Abstract
Aims
Because ceramide accumulates in several forms of cardiovascular disease and ceramide-induced apoptosis may involve the volume-sensitive Cl− current, ICl,swell, we assessed whether ceramide activates ICl,swell.
Methods and results
ICl,swell was measured in rabbit ventricular myocytes by whole-cell patch clamp after isolating anion currents. Exogenous C2-ceramide (C2-Cer), a membrane-permeant short-chain ceramide, elicited an outwardly rectifying Cl− current in both physiological and symmetrical Cl− solutions that was fully inhibited by DCPIB, a specific ICl,swell blocker. In contrast, the metabolically inactive C2-Cer analogue C2-dihydroceramide (C2-H2Cer) failed to activate Cl− current. Bacterial sphingomyelinase (SMase), which generates endogenous long-chain ceramides as was confirmed by tandem mass spectrometry, also elicited an outwardly rectifying Cl− current that was inhibited by DCPIB and tamoxifen, another ICl,swell blocker. Bacterial SMase-induced current was partially reversed by osmotic shrinkage and fully suppressed by ebselen, a scavenger of reactive oxygen species. Outward rectification with physiological and symmetrical Cl− gradients, block by DCPIB and tamoxifen, and volume sensitivity are characteristics that identify ICl,swell. Insensitivity to C2-H2Cer and block by ebselen suggest involvement of ceramide signalling rather than direct lipid-channel interaction.
Conclusion
Exogenous and endogenous ceramide elicited ICl,swell in ventricular myocytes. This may contribute to persistent activation of ICl,swell and aspects of altered myocyte function in cardiovascular diseases associated with by ceramide accumulation.
Keywords: Cl channel; Ceramide; Sphingomyelinase; ICl,swell; VRAC
1. Introduction
Volume-sensitive Cl− current, ICl,swell, is elicited in cardiac myocytes by osmotic swelling, hydrostatic inflation, and β1 integrin stretch, and in several models of cardiac disease. In turn, ICl,swell modulates cardiac electrical activity, cell volume, and apoptosis, and is implicated in ischaemic preconditioning.1–3 Regulation of ICl,swell is complex and involves a number of signalling pathways. Recently, reactive oxygen species (ROS) were identified as a downstream effector, and exogenous H2O2 elicits ICl,swell in cardiomyocytes4–6 and other cells.7–9 Upstream signalling molecules include Src family kinases,10–12 focal adhesion kinase,12,13 protein tyrosine kinase,14 angiotensin II (Ang II),4,6 epidermal growth factor receptor (EGFR) kinase,11 and phosphoinositide 3-kinase (PI-3K).5,6 Protein kinase C (PKC) also is implicated, although its role is controversial because it appears to inhibit15 or activate ICl,swell.15,16
Many of the signalling cascades that activate ICl,swell overlap those involved in sphingolipid signalling,17–19 raising the possibility that certain sphingolipids might regulate ICl,swell. Sphingolipids are a class of phospholipids defined by the presence of an amide-linked fatty acid, a free hydroxyl group at position 3, and a trans double-bond between carbons 4 and 5. Initially, sphingolipids were considered membrane structural components without further function. More recently, sphingolipids, specifically ceramide and sphingosine, were recognized as bioactive molecules that participate in a number of signalling cascades and mediate apoptosis, mitogenesis, and other cellular processes. Alterations in sphingolipid metabolism are implicated in cardiovascular diseases, including congestive heart failure, atherosclerosis, and ischaemia/reperfusion injury.18,20 Sphingosine kinase, which phosphorylates the ceramide metabolite sphingosine, mediates Ang II-induced PI-3K activation21 and EGFR upregulation22 in vascular smooth muscle cells. Exogenous ceramide elicits ROS production via NADPH oxidase in bovine coronary artery cells23 and the mitochondrial electron transport chain in rat liver24 and heart.25 Moreover, ICl,swell is postulated to control the ceramide-induced apoptotic volume decrease (AVD) in cardiomyocytes,26 but effects of ceramide on ICl,swell were not assessed.
This study tested whether ceramide activates ICl,swell in ventricular myocytes. Under isosmotic conditions, exogenous, short-chain ceramide elicited an outwardly rectifying Cl− current in both physiological and symmetrical Cl− gradients that was suppressed by DCPIB, a highly selective ICl,swell blocker. Bacterial sphingomyelinase (SMase), which generates endogenous long-chain ceramides, also elicited an outwardly rectifying Cl− current that was inhibited by DCPIB and tamoxifen, a second ICl,swell blocker. Finally, osmotic shrinkage partially reversed and the ROS scavenger ebselen fully reversed SMase-induced current. These data suggest that ceramide evokes ICl,swell in cardiac myocytes. This may contribute to the persistent activation of ICl,swell in cardiovascular diseases marked by ceramide accumulation.
2. Methods
This study conforms to the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85–23, 1996) and was approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee (AM10290).
2.1. Cell isolation and experimental solutions
Ventricular myocytes were isolated from adult New Zealand white rabbits (∼3 kg) by an enzymatic dissociation procedure.13 Complete cell isolation methods are given in Supplementary material online.
