Abstract
Aims
On the basis of our previous reports that cardioprotection induced by ischaemic preconditioning induces autophagy and that resveratrol, a polyphenolic antioxidant present in grapes and red wine induces preconditioning-like effects, we sought to determine if resveratrol could induce autophagy.
Methods and results
Resveratrol at lower doses (0.1 and 1 µM in H9c2 cardiac myoblast cells and 2.5 mg/kg/day in rats) induced cardiac autophagy shown by enhanced formation of autophagosomes and its component LC3-II after hypoxia–reoxygenation or ischaemia–reperfusion. The autophagy was attenuated with the higher dose of resveratrol. The induction of autophagy was correlated with enhanced cell survival and decreased apoptosis. Treatment with rapamycin (100 nM), a known inducer of autophagy, did not further increase autophagy compared with resveratrol alone. Autophagic inhibitors, wortmannin (2 µM) and 3-methyladenine (10 mM), significantly attenuated the resveratrol-induced autophagy and induced cell death. The activation of mammalian target of rapamycin (mTOR) was differentially regulated by low-dose resveratrol, i.e. the phosphorylation of mTOR at serine 2448 was inhibited, whereas the phosphorylation of mTOR at serine 2481 was increased, which was attenuated with a higher dose of resveratrol. Although resveratrol attenuated the activation of mTOR complex 1, low-dose resveratrol significantly induced the expression of Rictor, a component of mTOR complex 2, and activated its downstream survival kinase Akt (Ser 473). Resveratrol-induced Rictor was found to bind with mTOR. Furthermore, treatment with Rictor siRNA attenuated the resveratrol-induced autophagy.
Conclusion
Our results indicate that at lower dose, resveratrol-mediated cell survival is, in part, mediated through the induction of autophagy involving the mTOR-Rictor survival pathway.
Keywords: Autophagy, Cell survival, Rictor, mTOR, Resveratrol, Cardioprotection
1. Introduction
As cardiac myocytes are terminally differentiated, cellular degradation via ubiquitin-proteasomal pathway and/or autophagy may play an important role in the homeostasis of cardiac cells.1 Autophagy is a catabolic process through which cells’ own components are degraded using the lysosomal machineries. In normal conditions, autophagy occurs at low levels for the turnover of damaged or long-lived proteins, macromolecules, and organelles like mitochondria, ribosomes, endoplasmic reticulum, and peroxisomes.2 Autophagy provides a necessary source of energy for the cardiac myocytes during early neonatal starvation period.3 However, autophagy is shown to be the main mechanism causing cell death leading to the progression from compensated hypertrophy to heart failure and left ventricular systolic dysfunction in pressure-overloaded human heart.4,5 Also, in dilated cardiomyopathy patients, autophagy is associated with the degradation of damaged intracellular organelles leading to the destruction of cardiomyocytes.6 Moreover, basal level of autophagy is triggered in pressure-overloaded myocardium, a major risk factor for cardiac hypertrophy and heart failure.7 In spite of this, autophagy is shown to protect the myocardium and cardiac cells against ischaemia–reperfusion (IR) injury,8,9 and recently, we have shown that ischaemic preconditioning, a state-of-the-art technique for the protection of myocardium induces cardiac autophagy.8,10
Recently, autophagy has been found to be regulated by redox signalling.11,12 As resveratrol, a polyphenolic phytoalexin found in grapes, wines, peanuts, and several other fruits and vegetables, has been found to precondition the ischaemic myocardium by redox signalling,13 we used this compound to explore the mechanism of cardiac autophagy induced by IR. Previously, we have shown that resveratrol (3,4′,5-trihydroxy-trans-stilbene) protects the cardiovascular system by diverse mechanisms, mainly by the inhibition of apoptotic cell death at very low concentrations.14 In another study, we found that resveratrol provides cardioprotection via redox signalling and is likely to play a role in switching IR-induced death signals into survival signals through the activation of Akt and Bcl-2.15 Recently, resveratrol was found to induce caspase-independent cancer cell death through autophagocytosis.16,17 As resveratrol generates a survival signal at a relatively low concentration, we hypothesized that resveratrol might induce autophagy for the protection of myocardium against IR injury. To test this hypothesis, we examined the effects of resveratrol at different doses on the induction of the autophagy. Our results confirmed for the first time that resveratrol induces cell survival through the induction of autophagy at a low concentration both in H9c2 cardiac myoblast cells and in the rat myocardium, and the autophagy in part is mediated through the activation of mammalian target of rapamycin (mTOR)-Rictor (mTOR complex 2, mTORC2) survival pathway.
2. Methods
2.1. Animals
All animals used in this study received humane care in compliance with the regulations relating to animals and experiments involving animals and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, NIH Publication, 1996 edition, and all the protocols were approved by the Institutional Animal Care Committee of University of Connecticut Health Center, Farmington, CT, USA.
Male Sprague–Dawley rats weighing between 250 and 300 g were fed ad libitum regular rat chow with free access to water until the start of the experimental procedure. Animals were gavaged with one of the three different doses of resveratrol (2.5, 25, or 100 mg/kg/day) for 10 days. Wortmannin (15 µg/kg) was injected into rats via intraperitoneal route 30 min before isolation of heart.
2.2. Cell culture
Rat myocardium-derived H9c2 cardiac myoblast cell line was in Dulbecco's Modified Eagle's Medium (Invitrogen, Grand Island, NY) containing 4 mM l-glutamine, 4.5 g/L glucose, and 10% foetal bovine serum (Invitrogen). Treatment with rapamycin (100 nM), wortmannin (2 µM), and 3-methyladenine (10 mM) were accomplished by adding these compounds 15 min before resveratrol treatment. One of the three different doses of resveratrol (0.1, 1, and 100 µM) was added 1 h before hypoxia–reoxygenation (HR). Experimental procedures are depicted in Supplementary material online, Figure S1.
2.3. In vitro siRNA transfection
In vitro siRNA transfection of Rictor and control siRNAs (Santa Cruz Biotechnology) into H9c2 cells was performed using TransPass R2 Transfection Reagent (New England BioLabs) as described earlier.8
2.4. HR in cell culture
H9c2 culture plates were subjected to 30 min of hypoxia followed by 1 h of reoxygenation as mentioned in our previous study.8
2.5. Immunofluorescence staining in H9c2 cells
Immunofluorescence staining with LC3-II antibody followed by confocal microscopy was performed as mentioned earlier.8
2.6. Autophagosome assay
Labelling of autophagic vacuoles with monodansylcadaverine followed by fluorescence photometry was performed.18 Details are given in Supplementary material online, Supplementary Method.
