Abstract
Lytic bone diseases and in particular osteoporosis are common age-related diseases characterized by enhanced bone fragility due to loss of bone density. Increasingly, osteoporosis poses a major global health-care problem due to the growth of the elderly population. Recently, it was found that the gene regulatory transcription factor CCAAT/enhancer binding protein beta (C/EBPβ) is involved in bone metabolism. C/EBPβ occurs as different protein isoforms of variable amino terminal length, and regulation of the C/EBPβ isoform ratio balance was found to represent an important factor in osteoclast differentiation and bone homeostasis. Interestingly, adjustment of the C/EBPβ isoform ratio by the process of translational control is downstream of the mammalian target of rapamycin kinase (mTOR), a sensor of the nutritional status and a target of immunosuppressive and anticancer drugs. The findings imply that modulating the process of translational control of C/EBPβ isoform expression could represent a novel therapeutic approach in osteolytic bone diseases, including cancer and infection-induced bone loss.
Keywords: C/EBPβ, MafB, Osteoporosis, Rapamycin, Cancer, Leukemia
Introduction
Regulating bone mass, bone turnover (remodeling), and bone integrity requires a tight coupling between the two major bone cell types, the bone-forming osteoblasts, and the bone-resorbing osteoclasts [1]. Maintenance of a correct balance between the functions of both cell types is crucial during the phase of skeletal development and retaining bone homeostasis during adulthood. Pathological bone loss may occur when the cross talk between osteoblasts and osteoclasts is disturbed. This is often due to increased osteoclastic activity and diminished osteoblast activity, as observed in age-related and secondary osteoporosis or in inflammation-induced (e.g., in rheumatoid arthritis) or cancer-induced bone loss (reviewed in [2]). Although therapeutic strategies to treat osteoporosis have been developed, increasing knowledge on the regulation of osteoclastogenesis may facilitate identification of novel therapeutic targets and rational approaches.
Recently, a novel player in the regulation of osteoclast differentiation has been identified as the transcription factor CCAAT/enhancer binding protein beta (C/EBPβ) [3]. C/EBPβ was found to affect both osteoblast activity in bone formation and osteoclast activity in bone resorption. These insights were obtained by combining studies of recombinant genetic mouse models and cell biological, pharmacological, and gene expression-profiling approaches. Briefly, the distinct C/EBPβ isoforms were found to differentially regulate expression of the downstream transcription factor MafB that acts like a brake to restrict osteoclastic differentiation and osteolytic activity [4]. C/EBPβ is normally expressed as two long transactivator isoforms, termed “LAP*/LAP” or as a truncated repressor, termed “LIP” (for the sake of simplicity, we will not discriminate between both activator isoforms, although LAP* displays additional epigenetic features in comparison to LAP). The balance between LAP*/LAP and LIP is adjusted by the mammalian target of rapamycin kinase (mTOR) pathway, which acts as a sensor to integrate growth and nutrient stimuli. The LAP C/EBPβ isoform enhances expression of MafB that subsequently blunts osteoclastogenesis, whereas the truncated LIP C/EBPβ isoform enhances osteoclastogenesis by decreasing expression of MafB [3].
C/EBPβ
The transcription factor C/EBPβ belongs to the gene regulator family of the CCAAT/enhancer binding proteins, which consists of six members: α, β, δ, γ, ε, and ζ. C/EBPs are characterized by highly conserved carboxyl terminal “basic leucine zipper domains” (bZip), containing an alpha helical basic DNA binding domain and a leucine zipper coiled-coil dimerization domain. The N-terminal domains of C/EBPs are more variable and only partially conserved. The two main members C/EBPα and C/EBPβ are partially redundant during embryogenesis, and after birth, are involved in cell growth, proliferation, and differentiation in several tissues [5]. Both C/EBPα and C/EBPβ are encoded by intronless genes and are present as single messenger RNAs (mRNAs). Nevertheless, amino terminally truncated protein isoforms may be generated by a process known as alternative translation initiation [6]. Thus, the protein isoforms display different domains and exert distinct biological functions [7].
Alternative initiation of C/EBP translation is mediated by small “upstream open reading frames” (uORF) that act as regulatory elements to sense the activity of the translation machinery and to direct initiation to alternative start codons. A key signaling component of regulated translational initiation is the mTOR. The activity of mTOR depends on nutritional signals, including glucose and amino acid availability, and it also responds to extracellular signals such as growth factors and insulin [8]. The macrolide antibiotic rapamycin specifically inhibits the mTOR kinase, lowering the activity of the critical eukaryotic translation initiation factor 4E (eIF4E). Low eIF4E activity abolishes translation reinitiation, and in the case of C/EBPβ, diminishes expression of LIP. This results in a higher LAP to LIP ratio of the C/EBPβ isoforms and alters expression of C/EBPβ target genes (Fig. 1) [6].
