Summary
T cell receptor (TCR)-mediated cytoskeletal reorganization is considered to be Arp2/3-dependent. We therefore examined Arp2/3- and formin-dependent cytoskeletal processes during T cell activation. We demonstrate that without Arp2/3-mediated actin nucleation, stimulated T cells cannot form an F-actin-rich lamellipod, but instead produce polarized filopodia-like structures. Moreover, the microtubule-organizing center (MTOC), which rapidly reorients to the immunological synapse through an unknown mechanism, polarizes in the absence of Arp2/3. Conversely, the actin-nucleating formins, DIA1 and FMNL1, do not affect TCR-stimulated F-actin-rich structures, but instead display unique patterns of centrosome co-localization and control TCR-mediated MTOC polarization. Significantly, depletion of FMNL1 or DIA1 in cytotoxic lymphocytes abrogates cell-mediated killing. Altogether, our results identify Arp2/3-independent cytoskeletal reorganization events in T lymphocytes, and indicate that formins are essential cytoskeletal regulators of centrosome polarity in T cells.
Introduction
Within minutes following antigen-presenting cell (APC) recognition, T cells undergo cytoskeletal polarization, involving formation of an F-actin-rich lamellipodium (Bunnell et al., 2001) and MTOC reorientation to the immune synapse (IS) (Kupfer et al., 1987). Although the mechanisms controlling polarization of these cytoskeletal elements remain unclear, this restructuring is essential for T cell function (Vicente-Manzanares and Sanchez-Madrid, 2004). In addition, the speed with which cytoskeletal reorganization occurs makes the T cell-APC recognition process a unique model for studying the molecular mechanisms controlling cytoskeletal polarization.
TCR-mediated MTOC (or centrosome) reorientation has been suggested to be regulated by only a few signaling molecules (Ardouin et al., 2003; Combs et al., 2006; Kuhne et al., 2003; Martin-Cofreces et al., 2006; Serrador et al., 2004; Stowers et al., 1995). Functionally, MTOC polarization is thought to control the positioning of the secretory apparatus for directed release of lymphokines in T-helper cells (Kupfer et al., 1991), or cytotoxins in cytolytic cells (Kupfer and Dennert, 1984). Also, centrosome–plasma membrane contact in T cells is thought to be necessary for directed secretion and to be F-actin-dependent (Stinchcombe et al., 2006). However, the putative actin regulators controlling this centrosome polarization remain to be identified.
Several studies making use of pharmacologic agents have demonstrated that F-actin reorganization is crucial for T cell activation (Campi et al., 2005; Holsinger et al., 1998; Valitutti et al., 1995). The Arp2/3 complex, which is directly stimulated by activators such as WASp and WAVE, nucleates F-actin into an expanding array of individual filaments branching off one another. This Arp2/3-generated F-actin meshwork is proposed to produce sheet-like lamellipodia and spike-like filopodia. Even though filopodia are predicted to arise from the Arp2/3-dependent dendritic network through selective bundling of Arp2/3-generated filaments (Biyasheva et al., 2004), this idea remains controversial (Faix and Rottner, 2006). In fact, it is now suggested that the Arp2/3 complex is dispensable for filopodia formation (Steffen et al., 2006). Indeed, genetic evidence in yeast, Drosophila, and C. elegans reveals that Arp2/3 is not the sole nucleator for all F-actin-containing structures (Evangelista et al., 2002; Hudson and Cooley, 2002; Severson et al., 2002; Tolliday et al., 2002). This suggests that F-actin nucleators are specialized, producing specific architectural frameworks that coordinate distinct cellular functions.
Formin family proteins can also nucleate F-actin, and unlike the Arp2/3 complex, formins nucleate linear F-actin filaments and are proposed to generate unbranched structures, such as actin cables, filopodia and stress fibers (Faix and Grosse, 2006). Formins are conserved modular proteins sharing characteristic formin homology (FH) domains (Higgs, 2005). The FH1 domain interacts with the G-actin-binding protein profilin, providing G-actin for filament assembly, while the adjacent FH2 domain nucleates F-actin. In yeast, formins participate in microtubule organization, spindle positioning, polarized cell growth, and contractile ring formation during cytokinesis (Faix and Grosse, 2006). However, the functions of mammalian formins are not well understood, with recent evidence suggesting roles in cell motility, adhesion, and microtubule capture and stabilization (Faix and Grosse, 2006; Kobielak et al., 2004; Wen et al., 2004). In fact, there appears to be a strong, yet ill-defined, link emerging between mammalian formins and the microtubule cytoskeleton.
Here we examined the contributions of the Arp2/3 complex and formins to cytoskeletal regulation during T cell activation. We found that Arp2/3-depleted cells, which could not form F-actin-rich lamellipodia, still polarized actin-based filopodia and were even capable of MTOC polarization. In contrast, the formins, Diaphanous-1 (DIA1) and Formin-like-1 (FMNL1), do not regulate F-actin accumulation at the IS, but instead co-localize with the centrosome and control MTOC polarization and cell-mediated killing. Thus, we find that the Arp2/3 complex and formins distinctly regulate the T cell cytoskeleton, and identify the formins as essential regulators of microtubule and centrosome polarity during T cell activation.
Results
TCR stimulation leads to distinct Arp2/3-independent F-actin structures
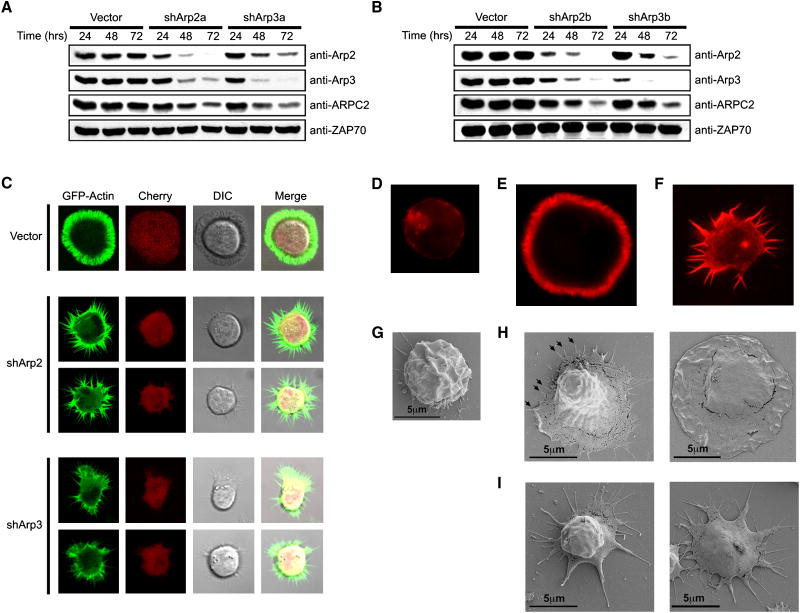
In order to examine the role of the Arp2/3 complex in T cells we generated short-hairpin RNA (shRNA) vectors to silence both Arp2 and Arp3. Transfection of these vectors into Jurkat T cells led to a substantial depletion of Arp2 and Arp3 (Figures 1A-B). Consistent with studies in non-hematopoietic cells (Steffen et al., 2006), suppression of either Arp2 or Arp3 with each individual targeting vector led to a decrease in expression of other Arp2/3 complex components (Figures 1A-B). Thus, the integrity of the Arp2/3 complex as a whole is dependent on the presence of either Arp2 or Arp3 in T cells.
Figure 1. TCR stimulation leads to distinct Arp2/3-independent F-actin structures.
(A, B) Jurkat cells were transfected with shRNA vectors against Arp2 and Arp3, and analyzed over time via immunoblot for Arp2/3 expression. (C) Jurkat cells expressing GFP-actin (green) were transfected with control, shArp2-mCherry, or shArp3-mCherry vectors (red) and stimulated on anti-TCR coated coverslips. Representative confocal images are shown. (D and G) Control vector-transfected Jurkat cells were bound to poly-L-Lysine coated coverslips. Control-transfected (E and H) or shArp2-transfected (F and I) Jurkat cells were spread onto poly-L-Lysine/anti-TCR coated coverslips. The cells from D-F were then stained with phalloidin. The cells from G-I were then analyzed by SEM. In H and I, the left image is predicted to be early in the spreading process, whereas the right image is late stage.
Since the Arp2/3 complex is considered essential for TCR-mediated F-actin reorganization, we first examined the morphology of TCR-stimulated Arp2/3-depleted GFP-actin-expressing Jurkat cells spreading following TCR ligation (Bunnell et al., 2001). GFP-actin Jurkat cells were transfected with shRNA vectors containing a separate mCherry transcriptional cassette (Nolz et al., 2006). Control mCherry-expressing cells spread onto anti-TCR-coated coverslips in an ordered fashion, forming a round, actin-rich lamellipod (Figures 1C; Supplemental Movie S1). Spreading was maximal by 5 minutes after initial coverslip contact, with the central area corresponding to the cell body visually devoid of GFP-actin (Figure 1C). This was followed by a 15-20 minute retraction phase, characterized by disassembly of the lamellipod and the return of GFP-actin to the cell body (Supplemental Movie S1). DIC images indicated retrograde flow and ruffling of the extended lamellipod throughout the spreading process (Supplemental Movies S1).