2.7. Cell death assay
Cell death analysis was performed with the culture medium at the end of experimentation using LDH Cytotoxicity Assay kit (Cayman Chemical Company, Ann Arbor, MI, USA) as described by the manufacturer.
2.8. Cell survival assay
Viability of cells was studied using MTT Cell Proliferation Assay kit (Cayman Chemical Company) as described by the manufacturer.
2.9. Immunoprecipitation
Cells were lysed in RIPA buffer and the total cell lysate containing 500 µg of total protein was immunoprecipitated with mTOR and Protein A Sepharose beads (Zymed, San Francisco, CA, USA) as mentioned earlier.8
2.10. Isolated heart preparation
Isolated working and non-working heart preparations were performed using Langendorff perfusion apparatus as mentioned earlier.8 Functional parameters were measured at the baseline level and during the experiments. Experimental procedures are depicted in Supplementary material online, Figure S1.
2.11. Western blot analysis
Total cell lysate from cell culture or cytosolic extract from rat heart tissue or immunoprecipitated samples were separated in SDS–PAGE and transferred to nitrocellulose filters and analysed by western blotting as mentioned earlier.8
2.12. Transmission electron microscopy
Small left ventricular heart tissue samples of about 1 mm3 were fixed by immersion in 4% glutaraldehyde. Small fragments of myocardium were processed for transmission electron microscopy (TEM) according to routine procedures, as we previously described.19,20
2.13. Assessment of apoptotic cell death
transferase dUTP nick end labelling (TUNEL) apoptotic analysis was performed using DeadEnd™ Fluorimetric TUNEL System (Promega, Madison, WI, USA) as described by the manufacturer.
2.14. Statistical analysis
All values are expressed as the mean ± standard error of mean. One-way analysis of variance test followed by Bonferoni's correction was first carried out to test for any differences between the mean values of all groups. Student's t-test was performed to compare the difference between control and Rictor siRNA-treated groups in Figure 6. The results were considered significant, if P < 0.05.
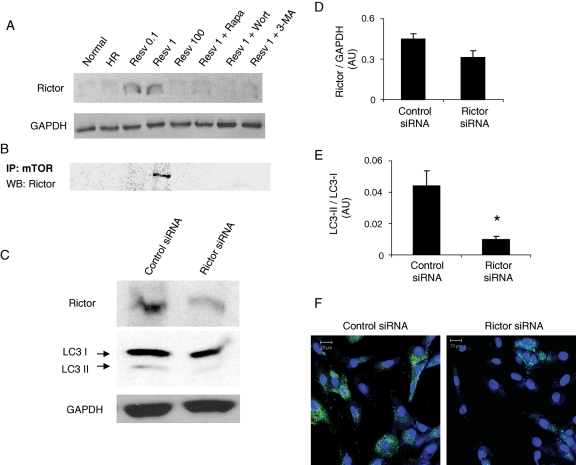
Figure 6.
Resveratrol-mediated autophagy induces mTORC2 and dependent on Rictor. (A) Western immunoblotting was performed with H9c2 cell (3 × 105) lysate obtained as mentioned in Figure 1 for the expression of Rictor. (B) H9c2 cell (3 × 105) lysates were immunoprecipitated with mTOR antibody followed by western immunoblotting for the detection of Rictor. (C) H9c2 cardiac myoblast cells (6 × 104) were transfected with either control or Rictor siRNA followed by treatment with 1 µM resveratrol and subjected to HR as mentioned in Methods. Total cell lysates were used for studying the expression of Rictor and LC3 I and II by western immunoblotting. (D and E) Bar graphs showing the quantification of Rictor/GAPDH (C) and LC3-II/LC3-I (D) using the immunoreactive bands with QuantiOne imaging software (Biorad). (F) Confocal immunofluorescent microscopic images of H9c2 cells (plated at the concentration of 500 cells/cm2 in a chambered glass slide) showing the expression of LC3-II in control or Rictor siRNA-treated cells. Figures are representative images of at least three different samples, and each experiment was repeated at least thrice. Results are expressed as mean ± SEM. *P<0.05 vs. control siRNA.
3. Results
3.1. Resveratrol treatment induces autophagy in H9c2 cardiac cells
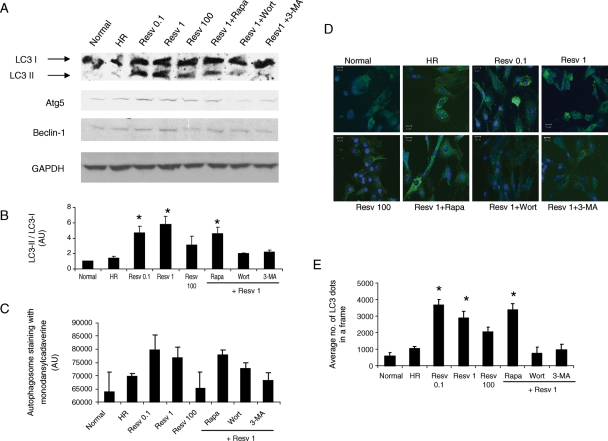
Thirty minutes of hypoxia followed by 1 h of reoxygenation in H9c2 cells to some extent induced autophagy (Figure 1A). Treatment with low doses of resveratrol (0.1 and 1 µM) followed by HR strongly induced autophagy. The induction of autophagy was confirmed by the increased protein expression of LC3-II, an autophagosomal membrane component, and the enhanced ratio of LC3-II/LC3-I (Figure 1B). Moreover, the markers of autophagy such as Atg5 and Beclin-1 were also enhanced in low doses of resveratrol-treated cells (Figure 1A). The amount of autophagic vacuole formation evaluated by staining the cells with monodansylcadaverine followed by spectrofluorometric analysis showed that autophagosome vacuoles formation was enhanced under low doses of resveratrol treatment (Figure 1C). Confocal fluorescent microscopic analysis further showed that the expression of LC3-II was enhanced in low doses of resveratrol-treated cells (Figure 1D and E). However, high dose of resveratrol (100 µM) reduced the extent of autophagy (Figure 1A–E). Treatment with rapamycin, an inhibitor of mTOR known to induce autophagy, did not significantly induce the autophagy compared with low-dose (1 µM) resveratrol alone (Figure 1A–E). However, treatment with inhibitors of autophagy such as wortmannin and 3-methyladenine significantly inhibited the low dose (1 µM) resveratrol-induced autophagy (Figure 1A–E).
