells/mm; 20.87 ± 0.73 vs. 27.09 ± 0.56 positive cells/mm, P < 0.0001 for both). No significant difference was detected in the expression of VEGF-A and its receptors (FLK-1 and FLT-1) between IVTA-treated and sham-treated nondiabetic retinas (PVEGF-A = 0.2641, PFLK-1 = 0.4792, and PFLT-1 = 0.5222) (Fig. 2A–C).
FIG. 2.
Quantification analysis of VEGF-A, FLK-1, and FLT-1 distribution in the retina from different treatment groups. Number of positive cells for VEGF-A (A), FLK-1 (B), and FLT-1 (C) per millimeter (means ± SEM, n = 8). ***P < 0.001.
Retinal thickness analysis.
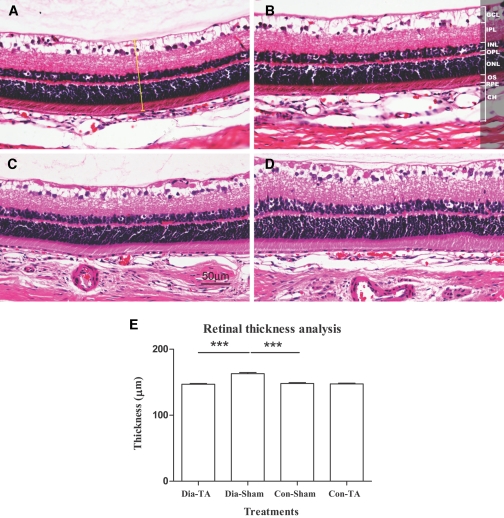
There was no significant difference in retinal thickness between IVTA- and sham-treated control eyes (147.28 ± 1.23 vs. 147.60 ± 1.40 μm, P = 0.8627) (Fig. 3A and B). There was a 10% reduction in the thickness of the IVTA-treated diabetic retinas compared with the sham-treated diabetic retinas (148.20 ± 1.06 vs. 163.25 ± 1.78 μm, P < 0.001) (Fig. 3C–E). Sham-treated diabetic retinas were significantly thicker than sham-treated nondiabetic retinas (163.25 ± 1.78 vs. 147.60 ± 1.40 μm, P < 0.001) (Fig. 3E).
FIG. 3.
Retinal thickness was measured on H&E-stained sections at the similar location. The micrograph shows representative samples from IVTA-treated nondiabetic retina (A), sham-treated nondiabetic retina (B), IVTA-treated diabetic retina (C), and sham-treated diabetic retina (D). The yellow bar in A demonstrates one measurement of retinal thickness. E: Table showing mean (± SEM) retinal thickness (*** P < 0.001). Original magnifications ×200. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; RPE, retinal pigment epithelium layer; OS, inner and outer photoreceptor segment; CH, choroidal layer. To view a high-quality digital representation of this image, go to http://dx.doi.org/db07-0982.
The effect of IVTA on albumin leakage.
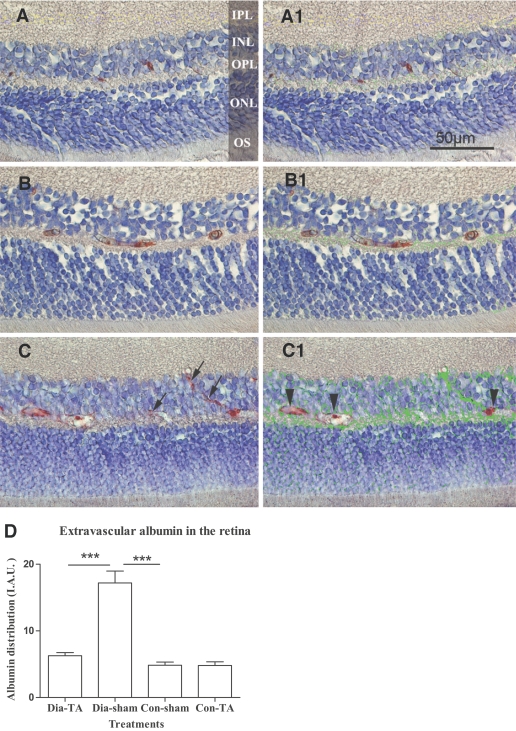
Extravascular albumin was scarcely detected in the inner nuclear layer, outer nuclear layer, and outer plexiform layer of nondiabetic retinas (Fig. 4A and A1). In IVTA-treated and sham-treated diabetic rats, albumin leakage was observed mainly in the inner nuclear, outer plexiform, and outer nuclear layers (Fig. 4B, B1, C, and C1). There was 3.6-fold higher albumin expression in the sham-treated diabetic retinas than the nondiabetic controls (17.20 ± 1.78 vs. 4.84 ± 1.58 IAU, P < 0.0001) (Fig. 4D). However, albumin expression in the extravascular space was significantly reduced by 63.5% in IVTA-treated diabetic retinas compared with that in sham-treated diabetic retinas (6.27 ± 0.46 vs. 17.20 ± 1.78 IAU, P < 0.0001) (Fig. 4D). There was no difference between the IVTA-treated nondiabetic retinas versus sham-treated nondiabetic retinas (4.81 ± 1.58 vs. 4.84 ± 1.48 IAU, P = 0.9590) (Fig. 4D).
