Abstract
Malfunction of mitochondrial complex I caused by nuclear gene mutations causes early-onset neurodegenerative diseases. Previous work using cultured fibroblasts of complex-I-deficient patients revealed elevated levels of reactive oxygen species (ROS) and reductions in both total Ca2+ content of the endoplasmic reticulum (ERCa) and bradykinin(Bk)-induced increases in cytosolic and mitochondrial free Ca2+ ([Ca2+]C; [Ca2+]M) and ATP ([ATP]C; [ATP]M) concentration. Here, we determined the mitochondrial membrane potential (Δψ) in patient skin fibroblasts and show significant correlations with cellular ROS levels and ERCa, i.e., the less negative Δψ, the higher these levels and the lower ERCa. Treatment with 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox) normalized Δψ and Bk-induced increases in [Ca2+]M and [ATP]M. These effects were accompanied by an increase in ERCa and Bk-induced increase in [Ca2+]C. Together, these results provide evidence for an integral role of increased ROS levels in complex I deficiency and point to the potential therapeutic value of antioxidant treatment.
Keywords: Reactive oxygen species, Oxidative stress, Mitochondrial membrane potential, Endoplasmic reticulum Ca2+ content, Mitochondrial disease, Antioxidant, Human skin fibroblasts
Introduction
Mitochondria are double membrane-bound organelles that do not only constitute the ‘cellular power plants’ but also are crucially involved in survival, apoptosis, redox control, Ca2+ homeostasis, and many metabolic and biosynthetic pathways [1]. To fulfill these diverse functions, mitochondria require a highly negative membrane potential across their inner membrane (∆ψ), which is maintained by the action of the three proton pumps (complexes I, III, and IV) of the electron transport chain. Together with F0/F1-ATP-synthase (complex V), which uses the resulting electrochemical proton gradient to generate ATP, and complex II of the electron transport chain, which oxidizes FADH2, these proton pumps constitute part of the oxidative phosphorylation (OXPHOS) system, which accounts for the majority of cellular ATP production.
Complex I (EC 1.6.5.3) is the largest OXPHOS complex and clinical presentations associated with its deficiency (MIM 252010) are heterogeneous ranging from severe multi-system diseases with neonatal death (e.g., Leigh Disease) to isolated myopathies [2]. Complex I consists of seven subunits encoded by the mitochondrial DNA and 38 by that of the nucleus [3]. As to the nuclear genome, disease-causing mutations have been identified in the genes of core subunits NDUFV1, NDUFV2, NDUFS1, NDUFS2, NDUFS3, NDUFS7, and NDUFS8 [4–9], supernumerary subunits NDUFS4, NDUFS6, NDUFA11, and NDUFA2 [10–13], and complex I assembly factors NDUFAF2, NDUFAF1, and C6orf66 [14–16].
Detailed knowledge of the cytopathological consequences of complex I deficiency is prerequisite to understanding the clinical presentations and their heterogeneity, and developing therapeutic strategies [17]. To gain this knowledge, we study skin fibroblasts from patients with mutations in nuclear complex I genes. We demonstrated that these mutations can be associated with a reduction in Ca2+ content of the endoplasmic reticulum (ERCa; [18, 19]). Because OXPHOS depends on the electrochemical proton gradient, the latter might be altered in patient cells, leading to a reduced supply of ATP to ER Ca2+-pumps, thus explaining the observed reduction in resting ERCa. In agreement with this idea, we showed before that chronic inhibition of complex I by rotenone reduced ∆ψ and decreased ERCa in healthy fibroblasts [20]. We furthermore showed that a reduction in ERCa was paralleled by decreased amplitudes of the bradykinin (Bk)-induced increases in [Ca2+]C, [Ca2+]M, [ATP]C and [ATP]M [19]. Finally, we demonstrated that these aberrations were accompanied by a decrease in the rate of cytosolic Ca2+ removal during the decay phase of the Bk-induced [Ca2+]C transient [19]. Because Bk stimulates the release of Ca2+ from the ER into the cytosol from which Ca2+ is taken up by mitochondria to stimulate the OXPHOS process, these data suggest that the reduction in resting ERCa is responsible for the decrease in Bk-stimulated mitochondrial ATP production, which, in turn, results in a lower cytosolic Ca2+ removal rate. Evidence comes from experiments with, on the one hand, an inhibitor of mitochondrial Na+–Ca2+ exchange, which was shown to normalize both the Bk-induced increase in [ATP]M and the rate of cytosolic Ca2+ removal in patient fibroblasts [18], and on the other hand a mitochondrial Ca2+ buffer, which decreased both parameters in fibroblasts of a healthy individual [21].
Apart from alterations in intracellular Ca2+ handling we observed elevated levels of reactive oxygen species (ROS) in fibroblasts of patients with a nuclear complex I gene defect [22, 23]. Because elevated ROS levels have been associated with a less negative ∆ψ [24, 25], and because a less negative ∆ψ might decrease mitochondrial ATP-dependent Ca2+ uptake by the ER (see above), we here investigated the relationship between ∆ψ, elevated ROS levels and ERCa in these patient fibroblasts.
