Abstract
Microbial communities typically vary in composition and structure over space and time. Little is known about the inherent characteristics of communities that govern various drivers of these changes, such as random variation, changes in response to perturbation, or susceptibility to invasion. In this study, we use 16S ribosomal RNA gene sequences to describe variation among bacterial communities in the midguts of cabbage white butterfly (Pieris rapae) larvae and examine the influence of community structure on susceptibility to invasion. We compared communities in larvae experiencing the same conditions at different times (temporal variation) or fed different diets (perturbation). The most highly represented phylum was Proteobacteria, which was present in all midgut communities. The observed species richness ranged from six to 15, and the most abundant members affiliated with the genera Methylobacteria, Asaia, Acinetobacter, Enterobacter, and Pantoea. Individual larvae subjected to the same conditions at the same time harbored communities that were highly similar in structure and membership, whereas the communities observed within larval populations changed with diet and over time. In addition, structural changes due to perturbation coincided with enhanced susceptibility to invasion by Enterobacter sp. NAB3R and Pantoea stewartii CWB600, suggesting that resistance to invasion is in part governed by community structure. These findings along with the observed conservation of membership at the phylum level, variation in structure and membership at lower taxonomic levels, and its relative simplicity make the cabbage white butterfly larval community an attractive model for studying community dynamics and robustness.
Electronic supplementary material
The online version of this article (doi:10.1007/s00248-009-9595-8) contains supplementary material, which is available to authorized users.
Introduction
Microbial communities are dynamic, often experiencing changes in composition and structure. Changes can result from alterations in nutrient availability, physical aspects of the environment, and proximity to other organisms [1–4]. Many communities, such as those inhabiting lakes, soil, insects, humans, and other animals, experience temporal changes associated with factors such as season, nutrient availability, and host development [5–10].
In addition to cyclical or programmed influences, sudden disturbances can also alter community composition. In animal-associated communities, a common disturbance is change in host diet. Plant- vs nonplant-based diets, differences in plant species, fiber content and type, and fat source all have been implicated in changes in gut community composition [11–15]. The addition of antibiotics to diet and intravenous administration also alter community composition [16–18]. While the impact of antibiotics on the human gastrointestinal microbial community has attracted interest as a medical issue, antibiotics also provide a tool for exploring the ecology of animal gut-associated communities.
One of the most important ecological processes is invasion, arising from both natural and anthropogenic introductions. The process of biological invasion can be modeled in gut communities. Biological invasion theory attempts to predict invasion patterns, characteristics of successful invaders, characteristics of communities susceptible to invasion, consequences of invasion, and processes driving establishment [19, 20]. General theories of invasibility are needed, both to develop a more proactive and predictive approach to the increased frequency of biological invasions and to help guide the strategies and success of deliberate introductions.
In some situations, microbial invasions are associated with damage to an ecosystem (i.e., reference [21] and [22]), and in others, invasion is desired. For example, bacteria comprising probiotic preparations and disease-suppressive bacterial biocontrol agents for crop health must invade a community to provide a benefit [23, 24]. The ability to resist invasion by exogenous bacteria, also known as the barrier effect or colonization resistance, is a central attribute of the microbial communities in the human gastrointestinal tract and vagina [25–27]. Despite the importance of invasions, community susceptibility to them is not well understood [28, 29].
Here, we present the lepidopteran midgut as a potential model system for studying ecological processes in animal-associated gastrointestinal communities. Recently, the midgut of Lymantria dispar, the gypsy moth, was shown to contain a simple bacterial community of approximately seven members [30]. Because of its relative simplicity, the community in the lepidopteran midgut is attractive as a potential model system. The lepidopteran species used in this study, Pieris rapae, the cabbage white butterfly, has several practical attributes, including ease of care, handling, and manipulation, and a short life cycle that facilitates multigenerational studies. Although there has been much work examining the digestive physiology of the cabbage white butterfly (i.e., the maintenance of a slightly alkaline gut pH and the presence of endopeptidases), there has been little work exploring the gut microbiota [31, 32]. A previous microbiological study of this insect was limited to culture-based analysis of the adult alimentary tract [33].
The goals of this study were to (1) characterize the species richness and composition of the midgut bacterial community of cabbage white butterfly larvae using culture-independent methods, (2) investigate the effects of time, diet, and antibiotics on this community, and (3) explore the cabbage white butterfly midgut as a model for studying aspects of inherent community features, such as robustness. Robustness is a comprehensive term used to describe the extent to which a community exhibits temporal stability (constancy in structure over time; [34]), resistance (ability to resist change following perturbation; [35, 36]), and resilience (ability to return to an initial structure following perturbation; [37]. Two aspects of robustness, temporal stability and resistance (to dietary perturbation and invasion), were examined in this study.
Methods
Treatment and Rearing of Larvae
Cabbage white butterfly eggs were obtained from Carolina Biological Supply Company (Burlington, NC, USA) and soaked in a solution of 1% Tween and 2% bleach for 3 min and then rinsed in sterile distilled water. This treatment was used to reduce the presence of fungi and bacteria on the surface of the egg capsule. The eggs were then dried in a sterile hood and placed in a sterilized Petri dish with either unamended sterilized standard gypsy moth artificial diet (MP Biomedical, Irvine, CA, USA), sterile artificial diet amended with a penicillin and streptomycin cocktail (Sigma-Aldrich Biotechnology, St. Louis, MO, USA) at 10 units per milliliter and 10 μg/ml, respectively, and sterilized artificial diet amended with sinigrin (Sigma-Aldrich Biotechnology), a major phytochemical component of Brussels sprouts, at a concentration of 3.0 mg/ml, which is comparable to the concentration in fresh Brussels sprouts [38] or Brussels sprout leaves. Larvae were reared to fourth instar in growth chambers with 16 h/8 h (light/dark) photoperiods at 27 °C. Petri dishes containing larvae were opened only inside a sterile hood for feeding and cleaning purposes.
Preparation of Diet
A denatured wheat germ diet used to rear many Lepidoptera, such as gypsy moth, was prepared as directed by the manufacturer's instructions and autoclaved (MP Biomedicals, Irvine, CA, USA). Diet was cooled, and treatments were incorporated (compounds and concentrations described above). Diet samples were plated periodically to confirm the absence of culturable bacteria and fungi. Conventionally raised Brussels sprouts were obtained from a local grocery store. Brussels sprout leaves were separated and sonicated for 60 s in 5% bleach, washed in 5% bleach for 5 min, and rinsed twice in sterile distilled water for 2 min. Efforts to culture bacteria from treated Brussels sprout leaves were unsuccessful, thereby confirming effectiveness of bleach treatment. Leaves were then dried in a sterile hood and stored for up to 5 days in parafilm-sealed sterile Petri dishes at 4 °C until use.
Sampling and Dissection
Fourth instar larvae were used in all experiments. Larvae were placed in sterilized Petri dishes and starved for 4 to 6 h before dissection to reduce food content in the midgut and the presence of transient bacteria. Dissections were performed as described previously [30], and guts were stored at −20 °C prior to DNA isolation or used immediately when bacterial cultivation was necessary.
Bacterial Cultivation
Bacteria were isolated from midguts as described previously [30]. Briefly, midguts were sonicated for 60 s and plated on 1/10th strength tryptic soy agar or 1/10th tryptic soy agar amended with 25 μg/ml nalidixic acid when appropriate.
Analysis of 16S Ribosomal RNA Genes
DNA was extracted from pools of ten or from individual midguts as described previously [30]. Individual guts were sampled to determine intrinsic variability between insects reared under the same conditions at the same time. Amplification of 16S ribosomal RNA (rRNA) genes was performed as described previously using primers 27F and 1492R [39]. Polymerase chain reaction (PCR) products were then ligated into pGEM-T (Promega Corporation, Madison, WI, USA) according to the manufacturer's directions. Escherichia coli JM109 (Promega Corporation) was transformed with the ligation mix according to the manufacturer's instructions. Clones were grown in Luria–Bertani broth containing 100 μg/mL ampicillin. The 16S rRNA genes were amplified in PCR reactions containing standard vector primers M13F and M13R. PCR products were purified using the AMPure magnetic bead system (Agencourt Bioscience, Beverly, MA, USA). Sequencing reactions were conducted as described previously using the BigDye V3.1 reaction mix (Applied Biosystems, Foster City, CA, USA) and primers 27F for onefold coverage or 27F and 907R for twofold coverage of ∼500–900 bp of the 5′ end of the 16S rRNA gene [30, 39]. The reverse sequences obtained using the 907R primer differed from the forward sequences, on average, by less than 1%; therefore, reverse sequences were not included in the analysis of most of the experiments. Products were then purified using the CleanSEQ magnetic bead system (Agencourt Bioscience) and analyzed at the University of Wisconsin-Madison Biotechnology Center.