FIG. 4.
Immunohistochemical analysis of retinal albumin distribution. Albumin was detected (in red) within the vessels in sham-treated nondiabetic retinas (A). A similar pattern of albumin distribution was observed in IVTA-treated diabetic retina, but with slightly higher expression in extra vascular space (B). Diffuse extravascular albumin was observed in the inner nuclear, outer plexiform, and outer nuclear layers in sham-treated diabetic retina (C). Extravasated albumin (arrows in C) was marked green in panel A1, B1, and C1 (corresponding to panels A, B, and C) by IPP4.5. Intravascular albumin was excluded by the analysis (arrow heads in C1). D: Quantification of albumin distribution/leakage in the four treatment groups. Values were presented as IAU. Data are means ± SEM, n = 8; *** P < 0.001. Original magnifications ×400. To view a high-quality digital representation of this image, go to http://dx.doi.org/db07-0982.
DISCUSSION
In this study, we have demonstrated that IVTA inhibits the increased expression of Vegf-a and Flk-1 in early diabetic rat retinas. By contrast, Flt-1 expression was decreased in early diabetic retinas but was upregulated following IVTA treatment. A strong correlation of albumin leakage and retinal thickness with the expression of Vegf-a and its receptors was observed. These findings indicate that one likely mechanism of action of IVTA on diabetic macular edema is through the differential regulation of VEGF-A and its receptors.
VEGF-A is a major regulator of angiogenesis and vascular permeability in development and injury. The involvement of one of its receptors, FLK-1, in angiogenesis has been widely demonstrated (11), but few studies have addressed its role as a mediator of the BRB permeability. Ojima et al. (25) demonstrated that VEGF-induced FLK-1 phosphorylation and its downstream signaling cascade correlated with BRB breakdown and was inhibited by EphA2/ephrinA1 both in vitro and in vivo. In our study, the direct relationship between the expression of FLK-1 and the vascular permeability/breakdown of the BRB was further investigated in the diabetic retina. The overexpression of VEGF-A and FLK-1 that was found in the diabetic retina in this study, in association with increased albumin leakage, supports the hypothesis that FLK-1 is another important permeability factor and regulator of BRB dysfunction in diabetes. These data are consistent with a report that FLK-1 plays a critical role in breakdown of the brain barrier (26).
The expression of another VEGF receptor, FLT-1, was unexpectedly decreased in diabetic retinas and increased following IVTA treatment. This may be a result of competition between FLK-1 and FLT-1. FLT-1 on endothelial cells may function as a negative regulator of FLK-1, acting as a so-called “decoy” receptor that regulates the activity of VEGFs on vascular endothelium by sequestering VEGF-A and thus rendering it less available to bind to FLK-1 (27). It is speculated that triggering of FLT-1 induces weak or undetectable ligand-dependent tyrosine phosphorylation in porcine aortic endothelial cells, whereas FLK-1 binding induces a strong response (28). Furthermore, VEGF-A mutants that bind selectively to FLK-1 are able to induce mitogenesis and chemotaxis in endothelial cells, and vascular permeability and angiogenesis in vivo, whereas VEGF-A mutants that selectively bind to FLT-1 do not have the activities stated above (29,30). In contrast to our finding, increased expression of Flt-1 in the 6-month STZ-induced diabetic rat retinas was reported by Hammes et al. (31). In their study, increased Flt-1 mRNA levels detected by in situ hybridization were found in the ganglion cell and both nuclear layers of diabetic samples only. This apparent discrepancy may be explained by the different durations of diabetes studied.