In contrast to control transfectants, shArp2- or shArp3-transfected cells did not form lamellipodia, but instead displayed a dramatically different phenotype, extending long, dynamic actin-rich filopodia in response to TCR stimulation (Figure 1C; Supplemental Movies S2-3). These structures were not as long-lived as the lamellipod and were not uniformly produced, with cells displaying distinct morphologies (Figure 1C). Also, Arp2/3-depleted cells retained substantial GFP-actin in their cell bodies, yet were seemingly capable of flattening against the activating coverslips (Supplemental Movies S2-3). In contrast to Arp2-suppressed cells, shArp3-transfected cells transiently displayed periodic membrane bursts occurring between adjacent filopodial structures (Supplemental Movie S3), which is most likely due to inefficient suppression of Arp3. Phalloidin staining of control and Arp2-suppressed T cells indicated that the GFP-actin enrichment seen in Figure 1C indeed correlated with F-actin polymerization (Figures 1D-F), suggesting that Arp2/3-independent actin reorganization is responsible for microspike formation. In fact, shArp2-transfected cells no longer produced filopodia upon cytochalasin treatment (not shown), but did form them upon colchicine treatment (not shown), indicating an actin-dependent yet microtubule-independent mechanism for formation of these filopodia.
In order to more closely visualize the morphological differences between control and Arp2-suppressed cells, we utilized scanning electron microscopy (SEM). Unstimulated control cells did not undergo morphological alterations in response to the coverslip (Figure 1G), while anti-TCR stimulated control cells extended flat sheet-like structures, which apparently displayed filopodia protruding at the leading edge (Figure 1H, Left). After spreading completely, control cells were dramatically flattened against the activating coverslip, with apparent ruffling at the periphery (Figure 1H, Right). Arp2-suppressed cells sent out filopodial appendages, which only extended from the area in contact with the coverslip (Figure 1I, Left). SEM also confirmed that shArp2-transfected cells indeed flattened against the coverslip (Figure 1I, Right). These data indicate that TCR-induced F-actin polymerization arises independently of the Arp2/3 complex, minimally in the form of microspikes.
Arp2/3-independent filopodia polarize during APC recognition
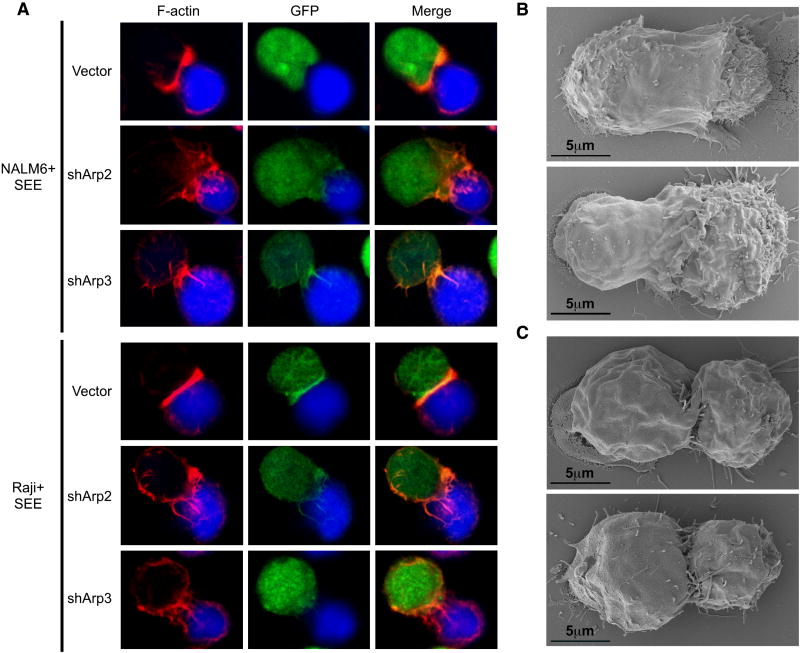
Due to the altered morphology of activated Arp2/3-suppressed cells, we examined if they could respond to APCs with polarized F-actin. To analyze this physiological response, control and suppressed T cells were allowed to form conjugates with superantigen (SEE)-pulsed NALM6 or Raji B cells. As expected, control GFP+ cells flattened against the stimulating NALM6 cells, showing a robust band of polarized F-actin at the IS (Figure 2A). Interestingly, conjugates were found between GFP+ Arp2- and Arp3-suppressed cells and SEE-pulsed NALM6 cells, and upon analysis, these pairs frequently displayed polarized F-actin. However, similar to the filopodial structures observed in shArp2/3-suppressed cells activated on coverslips, shArp2- and shArp3-depleted cells produced disorganized F-actin rich projections over the B cell surface (Figure 2A). To determine if costimulation altered this response by Arp2- and Arp3-depleted cells, we also used SEE-pulsed RAJI cells, which express B7, the costimulatory ligand that binds CD28 on T cells. We found that although control GFP+ cells flattened tightly against the RAJI cells, the Arp2- and Arp3-suppressed T cells responded with similar polarized F-actin-based protrusions (Figure 2A).
Figure 2. Arp2/3-independent filopodia polarize during APC recognition.
(A) Jurkat cells were transfected with control, shArp2-GFP, or shArp3-GFP vectors (green). Each population was conjugated with SEE-pulsed/CMAC-stained NALM6 or Raji cells (blue) and stained with phalloidin (red). Control-transfected (B) or shArp2-transfected (C) Jurkat cells were conjugated with SEE-pulsed Raji cells and analyzed by SEM.
We next formed conjugates between control or shArp2-transfected T cells and SEE-pulsed Raji cells, and imaged them by SEM (representative conjugates are shown in Figures 2B-C). In many cases, control cells maintained strong contacts with APCs, with the forming lamellipod extending over the APC surface (Figure 2B). In contrast, Arp2-knockdown T cells did not produce lamellipodia or display impressive morphological alterations. Actually, these cells showed less contact with APCs, extending ‘finger-like’ projections throughout the cell-cell contact zone (Figure 2C). These structures are also apparent in confocal images with shArp2/shArp3-suppressed cells (Figure 2A). These data further support that polarized F-actin reorganization at the IS is not exclusively regulated by the Arp2/3 complex.
TCR-mediated MTOC polarization is Arp2/3-independent
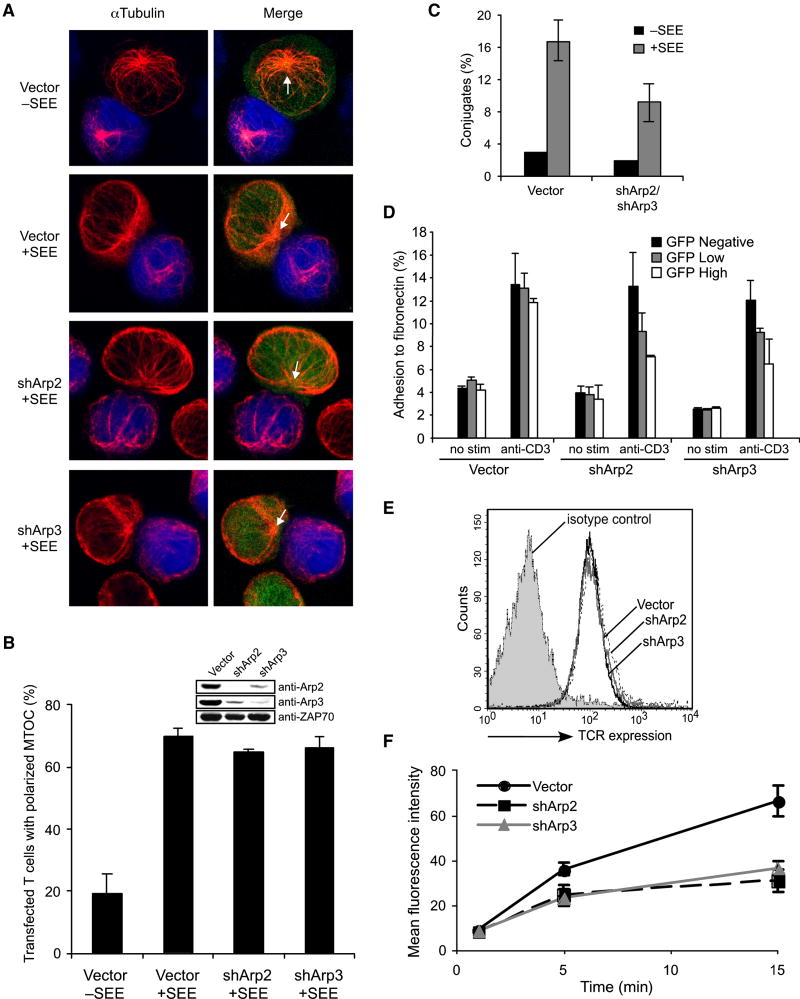
Since Arp2/3-depleted T cells retained the ability to polarize F-actin structures, we next examined MTOC reorientation toward APCs, a hallmark of T cell polarization. We found that TCR-mediated MTOC repositioning is Arp2/3-independent, with shArp2-GFP and shArp3-GFP transfected cells efficiently polarizing their MTOC and microtubule system to face APCs (Figures 3A and 3B). Thus, while depletion of the Arp2/3 complex regulates activated T cell morphology, it does not control the formation or polarization of all F-actin-based structures or MTOC reorientation toward the IS.