Bath and pipette solutions were designed to isolate Cl− current. Isosmotic bath solution (1 T; 300 mOsm/kg; T, times isosmotic) contained (in mM): 90 N-methyl-d-glucamine-Cl, 3 MgCl2, 10 HEPES, 10 glucose, 5 CsCl, 0.5 CdCl2, 70 mannitol (pH 7.4, adjusted CsOH). Hyperosmotic bath solution (1.5 T, 450 mOsm/kg) had the same composition except for an additional 150 mM mannitol, and hypo-osmotic bath solution (0.7 T, 230 mOsm/kg) contained no mannitol. For experiments with SMase, 6 mM MgCl2 was added to augment enzymatic activity, with mannitol concentrations adjusted accordingly. Pipette solution contained (in mM): 110 Cs-Aspartate, 20 TEA-Cl, 5 Mg-ATP, 0.1 Tris–GTP, 0.15 CaCl2, 8 Cs2-EGTA, 10 HEPES (pH 7.1, adjusted with CsOH). To make symmetrical Cl− pipette solution, 82 mM CsCl replaced an equal amount of Cs-Aspartate. Osmolarity was verified by freezing-point depression.
Stock solutions of d-erythro-C2-ceramide (C2-Cer; 5 mM; Biomol), d-erythro-C2-dihydroceramide (C2-H2Cer; 5 mM; Biomol), ebselen (15 mM, Calbiochem), DCPIB (20 mM; Tocris) in DMSO, and tamoxifen (20 mM; Sigma-Aldrich) in ethanol were frozen (−20°C) in aliquots until use. Stock solutions of Mg2+-dependent, neutral bacterial SMase, also known as SMase C, from Bacillus cereus (50 U/mL, in H2O; Sigma-Aldrich) were stored in aliquots at 4°C until use.
Endogenous synthetic short-chain C2-Cer was employed because it is membrane-permeant and is soluble in serum-free experimental solutions without forming micelles. In contrast, bacterial SMase generates native long-chain ceramides from membrane SMase and may better represent ceramide accumulation in physiological and pathophysiological settings.
2.2. Electrophysiological recordings
Ventricular myocytes were scattered on a glass-bottomed chamber and on an inverted light microscope (Nikon) with Hoffman modulation optics and a high-resolution video camera to visualize cells. Cells were suprafused with bath solution at 2−3 mL/min at 22–23°C. Pipettes were pulled from 7740 thin-walled borosilicate tubing (Sutter) and fire-polished to a final tip diameter of ∼3 μm with a resistance in bath solution of 2–4 MΩ. Whole-cell currents were recorded using an Axopatch 200B amplifier and Digidata 1322A (Axon). A 3 M KCl agar bridge served as ground. Seal resistances of 2–20 GΩ were obtained typically, and membrane capacitance was measured routinely. Membrane potential was corrected for measured liquid junction potential in all experiments, and myocytes were dialysed with pipette solution for 8–10 min prior to the start of recording. Voltage clamp protocols and data acquisition were controlled by pClamp 8.2. Successive 500 ms steps were from a holding potential of −60 mV to test potentials from −100 to 60 mV in 10 mV increments. Membrane currents were low-pass-filtered at 2 kHz and digitized at 5 kHz. Representative traces were low-pass-filtered at 500 Hz for presentation, and displayed I–V curves are from corresponding current traces. Currents were not leak-corrected. To minimize variability, experiments used cells as their own controls.
2.3. Lipid analysis by tandem mass spectrometry
Cells in 1 T bath solution were treated with bacterial SMase (0.03 U/mL, 15 min), or left untreated. Lipids were extracted and assayed as described,27,28 with slight modification. Sphingosine, sphinganine, sphingosine-1-phosphate sphinganine-1-phosphate, and ceramide-1-phosphate were quantified via reversed-phase HPLC ESI-MS/MS using a Discovery C18 column attached to a Shimadzu HPLC (20AD series) and mass spectrometric analysis using a 4000 Q-Trap (Applied Biosystems).27 Ceramides, sphingomyelins, and monohexosyl ceramides were quantified via normal-phase HPLC ESI-MS/MS using an amino column (Sigma).28 Complete methods for lipid analysis are given in Supplementary material online.
2.4. Statistics
Summary data are reported as mean ± SEM; n denotes the number of cells. Mean currents are expressed as current density (pA/pF), and selected paired comparisons are expressed as a percentage or as intervention-induced difference currents. Statistical analysis was executed using SigmaStat 3.11 (Systat). Except as noted, a one-way or one-way repeated measures ANOVA was performed followed by a Student–Newman–Keuls test. P < 0.05 was taken as significant. Non-linear curve fits were done in SigmaPlot 10.0 (Systat).
3. Results
3.1. Exogenous ceramide activates a Cl− current resembling ICl,swell
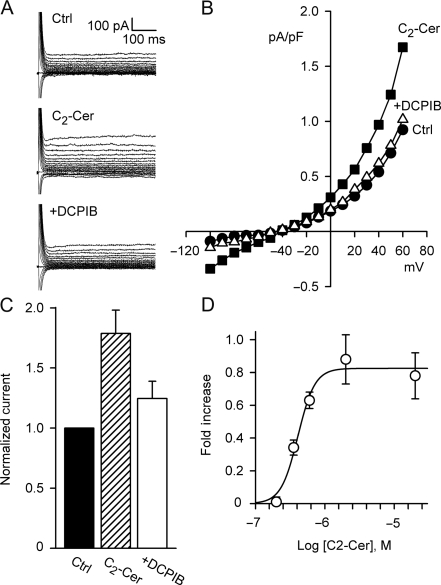
C2-Cer (2 μM, 10–12 min), a membrane-permeant, short-chain ceramide analogue, activated an outwardly rectifying Cl− current with a reversal potential near the Cl− equilibrium potential (ECl), −43 mV (Figure 1). Current at 60 mV increased by 0.70 ± 0.09 pA/pF (n = 15, P < 0.001), from 0.94 ± 0.13 to 1.57 ± 0.22 pA/pF, and a C2-Cer-induced current was observed in >90% of cells tested. Addition of DCPIB (10 μM, 12–15 min), a highly selective ICl,swell blocker, inhibited C2-Cer-induced Cl− current by 76 ± 8% (n = 6, P < 0.001) in the continued presence of C2-Cer, and there was no significant difference between the DCPIB-inhibited and control currents. Furthermore, C2-Cer-induced current was steeply concentration-dependent with an EC50 of 0.41 μM and a Hill coefficient of 3.6. The physiological range for native ceramide in many cell types is 1–5 μM,29 although local concentrations under some conditions may be greater19; because C2-Cer is a short-chain synthetic ceramide, its concentration dependence may not match that of native ceramides. No change in membrane capacitance was observed in individual cells treated with C2-Cer. Under control conditions, background current usually displayed modest outward rectification, and its amplitude varied from cell to cell. Such variation was noted previously and likely reflects partial activation of ICl,swell under control conditions.
