Abstract
Purpose of review
The past year has marked a period of growing awareness of the need for improved diagnosis and treatment in children with arterial ischemic stroke (AIS) and cerebral sinovenous thrombosis (CSVT). Here we review these conditions, highlighting the importance of the intersection between hematologic abnormalities and pediatric stroke as they impact clinical management.
Recent findings
Recent multicenter cohort studies are beginning to clarify the incidence, risk factors, clinical course and outcomes of AIS and CSVT in children. Key findings include: 1)diagnosis rests on adequate neuroimaging, and is often delayed > 24 hours after symptom onset, 2) multiple risk factors and inciting events are often involved, 3)one or more prothrombotic risk factors are common, 4) recurrence is common and 5) selected groups of patients benefit from anticoagulation, and less frequently, thrombolytic therapies.
Summary
Progress in caring for children with AIS and CSVT require greatly improved awareness of cerebrovascular disease among primary providers who are most often the first point of contact, more rapid and specific diagnosis using appropriate advanced neuroimaging technologies, comprehensive hematologic evaluation for inherited and acquired thrombophilias, and multidisciplinary approaches to treatment. Additional large cohort studies and clinical trials are greatly needed to further clarify these issues.
Keywords: prothrombotic condition, magnetic resonance imaging, vasculopathy, recurrence, antithrombotic treatment
Introduction
Arterial ischemic stroke (AIS) and cerebral sinus venous thrombosis (CSVT) cause significant morbidity and mortality in children. The etiologies and clinical presentation in children are diverse and vastly different from adults. At the Children's Hospital of Philadelphia and other pediatric stroke centers, we employ a multidisciplinary approach for managing all children with cerebrovascular disorders. Affected children are evaluated by a team that includes neurologists with expertise in cerebrovascular disease, hematologists with expertise in coagulation disorders, neuroradiologists, rehabilitation experts, and intensivists. Patients are seen by an interactive team initially and reviewed at a weekly neurovascular conference. Specialized nurses working with the thrombosis clinic educate families on practical issues of anticoagulation. We believe that this approach optimizes patient outcomes. Here were review pediatric stroke/sinus clot emphasizing the current clinical issues and illustrating the intersection between neurologic and hematologic factors as pathophysiol determinants of these disorders. Accordingly, collaborations between these two subspecialties has led to an improvement in our understanding and care of affected children. In this review we focus on recent advances that illustrate the importance of early recognition of pediatric cerebrovascular disease, comprehensive diagnostic evaluation, and prompt treatment. We highlight the importance of intersecting hematologic abnormalities and cerebrovascular disease in children and review the current published consensus-based treatment guidelines.
Cerebral Sinus Venous Thrombosis
Case report: An 18 month old male child with a history of iron deficiency anemia developed progressive lethargy and vomiting over several days, culminating in flaccid quadriparesis. Brain MRI and MRV confirmed CSVT with subcortical white matter infarction (Fig. 1). Lumbar puncture revealed elevated opening pressure (55 mm H2O), normal cerebrospinal fluid cell count, protein and glucose. He received IV hydration and systemic anticoagulation with heparin. Laboratory studies showed Hgb 7.5 with iron deficiency, and a positive lupus anticoagulant, initially indicated by prolonged partial thromboplastin time (PTT) and confirmed by prolonged Dilute Russel Viper Venom Time (DRVVT). After starting heparin, mental status improved promptly, and the quadriparesis resolved steadily over 4 weeks. After 3 months of anticoagulation, repeat MRI/MRV showed recanalization of thrombosed sinuses. At follow-up 2 years later he appeared neurologically normal.
Fig. 1.
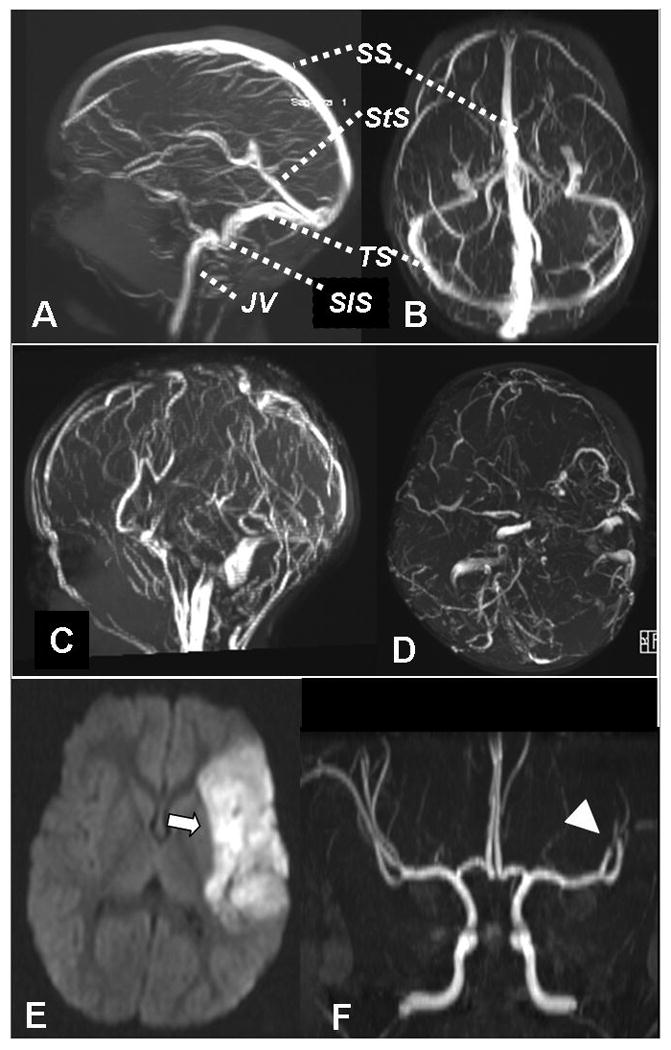
Normal MR venogram, sagittal view (A), axial view (B). Case of CSVT, acute MR venogram sagittal (C) and axial (D), showing absent flow signal in SS, both TS & StS. SS sagittal sinus, StS straight sinus, TS transverse sinus, SiS sigmoid sinus, JV jugular vein. Case of AIS, brain MRI diffusion weighted image (E) showing left middle cerebral artery (MCA) acute infarct (→), MRA (F) with abrupt vessel cutoff in distal left MCA branches (arrowhead), typical of thromboembolic occlusion.
