Abstract
We present a high throughput shotgun mass spectrometry workflow using a bidimensional peptide fractionation procedure consisting of isoelectric focusing and RP-HPLC prior to mass spectrometric analysis, with the aim of optimizing peptide separation and protein identification. As part of the workflow we used the ‘Isotope-Coded Protein Labeling’ (ICPL) method for accurate relative quantitation of protein expression. Such workflow was successfully applied to a comparative proteome analysis of schizophrenia versus healthy control brain tissues and can be an alternative to proteome researches.
Keywords: Shotgun mass spectrometry, MALDI-TOF, HPLC, IEF, Proteomics
Proteomics studies of cells and organisms grow continuously at exponential rates. A number of different approaches and methods have been described to achieve comprehensive proteome analysis [8]. Although two-dimensional gel electrophoresis (2-DE) is still used in many proteome studies, the use of shotgun mass spectrometry (shotgun-MS), introduced by Link et al. [5], in combination with stable isotope labeling methods has received increased popularity in recent years. Shotgun-MS in combination with chromatographic separation methods can overcome some of the 2-DE limitations such as the bias towards the most expressed proteins, the identification of proteins with extreme MWs and pIs [3] as well as low abundant proteins such as transcription factors and membrane receptors, which often play important roles disease-associated mechanisms [17, 18].
A shotgun-MS approach basically consists in the separation of peptides resulting from protein digestion followed by tandem mass spectrometry (MS/MS) analysis. Computational algorithms allow the automatic assignment of MS/MS spectra by matching the raw data with the predicted fragment spectra contained in the protein sequence databases [4]. Stable isotope labeling methods such as ‘Isotope-Coded Affinity Tags’ (ICAT) [2], ‘Isobaric Tags for Relative and Absolute Quantification’ (iTRAQ) [14] and ‘Isotope-Coded Protein Labeling’ (ICPL) [15] can be used in combination with shotgun-MS in order to compare the proteomes of interest.
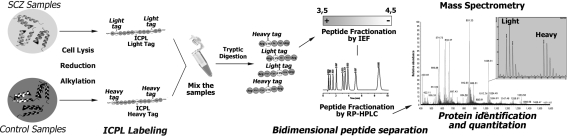
Here we present an example of a shotgun-MS workflow for proteome analysis by bidimensional peptide separation consisting of isoelectric focusing (IEF) and nano-reverse phase liquid chromatography (RP-HPLC) followed by tandem MALDI mass spectrometry (MALDI-TOF/TOF) for peptide sequencing and protein identification. This proteomic platform is suited for the integration of a stable isotope labeling method, such as ICPL, for relative quantitative proteome analysis. We have successfully used this workflow for the proteome analysis of schizophrenia (SCZ) and control human brains (Fig. 1).
Fig. 1.
Shotgun-MS workflow for the comparison of human SCZ and control brain samples. The SCZ and control proteins are modified by heavy and light ICPL reagents, respectively, mixed in equal amounts and then digested with trypsin. The tryptic peptides are separated by IEF and RP-HPLC and then analyzed by mass spectrometry. The mass spectrometry peptide signal intensities enable protein identification and relative quantitation
Brain proteins were extracted as described [6]. Following the ICPL kit protocol (Serva Electrophoresis, Heidelberg, Germany), a total of 100 μg protein from SCZ or control samples were reduced by adding dithiothreitol reagent and the resultant free thiol groups were alkylated with 0.4 M iodoacetamide. Excess of iodoacetamide was quenched by adding 0.5 M N-acetylcysteine. For protein labeling a ten-fold molar excess of light and heavy Nic-NHS reagent tags for control and SCZ samples, respectively, were added and incubated for 2 h at RT. Hydroxylamine (1.5 M) was added to the sample to inactivate the remaining Nic-NHS reagents. Equal amounts of light and heavy labeled samples were then combined. The combined protein samples were subsequently digested with trypsin at a 1:50 ratio (protein/enzyme) in 200 mM NH4HCO3 (pH 8.3) at 37 °C for 4 h. The resultant peptides were fractionated by IEF on immobilized pH gradient strip (IPG strip, 17 cm, 3.5–4.5 range). The strip was rehydrated for 12 h and focusing was performed for 8 h at 10,000 V constant voltage. The strip was manually cut in 47 pieces and the peptides extracted with 1% formic acid. Each extracted peptide sample was further fractionated on a nano RP-C18 monolithic column (200 um id. × 5 cm, Dionex, Sunnyvale, CA) at a flow rate of 4 μL/min using a 40 min gradient from 10 to 100% of solvent B (ACN; 0.1% TFA) on a micro-LC-System (HP1100 Agilent Technologies, Waldbronn, Germany). The eluted peptides were collected onto Prespotted AnchorChip sample target (Bruker Daltonics, Bremen, Germany) using a PROTEINEER-FC robot (Bruker Daltonics).