Figure 3. Arp2/3-dependent F-actin polymerization does not control MTOC polarity, but regulates integrins and TCR internalization.
(A) Jurkat cells were transfected with control, shArp2-GFP, or shArp3-GFP vectors (green). Each population was conjugated with unpulsed or SEE-pulsed/CMAC-stained Raji cells (blue), labeled with anti-αTubulin (red), and scored for MTOC polarization (B). Arrows indicate MTOC position. (C) Jurkat cells were transfected with control or with both shArp2-GFP and shArp3-GFP vectors (green), and incubated with unpulsed or SEE-pulsed/PKH26-stained NALM6 B cells (red). Conjugation efficiency was determined by flow cytometry. (D) Jurkat cells were transfected as in A and analyzed for fibronectin binding. The percent adhesion (GFP-negative, low, and high) in each sample was determined by flow cytometry. (E) Jurkat cells were transfected as in A, stained on ice with anti-CD3-PE and cell surface expression of the TCR was analyzed on GFP+ cells. (F) Jurkat cells were transfected as in A, stained on ice with anti-CD3-PE, cross-linked for the indicated times at 37°C, incubated in cold stripping buffer (removing surface antibodies), and analyzed by flow cytometry for TCR internalization in GFP+ cells. Bars in B,C,D, and F represent mean ±StDev from three independent experiments.
Arp2/3-dependent F-actin regulates integrin activation and TCR internalization
TCR-mediated β2-integrin activation is considered actin-dependent and is essential for T cell-APC conjugation (Kinashi, 2005). To examine whether β2-integrin activation directly requires Arp2/3-mediated F-actin, we analyzed conjugate formation without Arp2/3. We found that shArp2/shArp3-transfected cells formed conjugates with SEE-pulsed B cells less frequently than control transfectants (Figure 3C), and that single Arp2 or Arp3 suppression yielded similar results (not shown). Since β2-integrin-mediated conjugation was Arp2/3-dependent, we next investigated whether TCR-stimulated shArp2- or shArp3-transfected cells were capable of binding fibronectin, a β1-integrin-dependent event. As shown in Figure 3D, GFP-expressing Arp2 and Arp3 knockdown cells also demonstrated diminished TCR-mediated fibronectin adhesion.
Additionally, the Arp2/3 complex may participate in receptor internalization (Engqvist-Goldstein and Drubin, 2003). As shown in Figure 3E, basal TCR surface levels were unaffected without Arp2/3. However, Arp2/3-depleted cells displayed significant TCR internalization defects (Figure 3F), even in spite of the fact that microtubules, which are suggested to regulate TCR internalization (Barr et al., 2006), still polarize in the absence of Arp2/3. We next analyzed the ability of shArp2- and shArp3-transfected cells to signal efficiently following TCR ligation. So far, the role of the actin cytoskeleton in regulating calcium-related signaling events and mitogen-activated protein kinases (MAPKs) in lymphocytes has been controversial (Hao and August, 2005; Holsinger et al., 1998; Rivas et al., 2004; Valitutti et al., 1995). Interestingly, our studies indicate that Arp2- and Arp3-suppressed Jurkat cells display normal TCR-mediated PLC-γ1 phosphorylation and Ca2+ mobilization (Supplemental Figure 1A and 1B). Also, we found prolonged ERK phosphorylation in the absence of Arp2/3 (Supplemental Figure 1A), which may be a direct result of diminished TCR internalization. Thus, although Arp2/3 is not required for MTOC and F-actin polarization or certain TCR-mediated signaling events, integrin activation and TCR internalization are diminished. This indicates that F-actin generated independently of Arp2/3 may have distinct roles from Arp2/3-nucleated F-actin during T cell activation.
Formins display distinct cytoskeletal co-localization in T cells
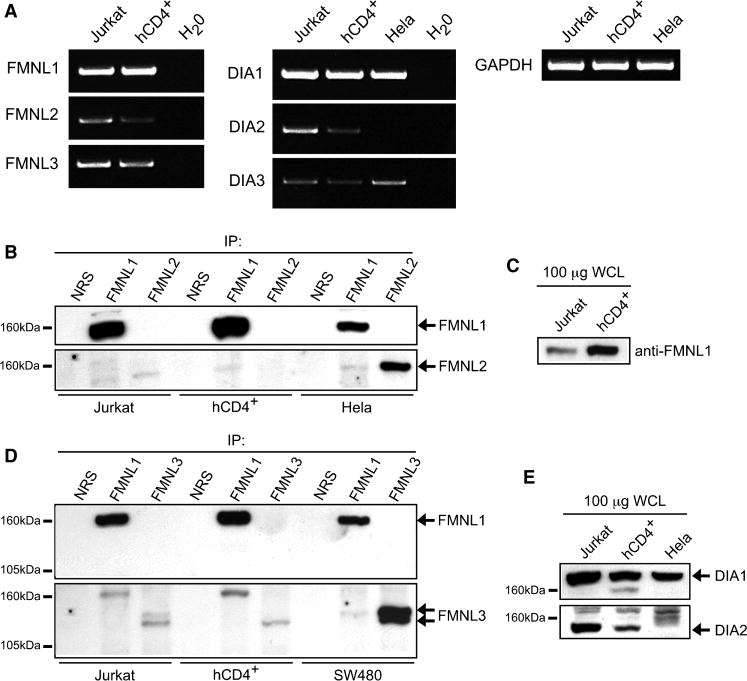
To identify potential Arp2/3-independent mechanisms for F-actin nucleation, we examined formins because of their suggested role in filopodia formation. Formin expression in T cells has not been analyzed, although lymphocytes have been demonstrated to express FMNL1 (Favaro et al., 2006). Indeed, we found FMNL1 mRNA and protein was expressed in both Jurkat and peripheral blood human CD4+ (hCD4+) T cells (Figure 4A and 4B), with primary T cells expressing more protein than Jurkat cells (Figure 4C). In contrast, while mRNA for the other formin-like family members (FMNL2 and FMNL3) could be detected, very little protein was immunoprecipitated from Jurkat or hCD4+ T cell lysates (Figure 4B and 4D). However, we detected FMNL2 and FMNL3 protein in non-immune cell types (Figure 4B and 4D). Additionally, of the Diaphanous family members (DIA1, DIA2 and DIA3), DIA1 appeared to be the predominant isoform expressed in T cells (Figure 4A and 4E).
Figure 4. Formins are expressed in T cells.
(A) RT-PCR for mRNA expression of FMNL1-3 and DIA1-3. Formins were immunoprecipitated (B and D) or immunoblotted in whole cell lysates (C and E) to analyze expression.
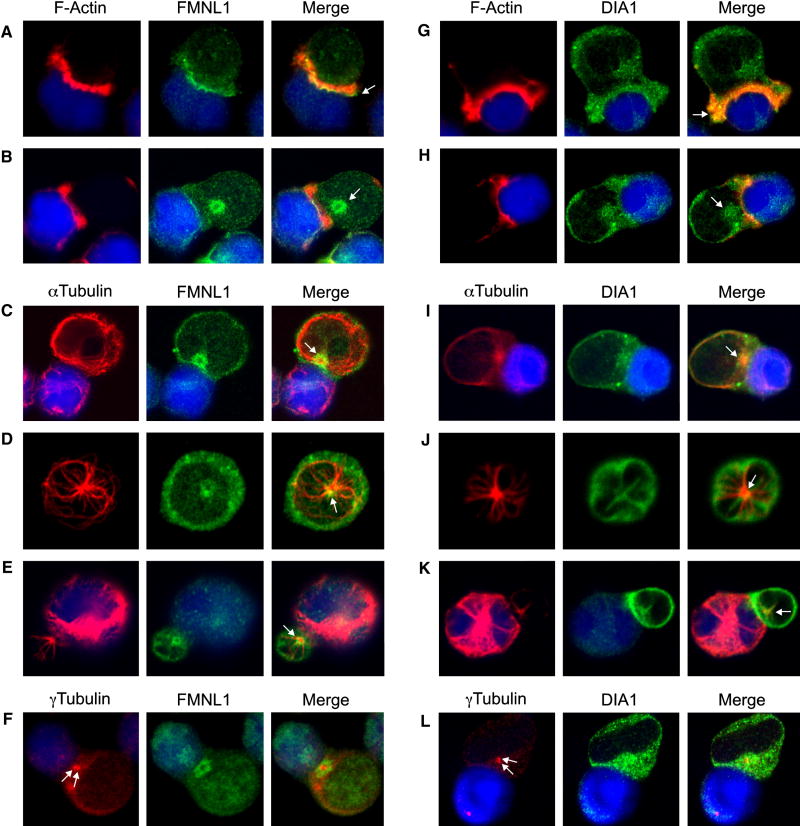
We next analyzed the localization of these formins by immunofluorescence. We found fringe-like localization of FMNL1 at the leading edge of the lamellipod in spreading T cells (Supplemental Figure 2B). This FMNL1 accumulation was also apparent at the edge of F-actin structures, which formed during APC recognition (Figure 5A), along with a distinct, ‘ring-like’ localization of FMNL1 within the T cell body (Figure 5B). Upon further analysis, we found that this FMNL1 ring reoriented along with the MTOC to face stimulating APCs at later timepoints (Figure 5C). Moreover, a similar FMNL1 ring polarized with the MTOC in hCD4+ T cells (Figure 5D and 5E). Interestingly, we identified that the centrosome was positioned precisely within the center of this FMNL1 ring structure (Figure 5F).