Figure 1.
C2-Cer elicited a Cl− current that resembled ICl,swell. (A) Families of currents under control conditions (Ctrl), after C2-Cer exposure (2 µM, 10 min), and after addition of DCPIB (+DCPIB; 10 μM) in continued presence of C2-Cer. Holding potential, −60 mV; test potentials, −100 to 60 mV. (B) Current–voltage (I–V) relationships for A. (C) Normalized currents at 60 mV. C2-Cer increased Cl− current by 0.70 ± 0.09 pA/pF (n = 14, P < 0.001). C2-Cer-induced current was inhibited by 76 ± 8% (n = 6, P < 0.001) by the ICl,swell-specific inhibitor DCPIB; current after DCPIB was not different than control. (D) C2-Cer-induced currents at 0.2 (n = 3), 0.36 (n = 3), 0.6 (n = 3), 2 (n = 14), and 20 μM (n = 4) and fit (solid line) to EC50 of 0.41 μM and Hill coefficient of 3.6.
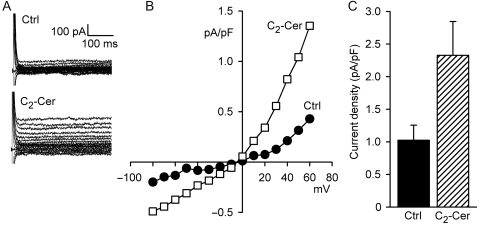
Outward rectification in symmetrical Cl− solutions is a characteristic of ICl,swell that distinguishes it from several other Cl− currents, including CFTR and Ca2+-activated Cl− currents.3 Under symmetrical Cl− conditions (Figure 2), C2-Cer (2 μM, 10–12 min) elicited current that outwardly rectified and reversed at 0 mV. At 60 mV, C2-Cer increased current density by 1.30 ± 0.32 pA/pF (n = 6, P < 0.01), from 1.03 ± 0.23 to 2.33 ± 0.52 pA/pF. Taken together, outward rectification in physiological and symmetrical Cl− and block by DCPIB are diagnostic for ICl,swell.
Figure 2.
C2-Cer (2 μM, 10 min) activated outwardly rectifying Cl− current in symmetrical Cl−. (A) Families of currents and (B) I–V relationships. C2-Cer-induced current reversed near 0 mV. (C) C2-Cer-induced current at 60 mV was 1.30 ± 0.35 pA/pF (n = 6, P < 0.01). Outward rectification in symmetrical Cl− and block by DCPIB (Figure 1) indicate C2-Cer activated ICl,swell.
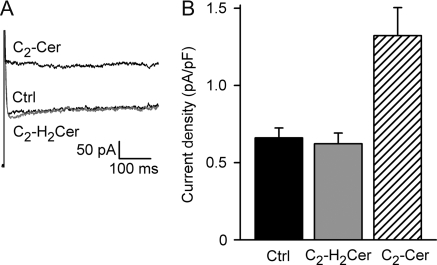
Alterations in membrane curvature due to asymmetric insertion of amphipaths into the plasmalemma outer or inner leaflets mimic changes in cell volume and activate ICl,swell.30 To exclude the possibility that C2-Cer activated ICl,swell via alteration of membrane curvature or other non-specific mechanisms, we used C2-H2Cer, a C2-Cer analogue that is inactive in ceramide signalling31 but should exert similar mechanical effects on membranes. As depicted in Figure 3, C2-H2Cer failed to activate current above control (n = 6, P = 0.94). To verify the presence of ICl,swell in cells unresponsive to C2-H2Cer, C2-Cer was then added in four experiments. C2-Cer evoked ICl,swell in each of these previously unresponsive cells (n = 4, P < 0.01). Activation by C2-Cer but not C2-H2Cer suggests ICl,swell was elicited via normal ceramide pathways rather than by a non-specific mechanism.
Figure 3.
C2-H2Cer, a metabolically inactive C2-Cer analogue, did not alter membrane current, but C2-Cer elicited ICl,swell in the same cell. (A) Typical currents at 60 mV. (B) Cl− current densities in control and with C2-H2Cer and C2-Cer (both: 2 μM, 10 min). C2-H2Cer was ineffective (−4 ± 5%, n = 6, NS), whereas C2-Cer subsequently activated current in all four cells tested (P < 0.01). The data suggest C2-Cer elicited ICl,swell via its normal pathway rather than by non-specific mechanisms.