Phylogenetic Analysis and Temporal Stability of Community
Sequences were initially analyzed in SeqMan (DNASTAR, Madison, WI), rapidly aligned using the align tool of the Greengenes web application (www.greengenes.lbl.gov) [40] and then manually aligned further using ARB (www.arb-home.de) [41, 42]. Bellerophon (http://foo.maths.uq.edu.au/∼huber/bellerophon.pl) [43] was used to detect chimeras using the Huber–Hugenholtz correction; poor quality and chimeric sequences were removed from the group to be analyzed further. Distance matrices were generated using the Jukes–Cantor correction in ARB and used in the subsequent analyses. DOTUR (http://schloss.micro.umass.edu/software/dotur.html) [44] was used to assign sequences to operational taxonomic units (OTUs) and to calculate diversity indices for each of the 14 libraries constructed. Sequences that were in the same OTU0.03 (identity ≥97%) were considered to be from the same species, and sequences with an identity of less than 97% were considered to be different species [44]. Sequences that were in the same OTU0.20 (identity ≥80%) were considered to be from the same phylum [44]. BLASTN (http://blast.ncbi.nlm.nih.gov/Blast.cgi) [41] was used to compare sequences in OTUs to sequences in GenBank and to assign identity. The sequences were assigned to phylogenetic divisions and/or species based on those results. An estimate of library coverage was determined using the formula
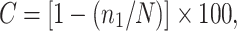 |
where C is the percent coverage, n 1 is the number of sequences appearing once, and N is the total number of sequences in the library [45] for each library. Descriptive characteristics of each library are summarized in Table 1. SONS (http://schloss.micro.umass.edu/software/sons.html) [46] was used to calculate indices that measure similarity in structure (θ, Yue and Clayton index) and the ratio of shared to unshared species (Jaccard's index). The Yue and Clayton index, θ, measures structural similarity by calculating proportions of the community represented by shared and unshared species and placing more weight on shared species that are similar in abundance than those of dissimilar abundance [47]. We used the Lehman and Tilman measure of total community temporal stability, S T, as
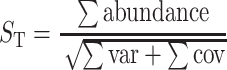 |
[48]. Instead of using absolute abundances as was previously described [48], we calculated S T using relative abundances (proportions of libraries represented by each OTU0.03). Sums were calculated for the mean proportion, and variance for each OTU0.03 represented in at least one of the libraries as well as the covariance for each pair of OTU0.03s.
Table 1.
Characteristics of 16S rRNA gene libraries constructed from midgut communities in cabbage white butterfly larvae reared
| Experiment | Library name | Diet | Date of sampling | Sample composition | No. of sequences | No. of OTUs observeda | Chao estimate OTUs (95% confidence interval) | Good's coverage | Shannon–Weaver diversity index (95% confidence interval) |
|---|---|---|---|---|---|---|---|---|---|
| Effect of diet | BL-1 | Artificial | 1/05 | Pool—10 guts | 79 | 7 | 7.4 (7.0–20) | 97% | 1.2 (0.9–1.4) |
| BL-2 | Sinigrin | 1/05 | Pool—10 guts | 59 | 9 | 10 (9.1–20) | 93% | 1.7 (1.4–1.9) | |
| BL-3 | Brussels sprouts | 1/05 | Pool—10 guts | 69 | 7 | 7.3 (7.0–16) | 97% | 0.7 (0.4–1.0) | |
| Effects of antibiotics | BL-4 | Artificial | 11/05 | Pool—10 guts | 99 | 11 | 14 (11–34) | 96% | 1.7 (1.6–1.9) |
| BL-5 | PenStrep | 11/05 | Pool—10 guts | 100 | 15 | 43 (22–120) | 93% | 2.0 (1.8–2.2) | |
| BL-6 | PenStrep » artificial | 11/05 | Pool—10 guts | 98 | 10 | 13 (10–33) | 97% | 1.4 (1.2–1.6) | |
| Larva-to-larva variation | BL-7 | Artificial | 12/05 | Individual gut | 69 | 9 | 38 (18–115) | 94% | 0.8 (0.4–1.1) |
| BL-8 | Artificial | 12/05 | Individual gut | 48 | 6 | 9.0 (6.4–31) | 98% | 0.9 (0.6–1.2) | |
| BL-9 | Artificial | 12/05 | Individual gut | 56 | 8 | 16 (10–52) | 98% | 0.9 (0.6–1.3) | |
| Batch-to-batch variation | BL-10 | Artificial | 3/04 | Pool—10 guts | 136 | 6 | 7.0 (6.1–20) | 99% | 0.9 (0.6–1.1) |
| BL-11 | Artificial | 11/04 | Pool—10 guts | 146 | 9 | 9.5 (9.0–17) | 99% | 0.6 (0.5–0.8) | |
| Larva-to-larva variation | BL-12 | Artificial | 8/06 | Individual gut | 77 | 8 | 8.0 (8.0–8.0) | 99% | 1.6 (1.4–1.8) |
| BL-13 | Artificial | 8/06 | Individual gut | 82 | 8 | 14 (12–33) | 96% | 1.4 (1.2–1.6) | |
| BL-14 | Artificial | 8/06 | Individual gut | 76 | 10 | 14 (11–34) | 95% | 1.7 (1.5–1.9) |
Artificial sterile artificial diet, Sinigrin sterile artificial diet amended with sinigrin, PenStrep sterile artificial diet amended with penicillin and streptomycin, PenStrep » artificial PenStrep transferred to sterile artificial diet
aAll sequences within each OTU differ by a sequence divergence of 3% or less
Susceptibility to Invasion
To assess the effects of diet and antibiotics on invasion, we placed larvae in sterile Petri dishes without food for 4 to 6 h, after which they were fed a diet disk inoculated with approximately 1.0 × 107 colony-forming units (CFUs) of one bacterial strain (Table 2). Each strain was chosen because of its relationship with the cabbage white butterfly midgut community. Pantoea stewartii CWB600 is native to the cabbage white butterfly midgut environment but is exogenous to the sterile artificial diet community (Table 2). This strain was also naturally resistant to penicillin and streptomycin (data not shown). Pantoea sp. CWB304 is native to the communities in larvae reared on sterile artificial diet community and Brussels sprouts and served as a colonization control [49] (Table 2). Two strains were chosen because they were completely exogenous to the cabbage white butterfly midgut community. Enterobacter sp. NAB3R was isolated from the gypsy moth and Bacillus cereus UW85 was originally isolated from soil [30, 50]. Interestingly, B. cereus UW85 also produces zwittermicin, a broad-spectrum antibiotic [51]. Dissections were performed as previously described 24 h after the bacterial feeding [30]. Experiments were conducted at least three times, and bacterial counts were log transformed to reduce heteroscedasity and analyzed by analysis of variance. When transformations were inappropriate, the Kruskal–Wallis and Mann–Whitney tests were used to determine significance. Statistical computations were conducted using Minitab Statistical Software (Minitab, Inc., State College, PA) and R version 2.4.1 (http://www.r-project.org/) [52].
Table 2.