Mass spectra were acquired fully automatically using an Ultraflex I MALDI-TOF/TOF mass spectrometer (Bruker Daltonics) equipped with a nitrogen laser. Measurements were performed in positive reflector mode and a 20,000 V acceleration voltage. WARP-LC 1.1 software (Bruker Daltonics) was used for the spectra acquisition and the automatic selection of peptide signals for the subsequent MS/MS analysis. The ICPL-labeled peptides were automatically selected for the MS/MS analysis independently of their Heavy/Light (H/L) ratio. Acquired MS and MS/MS spectra were sent by the WARP-LC 1.1 to Biotools software 3.1 (Bruker Daltonics) as combined peak lists and searched against the NCBI database using an in-house version of MASCOT 2.1 (Matrix Science, London, UK) with the following parameter settings: Homo sapiens as organism, trypsin as enzyme, carbamidomethylation as fixed modification, oxidized methionine and heavy and light ICPL labeling of lysine residue and N-terminal protein as variable modifications. The determination of ICPL-labeled peptide pair (heavy and light) ratios was performed by WARP-LC 1.1 Protein Browser (Bruker Daltonics), by comparing the relative heavy and light cluster signal intensities. The technical parameters and the instrument settings for peptide separation and MS measurements reported here are sample- and instrument-dependent.
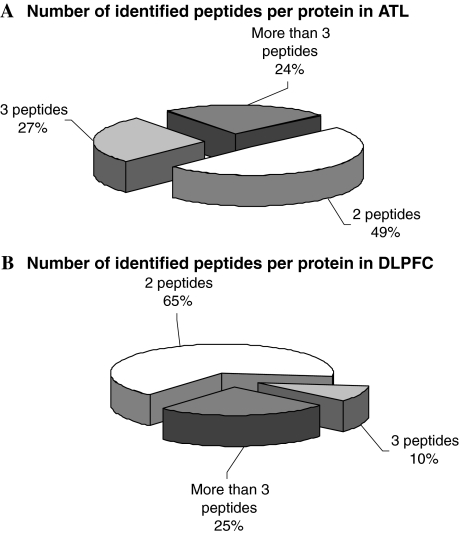
The described shotgun-MS workflow in combination with the ICPL labeling method was applied to the proteome analysis of anterior temporal lobe (ATL) and dorsolateral prefrontal cortex (DLPFC) brain regions from SCZ and control patients [9, 10]. In the ATL proteome analysis, 837 peptides were identified, leading to the identification of 479 proteins, 37 of which were differentially expressed according the statistical analysis (t-test) performed by WARP-LC 1.1, considering the signal intensities and number of identified peptides per protein. In the DLPFC proteome analysis, we identified 2,541 peptides which led to the identification of 1,261 proteins, 84 of which were differentially expressed, using the same statistical analysis described above. In both comparative proteome analyses, “one-hit wonder” proteins were not considered as identified (Fig. 2). The proteins we found to be differentially expressed between SCZ and controls not only revealed new interesting data but also confirmed previous transcriptome and proteome results obtained by other groups [1, 12, 13, 16].
Fig. 2.
Number of identified peptides per protein obtained using our shotgun-MS workflow for the analysis of ATL (A) and in DLPFC (B) brain tissue samples from SCZ patients and controls
The combination of IEF and RP-HPLC for tryptic peptide fractionation prior to MS provides a powerful tool for proteome profiling and biomarker discovery. The presented ICPL shotgun-MS method has enabled the identification of regulated and non-regulated proteins in the same experiment while in 2-DE comparative analyses usually only the differentially expressed proteins are selected and identified.