Fig. 1.
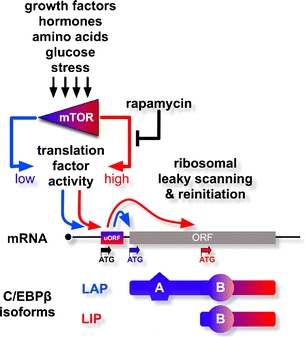
Translational control and C/EBPβ isoform regulation. Growth factor, hormone, nutrition, and stress signals are integrated by the mammalian mTOR kinase that monitors the growth and differentiation status of a cell. The mTOR kinase (low activity (blue); high activity (red)) regulates the function of the translation initiation machinery. The cis-regulatory upstream open reading frame uORF in the C/EBPβ mRNA senses the activity of the translation machinery and directs initiation, leaky scanning, and reinitiation. High activity of the translation machinery directs reinitiation and production of the truncated “repressor” isoform LIP of C/EBPβ. Lowering the activity of the initiation machinery results in leaky scanning over the uORF ATG and translation initiation at the first ATG, resulting in the production of the long “activator” isoform LAP. Rapamycin inhibits mTOR signaling, resulting in the production of mainly the LAP isoform
C/EBPβ is involved in the differentiation of a large variety of cell types, including keratinocytes, hepatocytes, mammary epithelial cells, ovarian luteal cells, adipocytes, B cells, and macrophages. Moreover, C/EBPβ regulates cell survival, apoptosis, metabolism, inflammation, and tumorigenic transformation [5, 7, 9]. C/EBPβ is expressed as three different protein isoforms differing in their N-terminal domains, LAP*, LAP (both are transcriptional activators), and LIP (a transdominant repressor), each displaying unique functions in various cellular processes. The diversity of C/EBPβ’s functional properties is further increased by signaling-dependent posttranslational modifications that regulate the biological activity and the subcellular distribution of C/EBPβ [7]. Action of C/EBPβ requires dimerization through the bZip domain, either with another C/EBPβ molecule or through heterodimerization with any of the other C/EBP family members or some transcription factors with compatible bZip domains.
C/EBPβ controls bone cells
C/EBPβ has been suggested to act as a scaffold protein in the assembly of osteogenic transcription factors [9], such as with Runx2 or ATF4, thereby enhancing osteoblast differentiation [10, 11]. Analyses of C/EBPβ-deficient mice confirmed a role of C/EBPβ in osteoblast differentiation and bone formation: In the absence of C/EBPβ, bone mass is decreased [12] due to decreased osteoblast differentiation and function [11]; however, this appeared to entail also noncell autonomous effects [3, 13]. C/EBPβ deficiency also resulted in growth retardation during embryogenesis [11, 14] and at postnatal stages [3], due to diminished chondrocyte differentiation [11, 14]. C/EBPβ activates the cyclin-dependent kinase inhibitor p57kip in chondrocytes that restricts chondrocyte proliferation and stimulates the transition to hypertrophic differentiation [14].
Surprisingly, expression of the (repressor) LIP isoform instead of the entire C/EBPβ gene from its endogenous locus in “knock-in” mouse mutants restored bone formation and rescued the growth retardation observed in C/EBPβ-deficient animals [3]. Moreover, LIP enhanced osteoblast differentiation and function [3], possibly by acting as a coactivator for Runx2 [10].
Besides cell autonomous effects, C/EBPβ is possibly also involved in paracrine action and the coupling between osteoblasts and osteoclasts. C/EBPβ and its related family member C/EBPδ induce IGF-I expression [15–18] that represents an important anabolic factor for bone formation. Similar to TGFβ [19], IGF-I is released from the bone matrix by osteoclasts and may then stimulate osteoblasts [20]. In addition, coupling between osteoclasts and osteoblasts is thought to involve the cytokines interleukin 6 (IL-6) and tumor necrosis factor alpha (TNFα) [20] that also represent targets of C/EBPβ in osteoclasts [3].