3.2. Endogenous ceramide generation is sufficient to activate ICl,swell
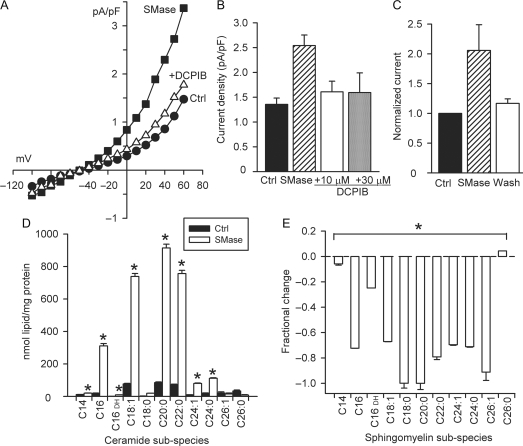
Bacterial SMase is a neutral, Mg2+-dependent enzyme that acts specifically at the plasmalemma to convert sphingomyelin to long-chain ceramides that are native to the cell. Bacterial SMase (0.03 U/mL, 15–18 min), like exogenous C2-Cer, evoked an outwardly rectifying Cl− current in >90% of cells tested, and current at 60 mV increased by 1.01 ± 0.05 pA/pF (n = 75, P < 0.001), from 1.22 ± 0.07 to 2.23 ± 0.10 pA/pF (Figure 4Aand B). SMase-induced current was reversible with 20 min of washout in control bath solution in each of the cells tested (n = 3, P < 0.05) (Figure 4C). No change in membrane capacitance was observed with bacterial SMase treatment. As expected and confirmed by tandem mass spectrometry, bacterial SMase increased myocyte ceramides and decreased sphingomyelins under the same experimental conditions (Figure 4Dand E).
Figure 4.
Bacterial SMase reversibly activated ICl,swell. (A) I–V relationships for Cl− current elicited by SMase (0.03 U/mL, 15−18 min) and inhibition by DCPIB (10 μM). (B) SMase increased Cl− current by 1.1 ± 0.1 pA/pF at 60 mV (n = 30), and DCPIB (10 or 30 μM) suppressed 78 ± 6% (n = 7) or 81 ± 6% (n = 4), respectively (P < 0.01 for both). (C) Effect of SMase reversed on washout (18−20 min, n = 3, P < 0.05). (D and E) Exposure to SMase (20 min) generated endogenous long-chain ceramides and depleted a substantial fraction of sarcolemmal sphingomyelins (n = 6; *P < 0.05). For ceramides and sphingomyelins, each lipid species was compared with control using a three-way ANOVA based on two separate experimental data sets, each analysed in triplicate.
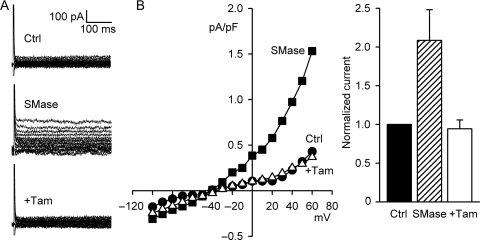
Two blockers of ICl,swell inhibited bacterial SMase-induced current. DCPIB suppressed 78 ± 6% (10 μM, n = 7, P < 0.01) of the current, and the remaining current was not significantly different than control (Figure 4B). Increasing DCPIB to 30 μM did not reduce the SMase-induced current further (81 ± 6%; n = 4, P = 0.86 vs. 10 μM DCPIB). Tamoxifen (10 μM, 5–8 min) also was effective in blocking the SMase-induced Cl− current (Figure 5); it decreased current by 116 ± 16% at 60 mV (n = 5, P < 0.01). Block of SMase-induced current by DCPIB and tamoxifen confirms its attribution to ICl,swell.
Figure 5.
Tamoxifen (Tam) inhibited SMase-induced ICl,swell. (A) Currents before and after treatment with SMase (0.03 U/mL, 15−18 min) and after the addition of Tam (10 µM). (B) I–V relationships. (C) Tam fully blocks SMase-induced Cl− current (116 ± 16%, n = 5, P < 0.01).
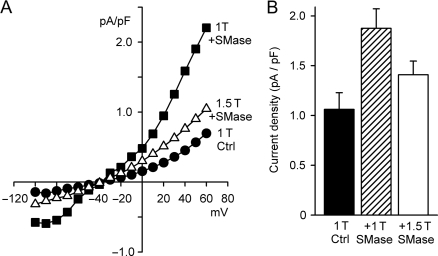
The volume-sensitivity of Cl− current elicited by bacterial SMase was tested by exposure to hyperosmotic (1.5 T) bathing solution in the continued presence of SMase (Figure 6). Cell shrinkage for 15 min inhibited SMase-induced current by 43 ± 8% (n = 6, P < 0.02), from 1.88 ± 0.20 to 1.41 ± 0.14 pA/pF at 60 mV (Figure 6B). SMase-induced current in 1.5 T bath solution remained, however, significantly greater than control (n = 6, P < 0.02). Partial inhibition by hyperosmotic cell shrinkage indicates that the activation of SMase-induced current had both volume-sensitive and volume-independent components.
Figure 6.
Osmotic shrinkage partially inhibited SMase-induced ICl,swell. (A) I–V relationships before (1 T Ctrl) and after (1 T + SMase) exposure to SMase (0.03 U/mL, 18 min) in isosmotic bath solution, and then, after shrinking the same cell in hyperosmotic bath solution containing SMase (1.5 T + SMase; 0.03 U/mL, 15 min). (B) Current densities at 60 mV before and after treatment with SMase in 1 T and 1.5 T bath solutions. Cell shrinkage in 1.5 T partially inhibited the SMase-induced Cl− current (43 ± 8%, n = 6, P < 0.02). This suggested that SMase elicits ICl,swell via volume-dependent and volume-independent pathways.