Diabetic macular edema is consistently reduced after IVTA in human clinical trials, as is sensitively demonstrated by Optical Coherence Tomography (32). In this study, the reduction of VEGF-A levels by IVTA treatment that we observed is consistent with a reduction in levels of extravasated albumin from the retinal vessels. This provides strong evidence that anti-VEGF therapy is an effective strategy for diabetic BRB dysfunction. Indeed, a recent phase II study suggested that the VEGF-A inhibitor, pegaptanib sodium, may also be efficacious in the treatment of diabetic macular edema (12).
Two hypotheses have been proposed to explain the clinically established beneficial effect of corticosteroids on diabetic macular edema and the restoration of the BRB. Firstly, corticosteroids may inhibit VEGF-A signaling by blocking cytokine production, inducing leukocyte apoptosis (33), and, secondly, corticosteroids can directly induce the barrier properties of interendothelial tight junction complexes (34). A recent study suggests that corticosteroids may have relatively rapid effects, such as a decrease in paracellular flux and an increase in transepithelial resistance, which correlates with an increase in tight junction immunoreactivity at the cell border. A concomitant decrease in occludin phosphorylation, potentially countering the effects of VEGF-A, has also been noted (35). Steroid treatment also appears to increase the expression of at least some tight junction-associated proteins (36).
By providing a deeper understanding of the mechanisms of action of intravitreal steroids on retinal disease, the results of this study may be used to refine their clinical use. In a placebo-controlled, randomized clinical trial, IVTA treatment doubled the chance of vision improvement and halved the risk of further loss in eyes with advanced diabetic macular edema. However, there was a significant risk of local adverse events, with 55% of eyes requiring surgery to remove cataract over 2 years and 44% requiring eye drops to control elevated intraocular pressure (18). Anti-VEGF antibodies are another potential treatment for diabetic retinopathy. Ranibizumab (37,38) and pegaptanib sodium (13) have already been approved in the U.S. for treatment of subretinal neovascularization; they are currently in advanced clinical development for diabetic retinopathy, and more specific VEGF inhibitors are in the pipeline. Presently, one of the disadvantages of these drugs is that they need to be injected every 4–6 weeks, as compared with every 6 months for triamcinolone (18). However, it is likely that longer-acting preparations will appear in due course. While VEGF inhibitors do not cause corticosteroid-associated side effects, they may confer an increased risk of systematic vascular adverse events (39). There is also a suspicion that VEGF inhibitors may eventually cause retinal atrophy by blocking the cytokine's recently discovered neuroprotective action (40). On the other hand, an increase in systemic adverse events has not been detected in patients receiving intravitreal steroid therapy, and there is some evidence to suggest that steroids may have a protective effect on photoreceptors (41), which could potentially offset any neurotoxic effect it might have through inhibiting VEGF-A. How the known adverse events of steroids will balance against the as yet largely unknown safety profile of specific VEGF inhibitors is a subject that warrants careful clinical research in future, as does the issue as to whether a long-acting steroid combined with a specific VEGF inhibitor in low doses might deliver the safest and most efficacious clinical response.
The integrity of the BRB can be assessed by various techniques, each of which has advantages and limitations (42). Extravasated albumin has been demonstrated to be a precise indicator of the permeability of the blood-retinal and blood-brain barriers (21,43). IPP is a sensitive image analysis system that has been used to quantify subtle changes in a number of studies (24,44). In this study, immunolocalization of extravasated albumin was identified at the light microscopic level and quantified using IPP, providing a useful technique with which to study the effect of IVTA on DR.
Rodent models do not mimic all aspects of human diabetes, but such models are essential for the development of new therapeutic approaches. While the short duration of diabetic injury in this model (3.5 weeks) imposes some limitations on the interpretation of the data, similar findings relating to the function of VEGF-A and its receptors on the BRB breakdown have also been observed in humans with diabetes, providing justification for our studies in rodents (45).
