Abstract
Aims
β-Adrenergic receptor (β-AR) stimulation induces apoptosis in adult rat ventricular myocytes (ARVMs) via the activation of glycogen synthase kinase-3β (GSK-3β) and mitochondrial pathways. However, β-AR stimulation induces apoptosis only in a fraction (∼15–20%) of ARVMs. We hypothesized that ARVMs may secrete/release a survival factor(s) which protects 80–85% of cells from apoptosis.
Methods and results
Using two-dimensional gel electrophoresis followed by MALDI TOF and MS/MS, we identified ubiquitin (Ub) in the conditioned media of ARVMs treated with β-AR agonist (isoproterenol). Western blot analysis confirmed increased Ub levels in the conditioned media 3 and 6 h after β-AR stimulation. Inhibition of β1-AR and β2-AR subtypes inhibited β-AR-stimulated increases in extracellular levels of Ub, whereas activation of adenylyl cyclase using forskolin mimicked the effects of β-AR stimulation. Incubation of cells with exogenous biotinylated Ub followed by western blot analysis of the cell lysates showed uptake of extracellular Ub into cells, which was found to be higher after β-AR stimulation (1.9 ± 0.4-fold; P < 0.05 vs. control, n = 6). Pre-treatment with Ub inhibited β-AR-stimulated increases in apoptosis. Inhibition of phosphoinositide 3-kinase using wortmannin and LY-294002 prevented anti-apoptotic effects of extracellular Ub. Ub pre-treatment inhibited β-AR-stimulated activation of GSK-3β and c-Jun N-terminal kinase (JNK) and increases in the levels of cytosolic cytochrome c. The use of methylated Ub suggested that the anti-apoptotic effects of extracellular Ub are mediated via monoubiquitination.
Conclusion
β-AR stimulation increases levels of Ub in the conditioned media. Extracellular Ub plays a protective role in β-AR-stimulated apoptosis, possibly via the inactivation of GSK-3β/JNK and mitochondrial pathways.
Keywords: Ubiquitin, Apoptosis, Myocytes, Heart, GSK-3β, JNKs
1. Introduction
Apoptosis occurs in the myocardium of patients with end-stage heart failure and myocardial infarction, and in animal models of myocardial hypertrophy and failure.1 Stimulation of β-adrenergic receptor (β-AR) induces apoptosis in cardiac myocytes in vitro and in vivo.2–6 β-AR-stimulated apoptosis in adult rat ventricular myocytes (ARVMs) is demonstrated to occur via the c-Jun NH2-terminal kinase (JNK)-dependent mitochondrial death pathway.7 Recently, we provided evidence that β-AR stimulation increases glycogen synthase kinase-3β (GSK-3β) activity, and activation of GSK-3β plays a pro-apoptotic role in β-AR-stimulated apoptosis via the involvement of the mitochondrial death pathway.8
Ubiquitin (Ub) is a highly conserved low molecular weight (8.5 kDa) protein of 76 amino acid residues found in all eukaryotic cells. The most important intracellular function of Ub is to regulate protein turnover and to protect the cells from damaged or misfolded proteins by the Ub–proteosome pathway.9 Ub–proteosome system may regulate receptor internalization, hypertrophic response, apoptosis, and tolerance to ischaemia and reperfusion in cardiac myocytes.10 Ub is normally present in trace amounts in body fluids. Elevated levels of Ub are described in the serum or plasma of patients with parasitic and allergic diseases,11 alcoholic liver disease,12 type 2 diabetes,13 β2-microglobulin amyloidosis,14 and chronic haemodialysis patients.15 Patients with traumatic brain injury are shown to have increased Ub levels in the cerebrospinal fluid.16 Extracellular Ub is proposed to have pleiotropic functions including regulation of immune response, anti-inflammatory, and neuroprotective activities,17–20 as well as growth regulation and apoptosis control in haematopoetic cells.21 The biological functions of extracellular Ub, however, remain poorly understood. In particular, the mechanism of action of extracellular Ub in cell survival or apoptosis in cardiac myocytes has not yet been explored.
β-AR stimulation induces apoptosis only in a fraction of ARVMs (∼15–20%), although all the cells are exposed to the same stimulus. On the basis of this observation, we hypothesized that ARVMs may secrete some survival factor(s) which may protect ∼80–85% of ARVMs from β-AR-stimulated apoptosis. This hypothesis led us to search for survival factor(s) in the conditioned media. Using two-dimensional (2D) gel electrophoresis followed by MALDI TOF and MS/MS, we identified Ub in the conditioned media of ARVMs. β-AR stimulation increased levels of extracellular Ub in the media. Importantly, we provide evidence that extracellular Ub plays a protective role in β-AR-stimulated apoptosis, possibly via the inactivation of GSK-3β/JNK and mitochondrial death pathways.
2. Methods
2.1. Cell isolation and culture
Calcium-tolerant ARVMs were isolated from the hearts of adult male Sprague–Dawley rats (200–240 g) as described.22,23 ARVMs were plated in Dulbecco's modified Eagle's medium (DMEM; Mediatech) supplemented with HEPES (25 mM), bovine serum albumin (BSA, 0.2%), creatine (5 mM), l-carnitine (2 mM), taurine (5 mM), and 0.1% penicillin–streptomycin at a density of 30–50 cells/mm2 on 100 mm tissue culture dishes (Fisher Scientific) or coverslips pre-coated with laminin (1 µg/cm2). The chemicals, if not mentioned otherwise, were purchased from Sigma-Aldrich, USA. The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). The animal protocol was approved by the University Committee on Animal Care.
2.2. Cell treatment
ARVMs, cultured for 24 h, were treated with isoproterenol (ISO, 10 µM), angiotensin II (AngII, 1 or 100 nM), l-norepinephrine (NE; 10 µM), forskolin (FSK, 10 µM; Calbiochem), or hydrogen peroxide (100 µM) for indicated time points. For treatment with ISO and NE, dishes were supplemented with ascorbic acid (100 µM). To selectively stimulate β-AR using NE, prazosin (PZ, 100 nM) was added 30 min prior to NE. Ub (10 µg/mL), biotinylated Ub (BiotUb, 0.5 µg/mL, Boston Biochem), methylated Ub (MeUb, 10 µg/ml, Boston Biochem), wortmannin (WORT, 0.5 µM), LY-294002 (LY, 1 µM; Sigma), CGP 20712A (CGP, 0.3 µM; Sigma), or ICI 118,551 (ICI, 0.1 µM; Sigma) was added for 30 min prior to ISO treatment.