C/EBPβ as a switch in osteoclast differentiation
The first observations suggesting a connection between C/EBPβ and osteoporosis were made over a decade ago in connection with the cytokine IL6. C/EBPβ, in synergy with NFκB, was found to act downstream of estrogen, involving binding of the estrogen receptor and resulting in suppression of IL6 gene transcription in osteoblasts [21]. This is of clinical relevance as decreased estrogen levels are one of the major causes of postmenopausal osteoporosis, which results in increased IL-6 that activates osteoclasts and thus bone resorption [22, 23]. Indeed, C/EBPβ-deficient mice show a similar general pathology as mice overexpressing IL6 [24] and with bone loss as a consequence of enhanced activity of osteoclasts [3]. Macrophage defects observed in C/EBPβ-deficient mice [25] were the first indication of a possible role of C/EBPβ in the bone-resorbing osteoclast, which shares a monocytic precursor cell with macrophages.
In contrast to macrophages, osteoclast precursors fuse into large polykaryons to generate mature osteoclasts that attach to the bone surface and resorb bone matrix. Both differentiation and osteolytic activity of osteoclasts requires macrophage colony-stimulating factor (M-CSF) and membrane-bound receptor activator of NFκB ligand (RANK-L), which are both produced by osteoblasts and by stromal cells. Osteoblasts also produce osteoprotegerin, a decoy receptor of RANK-L, thereby counteracting and fine tuning the effect of RANK-L in osteoclastogenesis. These cytokines activate downstream osteoclastic transcription factors, including NFATc1, Mitf, and c-Fos that affect proliferation, differentiation, and survival of osteoclasts [2, 26].
Absence of C/EBPβ or expression of only the LIP isoform was observed to strongly enhance osteoclast differentiation. This suggested that the absence of LAP would cause enhanced osteoclastogenesis, and LAP would induce a gene whose product blocks osteoclast differentiation. Such an inhibitor was identified as MafB, a transcription factor recently found to be involved in osteoclastogenesis [4] and a target gene of LAP [3].
MafB is also a bZIP transcription factor that, however, belongs to a distinct family. MafB is important in several developmental processes [27] and in oncogenesis [28]. In the hematopoietic system, the function of MafB is restricted to the monocytic lineage [29]. Similarly to C/EBPβ, the expression of MafB starts at the myeloblast stage and strongly increases during macrophage differentiation [29]. Although not strictly required for macrophage differentiation, absence of MafB in monocytes results in an altered phenotype. Absence of MafB reduces F4/80 levels in macrophages [30]. Upon M-CSF treatment, MafB-deficient macrophages form multiple extensive cellular extrusions, often branched, known as filopodia [31], a process that also occurs in osteoclasts and that is required for cell migration [32].
MafB directs macrophage versus osteoclast differentiation [4], and recently, MafB was reported to restrict M-CSF receptor-induced activation of the PU.1 gene [33] that encodes an early and essential myeloid and osteoclastic transcription factor [26]. No bone phenotype has been reported of MafB knockout mice so far, possibly due to their early postnatal lethality [27]. However, the MafB protein interacts with and thereby attenuates the activity of the osteoclastogenic transcription factors NFATc1, Mitf, and c-Fos [4]. The long C/EBPβ isoform LAP induces MafB expression and thus inhibits osteoclastogenesis, whereas absence of LAP (such as in C/EBPβ-deficient animals or in knockin mutants expressing only the inhibitory LIP isoform) results in diminished MafB expression. Lack of MafB enhances the functionality of NFATc1, its downstream target OSCAR, and probably other factors, including Mitf and c-Fos, to augment expression of osteoclast genes, including the cell fusion-promoting genes DC-STAMP, ATP6v0d2, and TNFα [34]. The opposing roles of the different C/EBPβ protein isoforms in MafB expression suggest that the C/EBPβ isoform ratio plays a fundamental role as an upstream regulator in the initiation of osteoclastogenesis and connects MafB expression and osteoclastogenesis to the mTOR pathway (Fig. 2).
Fig. 2.