Materials and methods
Cell lines
Fibroblasts were obtained following informed parental consent and according to the relevant Institutional Review Boards from skin biopsies of four healthy subjects and ten patients in whom an isolated complex I deficiency was confirmed in both muscle tissue and cultured fibroblasts (Table 1). Activity measurements were performed as described previously [26]. The activity of the complex was normalized against that of cytochrome c oxidase, which was in the control range, and expressed as percentage of the lowest control (0.11 mU/mU cytochrome c oxidase; [26]). All patients were negative with respect to mitochondrial DNA alterations previously associated with complex I deficiency. Fibroblasts were cultured in HEPES (25 mM)-buffered M199 medium (Invitrogen) supplemented with 10% (v/v) fetal calf serum, 5 mg/l Tween 20, 100 IU/ml penicillin and 100 IU/ml streptomycin.
Table 1.
Characteristics of control and patient fibroblasts
| Cell linea | Mutation | CI act.b | Mean TMRM pixel intensityc | ER Ca2+ contentd | Rate of CM-DCF formatione | |
|---|---|---|---|---|---|---|
| Gene | Protein | |||||
| C-5120 | None | None | 113 | 100 ± 0.2 (N = 1033) | 100 ± 1 (N = 81) | 100 ± 2 (N = 412) |
| C-4996 | None | None | N.d. | 100 ± 0.7 (N = 134) | N.d. | N.d. |
| C-5118 | None | None | 103 | 99 ± 0.7 (N = 115) | 102 ± 8 (N = 20) | 125 ± 13 (N = 61) |
| C-5119 | None | None | 105 | 99 ± 1.1 (N = 65) | 102 ± 6 (N = 15) | 100 ± 11 (N = 48) |
| S1-6173 | NDUFS1 | R557X/D618N | 31 | 92 ± 0.8 (N = 120) | 82 ± 4 (N = 29) | 171 ± 13 (N = 87) |
| S2-5067 | NDUFS2 | P229Q | 36 | 94 ± 1.2 (N = 105) | 94 ± 4 (N = 70) | 351 ± 41 (N = 28) |
| S2-5170 | NDUFS2 | R228Q | 39 | 99 ± 0.6 (N = 126) | 95 ± 2 (N = 23) | 169 ± 7 (N = 124) |
| S4-4608 | NDUFS4 | K158fs | 75 | 87 ± 0.8 (N = 112) | 96 ± 4 (N = 44) | 289 ± 46 (N = 29) |
| S4-5260 | NDUFS4 | R106X | 36 | 92 ± 0.9 (N = 110) | 77 ± 4 (N = 40) | 187 ± 12 (N = 160) |
| S4-5737 | NDUFS4 | VPEEH167/VEKSIstop | 53 | 95 ± 0.6 (N = 160) | 87 ± 6 (N = 22) | 202 ± 12 (N = 98) |
| S7-5175 | NDUFS7 | V122M | 68 | 91 ± 0.4 (N = 384) | 73 ± 2 (N = 16) | 212 ± 15 (N = 76) |
| S8-6603 | NDUFS8 | R94C | 18 | 89 ± 0.8 (N = 121) | 85 ± 6 (N = 17) | 275 ± 20 (N = 117) |
| V1-5866 | NDUFV1 | R59X/T423M | 64 | 94 ± 0.8 (N = 68) | 87 ± 3 (N = 43) | 135 ± 12 (N = 49) |
| V1-5171 | NDUFV1 | R59X/T423M | 73 | 92 ± 1 (N = 104) | 96 ± 3 (N = 48) | 242 ± 19 (N = 78) |
Statistics: values significantly different from control C-5120 (p < 0.05) are depicted in italics
N indicates the number of cells analyzed, N.d. not determined, C indicates control cells
aNumerals indicate the designation of the cell lines within the Nijmegen Center for Mitochondrial Disorders (NCMD)
bDetermined relative to complex IV and expressed as percentage of lowest control [35]
cExpressed as % of the value for control C-5120
dERCa, expressed as % of the value for control C-5120 [23, 25]
Fluorescence imaging of ∆ψ
∆ψ was measured by digital imaging microscopy of fibroblasts loaded with tetramethyl rhodamine methyl ester (TMRM) as described in detail before [27]. During measurements, cells were maintained in a HEPES–Tris medium (pH 7.4) containing 132 mM NaCl, 4.2 mM KCl, 5.5 mM d-glucose, 1 mM MgCl2, 1 mM CaCl2 and 10 mM HEPES.
Luminescence monitoring of [Ca2+]M and [ATP]M
[Ca2+]M and [ATP]M were determined by luminometry of fibroblasts transduced with a baculovirus encoding mitochondrial targeted forms of wild-type aequorin (mtAEQ) and luciferase (mtLUC) as described previously [18, 19, 21]. During [ATP]M measurements, cells were superfused with HEPES/Tris medium containing 5 μM beetle luciferin (Promega, Madison, WI). Prior to [Ca2+]M measurements, mtAEQ was reconstituted with 5 μM native coelenterazine (Promega) in serum-free M199 medium for 1 h at 37°C. The wild-type aequorin-native coelenterazine pair measures Ca2+ concentrations in the 0.1–10 µM range and mtAEQ photon emission was converted off-line to Ca2+ concentration by using of a computer algorithm based on the Ca2+ response curve of wild-type aequorin [28]. Bk was added through the superfusion medium.