2.3. Two-dimensional, MALDI TOF, and MS/MS
ARVMs, cultured for 24 h, were washed three times with serum-free media to remove non-adherent cells. The cells were then treated with ISO (10 µM) in serum-free media for 3, 6, and 24 h. The collected conditioned media were centrifuged to remove non-adherent cells and lyophilized to dryness. The pellet was dissolved in 2D sample buffer (7 M urea, 2 M thiourea, 4% CHAPS, 0.5% Triton X-100, 10 mM DTT, and 0.5% carrier ampholyte, pH 5–8). Isoelectric focusing was performed using ReadyStrip IPG strips of pH range 5–8 (Bio-Rad). The second dimension was accomplished using a Criterion cell and 8–16% precast polyacrylamide gels (Bio-Rad). The gels were stained with SYPRO Ruby fluorescent stain and photographed. The protein spots of interest were identified by MALDI TOF and MS/MS (Applied Biosystems).
2.4. Adenovirus infection
ARVMs were infected with adenoviruses expressing a constitutively active form of GSK-3β (S9A-GSK; courtesy of Dr Morris J. Birnbaum, Howard Hughes Medical Institute, University of Pennsylvania—School of Medicine, Philadelphia, PA, USA), or green fluorescence protein (GFP) at a multiplicity of infection of 50–100 for a total period of 48 h.
2.5. Terminal deoxynucleotidyl transferase-mediated nick end-labelling assay
Terminal deoxynucleotidyl transferase-mediated nick end-labelling (TUNEL) staining was performed on ARVMs plated on thermanox coverslips using in situ death detection kit according to the manufacturer's instructions (Roche Molecular Biochemicals). The percentage of TUNEL-positive cells (relative to total ARVMs) was determined by counting ∼200 cells in 10 randomly chosen fields per coverslip for each experiment.
2.6. Analysis of cytosolic cytochrome c
Cytosolic fractions were prepared as described.8 The cytosolic fractions were analysed by western blot using anti-cytochrome c antibody (Santa Cruz).
2.7. Western blot analyses
ARVMs were lysed in cell lysis buffer [10 mM Tris–HCl (pH 7.4), 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 0.2 mM sodium orthovanadate, 0.5% Nonidet P-40, 1% Triton X-100, and 1 mM phenylmethylsulfonyl fluoride]. To measure the levels of Ub in the conditioned media, conditioned media collected at the end of treatment period were centrifuged at 1000 g for 5 min and freeze-dried. The samples were reconstituted in distilled water (500 µL) and subjected to several rounds of desalting using Microcon centrifugal filter devices (Millipore). Equal amounts of total proteins (50–100 µg) from the cell lysates or conditioned media (0.5–5 µg) were analysed by western blot as described.24 The primary antibodies used were Ub, P-JNK, or cytochrome c (Santa Cruz). The membranes were stripped and probed with anti-GAPDH antibody (Santa Cruz) to normalize protein loading. To detect biotin-labelled Ub, the membranes were incubated overnight in extra-avidin peroxidase (Sigma; 1:2000 dilution in TBST). The biotin-labelled Ub was visualized using chemiluminescence reagents.
2.8. Measurement of GSK-3β activity
GSK-3β activity was measured by immune-complex kinase assay using phosphoglycogen synthase peptide-2 (Upstate) as described.25
2.9. Plasma membrane integrity
Integrity of plasma membrane was measured using propidium iodide (PI)/Hoechst and Trypan Blue staining.
2.10. Statistical analyses
All data are expressed as mean ± SE. Statistical analysis was performed using the Student's t-test or a one-way analysis of variance followed by the Student–Newman–Keuls test. Probability (P) values of <0.05 were considered to be significant. The number (n) indicates the number of biological replicates.
3. Results
3.1. β-AR stimulation increases levels of Ub in the conditioned media
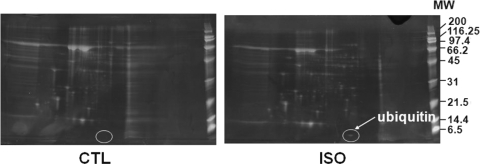
Visual analysis of SYPRO Ruby fluorescent-stained 2D images revealed that β-AR stimulation increased intensities of several protein spots. There was a consistent presence of a low molecular weight protein in ISO-treated samples at all time points (3, 6, and 24 h). Figure 1 represents a proteome map of conditioned media from control (CTL) and ISO-treated (3 h) samples. MALDI TOF and MS/MS identified this protein as Ub with 99.56% confidence. The amino acid sequence, EGIPDDQQR, matched completely with the amino acid sequence of Ub (from residues 30–38).
Figure 1.
Proteome map of conditioned media from CTL and ISO-treated samples. ARVMs were treated with ISO (10 µM) for 3 h. Proteins from the concentrated conditioned media were resolved by 2D gel electrophoresis, stained with SYPRO Ruby fluorescent stain, and photographed. The circled protein was identified as Ub by MALDI TOF and MS/MS. CTL, control; MW, molecular weight ladder (kDa).
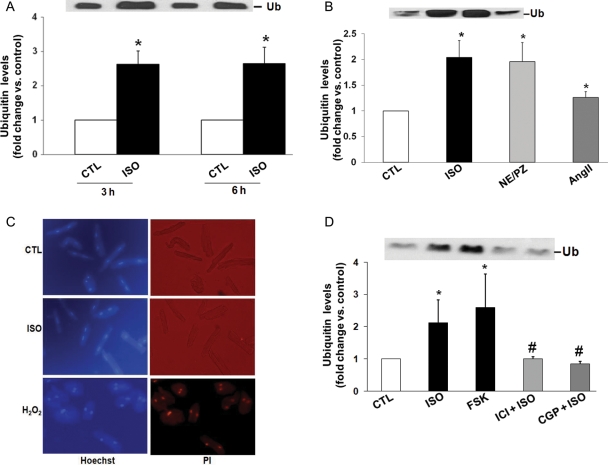
To confirm that β-AR stimulation indeed increases levels of extracellular Ub, ARVMs were washed with serum-free DMEM and treated with ISO for 3 and 6 h. Analysis of concentrated conditioned media by western blot using anti-Ub antibodies detected the presence of extracellular Ub with an apparent molecular weight of 8.5 kDa in the conditioned media of ARVMs (Figure 2A). Levels of extracellular Ub were increased by 2.6 ± 0.4-fold (P < 0.01, n = 7) and 2.6 ± 0.5-fold (P < 0.05, n = 4) 3 and 6 h after β-AR stimulation, respectively. Western blot analysis of cell lysates indicated no significant change in the intracellular levels of Ub (fold change vs. CTL; 0.93 ± 0.1; P = NS vs. CTL; n = 5).
Figure 2.