Figure 1.
Resveratrol induces autophagy. H9c2 cardiac myoblast cells (3 × 105 in a 10 cm dish) were treated with resveratrol (Resv) at three different doses (0.1, 1, and 100 µM) or resveratrol (0.1 µM) along with either rapamycin (Rapa, 100 nM) or wortmannin (Wort, 2 µM) or 3-methyladenine (3-MA, 10 mM) treatment followed by hypoxia and reoxygenation (HR). (A) Western immunoblotting was performed with H9c2 cell lysate for the expression of autophagic marker proteins. (B) Quantification of LC3-II/LC3-I was performed using the immunoreactive bands with QuantiOne® imaging software (Biorad). (C) H9c2 cells (6 × 104 in a 12-well plate) were stained with monodansylcadaverine, and the fluorescent intensity was measured in spectroflurometer. (D) Confocal fluorescent microscopic images of H9c2 cells (plated at the concentration of 500 cells/cm2 in a chambered glass slide) showing the staining of LC3-II (green, Alexa Fluor 488) and the nucleus (blue, Topro-3-iodide). (E) Quantification of the staining of LC3-II particles obtained from confocal fluorescent microscopic images (triplicates of 10 random fields from each group) was performed with Adobe Photoshop® software. Figures are representative images of at least three different samples, and each experiment was repeated at least thrice. Results are expressed as mean ± SEM. *P < 0.05 vs. HR, Wort + Resv 1 and 3-MA + Resv 1.
3.2. Low doses of resveratrol-mediated autophagy enhance cell survival
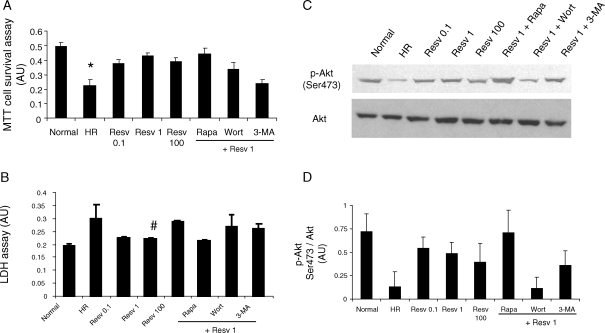
When autophagy was induced with low doses of resveratrol, the cell survival as indicated by MTT cell survival assay was significantly increased than HR (Figure 2A). A high dose of resveratrol slightly reduced the cell survival. Pre-treatment of cells with rapamycin alone increased the cell survival compared with HR alone (data not shown); however, pre-treatment with both rapamycin followed by 1 µM resveratrol did not significantly alter the cell survival than the resveratrol alone (Figure 2A). When autophagy was inhibited with inhibitors of autophagy, the low-dose resveratrol-induced cell survival was attenuated (Figure 2A).
Figure 2.
Resveratrol-mediated autophagy induces cell survival. H9c2 cells [1 × 104 in a 96-well plate for (A and B) and 3 × 105 in a 10 cm dish for (C and D)] were cultured as mentioned in Figure 1. (A) MTT cell survival assay was performed with MTT Cell Proliferation Assay kit (Cayman Chemical). (B) The release of lactate dehydrogenase (LDH) as a marker of cell death was performed with LDH Cytotoxicity Assay kit (Cayman Chemical). Western immunoblotting (C) and its quantification (D) with QuantiOne® software were performed with H9c2 cell lysate for the expression of cell survival marker. Figures are representative images of at least three different samples, and each experiment was repeated at least thrice. Results are expressed as mean ± SEM. *P < 0.05 vs. Normal, Resv 1, and Rapa + Resv 1; #P < 0.05 vs. HR.
The pre-treatment of cells with low doses resveratrol significantly reduced the cell death as indicated by the reduced lactate dehydrogenase (LDH) activity in the medium (Figure 2B). The LDH activity was higher with high dose of resveratrol treatment. Pre-treatment of cells with rapamycin alone decreased the LDH release than HR (data not shown); however, pre-treatment with rapamycin followed by 1 µM resveratrol did not significantly alter the LDH release compared with resveratrol alone (Figure 2B). Inhibition of autophagy by treatment with 3-methyladenine and wortmannin elevated the release of LDH compared with low-dose resveratrol-treated samples indicating the incidence of cell death (Figure 2B).
Further, HR in cardiac myoblast cells decreased the Ser 473 phosphorylation of Akt, a survival signalling kinase. Pre-treatment of cells with low doses of resveratrol followed by HR increased the activation of Akt during which the autophagy was enhanced (Figure 2C and D). Though dual treatment of cells with rapamycin and resveratrol did not enhance autophagy compared with low doses of resveratrol alone, the level of phosphorylation of Akt was enhanced in dual-treated cells than low-dose resveratrol-treated cells alone (Figure 2C and D). Both the inhibitors of autophagy inhibited the activation of Akt (Figure 2C and D).