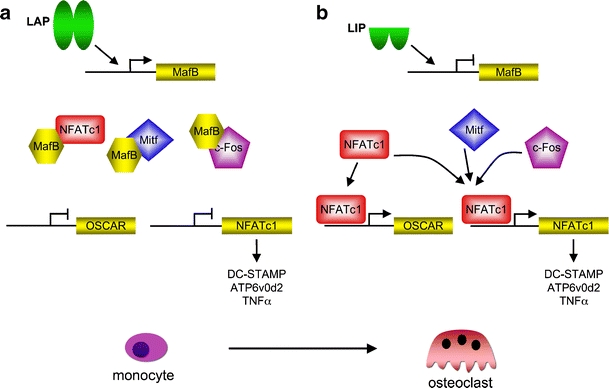
C/EBPβ as a master switch in osteoclast differentiation. a The LAP isoform of C/EBPβ induces expression of MafB. MafB binds to and inactivates the osteoclastic transcription factors c-Fos, Mitf, and NFATc1. Inactivation of these key transcription factors prevents osteoclast differentiation by inhibition of osteoclast target gene expression of OSCAR and NFATc1, resulting in inhibition of NFATc1 target genes, including the cell fusion genes DC-STAMP, ATP6v0d2, and TNFα (partially derived from [4, 34]). b The LIP isoform of C/EBPβ inhibits MafB expression. As a result, MafB becomes limiting, and lack of MafB permits access of the osteoclast transcription factors (often in conjunction with NFATc1 as a major osteoclast transcription factor) to activate target genes and osteoclast differentiation
Implications for osteolytic diseases
Therapies of osteoporosis concentrate on the inhibition of the pathological bone resorption by osteoclasts using mainly bisphosphonates, but also estrogen-like drugs such as raloxifene, or strontium ranelate [1, 2, 35]. However, bisphosphonates inhibit the bone remodeling cycle, raising the need for the development of shorter-acting resorption inhibitors, which will not affect the bone-remodeling process itself too much to ensure continuous repair of microfractures and prevent additional weaknesses of the skeleton [2]. Therefore, novel therapeutic strategies are required to improve treatment strategies in osteoporosis and other lytic bone diseases. The findings of involvement of translational control of C/EBPβ isoforms in directing osteoclastogenesis may entail such novel targets for therapy. Translational control is affected by the antibiotic rapamycin that specifically inhibits mTOR signaling, resulting in a shift of the C/EBPβ isoform ratio toward the LAP isoform (Fig. 1). Rapamycin treatment thus favors LAP expression over LIP, which results in enhanced MafB gene activation and thus inhibition of both osteoclastogenesis and bone resorption. Inhibition of mTOR by rapamycin inhibits osteoclastogenesis not only in mouse [3, 36] but also in human cells [37], suggesting that the murine model may provide disease relevant data. Importantly, a derivative of rapamycin has already been shown to inhibit bone loss in an experimental rat model, where osteoporosis was induced by ovariectomy [37]. So far, however, no clinical data is available concerning the impact of rapamycin on bone. Taken together, these data suggest that rapamycin could potentially serve as a therapeutic agent in treating osteolytic diseases [38].
The mTOR signaling pathway is also important in the regulation of autophagy, a process recently proposed to be involved in osteoclast function [39, 40]. Rapamycin induces autophagy and therefore might play a role in late osteoclastogenesis. This would be in addition to the action of rapamycin early in osteoclast differentiation, where it inhibits differentiation [3]. Autophagy was suggested to decelerate aging processes [41], as also recently found for rapamycin that extends the life span of aged mice [42]. This may suggest that dampening mTOR signaling prevents age-related disease progression, including cancer.
Along these lines, the LIP isoform has been associated with enhanced proliferation of multiple myeloma and breast cancer and in Hodgkin and anaplastic large cell lymphoma. A rapamycin derivative was demonstrated to decrease tumor cell proliferation by abrogating LIP expression [43]. These observations further strengthen the notion that C/EBPβ is an important downstream target of mTOR, affecting cell proliferation and differentiation in diverse cell types. Moreover, rapamycin has been shown to inhibit tumor cell metastasis in an osteosarcoma mouse model [44]. Rapamycin has already been considered as a novel treatment in multiple myeloma, where it restrains tumor cell proliferation [45] and has antitumor activity, as reported in breast cancer patients where rapamycin was tested in clinical phase trials [46]. Induction of autophagy by rapamycin would, in addition, sensitize cancer cells to complementary treatments [47]. Both multiple myeloma and breast cancer generate bone metastases, causing local osteolytic lesions [48, 49]. Also, in rheumatoid arthritis, local bone loss occurs, and rapamycin was found to be beneficial due to its immunosuppressant characteristics [50, 51]. The observation that rapamycin also functions as an antiresorptive agent by modulating the C/EBPβ isoform ratio [3] may suggest a bipartite function [52] as tumor suppressor or immune suppressant and as an osteoclast regulator, as depicted in Fig. 3.
Fig. 3.