β-AR stimulation increases levels of extracellular Ub without affecting membrane integrity. (A) ARVMs were treated with ISO for 3 and 6 h. (B) ARVMs were treated with ISO, or norepinephrine + prazosin (NE/PZ; NE, 10 µM; PZ, 100 nM) or AngII (100 nM) for 3 h. Concentrated conditioned media were subjected to western blot analysis using anti-Ub antibodies. (A) *P < 0.01 vs. CTL; n = 4–7; (B) *P < 0.05 vs. CTL; n = 5. (C) ARVMs were treated with ISO or H2O2 for 3 h. The cells were stained with Hoechst 33258 (10 µM) and PI (10 µM) for 10 min, and visualized using fluorescent microscope and photographed. (D) ARVMs were treated with CGP or ICI for 30 min followed by the treatment with ISO for 3 h. To activate adenylyl cyclase, ARVMs were treated with FSK for 3 h. Concentrated conditioned media were subjected to western blot analysis using anti-Ub antibodies. *P < 0.01 vs. CTL; #P < 0.05 vs. ISO; n = 6.
To examine the effect of another β-AR agonist on the levels of Ub in the conditioned media, cells were treated with NE (10 µM) in the presence of α-AR antagonist PZ (100 nM) for 3 h. NE in the presence of PZ induces apoptosis to a similar extent as ISO in ARVMs.22 Analysis of conditioned media by western blot indicated that treatment with NE + PZ increased levels of extracellular Ub to a similar extent as ISO (Figure 2B). AngII, shown to induce cardiac myocyte apoptosis,26 also slightly but significantly increased extracellular levels of Ub (Figure 2B).
To rule out the possibility that increased levels of Ub in the conditioned media were due to the loss of plasma membrane integrity, live cells were stained with PI and Hoechst 33342 (Hoechst). Hoechst, readily taken up by all cells, is a UV-excitable nucleic acid stain with blue fluorescence. In contrast, PI only enters cells with compromised plasma membranes and stains nucleic acid to yield red fluorescence.27 To study plasma membrane integrity, 24 h plated ARVMs were treated with ISO (10 µM) for 3 h. As a positive control, ARVMs were treated with H2O2 (100 µM) for 3 h. H2O2 is shown to induce cytotoxicity in various cells, including cardiac myocytes.28,29 The cells were then incubated with PI (10 µM) and Hoechst (10 µM) for 10 min. Live (unfixed) cells were visualized using fluorescent microscope (Nikon Eclipse TE2000-S) and photographed. CTL and ISO-treated cells exhibited blue nuclear staining for Hoechst, while exhibiting only rare positive PI-stained cells (Figure 2C). H2O2 treatment clearly increased the number of PI-stained cells (>90%; red nuclear staining). Similar data were obtained using Trypan Blue staining (data not shown). These data suggest that the increased presence of Ub in the conditioned media following β-AR stimulation is unlikely due to the loss of membrane integrity.
3.2. Involvement of β-AR subtypes and adenylyl cyclase
Stimulation of β1-AR increases apoptosis, whereas that of β2-AR inhibits apoptosis.30 To study the involvement of β1- or β2-AR subtype in the increased levels of Ub in the conditioned media, ARVMs were pre-treated with CGP (0.3 µM; β1-AR-selective antagonist) or ICI (0.1 µM; β2-AR-selective antagonist) for 30 min followed by treatment with ISO for 3 h. Analysis of concentrated conditioned media by western blot using anti-Ub antibodies demonstrated that both CGP and ICI significantly inhibit β-AR-stimulated increases in the levels of extracellular Ub (fold change vs. CTL; ISO, 2.3 ± 0.8*; ICI + ISO, 1.0 ± 0.06#; CGP + ISO, 0.9 ± 0.09#; *P < 0.05 vs. CTL; #P < 0.05 vs. ISO; n = 6; Figure 2D). Treatment with FSK (10 µM) for 3 h also increased extracellular levels of Ub (Figure 2D).
3.3. β-AR stimulation increases cellular interaction/uptake of extracellular Ub
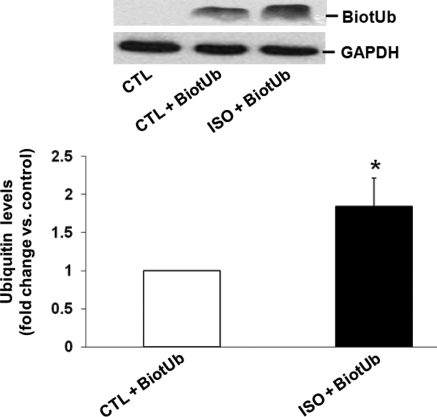
To investigate cellular interaction/uptake of extracellular Ub, ARVMs were pre-treated with BiotUb (0.5 µg/mL) in the presence of unlabelled Ub (10 µg/mL) for 30 min followed by treatment with ISO for 3 h. Cells incubated only with unlabelled Ub served as controls. Analysis of cell lysates by western blot using extravidin peroxidase to identify biotin-labelled Ub showed a clear band for Ub with apparent molecular weight of ∼8.5 kDa in the cell lysates prepared from ARVMs treated with BiotUb. Interestingly, the intensity of this band was significantly enhanced following β-AR stimulation (1.85 ± 0.37-fold vs. CTL; *P < 0.05; n = 5; Figure 3). No bands were detectable in cells incubated only with unlabelled Ub (Figure 3; CTL). This finding suggests that cellular interaction/uptake of exogenous Ub is possible in AVRMs, and that β-AR stimulation enhances the cellular interaction/uptake of exogenous Ub.
Figure 3.
β-AR stimulation increases cellular interaction/uptake of Ub. ARVMs were pre-treated with BiotUb (0.5 µg/mL) together with Ub (10 µg/mL) for 30 min before treatment with ISO (3 h). Cell lysates were analysed by western blot using extravidin peroxidase to measure the levels of BiotUb. GAPDH immunostaining indicates protein loading. *P < 0.05 vs. CTL; n = 6.