3.3. Resveratrol induces autophagy in the myocardium
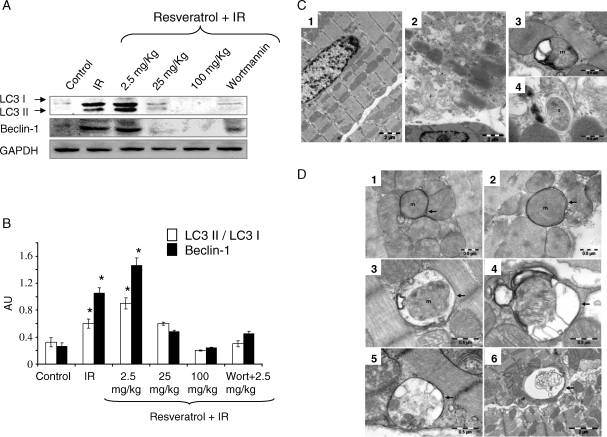
In accordance with our previous study results,14 the cardiac functional parameters like left ventricular developed pressure (LVDP), and its first derivative of LVmaxdp/dt and aortic flow were significantly improved during the 2 h of reperfusion period for the low-dose resveratrol (2.5 mg/kg/day) treated hearts compared with the control hearts (see Supplementary material online, Figure S2). However, treatment with high dose of resveratrol (100 mg/kg/day) and treatment with autophagic inhibitor wortmannin attenuated the low-dose resveratrol-induced improvement in the cardiac function (see Supplementary material online, Figure S2). Treatment of rats with resveratrol at the dose of 2.5 mg/kg/day for 10 days induced the autophagy shown by the induction of LC3-II, LC3-II/LC3-I, and Beclin-1 (Figure 3A and B). However, high doses of resveratrol (25 and 100 mg/kg/day) decreased the expression of LC3-II and Beclin-1 (Figure 3A and B). Single intraperitoneal injection of wortmannin (15 µg/kg), an inhibitor of autophagy, attenuated the low-dose resveratrol (2.5 mg/kg/day)-induced autophagy (Figure 3A and B). TEM images of the myocardium showed the presence of increased number of double membrane structured autophagosomes in low-dose resveratrol-treated animal groups (Figure 3C and D). The average number of autophagosomes found through TEM is positively correlated with the LC3-II protein expression in the myocardium. Though it is not statistically significant, the average number of autophagosomes per 2500 µm2 is 28 and 15 for low-dose resveratrol and wortmannin treated samples, respectively. The average diameter of autophagosomes is 0.96 ± 0.43 µm (min = 0.53 µm and max = 2.40 µm) and 0.92 ± 0.49 µm (min = 0.26 µm and max = 2.08 µm) for resveratrol and wortmannin treated samples, respectively.
Figure 3.
Resveratrol induces autophagy in vivo. (A) Western immunoblotting was performed with left ventricular tissue lysate of rats treated with resveratrol as mentioned in Methods for the expression of autophagic marker proteins. (B) Bar graph showing the quantification of the immunoreactive bands obtained as above. (C) Transmission electron micrograph of left ventricular tissue sections (1–4) of control (1) and ischaemia–reperfusion (3 and 4) group. Images show the normal structure (1) and ischaemia–reperfusion-induced cellular lyses, fibrils disorganization, and mitochondrial injuries (2). Autophagosomes in (3) and (4) contain mitochondria (m) and cytoplasm with glycogen (g), respectively. (D) Transmission electron micrographs of the myocardium of resveratrol (2.5 mg/kg)-treated samples showing autophagosomes surrounded by double membranes (arrows). TEM images show early autophagic vacuoles contain still identifiable organelles (1–3) and late autophagosomes containing lamellar and vesicular structures (4–6). Autophagosomes preferentially contain mitochondria (m) enclosed by distinctive double membrane (arrows). Figures are representative images of at least three different heart samples, and each experiment was repeated at least thrice. Results are expressed as mean ± SEM. *P < 0.05 vs. control, 25, 100 mg/kg resveratrol, and Wort + 2.5 mg/kg resveratrol.
3.4. Resveratrol-mediated autophagic induction is negatively correlated with the induction of apoptosis
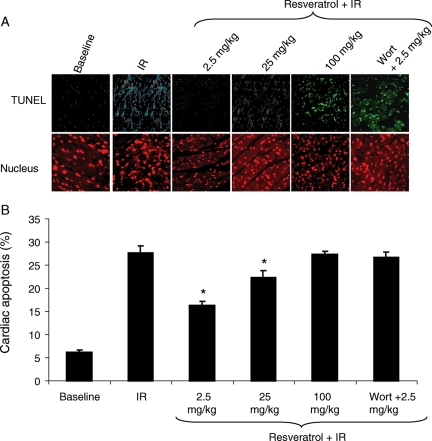
Apoptosis analyses performed via terminal deoxynucleotidyl TUNEL staining indicate that the percentage of apoptotic cells was negatively correlated with the induction of autophagy in the myocardium (Figure 4A and B), i.e. when autophagy was highly induced as in the case of low dose of resveratrol treatment, the apoptosis was minimum. Whereas, when the autophagy was reduced as in the case of high-dose resveratrol and wortmannin-treated hearts, the percentage of apoptosis was increased (Figure 4A and B).
Figure 4.
Low-dose resveratrol treatment attenuates apoptosis. Terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) immunofluorescence analysis of left ventricular tissue sections of rats treated with resveratrol as mentioned in Methods. (A) Fluorescent microscopic images showing the TUNEL staining (green) and nuclear staining (red, propidium iodide). (B) Quantification of cardiac apoptosis using the TUNEL stained images with Adobe Photoshop® software. Values are mean ± SEM. Figures are representative images of at least three different heart samples, and each experiment was repeated at least thrice. Results are expressed as mean ± SEM. *P < 0.05 vs. IR, 100 mg/kg resveratrol, and Wort + 2.5 mg/kg resveratrol.
3.5. Treatment with resveratrol dose dependently inhibits mTOR complex 1
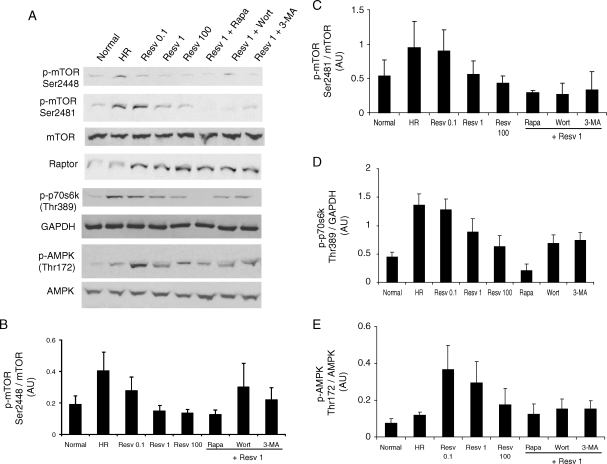
The protein kinase mTOR plays an essential role in sensing and responding to nutrients, stress, and intracellular energy state. mTOR signalling negatively regulates autophagy, i.e. the phosphorylation and activation of mTOR is inhibited when autophagy is induced.11 HR in cardiac myoblast cells induced the phosphorylation of mTOR at both serine 2448 and serine 2481 sites (Figure 5A–C). Under resveratrol treatment, mTOR was differentially regulated, i.e. phosphorylation of mTOR at serine 2448 was inhibited, whereas the phosphorylation of mTOR at serine 2481 was increased, and it was attenuated with the high-dose resveratrol (Figure 5A–C). As expected, treatment with rapamycin alone (data not shown) or along with resveratrol treatment inhibited the activation of mTOR phosphorylation at both serine 2448 and serine 2481 sites (Figure 5A–C). Treatment with either inhibitor of autophagy such as 3-methyladenine or wortmannin alone did not alter the phosphorylation of mTOR than that of normal (data not shown). Furthermore, treatment with 3-methyladenine or wortmannin along with resveratrol also inhibited the phosphorylation of mTOR at both serine 2448 and serine 2481 sites (Figure 5A–C).