3.3. ROS mediate bacterial SMase-induced activation of ICl,swell
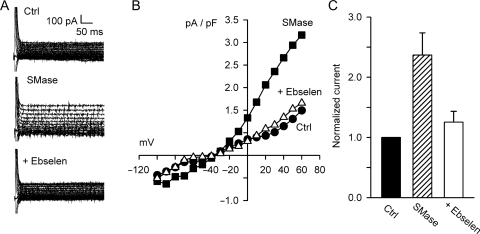
Previously, we demonstrated that H2O2 is a downstream mediator of ICl,swell activation and exogenous H2O2 elicits ICl,swell even under hyperosmotic conditions.4,6 As shown in Figure 7, ebselen (20 μM, 5 min), a cell-permeable glutathione peroxidase mimetic that converts H2O2 to H2O, inhibited SMase-induced Cl− current by 124 ± 39% (n = 5, P < 0.01) from 2.46 ± 0.43 pA/pF to 1.56 ± 0.33 pA/pF at 60 mV. There was no difference in Cl− currents under control conditions (1.59 ± 0.55 pA/pF) and after the addition of ebselen (P = 0.87). This demonstrates that the SMase-induced Cl− current is mediated by ROS.
Figure 7.
Bacterial SMase-induced Cl− current was inhibited by ebselen. (A) Currents before and after treatment with SMase (0.03 U/mL, 15−18 min) and after the addition of ebselen (20 µM, 5 min). (B) I–V relationships. (C) Ebselen, a glutathione peroxidase mimetic that scavenges H2O2, fully blocked SMase-induced Cl− current at 60 mV (n = 5, P < 0.01). These data suggest the SMase-induced Cl− current is elicited by H2O2, a downstream mediator of ICl,swell.4,6
3.4. Differences in time course of activation due to exogenous and endogenous ceramides
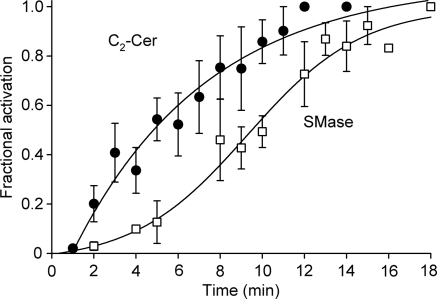
Figure 8 compares the time course of activation of ICl,swell by C2-Cer and bacterial SMase. The C2-Cer-induced difference current was fit by a single exponential function with a time constant of 6.4 ± 1.6 min (R2 = 0.93, n = 11), equivalent to a t1/2 of 4.8 ± 1.2 min. In contrast, SMase-induced difference current was fit by a sigmoid function with a t1/2 of 9.3 ± 0.6 min (R2 = 0.99, n = 10). The magnitude of the current turned on at 60 mV by C2-Cer, bacterial SMase, and osmotic swelling (i.e. test—control) also were compared. The C2-Cer-induced current (0.70 ± 0.09 pA/pF; n = 14) was significantly different than that evoked by bacterial SMase (1.01 ± 0.05 pA/pF; n = 75, P < 0.02) or by hypo-osmotic cell swelling in 0.7 T bath solution (1.22 ± 0.17 pA/pF, data not shown; n = 6, P < 0.05), whereas the SMase- and swelling-induced currents were indistinguishable (P = 0.24).
Figure 8.
Time course of ICl,swell activation by C2-Cer and bacterial SMase. C2-Cer data were fit by an exponential function with a time constant of 6.4 ± 1.6 min (R2 = 0.98, n = 11), equivalent to a t1/2 of 4.8 ± 1.2 min. SMase data were fit by a sigmoid function with a t1/2 = 9.3 ± 0.6 min (R2 = 0.99, n = 10). Soluble C2-Cer may reach the site of activation of ICl,swell more quickly than long-chain endogenous ceramides that must first be produced by SMase. Alternatively, ceramides with different chain lengths may activate different sites in the signalling cascade.
4. Discussion
Exogenous C2-Cer and endogenous long-chain ceramides generated by bacterial SMase activated currents that reversed near ECl, exhibited outward rectification in physiological and symmetrical Cl− gradients, were partially inhibited by hyperosmotic shrinkage, and were suppressed by the ROS scavenger ebselen. These biophysical features matched those of volume-sensitive ICl,swell,1,3,30 and ROS are required for ICl,swell activity in heart and other tissues.4–9 Additionally, block by DCPIB and tamoxifen strongly implicated ICl,swell. Tamoxifen may suppress ICl,swell by scavenging ROS and inhibiting mitochondrial complex I,32 whereas the mechanism of block by DCPIB is unknown. Although several independent lines of evidence support the conclusion that ceramides activate ICl,swell, we cannot rigorously exclude the possibility that short-chain and native ceramides form plasmalemmal pores that fortuitously share multiple characteristics with ICl,swell. C2-Cer and C16-Cer produce pores with very high conductances, up to 200 nS, in mitochondrial outer membranes and lipid bilayers,33 but the resulting currents are far too large to explain those described here.
Swelling in 0.7 T gives nearly full activation of ICl,swell in ventricular myocytes,34 and the magnitude of the current elicited by bacterial SMase and hypo-osmotic swelling were not distinguishable. In contrast, 2 μM C2-Cer evoked a significantly smaller current (∼70% of SMase- and 60% of 0.7 T-induced currents) that activated more rapidly, and increasing C2-Cer from 2 to 20 μM did not elicit additional current. These differences may reflect, in part, that C2-Cer must permeate the sarcolemma to reach its target(s) and that SMase must first hydrolyse sarcolemmal sphingomyelin to native long-chain ceramides, which also must reach target(s). It also is possible that synthetic short-chain and native long-chain ceramides work via distinct pathways or differ in their efficacy to stimulate processes causing ICl,swell activation. That hyperosmotic shrinkage in 1.5 T only partially inhibited SMase-induced current may suggest that it acts at multiple sites and one is downstream from the site controlled by shrinkage. Insensitivity of ICl,swell to osmotic shrinkage when elicited by a downstream effector is not unique. We previously showed that H2O2-induced ICl,swell is insensitive to osmotic shrinkage.6
Effects of sphingolipids on sarcolemmal channel function have been explored only recently. Prolonged (>10 h) C2-Cer and bacterial SMase exposure downregulates hERG K+ channels via a pathway involving ROS,35,36 and CFTR is inhibited more rapidly (<60 min).37 These effects appear to be PKA- and PKC-independent. d'Anglemont de Tassigny et al.26 found that ICl,swell is required for the AVD in cardiomyocytes and hypothesized that ICl,swell is activated in C2-Cer-induced apoptosis. Although outwardly rectifying Cl− currents were observed during doxorubicin-induced apoptosis, these authors did not establish a link between ceramide and ICl,swell activation.