3.4. Ub inhibits β-AR-stimulated apoptosis via the involvement of phosphoinositide 3-kinase pathway
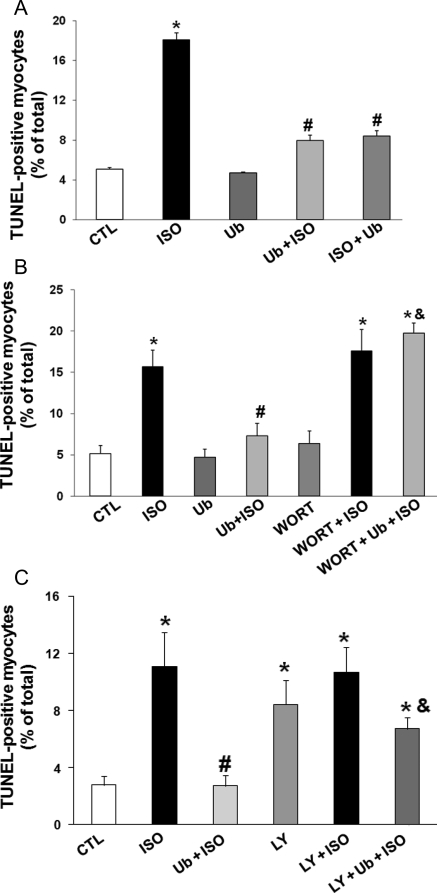
To study the role of extracellular Ub in β-AR-stimulated apoptosis, ARVMs were pre-treated with purified bovine Ub at concentrations ranging from 0.01 to 10 µg/mL for 30 min followed by treatment with ISO for 24 h. Analysis of apoptosis using TUNEL staining assay indicated that lower concentrations of Ub (0.01, 0.1, and 1 µg/mL) partially inhibit β-AR-stimulated apoptosis (data not shown). However, at a concentration of 10 µg/mL, Ub almost completely inhibited β-AR-stimulated apoptosis (per cent apoptosis; CTL, 5.1 ± 0.2; ISO, 18.1 ± 0.7*; Ub + ISO, 7.97 ± 0.55#; *P < 0.05 vs. CTL, #P < 0.05 vs. ISO; n = 3–6; Figure 4A). Protective effects of Ub were preserved even when cells were treated with Ub (10 µg/mL) 30 min after β-AR stimulation (ISO + Ub, Figure 4A). BSA (10 µg/mL) had no effect on β-AR-stimulated apoptosis (data not shown).
Figure 4.
Extracellular Ub inhibits β-AR-stimulated apoptosis. (A) ARVMs were pre-treated with Ub (10 µg/mL) for 30 min followed by treatment with ISO (Ub + ISO) or pre-treated with ISO for 30 min followed by treatment with Ub (ISO + Ub) for 24 h. The number of apoptotic cells was measured using TUNEL-staining assay. *P < 0.01 vs. CTL; #P < 0.01 vs. ISO; n = 3–6. (B) ARVMs were pre-treated with WORT (0.5 µM) for 30 min followed by treatment with Ub (10 µg/mL; 30 min) and ISO (10 µM) for 24 h. The number of apoptotic cells was measured using TUNEL-staining assay. *P < 0.05 vs. CTL; #P < 0.05 vs. ISO; &P < 0.05 vs. Ub + ISO; n = 3–5. (C) ARVMs were pre-treated LY for 30 min followed by treatment with Ub (10 µg/mL; 30 min) and ISO (10 µM) for 24 h. The number of apoptotic cells was measured using TUNEL-staining assay. *P < 0.05 vs. CTL; #P < 0.05 vs. ISO; &P < 0.05 vs. Ub + ISO; n = 4–6.
Recently, we have provided evidence that inhibition of phosphoinositide 3 (PI3)-kinase inhibits the protective effects of β1-integrin in β-AR-stimulated apoptosis.8 To determine whether protective effects of extracellular Ub are mediated via the activation of PI3-kinase, cells were pre-treated for 30 min with WORT (0.5 µM) or LY (1 µM; Sigma) followed by treatment with Ub (10 µg/mL; 30 min) and ISO (10 µM; 24 h). Analysis of apoptosis using TUNEL-staining assay showed that wortmannin prevents the anti-apoptotic effects of Ub (per cent apoptosis, Ub + ISO, 7.31 ± 1.51#; Ub + ISO + WORT, 19.72 ± 1.24&; #P < 0.05 vs. ISO; &P < 0.05 vs. Ub + ISO; n = 3–5, Figure 4B). Similar data were obtained using pre-treatment with LY (per cent apoptosis, CTL, 2.5 ± 0.7; ISO, 10.7 ± 2.8*; Ub + ISO, 2.5 ± 1.0#; Ub + ISO + LY, 6.8 ± 1.0&; *P < 0.05 vs. CTL; #P < 0.05 vs. ISO; &P < 0.05 vs. Ub + ISO; n = 3–5, Figure 4C).
3.5. Extracellular Ub inhibits β-AR-stimulated activation of GSK-3β and JNKs, and GSK-3β acts upstream in the activation of JNKs
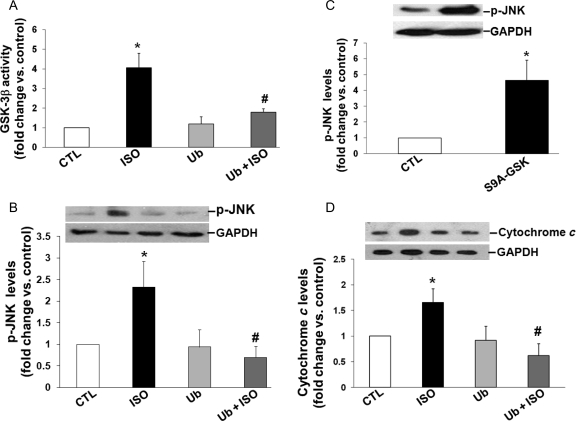
We have provided evidence that β-AR-stimulated activation of GSK-3β plays a pro-apoptotic role.8 To study whether extracellular Ub inhibits β-AR-stimulated activation of GSK-3β activity, ARVMs were pre-treated with Ub (10 µg/mL) for 30 min followed by treatment with ISO for 15 min. GSK-3β activity was measured in total cell lysates using immune-complex kinase assay.8 This analysis indicated increased GSK-3β activity following β-AR stimulation. This increase in GSK-3β activity was inhibited by Ub pre-treatment (fold change vs. CTL; ISO, 4.1 ± 0.7*; Ub, 1.2 ± 0.4; Ub + ISO, 1.8 ± 0.2#; *P < 0.05 vs. CTL; #P < 0.05 vs. ISO; n = 3; Figure 5A).
Figure 5.
Extracellular Ub inhibits β-AR-stimulated activation of GSK-3β and JNKs and increases in the levels of cytosolic cytochrome c. (A and B) ARVMs were treated with Ub (10 µg/mL) for 30 min before treatment with ISO (15 min). Cell lysates were analysed by immune-complex kinase assay to measure GSK-3β activity (A) or by western blot using phospho-specific anti-JNK antibodies (B). *P < 0.05 vs. CTL; n = 3; #P < 0.05 vs. ISO; n = 3–5. (C) ARVMs were infected with adenoviruses expressing constitutively active GSK-3β (S9A-GSK) for 48 h. Cell lysates were analysed by western blot using phospho-specific anti-JNK antibodies. GAPDH immunostaining indicates protein loading. *P < 0.05 vs. CTL. (D) ARVMs were pre-treated with Ub (10 µg/mL) for 30 min followed by treatment with ISO (10 µM, 6 h). Cytosolic cytochrome c levels were measured using western blot analysis. GAPDH immunostaining indicates protein loading. *P < 0.05 vs. CTL, #P < 0.05 vs. ISO; n = 4.