Using shotgun-MS [10] we were able to identify 84 differentially expressed proteins statistically significant (as described above) in DLPFC between SCZ patients and controls. These proteins are involved in 11 biological processes. Using 2-DE comparative analysis of the same brain region, the volumes of all protein spots in SCZ and controls gels were determined and corresponding spots were matched for all 2-DE profiles. Next, the spot volumes were analyzed by t-test, revealing 24 statistically significant differences in the protein expression, involved in 6 biological processes [11]. These results confirm the notion that shotgun-MS is more efficient in the higher detection differentially expressed proteins as well as in the identification of low abundant proteins, which may play important roles in brain diseases [7]. Whereas only 2 out of 24 (8.3%) differentially regulated proteins found by 2-DE were membrane proteins, with shotgun-MS 190 (15.1%) of the expressed proteins were identified as membrane proteins, even though we did not apply membrane protein enrichment protocols. Yet, by 2-DE, 8.3% were extracellular proteins, and the remaining 83.4% were cytoplasmatic proteins, while by shotgun-MS, not only extracellular proteins were identified, but also nuclear (15.3%) and vesicular proteins (6.8%) were revealed. Furthermore, it is known that the determination of quantitative protein expression differences using stable isotope methods is more accurate than the determination by densitometric measurements of 2-DE spots [4, 8].
Analyzing our shotgun-MS data, we have observed an issue concerning the number of identified peptides per protein. In the DLPFC and ATL data analysis, 65% and 49% of proteins, respectively, were identified by only 2 peptides (Fig. 2). This is probably due to the use of the ICPL methodology. Since the ICPL tag modifies lysine residues in the intact proteins, the subsequent trypsin digestion occurs only at arginine residues generating long peptides whose fragmentation by MS/MS is incomplete. As a consequence a lower number of peptides is identified compared to tryptic digests where cleavage occurs at both lysine and arginine residues. A solution to this issue could be the use of the selected stable isotope labeling method at peptide level.
For the proteome analysis of SCZ thalamus, we have integrated in our shotgun-MS workflow the iTRAQ technology, which labels the lysine residues and the N-termini of the proteolytic peptides. As a result, we observed an increased number of identified peptides per protein and an increased number of identified proteins (data to be published).
The presented shotgun-MS workflow can overcome the issue of the high proteome complexity by using multidimensional separation with IEF and RP-HPLC for tryptic peptides fractionation. The combination of the orthogonal and multidimensional separation methods reduces peptide complexity to a level that allows a successful MS analysis. Moreover, the techniques used in this workflow are automated and enable high-throughput experiments. Furthermore, the shotgun-MS workflow described here is compatible with the use of stable isotope reagents.
Although the search for the best method for representing proteomes is still on-going, we have presented here a shotgun-MS workflow that can be applied to any kind of proteome as an alternative and/or complement to the 2-DE-based proteome analysis.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviation List
- 2-DE
Two-dimensional gel electrophoresis
- ACN
Acetonitrile
- ATL
Anterior temporal lobe
- DLPFC
Dorsolateral prefrontal cortex
- HPLC
High performance liquid chromatography
- ICAT
Isotope-Coded Affinity Tags
- ICPL
Isotope-Coded Protein Labeling
- IEF
Isoelectric focusing
- IPG
Immobilized pH gradient
- iTRAQ
Isobaric Tags for Relative and Absolute Quantification
- MALDI
Matrix-Assisted Laser Desorption/Ionization
- MS/MS
Tandem mass spectrometry
- MS
Mass spectrometry
- MW
Molecular weight
- pI
Isoelectric point
- RP-HPLC
Reverse phase—high performance liquid chromatography
- SCZ
Schizophrenia
- Shotgun-MS
Shotgun mass spectrometry
- TFA
Trifluoroacetic acid
- TOF
Time of flight
Contributor Information
Christoph W. Turck, Phone: +49-89-30622-317, FAX: +49-89-30622-610, Email: turck@mpipsykl.mpg.de
Daniel Martins-de-Souza, Email: martins@mpipsykl.mpg.de.