Figure 5. FMNL1 and DIA1 display distinct cytoskeletal co-localization in T cells.
Jukat cells were conjugated to SEE-pulsed/CMAC-stained Raji cells (blue) and co-stained with phalloidin and either anti-FMNL1 (A and B) or anti-DIA1 (G and H). Jurkat-Raji conjugates were co-stained with anti-αTubulin and either anti-FMNL1 (C) or anti-DIA1 (I). hCD4+ T cells were co-stained with anti-αTubulin and either anti-FMNL1 (D) or anti-DIA1 (J). hCD4+ T cells were conjugated to superantigen cocktail-pulsed Raji cells and stained with anti-αTubulin and either anti-FMNL1 (E) or anti-DIA1 (K). Jurkat-Raji conjugates were co-stained with antiγTubulin to label the centrosome (arrows) and either anti-FMNL1 (F) or anti-DIA1 (L). Arrows in A-E and G-K indicate points of distinct formin localization.
In contrast to FMNL1, DIA1 was enriched in a fine line surrounding the lamellipod of spreading T cells (Supplemental Figure 2A). In conjugates, DIA1 did not display remarkable accumulation with F-actin, although was within F-actin-rich areas (Figure 5G). However, like FMNL1, DIA1 displayed a distinct point of localization within the T cell body (Figure 5G and 5H), and upon closer analysis appeared to be in a “starburst” pattern overlaying the MTOC and microtubules (Figure 5I). Additionally, DIA1 co-localized with the MTOC in primary hCD4+ T cells (Figure 5J and 5K) and was also found surrounding the centrosome (Figure 5L). Moreover, DIA1 localized throughout the Arp2/3-independent filopodia, whereas FMNL1 was frequently enriched at the tips (Supplememental Figure 2). In contrast to DIA1, DIA2 was not found to localize with F-actin-rich T cell lamellipodia or filopodia (not shown), but did similarly surround the centrosome (Supplememental Figure 3).
FMNL1 and DIA1 do not control TCR-induced F-actin accumulation at the IS
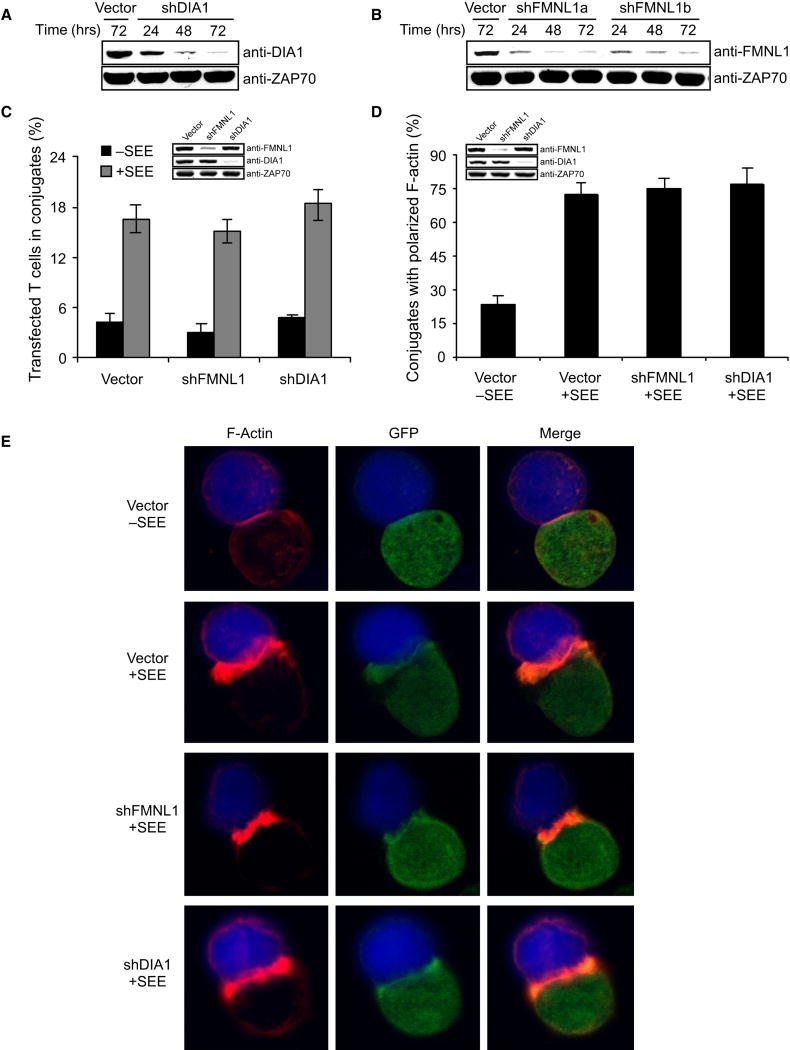
Since FMNL1 and DIA1 displayed unique cytoskeletal localization patterns and potentially regulate Arp2/3-independent F-actin polymerization, we generated shRNA-targeting vectors against FMNL1 and DIA1 (Figure 6A-B). Use of these suppression vectors abrogated centrosomal staining by both anti-FMNL1 and anti-DIA1, demonstrating their high specificity (Supplemental Figure 4). We next examined whether suppression of these formins affected Arp2/3-independent filopodia formation. Like control, shFMNL1a-mCherry, shFMNL1b-mCherry or shDIA1-mCherry transfected cells spread normally (Supplemental Figure 5A-D). Likewise, GFP-actin cells co-transfected with shArp2-mCherry and shFMNL1 or shDIA1 vectors (individually or combined) produced microspikes upon activation (Supplemental Figure 5E-I). Also, shFMNL1- and shDIA1-transfected cells efficiently formed conjugates and displayed normal F-actin accumulation at the IS (Figure 6C-E). Moreover, FMNL1- and DIA1-depleted T cells show normal PLC-γ1, ERK and calcium responses following TCR stimulation (Supplemental Figure 1C and 1D). Thus, although these formins localize in the lamellipod of spreading cells and to the filopodia of Arp2/3-suppressed cells, their depletion does not affect the formation of these F-actin structures or signaling. This suggests that FMNL1 and DIA1 function distinctly, as they do not impact Arp2/3-regulated cellular processes, such as lamellipod formation and integrin activation.
Figure 6. FMNL1 and DIA1 do not regulate integrin activation or the accumulation of F-actin at the IS.
(A) Jurkat cells were transfected with shDIA1 (A) or shFMNL1 (B) vectors and lysates were immunoblotted as indicated. (C) Jurkat cells were transfected with control, shDIA1-GFP or shFMNL1-GFP vectors (green), incubated with unpulsed or SEE-pulsed/PKH26-stained NALM6 B cells (red), and the % of GFP+ T cells in conjugates was determined by flow cytometry. (D and E) Jurkat cells were transfected as in C, conjugated with unpulsed or SEE-pulsed Raji cells (blue), labeled with phalloidin, and scored for F-actin at the IS (D). Bars in C and D represent mean ±StDev from three independent experiments.
As a means to further analyze if formins produce Arp2/3-independent filopodia, we studied the role of lymphocyte-expressed ENA/VASP proteins (EVL and VASP). The ENA/VASP family of proteins are proposed to be necessary for formin-dependent filopodia formation through their bundling of formin-mediated F-actin (Schirenbeck et al., 2006). In spreading cells EVL was accumulated at the very leading edge and co-localized with F-actin within the lamellipod, whereas VASP, primarily localized only to the edge of the lamellae (Supplemental Figure 6). In addition, EVL was within Arp2/3-independent filopodia, while VASP localized to the tips (Supplemental Figure 6). Interestingly, shEVL-mCherry, shVASP-mCherry as well as doubly shEVL-mCherry/shVASP-mCherry transfected cells spread normally (Supplemental Figure 7A-D). Likewise, GFP-actin cells co-transfected with shArp2-mCherry and shEVL or shVASP vectors (individually or both) efficiently formed filopodia (Supplemental Figure 7E-I). Thus, Arp2/3-independent filopodia formation in T cells is also EVL-and VASP-independent.