β-AR-stimulated activation of JNK pathway is demonstrated to play a pro-apoptotic role in β-AR-stimulated apoptosis.7 To determine whether extracellular Ub inhibits β-AR-stimulated activation of JNK, cell lysates (prepared as above) were analysed by western blot using phospho-specific JNK antibodies. This analysis showed increased JNK phosphorylation following β-AR stimulation. Ub pre-treatment completely reversed the β-AR-stimulated increases in JNK phosphorylation (fold change vs. CTL; ISO, 2.2 ± 0.5*; Ub, 1.0 ± 0.3; Ub + ISO, 0.6 ± 0.2#; *P < 0.05 vs. CTL; #P < 0.05 vs. ISO; n = 6; Figure 5B). Collectively, these data suggest that extracellular Ub exerts its anti-apoptotic effects via the inhibition of GSK-3β and JNK pathways.
In cells of non-cardiac origin, GSK-3β is suggested to act upstream in the activation of JNKs.31,32 To evaluate the possibility that GSK-3β acts upstream of JNKs in ARVMs, cells were infected with adenoviruses overexpressing a constitutively active form (S9A) of GSK-3β or GFP (CTL) for 48 h. The cell lysates were analysed by western blot using phospho-specific JNK antibodies. Overexpression of constitutively active GSK-3β clearly increased JNK phosphorylation when compared with CTL (*P < 0.01 vs. CTL; n = 4; Figure 5C).
3.6. Extracellular Ub inhibits β-AR-stimulated increases in the levels of cytochrome c
Activation of GSK-3β and JNKs plays a pro-apoptotic role in β-AR-stimulated apoptosis via the involvement of mitochondrial death pathway.7,8 To determine whether extracellular Ub affects the mitochondrial death pathway, ARVMs were pre-treated with Ub (10 µg/mL) for 30 min followed by treatment with ISO (10 µM) for 6 h. Cytosolic fractions were analysed by western blot using anti-cytochrome c antibodies. As shown previously,8 β-AR stimulation increased the levels of cytosolic cytochrome c (Figure 5D). Pre-treatment with extracellular Ub almost completely inhibited β-AR-stimulated increases in cytosolic cytochrome c (fold change vs. CTL; ISO, 1.65 ± 0.26*; Ub + ISO, 0.62 ± 0.23#; *P < 0.05 vs. CTL, #P < 0.05 vs. ISO; n = 4; Figure 5D).
3.7. Anti-apoptotic effects of extracellular Ub are exerted via monoubiquitination
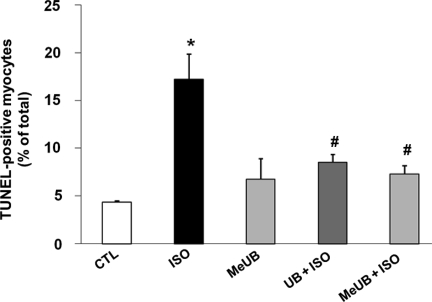
There is growing evidence that while formation of multi-Ub chains targets proteins for destruction by the proteosomal complex, monoubiquitination mediates more diverse functions such as protein transport and transcription regulation.33–35 To investigate whether anti-apoptotic signalling by extracellular Ub is brought about by monoubiquitination of cellular proteins, we used a methylated analogue of Ub (MeUb). In MeUb, methyl groups are covalently attached to lysine residues, thereby preventing polyubiquitination. To study the role of MeUb in β-AR-stimulated apoptosis, ARVMs were pre-treated with MeUb (10 µg/mL) for 30 min followed by treatment with ISO (10 µM, 24 h). Analysis of apoptosis using TUNEL-staining assay indicated that MeUb inhibits β-AR-stimulated apoptosis to a similar extent as Ub (per cent apoptosis; CTL, 4.4 ± 0.2; ISO, 17.3 ± 2.6*; Ub + ISO, 8.5 ± 0.8#; MeUb + ISO, 7.3 ± 0.9#; *P < 0.05 vs. CTL, #P < 0.05 vs. ISO; n = 4, Figure 6). These data suggest that the protective effects of extracellular Ub are exerted by monoubiquitination of cellular proteins.
Figure 6.
Anti-apoptotic effects of extracellular Ub are mediated via monoubiquitination. ARVMs were pre-treated with MeUb (10 µg/mL) for 30 min followed by treatment with ISO (24 h). The numbers of apoptotic cells were measured using TUNEL-staining assay. *P < 0.05 vs. CTL; #P < 0.05 vs. ISO; n = 4.
4. Discussion
Stimulation of β-AR induces apoptosis in cardiac myocytes in vitro and in vivo.2–6 In vitro, β-AR stimulation increases apoptosis in only a fraction of cardiac myocytes. This observation led us to search for potential anti-apoptotic proteins present in the conditioned media following β-AR stimulation. This study is the first to describe the presence of Ub in the conditioned media of adult cardiac myocytes and its role in cardiac myocyte apoptosis. The major new findings of the present study are: (i) β-AR stimulation increases extracellular levels of Ub in ARVMs; (ii) extracellular Ub plays a protective role in β-AR-stimulated apoptosis; (iii) extracellular Ub inhibits β-AR-stimulated activation of GSK-3β and JNKs and increases in the levels of cytosolic cytochrome c; (iv) GSK-3β acts upstream in the activation of JNKs; and (v) protective effects of extracellular Ub are mediated, at least in part, via monoubiquitination of cellular proteins.
The most important intracellular function of Ub is to regulate protein turnover by the Ub–proteosome pathway.21 Ub is a normal constituent of plasma. Increased systemic levels of Ub in the plasma are observed during several diseases.11–14,16,21 Using cell transfection assay, Daino et al.21 demonstrated increased secretion of intracellularly synthesized Ub in the conditioned media of murine pro-B (Ba/F3) and human kidney (293T) cell lines. Extracellular levels of Ub were also found to be increased in packed red blood cells during storage.36 Using a proteomic approach and western blot analyses, we provide evidence for the first time that Ub is present in the conditioned media of ARVMs. β-AR stimulation using two different stimuli, ISO and NE + PZ, increased the levels of Ub in the conditioned media. At least two factors may be responsible for the increased extracellular levels of Ub in the conditioned media: loss of membrane-integrity and/or increased secretion of Ub following β-AR stimulation. Using PI- and Trypan Blue-exclusion assays, we provide evidence that β-AR-stimulated loss of membrane integrity may not be responsible for the increased extracellular levels of Ub. Furthermore, an increased rate of cardiac myocyte apoptosis starts to become apparent 6 h after β-AR stimulation,22 while the increased levels of Ub in the conditioned media are readily detectable 3 h after β-AR stimulation. These observations point towards the possibility that increased secretion is the most probable mechanism leading to enhanced levels of Ub in the conditioned media following β-AR stimulation. This possibility is supported by the observation that stimulation of adenylyl cyclase also increases extracellular levels of Ub. ICI and CGP each inhibited β-AR-stimulated increase in extracellular Ub, suggesting involvement of both β1- and β2-AR subtypes in Ub secretion. The ability of β1-AR to increase extracellular levels of Ub suggests that this subtype can activate both apoptotic and anti-apoptotic pathways. Of note, treatment with another pro-apoptotic factor, AngII, also increases levels of extracellular Ub, although to a lesser extent than β-AR agonists.