Fluorescence imaging of [Ca2+]C
[Ca2+]C was measured by digital imaging microscopy of fibroblasts loaded with fura-2 as previously described [18, 19, 21]. Bk, applied through the superfusion medium, evoked a transient increase in fura-2 fluorescence emission ratio. The rate of Ca2+ removal was determined by fitting the decay phase of the transient to a mono-exponential equation as described before [18, 19, 21].
Determination of ERCa
ERCa was determined by digital imaging microscopy of fibroblasts loaded with fura-2 as described before [18, 19, 21]. Briefly, cells were challenged with 2′,5′-di(tert-butyl)-1,4-benzohydroquinone (BHQ; 10 µM), a specific inhibitor of the ER Ca2+ ATPase, in the absence of external Ca2+ (no Ca2+ added to the extracellular medium and in the presence of 0.5 mM EGTA). Under these conditions, Ca2+ that passively leaks out of the ER is not resequestered, resulting in a rapid increase in [Ca2+]C. The peak increase in fluorescence emission ratio after treatment with BHQ was used as a measure of ERCa.
Chemicals and statistics
Culture material and TMRM were from Invitrogen (Breda, The Netherlands). Trolox was from Fluka (Buchs, Switzerland). All other reagents were from Sigma (Zwijndrecht, the Netherlands). Values from multiple experiments were expressed as average ± S.E.M and statistical significances were assessed by Student's t-test.
Results
∆ψ is reduced in complex-I-deficient patient fibroblasts
Using our recently developed method for microscopic quantification of ∆ψ in cultured human skin fibroblasts [13, 27, 29], we here show that ∆ψ is variably decreased in fibroblast lines of ten complex-I-deficient patients compared to four healthy individuals (Fig. 1a). For each cell line, the average TMRM intensity per mitochondrial pixel is expressed relative to that of C-5120 healthy fibroblasts measured on the same day. Linear regression analysis revealed the absence of any correlation between ∆ψ and residual complex I activity.
Fig. 1.
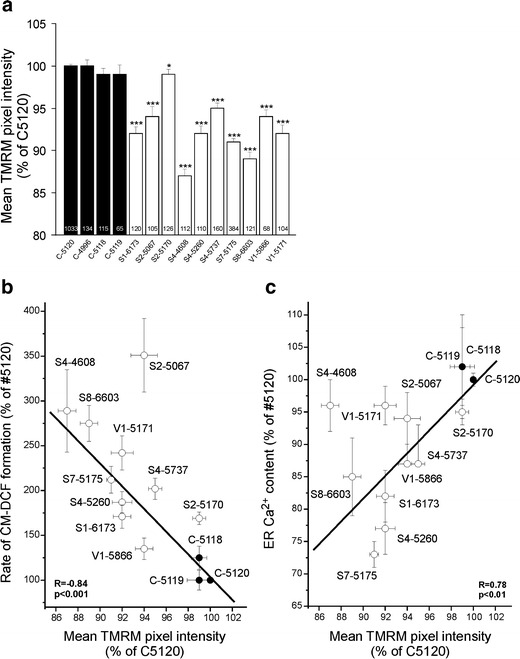
Mitochondrial membrane potential is reduced in complex-I-deficient patient fibroblasts and correlates with cellular ROS levels and ER Ca2+ content. a Mean TMRM pixel intensity in healthy and complex-I-deficient human skin fibroblasts. In each experiment, the mean value obtained with healthy control fibroblasts (C-5120) was set at 100%, to which all values were related. Significant differences with C-5120 are indicated by *p < 0.05 and ***p < 0.001. Error bars indicate SEM. Numerals within bars indicate the number of individual cells analyzed. b Linear relationship between mean TMRM pixel intensity and cellular reactive oxygen species (ROS) levels, indicated by the rate of oxidative formation of fluorescent 5-(and -6)-chloromethyl-2′,7′-dichlorofluorescein (CM-DCF), for fibroblast lines of three healthy subjects (C-5120, C-5118, C-5119; closed circles) and ten patients (S7-5175, S2-5170, S8-6603, S1-6173, S2-5737, V1-5171, V1-5866, S4-4608, S4-5260, S2-5067; open circles). c Linear relationship between mean TMRM pixel intensity and endoplasmic reticulum Ca2+ content (ERCa). CM-DCF and ERCa data were taken from [19, 20, 23, 31]
∆ψ is related to cellular ROS levels and ERCa
We showed previously that cellular ROS levels are elevated to a variable extent in our whole cohort of complex-I-deficient patients (Table 1; [22, 23]). Linear regression analysis revealed a negative correlation between ROS levels and ∆ψ (Fig. 1b). We have also shown that, similar to cellular ROS levels, ERCa is variably decreased in these patient fibroblasts (Table 1; [19]). Again a significant, this time positive correlation was observed (Fig. 1c).