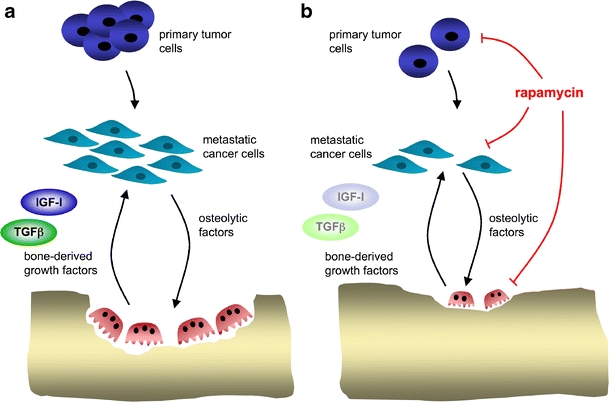
Dual effect of rapamycin on cancer-induced bone loss. a In this hypothetical model, several types of tumor cells with high LIP expression (breast, prostate, lung, multiple myeloma) preferentially generate osteolytic metastasis by activating osteoclasts to release tumor-promoting growth factors (e.g., TGFβ, IGF-I). This results in a continuous cycle of stimulation of metastatic cells and bone resorption (derived from [56]). b The vicious circle between tumor and stroma (osteoclasts) may be interrupted by drugs that impinge on translational control, such as rapamycin or its derivatives
Rapamycin’s immunosuppressant action due to modulation of regulatory T cells might raise concerns when used for other purposes than immunosuppression, e.g., as in cancer patients or elderly with osteolytic diseases. Problems in wound healing [53] and occurrence of anemia [54] have been reported, although no increase in infectious complications are also reported [55].
It is interesting to note that the downstream C/EBPβ target MafB has also been found to be deregulated in the majority of multiple myeloma cases [28]. Moreover, C/EBPβ is known to play a central role in inflammation. Both processes have a strong impact on osteoclastogenesis and can induce pathological bone resorption. The recent identification of the role of the C/EBPβ isoform ratio in the control of osteoclast differentiation and bone resorption may link the pathology of these lytic bone diseases to the translational control of a distinct gene regulator and may thus open new avenues for novel therapeutic approaches.
The usage of rapamycin to direct translational control might have several potential benefits to treat osteolytic-associated diseases, as it can attack these diseases at different levels. Rapamycin treatment, combined with restraining osteoclast differentiation, may have combinatorial functions in treating osteolytic diseases. However, one has to take into consideration that inhibition of the mTOR pathway could have adverse side effects, requiring the development of novel rapamycin analogs or novel tissue-specific mTOR inhibitors displaying less side effects.
Acknowledgments
The authors are grateful to Dr. J Tuckermann (Leibnitz Institute for Age Research, Jena, Germany) for critically reading the manuscript. The authors are also thankful to the members of the Leutz laboratory for helpful discussions. The authors apologize to all those authors whose work was not cited in this minireview due to space limitations. This work was supported by the Berliner Krebsgesellschaft (LEFF200708). The authors declare that they have no competing financial interests.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13:791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- 2.Novack DV, Teitelbaum SL. The osteoclast: friend or foe? Annu Rev Pathol. 2008;3:457–484. doi: 10.1146/annurev.pathmechdis.3.121806.151431. [DOI] [PubMed] [Google Scholar]
- 3.Smink JJ, Begay V, Schoenmaker T, Sterneck E, de Vries TJ, Leutz A. Transcription factor C/EBPbeta isoform ratio regulates osteoclastogenesis through MafB. Embo J. 2009;28:1769–1781. doi: 10.1038/emboj.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim K, Kim JH, Lee J, Jin HM, Kook H, Kim KK, Lee SY, Kim N. MafB negatively regulates RANKL-mediated osteoclast differentiation. Blood. 2007;109:3253–3259. doi: 10.1182/blood-2006-09-048249. [DOI] [PubMed] [Google Scholar]
- 5.Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J. 2002;365:561–575. doi: 10.1042/BJ20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calkhoven CF, Muller C, Leutz A. Translational control of C/EBPalpha and C/EBPbeta isoform expression. Genes Dev. 2000;14:1920–1932. [PMC free article] [PubMed] [Google Scholar]
- 7.Zahnow CA. CCAAT/enhancer-binding protein beta: its role in breast cancer and associations with receptor tyrosine kinases. Expert Rev Mol Med. 2009;11:e12. doi: 10.1017/S1462399409001033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corradetti MN, Guan KL. Upstream of the mammalian target of rapamycin: do all roads pass through mTOR? Oncogene. 2006;25:6347–6360. doi: 10.1038/sj.onc.1209885. [DOI] [PubMed] [Google Scholar]
