Abstract
There has been growing interest in targeting myocardial substrate metabolism for the therapy of cardiovascular and metabolism diseases. This is largely based on the observation that cardiac metabolism undergoes significant changes during both physiological and pathological stresses. In search for an effective therapeutic strategy, recent studies have focused on the functional significance of the substrate switch in the heart during stress conditions, such as cardiac hypertrophy and failure, using both pharmacological and genetic approaches. The results of these studies indicate that both the capacity and the flexibility of the cardiac metabolic network are essential for normal function; thus, their maintenance should be the primary goal for future metabolic therapy.
Introduction
The heart requires a continually high level of energy supply to maintain its mechanical function throughout life. The amount of ATP generated and consumed by a human heart daily is over 15 times its own weight (Ingwall 2002) and is primarily generated through complex metabolic pathways that supply carbon substrates to the mitochondria for oxidative phosphorylation (Figure 1). Mitochondria occupy ~30% of the volume of a cardiac myocyte, ensuring the great oxidative capacity of the system. To meet the high energetic demand, the cardiac metabolic network has developed into an extremely versatile system, capable of metabolizing all carbon substrates, i.e. lipids, carbohydrates, and amino acids, for energy production.
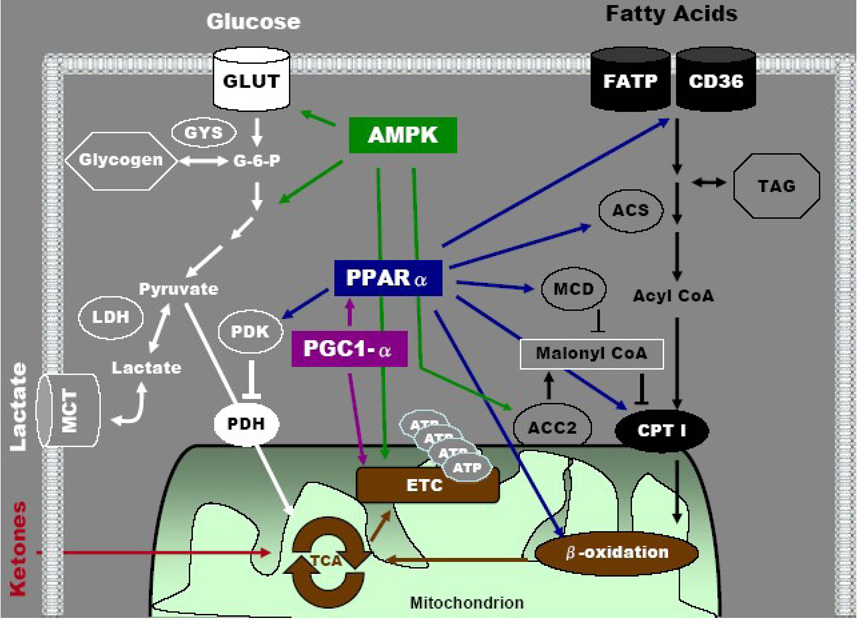
Figure 1.
Cardiac metabolic network for substrate utilization. Highlighted in solid colors are key regulatory sites for carbohydrates and lipids metabolism. The molecular targets of several transcriptional and/or signaling pathways in the regulation of substrate selection are also illustrated. ACC2: acetyl CoA carboxylase 2; ACS: acyl-coA synthetase; AMPK: adenosine monophosphate-activated protein kinase; ATP: adenosine triphosphate; CD36: cluster of differentiation 36 (fatty acid transporter); CPT1: carnitine palmitoyl transferase I; ETC: electron transport chain; FATP: fatty acid transport protein; G-6-P: glucose-6-phosphate; GLUT: glucose transporter; GYS: glycogen synthase; LDH: lactate dehydrogenase; MCD: malonyl CoA decarboxylase; MCT: monocarboxylate transporter; PDH: pyruvate dehydrogenase; PDK: pyruvate dehydrogenase kinase; PGC1-α: peroxisome proliferator-activated receptor-gamma coactivator 1 alpha; PPARα: peroxisome proliferator-activated receptor alpha; TAG: triacylglycerol; TCA: tricarboxylic acid cycle.
An important feature of cardiac metabolism is that it is highly adaptable throughout the life cycle as well as under physiological or pathological stressors. In utero, the fetal heart relies on carbohydrate substrates for ATP generation (Fisher 1984). As the heart matures, in parallel to the increase of mitochondrial volume and higher circulating fatty acids levels, fatty acids become the dominant energy substrate (Lopaschuk et al. 1994). During conditions of fasting or diabetes, the adult heart can become even more dependant on fatty acids (Belke et al. 2000, Mazumder et al. 2004). This is in contrast to the hypoxic or failing heart, where the relative use of carbohydrate, especially glucose, is increased (Allard et al. 1997, Barger et al. 1999, Tian 2003). Although much attention has focused on the use of glucose and fatty acids in cardiac metabolism, the heart is also capable of utilizing ketones, lactate, and endogenous substrates, i.e. glycogen and triglyceride, as fuel (Figure 1). These observations underscore the flexibility of cardiac metabolism in response to a variety of environmental changes. Studies in the last decade have revealed a number of mechanisms that remodel the metabolic pathways at the molecular level to allow such adaptations, e.g. PPARs, AMPK and PGC-1 (Figure 1). As our understanding of the molecular mechanisms regulating metabolic flexibility advances, it becomes increasingly attractive to consider metabolic modulations as means to maintain or improve cardiac function under pathological conditions.