Bacterial strains used in this study
| Strains | Description | Source/reference | Medium |
|---|---|---|---|
| Pantoea stewartii CWB600 | Isolate from midguts of cabbage white butterfly larvae fed Brussels sprouts | This study | 1/10th TSB |
| Bacillus cereus UW85 | Zwittermicin-producing isolate from soil with biocontrol activity | [21] | 1/10th TSB |
| Enterobacter sp. NAB3R | RifR spontaneous mutant of isolate from midguts of gypsy moth larvae fed sterile artificial diet | [70] | 1/10th TSB |
| Pantoea sp. CWB304 | NalR spontaneous mutant of isolate from midguts of cabbage white butterfly larvae fed artificial diet | [61] | 1/10th TSB + Nal |
Rif rifampicin, Nal nalidixic acid, 1/10th TSB one tenth strength tryptic soy broth
Nucleotide Accession Numbers
16S rRNA gene sequences from this study were deposited in GenBank and are available through the accession numbers DQ342363–DQ343128, DQ349068–DQ349097, DQ537959–DQ538132, EU352364–EU352599, and EU984512.
Results
Species- and Phylum-Level Composition
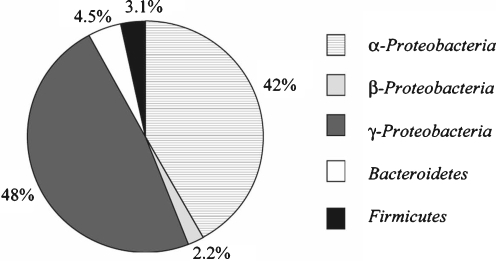
The bacterial community in the cabbage white butterfly midgut contained 103–106 CFUs per gut (data not shown) and is dominated by organisms whose 16S rRNA genes affiliated with those of the Proteobacteria with additional sequences from the Firmicutes and Bacteroidetes (Fig. 1). The most abundant sequences affiliated with the genera Methylobacteria, Asaia, Enterobacter, Acinetobacter, and Pantoea (Table 3). Less prevalent sequences affiliated with the genera Escherichia, Roseomonas, Lactobacillus, Enterococcus, Staphylococcus, Acidovorax, Pseudomonas, Rhizobium, Bacillus, Imtechium, Moraxella, Ralstonia, Hymenobacter, Flavobacterium, Propionibacterium, Nevskia, Corynebacterium, and Comamonas (Supplementary Table 1). Eight species affiliates (OTUs that affiliated with specific species) were considered predominant because they were present in at least 50% of the libraries and/or represented the most sequences in at least one library (Table 3). Seven of the eight dominant species affiliates grouped with Proteobacteria and one grouped with Bacteroidetes. Other phyla represented among the less prevalent species affiliates included Firmicutes, Actinobacteria, and Chloroflexi (Fig. 1). Observations at the phylum level and subphylum level revealed differences in the distribution between the α- and γ-Proteobacteria subphyla (Fig. 1 and Table 3). Three of the seven Proteobacteria affiliates that were dominant community members were α-Proteobacteria, and four were γ-Proteobacteria.
Figure 1.
Average distribution of clones affiliated with the α-, β-, and γ-Proteobacteria subphyla and other phyla identified in cabbage white butterfly midgut bacterial communities. Graph excludes Actinobacteria and Chloroflexi, which were each detected once in one sample (0.13%)
Table 3.
Predominant species in the larval midgut community of the cabbage white butterfly
| Phylogenetic division | Representative clone GenBank accession no. | Closest culturable match GenBank; accession no.; % identity | Proportion of libraries containing OTU (%) | Representation of OTU in library (%) | Proportion of libraries dominated by OTU (%) |
|---|---|---|---|---|---|
 Proteobacteria Proteobacteria |
DQ342928 | Phenanthrene-degrading bacterium; AY177358; 99% | 91 | 34 | 36 |
| DQ342958 | Roseomonas gilardii AY150045; 100% | 64 | 3 | 0 | |
| DQ342728 | Asaia sp. SF2.1; AB025929; 99% | 55 | 5 | 9 | |
| γ-Proteobacteria | DQ342888 | Enterobacter cloacae; AB244457; 99% | 73 | 24 | 27 |
| DQ342721 | Acinetobacter sp. pheno2; APH278311; 99% | 73 | 10 | 18 | |
| DQ342869 | Escherichia coli O157:H7; 99% | 64 | 3 | 0 | |
| DQ342866 | Pantoea sp. PPE7; AY501386; 97% | 36 | 7 | 9 | |
| Bacteroidetes | DQ342843 | Flavobacteriaceae bacterium YMS-2; EF017801; 99% | 64 | 4 | 0 |
OTUs were present in ≥50% of libraries or were the most abundant OTU in a library
Batch-to-Batch and Larva-to-Larva Variation, and Temporal Stability
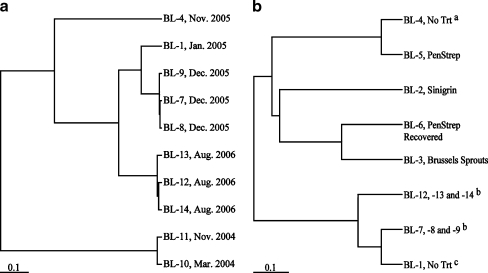
In order to assess stability of the gut flora in an insect colony over time and compare batch-to-batch variation, midgut communities from the lab colony larvae were sampled at two dates, March 2004 (BL-10) and November 2004 (BL-11). The communities were similar in both structure and membership at the species level (Fig. 2a and Table 4). In contrast, the structures of communities from larvae reared from commercially obtained eggs sampled in January (BL-1) and November 2005 (BL-4) were not similar in structure or membership at the species level (Fig. 2a, Table 4, and Supplementary Table 1).
Fig. 2.
Comparisons of community structure as determined by Yue and Clayton (θ) indices at OTU0.03. a Batch-to-batch and larva-to-larva structural variation. Unweighted pair-group method using arithmetic mean (UPGMA) clustering of communities from larvae reared on sterile artificial diet only. Library names are followed by date of sampling. b Effects of diet on community structure. UPGMA clustering of communities in larvae reared on sterile artificial diet (No Trt), Brussels sprouts, sinigrin, penicillin and streptomycin (PenStrep), or penicillin and streptomycin and then transferred to sterile artificial diet (PenStrep Recovered). Reference bar length corresponds to a distance of 0.10 (distance = 1−θ). No treatment control for antibiotics experiment (a); sequences from libraries constructed from individual larvae reared on sterile artificial diet were pooled and treated as one library (b); no treatment control for diet experiment (c)
Table 4.
Pairwise comparisons of structural similarity between batches of larvae and individual larvae fed sterile artificial diet
| Libraries compared | Yue and Clayton indexa | Jaccard's index |
|---|---|---|
| Batches of larvae | ||
| BL-1 (Jan. 2005) × BL-4 (Nov. 2005) | 0.24 (0.06)b | 0.24 |
| BL-10 (Mar. 2004) × BL-11 (Nov. 2004) | 0.97 (0.01) | 0.12 |
| Combined BL-7, 8 and 9 (Dec. 2005) × combined BL-12,13, and 14 (Aug. 2006)c | 0.65 (0.06)b | 0.32 |
| Individual larvae | ||
| BL-7 (larva 1-Dec. 2005) × BL-8 (larva 2-Dec. 2005) | 0.99 (0.01) | 0.23 |
| BL-7 (larva 1-Dec. 2005) × BL-9 (larva 3-Dec. 2005) | 0.99 (0.01) | 0.5 |
| BL-8 (larva 2-Dec. 2005) × BL-9 (larva 3-Dec. 2005) | 0.98 (0.01) | 0.4 |
| BL-12 (larva 1-Aug. 2006) × BL-13 (larva 2-Aug. 2006) | 0.96 (0.05) | 0.45 |
| BL-12 (larva 1-Aug. 2006) × BL-14 (larva 3-Aug. 2006) | 0.98 (0.02) | 0.58 |
| BL-13 (larva 2-Aug. 2006) × BL-14 (larva 3-Aug. 2006) | 0.98 (0.02) | 0.43 |
Larvae within a batch hatched from eggs from the same source were reared at the same time and on the same diet and sampled at the same time
aYue and Clayton index provided with 95% confidence interval in parentheses
bThe value differs significantly from 1.0. Similarity increases as value approaches 1.0 for both indices
cLibraries BL-7, BL-8, and BL-9 and BL-12, BL-13, and BL-14 were constructed from individual midguts. The resulting sequences were combined into two groups based on batch (Dec. 2005 or Aug. 2006) and then compared to each other
There was high similarity among communities in the midguts of the individual larvae that were fed the same diet, sampled at the same time, and sampled on an individual basis (BL-7, BL-8, and BL-9 sampled in December 2005 and BL-12, BL-13, and BL-14 sampled in August 2006). Species affiliates detected in at least two of the three communities sampled in December 2005 included Methylobacterium, Asaia, Acinetobacter, Ralstonia, and Staphylococcus affiliates as well as an OTU that affiliated closely with the Cytophaga–Flavobacterium–Bacteroides division (Supplementary Table 1). At least two of the three communities sampled in August 2006 contained the same Methylobacterium, Ralstonia, and Staphylococcus affiliates listed above as well as Propionibacterium and Corynebacterium affiliates (Supplementary Table 1). These communities also shared similar levels of diversity and were almost identical structurally (Fig. 2a, Table 4).