- 9.Nerlov C. The C/EBP family of transcription factors: a paradigm for interaction between gene expression and proliferation control. Trends Cell Biol. 2007;17:318–324. doi: 10.1016/j.tcb.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Hata K, Nishimura R, Ueda M, Ikeda F, Matsubara T, Ichida F, Hisada K, Nokubi T, Yamaguchi A, Yoneda T. A CCAAT/enhancer binding protein beta isoform, liver-enriched inhibitory protein, regulates commitment of osteoblasts and adipocytes. Mol Cell Biol. 2005;25:1971–1979. doi: 10.1128/MCB.25.5.1971-1979.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tominaga H, Maeda S, Hayashi M, Takeda S, Akira S, Komiya S, Nakamura T, Akiyama H, Imamura T. CCAAT/enhancer-binding protein beta promotes osteoblast differentiation by enhancing Runx2 activity with ATF4. Mol Biol Cell. 2008;19:5373–5386. doi: 10.1091/mbc.E08-03-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staiger J, Lueben MJ, Berrigan D, Malik R, Perkins SN, Hursting SD, Johnson PF. C/EBPbeta regulates body composition, energy balance-related hormones and tumor growth. Carcinogenesis. 2009;30:832–840. doi: 10.1093/carcin/bgn273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zanotti S, Stadmeyer L, Smerdel-Ramoya A, Durant D, Canalis E. Misexpression of CCAAT/enhancer binding protein beta (C/EBPb) causes osteopenia. J Endocrinol. 2009;201:263–274. doi: 10.1677/JOE-08-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirata M, Kugimiya F, Fukai A, Ohba S, Kawamura N, Ogasawara T, Kawasaki Y, Saito T, Yano F, Ikeda T, Nakamura K, Chung UI, Kawaguchi H. C/EBPbeta promotes transition from proliferation to hypertrophic differentiation of chondrocytes through transactivation of p57. PLoS ONE. 2009;4:e4543. doi: 10.1371/journal.pone.0004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wessells J, Yakar S, Johnson PF. Critical prosurvival roles for C/EBP beta and insulin-like growth factor I in macrophage tumor cells. Mol Cell Biol. 2004;24:3238–3250. doi: 10.1128/MCB.24.8.3238-3250.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang W, Rewari A, Centrella M, McCarthy TL. Fos-related antigen 2 controls protein kinase A-induced CCAAT/enhancer-binding protein beta expression in osteoblasts. J Biol Chem. 2004;279:42438–42444. doi: 10.1074/jbc.M405549200. [DOI] [PubMed] [Google Scholar]
- 17.Umayahara Y, Billiard J, Ji C, Centrella M, McCarthy TL, Rotwein P. CCAAT/enhancer-binding protein delta is a critical regulator of insulin-like growth factor-I gene transcription in osteoblasts. J Biol Chem. 1999;274:10609–10617. doi: 10.1074/jbc.274.15.10609. [DOI] [PubMed] [Google Scholar]
- 18.Umayahara Y, Ji C, Centrella M, Rotwein P, McCarthy TL. CCAAT/enhancer-binding protein delta activates insulin-like growth factor-I gene transcription in osteoblasts. Identification of a novel cyclic AMP signaling pathway in bone. J Biol Chem. 1997;272:31793–31800. doi: 10.1074/jbc.272.50.31793. [DOI] [PubMed] [Google Scholar]
- 19.Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z, Zhao L, Nagy TR, Peng X, Hu J, Feng X, Van Hul W, Wan M, Cao X. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. 2009;15:757–765. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin TJ, Sims NA. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol Med. 2005;11:76–81. doi: 10.1016/j.molmed.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Stein B, Yang MX. Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-kappa B and C/EBP beta. Mol Cell Biol. 1995;15:4971–4979. doi: 10.1128/mcb.15.9.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clowes JA, Riggs BL, Khosla S. The role of the immune system in the pathophysiology of osteoporosis. Immunol Rev. 2005;208:207–227. doi: 10.1111/j.0105-2896.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 23.Manolagas SC. The role of IL-6 type cytokines and their receptors in bone. Ann N Y Acad Sci. 1998;840:194–204. doi: 10.1111/j.1749-6632.1998.tb09563.x. [DOI] [PubMed] [Google Scholar]