Figure 5.
Resveratrol-mediated autophagy attenuates mTORC1, but activates AMPK. H9c2 cells (3 × 105 in a 10 cm dish) were cultured as mentioned in Figure 1. (A) Western immunoblotting was performed with H9c2 cell lysate for the activation of mammalian target of rapamycin (mTOR) and AMP-activated protein kinase (AMPK). Quantification of the ratio of (B) phospho mTOR(Ser2448)/mTOR, (C) phospho mTOR(Ser2481)/mTOR, and (D) phospho-AMPK/AMPK using the respective immunoreactive bands with QuantiOne® imaging software (Biorad). Figures are representative images of at least three different samples, and each experiment was repeated at least thrice. Results are expressed as mean ± SEM.
mTOR assembles into two functionally distinct complexes, i.e. mTOR complex 1 (mTORC1) and mTORC2. Raptor, a component of mTORC1 is elevated in all resveratrol-treated samples irrespective of other treatments like rapamycin, wortmannin, or 3-methyladenine. To further analyse the role of mTORC1, we analysed the activation of p70s6 kinase (phosphorylation at Thr389), a direct down-stream target of mTORC1. p70s6 kinase was highly active during HR, and the activation of p70s6 kinase was dose dependently reduced by resveratrol treatment (Figure 5A and D). Rapamycin abolished the p70s6 kinase activation, whereas the treatment with wortmannin or 3-methyladenine partially inhibited the p70s6 kinase activation (Figure 5A and D).
AMP-activated protein kinase (AMPK) serves as a general integrator of metabolic responses to changes in energy availability and is activated in response to elevations of the AMP/ATP ratio.21 Low doses of resveratrol treatment extensively induced the activation of AMPK (phosphorylation at Thr172), compared with HR alone or high dose of resveratrol (Figure 5A and E). Treatment with rapamycin, 3-methyladenine, or wortmannin attenuated low-dose resveratrol-induced AMPK activation (Figure 5A and E).
3.6. Low-dose resveratrol treatment selectively induces mTOR complex 2 leading to autophagy induction
Rictor, the main component of mTORC2 was found to be induced only with low-dose resveratrol followed by HR (Figure 6A). However, HR alone or high-dose resveratrol did not induce Rictor (Figure 6A). Induction of autophagy with rapamycin and also inhibition of autophagy with wortmannin or 3-methyladenine abolished the low-dose resveratrol-induced Rictor expression (Figure 6A). Moreover, treatment with low-dose resveratrol without HR or treatment with rapamycin alone followed by HR did not induce Rictor expression (data not shown). These results strongly indicate that only low-dose resveratrol under HR condition induced the expression of Rictor, a component of mTORC2 known to activate cell survival via phosphorylation of Ser 473 and activation of Akt.22
In order to study whether the low-dose resveratrol-induced Rictor bind with mTOR, we immunoprecipitated the total cell lysate with mTOR antibody and the immunoprecipitate was analysed by western immunoblotting. The results showed that low-dose resveratrol-induced Rictor bound with mTOR in the total cell lysate (Figure 6B).
Recently, we have shown that the induction of autophagy by ischaemic preconditioning led to cell survival and that low-dose resveratrol is known to induce ischaemic-preconditioning like effects.8,14 On the basis of the above results, we hypothesized that the activation of Rictor-mediated mTORC2 survival pathway by low-dose resveratrol treatment under HR might be involved in the induction of autophagy. To test the above hypothesis, we transfected H9c2 cardiac myoblast cells with either Rictor siRNA or control siRNA as mentioned in Methods. The transfection efficiency in H9c2 cells was analysed by studying the expression of Rictor under low-dose resveratrol (1 µM) treatment followed by HR. Western immunoblotting analyses indicate that Rictor siRNA treatment considerably reduced the expression of Rictor in comparison to control siRNA-treated cells (Figure 6C and D). At the same time, the expression of LC3-II was significantly reduced in Rictor siRNA-treated cells compared with control siRNA-treated cells as shown by western immunoblotting and confocal immunofluorescence analysis (Figure 6C, E, and F). These results suggest that Rictor-mediated mTORC2 may participate in the induction of autophagy.
4. Discussion
The main findings of the current study are (i) low doses of resveratrol treatment induces autophagy in parallel to the enhanced cell survival and decreased cell death indicating the protection of cardiac cells against IR injury, (ii) low doses of resveratrol treatment specifically induces Rictor, a component of mTORC2, whereas mTORC1 is inhibited during the induction of autophagy.