This review will focus on recent advances in the understanding of the functional significance of alterations in the myocardial substrate utilization that accompany cardiac hypertrophy/heart failure and obesity or diabetes. In addition, metabolic manipulations that have been attempted for these conditions in animal models and patients will be discussed.
The Fetal Metabolic Profile in Cardiac Hypertrophy and Failure
It has been widely recognized that pathological hypertrophy is associated with the reappearance of fetal gene expression pattern (Buttrick et al. 1994). The metabolic profile of the hypertrophied heart also reverts to the fetal pattern, showing decreased fatty acid oxidation and increased reliance on carbohydrate fuel sources (Barger et al. 1999, Razeghi et al. 2001). The switch in the metabolic profile in animal models of heart failure is associated with downregulation of PPARα and upregulation of enzymes involved in glucose utilization (Tian 2003). As pathological hypertrophy progresses to heart failure, the shift of substrate preference to glucose is closely associated with impairment of myocardial energetics and loss of contractile reserve (Neubauer 2007). These observations have raised the question whether the metabolic switch towards glucose is maladaptive for cardiac hypertrophy and failure.
To determine a causal relationship between altered cardiac metabolism and the development of heart failure, genetically altered mouse models have been used to recapitulate or manipulate the metabolic phenotype in cardiac hypertrophy and failure (Table 1). Due to the space restraint, the discussion will be limited to a few models. We utilized transgenic mice expressing the insulin-independent glucose transporter (GLUT1) in the heart that led to increased glucose uptake, glycolysis, and glucose oxidation, with decreases in fatty acid oxidation in the heart (Liao et al. 2002, Luptak et al. 2005). Despite the fetal-like cardiac metabolic profile, the GLUT1 mice lived a normal life span with unaltered cardiac function, and when subjected to pressure overload by ascending aortic constriction, were protected against contractile dysfunction and LV dilation (Liao et al. 2002, Luptak et al. 2007). These studies demonstrate that increased reliance on glucose per se is not detrimental to the heart.
Table 1.
Mouse models of altered cardiac metabolism. LVH: left ventricular hypertrophy; HF: heart failure; GOX: glucose oxidation; FAO: fatty acid oxidation; GLUT1: glucose transporter 1; PPARα: peroxisome proliferator-activated receptor alpha; −/−: knockout; PGC1-α: peroxisome proliferator-activated receptor-gamma coactivator 1 alpha; PDK4: pyruvate dehydrogenase kinase 4; CIRKO: cardiomyocyte-selective insulin receptor knockout; MCD: malonyl CoA decarboxylase; GLUT4: glucose transporter 4; GLUT4H: cardiac-specific glucose transporter 4; M/MtCK: muscle and mitochondrial isoforms of creatine kinase. CD36: cluster of differentiation 36 (fatty acid transporter); FATP1: fatty acid transport protein 1.
| Mouse Model | Metabolic Profile | Cardiac Phenotype | References |
|---|---|---|---|
|
LVH/HF in Wild-type |
Switch to fetal metabolic profile with ↑ GOX and glycolysis and ↓ FAO |
Decreased function, energetics, and switch to fetal metabolic phenotype |
(Allard et al. 1997, Barger et al. 1999, Tian 2003) |
|
GLUT1 overexpression |
↑ GOX and glycolysis with ↓ FAO | Improved function, energetics, and survival | (Liao et al. 2002, Luptak et al. 2005) |
|
PPARα overexpression |
↑ FAO at the expense of glucose | Development of cardiomyopathy | (Finck et al. 2002) |
| PPARα null | ↓ FAO with ↑ GOX and lactate oxidation | Impaired function and energetics with increased workload, age-associated development of cardiac fibrosis |
(Watanabe et al. 2000, Campbell et al. 2002, Luptak et al. 2005, Loichot et al. 2006) |
| PGC1α −/− | ↓ FAO | Reduced function at baseline and impaired with physiological challenge; accelerated heart failure |
(Arany et al. 2005, Arany et al. 2006) |
|
PGC1α overexpression |
↑ in mitochondria number with abnormal ultrastructure |
Development of cardiomyopathy | (Lehman et al. 2000, Russell et al. 2004) |
|
PDK4 overexpression |
↑ in FAO and ↓ GOX | Exacerbation of heart failure in cardiomyopathy model; decreased survival |
(Zhao et al. 2008) |
| PDK4 −/− | No change in GOX or glycolysis | Preserved cardiac function and decreased fibrosis | (Wende 2009) |
| CIRKO | ↑ GOX and glycolysis with ↓ FAO | Reduced heart mass with lower cardiac function | (McQueen et al. 2005, Sena et al. 2009) |
| MCD −/− | No change in metabolism at baseline; ↑ GOX during reperfusion |
Normal cardiac function at baseline; improved function after ischemia |
(Dyck et al. 2006) |
| GLUT4-null | Normal glucose uptake with ↓ fatty acid metabolic genes |
Marked cardiac hypertrophy and fibrosis; reduced longevity |
(Katz et al. 1995, Stenbit et al. 2000) |
| GLUT4H −/− | ↑ basal glucose uptake and ↓ insulin- stimulated glucose uptake |
Modest cardiac hypertrophy with preserved function, poor response to ischemia/reperfusion |
(Abel et al. 1999, Tian et al. 2001) |