This study adduces data that support the hypothesis that intravitreal corticosteroid treatment improves diabetic retinal edema by regulating the pathological expression of VEGF-A and its receptors. We also show that IVTA does not alter physiological VEGF-A, FLK-1, and FLT-1 levels in the normal retina. This raises the possibility that combination therapy of low-dose anti-VEGF drugs and steroids may offer advantages over anti-VEGF drugs alone for the treatment of DR that is resistant to conventional management.
ACKNOWLEDGMENTS
X.Z. is supported by a “University of Sydney–China Scholarship Council” scholarship. The study was also supported by the Lowy Medical Research Institute.
Preliminary data were presented at the Australian Ophthalmic and Vision Science Meeting, December 2005.
The authors appreciate the technical assistance provided by the Histopathology Laboratory, Discipline of Pathology, University of Sydney.
M.C.G. is included as an inventor on patents relating to the formulation of triamcinolone for ocular use and its use for the treatment of retinal neovascularization, but not macular edema. M.C.G. is an advisor and receives research support from Allergan (Posurdex).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
- AMD
- age-related macular degeneration
- AEC
- 3-amino-9-ethylcarbazole chromogen
- BRB
- blood-retinal barrier
- DAB
- diaminobenzidine
- DR
- diabetic retinopathy
- FLK
- fetal liver kinase
- FLT
- fms-like tyrosine kinase
- IAU
- image arbitrary unit
- IPP
- Image-Pro Plus
- IVTA
- intravitreal injection of triamcinolone acetonide
- PFA
- paraformaldehyde
- qPCR
- quantitative polymerase chain reaction
- REST
- relative expression software tool
- STZ
- streptozotocin
- TA
- triamcinolone acetonide
- TBS
- Tris-buffered saline
- UDG
- uracil-DNA glycosylase
- VEGF
- vascular endothelial growth factor.
REFERENCES
- 1.Kohner EM: Diabetic retinopathy and high blood pressure: defining the risk. Am J Hypertens 10:181S–183S, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Group ETDRSR: Effects of asprin treatment on diabetic retinopathy ETDRS report number 8. Opthalmology 98 (Suppl.):757–765, 1991 [PubMed] [Google Scholar]
- 3.Sander B, Larsen M, Moldow B, Lund-Andersen H: Diabetic macular edema: passive and active transport of fluorescein through the blood-retina barrier. Invest Ophthalmol Vis Sci 42:433–438, 2001 [PubMed] [Google Scholar]
- 4.Engler CB, Sander B, Larsen M, Koefoed P, Parving HH, Lund-Andersen H: Probenecid inhibition of the outward transport of fluorescein across the human blood-retina barrier. Acta Ophthalmol (Copenh) 72:663–667, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Vinores SA, McGehee R, Lee A, Gadegbeku C, Campochiaro PA: Ultrastructural localization of blood-retinal barrier breakdown in diabetic and galactosemic rats. J Histochem Cytochem 38:1341–1352, 1990 [DOI] [PubMed] [Google Scholar]
- 6.Xu Q, Qaum T, Adamis AP: Sensitive blood-retinal barrier breakdown quantitation using Evans blue. Invest Ophthalmol Vis Sci 42:789–794, 2001 [PubMed] [Google Scholar]
- 7.Qaum T, Xu Q, Joussen AM, Clemens MW, Qin W, Miyamoto K, Hassessian H, Wiegand SJ, Rudge J, Yancopoulos GD, Adamis AP: VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci 42:2408–2413, 2001 [PubMed] [Google Scholar]
- 8.Mathews MK, Merges C, McLeod DS, Lutty GA: Vascular endothelial growth factor and vascular permeability changes in human diabetic retinopathy. Invest Ophthalmol Vis Sci 38:2729–2741, 1997 [PubMed] [Google Scholar]
- 9.Schwesinger C, Yee C, Rohan RM, Joussen AM, Fernandez A, Meyer TN, Poulaki V, Ma JJ, Redmond TM, Liu S, Adamis AP, D'Amato RJ: Intrachoroidal neovascularization in transgenic mice overexpressing vascular endothelial growth factor in the retinal pigment epithelium. Am J Pathol 158:1161–1172, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mukhopadhyay D, Nagy JA, Manseau EJ, Dvorak HF: Vascular permeability factor/vascular endothelial growth factor-mediated signaling in mouse mesentery vascular endothelium. Cancer Res 58:1278–1284, 1998 [PubMed] [Google Scholar]