- 24.Screpanti I, Romani L, Musiani P, Modesti A, Fattori E, Lazzaro D, Sellitto C, Scarpa S, Bellavia D, Lattanzio G, et al. Lymphoproliferative disorder and imbalanced T-helper response in C/EBP beta-deficient mice. Embo J. 1995;14:1932–1941. doi: 10.1002/j.1460-2075.1995.tb07185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poli V. The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J Biol Chem. 1998;273:29279–29282. doi: 10.1074/jbc.273.45.29279. [DOI] [PubMed] [Google Scholar]
- 26.Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4:638–649. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- 27.Blanchi B, Kelly LM, Viemari JC, Lafon I, Burnet H, Bevengut M, Tillmanns S, Daniel L, Graf T, Hilaire G, Sieweke MH. MafB deficiency causes defective respiratory rhythmogenesis and fatal central apnea at birth. Nat Neurosci. 2003;6:1091–1100. doi: 10.1038/nn1129. [DOI] [PubMed] [Google Scholar]
- 28.Eychene A, Rocques N, Pouponnot C. A new MAFia in cancer. Nat Rev Cancer. 2008;8:683–693. doi: 10.1038/nrc2460. [DOI] [PubMed] [Google Scholar]
- 29.Kelly LM, Englmeier U, Lafon I, Sieweke MH, Graf T. MafB is an inducer of monocytic differentiation. Embo J. 2000;19:1987–1997. doi: 10.1093/emboj/19.9.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moriguchi T, Hamada M, Morito N, Terunuma T, Hasegawa K, Zhang C, Yokomizo T, Esaki R, Kuroda E, Yoh K, Kudo T, Nagata M, Greaves DR, Engel JD, Yamamoto M, Takahashi S. MafB is essential for renal development and F4/80 expression in macrophages. Mol Cell Biol. 2006;26:5715–5727. doi: 10.1128/MCB.00001-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aziz A, Vanhille L, Mohideen P, Kelly LM, Otto C, Bakri Y, Mossadegh N, Sarrazin S, Sieweke MH. Development of macrophages with altered actin organization in the absence of MafB. Mol Cell Biol. 2006;26:6808–6818. doi: 10.1128/MCB.00245-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faccio R, Takeshita S, Colaianni G, Chappel J, Zallone A, Teitelbaum SL, Ross FP. M-CSF regulates the cytoskeleton via recruitment of a multimeric signaling complex to c-Fms Tyr-559/697/721. J Biol Chem. 2007;282:18991–18999. doi: 10.1074/jbc.M610937200. [DOI] [PubMed] [Google Scholar]
- 33.Sarrazin S, Mossadegh-Keller N, Fukao T, Aziz A, Mourcin F, Vanhille L, Kelly Modis L, Kastner P, Chan S, Duprez E, Otto C, Sieweke MH. MafB restricts M-CSF-dependent myeloid commitment divisions of hematopoietic stem cells. Cell. 2009;138:300–313. doi: 10.1016/j.cell.2009.04.057. [DOI] [PubMed] [Google Scholar]
- 34.Kim K, Lee SH, Ha Kim J, Choi Y, Kim N. NFATc1 induces osteoclast fusion via up-regulation of Atp6v0d2 and the dendritic cell-specific transmembrane protein (DC-STAMP) Mol Endocrinol. 2008;22:176–185. doi: 10.1210/me.2007-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook P, Cooper C. Osteoporosis. Lancet. 2006;367:2010–2018. doi: 10.1016/S0140-6736(06)68891-0. [DOI] [PubMed] [Google Scholar]
- 36.Glantschnig H, Fisher JE, Wesolowski G, Rodan GA, Reszka AA. M-CSF, TNFalpha and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ. 2003;10:1165–1177. doi: 10.1038/sj.cdd.4401285. [DOI] [PubMed] [Google Scholar]
- 37.Kneissel M, Luong-Nguyen NH, Baptist M, Cortesi R, Zumstein-Mecker S, Kossida S, O’Reilly T, Lane H, Susa M. Everolimus suppresses cancellous bone loss, bone resorption, and cathepsin K expression by osteoclasts. Bone. 2004;35:1144–1156. doi: 10.1016/j.bone.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Boyce BF, Xing L, Yao Z, Shakespeare WC, Wang Y, Metcalf CA, 3rd, Sundaramoorthi R, Dalgarno DC, Iuliucci JD, Sawyer TK. Future anti-catabolic therapeutic targets in bone disease. Ann N Y Acad Sci. 2006;1068:447–457. doi: 10.1196/annals.1346.042. [DOI] [PubMed] [Google Scholar]
- 39.Helfrich MH, Hocking LJ. Genetics and aetiology of Pagetic disorders of bone. Arch Biochem Biophys. 2008;473:172–182. doi: 10.1016/j.abb.2008.02.045. [DOI] [PubMed] [Google Scholar]