Modification of direct interactions between membrane lipids and channel proteins has been invoked to explain altered gating of KV channels38,39 and CFTR inhibition40 after SMase D treatment. SMase D depletes membrane sphingomyelin without stimulating ceramide signalling; it produces choline and ceramide-1-phosphate, whereas bacterial SMase (SMase C) generates phosphocholine and ceramide. Such depletion of membrane lipids is not likely to explain the present results, however. C2-Cer and bacterial SMase both activated ICl,swell, whereas C2-Cer will favour, if anything, an increase in sphingolipids rather than depletion.
The lack of an effect of metabolically inactive C2-H2Cer supports the hypothesis that both C2-Cer and endogenous ceramides generated by bacterial SMase act via one or more ceramide signalling cascades rather than by a non-specific mechanism.31 Furthermore, block of SMase-induced current by ebselen strongly suggests ROS, most likely H2O2, are an intermediate. Amplification by a signalling cascade may contribute to the strong concentration dependence of current activation. In cardiomyocytes, ROS produced by NADPH oxidase4–6 and mitochondria41 are essential downstream effectors of ICl,swell activation by osmotic swelling, integrin stretch, and growth factors, and exogenous H2O2 elicits ICl,swell in cardiomyocytes and other tissues.7,8 Ceramides also produce ROS. For example, apoptosis triggered by ceramide is accompanied by mitochondrial ROS production,18,29 and ceramide is involved in NADPH oxidase activation in rat mesangial and bovine coronary artery smooth muscle cells.23,42
Native ceramides generated by bacterial SMase may not be the ultimate sphingolipid mediator of ICl,swell. Both ceramide and its metabolite, S1P, are potent lipid second messengers, often with opposing effects on signalling and a cell's fate via the ceramide/S1P rheostat.17,19 In contrast, metabolites are unlikely to be required to explain the action of synthetic C2-Cer because it does not undergo metabolism by the cellular ceramide pathway.43
ICl,swell is persistently activated in models of dilated cardiomyopathy1 and is involved in the AVD7,8 that precedes apoptotic cell death in normal development, ischaemia, or heart failure. The sphingomyelin/ceramide pathway is activated in vivo during ischaemia/reperfusion20,44,45 and heart failure,20,45,46 and the oxidation of sphingolipids is implicated in atherosclerotic plaque formation.18 The data presented here show a link between intracardiac ceramide accumulation and ICl,swell activation that may be important for understanding these cardiovascular disease states. Because ICl,swell outwardly rectifies, its activation tends to shorten action potential duration and depolarize resting membrane potential. Nevertheless, effects on other ion channels must be assessed to evaluate the consequences of ceramide accumulation on cardiac electrophysiology.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by grants to C.M.B. (American Heart Association, 0855044E; and A.D. Williams Foundation) and to C.E.C. (Veteran's Administration, VA Merit Review I and Research Career Scientist Award; National Institutes of Health, HL072925 and CA117950).
References
- 1.Baumgarten CM, Browe DM, Ren Z. Swelling- and stretch-activated chloride channels in the heart: regulation and function. In: Kamkin A, Kiseleva I, editors. Mechano-Electric Feedback in the Heart: Fundamental and Clinical Aspects. Moscow: Academic Book; 2005. pp. 79–102. [PubMed] [Google Scholar]
- 2.Duan DY, Liu LL, Bozeat N, Huang ZM, Xiang SY, Wang GL, et al. Functional role of anion channels in cardiac diseases. Acta Pharmacol Sin. 2005;26:265–278. doi: 10.1111/j.1745-7254.2005.00061.x. [DOI] [PubMed] [Google Scholar]
- 3.Hume JR, Duan D, Collier ML, Yamazaki J, Horowitz B. Anion transport in heart. Physiol Rev. 2000;80:31–81. doi: 10.1152/physrev.2000.80.1.31. [DOI] [PubMed] [Google Scholar]
- 4.Browe DM, Baumgarten CM. Angiotensin II (AT1) receptors and NADPH oxidase regulate Cl− current elicited by β1 integrin stretch in rabbit ventricular myocytes. J Gen Physiol. 2004;124:273–287. doi: 10.1085/jgp.200409040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Browe DM, Baumgarten CM. EGFR kinase regulates volume-sensitive chloride current elicited by integrin stretch via PI-3K and NADPH oxidase in ventricular myocytes. J Gen Physiol. 2006;127:237–251. doi: 10.1085/jgp.200509366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren Z, Raucci FJ, Jr, Browe DM, Baumgarten CM. Regulation of swelling-activated Cl− current by angiotensin II signalling and NADPH oxidase in rabbit ventricle. Cardiovasc Res. 2008;77:73–80. doi: 10.1093/cvr/cvm031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varela D, Simon F, Riveros A, Jorgensen F, Stutzin A. NADPH oxidase-derived H2O2 signals chloride channel activation in cell volume regulation and cell proliferation. J Biol Chem. 2004;279:13301–13304. doi: 10.1074/jbc.C400020200. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu T, Numata T, Okada Y. A role of reactive oxygen species in apoptotic activation of volume-sensitive Cl− channel. Proc Natl Acad Sci USA. 2004;101:6770–6773. doi: 10.1073/pnas.0401604101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haskew-Layton RE, Mongin AA, Kimelberg HK. Hydrogen peroxide potentiates volume-sensitive excitatory amino acid release via a mechanism involving Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 2005;280:3548–3554. doi: 10.1074/jbc.M409803200. [DOI] [PubMed] [Google Scholar]