As controls, the temporal stabilities of communities sampled at the same time were calculated. As expected, when the temporal stability of communities in larvae sampled from the same batch was calculated, the stability was high (S T = 19.6 and 21.6 for BL 7–9 and BL 12–14, respectively). The temporal stability of all communities, across all batches (BL 1–9, 12–14) was relatively low (S T = 2.39).
Resistance to Diet- and Antibiotic-Induced Changes
Larvae were reared from hatching on sterile artificial diet (BL-1; the control community to which others would be compared), sterile artificial diet amended with sinigrin (BL-2), or Brussels sprouts (BL-3). All communities contained sequences whose closest cultured matches in GenBank were Methylobacterium sp. PB133 (99% similarity) and Roseomonas gilardii strain ATCC49956 (100% similarity). The addition of sinigrin to artificial diet, or substituting Brussels sprouts for artificial diet, resulted in a different midgut community composition and structure (Fig. 2b, Table 5, and Supplementary Table 1). The compositions of the communities in the larvae reared on Brussels sprouts or sinigrin were more similar to each other than either was to those in larvae reared on unamended diet (Supplementary Table 1). For example, addition of sinigrin to artificial diet or substitution with Brussels sprouts resulted in the presence of an Enterobacter species that was not detected in the communities of larvae fed unamended diet and in the tenfold reduction of the representation of a Methylobacterium species that had been the dominant member of the control communities (Supplementary Table 1). Despite differences in total composition, there was overlap among some members of the three communities (Supplementary Table 1).
Table 5.
Pairwise comparisons of structural similarity of communities from larvae fed different diets
| Libraries compared | Yue and Clayton indexa | Jaccard's index |
|---|---|---|
| BL-1 (unamended) × BL-2 (sinigrin) | 0.08 (0.04)b | 0.22 |
| BL-1 (unamended) × BL-3 (Brussels sprouts) | 0.05 (0.03)b | 0.36 |
| BL-2 (sinigrin) × BL-3 (Brussels sprouts) | 0.42 (0.11)b | 0.28 |
| BL-4 (unamended) × BL-5 (penicillin and streptomycin) | 0.88 (0.06) | 0.5 |
| BL-4 (unamended) × BL-6 (penicillin and streptomycin recovered) | 0.26 (0.06)b | 0.57 |
| BL-5 (penicillin and streptomycin) × BL-6 (penicillin and streptomycin recovered) | 0.37 (0.08)b | 0.53 |
Unamended larvae were fed unamended sterile artificial diet, sinigrin larvae were fed sterile artificial diet amended with sinigrin at 3.0 mg/mL, Brussels sprouts larvae were fed Brussels sprouts, penicillin and streptomycin larvae were fed sterile artificial diet amended with penicillin and streptomycin at 10 units/ml and 10 mg/ml, respectively, penicillin and streptomycin recovered larvae were fed penicillin and streptomycin and then transferred to unamended sterile artificial diet 24 h before they molted to fourth instar
aYue and Clayton index provided with 95% confidence interval in parentheses
bThe value is significantly different from 1.0. Similarity increases as value approaches 1.0 for both indices
Resistance to antibiotic-induced changes was assessed by rearing larvae on untreated sterile artificial diet, or sterile artificial diet amended with antibiotics, and sampling the communities. Two experiments were conducted. Trial 1 revealed the communities in these larvae were similar in composition, but the addition of antibiotics resulted in the detection of previously unobserved species (e.g., an affiliate of Pantoea sp. PPE7, Supplementary Table 1), and an increase of the Chao1 estimated richness from 14 (11–34) to 43 (22–120) (BL-4 and BL-5; Table 1). Testing whether communities could recover by transferring larvae from diet that contained antibiotics to diet that did not contain antibiotics (BL-6) resulted in a community that was significantly different from the communities in larvae fed only antibiotic-containing diet (θ = 0.26, s.e. = 0.06) and in larvae fed only unamended diet (θ = 0.37, s.e. = 0.08; Table 5). The recovery period also resulted in the increased detection of a Pantoea species that was previously detected at low levels only in the communities of larvae fed antibiotics (Fig. 3) and in the twofold reduction of an Acinetobacter species that represented about 30% of the antibiotic and control communities (Supplementary Table 1).
Figure 3.
Distribution of predominant γ-Proteobacteria in libraries constructed from larvae reared on unamended sterile artificial diet (BL-4), sterile artificial diet amended with penicillin and streptomycin (BL-5), or sterile artificial diet amended with penicillin and streptomycin and then transferred to unamended artificial diet (BL-6)
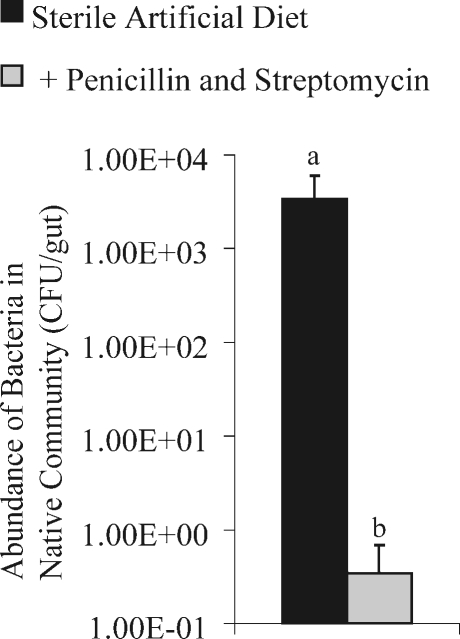
In trial 2, the addition of antibiotics increased the Chao1 estimated species richness from 8.25 (8.0–12.8) to 15.3 (12.5–34.1), similar to what was observed in trial 1, although less dramatic. Unlike the treatment of communities sampled in trial 1, in trial 2, antibiotics significantly altered the structure of community (θ = 0.07, s.e. = 0.02). The dominant member of the control community was an Asaia species, but the dominant member in the microbial community within larvae fed antibiotics was an Acidovorax species. Additionally, antibiotics in artificial diet reduced the abundance of culturable bacteria by 1,000-fold (Fig. 4).
Figure 4.
Effect of antibiotics on abundance of culturable bacteria. Midguts from cabbage white butterfly larvae fed untreated sterile artificial diet (n = 24) or penicillin and streptomycin (n = 29) were plated on one tenth strength tryptic soy agar. Error bars are standard error mean. Different letters indicate significant difference (Kruskal–Wallis and Mann–Whitney; P < 0.05)
Resistance to Invasion
Establishment by Pantoea stewartii CWB600 was enhanced in cabbage white butterfly larval midguts by feeding antibiotics (Mann–Whitney test, P < 0.01; Fig. 5a). When compared to feeding on untreated sterile artificial diet and feeding on Brussels sprouts, feeding on sinigrin increased community susceptibility to establishment by Enterobacter sp. NAB3R (Mann–Whitney test, P < 0.01; Fig. 5b). A similar pattern was observed when Pantoea sp. CWB304 was introduced to communities of larvae fed the diets above, although establishment was not increased significantly (Mann–Whitney test, P = 0.13 and 0.27, respectively, Fig. 5b).