Autophagy is a self-clearing process to remove the damaged proteins or organelles, an alternate mechanism for proteasomal degradation, which can generate a survival signal, as in the case of myocardial ischaemia.2,23 Although autophagy was initially believed to be involved in a non-apoptotic form of programmed cell death, recent studies have changed this concept by demonstrating that autophagy can also cause cell survival. However, Matsui et al.24 have shown that in the case of myocardial ischaemic injury, autophagy causes cell survival, whereas the reperfusion injury causes cell death. In our own study, we found that myocardial ischaemic preconditioning induces autophagy for the protection of myocardium through the induction of BAG-1 survival protein.8 As low-dose resveratrol-mediated survival signal is realized by its ability to induce pharmacological preconditioning,13,15 we tested the ability of resveratrol to induce autophagy in the myocardium. Our results show for the first time that low doses of resveratrol induce cardiac autophagy and generate a survival signal in H9c2 cardiac myoblast cells as well as in the myocardium, and the inhibition of autophagy diminished the resveratrol-mediated cardioprotection. Our results are consistent with our previous reports that resveratrol at low dose (2.5 mg/kg/day) protects the myocardium from IR injury by reducing myocardial infarct size via the activation of Akt.14 Treatment with rapamycin alone (data not shown) or treatment with rapamycin followed by low dose (1 µM) of resveratrol enhanced the autophagic puncta (Figure 1D and E) and further enhanced the autophagosome formation (Figure 2C) measured by staining with monodansylcadaverine, which is shown to be specific for autophagosomes as described previously by Iwai-Kanai et al.25 Further, treatment with 3-methyladenine or wortmannin, which inhibit the initial steps of autophagic process, attenuated the autophagic puncta and autophagosome formation (Figure 1C–E) induced by low-dose resveratrol treatment. These results suggest that the entire process of autophagy is upregulated after low-dose of resveratrol treatment. Although high dose of resveratrol treatment in H9c2 cells decreased the autophagy, it did not alter the cell survival as observed with inhibitors of autophagy (Figure 2). These in vitro results suggest that the resveratrol-mediated autophagy only partly contribute for the cell survival under the given set of experimental conditions. However, our in vivo data (Figures 3 and 4) show that high dose of resveratrol decreased the autophagy and at the same time induced cell death. Further, it should be noted that even though cardiac IR alone markedly induced autophagy, HR alone in H9c2 cells did not. This discrepancy could be due to the difference between the ex vivo and in vitro experimental conditions.
In order to study the mechanism of resveratrol-induced autophagy, we examined the activation of mTOR, a molecule known to be repressed during autophagic induction. TOR not only can act as an amino acid sensor, but also as a sensor of ATP.26 mTOR assembles into two functionally distinct complexes: mTORC1 and mTORC2. mTORC1 comprises mTOR, Raptor, mLST8, and PRAS40, which represses mTORC1 activity. mTORC2 comprises mTOR, Rictor (rapamycin-insensitive companion of mTOR), SIN1, and mLST8.27,28 Rapamycin binds with FKBP12 and specifically inhibits mTORC1.27 However, studies have shown that long-term treatment with rapamycin also inhibit the functions of mTORC2.27,29 Though inhibition of mTOR with rapamycin has been shown to induce autophagy in many cell types,30–33 in our study, rapamycin treatment did not induce further autophagy than that induced by low dose of resveratrol alone. These results indicate that resveratrol-mediated autophagy could also follow the same pathway as rapamycin through the inhibition of mTOR. However, our results show that mTOR was differentially regulated when autophagy was induced by low dose of resveratrol, i.e. phosphorylation of mTOR at serine 2448 was inhibited, whereas the phosphorylation of mTOR at serine 2481 was elevated. Our results are in accordance with a recent study, where resveratrol has been shown to inhibit oxidized LDL-induced PI3K/Akt-mediated phosphorylation of mTOR (Ser2448) and its downstream molecule p70s6k in rabbit femoral smooth muscle cells.34 However, p70s6k is needed for the entire process of autophagy, and it must be activated first for the maximal activation of autophagy.35 Rictor, a component of mTORC2 was induced by low-dose resveratrol treatment, and it was found to be associated with mTOR shown by immunoprecipitation experiments. Rictor is primarily responsible for the phosphorylation of Akt on Ser 47327 as observed in our study showing the induction of Rictor and simultaneous activation of Akt at Ser 473 (Figures 2 and 6). Akt phosphorylates a number of substrates involved in regulating cell survival, growth, proliferation, and metabolism.36 As our results showed the positive correlation between the induction of Rictor, autophagy, and cell survival with low dose of resveratrol, we speculate whether mTORC2 could play any role in the induction of autophagy. To test this, we suppressed the expression of Rictor by Rictor siRNA treatment in cardiac myoblast cells, and this resulted in the suppression of autophagy induced by low dose of resveratrol followed by HR. In contrast, shRNA-mediated knockdown of Rictor induced autophagy in skeletal muscle cells.37 Taken together, our results indicate that low-dose resveratrol-mediated cell survival is at least in part mediated through induction of autophagy, which may in part, depend on the activation of mTORC2 survival pathway.
AMPK is a sensor of energy molecule ATP and is activated when the ratio of ATP/ADP is decreased.38–40 AMPK activation is shown to be dominantly inhibiting mTOR via two mechanisms: (i) by phosphorylation of TSC2 at Ser 1345 leading to the stimulation of GTPase activator protein (GAP) activity and (ii) by phosphorylation and inhibition of Raptor. Recently, it has been shown that glucose deprivation in cardiac cells and cardiac ischaemia activates AMPK leading to the activation of autophagy.41,42 Activated AMPK dominantly suppresses the effect of Akt on the activation of mTORC1,43,44 at the same time Akt activity negatively regulates AMPK phosphorylation.45 Activation of Akt can activate mTORC1 in two ways: (i) by phosphorylation of TSC2 causing the inhibition of GAP domain of TSC2 leading to the activation of Rheb and mTORC1 and (ii) by phosphorylation of PRAS40 and relieving its inhibitory effect on mTORC1. On the basis of the above studies, we presume that low-dose resveratrol-mediated activation of AMPK could suppress the effect of Akt on the activation of mTORC1.
In this study, rapamycin treatment inhibited the resveratrol-induced expression of Rictor, as rapamycin treatment can disrupt the interaction between Rictor and mTOR and can reduce the basal mTORC2 activity.34 Prolonged rapamycin treatment increased the phosphorylation of Ser 473 despite a severe disruption of mTORC2 stability.46 Inhibition of mTOR signalling by rapamycin has been shown to increase the Akt phosphorylation.47 Also, in our study, rapamycin treatment increased the Ser 473 phosphorylation of Akt than low-dose resveratrol treatment alone. Thus, rapamycin treatment-induced activation of Akt through an unknown mechanism could probably involve in the cell survival seen in our present study as shown in Figure 3.
Beclin1/Atg6, the first mammalian protein described to mediate autophagy by forming a complex with Vps34, a class III PI3K, positively regulate autophagy via formation of autophagosomes and initiation of autophagy.48 PI3K-III inhibitors such as 3-methyladenine, wortmannin, and LY294002 interfere with this pathway.49 Resveratrol was shown to induce cell death in ovarian cancer cells through a mechanism distinct from apoptosis,50 and induced Beclin-1-independent autophagy in breast cancer cells,51 whereas Beclin-1-dependent autophagy was found in colorectal cancer cells.17 To the best of our knowledge, this is the first study to show that resveratrol induces autophagy in the myocardium and in cardiac myoblast cells in Beclin-1-dependent manner. Inhibition of autophagy with 3-methyladenine and wortmannin, which is also known to inhibit both mTORC1 and mTORC2,52,53 inhibited resveratrol-mediated autophagy and abolished the cardioprotective abilities of resveratrol. Though the complex interaction and the positive and negative feedback regulation among PI3 kinase, Akt, mTOR, and AMPK are known to some extent, the exact mechanism through which 3-methyladenine and wortmannin causes the inactivation of mTOR and p70s6k is unclear.