Biological functions of extracellular Ub are not yet completely understood. A limited number of studies suggest pleiotropic effects of extracellular Ub on immune function, cytokine production, and host defence mechanisms.17–21 Majetschak et al.18,19 reported that Ub is released into the extracellular space in a number of critical conditions and acts as a cytokine-like protein playing an important role in the regulation of cellular immune responses. In animal models, exogenous Ub decreased tumour necrosis factor-α production after trauma and endotoxic shock.19 Recently, Ub treatment was shown to enhance the Th2 cytokine response in post-ischaemic lungs during reperfusion, reduce lung oedema formation, and improve pulmonary function during lung ischaemia–reperfusion injury, suggesting anti-inflammatory properties of Ub.37 A decapeptide of Ub with amino acid sequence LEDGRTLSDY (located in the external loop of the molecule) is demonstrated to have immunosuppressive effects on cellular and humoral immune responses, comparable to cyclosporine. Cyclization of this peptide, which was designed to mimic the conformation in the native protein, also selectively suppressed the cellular immune response in vivo.38 It is interesting to note that this Ub decapeptide contains a retro-RGD sequence. The RGD sequence is known to be important in extracellular matrix proteins for cell adhesion interactions.39 Very little is known about the role of extracellular Ub in the regulation of cell survival and apoptosis. Exogenous Ub is shown to prolong skin graft survival.40 In haematopoietic cells, extracellular Ub induced apoptosis via the involvement of STAT3 degradation by proteosome pathway.21 In contrast, we observed that extracellular Ub plays an anti-apoptotic role in β-AR-stimulated apoptosis in ARVMs. Exogenously added Ub dose-dependently inhibited β-AR-stimulated apoptosis in ARVMs. These studies point towards the possibility the effects of extracellular Ub on cell survival and apoptosis may be cell-type specific.
Using N-terminal biotin-labelled Ub, we provide evidence for the cellular interaction/uptake of exogenous Ub. Furthermore, this interaction/uptake is enhanced ∼1.9-fold upon β-AR stimulation. Uptake of N-terminal fluorescein-labelled Ub into human peripheral blood mononuclear cells and monocytic leukaemia cells has been demonstrated using fluorescence and confocal microscopy.41 Inflammatory stimuli increased uptake of labelled Ub up to two-fold. In the meantime, a significant decrease in Ub–protein conjugates was found in human peripheral blood mononuclear cells during sepsis.42 These data point to the possibility that uptake of extracellular Ub could be directed towards restoring intracellular Ub equilibrium. Apoptotic myocytes may secrete Ub, hence increasing concentrations of extracellular Ub. This, in turn, may enhance cellular interaction/uptake of Ub and protect the remaining non-apoptotic cells against β-AR-stimulated apoptosis.
Intracellular signals initiated by extracellular Ub are not yet known. Recently, extracellular Ub was shown to conjugate to intracellular proteins in leukaemia cell lines.41 The identity of these Ub conjugating proteins remains unknown. β-AR-stimulated activation of JNKs plays a pro-apoptotic role via the involvement of the mitochondrial death pathway.7 Previously, we have shown that β-AR stimulation increases GSK-3β activity, and that GSK-3β plays a pro-apoptotic role in β-AR-stimulated apoptosis via the involvement of mitochondrial death pathway.8 We hypothesized that by analogy with non-cardiac cells,31,32 GSK-3β acts upstream in the activation of JNKs in cardiac myocytes. Indeed, expression of constitutively active GSK-3β in ARVMs increased JNKs phosphorylation, suggesting that GSK-3β acts upstream in the activation of JNKs. Using an immune-complex kinase assay and western blot analyses, we show that exogenous Ub inhibits β-AR-stimulated activation of both GSK-3β and JNKs. Exogenous Ub also inhibited β-AR-stimulated increases in cytosolic cytochrome c, a biomarker of mitochondrial death pathway of apoptosis. Activation of PI3-kinase-Akt plays an important role in the regulation of GSK-3β activity.43 We have shown that β1-integrin-mediated activation of PI3-kinase inhibits β-AR-stimulated activation of GSK-3β.8 Inhibition of PI3-kinase using WORT or LY reversed anti-apoptotic effects of Ub. Collectively, these data suggest that Ub activates PI3-kinase and inhibits β-AR-stimulated activation of GSK-3β and JNKs and mitochondrial death pathway of apoptosis.
Monoubiquitination, contrary to polyubiquitination, performs a variety of regulatory functions such as transcriptional regulation, internalization signalling in endocytosis, and virus budding.33–35 The mechanism by which monoubiquitin exerts these functions remains generally unknown. It has been hypothesized that monoubiquitination of proteins modifies their conformation or oligomeric state.33 In the present work, we demonstrated that MeUb, incapable of forming polyubiquitin chains, inhibits β-AR-stimulated apoptosis in cardiac myocytes. These data suggest that the anti-apoptotic effects of exogenous Ub in cardiac myocytes are exerted by monoubiquitination of cellular proteins.
The data presented here are novel and of significant interest since stimulation of β-AR increases extracellular levels of Ub and extracellular Ub plays a protective role against β-AR-stimulated apoptosis. We also provide evidence that the anti-apoptotic effects of extracellular Ub are mediated via the involvement of GSK-3β–JNKs pathway. A clear understanding of the signalling mechanisms leading to Ub-mediated activation of PI3-kinase and inactivation of GSK-3β–JNKs pathway may uncover novel strategies for the treatment of heart failure.
Funding
This work is supported by National Institutes of Health (Grant numbers HL-071519, HL-091405, and HL-092459) and a Merit Review Grant from the Department of Veterans Affairs.
Acknowledgement
Technical help received from Barbara A. Connelly is appreciated.
Conflict of interest: none declared.