- 10.Browe DM, Baumgarten CM. Acetylcholine activates the swelling-activated chloride current, ICl,swell in rabbit ventricular myocytes by opening mitochondrial KATP channels. (Abstract) Biophys J. 2005;88:289a. [Google Scholar]
- 11.Du XL, Gao Z, Lau CP, Chiu SW, Tse HF, Baumgarten CM, et al. Differential effects of tyrosine kinase inhibitors on volume-sensitive chloride current in human atrial myocytes: evidence for dual regulation by Src and EGFR kinases. J Gen Physiol. 2004;123:427–439. doi: 10.1085/jgp.200409013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh KB, Zhang J. Regulation of cardiac volume-sensitive chloride channel by focal adhesion kinase and Src kinase. Am J Physiol Heart Circ Physiol. 2005;289:H2566–H2574. doi: 10.1152/ajpheart.00292.2005. [DOI] [PubMed] [Google Scholar]
- 13.Browe DM, Baumgarten CM. Stretch of β1 integrin activates an outwardly rectifying chloride current via FAK and Src in rabbit ventricular myocytes. J Gen Physiol. 2003;122:689–702. doi: 10.1085/jgp.200308899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorota S. Tyrosine protein kinase inhibitors prevent activation of cardiac swelling-induced chloride current. Pflugers Arch. 1995;431:178–185. doi: 10.1007/BF00410189. [DOI] [PubMed] [Google Scholar]
- 15.Duan D, Cowley S, Horowitz B, Hume JR. A serine residue in ClC-3 links phosphorylation-dephosphorylation to chloride channel regulation by cell volume. J Gen Physiol. 1999;113:57–70. doi: 10.1085/jgp.113.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong W, Xu H, Shimizu T, Morishima S, Tanabe S, Tachibe T, et al. ClC-3-independent, PKC-dependent activity of volume-sensitive Cl channel in mouse ventricular cardiomyocytes. Cell Physiol Biochem. 2004;14:213–224. doi: 10.1159/000080330. [DOI] [PubMed] [Google Scholar]
- 17.Spiegel S, Milstien S. Sphingosine 1-phosphate, a key cell signaling molecule. J Biol Chem. 2002;277:25851–25854. doi: 10.1074/jbc.R200007200. [DOI] [PubMed] [Google Scholar]
- 18.Levade T, Auge N, Veldman RJ, Cuvillier O, Negre-Salvayre A, Salvayre R. Sphingolipid mediators in cardiovascular cell biology and pathology. Circ Res. 2001;89:957–968. doi: 10.1161/hh2301.100350. [DOI] [PubMed] [Google Scholar]
- 19.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 20.Chatterjee S, Kolmakova A, Miller M. The role of the phospholipid sphingomyelin in heart disease. Curr Opin Investig Drugs. 2006;7:219–228. [PubMed] [Google Scholar]
- 21.Mulders AC, Hendriks-Balk MC, Mathy MJ, Michel MC, Alewijnse AE, Peters SL. Sphingosine kinase-dependent activation of endothelial nitric oxide synthase by angiotensin II. Arterioscler Thromb Vasc Biol. 2006;26:2043–2048. doi: 10.1161/01.ATV.0000237569.95046.b9. [DOI] [PubMed] [Google Scholar]
- 22.Tanimoto T, Lungu AO, Berk BC. Sphingosine 1-phosphate transactivates the platelet-derived growth factor beta receptor and epidermal growth factor receptor in vascular smooth muscle cells. Circ Res. 2004;94:1050–1058. doi: 10.1161/01.RES.0000126404.41421.BE. [DOI] [PubMed] [Google Scholar]
- 23.Zhang DX, Zou AP, Li PL. Ceramide-induced activation of NADPH oxidase and endothelial dysfunction in small coronary arteries. Am J Physiol Heart Circ Physiol. 2003;284:H605–H612. doi: 10.1152/ajpheart.00697.2002. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Ruiz C, Colell A, Mari M, Morales A, Fernandez-Checa JC. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J Biol Chem. 1997;272:11369–11377. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- 25.Gudz TI, Tserng KY, Hoppel CL. Direct inhibition of mitochondrial respiratory chain complex III by cell-permeable ceramide. J Biol Chem. 1997;272:24154–24158. doi: 10.1074/jbc.272.39.24154. [DOI] [PubMed] [Google Scholar]
- 26.d'Anglemont de Tassigny A, Souktani R, Henry P, Ghaleh B, Berdeaux A. Volume-sensitive chloride channels (ICl,vol) mediate doxorubicin-induced apoptosis through apoptotic volume decrease in cardiomyocytes. Fundam Clin Pharmacol. 2004;18:531–538. doi: 10.1111/j.1472-8206.2004.00273.x. [DOI] [PubMed] [Google Scholar]