Chronic Trolox treatment normalizes ∆ψ
Based on the significant correlation between ROS levels and ∆ψ, as shown in Fig. 1b, we concluded that lowering of oxidant levels may restore the electrochemical proton gradient across the inner mitochondrial membrane. We previously demonstrated that chronic treatment of patient fibroblasts with Trolox, a water-soluble analogue of vitamin E, reduced ROS levels and increased the amount of fully assembled complex I [30]. Figure 2 shows that chronic Trolox (300 µM, 72 h) fully restored ∆ψ in S4-5260 and S7-5175 patient cells, which were among those displaying the largest decrease in ∆ψ (Fig. 1a). Trolox did not affect ∆ψ in healthy fibroblasts.
Fig. 2.
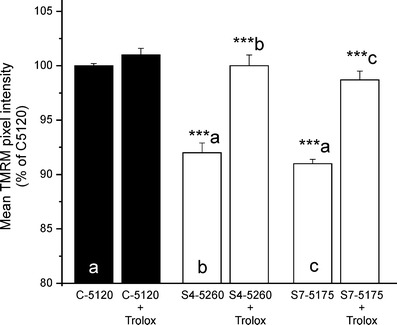
Trolox treatment restores the mitochondrial membrane potential in complex-I-deficient patient fibroblasts. Mean TMRM pixel intensity in healthy (C-5120; black bars) and patient (S4-5260, S7-5175; open bars) fibroblasts cultured for 72 h in the absence or presence of Trolox (300 µM). In each experiment, the mean value obtained with vehicle-treated healthy fibroblasts was set at 100%, to which all values were related. (a) Significantly different from vehicle-treated C-5120. (b) Significantly different from vehicle-treated S4-5260. (c) Significantly different from vehicle-treated S7-5175. The values presented are the mean ± SEM of 100–200 individual cells. (***p < 0.001)
Chronic Trolox treatment dose-dependently increases ERCa
A reduced ∆ψ may have severe consequences for mitochondrial energy metabolism and thus for cellular processes, such as ER Ca2+ uptake, that depend hereupon. This prompted us to investigate the possibility that Trolox may restore ERCa in our patient fibroblasts. Indeed, chronic Trolox (72 h) dose-dependently increased ERCa to ∼135% of the value obtained with untreated C-5120 healthy cells in both these and S4-5260 patient fibroblasts (Fig. 3). The half-maximal effect was reached at ∼85 µM Trolox. In sharp contrast, acute application of Trolox (500 µM; 30 min) neither changed ERCa in healthy (n = 22 cells) nor increased it in patient (n = 21 cells) fibroblasts (data not shown). The S4-5260 patient cell line was selected because it was among those with the largest decrease in ∆ψ and ERCa.
Fig. 3.
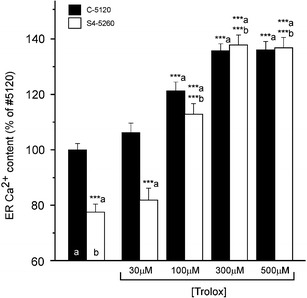
Trolox treatment dose-dependently increases the ER Ca2+ content in complex-I-deficient patient fibroblasts and healthy fibroblasts. Endoplasmic reticulum Ca2+ content (ERCa) in healthy (C-5120; open bars) and patient (S4-5260; black bars) fibroblasts cultured for 72 h in the absence or presence of the indicated concentration of Trolox. For each experimental condition, the values presented are the average ± SEM of 20–50 fibroblasts. (a) Significantly different from vehicle-treated healthy fibroblasts. (b) Significantly different from corresponding vehicle-treated patient fibroblasts (***p < 0.001)
Chronic Trolox treatment restores aberrant Ca2+ and ATP handling during bradykinin stimulation
In a final series of experiments, we assessed the effect of chronic Trolox on the Bk-induced increase in [Ca2+]C, the ensuing increases in [Ca2+]M and [ATP]M and the rate of cytosolic Ca2+ removal. Figure 4a shows that chronic Trolox dose-dependently increased the amplitude of the Bk (1 µM)-induced increase in [Ca2+]C to ∼125% of that in untreated C-5120 healthy cells in both these and S4-5260 patient cells. The half-time of Ca2+ removal (t 1/2) decreased from 22.5 ± 0.8 s (n = 28 cells) and 14.6 ± 0.6 s (n = 28 cells) for untreated and Trolox-treated patient fibroblasts, respectively. This means that Trolox also normalized the slower cytosolic Ca2+ removal rate in patient cells. Trolox did not alter t 1/2 in healthy fibroblasts (t 1/2 = 14.2 ± 0.8 s and 14.5 ± 0.6 s for 24 untreated and 31 Trolox-treated healthy cells, respectively).
Fig. 4.