| M/MtCK −/− | ↓CK activity and ↓phosphocreatine levels | Impaired energetics with ↑ workload; development of hypertrophy and LV dilation |
(Saupe et al. 1998, Nahrendorf et al. 2005) |
| CD36 −/− | ↑ GOX and ↓ FAO | ↓ lipid accumulation, Improves lipotoxic cardiomyopathy; No change or decreased function after ischemia |
(Irie et al. 2003, Kuang et al. 2004, Koonen et al. 2007, Yang et al. 2007) |
However, as discussed previously, increased glucose utilization has been shown to be associated with impaired myocardial energetics in the hypertrophied and failing heart. Similarly, PPARα-null hearts, which had permissive increases in glucose oxidation as a result of impaired fatty acid oxidation, also failed to maintain myocardial energetics and function during a high workload challenge (Luptak et al. 2005). In addition, the PPAR-null mice develop cardiomyopathy at an old age (Watanabe et al. 2000). Interestingly, the loss of energetic and contractile reserves in the PPAR-null heart could be rescued by overexpressing GLUT1, which markedly expanded the capacity for glucose uptake and utilization (Luptak et al. 2005). These findings suggest that the inherent capacity for glucose utilization in an adult heart, when fatty acid oxidation is severely impaired, is insufficient for sustaining normal energy supply under stress. Overexpression of GLUT1 under these conditions expanded the capacity and provided the optimal substrate in the face of impaired ability to oxidize fatty acids. There has been significant amount of evidence suggesting that the failing heart is insulin resistant (Witteles et al. 2004, Ashrafian et al. 2007). Since the capacity for glucose uptake and utilization in an adult heart is highly insulin-dependent, impaired insulin signaling in combination with decreased fatty acid oxidation, can result in severe limitations of substrate oxidation in heart failure. The benefit of overexpressing GLUT1, an insulin-independent glucose transporter, may be partially attributable to the relief of limitations in substrate supply associated with insulin resistance. Thus, for failing hearts, an adaptive metabolic profile must be able to fully support the energetic demand among other metabolic considerations.
Pharmacological Approaches to Optimize Cardiac Metabolism in Heart Failure
Along the line of promoting glucose utilization of the heart, an established protein target is the muscle form of the carnitine-palmitoyl transferase-I (CPT-1, Figure 1), the enzyme responsible for the uptake of long-chain fatty acids into the mitochondria. Several CPT-1 inhibitors, oxfenicine, etomoxir, and perhexiline, have been shown to partially reduce fatty acid oxidation and promote glucose oxidation of the heart. In rodent and large animal models of heart failure, these compounds delayed the onset of decompensated failure while preventing the transcriptional down-regulation of key enzymes in cardiac energy metabolism (Lionetti et al. 2005) and improving the rate of sarcoplasmic reticulum calcium uptake (Rupp et al. 2000). Furthermore, a recent study showed that short-term treatment with perhexiline, in addition to standard medication, improved cardiac function and peak exercise oxygen consumption in chronic heart failure patients. Other partial fatty acid oxidation inhibitors, e.g. trimetazidine, also showed benefit in a small study of elderly heart failure patients with coronary heart disease (Vitale et al. 2004). One potential mechanism for the benefit of replacing fatty acid oxidation with glucose is the higher oxygen efficiency during ATP synthesis. Theoretically, glucose oxidation requires 11–13% less oxygen than fatty acid oxidation for ATP synthesis (Opie 2004). However, acute depletion of free fatty acid supply to the heart resulted in ~25% oxygen sparing in normal mouse and human hearts (How et al. 2005, Tuunanen et al. 2006), consistent with the notion that high levels of fatty acids may stimulate mitochondrial uncoupling proteins resulting in a decline in oxygen efficiency beyond what is expected from the P:O ratio (Boudina et al. 2007).
A different class of molecules, such as glucagon-like peptide (GLP), promotes myocardial glucose utilization via stimulation of insulin secretion and its insulin-mimetic effects. GLP stimulates insulin signaling, enhances myocardial glucose uptake and reduces circulating fatty acid levels, and hence promotes glucose utilization via a distinct mechanism from the partial inhibition of fatty acid oxidation (D'Alessio et al. 1994). Both animal experiments and clinical studies using GLP for short-term treatment have demonstrated improvement of cardiac function in heart failure (Nikolaidis et al. 2004, Sokos et al. 2006, Poornima et al. 2008). Taken together, pharmacological treatments that enhance cardiac glucose utilization appear to protect against the progression of heart failure, although the molecular mechanisms remain to be fully defined. An outstanding challenge is to determine whether such metabolic modulations alter the long-term clinical outcome, i.e. survival rate in heart failure patients.