FMNL1 and DIA1 regulate MTOC polarization
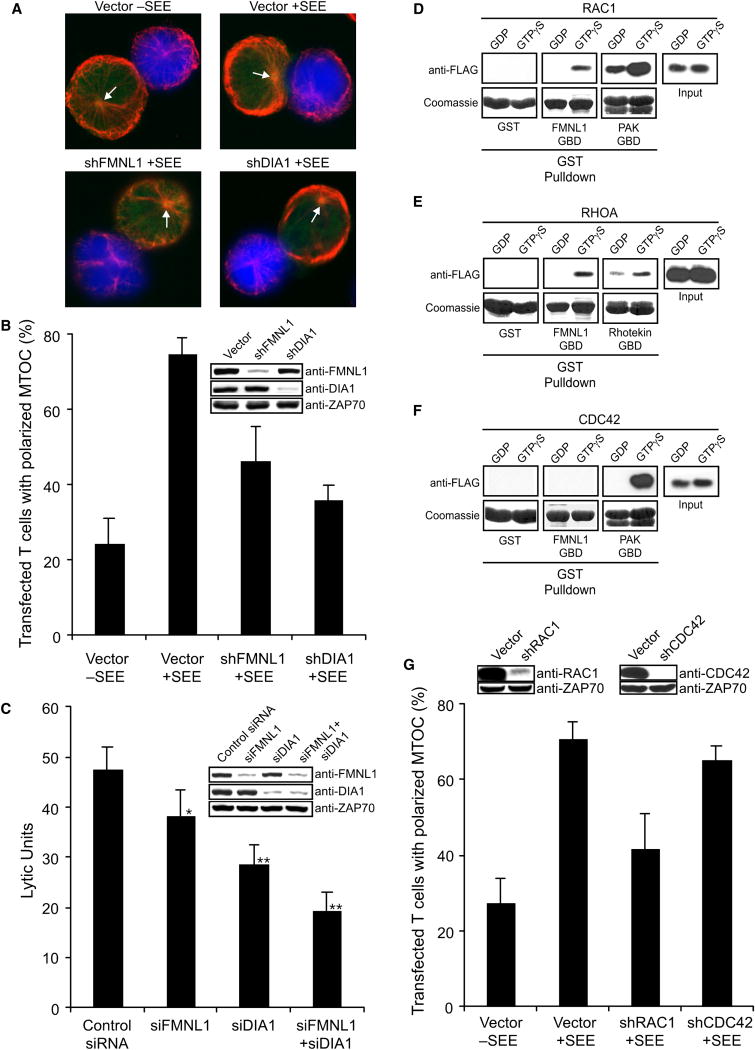
Recent evidence implicates F-actin reorganization in establishing T cell centrosomal polarity (Stinchcombe et al., 2006). Given that formins co-localize with the T cell centrosome, we examined their role in TCR-mediated MTOC polarization. In contrast to Arp2/3 depletion, which did not affect MTOC reorientation (Figure 3A-B), loss of FMNL1 or DIA1 did reduce MTOC polarization (Figure 7A and 7B). Thus, although FMNL1 and DIA1 do not affect the formation of F-actin-based structures at the IS, they do regulate centrosome polarization in T cells.
Figure 7. FMNL1 and DIA1 control TCR-mediated MTOC polarization.
(A) Jurkat cells were transfected with shRNA-GFP vectors as indicated and conjugated with unpulsed or SEE-pulsed/CMAC-stained Raji B cells (blue). The conjugates were stained with anti-αTubulin (red) and scored for MTOC polarization (B). Arrows indicate MTOC position. (C) hCD8+ T cells were transfected with siRNA against FMNL1 and/or DIA1 and then used in a redirected cytotoxicity assay. Data were analyzed using the Student’s t-test, *P<0.05 and **P<0.0002. (D) Purified FLAG-tagged Rac1 (D), Cdc42 (E) or RhoA (F) was loaded with GDP or GTPγS, and then analyzed for binding to GST control or GST-FMNL1 GBD (GTPase binding domain). GST-PAK-GBD was used as a positive control for binding to GTP-loaded Rac1 and Cdc42, whereas Rhotekin-GBD was used for RhoA. (G) Jurkat cells were transfected with the indicated shRNA-GFP vectors and then analyzed for MTOC polarization as in A and B. Bars in B, C, and G represent mean ±StDev from three independent experiments.
Since centrosome polarization is essential for the directed release of granules during cytotoxic T cell (CTL)-mediated killing, we were next interested in the ability of siFMNL1- and siDIA1-transfected primary CD8+ T cell clones to kill target cells. Like hCD4+ T cells, hCD8+ T cells express FMNL1 and DIA1, which co-localizes with the MTOC (Supplemental Figure 8). However, we found that primary human T cells (CD4+ and CD8+) also highly express a smaller 80kDa variant of DIA1 (Supplemental Figure 8C), which along with the larger variant was specifically depleted with siDIA1 (Figure 7C). Interestingly, CTLs did not express DIA2 (Supplemental Figure 8C). In a redirected cytotoxicity assay we found decreased target cell lysis by FMNL1- and DIA1-depleted CTLs, while combined suppression of these formins significantly reduced CTL-mediated killing (Figure 7C). This suggests that the diminished centrosomal polarization in the absence of FMNL1 and DIA1 negatively affects CTL function.
So far, regulation of TCR-mediated MTOC polarization has been shown to involve FYN, ZAP70, LAT, SLP76, and VAV1 (Ardouin et al., 2003; Kuhne et al., 2003; Martin-Cofreces et al., 2006). These components are known to be required for numerous signaling pathways downstream of the TCR including the activation of Rho family GTPases. Importantly, regulation of the formin proteins is primarily thought to occur through interactions with these small GTPases, with DIA1 being regulated by RhoA-C (Faix and Grosse, 2006), and FMNL1 predicted to be Rac1-regulated (Yayoshi-Yamamoto et al., 2000). However, we find that in addition to binding GTP-bound Rac1 (Figure 7D), FMNL1 also directly and specifically interacts with GTP-RhoA (Figure 7E), but not GTP-Cdc42 (Figure 7F). It is surprising that DIA1 and FMNL1 are potentially regulated through interactions with Rho and Rac, yet MTOC polarization in T cells has been suggested to be Cdc42-dependent (Stowers et al., 1995). However, this Cdc42-dependency for MTOC polarity was shown utilizing overexpression of dominant-negative Cdc42, which may have pleiotropic affects on signaling. Thus, we specifically depleted Rac1 and Cdc42 using shRNA and analyzed MTOC polarization in order to examine their role in TCR-mediated centrosome polarity. Interestingly, Rac1 suppression, but not Cdc42 suppression, resulted in diminished MTOC polarization in Jurkat cells (Figure 7G). This suggests that Rac1, possibly through its affects on FMNL1, is directing TCR-mediated movement of the MTOC to the IS.
Discussion
In this study, we found that TCR-stimulated Arp2/3-depleted cells could still polarize F-actin in the form of filopodial protrusions, and were even capable of MTOC polarization. In contrast, DIA1 and FMNL1, did not regulate F-actin polarization but instead controlled MTOC polarity. Thus, the Arp2/3 complex does not exclusively govern TCR-mediated cytoskeletal reorganization.
Upon stimulation, Arp2/3-suppressed T cells form atypical filopodia. However, these structures probably normally arise during activation, but are obscured by global actin reorganization events within the Arp2/3-driven meshwork. Indeed, Arp3-suppressed T cells displayed remnants of lamellipod formation, which transiently originated and spread between Arp2/3-independent spikes. Also, SEM images revealed apparent filopodial tips protruding from the lamellipod edge in spreading cells. In fact, microspikes have been described buried within the lamelipodial meshwork in fibroblasts (Small, 1981). Thus, Arp2/3-independent filopodia formation might concomitantly direct Arp2/3-mediated lamellipod formation, and is likely to be mechanistically important in cooperating with Arp2/3 for lamellipodial production.
Interestingly, it was recently reported that depletion of HEM-1, a member of the WAVE2 complex, induced similar microspikes at the leading edge of stimulated HL-60 cells (Weiner et al., 2006). Our data predicts that this is due to diminished WAVE2-dependent Arp2/3 nucleation and does not result from an independent HEM-1 regulated event. However, we have reported that WAVE2 depletion abrogates T cell spreading and does not lead to the generation of microspikes (Nolz et al., 2006). This may result from the inability of WAVE2-suppressed T cells to efficiently mobilize Ca2+ through CRAC channels (Nolz et al., 2006), since Arp2/3-independent filopodia formation is calcium-dependent (data not shown). Alternatively, the WAVE2 complex may be necessay for other actin nucleators to function or localize in T cells. Indeed, HEM-1 was found to associate with formin family members (Weiner et al., 2006). Moreover, we find WAVE2 localized to the edges of microspikes produced in Arp2/3-depleted T cells, as it does at the edge of the T cell lamellipod (Supplemental Figure 9)(Nolz et al., 2006; Zipfel et al., 2006). Thus, WAVE2 sits poised on the edges of filopodia and may even participate in their formation independently of Arp2/3, possibly through an association with formins or other actin nucleating complexes. Then WAVE2 could promote the Arp2/3-dependent polymerization required for filling between microspikes, creating lamellipodia. Interestingly, we do not see WASp or WIP localizing to these same filopodia (not shown), suggesting that Arp2/3 actin generated by WASp/WIP mat be involved in distinct cellular events than those generated by the WAVE2 complex.