References
- 1.Andreka P, Nadhazi Z, Muzes G, Szantho G, Vandor L, Konya L, et al. Possible therapeutic targets in cardiac myocyte apoptosis. Curr Pharm Des. 2004;10:2445–2461. doi: 10.2174/1381612043383908. [DOI] [PubMed] [Google Scholar]
- 2.Singh K, Xiao L, Remondino A, Sawyer DB, Colucci WS. Adrenergic regulation of cardiac myocyte apoptosis. J Cell Physiol. 2001;189:257–265. doi: 10.1002/jcp.10024. [DOI] [PubMed] [Google Scholar]
- 3.Iwai-Kanai E, Hasegawa K, Araki M, Kakita T, Morimoto T, Sasayama S. Alpha- and beta-adrenergic pathways differentially regulate cell type-specific apoptosis in rat cardiac myocytes. Circulation. 1999;100:305–311. doi: 10.1161/01.cir.100.3.305. [DOI] [PubMed] [Google Scholar]
- 4.Zaugg M, Xu W, Lucchinetti E, Shafiq SA, Jamali NZ, Siddiqui MA. Beta-adrenergic receptor subtypes differentially affect apoptosis in adult rat ventricular myocytes. Circulation. 2000;102:344–350. doi: 10.1161/01.cir.102.3.344. [DOI] [PubMed] [Google Scholar]
- 5.Shizukuda Y, Buttrick PM, Geenen DL, Borczuk AC, Kitsis RN, Sonnenblick EH. Beta-adrenergic stimulation causes cardiocyte apoptosis: influence of tachycardia and hypertrophy. Am J Physiol. 1998;275:H961–H968. doi: 10.1152/ajpheart.1998.275.3.H961. [DOI] [PubMed] [Google Scholar]
- 6.Colucci WS, Sawyer DB, Singh K, Communal C. Adrenergic overload and apoptosis in heart failure: implications for therapy. J Card Fail. 2000;6:1–7. [PubMed] [Google Scholar]
- 7.Remondino A, Kwon SH, Communal C, Pimentel DR, Sawyer DB, Singh K, et al. Beta-adrenergic receptor-stimulated apoptosis in cardiac myocytes is mediated by reactive oxygen species/c-Jun NH2-terminal kinase-dependent activation of the mitochondrial pathway. Circ Res. 2003;92:136–138. doi: 10.1161/01.res.0000054624.03539.b4. [DOI] [PubMed] [Google Scholar]
- 8.Menon B, Johnson JN, Ross RS, Singh M, Singh K. Glycogen synthase kinase-3beta plays a pro-apoptotic role in beta-adrenergic receptor-stimulated apoptosis in adult rat ventricular myocytes: role of beta1 integrins. J Mol Cell Cardiol. 2007;42:653–661. doi: 10.1016/j.yjmcc.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- 10.Zolk O, Schenke C, Sarikas A. The ubiquitin–proteasome system: focus on the heart. Cardiovasc Res. 2006;70:410–421. doi: 10.1016/j.cardiores.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 11.Asseman C, Pancre V, Delanoye A, Capron A, Auriault C. A radioimmunoassay for the quantification of human ubiquitin in biological fluids: application to parasitic and allergic diseases. J Immunol Methods. 1994;173:93–101. doi: 10.1016/0022-1759(94)90286-0. [DOI] [PubMed] [Google Scholar]
- 12.Takagi M, Yamauchi M, Toda G, Takada K, Hirakawa T, Ohkawa K. Serum ubiquitin levels in patients with alcoholic liver disease. Alcohol Clin Exp Res. 1999;23:76S–80S. doi: 10.1111/j.1530-0277.1999.tb04539.x. [DOI] [PubMed] [Google Scholar]
- 13.Akarsu E, Pirim I, Capoglu I, Deniz O, Akcay G, Unuvar N. Relationship between electroneurographic changes and serum ubiquitin levels in patients with type 2 diabetes. Diabetes Care. 2001;24:100–103. doi: 10.2337/diacare.24.1.100. [DOI] [PubMed] [Google Scholar]
- 14.Okada M, Miyazaki S, Hirasawa Y. Increase in plasma concentration of ubiquitin in dialysis patients: possible involvement in beta 2-microglobulin amyloidosis. Clin Chim Acta. 1993;220:135–144. doi: 10.1016/0009-8981(93)90042-3. [DOI] [PubMed] [Google Scholar]
- 15.Akarsu E, Pirim I, Selcuk NY, Tombul HZ, Cetinkaya R. Relation between serum ubiquitin levels and KT/V in chronic hemodialysis patients. Nephron. 2001;88:280–282. doi: 10.1159/000046005. [DOI] [PubMed] [Google Scholar]
- 16.Majetschak M, King DR, Krehmeier U, Busby LT, Thome C, Vajkoczy S, et al. Ubiquitin immunoreactivity in cerebrospinal fluid after traumatic brain injury: clinical and experimental findings. Crit Care Med. 2005;33:1589–1594. doi: 10.1097/01.ccm.0000169883.41245.23. [DOI] [PubMed] [Google Scholar]
- 17.Pancre V, Pierce RJ, Fournier F, Mehtali M, Delanoye A, Capron A, et al. Effect of ubiquitin on platelet functions: possible identity with platelet activity suppressive lymphokine (PASL) Eur J Immunol. 1991;21:2735–2741. doi: 10.1002/eji.1830211113. [DOI] [PubMed] [Google Scholar]
- 18.Majetschak M, Krehmeier U, Bardenheuer M, Denz C, Quintel M, Voggenreiter G, et al. Extracellular ubiquitin inhibits the TNF-alpha response to endotoxin in peripheral blood mononuclear cells and regulates endotoxin hyporesponsiveness in critical illness. Blood. 2003;101:1882–1890. doi: 10.1182/blood-2002-03-0918. [DOI] [PubMed] [Google Scholar]
- 19.Majetschak M, Cohn SM, Nelson JA, Burton EH, Obertacke U, Proctor KG. Effects of exogenous ubiquitin in lethal endotoxemia. Surgery. 2004;135:536–543. doi: 10.1016/j.surg.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Griebenow M, Casalis P, Woiciechowsky C, Majetschak M, Thomale UW. Ubiquitin reduces contusion volume after controlled cortical impact injury in rats. J Neurotrauma. 2007;24:1529–1535. doi: 10.1089/neu.2007.0306. [DOI] [PubMed] [Google Scholar]
- 21.Daino H, Matsumura I, Takada K, Odajima J, Tanaka H, Ueda S, et al. Induction of apoptosis by extracellular ubiquitin in human hematopoietic cells: possible involvement of STAT3 degradation by proteasome pathway in interleukin 6-dependent hematopoietic cells. Blood. 2000;95:2577–2585. [PubMed] [Google Scholar]