The Fatty Acid Paradox
Despite the overwhelming evidence suggesting the benefit of increasing glucose utilization in the failing heart, a recent clinical study showed that fatty acid oxidation remains essential for cardiac function. Acipimox, a nicotinic acid derivative with profound anti-lipolytic effects, was used to acutely lower serum fatty acid levels and hence the rate of fatty acid uptake in the heart. In patients with dilated cardiomyopathy acipimox promoted glucose oxidation but caused significant falls of cardiac work and efficiency (Tuunanen et al. 2006). In a mouse model deficient of lipoprotein-lipase in the heart, cardiac dysfunction was observed despite the upregulation of myocardial glucose utilization (Augustus et al. 2006), suggesting a critical role of lipase-derived fatty acids in cardiac metabolism. In addition, it has been shown that endogenous triglyceride metabolism in the failing heart is impaired (O'Donnell et al. 2008), indicating that the turnover of the triglyceride pool may also represent a novel target for metabolic intervention.
Given the predominance of fatty acid oxidation in cardiac energy supply, normalization of fatty acid oxidation in the failing heart seems to be a logical strategy. However, increasing fatty acid oxidation via pharmacological intervention in heart failure has yielded conflicting results. Chronic activation of peroxisome proliferator-activated receptor-alpha (PPARα) with fenofibrate in rats post MI or in dogs with pacing-induced heart failure maintained the fatty acid oxidation gene profile but had modest benefits on the development of heart failure (Morgan et al. 2006, Labinskyy et al. 2007). Conversely, Young, et al. (2001) demonstrated that although PPARα agonist treatment after aortic banding prevented down-regulation of fatty acid oxidation genes, it failed to correct cardiac dysfunction. Furthermore, PPARα agonism has been shown to worsen post-ischemic injury (Sambandam et al. 2006, Hafstad et al. 2009). Recently, an interesting series of studies suggested that high fat diet protected against the development of heart failure in a variety of animal models (Okere et al. 2006, Chess et al. 2009, Rennison et al. 2009). The mechanisms underlying the benefits of high fat diet are unknown, but the observation again challenges the concept that fatty acids are detrimental to the failing heart.
Substrate Preference Switch versus Maintaining Metabolic Capacity and Flexibility
Concerns of excessive fatty acid oxidation were raised in hearts with ischemia-reperfusion injury and in cardiac dysfunction observed in animals or patients with obesity and diabetes (Kudo et al. 1995, Aasum et al. 2003, Buchanan et al. 2005). Under both conditions, the inefficiency of cardiac work and impaired contractile function associated with high fatty acid oxidation can be corrected by promoting glucose oxidation (Bersin et al. 1997, Hafstad et al. 2007). The notion that increased glucose metabolism is protective against the ischemia-reperfusion injury is further supported by the observations that overexpressing GLUT1 protects the ischemic heart, whereas deletion of insulin-sensitive glucose transporter (GLUT4) is detrimental (Tian et al. 2001, Luptak et al. 2007).
The results of targeting substrate preference in diabetic hearts are quite mixed. Treatment with PPARα or PPARγ activators in animal models of diabetes reduced fatty acid oxidation and increased glycolysis and glucose oxidation, but yielded inconsistent outcomes with regard to functional improvement (Aasum et al. 2002, Carley et al. 2004, How et al. 2007). An important consideration here is that the commonly used type 2 diabetes rodent models (also used in these studies) have defects in leptin signaling, which causes cardiomyopathy independent of substrate metabolism (Barouch et al. 2003). Nevertheless, in type 1 diabetes model treatments with ACE inhibitors or β-blockers increased cardiac glucose utilization, decreased fatty acid oxidation, and improved cardiac function, although the causal relationship could not be defined in these studies (Arikawa et al. 2007, Sharma et al. 2008).
In a recent study (Yan et al. 2009), the cardiac-specific GLUT1 transgenic mouse, which had demonstrated increased myocardial glucose uptake and oxidation, was fed a high fat diet (HFD). The wild-type animals on HFD demonstrated the expected increase in cardiac fatty acid oxidation, while the GLUT1 transgenic hearts maintained high glucose oxidation despite comparable levels of obesity and insulin resistance in both genotypes. The resistance to increased fatty acid oxidation in the GLUT1 transgenic heart was attributed to a number of glucose-dependent changes in the gene expression that restrict fatty acid oxidation and promote glucose oxidation. Surprisingly, the protection against the high fatty acid oxidation was associated with elevated oxidative stress and cardiac dysfunction in GLUT1 transgenic mice with diet-induced obesity. This was unexpected, given the mounting evidence suggesting that a switch of substrate utilization towards glucose would be beneficial. An important point raised by the study is that the molecular remodeling caused by excessive reliance on one substrate (glucose in the case of GLUT1 mice and fatty acids in the case of obesity and diabetes) compromises the flexibility of the metabolic network and prevents the heart from utilizing the most efficient substrate.