Figure 5.
Effects of antibiotics and diet on establishment of invaders in cabbage white butterfly midgut communities. a Larvae fed unamended sterile artificial diet (n = 36) or penicillin and streptomycin (100 units/ml and 100 μg/ml, respectively; n = 33) from hatching were fed Pantoea sp. CWB600. b Larvae fed unamended sterile artificial diet (n = 27) or sterile artificial diet amended with sinigrin (3.0 mg/ml; n = 18) or Brussels sprouts (n = 23) from hatching were fed Pantoea sp. CWB304. Error bars are standard error mean. Different letters indicate that values differ significantly (Kruskal–Wallis and Mann–Whitney; P values <0.05
Discussion
Here, we explore robustness of the cabbage white butterfly midgut bacterial community as a potential model system for studying both specific aspects of microbial ecology and general theories of biological invasion. We calculated variation in community structure over time, examined the effects of diet and antibiotics on this community, and explored the community's susceptibility to invasion.
Steinhaus observed that the alimentary tract of adult cabbage white butterflies contained Enterobacter spp. (called Aerobacter spp. in 1941), as well as a Flavobacterium sp. [33]. We likewise detected these members and further determined that the larval stage of this insect contains several additional genera. The community is similar in composition to those found in other insects that have recently been characterized using culture-independent techniques. For example, Enterobacter and Pantoea have been identified in numerous insects including gypsy moths (Lepidoptera: Lymantriidae), ant lions (Neuroptera: Myrmeleontidae), biting midges (Diptera: Ceratopogonidae), stable flies (Diptera:Muscidae), grasshoppers (Orthoptera:Acrididae), mosquitoes (Diptera:Culicidae), and thrips (Thysanoptera: Thripidae) as well as the cabbage white butterfly (Lepidoptera: Pieridae) [30, 53–56]. Other members of the cabbage white butterfly larval midgut community that are also found in other insects are Bacillus, Acinetobacter, Lactobacillus, and Pseudomonas spp. [57–60].
In addition to identifying the compositional similarities that exist between the bacterial communities in cabbage white butterfly and other insects, this study also revealed that the cabbage white butterfly midgut bacterial community exhibits temporal instability at the species level and conservation of membership at the phylum level. Similar patterns of change and conservation of membership that are linked to a specific phylogenetic level occur in other communities as well. The human colon, for example, experiences changes in membership at the species level throughout the lifetime of an individual, between individuals, and in response to diet, but phylum-level and often genus-level memberships are consistent [61–67]. This sort of phylum-level stability is also exhibited in the gypsy moth, in which species composition changes with diet, while the γ-Proteobacteria and Firmicutes phyla are present in under all conditions [30]. This trend toward consistency at higher taxonomic levels and flexibility at lower levels may indicate that the overall function of a community is often more important than the presence of particular members. This explanation has been suggested for reported variations among human gastrointestinal microbial communities [68]. In this case, multiple members within certain groups of bacteria, i.e., the α-Proteobacteria, might be equally able to meet particular functional requirements.
Functional requirements of communities may also play a role in the observation that host plants and phytochemicals, specifically Brussels sprouts and sinigrin, alter community structure in cabbage white butterfly larvae midguts. Our hypothesis was that amending sterile artificial diet with sinigrin, a major component of Brussels sprouts, would alter the community to resemble that in larvae fed Brussels sprouts. In the wild, the cabbage white butterfly midgut community is consistently exposed to sinigrin, which is a glucosinolate—a class of phytochemicals whose breakdown products have antimicrobial properties [69–73]. Sinigrin resulted in a community that was more similar to the community Brussels sprouts-fed larvae than the sterile artificial diet-fed larvae, but was significantly different from both. Relatives of several cabbage white butterfly community members are able degrade sinigrin and utilize the end products of insect-mediated sinigrin degradation suggesting the possibility that certain community members assist in degradation of sinigrin and benefit from it [74–80]. Future work including the functional analyses of the community in larvae-fed sinigrin would likely reveal that some proportion of this community is able to degrade and/or utilize sinigrin.
The presence of antibiotics in the artificial diet perturbed the community structure and resulted in changes in susceptibility to invasion, as did the presence of sinigrin. Our results provide experimental support for the view that perturbation can increase the susceptibility of communities to invasion [81, 82]. Both theoretical treatments and correlative analyses have argued for the importance of this relationship, but more widespread acceptance and implementation of corresponding management tactics have lagged pending more direct evidence. The manipulative experiments on insect gut communities described here will hopefully help bridge these various approaches and scales. It is not yet possible to quantify the generality of our results, but it is informative that similar conclusions emerge from studies of macroscale (e.g., lagoon, grassland) [81–84] and microscale (e.g., gut microbial) communities.
These results reinforce the importance of community structure and microbe–microbe interactions and support the view that the presence or absence of only a few members may influence community resistance and susceptibility to invasion. This raises another point of interest that has been explored in macroscale communities—the impact of invaders on existing community interactions [85, 86]. Because of this community's relatively low richness, it would be amenable to studies that seek to introduce an exogenous species and monitor the resulting changes in the community.
Our experiments with antibiotics indicate that perturbation can alter the relative abundance of various community members. Specifically, we detected community members after antibiotic treatment that were not detectable before treatment. The antibiotic might have reduced the population sizes of some members that normally dominate the community, enabling rare members to fill the vacated niche. For example, a Pantoea affiliate that was not detected in the artificial diet community (trial 1, BL-4) and represented a small fraction of the community in antibiotic-fed larvae became the numerically dominant member (51%) of the recovered community (Fig. 3). Similarly, the population of this Pantoea affiliate increased to 20% of the community following larval consumption of Brussels sprouts. Antibiotic treatment also increased the number of species present at low abundance, producing higher species richness. The results of both studies indicate that this community may have low resistance to structural change when confronted with antibiotic exposure, and trial 1 suggests that the community may lack short-term structural resilience after antibiotic exposure. A longer recovery period might reveal that the community has the capability to return to a structure similar to its native state as is the case for other microbial communities (i.e., [18]). The differences we observed between our antibiotic trials, as well as the differences among batches of larvae suggest that the assembly and reassembly processes are complex and include both elements of randomness and underlying species interactions, host–microbial relationships, and external drivers not yet understood. These features make the cabbage white butterfly community a rich opportunity for studying secondary succession, the process by which a community reestablishes following a disturbance [87].
Model systems have proven to be essential to understanding microbial interactions [88]. For example, study of the squid–Vibrio symbiosis led to the discovery of bacterial quorum sensing [89–92]. The termite hindgut has been a fruitful source of information about metabolic processes in communities, including the demonstration of microbe-regulated oxygen and hydrogen gradients and the linking of function and spatial organization to specific organisms [93, 94]. Each of these systems has characteristics that make it amenable to the kinds of studies that led to development of new principles in microbial ecology. We present the cabbage white butterfly larval community as a relatively simple, easily manipulatable, multispecies community in which to test ecological hypotheses about interspecies interactions and community robustness.
In this study, we initiated the evaluation of robustness of the bacterial community in the cabbage white butterfly larval midgut by measuring temporal stability, resistance, and resilience. Further exploration of robustness will generate principles that govern the dynamics of this community and perhaps others. Understanding the determinants of robustness will also require development of improved statistical tools to quantify it. Because the lepidopteran larval gut presents a community that is relatively simple, tractable, and easy to manipulate, it is ideal for building and testing statistical models and investigating ecological events such as succession and invasion and the basis for robustness.
Electronic Supplementary Materials
Below is the link to the electronic supplementary material.