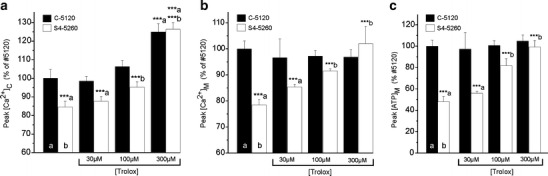
Trolox treatment restores Ca2+ and ATP handling in patient fibroblasts. a Dose-dependence of the effect of Trolox treatment for 72 h on the Bk-induced increase in [Ca2+]C in healthy (C-5120; closed bars) and patient (S4-5260; open bars) fibroblasts. b The same for [Ca2+]M and c the same for [ATP]M. The values presented are the mean ± SEM of 20–30 cells (a) or five to 15 coverslips (b and c). For each parameter, the mean value obtained with vehicle-treated healthy fibroblasts was set at 100%, to which all values were related. (a) Significantly different from vehicle-treated C-5120. (b) Significantly different from corresponding vehicle-treated S4-5260 (***p < 0.001)
Chronic Trolox did not affect the Bk-induced peak increase in [Ca2+]M in healthy fibroblasts (Fig. 4b). In S4-5260 patient cells, however, chronic Trolox dose-dependently increased this parameter to the same value as in untreated healthy cells. Chronic Trolox was also without affect on the Bk-induced peak [ATP]M increase in healthy cells, whereas it normalized this parameter in patient cells (Fig. 4c).
Discussion
NADH:ubiquinone oxidoreductase or complex I forms a major entry point of electrons into the mitochondrial OXPHOS system and its malfunction is associated with a wide variety of clinical presentations [2]. To understand the cellular mechanisms underlying these syndromes, we study cultured skin fibroblasts of a unique cohort of patients with inherited complex I deficiency of nuclear origin [20, 31, 32]. One of our most striking findings is that the amount of fully assembled complex I is markedly decreased in patient fibroblasts, indicating that in addition to a likely decrease in intrinsic catalytic activity also its assembly and/or stability is compromised [22, 30, 32]. Other important findings include increased ROS levels and disturbances of Ca2+ and ATP homeostasis both at rest and during stimulation [20, 31]. It is presently unknown how these changes are exactly brought about in these patient fibroblasts.
Here, we show that ∆ψ is significantly reduced in our cohort of patient fibroblast lines. At first glance, the most likely explanation would be a decrease in proton translocation. However, the OXPHOS system is most probably not working at its maximum capacity in resting fibroblasts and may, therefore, very well be able to maintain ∆ψ even when the maximum capacity of its first complex is decreased due to a lower expression and/or decreased intrinsic catalytic activity. Given the strong correlation between ROS levels and ∆ψ, i.e., the higher these levels, the less negative ∆ψ, the above finding could also be explained in that increased ROS levels induce a proton leak [33]. The relevance of the consequent ∆ψ decrease would be to try and prevent excessive ROS production. We also show that ∆ψ and ERCa are correlated, i.e., the less negative ∆ψ, the lower ERCa. Because ∆ψ is an important determinant for the rate of mitochondrial ATP production, this result is in agreement with our idea that fueling of ER Ca2+-pumps with mitochondrial ATP is reduced in resting patient fibroblasts. Importantly, our previous results argue against the idea that the reduction in ERCa is due to increased ROS-induced Ca2+ leak [18].
The other major finding is that chronic Trolox normalizes ∆ψ in patient fibroblasts without affecting this parameter in healthy cells. This result is consistent with the above-described mechanism in which elevated ROS levels cause an increase in mitochondrial proton leak. Additional support comes from work demonstrating that Trolox is an efficient inhibitor of such a proton leak [34]. Normalization of ∆ψ likely restores cellular processes that are disturbed by its disease-associated reduction. Among these processes, adequate delivery of mitochondrial ATP to ER Ca2+-pumps is of crucial relevance. Strikingly, chronic Trolox increased ERCa in both healthy and patient fibroblasts. Although the increase in healthy cells was unexpected in view of the lack of effect of chronic Trolox on ∆ψ, it should be noted that the increase in ERCa observed with 100 µM Trolox was much more pronounced in patient cells, suggesting a dual mechanism involving normalization of ∆ψ (only in patient cells) and modification of a hitherto unknown process (both in healthy and patient cells). Such a dual mechanism is consistent with the idea that elevated ROS levels hamper proper fueling of ER Ca2+-pumps primarily by their effect on ∆ψ. Future research will reveal whether the ∆ψ-independent mechanism involves a decrease in passive Ca2+ leak, an increase in Ca2+ buffering capacity in the lumen of the ER and/or an increase in glycolytic ATP-dependent Ca2+ uptake.
The present result that the effect of chronic Trolox on ERCa was paralleled by a similar effect on the Bk-induced increase in [Ca2+]C is consistent with the linear relationship between both parameters in our cohort of patient fibroblast lines [19]. Similar to our finding, Trolox enhanced the bombesin-induced increase in [Ca2+]C in skin fibroblasts of patients with genetic and non-genetic forms of Alzheimer's disease [35]. Although chronic Trolox markedly enhanced the Bk-induced increase in [Ca2+]C in both healthy and patient fibroblasts, it did not affect the consequent increase in [Ca2+]M in healthy cells, whereas it dose-dependently normalized this parameter in patient cells. Calibration of the mtAEQ signal revealed that Bk increased [Ca2+]M to ∼4.5 µM [18, 19, 21], whereas the maximum Ca2+ concentration that can be measured is 10 µM [28]. Therefore, the results obtained suggest that the capacity of mitochondria to take up Ca2+ is restricted to a maximum that is reached with 1 µM Bk and cannot be exceeded even when the Bk-induced increase in [Ca2+]C is further increased by the action of Trolox. Additional evidence for restricted Ca2+ uptake capacity was obtained in experiments with an inhibitor of mitochondrial Na+–Ca2+ exchange. As expected, this inhibitor, CGP-37157, did not alter the Bk-induced increase in [Ca2+]C, whereas it normalized the Bk-induced increase in [Ca2+]M in patient cells [18, 21]. Strikingly, CGP-37157 was without effect in healthy control cells, which is in agreement with the idea that 1 µM Bk maximally increases [Ca2+]M in healthy fibroblasts.