FMNL1 and DIA1 localize within F-actin-rich structures, suggesting their involvement in F-actin polarization. Nevertheless, these formins do not appear to participate in actin dynamics (lamellipodia or filopodia) that lead to F-actin polarization at the IS. It remains possible that Arp2/3-independent filopodial formation results from another T cell expressed formin, or there is functional redundancy between different formin family members. It is also interesting that depletion of EVL and VASP, which are proposed to be required for the generation of formin-mediated filopodia (Schirenbeck et al., 2006), did not affect the formation of filopodia in the absence of Arp2/3. Thus, either formins do not require an association with these actin bundling proteins to promote the formation of filopodia in T cells, or formins are not the major regulator of microspike formation in Arp2/3-suppressed cells. Other actin nucleators, such as Spire proteins (Kerkhoff, 2006), could regulate these spikes.
Interestingly, although there may be functional compensation between formins in the generation of filopodia in the absence of Arp2/3, loss of FMNL1 or DIA1 individually affects MTOC reorientation, suggesting that, at least in this regard, they are not functionally redundant. In fact, it is clear that these formins display strikingly different patterns of centrosome co-localization, which may provide the basis for why they each independently regulate MTOC polarization. It is already thought that formins are essential for actin-dependent processes leading to polarity of the yeast MTOC (Faix and Grosse, 2006). Thus, microtubule regulation may be a general property of this protein family in eukaryotic cells, with a formin network controlling the actin-dependent processes that allow microtubule positioning. In fact, recent studies established the importance of formins in microtubule stabilization and suggest their role in migration through possible effects on microtubule polarization (Eng et al., 2006; Yamana et al., 2006). Therefore, even though the polarization of recognizable actin structures is unaffected by loss of FMNL1 and DIA1, we propose that these proteins each control distinct actin processes that are not as readily observed, but necessary for centrosome positioning.
Recently, it was suggested that the centrosome must contact the plasma membrane at the IS to allow T cell secretion (Stinchcombe et al., 2006). In this study, they proposed that the Cdc42-binding actin-regulatory protein IQGAP1, which interacts with microtubule plus end complexes, may provide the force for positioning the centrosome by linking actin-dependent processes to microtubule ends. Interestingly, formin family proteins may also bind microtubule plus end complexes (Faix and Grosse, 2006), and might provide the mechanical force needed for movement of these microtubule-regulating complexes. In support of this, we found that shRNA-depletion of IQGAP1 affects MTOC polarization (TSG and DDB unpublished observation), suggesting that these plus-end complexes are, in fact, essential for centrosome polarity in T cells. Thus, it is likely that formin-dependent and formin-independent processes acting along microtubules and directly at the MTOC will participate in TCR-mediated centrosome polarity and cell killing.
Surprisingly, although dominant-negative expression studies have indicated the importance of Cdc42 in MTOC polarization during T cell activation (Stowers et al., 1995), we find that depletion of Rac1, but not Cdc42 abrogates this process in T cells. While it is possible that the residual levels of Cdc42 following suppression are sufficient to drive MTOC reorientation, this finding is interesting because DIA1 is Rho-regulated, FMNL1 interacts with RhoA and Rac1, and IQGAP1 binds Rac1 (in addition to Cdc42). However, we must note, that in one report, FMNL1 was suggested to be Cdc42-regulated (Seth et al., 2006). In this study they could not detect GTP-Rac1 or GTP-Cdc42 binding to FMNL1, so they instead analyzed relief of FMNL1 auto-inhibition using a large molar excess of Cdc42 and truncated FMNL1 domains. Thus, this may not reflect a physiological mechanism of FMNL1 regulation. Further studies are needed to define the signaling pathways linking formin-mediated actin regulation to microtubule dynamics in T cells. In particular, the generation of reagents to specifically target and detect different members of the very homologous Rho family (RhoA-C) will help in dissecting the pathways linking formins to the TCR.
In contrast to MTOC polarization, β2-integrin activation appears to be specifically Arp2/3-dependent and does not rely on FMNL1 or DIA1. This is interesting because we have shown that WAVE2 controls integrin activation in T cells (Nolz et al., 2006), although WASp has been previously shown to be dispensible (Krawczyk et al., 2002). In fact, we observe a similar β2-integrin defect between Arp2/3- and WAVE2-suppressed cells, suggesting that WAVE2 regulation of β2-integrins is Arp2/3-dependent. However, the β1-integrin defect of WAVE2-depleted cells seems more severe than that seen with loss of Arp2/3 (not shown) (Nolz et al., 2006), and the explanation for this will require a better understanding of the contribution of WAVE2 and its interacting partners to actin reorganization. Interactions between the WAVE2 complex and formins may be important in this regard. In fact, it was recently demonstrated that formins might predominantly regulate β3-integrins (Butler et al., 2006), suggesting that integrin subsets might also be separately regulated through distinctly formed actin frameworks. Therefore, further dissection of the mechanisms by which distinct pools of F-actin are regulated to control both polarity and integrin function is necessary to fully understand these subtle complexities.
Altogether, we demonstrate that the Arp2/3 complex is not the sole regulator of TCR-mediated cytoskeletal dynamics, and find that formins control the dynamic centrosomal polarization necessary for T cell function and cellular cytotoxicity. Through the continued characterization of the distinct regulatory mechanisms that collaboratively control cytoskeletal restructuring, we can begin to functionally dissect the distinct contributions of the diverse F-actin architectural frameworks and their roles in establishing cell polarity.
Experimental Procedures
Reagents and Plasmids
Reagents were from Sigma unless otherwise specified. Anti-CD3 (OKT3) was from the Mayo Pharmacy and anti-ZAP70, anti-PLCγ1, and anti-WAVE2 have been previously described (Nolz et al., 2006; Ting et al., 1992; Williams et al., 1998). Anti-Arp3 and anti-Cdc42 (BD Transduction Laboratories), anti-Arp2 (clone H-84; Santa Cruz) and anti-ARPC2 and anti-Rac1 [clone 23A8] (Upstate Biotechnology) were used in this study. In addition, polyclonal antibodies against DIA1 and DIA2 (Bethyl Laboratories) were used. We obtained monoclonal anti-FLAG, anti-αTubulin and anti-γTubulin from Sigma (St Louis, MO). We also used antibodies against ERK1/2, pERK1/2 (T202/Y204) and pPLCγ1 (Y783) from Cell Signaling Technology. Antisera against FMNL1, FMNL2 and FMNL3 were generated through immunizing rabbits with KLH-conjugated synthetic peptides (Cocalico Biologicals; Supplemental Table 1). Antibodies against EVL(AA261-340) and VASP(AA231-325) were generated similarly, but with GST-fusion proteins. Anti-CD3εPE was from BD Immunocytometry Systems. The pFRT-H1p, pCMS3.eGFP.H1p, and pCMS3.mCherry.H1p shRNA vectors have been described (Gomez et al., 2005; Nolz et al., 2006). RT-PCR was performed as described (Nolz et al., 2006). See Supplemental Table 1 for RT-PCR primers and shRNA targeting sequences.
Cell Culture, Transfection, Immunoprecipitation, GST Pulldown and Immunoblot Analysis
Jurkat-E6, hCD4+, RAJI, NALM6, P815, and GFP-actin-Jurkat-E6 T cells were passaged as previously described (Gomez et al., 2006). Human CD8+ T cells were cloned and cultured as described (Billadeau et al., 2000). Jurkat cells were transfected with shRNA suppression vectors as described (Gomez et al., 2006). CD8+ T cells were transfected by electroporation at 295V using 3.5×106 cells and 600 pmol of siRNA per transfection. For single suppression, 300 pmol control siRNA and 300pmol specific siRNA was used and for double suppression 300 pmol of each specific siRNA was used together. For stimulation of Jurkat T cells, OKT3 mAb was used at 5 μg/ml. Immunoprecipitations (from 750 μg of protein) and lysates (75-100 μg of protein) were prepared and analyzed by immunoblot (Gomez et al., 2005). GST pulldowns were done using GTPase Binding Buffer (25 mM Tris pH7.5, 40 mM NaCl, 1 mM DTT, 30 mM MgCl2 and 0.5% NP-40). GST-fusion protein (25 μg) was bound to GSH-agarose (30 μl) followed by one wash. FLAG/His-tagged GTPases were purifed from insect cells using the FastBac system and Probond resin (Invitrogen), and loaded with GDP or GTPγS. For loading, 25 nM of purified GTPase was incubated with 50 μM GDP or GTPγS in GTPase Loading Buffer (20 mM Tris pH7.5, 50 mM NaCl, 1 mM EDTA, and 0.1% Triton) for 10 minutes at 37°C, and then MgCl2 was added to a final concentration of 2.5 mM. This GTPase-containing solution was then added to the GST-fusion protein bound agarose, rotated for 25 minutes at 4°C, and then washed two times.