In conclusion, the genetic and pharmacological studies show that the optimal cardiac function depends on the ability of the heart to utilize all carbon substrates. Thus, the ultimate goal of modulating cardiac metabolism for therapeutic purposes is not to shift the substrate utilization towards one end of the spectrum or the other but rather to sustain the flexibility of the network. Metabolic therapy in the future should include treatments that improve insulin sensitivity and sustain mitochondrial function hence satisfying the enormous energy requirement of the heart.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aasum E, Belke DD, Severson DL, et al. Cardiac function and metabolism in Type 2 diabetic mice after treatment with BM 17.0744, a novel PPAR-alpha activator. Am J Physiol Heart Circ Physiol. 2002;283:H949–H957. doi: 10.1152/ajpheart.00226.2001. [DOI] [PubMed] [Google Scholar]
- Aasum E, Hafstad AD, Severson DL, Larsen TS. Age-dependent changes in metabolism, contractile function, and ischemic sensitivity in hearts from db/db mice. Diabetes. 2003;52:434–441. doi: 10.2337/diabetes.52.2.434. [DOI] [PubMed] [Google Scholar]
- Abel ED, Kaulbach HC, Tian R, et al. Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. J Clin Invest. 1999;104:1703–1714. doi: 10.1172/JCI7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard MF, Henning SL, Wambolt RB, et al. Glycogen metabolism in the aerobic hypertrophied rat heart. Circulation. 1997;96:676–682. doi: 10.1161/01.cir.96.2.676. [DOI] [PubMed] [Google Scholar]
- Arany Z, He H, Lin J, et al. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab. 2005;1:259–271. doi: 10.1016/j.cmet.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Arany Z, Novikov M, Chin S, et al. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-gamma coactivator 1alpha. Proc Natl Acad Sci U S A. 2006;103:10086–10091. doi: 10.1073/pnas.0603615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arikawa E, Ma RC, Isshiki K, et al. Effects of insulin replacements, inhibitors of angiotensin, and PKCbeta's actions to normalize cardiac gene expression and fuel metabolism in diabetic rats. Diabetes. 2007;56:1410–1420. doi: 10.2337/db06-0655. [DOI] [PubMed] [Google Scholar]
- Ashrafian H, Frenneaux MP, Opie LH. Metabolic mechanisms in heart failure. Circulation. 2007;116:434–448. doi: 10.1161/CIRCULATIONAHA.107.702795. [DOI] [PubMed] [Google Scholar]
- Augustus AS, Buchanan J, Park TS, et al. Loss of lipoprotein lipase-derived fatty acids leads to increased cardiac glucose metabolism and heart dysfunction. J Biol Chem. 2006;281:8716–8723. doi: 10.1074/jbc.M509890200. [DOI] [PubMed] [Google Scholar]
- Barger PM, Kelly DP. Fatty acid utilization in the hypertrophied and failing heart: molecular regulatory mechanisms. Am J Med Sci. 1999;318:36–42. doi: 10.1097/00000441-199907000-00006. [DOI] [PubMed] [Google Scholar]
- Barouch LA, Berkowitz DE, Harrison RW, et al. Disruption of leptin signaling contributes to cardiac hypertrophy independently of body weight in mice. Circulation. 2003;108:754–759. doi: 10.1161/01.CIR.0000083716.82622.FD. [DOI] [PubMed] [Google Scholar]
- Belke DD, Larsen TS, Gibbs EM, Severson DL. Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am J Physiol Endocrinol Metab. 2000;279:E1104–E1113. doi: 10.1152/ajpendo.2000.279.5.E1104. [DOI] [PubMed] [Google Scholar]
- Bersin RM, Stacpoole PW. Dichloroacetate as metabolic therapy for myocardial ischemia and failure. Am Heart J. 1997;134:841–855. doi: 10.1016/s0002-8703(97)80007-5. [DOI] [PubMed] [Google Scholar]
- Boudina S, Sena S, Theobald H, et al. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007;56:2457–2466. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- Buchanan J, Mazumder PK, Hu P, et al. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology. 2005;146:5341–5349. doi: 10.1210/en.2005-0938. [DOI] [PubMed] [Google Scholar]
- Buttrick PM, Kaplan M, Leinwand LA, Scheuer J. Alterations in gene expression in the rat heart after chronic pathological and physiological loads. J Mol Cell Cardiol. 1994;26:61–67. doi: 10.1006/jmcc.1994.1008. [DOI] [PubMed] [Google Scholar]
- Campbell FM, Kozak R, Wagner A, et al. A role for peroxisome proliferator-activated receptor alpha (PPARalpha ) in the control of cardiac malonyl-CoA levels: reduced fatty acid oxidation rates and increased glucose oxidation rates in the hearts of mice lacking PPARalpha are associated with higher concentrations of malonyl-CoA and reduced expression of malonyl-CoA decarboxylase. J Biol Chem. 2002;277:4098–4103. doi: 10.1074/jbc.M106054200. [DOI] [PubMed] [Google Scholar]