References
- 1.Arion D, Unger T, Lewis DA, Levitt P, Mirnics K. Biol Psychiatry. 2007;62:711–721. doi: 10.1016/j.biopsych.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gygi SP, Rist B, Gerber SA, Turecek F, Gelb MH, Aebersold R. Nat Biotechnol. 1999;17:994–999. doi: 10.1038/13690. [DOI] [PubMed] [Google Scholar]
- 3.Gygi SP, Corthals GL, Zhang Y, Rochon Y, Aebersold R. Proc Natl Acad Sci USA. 2000;97:9390–9395. doi: 10.1073/pnas.160270797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haas W, Faherty BK, Gerber SA, Elias JE, Beausoleil SA, Bakalarski CE, Li X, Villén J, Gygi SP. Mol Cell Proteomics. 2006;5:1326–1337. doi: 10.1074/mcp.M500339-MCP200. [DOI] [PubMed] [Google Scholar]
- 5.Link AJ, Eng J, Schieltz DM, Carmack E, Mize GJ, Morris DR, Garvik BM, Yates JR., 3rd Nat Biotechnol. 1999;17:676–682. doi: 10.1038/10890. [DOI] [PubMed] [Google Scholar]
- 6.Martins-de-Souza D, Menezes de Oliveira B, dos Santos Farias A, Horiuchi RS, Crepaldi Domingues C, de Paula E, Marangoni S, Gattaz WF, Dias-Neto E, Camillo Novello J. Brief Funct Genomic Proteomic. 2007;6:70–75. doi: 10.1093/bfgp/elm009. [DOI] [PubMed] [Google Scholar]
- 7.Martins-de-Souza D, Gattaz WF, Schmitt A, Rewerts C, Maccarrone G, Dias-Neto E, Turck CW. J Sep Sci. 2008;31:3122–3126. doi: 10.1002/jssc.200800224. [DOI] [PubMed] [Google Scholar]
- 8.Martins de Souza D, Oliveira BM, Castro-Dias E, Winck FV, Horiuchi RS, Baldasso PA, Caetano HT, Pires NK, Marangoni S, Novello JC. Brief Funct Genomic Proteomic. 2008;7:312–321. doi: 10.1093/bfgp/eln023. [DOI] [PubMed] [Google Scholar]
- 9.Martins-de-Souza D, Gattaz WF, Schmitt A, Rewerts C, Marangoni S, Novello JC, Maccarrone G, Turck CW, Dias-Neto E. J Neural Transm. 2009;116:275–289. doi: 10.1007/s00702-008-0156-y. [DOI] [PubMed] [Google Scholar]
- 10.Martins-de-Souza D, Gattaz WF, Schmitt A, Rewerts C, Maccarrone G, Dias-Neto E, Turck CW. Eur Arch Psychiatry Clin Neurosci. 2009;259:151–163. doi: 10.1007/s00406-008-0847-2. [DOI] [PubMed] [Google Scholar]
- 11.Martins-de-Souza D, Gattaz WF, Schmitt A, Maccarrone G, Hunyadi-Gulyás E, Eberlin MN, Souza GH, Marangoni S, Novello JC, Turck CW, Dias-Neto E. J. Psychiatr Res. 2009;43(11):978–986. doi: 10.1016/j.jpsychires.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Middleton FA, Peng L, Lewis DA, Levitt P, Mirnics K. Neuropsychopharmacology. 2005;30:974–983. doi: 10.1038/sj.npp.1300674. [DOI] [PubMed] [Google Scholar]
- 13.Prabakaran S, Swatton JE, Ryan MM, Huffaker SJ, Huang JT, Griffin JL, Wayland M, Freeman T, Dudbridge F, Lilley KS, Karp NA, Hester S, Tkachev D, Mimmack ML, Yolken RH, Webster MJ, Torrey EF, Bahn S (2004) Mol Psychiatry 9(7):684–697, 643 [DOI] [PubMed]
- 14.Ross PL, Huang YN, Marchese JN, Williamson B, Parker K, Hattan S, Khainovski N, Pillai S, Dey S, Daniels S, Purkayastha S, Juhasz P, Martin S, Bartlet-Jones M, He F, Jacobson A, Pappin DJ. Mol Cell Proteomics. 2004;3:1154–1169. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt A, Kellermann J, Lottspeich F. Proteomics. 2005;5:4–15. doi: 10.1002/pmic.200400873. [DOI] [PubMed] [Google Scholar]
- 16.Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, Starkey M, Webster MJ, Yolken RH, Bahn S. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- 17.Washburn MP, Wolters D, Yates JR., III Nat Biotechnol. 2001;19:242–247. doi: 10.1038/85686. [DOI] [PubMed] [Google Scholar]
- 18.Wu L, Han DK. Expert Rev Proteomics. 2006;3:611–619. doi: 10.1586/14789450.3.6.611. [DOI] [PubMed] [Google Scholar]