Cytotoxicity Assays
The redirected 51Cr-release assays were performed as previously described (Billadeau et al., 2000). CD8+ T cells were used 48 hr post-transfection with siRNA, and were only able to kill P815 cells in the presence of OKT3 (1ng/well). In all cases, spontaneous release did not exceed 10% of maximum lysis. Lytic units were calculated based on 20% cytotoxicity (Pross et al., 1981).
Immunofluorescence and Live Cell imaging
Immunofluorescence of fixed Jurkat- or hCD4+-containing conjugates was performed as described (Gomez et al., 2005). For quantification, 50-100 conjugates were chosen randomly, and an individual blinded to the experiment scored conjugates consisting of one GFP+ T cell and one blue CMAC-stained B cell. Conjugates showing distinct labeling at the cell-cell contact site were scored positive. MTOC polarization studies were performed similarly, except conjugates were allowed to form for 30 minutes and scored positive if the MTOC (based on αTubulin staining) was against the cell-cell interface. For immunofluorescence of spreading cells, Jurkat cells were settled for 5 min onto poly-L-lysine coated coverslips (Gomez et al., 2006), which were pre-incubated with or without anti-CD3 (20 μg/ml in PBS) overnight at 4°C. For live cell imaging, Jurkat cells were imaged spreading onto anti-TCR-coated coverslips as described (Nolz et al., 2006).
Scanning Electron Microscopy (SEM)
For SEM, cells were prepared on glass coverslips as above (Gomez et al., 2005), but were placed in fixative (4% formaldehyde and 1% glutaraldehyde in sodium phosphate buffer, pH 7.3) at 4°C for up to 36 hours. The coverslips were treated with a series of solution exchanges, including two phosphate buffer washes followed by sequential 60% ethanol, 70% ethanol, 80% ethanol, 95% ethanol, and 100% ethanol washes. Coverslips were placed into a critical point dryer while in 100% ethanol for drying, sputter-coated with gold-palladium, and imaged on a Hitachi H-4700 SEM.
TCR Expression and Internalization
Jurkat cells were transfected with the indicated GFP-shRNA vectors, and basal TCR expression and TCR internalization were analyzed by flow cytometry gating on GFP+ cells as described (Gomez et al., 2005).
Adhesion Assays, Conjugate Analysis, and Calcium Mobilization
Adhesion assays were performed as described (Medeiros et al., 2005; Woods et al., 2001). Conjugate assays were performed as described (Gomez et al., 2005; Nolz et al., 2006). Calcium mobilization studies were done as previously described (Gomez et al., 2005).
Supplementary Material
Acknowledgments
We would like to thank Renee A Schoon and Christopher J. Dick for their help with the cytotoxicity assays. This work was supported by the Mayo Foundation, NIH grant R01-AI065474 to D.D.B., NIH grant F31-AI068624 to T.S.G., NIH grant R01-CA47752 to P.J.L., and NIH grants R01-AI038474 and R01-AI031126 to Y.S.
Footnotes
Competing Interest Statement The authors declare that they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ardouin L, Bracke M, Mathiot A, Pagakis SN, Norton T, Hogg N, Tybulewicz VL. Vav1 transduces TCR signals required for LFA-1 function and cell polarization at the immunological synapse. Eur J Immunol. 2003;33:790–797. doi: 10.1002/eji.200323858. [DOI] [PubMed] [Google Scholar]
- Barr VA, Balagopalan L, Barda-Saad M, Polishchuk R, Boukari H, Bunnell SC, Bernot KM, Toda Y, Nossal R, Samelson LE. T-cell antigen receptor-induced signaling complexes: internalization via a cholesterol-dependent endocytic pathway. Traffic. 2006;7:1143–1162. doi: 10.1111/j.1600-0854.2006.00464.x. [DOI] [PubMed] [Google Scholar]
- Billadeau DD, Mackie SM, Schoon RA, Leibson PJ. The Rho family guanine nucleotide exchange factor Vav-2 regulates the development of cell-mediated cytotoxicity. J Exp Med. 2000;192:381–392. doi: 10.1084/jem.192.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biyasheva A, Svitkina T, Kunda P, Baum B, Borisy G. Cascade pathway of filopodia formation downstream of SCAR. J Cell Sci. 2004;117:837–848. doi: 10.1242/jcs.00921. [DOI] [PubMed] [Google Scholar]
- Bunnell SC, Kapoor V, Trible RP, Zhang W, Samelson LE. Dynamic actin polymerization drives T cell receptor-induced spreading: a role for the signal transduction adaptor LAT. Immunity. 2001;14:315–329. doi: 10.1016/s1074-7613(01)00112-1. [DOI] [PubMed] [Google Scholar]
- Butler B, Gao C, Mersich AT, Blystone SD. Purified integrin adhesion complexes exhibit actin-polymerization activity. Curr Biol. 2006;16:242–251. doi: 10.1016/j.cub.2005.12.033. [DOI] [PubMed] [Google Scholar]
- Campi G, Varma R, Dustin ML. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J Exp Med. 2005;202:1031–1036. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs J, Kim SJ, Tan S, Ligon LA, Holzbaur EL, Kuhn J, Poenie M. Recruitment of dynein to the Jurkat immunological synapse. Proc Natl Acad Sci U S A. 2006 doi: 10.1073/pnas.0600914103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng CH, Huckaba TM, Gundersen GG. The Formin mDia Regulates GSK3{beta} through Novel PKCs to Promote Microtubule Stabilization but Not MTOC Reorientation in Migrating Fibroblasts. Mol Biol Cell. 2006 doi: 10.1091/mbc.E05-10-0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engqvist-Goldstein AE, Drubin DG. Actin assembly and endocytosis: from yeast to mammals. Annu Rev Cell Dev Biol. 2003;19:287–332. doi: 10.1146/annurev.cellbio.19.111401.093127. [DOI] [PubMed] [Google Scholar]
- Evangelista M, Pruyne D, Amberg DC, Boone C, Bretscher A. Formins direct Arp2/3-independent actin filament assembly to polarize cell growth in yeast. Nat Cell Biol. 2002;4:260–269. doi: 10.1038/ncb770. [DOI] [PubMed] [Google Scholar]
- Faix J, Grosse R. Staying in shape with formins. Dev Cell. 2006;10:693–706. doi: 10.1016/j.devcel.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Faix J, Rottner K. The making of filopodia. Curr Opin Cell Biol. 2006;18:18–25. doi: 10.1016/j.ceb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Favaro PM, Traina F, Vassallo J, Brousset P, Delsol G, Costa FF, Saad ST. High expression of FMNL1 protein in T non-Hodgkin’s lymphomas. Leuk Res. 2006;30:735–738. doi: 10.1016/j.leukres.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Gomez TS, Hamann MJ, McCarney S, Savoy DN, Lubking CM, Heldebrant MP, Labno CM, McKean DJ, McNiven MA, Burkhardt JK, Billadeau DD. Dynamin 2 regulates T cell activation by controlling actin polymerization at the immunological synapse. Nat Immunol. 2005;6:261–270. doi: 10.1038/ni1168. [DOI] [PubMed] [Google Scholar]
- Gomez TS, McCarney SD, Carrizosa E, Labno CM, Comiskey EO, Nolz JC, Zhu P, Freedman BD, Clark MR, Rawlings DJ, et al. HS1 functions as an essential actin-regulatory adaptor protein at the immune synapse. Immunity. 2006;24:741–752. doi: 10.1016/j.immuni.2006.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S, August A. Actin depolymerization transduces the strength of B-cell receptor stimulation. Mol Biol Cell. 2005;16:2275–2284. doi: 10.1091/mbc.E04-10-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs HN. Formin proteins: a domain-based approach. Trends Biochem Sci. 2005;30:342–353. doi: 10.1016/j.tibs.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Holsinger LJ, Graef IA, Swat W, Chi T, Bautista DM, Davidson L, Lewis RS, Alt FW, Crabtree GR. Defects in actin-cap formation in Vav-deficient mice implicate an actin requirement for lymphocyte signal transduction. Curr Biol. 1998;8:563–572. doi: 10.1016/s0960-9822(98)70225-8. [DOI] [PubMed] [Google Scholar]
- Hudson AM, Cooley L. A subset of dynamic actin rearrangements in Drosophila requires the Arp2/3 complex. J Cell Biol. 2002;156:677–687. doi: 10.1083/jcb.200109065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkhoff E. Cellular functions of the Spir actin-nucleation factors. Trends Cell Biol. 2006;16:477–483. doi: 10.1016/j.tcb.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Kinashi T. Intracellular signalling controlling integrin activation in lymphocytes. Nat Rev Immunol. 2005;5:546–559. doi: 10.1038/nri1646. [DOI] [PubMed] [Google Scholar]