- Carley AN, Semeniuk LM, Shimoni Y, et al. Treatment of type 2 diabetic db/db mice with a novel PPARgamma agonist improves cardiac metabolism but not contractile function. Am J Physiol Endocrinol Metab. 2004;286:E449–E455. doi: 10.1152/ajpendo.00329.2003. [DOI] [PubMed] [Google Scholar]
- Chess DJ, Khairallah RJ, O'Shea KM, et al. A High Fat Diet Increases Adiposity but Maintains Mitochondrial Oxidative Enzymes without Affecting Development of Heart Failure with Pressure Overload. Am J Physiol Heart Circ Physiol. 2009;297:H1585–H1593. doi: 10.1152/ajpheart.00599.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alessio DA, Kahn SE, Leusner CR, Ensinck JW. Glucagon-like peptide 1 enhances glucose tolerance both by stimulation of insulin release and by increasing insulin-independent glucose disposal. J Clin Invest. 1994;93:2263–2266. doi: 10.1172/JCI117225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck JR, Hopkins TA, Bonnet S, et al. Absence of malonyl coenzyme A decarboxylase in mice increases cardiac glucose oxidation and protects the heart from ischemic injury. Circulation. 2006;114:1721–1728. doi: 10.1161/CIRCULATIONAHA.106.642009. [DOI] [PubMed] [Google Scholar]
- Finck BN, Lehman JJ, Leone TC, et al. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109:121–130. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DJ. Oxygenation and metabolism in the developing heart. Semin Perinatol. 1984;8:217–225. [PubMed] [Google Scholar]
- Hafstad AD, Khalid AM, Hagve M, et al. Cardiac peroxisome proliferator-activated receptor-alpha activation causes increased fatty acid oxidation, reducing efficiency and post-ischaemic functional loss. Cardiovasc Res. 2009;83:519–526. doi: 10.1093/cvr/cvp132. [DOI] [PubMed] [Google Scholar]
- Hafstad AD, Khalid AM, How OJ, et al. Glucose and insulin improve cardiac efficiency and postischemic functional recovery in perfused hearts from type 2 diabetic (db/db) mice. Am J Physiol Endocrinol Metab. 2007;292:E1288–E1294. doi: 10.1152/ajpendo.00504.2006. [DOI] [PubMed] [Google Scholar]
- How OJ, Aasum E, Kunnathu S, et al. Influence of substrate supply on cardiac efficiency, as measured by pressure-volume analysis in ex vivo mouse hearts. Am J Physiol Heart Circ Physiol. 2005;288:H2979–H2985. doi: 10.1152/ajpheart.00084.2005. [DOI] [PubMed] [Google Scholar]
- How OJ, Larsen TS, Hafstad AD, et al. Rosiglitazone treatment improves cardiac efficiency in hearts from diabetic mice. Arch Physiol Biochem. 2007;113:211–220. doi: 10.1080/13813450701783281. [DOI] [PubMed] [Google Scholar]
- Ingwall JS. ATP and the heart. Boston: Kluwer Academic.; 2002. [Google Scholar]
- Irie H, Krukenkamp IB, Brinkmann JF, et al. Myocardial recovery from ischemia is impaired in CD36-null mice and restored by myocyte CD36 expression or medium-chain fatty acids. Proc Natl Acad Sci U S A. 2003;100:6819–6824. doi: 10.1073/pnas.1132094100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz EB, Stenbit AE, Hatton K, et al. Cardiac and adipose tissue abnormalities but not diabetes in mice deficient in GLUT4. Nature. 1995;377:151–155. doi: 10.1038/377151a0. [DOI] [PubMed] [Google Scholar]
- Koonen DP, Febbraio M, Bonnet S, et al. CD36 expression contributes to age-induced cardiomyopathy in mice. Circulation. 2007;116:2139–2147. doi: 10.1161/CIRCULATIONAHA.107.712901. [DOI] [PubMed] [Google Scholar]
- Kuang M, Febbraio M, Wagg C, et al. Fatty acid translocase/CD36 deficiency does not energetically or functionally compromise hearts before or after ischemia. Circulation. 2004;109:1550–1557. doi: 10.1161/01.CIR.0000121730.41801.12. [DOI] [PubMed] [Google Scholar]
- Kudo N, Barr AJ, Barr RL, et al. High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5'-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J Biol Chem. 1995;270:17513–17520. doi: 10.1074/jbc.270.29.17513. [DOI] [PubMed] [Google Scholar]
- Labinskyy V, Bellomo M, Chandler MP, et al. Chronic activation of peroxisome proliferator-activated receptor-alpha with fenofibrate prevents alterations in cardiac metabolic phenotype without changing the onset of decompensation in pacing-induced heart failure. J Pharmacol Exp Ther. 2007;321:165–171. doi: 10.1124/jpet.106.116871. [DOI] [PubMed] [Google Scholar]
- Lehman JJ, Barger PM, Kovacs A, et al. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000;106:847–856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao R, Jain M, Cui L, et al. Cardiac-specific overexpression of GLUT1 prevents the development of heart failure attributable to pressure overload in mice. Circulation. 2002;106:2125–2131. doi: 10.1161/01.cir.0000034049.61181.f3. [DOI] [PubMed] [Google Scholar]