- 27.Wijesinghe DS, Allegood JC, Gentile LB, Fox TE, Kester M, Chalfant CE. Use of high pressure liquid chromatography, electrospray ionization-tandem mass spectrometry for the analysis of ceramide-1-phosphate levels. J Lipid Res. 2009 doi: 10.1194/jlr.D000430. doi:10.1194/jlr.D000430. Published online ahead of print 4 August 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merrill AH, Jr, Sullards MC, Allegood JC, Kelly S, Wang E. Sphingolipidomics: high-throughput, structure-specific, and quantitative analysis of sphingolipids by liquid chromatography tandem mass spectrometry. Methods. 2005;36:207–224. doi: 10.1016/j.ymeth.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274:1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- 30.Tseng GN. Cell swelling increases membrane conductance of canine cardiac cells: evidence for a volume-sensitive Cl channel. Am J Physiol. 1992;262:C1056–C1068. doi: 10.1152/ajpcell.1992.262.4.C1056. [DOI] [PubMed] [Google Scholar]
- 31.Bielawska A, Crane HM, Liotta D, Obeid LM, Hannun YA. Selectivity of ceramide-mediated biology. Lack of activity of erythro-dihydroceramide. J Biol Chem. 1993;268:26226–26232. [PubMed] [Google Scholar]
- 32.Moreira PI, Custodio J, Moreno A, Oliveira CR, Santos MS. Tamoxifen and estradiol interact with the flavin mononucleotide site of complex I leading to mitochondrial failure. J Biol Chem. 2006;281:10143–10152. doi: 10.1074/jbc.M510249200. [DOI] [PubMed] [Google Scholar]
- 33.Siskind LJ, Kolesnick RN, Colombini M. Ceramide channels increase the permeability of the mitochondrial outer membrane to small proteins. J Biol Chem. 2002;277:26796–26803. doi: 10.1074/jbc.M200754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clemo HF, Stambler BS, Baumgarten CM. Swelling-activated chloride current is persistently activated in ventricular myocytes from dogs with tachycardia-induced congestive heart failure. Circ Res. 1999;84:157–165. doi: 10.1161/01.res.84.2.157. [DOI] [PubMed] [Google Scholar]
- 35.Bai Y, Wang J, Shan H, Lu Y, Zhang Y, Luo X, et al. Sphingolipid metabolite ceramide causes metabolic perturbation contributing to HERG K+ channel dysfunction. Cell Physiol Biochem. 2007;20:429–440. doi: 10.1159/000107527. [DOI] [PubMed] [Google Scholar]
- 36.Chapman H, Ramstrom C, Korhonen L, Laine M, Wann KT, Lindholm D, et al. Downregulation of the HERG (KCNH2) K+ channel by ceramide: evidence for ubiquitin-mediated lysosomal degradation. J Cell Sci. 2005;118:5325–5334. doi: 10.1242/jcs.02635. [DOI] [PubMed] [Google Scholar]
- 37.Ito Y, Sato S, Ohashi T, Nakayama S, Shimokata K, Kume H. Reduction of airway anion secretion via CFTR in sphingomyelin pathway. Biochem Biophys Res Commun. 2004;324:901–908. doi: 10.1016/j.bbrc.2004.09.134. [DOI] [PubMed] [Google Scholar]
- 38.Milescu M, Vobecky J, Roh SH, Kim SH, Jung HJ, Kim JI, et al. Tarantula toxins interact with voltage sensors within lipid membranes. J Gen Physiol. 2007;130:497–511. doi: 10.1085/jgp.200709869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramu Y, Xu Y, Lu Z. Enzymatic activation of voltage-gated potassium channels. Nature. 2006;442:696–699. doi: 10.1038/nature04880. [DOI] [PubMed] [Google Scholar]
- 40.Ramu Y, Xu Y, Lu Z. Inhibition of CFTR Cl− channel function caused by enzymatic hydrolysis of sphingomyelin. Proc Natl Acad Sci USA. 2007;104:6448–6453. doi: 10.1073/pnas.0701354104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Browe DM, Baumgarten CM. ACh activates volume-sensitive Cl current via mitochondrial complex III reactive oxygen species in rabbit ventricular myocytes. (Abstract) Biophys J. 2007;92(Suppl. 1):460a. [Google Scholar]
- 42.Yi F, Zhang AY, Janscha JL, Li PL, Zou AP. Homocysteine activates NADH/NADPH oxidase through ceramide-stimulated Rac GTPase activity in rat mesangial cells. Kidney Int. 2004;66:1977–1987. doi: 10.1111/j.1523-1755.2004.00968.x. [DOI] [PubMed] [Google Scholar]
- 43.Takeda S, Mitsutake S, Tsuji K, Igarashi Y. Apoptosis occurs via the ceramide recycling pathway in human HaCaT keratinocytes. J Biochem. 2006;139:255–262. doi: 10.1093/jb/mvj026. [DOI] [PubMed] [Google Scholar]
- 44.Bielawska AE, Shapiro JP, Jiang L, Melkonyan HS, Piot C, Wolfe CL, et al. Ceramide is involved in triggering of cardiomyocyte apoptosis induced by ischemia and reperfusion. Am J Pathol. 1997;151:1257–1263. [PMC free article] [PubMed] [Google Scholar]
- 45.Gulbins E, Li PL. Physiological and pathophysiological aspects of ceramide. Am J Physiol Regul Integr Comp Physiol. 2006;290:R11–R26. doi: 10.1152/ajpregu.00416.2005. [DOI] [PubMed] [Google Scholar]
- 46.Doehner W, Bunck AC, Rauchhaus M, von Haehling S, Brunkhorst FM, Cicoira M, et al. Secretory sphingomyelinase is upregulated in chronic heart failure: a second messenger system of immune activation relates to body composition, muscular functional capacity, and peripheral blood flow. Eur Heart J. 2007;28:821–828. doi: 10.1093/eurheartj/ehl541. [DOI] [PubMed] [Google Scholar]