- Kobielak A, Pasolli HA, Fuchs E. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat Cell Biol. 2004;6:21–30. doi: 10.1038/ncb1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk C, Oliveira-dos-Santos A, Sasaki T, Griffiths E, Ohashi PS, Snapper S, Alt F, Penninger JM. Vav1 controls integrin clustering and MHC/peptide-specific cell adhesion to antigen-presenting cells. Immunity. 2002;16:331–343. doi: 10.1016/s1074-7613(02)00291-1. [DOI] [PubMed] [Google Scholar]
- Kuhne MR, Lin J, Yablonski D, Mollenauer MN, Ehrlich LI, Huppa J, Davis MM, Weiss A. Linker for activation of T cells, zeta-associated protein-70, and Src homology 2 domain-containing leukocyte protein-76 are required for TCR-induced microtubule-organizing center polarization. J Immunol. 2003;171:860–866. doi: 10.4049/jimmunol.171.2.860. [DOI] [PubMed] [Google Scholar]
- Kupfer A, Dennert G. Reorientation of the microtubule-organizing center and the Golgi apparatus in cloned cytotoxic lymphocytes triggered by binding to lysable target cells. J Immunol. 1984;133:2762–2766. [PubMed] [Google Scholar]
- Kupfer A, Mosmann TR, Kupfer H. Polarized expression of cytokines in cell conjugates of helper T cells and splenic B cells. Proc Natl Acad Sci U S A. 1991;88:775–779. doi: 10.1073/pnas.88.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A, Swain SL, Singer SJ. The specific direct interaction of helper T cells and antigen-presenting B cells. II. Reorientation of the microtubule organizing center and reorganization of the membrane-associated cytoskeleton inside the bound helper T cells. J Exp Med. 1987;165:1565–1580. doi: 10.1084/jem.165.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Cofreces NB, Sancho D, Fernandez E, Vicente-Manzanares M, Gordon-Alonso M, Montoya MC, Michel F, Acuto O, Alarcon B, Sanchez-Madrid F. Role of Fyn in the rearrangement of tubulin cytoskeleton induced through TCR. J Immunol. 2006;176:4201–4207. doi: 10.4049/jimmunol.176.7.4201. [DOI] [PubMed] [Google Scholar]
- Medeiros RB, Dickey DM, Chung H, Quale AC, Nagarajan LR, Billadeau DD, Shimizu Y. Protein kinase D1 and the beta 1 integrin cytoplasmic domain control beta 1 integrin function via regulation of Rap1 activation. Immunity. 2005;23:213–226. doi: 10.1016/j.immuni.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Nolz JC, Gomez TS, Zhu P, Li S, Medeiros RB, Shimizu Y, Burkhardt JK, Freedman BD, Billadeau DD. The WAVE2 complex regulates actin cytoskeletal reorganization and CRAC-mediated calcium entry during T cell activation. Curr Biol. 2006;16:24–34. doi: 10.1016/j.cub.2005.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pross HF, Baines MG, Rubin P, Shragge P, Patterson MS. Spontaneous human lymphocyte-mediated cytotoxicity against tumor target cells. IX. The quantitation of natural killer cell activity. J Clin Immunol. 1981;1:51–63. doi: 10.1007/BF00915477. [DOI] [PubMed] [Google Scholar]
- Rivas FV, O’Keefe JP, Alegre ML, Gajewski TF. Actin cytoskeleton regulates calcium dynamics and NFAT nuclear duration. Mol Cell Biol. 2004;24:1628–1639. doi: 10.1128/MCB.24.4.1628-1639.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirenbeck A, Arasada R, Bretschneider T, Stradal TE, Schleicher M, Faix J. The bundling activity of vasodilator-stimulated phosphoprotein is required for filopodium formation. Proc Natl Acad Sci U S A. 2006;103:7694–7699. doi: 10.1073/pnas.0511243103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrador JM, Cabrero JR, Sancho D, Mittelbrunn M, Urzainqui A, Sanchez-Madrid F. HDAC6 deacetylase activity links the tubulin cytoskeleton with immune synapse organization. Immunity. 2004;20:417–428. doi: 10.1016/s1074-7613(04)00078-0. [DOI] [PubMed] [Google Scholar]
- Seth A, Otomo C, Rosen MK. Autoinhibition regulates cellular localization and actin assembly activity of the diaphanous-related formins FRLalpha and mDia1. J Cell Biol. 2006;174:701–713. doi: 10.1083/jcb.200605006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson AF, Baillie DL, Bowerman B. A Formin Homology protein and a profilin are required for cytokinesis and Arp2/3-independent assembly of cortical microfilaments in C. elegans. Curr Biol. 2002;12:2066–2075. doi: 10.1016/s0960-9822(02)01355-6. [DOI] [PubMed] [Google Scholar]
- Small JV. Organization of actin in the leading edge of cultured cells: influence of osmium tetroxide and dehydration on the ultrastructure of actin meshworks. J Cell Biol. 1981;91:695–705. doi: 10.1083/jcb.91.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen A, Faix J, Resch GP, Linkner J, Wehland J, Small JV, Rottner K, Stradal TE. Filopodia Formation in the Absence of Functional WAVE- and Arp2/3-Complexes. Mol Biol Cell. 2006;17:2581–2591. doi: 10.1091/mbc.E05-11-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature. 2006;443:462–465. doi: 10.1038/nature05071. [DOI] [PubMed] [Google Scholar]
- Stowers L, Yelon D, Berg LJ, Chant J. Regulation of the polarization of T cells toward antigen-presenting cells by Ras-related GTPase CDC42. Proc Natl Acad Sci U S A. 1995;92:5027–5031. doi: 10.1073/pnas.92.11.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting AT, Karnitz LM, Schoon RA, Abraham RT, Leibson PJ. Fc gamma receptor activation induces the tyrosine phosphorylation of both phospholipase C (PLC)-gamma 1 and PLC-gamma 2 in natural killer cells. J Exp Med. 1992;176:1751–1755. doi: 10.1084/jem.176.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolliday N, VerPlank L, Li R. Rho1 directs formin-mediated actin ring assembly during budding yeast cytokinesis. Curr Biol. 2002;12:1864–1870. doi: 10.1016/s0960-9822(02)01238-1. [DOI] [PubMed] [Google Scholar]
- Valitutti S, Dessing M, Aktories K, Gallati H, Lanzavecchia A. Sustained signaling leading to T cell activation results from prolonged T cell receptor occupancy. Role of T cell actin cytoskeleton. J Exp Med. 1995;181:577–584. doi: 10.1084/jem.181.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Sanchez-Madrid F. Role of the cytoskeleton during leukocyte responses. Nat Rev Immunol. 2004;4:110–122. doi: 10.1038/nri1268. [DOI] [PubMed] [Google Scholar]
- Weiner OD, Rentel MC, Ott A, Brown GE, Jedrychowski M, Yaffe MB, Gygi SP, Cantley LC, Bourne HR, Kirschner MW. Hem-1 complexes are essential for Rac activation, actin polymerization, and myosin regulation during neutrophil chemotaxis. PLoS Biol. 2006;4:e38. doi: 10.1371/journal.pbio.0040038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Eng CH, Schmoranzer J, Cabrera-Poch N, Morris EJ, Chen M, Wallar BJ, Alberts AS, Gundersen GG. EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat Cell Biol. 2004;6:820–830. doi: 10.1038/ncb1160. [DOI] [PubMed] [Google Scholar]
- Williams BL, Schreiber KL, Zhang W, Wange RL, Samelson LE, Leibson PJ, Abraham RT. Genetic evidence for differential coupling of Syk family kinases to the T-cell receptor: reconstitution studies in a ZAP-70-deficient Jurkat T-cell line. Mol Cell Biol. 1998;18:1388–1399. doi: 10.1128/mcb.18.3.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods ML, Kivens WJ, Adelsman MA, Qiu Y, August A, Shimizu Y. A novel function for the Tec family tyrosine kinase Itk in activation of beta 1 integrins by the T-cell receptor. Embo J. 2001;20:1232–1244. doi: 10.1093/emboj/20.6.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamana N, Arakawa Y, Nishino T, Kurokawa K, Tanji M, Itoh RE, Monypenny J, Ishizaki T, Bito H, Nozaki K, et al. The Rho-mDia1 pathway regulates cell polarity and focal adhesion turnover in migrating cells through mobilizing Apc and c-Src. Mol Cell Biol. 2006;26:6844–6858. doi: 10.1128/MCB.00283-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayoshi-Yamamoto S, Taniuchi I, Watanabe T. FRL, a novel formin-related protein, binds to Rac and regulates cell motility and survival of macrophages. Mol Cell Biol. 2000;20:6872–6881. doi: 10.1128/mcb.20.18.6872-6881.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel PA, Bunnell SC, Witherow DS, Gu JJ, Chislock EM, Ring C, Pendergast AM. Role for the Abi/wave protein complex in T cell receptor-mediated proliferation and cytoskeletal remodeling. Curr Biol. 2006;16:35–46. doi: 10.1016/j.cub.2005.12.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.