- 22.Communal C, Singh K, Pimentel DR, Colucci WS. Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the beta-adrenergic pathway. Circulation. 1998;98:1329–1334. doi: 10.1161/01.cir.98.13.1329. [DOI] [PubMed] [Google Scholar]
- 23.Communal C, Colucci WS, Singh K. p38 mitogen-activated protein kinase pathway protects adult rat ventricular myocytes against beta-adrenergic receptor-stimulated apoptosis. Evidence for Gi-dependent activation. J Biol Chem. 2000;275:19395–19400. doi: 10.1074/jbc.M910471199. [DOI] [PubMed] [Google Scholar]
- 24.Menon B, Singh M, Ross RS, Johnson JN, Singh K. Beta-adrenergic receptor-stimulated apoptosis in adult cardiac myocytes involves MMP-2-mediated disruption of beta1 integrin signaling and mitochondrial pathway. Am J Physiol Cell Physiol. 2006;290:C254–C261. doi: 10.1152/ajpcell.00235.2005. [DOI] [PubMed] [Google Scholar]
- 25.Li M, Wang X, Meintzer MK, Laessig T, Birnbaum MJ, Heidenreich KA. Cyclic AMP promotes neuronal survival by phosphorylation of glycogen synthase kinase 3beta. Mol Cell Biol. 2000;20:9356–9363. doi: 10.1128/mcb.20.24.9356-9363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kajstura J, Cigola E, Malhotra A, Li P, Cheng W, Meggs LG, et al. Angiotensin II induces apoptosis of adult ventricular myocytes in vitro. J Mol Cell Cardiol. 1997;29:859–870. doi: 10.1006/jmcc.1996.0333. [DOI] [PubMed] [Google Scholar]
- 27.Sun XM, Snowden RT, Skilleter DN, Dinsdale D, Ormerod MG, Cohen GM. A flow-cytometric method for the separation and quantitation of normal and apoptotic thymocytes. Anal Biochem. 1992;204:351–356. doi: 10.1016/0003-2697(92)90251-2. [DOI] [PubMed] [Google Scholar]
- 28.Mehendale SR, Wang CZ, Shao ZH, Li CQ, Xie JT, Aung HH, et al. Chronic pretreatment with American ginseng berry and its polyphenolic constituents attenuate oxidant stress in cardiomyocytes. Eur J Pharmacol. 2006;553:209–214. doi: 10.1016/j.ejphar.2006.09.051. [DOI] [PubMed] [Google Scholar]
- 29.Kwon SH, Pimentel DR, Remondino A, Sawyer DB, Colucci WS. H(2)O(2) regulates cardiac myocyte phenotype via concentration-dependent activation of distinct kinase pathways. J Mol Cell Cardiol. 2003;35:615–621. doi: 10.1016/s0022-2828(03)00084-1. [DOI] [PubMed] [Google Scholar]
- 30.Communal C, Singh K, Sawyer DB, Colucci WS. Opposing effects of beta(1)- and beta(2)-adrenergic receptors on cardiac myocyte apoptosis: role of a pertussis toxin-sensitive G protein. Circulation. 1999;100:2210–2212. doi: 10.1161/01.cir.100.22.2210. [DOI] [PubMed] [Google Scholar]
- 31.Takada Y, Fang X, Jamaluddin MS, Boyd DD, Aggarwal BB. Genetic deletion of glycogen synthase kinase-3beta abrogates activation of IkappaBalpha kinase, JNK, Akt, and p44/p42 MAPK but potentiates apoptosis induced by tumor necrosis factor. J Biol Chem. 2004;279:39541–39554. doi: 10.1074/jbc.M403449200. [DOI] [PubMed] [Google Scholar]
- 32.Hongisto V, Smeds N, Brecht S, Herdegen T, Courtney MJ, Coffey ET. Lithium blocks the c-Jun stress response and protects neurons via its action on glycogen synthase kinase 3. Mol Cell Biol. 2003;23:6027–6036. doi: 10.1128/MCB.23.17.6027-6036.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hicke L. Protein regulation by monoubiquitin. Nat Rev Mol Cell Biol. 2001;2:195–201. doi: 10.1038/35056583. [DOI] [PubMed] [Google Scholar]
- 34.Schnell JD, Hicke L. Non-traditional functions of ubiquitin and ubiquitin-binding proteins. J Biol Chem. 2003;278:35857–35860. doi: 10.1074/jbc.R300018200. [DOI] [PubMed] [Google Scholar]
- 35.Sigismund S, Polo S, Di Fiore PP. Signaling through monoubiquitination. Curr Top Microbiol Immunol. 2004;286:149–185. doi: 10.1007/978-3-540-69494-6_6. [DOI] [PubMed] [Google Scholar]
- 36.Patel MB, Proctor KG, Majetschak M. Extracellular ubiquitin increases in packed red blood cell units during storage. J Surg Res. 2006;135:226–232. doi: 10.1016/j.jss.2006.04.037. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Covarrubias L, Manning EW, III, Sorell LT, Pham SM, Majetschak M. Ubiquitin enhances the Th2 cytokine response and attenuates ischemia–reperfusion injury in the lung. Crit Care Med. 2008;36:979–982. doi: 10.1097/CCM.0B013E318164E417. [DOI] [PubMed] [Google Scholar]
- 38.Szewczuk Z, Stefanowicz P, Wilczynski A, Staszewska A, Siemion IZ, Zimecki M, et al. Immunosuppressory activity of ubiquitin fragments containing retro-RGD sequence. Biopolymers. 2004;74:352–362. doi: 10.1002/bip.20084. [DOI] [PubMed] [Google Scholar]
- 39.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 40.Earle SA, El Haddad A, Patel MB, Ruiz P, Pham SM, Majetschak M. Prolongation of skin graft survival by exogenous ubiquitin. Transplantation. 2006;82:1544–1546. doi: 10.1097/01.tp.0000236057.56721.d0. [DOI] [PubMed] [Google Scholar]
- 41.Majetschak M, Ponelies N, Hirsch T. Targeting the monocytic ubiquitin system with extracellular ubiquitin. Immunol Cell Biol. 2006;84:59–65. doi: 10.1111/j.1440-1711.2005.01399.x. [DOI] [PubMed] [Google Scholar]
- 42.Ponelies N, Hirsch T, Krehmeier U, Denz C, Patel MB, Majetschak M. Cytosolic ubiquitin and ubiquitylation rates in human peripheral blood mononuclear cells during sepsis. Shock. 2005;24:20–25. doi: 10.1097/01.shk.0000164692.04026.76. [DOI] [PubMed] [Google Scholar]
- 43.Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]