Proportion of each library represented by members (OTU0.03) of the cabbage white butterfly midgut community (percentage) (PDF 1146 kb)
Acknowledgements
We thank Lisa Chanbusurakum, Stephanie Hicks, Eric Vasquez and Jane Remfert for technical assistance and Nichole Broderick for helpful discussions. This work was supported by the Howard Hughes Medical Institute; CJR was supported by the University of Wisconsin Biotechnology Training Grant NIGMS 5 T32 GM08349, the University of Wisconsin Advanced Opportunity Fellowship, a Ford Foundation Diversity Fellowship, and the UW-Madison College of Agricultural and Life Sciences.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Butler JL, Williams MA, Bottomley PJ, Myrold DD. Microbial community dynamics associated with rhizosphere carbon flow. Appl Environ Microbiol. 2003;69:6793–6800. doi: 10.1128/AEM.69.11.6793-6800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carrero-Colon M, Nakatsu CH, Konopka A. Effect of nutrient periodicity on microbial community Dynamics. Appl Environ Microbiol. 2006;72:3175–3183. doi: 10.1128/AEM.72.5.3175-3183.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiorboe T, Tang K, Grossart H-P, Ploug H. Dynamics of microbial communities on marine snow aggregates: colonization, growth, detachment, and grazing mortality of attached bacteria. Appl Environ Microbiol. 2003;69:3036–3047. doi: 10.1128/AEM.69.6.3036-3047.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vinas M, Sabate J, Espuny MJ, Solanas AM. Bacterial community dynamics and polycyclic aromatic hydrocarbon degradation during bioremediation of heavily creosote-contaminated soil. Appl Environ Microbiol. 2005;71:7008–7018. doi: 10.1128/AEM.71.11.7008-7018.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Höfle M, Haas H, Dominik K. Seasonal dynamics of bacterioplankton community structure in a eutrophic lake as determined by 5S rRNA analysis. Appl Environ Microbiol. 1999;65:3164–3174. doi: 10.1128/aem.65.7.3164-3174.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999;69:1035S–1045. doi: 10.1093/ajcn/69.5.1035s. [DOI] [PubMed] [Google Scholar]
- 7.Smalla K, Wieland G, Buchner A, Zock A, Parzy J, Kaiser S, Roskot N, Heuer H, Berg G. Bulk and rhizosphere soil bacterial communities studied by denaturing gradient gel electrophoresis: plant-dependent enrichment and seasonal shifts revealed. Appl Environ Microbiol. 2001;67:4742–4751. doi: 10.1128/AEM.67.10.4742-4751.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Wielen PW, Keuzenkamp DA, Lipman LJ, van Knapen F, Biesterveld S. Spatial and temporal variation of the intestinal bacterial community in commercially raised broiler chickens during growth. Microb Ecol. 2002;44:286–293. doi: 10.1007/s00248-002-2015-y. [DOI] [PubMed] [Google Scholar]
- 9.Vasanthakumar A, Delalibera I, Handelsman J, Klepzig KD, Schloss PD, Raffa KF. Characterization of gut-associated bacteria in larvae and adults of the southern pine beetle, Dendroctonus frontalis Zimmermann. Environ Entomol. 2006;35:1710–1717. doi: 10.1603/0046-225X(2006)35[1710:COGBIL]2.0.CO;2. [DOI] [Google Scholar]
- 10.Vila-Costa M, Pinhassi J, Alonso C, Pernthaler J, Simo R. An annual cycle of dimethylsulfoniopropionate-sulfur and leucine assimilating bacterioplankton in the coastal NW Mediterranean. Environ Microbiol. 2007;9:2451–2463. doi: 10.1111/j.1462-2920.2007.01363.x. [DOI] [PubMed] [Google Scholar]
- 11.Apajalahti JHA, Kettunen A, Bedford MR, Holben WE. Percent G + C profiling accurately reveals diet-related differences in the gastrointestinal microbial community of broiler chickens. Appl Environ Microbiol. 2001;67:5656–5667. doi: 10.1128/AEM.67.12.5656-5667.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apajalahti JHA, Kettunen H, Kettunen A, Holben WE, Nurminen PH, Rautonen N, Mutanen M. Culture-independent microbial community analysis reveals that inulin in the diet primarily affects previously unknown bacteria in the mouse cecum. Appl Environ Microbiol. 2002;68:4986–4995. doi: 10.1128/AEM.68.10.4986-4995.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill JE, Hemmingsen SM, Goldade BG, Dumonceaux TJ, Klassen J, Zijlstra RT, Goh SH, Van Kessel AG. Comparison of ileum microflora of pigs fed corn-, wheat-, or barley-based diets by chaperonin-60 sequencing and quantitative PCR. Appl Environ Microbiol. 2005;71:867–875. doi: 10.1128/AEM.71.2.867-875.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knarreborg A, Simon MA, Engberg RM, Jensen BB, Tannock GW. Effects of dietary fat source and subtherapeutic levels of antibiotic on the bacterial community in the ileum of broiler chickens at various ages. Appl Environ Microbiol. 2002;68:5918–5924. doi: 10.1128/AEM.68.12.5918-5924.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leser TD, Lindecrona RH, Jensen TK, Jensen BB, Moller K. Changes in bacterial community structure in the colon of pigs fed different experimental diets and after infection with Brachyspira hyodysenteriae. Appl Environ Microbiol. 2000;66:3290–3296. doi: 10.1128/AEM.66.8.3290-3296.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collier CT, Smiricky-Tjardes MR, Albin DM, Wubben JE, Gabert VM, Deplancke B, Bane D, Anderson DB, Gaskins HR. Molecular ecological analysis of porcine ileal microbiota responses to antimicrobial growth promoters. J Anim Sci. 2003;81:3035–3045. doi: 10.2527/2003.81123035x. [DOI] [PubMed] [Google Scholar]
- 17.Jernberg C, Löfmark S, Edlund C, Jansson JK. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME Jl. 2007;1:56–66. doi: 10.1038/ismej.2007.3. [DOI] [PubMed] [Google Scholar]
- 18.Young VB, Schmidt TM. Antibiotic-associated diarrhea accompanied by large-scale alterations in the composition of the fecal microbiota. J Clin Microbiol. 2004;42:1203–1206. doi: 10.1128/JCM.42.3.1203-1206.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shigesada N, Kawasaki K. Biological invasions : theory and practice. Oxford: Oxford University Press; 1997. p. 4. [Google Scholar]
- 20.Williamson M. Biological invasions. New York: Chapman; 1996. [Google Scholar]
- 21.Fox JG, Wang TC, Rogers AB, Poutahidis T, Ge Z, Taylor N, Dangler CA, Israel DA, Krishna U, Gaus K, Peek RM., Jr Host and microbial constituents influence Helicobacter pylori-induced cancer in a murine model of hypergastrinemia. Gastroenterology. 2003;124:1879–1890. doi: 10.1016/S0016-5085(03)00406-2. [DOI] [PubMed] [Google Scholar]
- 22.Hirano SS, Upper CD. Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae—a pathogen, ice nucleus, and epiphyte. Microbiol Mol Biol Rev. 2000;64:624–653. doi: 10.1128/MMBR.64.3.624-653.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed FE. Genetically modified probiotics in foods. Trends Biotechnol. 2003;21:491–497. doi: 10.1016/j.tibtech.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert GS, Clayton MK, Handelsman J, Parke JL. Use of cluster and discriminant analyses to compare rhizosphere bacterial communities following biological perturbation. Microb Ecol. 1996;32:123–147. doi: 10.1007/BF00185884. [DOI] [PubMed] [Google Scholar]
- 25.Guarner F. Enteric flora in health and disease. Digestion. 2006;73(Suppl 1):5–12. doi: 10.1159/000089775. [DOI] [PubMed] [Google Scholar]