In conclusion, our results indicate for the first time that low dose of resveratrol-induced cell survival is at least in part mediated through the induction of autophagy, which may, in part, depend on Rictor-mediated mTORC2 survival pathway.
Supplementary material
Supplementary Material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by National Heart, Lung and Blood Institute at the National Institutes of Health [HL 34360, HL 22559, and HL 33889].
References
- 1.Powell SR. The ubiquitin–proteasome system in cardiac physiology and pathology. Am J Physiol Heart Circ Physiol. 2006;291:H1–H19. doi: 10.1152/ajpheart.00062.2006. [DOI] [PubMed] [Google Scholar]
- 2.Gustafsson AB, Gottlieb RA. Recycle or die: the role of autophagy in cardioprotection. J Mol Cell Cardiol. 2008;44:654–661. doi: 10.1016/j.yjmcc.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, et al. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 4.Hein S, Arnon E, Kostin S, Schonburg M, Elsasser A, Polyakova V, et al. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation. 2003;107:984–991. doi: 10.1161/01.cir.0000051865.66123.b7. [DOI] [PubMed] [Google Scholar]
- 5.Hein S, Elsasser A, Kostin S, Zimmermann R, Schaper J. Functional disturbances due to structural remodeling in the failing human heart. Arch Mal Coeur Vaiss. 2002;95:815–820. [PubMed] [Google Scholar]
- 6.Shimomura H, Terasaki F, Hayashi T, Kitaura Y, Isomura T, Suma H. Autophagic degeneration as a possible mechanism of myocardial cell death in dilated cardiomyopathy. Jpn Circ J. 2001;65:965–968. doi: 10.1253/jcj.65.965. [DOI] [PubMed] [Google Scholar]
- 7.Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, et al. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest. 2007;117:1782–1793. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurusamy N, Lekli I, Gorbunov N, Gherghiceanu M, Popescu LM, Das DK. Cardioprotection by adaptation to ischemia augments autophagy in association with BAG-1 protein. J Cell Mol Med. 2008;13:375–389. doi: 10.1111/j.1582-4934.2008.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem. 2006;281:29776–29787. doi: 10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]
- 10.Gurusamy N, Lekli I, Gherghiceanu M, Popescu LM, Das DK. BAG-1 induces autophagy for cardiac cell survival. Autophagy. 2009;5:120–121. doi: 10.4161/auto.5.1.7303. [DOI] [PubMed] [Google Scholar]
- 11.Gurusamy N, Das DK. Autophagy, redox signaling and ventricular remodeling. Antioxid Redox Signal. 2009;11:1975–1988. doi: 10.1089/ars.2009.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das DK, Maulik N. Resveratrol in cardioprotection: a therapeutic promise of alternative medicine. Mol Interv. 2006;6:36–47. doi: 10.1124/mi.6.1.7. [DOI] [PubMed] [Google Scholar]
- 14.Dudley J, Das S, Mukherjee S, Das DK. Resveratrol, a unique phytoalexin present in red wine, delivers either survival signal or death signal to the ischemic myocardium depending on dose. J Nutr Biochem. 2009;20:443–452. doi: 10.1016/j.jnutbio.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Das S, Cordis GA, Maulik N, Das DK. Pharmacological preconditioning with resveratrol: role of CREB-dependent Bcl-2 signaling via adenosine A3 receptor activation. Am J Physiol Heart Circ Physiol. 2005;288:H328–H335. doi: 10.1152/ajpheart.00453.2004. [DOI] [PubMed] [Google Scholar]
- 16.Opipari AW, Jr, Tan L, Boitano AE, Sorenson DR, Aurora A, Liu JR. Resveratrol-induced autophagocytosis in ovarian cancer cells. Cancer Res. 2004;64:696–703. doi: 10.1158/0008-5472.can-03-2404. [DOI] [PubMed] [Google Scholar]
- 17.Trincheri NF, Follo C, Nicotra G, Peracchio C, Castino R, Isidoro C. Resveratrol-induced apoptosis depends on the lipid kinase activity of Vps34 and on the formation of autophagolysosomes. Carcinogenesis. 2008;29:381–389. doi: 10.1093/carcin/bgm271. [DOI] [PubMed] [Google Scholar]
- 18.Biederbick A, Kern HF, Elsasser HP. Monodansylcadaverine (MDC) is a specific in vivo marker for autophagic vacuoles. Eur J Cell Biol. 1995;66:3–14. [PubMed] [Google Scholar]
- 19.Gherghiceanu M, Hinescu ME, Popescu LM. Myocardial interstitial Cajal-like cells (ICLC) in caveolin-1 KO mice. J Cell Mol Med. 2009;13:202–206. doi: 10.1111/j.1582-4934.2008.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Popescu LM, Gherghiceanu M, Hinescu ME, Cretoiu D, Ceafalan L, Regalia T, et al. Insights into the interstitium of ventricular myocardium: interstitial Cajal-like cells (ICLC) J Cell Mol Med. 2006;10:429–458. doi: 10.1111/j.1582-4934.2006.tb00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samari HR, Seglen PO. Inhibition of hepatocytic autophagy by adenosine, aminoimidazole-4-carboxamide riboside, and N6-mercaptopurine riboside. Evidence for involvement of amp-activated protein kinase. J Biol Chem. 1998;273:23758–237563. doi: 10.1074/jbc.273.37.23758. [DOI] [PubMed] [Google Scholar]
- 22.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor–mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 23.Gustafsson AB, Gottlieb RA. Eat your heart out: role of autophagy in myocardial ischemia/reperfusion. Autophagy. 2008;4:416–421. doi: 10.4161/auto.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsui Y, Kyoi S, Takagi H, Hsu CP, Hariharan N, Ago T, et al. Molecular mechanisms and physiological significance of autophagy during myocardial ischemia and reperfusion. Autophagy. 2008;4:409–415. doi: 10.4161/auto.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwai-Kanai E, Yuan H, Huang C, Sayen MR, Perry-Garza CN, Kim L, et al. A method to measure cardiac autophagic flux in vivo. Autophagy. 2008;4:322–329. doi: 10.4161/auto.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neufeld TP. Contribution of Atg1-dependent autophagy to TOR-mediated cell growth and survival. Autophagy. 2007;3:477–479. doi: 10.4161/auto.4348. [DOI] [PubMed] [Google Scholar]