- Lionetti V, Linke A, Chandler MP, et al. Carnitine palmitoyl transferase-I inhibition prevents ventricular remodeling and delays decompensation in pacing-induced heart failure. Cardiovasc Res. 2005;66:454–461. doi: 10.1016/j.cardiores.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Loichot C, Jesel L, Tesse A, et al. Deletion of peroxisome proliferator-activated receptor-alpha induces an alteration of cardiac functions. Am J Physiol Heart Circ Physiol. 2006;291:H161–H166. doi: 10.1152/ajpheart.01065.2004. [DOI] [PubMed] [Google Scholar]
- Lopaschuk GD, Belke DD, Gamble J, et al. Regulation of fatty acid oxidation in the mammalian heart in health and disease. Biochim Biophys Acta. 1994;1213:263–276. doi: 10.1016/0005-2760(94)00082-4. [DOI] [PubMed] [Google Scholar]
- Luptak I, Balschi JA, Xing Y, et al. Decreased contractile and metabolic reserve in peroxisome proliferator-activated receptor-alpha-null hearts can be rescued by increasing glucose transport and utilization. Circulation. 2005;112:2339–2346. doi: 10.1161/CIRCULATIONAHA.105.534594. [DOI] [PubMed] [Google Scholar]
- Luptak I, Shen M, He H, et al. Aberrant activation of AMP-activated protein kinase remodels metabolic network in favor of cardiac glycogen storage. J Clin Invest. 2007;117:1432–1439. doi: 10.1172/JCI30658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder PK, O'Neill BT, Roberts MW, et al. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes. 2004;53:2366–2374. doi: 10.2337/diabetes.53.9.2366. [DOI] [PubMed] [Google Scholar]
- McQueen AP, Zhang D, Hu P, et al. Contractile dysfunction in hypertrophied hearts with deficient insulin receptor signaling: possible role of reduced capillary density. J Mol Cell Cardiol. 2005;39:882–892. doi: 10.1016/j.yjmcc.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Morgan EE, Rennison JH, Young ME, et al. Effects of chronic activation of peroxisome proliferator-activated receptor-alpha or high-fat feeding in a rat infarct model of heart failure. Am J Physiol Heart Circ Physiol. 2006;290:H1899–H1904. doi: 10.1152/ajpheart.01014.2005. [DOI] [PubMed] [Google Scholar]
- Nahrendorf M, Spindler M, Hu K, et al. Creatine kinase knockout mice show left ventricular hypertrophy and dilatation, but unaltered remodeling post-myocardial infarction. Cardiovasc Res. 2005;65:419–427. doi: 10.1016/j.cardiores.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Neubauer S. The failing heart--an engine out of fuel. N Engl J Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- Nikolaidis LA, Mankad S, Sokos GG, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–965. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- O'Donnell JM, Fields AD, Sorokina N, Lewandowski ED. The absence of endogenous lipid oxidation in early stage heart failure exposes limits in lipid storage and turnover. J Mol Cell Cardiol. 2008;44:315–322. doi: 10.1016/j.yjmcc.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okere IC, Young ME, McElfresh TA, et al. Low carbohydrate/high-fat diet attenuates cardiac hypertrophy, remodeling, and altered gene expression in hypertension. Hypertension. 2006;48:1116–1123. doi: 10.1161/01.HYP.0000248430.26229.0f. [DOI] [PubMed] [Google Scholar]
- Opie LH. Heart physiology : from cell to circulation. Philadelphia: Lippincott Williams & Wilkins; 2004. [Google Scholar]
- Poornima I, Brown SB, Bhashyam S, et al. Chronic Glucagon-like Peptide-1 (GLP-1) Infusion Sustains LV Systolic Function and Prolongs Survival in the Spontaneously Hypertensive Heart Failure Prone Rat. Circ Heart Fail. 2008;1:153–160. doi: 10.1161/CIRCHEARTFAILURE.108.766402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razeghi P, Young ME, Alcorn JL, et al. Metabolic gene expression in fetal and failing human heart. Circulation. 2001;104:2923–2931. doi: 10.1161/hc4901.100526. [DOI] [PubMed] [Google Scholar]
- Rennison JH, McElfresh TA, Chen X, et al. Prolonged exposure to high dietary lipids is not associated with lipotoxicity in heart failure. J Mol Cell Cardiol. 2009;46:883–890. doi: 10.1016/j.yjmcc.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp H, Vetter R. Sarcoplasmic reticulum function and carnitine palmitoyltransferase-1 inhibition during progression of heart failure. Br J Pharmacol. 2000;131:1748–1756. doi: 10.1038/sj.bjp.0703741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell LK, Mansfield CM, Lehman JJ, et al. Cardiac-specific induction of the transcriptional coactivator peroxisome proliferator-activated receptor gamma coactivator-1alpha promotes mitochondrial biogenesis and reversible cardiomyopathy in a developmental stage-dependent manner. Circ Res. 2004;94:525–533. doi: 10.1161/01.RES.0000117088.36577.EB. [DOI] [PubMed] [Google Scholar]