- 26.Vollaard EJ, Clasener HA. Colonization resistance. Antimicrob Agents Chemother. 1994;38:409–414. doi: 10.1128/aac.38.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winberg J, Herthelius-Elman M, Mollby R, Nord CE. Pathogenesis of urinary tract infection—experimental studies of vaginal resistance to colonization. Pediatr Nephrol. 1993;7:509–514. doi: 10.1007/BF00852528. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy TA, Naeem S, Howe KM, Knops JMH, Tilman D, Reich P. Biodiversity as a barrier to ecological invasion. Nature. 2002;417:636–638. doi: 10.1038/nature00776. [DOI] [PubMed] [Google Scholar]
- 29.Olyarnik SV, Bracken MES, Byrnes JE, Hughes AR, Hultgren KM, Stachowicz JJ (2009) Ecological factors affecting community invasibility: biological invasions in marine ecosystems, 215–238
- 30.Broderick NA, Raffa KF, Goodman RM, Handelsman J. Census of the bacterial community of the gypsy moth larval midgut by using culturing and culture-independent methods. Appl Environ Microbiol. 2004;70:293–300. doi: 10.1128/AEM.70.1.293-300.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berenbaum M. Adaptive significance of midgut pH in larval lepidoptera. Am Nat. 1980;115:138. doi: 10.1086/283551. [DOI] [Google Scholar]
- 32.Broadway RM. Characterization and ecological implications of midgut proteolytic activity in Larvalpieris rapae and Trichoplusia ni. J Chem Ecol. 1989;15:2101–2113. doi: 10.1007/BF01207441. [DOI] [PubMed] [Google Scholar]
- 33.Steinhaus EA. A study of the bacteria associated with thirty species of insects. J Bacteriol. 1941;42:757–790. doi: 10.1128/jb.42.6.757-790.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tilman D. The ecological consequences of changes in biodiversity: a search for general principles. Ecology. 1999;80:1455–1474. [Google Scholar]
- 35.McCann KS. The diversity—stability debate. Nature. 2000;405:228–233. doi: 10.1038/35012234. [DOI] [PubMed] [Google Scholar]
- 36.Pimm SL. The complexity and stability of ecosystems. Nature. 1984;307:321–326. doi: 10.1038/307321a0. [DOI] [Google Scholar]
- 37.Grimm V, Wissel C. Babel, or the ecological stability discussions: an inventory and analysis of terminology and a guide for avoiding confusion. Oecologia. 1997;109:323–334. doi: 10.1007/s004420050090. [DOI] [PubMed] [Google Scholar]
- 38.van Doorn HE, van Holst GJ, van der Kruk GC, Raaijmakers-Ruijs NCME, Postma E. Quantitative determination of the glucosinolates sinigrin and progoitrin by specific antibody ELISA assays in Brussels sprouts. J Agric Food Chem. 1998;46:793–800. doi: 10.1021/jf970523z. [DOI] [PubMed] [Google Scholar]
- 39.Lane DJ, Stackebrandt E, Goodfellow M. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucelic acid techniques in bacterial systematics. New York: Wiley; 1991. pp. 115–175. [Google Scholar]
- 40.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Altschul SF, Gish W, Miller W, Meyers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 42.Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar BA, Lai T, Steppi S, Jobb G, Forster W, Brettske I, Gerber S, Ginhart AW, Gross O, Grumann S, Hermann S, Jost R, Konig A, Liss T, Lussmann R, May M, Nonhoff B, Reichel B, Strehlow R, Stamatakis A, Stuckmann N, Vilbig A, Lenke M, Ludwig T, Bode A, Schleifer K-H. ARB: a software environment for sequence data. Nucleic Acids Res. 2004;32:1363–1371. doi: 10.1093/nar/gkh293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huber T, Faulkner G, Hugenholtz P (2004) Bellerophon: a program to detect chimeric sequences in multiple sequence alignments. Bioinformatics 20:2317–2319 [DOI] [PubMed]
- 44.Schloss PD, Handelsman J. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl Environ Microbiol. 2005;71:1501–1506. doi: 10.1128/AEM.71.3.1501-1506.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Good IJ. The population frequencies of species and the estimation of population parameters. Biometrika. 1953;40:237–264. [Google Scholar]
- 46.Schloss PD, Handelsman J. Introducting SONS, a tool that compares the membership of microbial communities. Appl Environ Microbiol. 2006;72:6773–6779. doi: 10.1128/AEM.00474-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yue JC, Clayton MK. A similarity measure based on species proportions. Comm Statist Theory Methods. 2005;34:2123–2131. doi: 10.1080/STA-200066418. [DOI] [Google Scholar]
- 48.Lehman CL, Tilman D. Biodiversity, stability, and productivity in competitive communities. Am Nat. 2000;156:534–552. doi: 10.1086/303402. [DOI] [PubMed] [Google Scholar]
- 49.Borlee BR, Geske GD, Robinson CJ, Blackwell HE, Handelsman J. Quorum-sensing signals in the microbial community of the cabbage white butterfly larval midgut. ISME J. 2008;2:1101–1111. doi: 10.1038/ismej.2008.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gilbert GS, Parke MK, Clayton MK, Handelsman J. Effects of an introduced bacterium on bacterial communities on roots. Ecology. 1993;74:840–854. doi: 10.2307/1940810. [DOI] [Google Scholar]
- 51.Silo-Suh LA, Lethbridge BJ, Raffel SJ, He H, Clardy J, Handelsman J. Biological activities of two fungistatic antibiotics produced by Bacillus cereus UW85. Appl Environ Microbiol. 1994;60:2023–2030. doi: 10.1128/aem.60.6.2023-2030.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ihaka R, Gentleman R. R: a language for data analysis and graphics. J Comput Graph Stat. 1996;5:299–314. doi: 10.2307/1390807. [DOI] [Google Scholar]
- 53.Hunt J, Charnley AK. Abundance and distribution of the gut flora of the desert locust, Schistocerca gregaria. J Invertebr Pathol. 1981;38:378–385. doi: 10.1016/0022-2011(81)90105-1. [DOI] [Google Scholar]
- 54.Mead LJ, Khachatourians GG, Jones GA. Microbial ecology of the gut in laboratory stocks of the migratory grasshopper, Melanoplus sanguinipes (fab) (Orthoptera: Acrididae) Appl Environ Microbiol. 1988;54:1174–1181. doi: 10.1128/aem.54.5.1174-1181.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Straif SC, Mbogo CN, Toure AM, Walker ED, Kaufman M, Toure YT, Beier JC. Midgut bacteria in Anopheles gambiae and An. Funestus (Diptera: Culicidae) from Kenya and Mali. J Med Entomol. 1998;35:222–226. doi: 10.1093/jmedent/35.3.222. [DOI] [PubMed] [Google Scholar]
- 56.Wells ML, Gitaitis RD, Sanders FH. Association of tobacco thrips, Frankliniella fusca (Thysanoptera: Thripidae) with two species of bacteria of the genus Pantoea. Ann Entomol Soc Am. 2002;95:719–723. doi: 10.1603/0013-8746(2002)095[0719:AOTTFF]2.0.CO;2. [DOI] [Google Scholar]
- 57.Geib SM, Jimenez-Gasco MDM, Carlson JE, Tien M, Hoover K. Effect of host tree species on cellulase activity and bacterial community composition in the gut of larval asian longhorned beetle. Environ Entomol. 2009;38:686–699. doi: 10.1603/022.038.0320. [DOI] [PubMed] [Google Scholar]
- 58.Schloss PD, Delalibera I, Handelsman J, Raffa KF. Bacteria associated with the guts of two wood-boring beetles: Anoplophora glabripennis and Saperda vestita (Cerambycidae) Environ Entomol. 2006;35:625–629. doi: 10.1603/0046-225X-35.3.625. [DOI] [Google Scholar]
- 59.Terenius O, Dantas de Oliveira C, Pinheiro WD, Tadei WP, James AA, Marinotti O. 16S rRNA gene sequences from bacteria associated with adult Anopheles darlingi (Diptera: Culicidae) mosquitoes. J Med Entomol. 2008;45:172–175. doi: 10.1603/0022-2585(2008)45[172:SRGSFB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 60.van der Hoeven R, Betrabet G, Forst S. Characterization of the gut bacterial community in Manduca sexta and effect of antibiotics on bacterial diversity and nematode reproduction. FEMS Microbiol Lett. 2008;286:249–256. doi: 10.1111/j.1574-6968.2008.01277.x. [DOI] [PubMed] [Google Scholar]