- 11.Cébe-Suarez AZ-FaKB-H: The role of VEGF receptors in angiogenesis; complex partnerships. Cell Mol Life Sci 63:601–615, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunningham ET, Jr, Adamis AP, Altaweel M, Aiello LP, Bressler NM, D'Amico DJ, Goldbaum M, Guyer DR, Katz B, Patel M, Schwartz SD: A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology 112:1747–1757, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Apte RS, Modi M, Masonson H, Patel M, Whitfield L, Adamis AP: Pegaptanib 1-year systemic safety results from a safety-pharmacokinetic trial in patients with neovascular age-related macular degeneration. Ophthalmology 114:1702–1712, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Jonas JB, Kreissig I, Sofker A, Degenring RF: Intravitreal injection of triamcinolone for diffuse diabetic macular edema. Arch Ophthalmol 121:57–61, 2003 [PubMed] [Google Scholar]
- 15.Martidis A, Duker JS, Greenberg PB, Rogers AH, Puliafito CA, Reichel E, Baumal C: Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology 109:920–927, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Edelman JL, Lutz D, Castro MR: Corticosteroids inhibit VEGF-induced vascular leakage in a rabbit model of blood-retinal and blood-aqueous barrier breakdown. Exp Eye Res 80:249–258, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Tamura H, Miyamoto K, Kiryu J, Miyahara S, Katsuta H, Hirose F, Musashi K, Yoshimura N: Intravitreal injection of corticosteroid attenuates leukostasis and vascular leakage in experimental diabetic retina. Invest Ophthalmol Vis Sci 46:1440–1444, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Gillies MC, Sutter FKP, Simpson JM, Larsson J, Ali H, Zhu M: Intravitreal triamcinolone for refractory diabetic macular edema: two-year results of a double-masked, placebo-controlled, randomized clinical trial. Ophthalmology 113:1533–1538, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Murata TNK, Khalil A, Ishibashi T, Inomata H, Sueishi K: The relation between expression of vasucular endothelial growth factor and breakdown of blood-retinal barrier in diabetic rat retina. Lab Invest 74:819–825, 1996 [PubMed] [Google Scholar]
- 20.Miyamoto K, Hiroshiba N, Tsujikawa A, Ogura Y: In vivo demonstration of increased leukocyte entrapment in retinal microcirculation of diabetic rats. Invest Ophthalmol Vis Sci 39:2190–2194, 1998 [PubMed] [Google Scholar]
- 21.Murata TIT, Inomata H: Immunohistochemical detection of blood-retinal barrier breakdown in steptozotocin-diabetic rats. Graefe's Archive for Clinical and Experimental Ophthalmology 231:175–177, 1993 [DOI] [PubMed] [Google Scholar]
- 22.Nicholls SJ, Cutri B, Worthley SG, Kee P, Rye KA, Bao S, Barter PJ: Impact of short-term administration of high-density lipoproteins and atorvastatin on atherosclerosis in rabbits. Arterioscler Thromb Vasc Biol 25:2416–2421, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Francisco JS, Moraes HP, Dias EP: Evaluation of the Image-Pro Plus 4.5 software for automatic counting of labeled nuclei by PCNA immunohistochemistry. Pesqui Odontol Bras 18:100–104, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Ramsay AJ, Husband AJ, Ramshaw IA, Bao S, Matthaei KI, Koehler G, Kopf M: The role of interleukin-6 in mucosal IgA antibody responses in vivo. Science 264:561–563, 1994 [DOI] [PubMed] [Google Scholar]
- 25.Ojima T, Takagi H, Suzuma K, Oh H, Suzuma I, Ohashi H, Watanabe D, Suganami E, Murakami T, Kurimoto M, Honda Y, Yoshimura N: EphrinA1 inhibits vascular endothelial growth factor-induced intracellular signaling and suppresses retinal neovascularization and blood-retinal barrier breakdown. Am J Pathol 168:331–339, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lafuente JV, Argandona EG, Mitre B: VEGFR-2 expression in brain injury: its distribution related to brain-blood barrier markers. J Neural Transm 113:487–496, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO: Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res 22:1–29, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH: Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem 269:26988–26995, 1994 [PubMed] [Google Scholar]