In agreement with the widely accepted notion that hormone-induced increases in [Ca2+]M stimulate mitochondrial ATP production [20], we show that chronic Trolox can normalize this process in patient fibroblasts without affecting it in healthy fibroblasts. Chronic Trolox normalized the rate of cytosolic Ca2+ removal during the decay phase of the Bk-induced [Ca2+]C transient in patient fibroblasts without affecting this parameter in healthy cells. The same observation was reached using CGP-37157 [18, 21], whereas the opposite was observed with the mitochondrial Ca2+ buffer Rhod-2 [21]. These results, together with the fact that the Bk-induced increase in [ATP]M is tightly correlated with the rate of cytosolic Ca2+ removal for the whole cohort of patient fibroblasts [19], clearly link these two processes.
In conclusion, the present results support the idea that fueling with mitochondrial ATP of cytosolic processes that are stimulated by hormone-induced increases in [Ca2+]C, such as exocytotic release of neurotransmitters in nerve cells and contraction and relaxation of muscle cells, may be hampered in complex I deficiency. It is these two cell types that are among the most vulnerable in this disease [2]. However, the most important conclusion of this study is that the aberrations in Ca2+-stimulated mitochondrial ATP production and the consequences thereof, such as observed in complex I deficiency, can be overcome with drugs that normalize the stimulus-induced increase in [Ca2+]M by either blocking mitochondrial Ca2+ export (CGP-37157) or increasing mitochondrial Ca2+ uptake (Trolox). It remains an important future task to translate this cellular information to clinical practice. Because patients with inborn errors of mitochondrial metabolism present with variable disease onset and a broad range of clinical symptoms, disease-adapted or even individualized treatment strategies might be required. In this context, Trolox and CGP-37157 constitute interesting new candidates to be evaluated as treatment options.
Acknowledgements
This work was supported by grants of the Netherlands Organization for Health Research and Development (903-46-176), the Netherlands Organization for Scientific Research (911-02-008) and the European Union's sixth Framework Program for Research, Priority 1 ‘Life sciences, genomics and biotechnology for health’ (LSHM-CT-2004-503116).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- Δψ
mitochondrial membrane potential
- Bk
bradykinin
- CI
complex I or NADH:ubiquinone oxidoreductase
- OXPHOS
oxidative phosphorylation
- ROS
reactive oxygen species
- TMRM
tetramethyl rhodamine methyl ester
- ER
endoplasmic reticulum
- ERCa
ER total Ca2+ content
- [Ca2+]C and [Ca2+]M
cytosolic and mitochondrial free Ca2+ concentration
- [ATP]M
mitochondrial ATP concentration
Footnotes
Felix Distelmaier and Henk-Jan Visch contributed equally to this paper.
Change history
6/10/2021
A Correction to this paper has been published: 10.1007/s00109-021-02057-3
References
- 1.Duchen MR. Mitochondria in health and disease: perspectives on a new mitochondrial biology. Mol Aspects Med. 2004;25:365–451. doi: 10.1016/j.mam.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Smeitink JAM, van den Heuvel L, DiMauro S. The genetics and pathology of oxidative phosphorylation. Nature Rev Genet. 2001;2:342–352. doi: 10.1038/35072063. [DOI] [PubMed] [Google Scholar]
- 3.Carroll J, Fearnley IM, Skehel JM, Shannon RJ, Hirst J, Walker JE. Bovine complex I is a component of 45 different subunits. J Biol Chem. 2006;281:32724–32727. doi: 10.1074/jbc.M607135200. [DOI] [PubMed] [Google Scholar]
- 4.Schuelke M, Smeitink J, Mariman E, Loeffen J, Plecko B, Trijbels F, Stöckler-Ipsiroglu S, van den Heuvel L. Mutant NDUFV1 subunit of complex I causes leukodystrophy and myoclonic epilepsy. Nature Genet. 1999;21:260–261. doi: 10.1038/6772. [DOI] [PubMed] [Google Scholar]