- 61.Bik EM, Eckburg PB, Gill SR, Nelson KE, Purdom EA, Francois F, Perez-Perez G, Blaser MJ, Relman DA. Molecular analysis of the bacterial microbiota in the human stomach. Proc Natl Acad Sci. 2006;103:732–737. doi: 10.1073/pnas.0506655103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hopkins MJ, Sharp R, Macfarlane GT. Age and disease related changes in intestinal bacterial populations assessed by cell culture, 16S rRNA abundance, and community cellular fatty acid profiles. Gut. 2001;48:198–205. doi: 10.1136/gut.48.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mitsuoka T (1992) Intestinal flora and aging. Nutr Rev 50: 438–438–446 [DOI] [PubMed]
- 65.Mueller S, Saunier K, Hanisch C, Norin E, Alm L, Midtvedt T, Cresci A, Silvi S, Orpianesi C, Verdenelli MC, Clavel T, Koebnick C, Zunft H-JF, Dore J, Blaut M. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl Environ Microbiol. 2006;72:1027–1033. doi: 10.1128/AEM.72.2.1027-1033.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 67.Woodmansey EJ. Intestinal bacteria and ageing. J Appl Microbiol. 2007;102:1178–1186. doi: 10.1111/j.1365-2672.2007.03400.x. [DOI] [PubMed] [Google Scholar]
- 68.Falk PG, Hooper LV, Midtvedt T, Gordon JI. Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiol Mol Biol Rev. 1998;62:1157–1170. doi: 10.1128/mmbr.62.4.1157-1170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brabban AD, Edwards C. The effects of glucosinolates and their hydrolysis products on microbial-growth. J Appl Bacteriol. 1995;79:171–177. doi: 10.1111/j.1365-2672.1995.tb00931.x. [DOI] [PubMed] [Google Scholar]
- 70.Brandi G, Amagliani G, Schiavano GF, De Santi M, Sisti M. Activity of Brassica oleracea leaf juice on foodborne pathogenic bacteria. J Food Prot. 2006;69:2274–2279. doi: 10.4315/0362-028x-69.9.2274. [DOI] [PubMed] [Google Scholar]
- 71.Delaquis PJ, Mazza G. Antimicrobial properties of isothiocyanates in food preservation. Food Technol. 1995;49:73–84. [Google Scholar]
- 72.Opler PA, Krizek GO. Butterflies east of the great plains. Baltimore: Johns Hopkins University Press; 1984. [Google Scholar]
- 73.Shofran BG, Purrington ST, Breidt F, Fleming HP. Antimicrobial properties of sinigrin and its hydrolysis products. J Food Sci. 1998;63:621–624. [Google Scholar]
- 74.Heinemann U, Kiziak C, Zibek S, Layh N, Schmidt M, Griengl H, Stolz ER. Conversion of aliphatic 2-acetoxynitriles by nitrile-hydrolysing bacteria. Appl Microbiol Biotechnol. 2003;V63:274–281. doi: 10.1007/s00253-003-1382-8. [DOI] [PubMed] [Google Scholar]
- 75.Kao CM, Chen KF, Liu JK, Chou SM, Chen SC. Enzymatic degradation of nitriles by Klebsiella oxytoca. Appl Microbiol Biotechnol. 2006;71:228–233. doi: 10.1007/s00253-005-0129-0. [DOI] [PubMed] [Google Scholar]
- 76.Kiziak C, Conradt D, Stolz A, Mattes R, Klein J. Nitrilase from Pseudomonas fluorescens ebc191: cloning and heterologous expression of the gene and biochemical characterization of the recombinant enzyme. Microbiology. 2005;151:3639–3648. doi: 10.1099/mic.0.28246-0. [DOI] [PubMed] [Google Scholar]
- 77.Oginsky EL, Stein AE, Greer MA. Myosinase activity in bacteria as demonstrated by the conversion of progoitrin to goitrin. P Soc Exp Biol Med. 1965;119:360–364. doi: 10.3181/00379727-119-30181. [DOI] [PubMed] [Google Scholar]
- 78.Palop ML, Smiths JP, ten Brink B. Degradation of sinigrin by Lactobacillus agilis strain r16. Int J Food Microbiol. 1995;26:219–229. doi: 10.1016/0168-1605(95)00123-2. [DOI] [PubMed] [Google Scholar]
- 79.Tani N, Ohtsuru M, Hata T. Isolation of myrosinase producing microorganism. Agric Biol Chem (Tokyo) 1974;38:1617–1622. [Google Scholar]
- 80.Wittstock U, Agerbirk N, Stauber EJ, Olsen CE, Hippler M, Mitchell-Olds T, Gershenzon J, Vogel H. Successful herbivore attack due to metabolic diversion of a plant chemical defense. Proc Natl Acad Sci. 2004;101:4859–4864. doi: 10.1073/pnas.0308007101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Elton CS. The ecology of invasions by animals and plants. London: Methuen; 1958. [Google Scholar]
- 82.Hobbs RJ, Huenneke LF. Disturbance, diversity, and invasion: implications for conservation. Conserv Biol. 1992;6:324–337. doi: 10.1046/j.1523-1739.1992.06030324.x. [DOI] [Google Scholar]
- 83.Ambrogi A. Biotic invasions in a Mediterranean lagoon. Biol Invasions. 2000;2:165–176. doi: 10.1023/A:1010004926405. [DOI] [Google Scholar]
- 84.Kneitel J, Perrault D. Disturbance-induced changes in community composition increase species invasion success. Community Ecol. 2006;7:245–252. doi: 10.1556/ComEc.7.2006.2.11. [DOI] [Google Scholar]
- 85.Padrón B, Traveset A, Biedenweg T, Diaz D, Nogales M, Olesen JM. Impact of alien plant invaders on pollination networks in two archipelagos. PLoS ONE. 2009;4:e6275. doi: 10.1371/journal.pone.0006275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Parker IM, Simberloff D, Lonsdale WM, Goodell K, Wonham M, Kareiva PM, Williamson MH, Von Holle B, Moyle PB, Byers JE, Goldwasser L. Impact: toward a framework for understanding the ecological effects of invaders. Biol Invasions. 1999;1:3–19. doi: 10.1023/A:1010034312781. [DOI] [Google Scholar]
- 87.Horn HS. The ecology of secondary succession. Annu Rev Ecol Syst. 1974;5:25–37. doi: 10.1146/annurev.es.05.110174.000325. [DOI] [Google Scholar]
- 88.Buckley MR. Microbial communities: from life apart to life together. Washington: American Academy of Microbiology; 2003. [Google Scholar]
- 89.Hooper LV. Bacterial contributions to mammalian gut development. Trends Microbiol. 2004;12:129–134. doi: 10.1016/j.tim.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 90.Koropatnick TA, Engle JT, Apicella MA, Stabb EV, Goldman WE, McFall-Ngai MJ. Microbial factor-mediated development in a host-bacterial mutualism. Science. 2004;306:1186–1188. doi: 10.1126/science.1102218. [DOI] [PubMed] [Google Scholar]
- 91.Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via paneth cells. Proc Natl Acad Sci USA. 2002;99:15451–15455. doi: 10.1073/pnas.202604299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Visick KL, Foster J, Doino J, McFall-Ngai M, Ruby EG. Vibrio fischeri lux genes play an important role in colonization and development of the host light organ. J Bacteriol. 2000;182:4578–4586. doi: 10.1128/JB.182.16.4578-4586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brune A, Friedrich M. Microecology of the termite gut: structure and function on a microscale. Curr Opin Microbiol. 2000;3:263–269. doi: 10.1016/S1369-5274(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 94.Warnecke F, Luginbuhl P, Ivanova N, Ghassemian M, Richardson TH, Stege JT, Cayouette M, McHardy AC, Djordjevic G, Aboushadi N, Sorek R, Tringe SG, Podar M, Martin HG, Kunin V, Dalevi D, Madejska J, Kirton E, Platt D, Szeto E, Salamov A, Barry K, Mikhailova N, Kyrpides NC, Matson EG, Ottesen EA, Zhang X, Hernandez M, Murillo C, Acosta LG, Rigoutsos I, Tamayo G, Green BD, Chang C, Rubin EM, Mathur EJ, Robertson DE, Hugenholtz P, Leadbetter JR. Metagenomic and functional analysis of hindgut microbiota of a wood-feeding higher termite. Nature. 2007;450:560–565. doi: 10.1038/nature06269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Proportion of each library represented by members (OTU0.03) of the cabbage white butterfly midgut community (percentage) (PDF 1146 kb)