- 27.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Martin DE, Hall MN. The expanding TOR signaling network. Curr Opin Cell Biol. 2005;17:158–166. doi: 10.1016/j.ceb.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 30.Goswami SK, Das DK. Autophagy in the myocardium: dying for survival? Exp Clin Cardiol. 2006;11:183–188. [PMC free article] [PubMed] [Google Scholar]
- 31.Buller CL, Loberg RD, Fan MH, Zhu Q, Park JL, Vesely E, et al. A GSK-3/TSC2/mTOR pathway regulates glucose uptake and GLUT1 glucose transporter expression. Am J Physiol Cell Physiol. 2008;295:C836–C843. doi: 10.1152/ajpcell.00554.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fingar DC, Richardson CJ, Tee AR, Cheatham L, Tsou C, Blenis J. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol Cell Biol. 2004;24:200–216. doi: 10.1128/MCB.24.1.200-216.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horman S, Beauloye C, Vertommen D, Vanoverschelde JL, Hue L, Rider MH. Myocardial ischemia and increased heart work modulate the phosphorylation state of eukaryotic elongation factor-2. J Biol Chem. 2003;278:41970–41976. doi: 10.1074/jbc.M302403200. [DOI] [PubMed] [Google Scholar]
- 34.Brito PM, Devillard R, Negre-Salvayre A, Almeida LM, Dinis TC, Salvayre R, et al. Resveratrol inhibits the mTOR mitogenic signaling evoked by oxidized LDL in smooth muscle cells. Atherosclerosis. 2009;205:126–134. doi: 10.1016/j.atherosclerosis.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 35.Scott RC, Schuldiner O, Neufeld TP. Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev Cell. 2004;7:167–178. doi: 10.1016/j.devcel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Armstrong SC. Protein kinase activation and myocardial ischemia/reperfusion injury. Cardiovasc Res. 2004;61:427–436. doi: 10.1016/j.cardiores.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 37.Attaix D, Bechet D. FoxO3 controls dangerous proteolytic liaisons. Cell Metab. 2007;6:425–427. doi: 10.1016/j.cmet.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Shang J, Chen LL, Xiao FX, Sun H, Ding HC, Xiao H. Resveratrol improves non-alcoholic fatty liver disease by activating AMP-activated protein kinase. Acta Pharmacol Sin. 2008;29:698–706. doi: 10.1111/j.1745-7254.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- 39.Penumathsa SV, Thirunavukkarasu M, Zhan L, Maulik G, Menon VP, Bagchi D, et al. Resveratrol enhances GLUT-4 translocation to the caveolar lipid raft fractions through AMPK/AKT/eNOS signaling pathway in diabetic myocardium. J Cell Mol Med. 2008;12:2350–2361. doi: 10.1111/j.1582-4934.2008.00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hwang JT, Kwon DY, Park OJ, Kim MS. Resveratrol protects ROS-induced cell death by activating AMPK in H9c2 cardiac muscle cells. Genes Nutr. 2008;2:323–326. doi: 10.1007/s12263-007-0069-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 42.Takagi H, Matsui Y, Hirotani S, Sakoda H, Asano T, Sadoshima J. AMPK mediates autophagy during myocardial ischemia in vivo. Autophagy. 2007;3:405–407. doi: 10.4161/auto.4281. [DOI] [PubMed] [Google Scholar]
- 43.Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277:23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- 44.Motoshima H, Goldstein BJ, Igata M, Araki E. AMPK and cell proliferation—AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol. 2006;574:63–71. doi: 10.1113/jphysiol.2006.108324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kovacic S, Soltys CL, Barr AJ, Shiojima I, Walsh K, Dyck JR. Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. J Biol Chem. 2003;278:39422–39427. doi: 10.1074/jbc.M305371200. [DOI] [PubMed] [Google Scholar]
- 46.Huang J, Manning BD. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem Soc Trans. 2009;37:217–222. doi: 10.1042/BST0370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 48.Zeng X, Overmeyer JH, Maltese WA. Functional specificity of the mammalian Beclin-Vps34 PI 3-kinase complex in macroautophagy versus endocytosis and lysosomal enzyme trafficking. J Cell Sci. 2006;119:259–270. doi: 10.1242/jcs.02735. [DOI] [PubMed] [Google Scholar]
- 49.Blommaart EF, Krause U, Schellens JP, Vreeling-Sindelarova H, Meijer AJ. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur J Biochem. 1997;243:240–246. doi: 10.1111/j.1432-1033.1997.0240a.x. [DOI] [PubMed] [Google Scholar]
- 50.Kueck A, Opipari AW, Jr, Griffith KA, Tan L, Choi M, Huang J, et al. Resveratrol inhibits glucose metabolism in human ovarian cancer cells. Gynecol Oncol. 2007;107:450–457. doi: 10.1016/j.ygyno.2007.07.065. [DOI] [PubMed] [Google Scholar]
- 51.Scarlatti F, Maffei R, Beau I, Codogno P, Ghidoni R. Role of non-canonical Beclin 1-independent autophagy in cell death induced by resveratrol in human breast cancer cells. Cell Death Differ. 2008;15:1318–1329. doi: 10.1038/cdd.2008.51. [DOI] [PubMed] [Google Scholar]
- 52.Lizcano JM, Alrubaie S, Kieloch A, Deak M, Leevers SJ, Alessi DR. Insulin-induced Drosophila S6 kinase activation requires phosphoinositide 3-kinase and protein kinase B. Biochem J. 2003;374:297–306. doi: 10.1042/BJ20030577. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Zhang HH, Lipovsky AI, Dibble CC, Sahin M, Manning BD. S6K1 regulates GSK3 under conditions of mTOR-dependent feedback inhibition of Akt. Mol Cell. 2006;24:185–197. doi: 10.1016/j.molcel.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]