- Sambandam N, Morabito D, Wagg C, et al. Chronic activation of PPARalpha is detrimental to cardiac recovery after ischemia. Am J Physiol Heart Circ Physiol. 2006;290:H87–H95. doi: 10.1152/ajpheart.00285.2005. [DOI] [PubMed] [Google Scholar]
- Saupe KW, Spindler M, Tian R, Ingwall JS. Impaired cardiac energetics in mice lacking muscle-specific isoenzymes of creatine kinase. Circ Res. 1998;82:898–907. doi: 10.1161/01.res.82.8.898. [DOI] [PubMed] [Google Scholar]
- Sena S, Hu P, Zhang D, et al. Impaired insulin signaling accelerates cardiac mitochondrial dysfunction after myocardial infarction. J Mol Cell Cardiol. 2009;46:910–918. doi: 10.1016/j.yjmcc.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V, Dhillon P, Wambolt R, et al. Metoprolol improves cardiac function and modulates cardiac metabolism in the streptozotocin-diabetic rat. Am J Physiol Heart Circ Physiol. 2008;294:H1609–H1620. doi: 10.1152/ajpheart.00949.2007. [DOI] [PubMed] [Google Scholar]
- Sokos GG, Nikolaidis LA, Mankad S, et al. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail. 2006;12:694–699. doi: 10.1016/j.cardfail.2006.08.211. [DOI] [PubMed] [Google Scholar]
- Stenbit AE, Katz EB, Chatham JC, et al. Preservation of glucose metabolism in hypertrophic GLUT4-null hearts. Am J Physiol Heart Circ Physiol. 2000;279:H313–H318. doi: 10.1152/ajpheart.2000.279.1.H313. [DOI] [PubMed] [Google Scholar]
- Tian R. Transcriptional regulation of energy substrate metabolism in normal and hypertrophied heart. Curr Hypertens Rep. 2003;5:454–458. doi: 10.1007/s11906-003-0052-7. [DOI] [PubMed] [Google Scholar]
- Tian R, Abel ED. Responses of GLUT4-deficient hearts to ischemia underscore the importance of glycolysis. Circulation. 2001;103:2961–2966. doi: 10.1161/01.cir.103.24.2961. [DOI] [PubMed] [Google Scholar]
- Tuunanen H, Engblom E, Naum A, et al. Free fatty acid depletion acutely decreases cardiac work and efficiency in cardiomyopathic heart failure. Circulation. 2006;114:2130–2137. doi: 10.1161/CIRCULATIONAHA.106.645184. [DOI] [PubMed] [Google Scholar]
- Vitale C, Wajngaten M, Sposato B, et al. Trimetazidine improves left ventricular function and quality of life in elderly patients with coronary artery disease. Eur Heart J. 2004;25:1814–1821. doi: 10.1016/j.ehj.2004.06.034. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Fujii H, Takahashi T, et al. Constitutive regulation of cardiac fatty acid metabolism through peroxisome proliferator-activated receptor alpha associated with age-dependent cardiac toxicity. J Biol Chem. 2000;275:22293–22299. doi: 10.1074/jbc.M000248200. [DOI] [PubMed] [Google Scholar]
- Wende A, Pires KM, Wayment B, Tuinei J, Jeoung NH, Litwin SE, Harris RA, Abel ED. American Heart Association Scientific Sessions. Orlando, Florida: 2009. Pyruvate dehydrogenase kinase 4 (PDK4) deficiency does not impair the cardiac metabolic or contractile response to pressure overload hypertrophy; p. 3889. [Google Scholar]
- Witteles RM, Tang WH, Jamali AH, et al. Insulin resistance in idiopathic dilated cardiomyopathy: a possible etiologic link. J Am Coll Cardiol. 2004;44:78–81. doi: 10.1016/j.jacc.2004.03.037. [DOI] [PubMed] [Google Scholar]
- Yan J, Young ME, Cui L, et al. Increased glucose uptake and oxidation in mouse hearts prevent high fatty acid oxidation but cause cardiac dysfunction in diet-induced obesity. Circulation. 2009;119:2818–2828. doi: 10.1161/CIRCULATIONAHA.108.832915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Sambandam N, Han X, et al. CD36 deficiency rescues lipotoxic cardiomyopathy. Circ Res. 2007;100:1208–1217. doi: 10.1161/01.RES.0000264104.25265.b6. [DOI] [PubMed] [Google Scholar]
- Young ME, Laws FA, Goodwin GW, Taegtmeyer H. Reactivation of peroxisome proliferator-activated receptor alpha is associated with contractile dysfunction in hypertrophied rat heart. J Biol Chem. 2001;276:44390–44395. doi: 10.1074/jbc.M103826200. [DOI] [PubMed] [Google Scholar]
- Zhao G, Jeoung NH, Burgess SC, et al. Overexpression of pyruvate dehydrogenase kinase 4 in heart perturbs metabolism and exacerbates calcineurin-induced cardiomyopathy. Am J Physiol Heart Circ Physiol. 2008;294:H936–H943. doi: 10.1152/ajpheart.00870.2007. [DOI] [PubMed] [Google Scholar]