- 29.Gille H, Kowalski J, Li B, LeCouter J, Moffat B, Zioncheck TF, Pelletier N, Ferrara N: Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2): a reassessment using novel receptor-specific vascular endothelial growth factor mutants. J Biol Chem 276:3222–3230, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Kliffen M, Sharma HS, Mooy CM, Kerkvliet S, de Jong PT: Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol 81:154–162, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammes HP, Lin J, Bretzel RG, Brownlee M, Breier G: Upregulation of the vascular endothelial growth factor/vascular endothelial growth factor receptor system in experimental background diabetic retinopathy of the rat. Diabetes 47:401–406, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Gillies MC, Sutter FK, Simpson JM, Larsson J, Ali H, Zhu M: Intravitreal triamcinolone for refractory diabetic macular edema: two-year results of a double-masked, placebo-controlled, randomized clinical trial. Ophthalmology 113:1533–1538, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Barnes PJ: Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci (Lond) 94:557–572, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Felinski EA, Antonetti DA: Glucocorticoid regulation of endothelial cell tight junction gene expression: novel treatments for diabetic retinopathy. Curr Eye Res 30:949–957, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Antonetti DA, Barber AJ, Hollinger LA, Wolpert EB, Gardner TW: Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1: a potential mechanism for vascular permeability in diabetic retinopathy and tumors. J Biol Chem 274:23463–23467, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Antonetti DA, Wolpert EB, DeMaio L, Harhaj NS, Scaduto RC, Jr.: Hydrocortisone decreases retinal endothelial cell water and solute flux coincident with increased content and decreased phosphorylation of occludin. J Neurochem 80:667–677, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Heier JS, Antoszyk AN, Pavan PR, Leff SR, Rosenfeld PJ, Ciulla TA, Dreyer RF, Gentile RC, Sy JP, Hantsbarger G, Shams N: Ranibizumab for treatment of neovascular age-related macular degeneration: a phase I/II multicenter, controlled, multidose study. Ophthalmology 113:642, e641–644, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Fung AE, Lalwani GA, Rosenfeld PJ, Dubovy SR, Michels S, Feuer WJ, Puliafito CA, Davis JL, Flynn HW, Jr, Esquiabro M: An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol 143:566–583, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Gillies MC, Tien YW: Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 356:747–750, 2007 [PubMed] [Google Scholar]
- 40.van Wijngaarden P, Coster DJ, Williams KA: Inhibitors of ocular neovascularization: promises and potential problems. JAMA 293:1509–1513, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Wenzel A, Grimm C, Seeliger MW, Jaissle G, Hafezi F, Kretschmer R, Zrenner E, Reme CE: Prevention of photoreceptor apoptosis by activation of the glucocorticoid receptor. Invest Ophthalmol Vis Sci 42:1653–1659, 2001 [PubMed] [Google Scholar]
- 42.Vinores S: Assessment of blood-retinal barrier integrity. Histol Histopath 10:141–154, 1995 [PubMed] [Google Scholar]
- 43.Bahcekapili N, Uzum G, Gokkusu C, Kuru A, Ziylan YZ: The relationship between erythropoietin pretreatment with blood-brain barrier and lipid peroxidation after ischemia/reperfusion in rats. Life Sci 80:1245–1251, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Nicholls SJ, Dusting GJ, Cutri B, Bao S, Drummond GR, Rye KA, Barter PJ: Reconstituted high-density lipoproteins inhibit the acute pro-oxidant and proinflammatory vascular changes induced by a periarterial collar in normocholesterolemic rabbits. Circulation 111:1543–1550, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Lip PL, Chatterjee S, Caine GJ, Hope-Ross M, Gibson J, Blann AD, Lip GY: Plasma vascular endothelial growth factor, angiopoietin-2, and soluble angiopoietin receptor tie-2 in diabetic retinopathy: effects of laser photocoagulation and angiotensin receptor blockade. Br J Ophthalmol 88:1543–1546, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]