- 5.Loeffen JL, Smeitink J, Triepels R, Smeets R, Schuelke M, Sengers R, Trijbels F, Hamel B, Mullaart R, van den Heuvel L. The first nuclear-encoded complex I mutation in a patient with Leigh syndrome. Am J Hum Genet. 1998;63:1598–1608. doi: 10.1086/302154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Triepels RH, van den Heuvel LP, Loeffen JL, Buskens CA, Smeets RJ, Rubio Gozalbo ME, Mariman EC, Wijburg FA, Barth PG, Trijbels JM, Smeitink JA. Leigh syndrome associated with a mutation in the NDUFS7 (PSST) nuclear encoded subunit of complex I. Ann Neurol. 1999;45:787–790. doi: 10.1002/1531-8249(199906)45:6<787::AID-ANA13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Benit P, Chretien D, Kadhom N, de Lonlay-Debeney P, Cormier-Daire V, Cabral A, Peudenier S, Rustin P, Munnich A, Rotig A. Large-scale deletion and point mutations of the nuclear NDUFV1 and NDUFS1 genes in mitochondrial complex I deficiency. Am J Hum Genet. 2001;68:1344–1352. doi: 10.1086/320603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benit P, Beugnot R, Chretien D, Giurgea I, De Lonlay-Debeney P, Issartel JP, Corral-Debrinski M, Kerscher S, Rustin P, Rotig A, Munnich A. Mutant NDUFV2 subunit of mitochondrial complex I causes early onset hypertrophic cardiomyopathy and encephalopathy. Hum Mutat. 2003;21:582–586. doi: 10.1002/humu.10225. [DOI] [PubMed] [Google Scholar]
- 9.Benit P, Slama A, Cartault F, Giurgea I, Chretien D, Lebon S, Marsac C, Munnich A, Rotig A, Rustin P. Mutant NDUFS3 subunit of mitochondrial complex I causes Leigh syndrome. J Med Genet. 2004;41:14–17. doi: 10.1136/jmg.2003.014316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van den Heuvel L, Ruitenbeek W, Smeets R, Gelman-Kohan Z, Elpeleg O, Loeffen J, Trijbels F, Mariman E, de Bruijn D, Smeitink J. Demonstration of a new pathogenic mutation in human complex I deficiency: a 5-bp duplication in the nuclear gene encoding the 18-kD (AQDQ) subunit. Am J Hum Genet. 1998;62:262–268. doi: 10.1086/301716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirby DM, Salemi R, Sugiana C, Ohtake A, Parry L, Bell KM, Kirk EP, Boneh A, Taylor RW, Dahl HH, Ryan MT, Thorburn DR. NDUFS6 mutations are a novel cause of lethal neonatal mitochondrial complex I deficiency. J Clin Invest. 2004;114:837–845. doi: 10.1172/JCI20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berger I, Hershkovitz E, Shaag A, Edvardson S, Saada A, Elpeleg O. Mitochondrial complex I deficiency caused by a deleterious NDUFA11 mutation. Ann Neurol. 2008;63:405–408. doi: 10.1002/ana.21332. [DOI] [PubMed] [Google Scholar]
- 13.Hoefs SJ, Dieteren CE, Distelmaier F, Janssen RJ, Epplen A, Swarts HG, Forkink M, Rodenburg RJ, Nijtmans LG, Willems PH, Smeitink JA, van den Heuvel LP. NDUFA2 complex I mutation leads to Leigh disease. Am J Hum Genet. 2008;82:1306–1315. doi: 10.1016/j.ajhg.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogilvie I, Kennaway NG, Shoubridge EA. A molecular chaperone for mitochondrial complex I assembly is mutated in a progressive encephalopathy. J Clin Invest. 2005;115:2784–2792. doi: 10.1172/JCI26020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunning CJ, McKenzie M, Sugiana C, Lazarou M, Silke J, Connelly A, Fletcher JM, Kirby DM, Thorburn DR, Ryan MT. Human CIA30 is involved in the early assembly of mitochondrial complex I and mutations in its gene cause disease. EMBO J. 2007;26:3227–3237. doi: 10.1038/sj.emboj.7601748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saada A, Edvardson S, Rapoport M, Shaag A, Amry K, Miller C, Lorberboum-Galski H, Elpeleg O. C6ORF66 is an assembly factor of mitochondrial complex I. Am J Hum Genet. 2008;82:32–38. doi: 10.1016/j.ajhg.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smeitink JAM, Zeviani M, Turnbull DM, Jacobs HT. Mitochondrial medicine: a metabolic perspective on the pathology of oxidative phosphorylation disorders. Cell Metab. 2006;3:9–13. doi: 10.1016/j.cmet.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Visch HJ, Rutter GA, Koopman WJ, Koenderink JB, Verkaart S, de Groot T, Varadi A, Mitchell KJ, van den Heuvel LP, Smeitink JA, Willems PHGM. Inhibition of mitochondrial Na+–Ca2+ exchange restores agonist-induced ATP production and Ca2+ handling in human complex I deficiency. J Biol Chem. 2004;279:40328–40336. doi: 10.1074/jbc.M408068200. [DOI] [PubMed] [Google Scholar]