- 40.DeSelm C, Miller B, Ross F, Virgin H, Teitelbaum S (2009) Autophagy proteins regulate vesicle secretion and bone resorption in the osteoclast. Abstract: ASBMR 31st Annual Meeting
- 41.Meijer AJ, Codogno P. Autophagy: regulation and role in disease. Crit Rev Clin Lab Sci. 2009;46:210–240. doi: 10.1080/10408360903044068. [DOI] [PubMed] [Google Scholar]
- 42.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jundt F, Raetzel N, Muller C, Calkhoven CF, Kley K, Mathas S, Lietz A, Leutz A, Dorken B. A rapamycin derivative (everolimus) controls proliferation through down-regulation of truncated CCAAT enhancer binding protein beta and NF-kappaB activity in Hodgkin and anaplastic large cell lymphomas. Blood. 2005;106:1801–1807. doi: 10.1182/blood-2004-11-4513. [DOI] [PubMed] [Google Scholar]
- 44.Wan X, Mendoza A, Khanna C, Helman LJ. Rapamycin inhibits ezrin-mediated metastatic behavior in a murine model of osteosarcoma. Cancer Res. 2005;65:2406–2411. doi: 10.1158/0008-5472.CAN-04-3135. [DOI] [PubMed] [Google Scholar]
- 45.Berenson JR, Yellin O. New drugs in multiple myeloma. Curr Opin Support Palliat Care. 2008;2:204–210. doi: 10.1097/SPC.0b013e3283090475. [DOI] [PubMed] [Google Scholar]
- 46.Chan S, Scheulen ME, Johnston S, Mross K, Cardoso F, Dittrich C, Eiermann W, Hess D, Morant R, Semiglazov V, Borner M, Salzberg M, Ostapenko V, Illiger HJ, Behringer D, Bardy-Bouxin N, Boni J, Kong S, Cincotta M, Moore L. Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol. 2005;23:5314–5322. doi: 10.1200/JCO.2005.66.130. [DOI] [PubMed] [Google Scholar]
- 47.Kim KW, Mutter RW, Cao C, Albert JM, Freeman M, Hallahan DE, Lu B. Autophagy for cancer therapy through inhibition of pro-apoptotic proteins and mammalian target of rapamycin signaling. J Biol Chem. 2006;281:36883–36890. doi: 10.1074/jbc.M607094200. [DOI] [PubMed] [Google Scholar]
- 48.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 49.Boyce BF, Yoneda T, Guise TA. Factors regulating the growth of metastatic cancer in bone. Endocr Relat Cancer. 1999;6:333–347. doi: 10.1677/erc.0.0060333. [DOI] [PubMed] [Google Scholar]
- 50.Bruyn GA, Tate G, Caeiro F, Maldonado-Cocco J, Westhovens R, Tannenbaum H, Bell M, Forre O, Bjorneboe O, Tak PP, Abeywickrama KH, Bernhardt P, van Riel PL. Everolimus in patients with rheumatoid arthritis receiving concomitant methotrexate: a 3-month, double-blind, randomised, placebo-controlled, parallel-group, proof-of-concept study. Ann Rheum Dis. 2008;67:1090–1095. doi: 10.1136/ard.2007.078808. [DOI] [PubMed] [Google Scholar]
- 51.Foroncewicz B, Mucha K, Paczek L, Chmura A, Rowinski W. Efficacy of rapamycin in patient with juvenile rheumatoid arthritis. Transpl Int. 2005;18:366–368. doi: 10.1111/j.1432-2277.2004.00070.x. [DOI] [PubMed] [Google Scholar]
- 52.Ory B, Moriceau G, Redini F, Heymann D. mTOR inhibitors (rapamycin and its derivatives) and nitrogen containing bisphosphonates: bi-functional compounds for the treatment of bone tumours. Curr Med Chem. 2007;14:1381–1387. doi: 10.2174/092986707780831159. [DOI] [PubMed] [Google Scholar]
- 53.Mills RE, Taylor KR, Podshivalova K, McKay DB, Jameson JM. Defects in skin gamma delta T cell function contribute to delayed wound repair in rapamycin-treated mice. J Immunol. 2008;181:3974–3983. doi: 10.4049/jimmunol.181.6.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hess G, Herbrecht R, Romaguera J, Verhoef G, Crump M, Gisselbrecht C, Laurell A, Offner F, Strahs A, Berkenblit A, Hanushevsky O, Clancy J, Hewes B, Moore L, Coiffier B. Phase III study to evaluate temsirolimus compared with investigator’s choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2009;27:3822–3829. doi: 10.1200/JCO.2008.20.7977. [DOI] [PubMed] [Google Scholar]
- 55.Hartford CM, Ratain MJ. Rapamycin: something old, something new, sometimes borrowed and now renewed. Clin Pharmacol Ther. 2007;82:381–388. doi: 10.1038/sj.clpt.6100317. [DOI] [PubMed] [Google Scholar]
- 56.Kingsley LA, Fournier PG, Chirgwin JM, Guise TA. Molecular biology of bone metastasis. Mol Cancer Ther. 2007;6:2609–2617. doi: 10.1158/1535-7163.MCT-07-0234. [DOI] [PubMed] [Google Scholar]