- 19.Visch HJ, Koopman WJ, Leusink A, van Emst-de Vries SE, van den Heuvel LP, Willems PH, Smeitink JA. Decreased agonist-stimulated mitochondrial ATP production caused by a pathological reduction in endoplasmic reticulum calcium content in human complex I deficiency. Biochim Biophys Acta. 2006;1762:115–123. doi: 10.1016/j.bbadis.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Willems PH, Valsecchi F, Distelmaier F, Verkaart S, Visch HJ, Smeitink JA, Koopman WJ. Mitochondrial Ca2+ homeostasis in human NADH:ubiquinone oxidoreductase deficiency. Cell Calcium. 2008;44:123–133. doi: 10.1016/j.ceca.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Visch HJ, Koopman WJ, Zeegers D, van Emst-de Vries SE, van Kuppeveld FJ, van den Heuvel LP, Smeitink JA, Willems PH. Ca2+ mobilizing agonists increase mitochondrial ATP production to accelerate cytosolic Ca2+ removal: aberrations in human complex I deficiency. Am J Physiol Cell Physiol. 2006;291:C308–C316. doi: 10.1152/ajpcell.00561.2005. [DOI] [PubMed] [Google Scholar]
- 22.Verkaart S, Koopman WJH, van Emst-de Vries SE, Nijmans LGJ, van den Heuvel LWPJ, Smeitink JAM, Willems PHGM. Superoxide production is inversely related to complex I activity in inherited complex I deficiency. Biochim Biophys Acta. 2007;1772:373–381. doi: 10.1016/j.bbadis.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Verkaart S, Koopman WJH, Cheek J, van Emst-de Vries SE, van den Heuvel LWPJ, Smeitink JAM, Willems PHGM. Mitochondrial and cytosolic thiol redox state are not detectably altered in isolated human NADH:ubiquinone oxidoreductase deficiency. Biochim Biophys Acta. 2007;1772:1041–1051. doi: 10.1016/j.bbadis.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Echtay KS, Murphy MP, Smith RA, Talbot DA, Brand MD. Superoxide activates mitochondrial uncoupling protein 2 from the matrix side. Studies using targeted antioxidants. J Biol Chem. 2002;277:47129–47135. doi: 10.1074/jbc.M208262200. [DOI] [PubMed] [Google Scholar]
- 25.Brookes PS. Mitochondrial H+ leak and ROS generation: an odd couple. Free Radic Biol Med. 2005;38:12–23. doi: 10.1016/j.freeradbiomed.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 26.Smeitink JAM, Sengers R, Trijbels F, van den Heuvel L. Human NADH:ubiquinone oxidoreductase. J Bioenerg Biomembr. 2001;33:259–266. doi: 10.1023/A:1010743321800. [DOI] [PubMed] [Google Scholar]
- 27.Distelmaier F, Koopman WJ, Testa ER, de Jong AS, Swarts HG, Mayatepek E, Smeitink JA, Willems PH. Life cell quantification of mitochondrial membrane potential at the single organelle level. Cytometry A. 2008;73A:129–138. doi: 10.1002/cyto.a.20503. [DOI] [PubMed] [Google Scholar]
- 28.Brini M, Marsault R, Bastianutto C, Alvarez J, Pozzan T, Rizzuto R. Transfected aequorin in the measurement of cytosolic Ca2+ concentration ([Ca2+]C). A critical evaluation. J Biol Chem. 1995;270:9896–9903. doi: 10.1074/jbc.270.17.9896. [DOI] [PubMed] [Google Scholar]
- 29.Komen JC, Distelmaier F, Koopman WJ, Wanders RJ, Smeitink J, Willems PH. Phytanic acid impairs mitochondrial respiration through protonophoric action. Cell Mol Life Sci. 2007;64:3271–3281. doi: 10.1007/s00018-007-7357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koopman WJH, Verkaart S, van Emst-de Vries SE, Grefte S, Smeitink JA, Nijtmans LG, Willems PH. Mitigation of NADH: Ubiquinone oxidoreductase deficiency by chronic Trolox treatment. Biochim Biophys Acta. 2008;1777:853–859. doi: 10.1016/j.bbabio.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 31.Koopman WJH, Verkaart S, Visch H, van Emst-de Vries SE, Nijtmans LGJ, Smeitink JAM, Willems PGHM. Human NADH:ubiquinone oxidoreductase deficiency: radical changes in mitochondrial morphology? Am J Physiol Cell Physiol. 2007;293:C22–C29. doi: 10.1152/ajpcell.00194.2006. [DOI] [PubMed] [Google Scholar]
- 32.Ugalde C, Janssen RJ, van den Heuvel LP, Smeitink JA, Nijtmans LG. Differences in assembly or stability of complex I and other mitochondrial OXPHOS complexes in inherited complex I deficiency. Hum Mol Genet. 2004;13:659–667. doi: 10.1093/hmg/ddh071. [DOI] [PubMed] [Google Scholar]
- 33.Brand MD, Buckingham JA, Esteves TC, Green K, Lambert AJ, Miwa S, Murphy MP, Pakay JL, Talbot DA, Echtay KS. Mitochondrial superoxide and aging: uncoupling-protein activity and superoxide production. Biochem Soc Symp. 2004;71:203–213. doi: 10.1042/bss0710203. [DOI] [PubMed] [Google Scholar]
- 34.Brookes PS, Land JM, Clark JB, Heales SJ. Peroxynitrite and brain mitochondria: evidence for increased proton leak. J Neurochem. 1998;70:2195–2202. doi: 10.1046/j.1471-4159.1998.70052195.x. [DOI] [PubMed] [Google Scholar]
- 35.Gibson GE, Zhang H, Xu H, Park LC, Jeitner TM. Oxidative stress increases internal calcium stores and reduces a key mitochondrial enzyme. Biochim Biophys Acta. 2002;1586:177–189. doi: 10.1016/s0925-4439(01)00091-6. [DOI] [PubMed] [Google Scholar]