Physiology
Cerebral venous outflow involves a network of cortical and deep veins draining to the dural sinuses, and then to the jugular veins. (Fig. 1 A&B). CSVT obstructs venous outflow, leading to central venous and intracranial hypertension. At the time of diagnosis multiple venous sites of thrombotic obstruction are found, most frequently the transverse and superior sagittal sinuses[1]. Thrombosis progresses to venous infarction in 40-57% of children, often hemorrhagic[2-4]. Diffuse cerebral edema with widespread infarction and hemorrhage can lead to herniation and death in the most severe cases, estimated at a frequency of 5-10%.
Epidemiology
While CSVT affects all ages, neonates are disproportionately at risk, accounting for 27-61% of CSVT in children [2,3,5-8]. CSVT is estimated to annually affect 2.6/100,000/yr neonates and 0.35/100,000 children[3,7].
Clinical Presentation
The clinical manifestations of CSVT are strongly influenced by age, thrombosis location, and the presence of a venous infarction. Symptoms are diverse and nonspecific, including most commonly the triad of headache, emesis and depressed mental status. Seizures are common, especially in neonates. Mental status changes are variable, ranging from sleepiness and confusion to deep coma. Signs include obtundation or coma, papilledema, cranial nerve abnormalities (6th nerve palsy), hemiparesis, quadriparesis, ataxia, and hyper-reflexia. In neonatal CSVT, the most common signs and symptoms are lethargy, vomiting, a full fontanelle, and seizures[3].
Etiology
CSVT in children often results from multiple risk factors and inciting events. One or more risk factors, which vary, with age can be identified in 71-100% children with CSVT [3-5,7-9]. In newborns, acute systemic illness with dehydration and infection are the most common predisposing conditions. Among previously healthy children, acute head and neck infections, dehydration, and iron deficiency anemia are common. Chronic diseases associated with childhood CSVT include inflammatory bowel disease, cancer, autoimmune disorders, and chronic liver or renal disease. A detailed discussion of causes of CSVT can be found in published reviews [10,11].
Prothrombotic abnormalities
Specific prothrombotic abnormalities that have been explored in pediatric CSVT include factor V Leiden and prothrombin gene mutations, protein C, S and antithrombin deficiencies, antiphospholipid antibodies, lipoprotein (a), methyl tetrahydrofolate reductase (MTHFR) mutations, homocysteine, fibrinogen, plasminogen, factor VIII and heparin cofactor II Pediatric CSVT cohort studies report prothrombotic abnormalities in 32 – 62% of cases, [3,4,7,9] compared to 15-29% in adults with CSVT[12,13]. Two small pediatric case control studies have examined the role of prothrombotic disorders in CSVT. Kenet et al found a prothrombotic condition in 42% of 46 children with CSVT, which was similar to the prevalence among 112 healthy controls[9] Heller et al compared prothrombotic risk factors in 149 pediatric patients with CSVT to 149 matched controls, and found a significant association of CSVT with factor V Leiden and lipoprotein (a), protein C or protein S deficiencies[7]. The prothrombin G20210A mutation was predictive of recurrence in a European cohort [14]. Conflicting results among existing studies indicate that more research to better define the role of specific thrombophilias in pediatric CSVT. The current Pediatric Stroke consensus-based guidelines from the American Heart Association propose that children with CSVT may benefit from a thorough investigation to identify potential underlying thrombophilic tendencies[10].
Diagnosis/Imaging
While CT imaging is fast and convenient, this modality may miss the diagnosis of CSVT in up to 40% of cases [2,3,15-17]. CSVT is best identified using MRI with MR venogram (MRV) [15,17,18]. CT venography is an alternative to MRI and MRV[15], but has the disadvantage of significant radation exposure. Ultrasound is convenient in infants with an open fontanel but is less sensitive than either CT or MRI[19].
Therapy
There have been no clinical trials evaluating the treatment of CSVT in children. Current approaches are extrapolated from studies in adults, which support the safety and efficacy of anticoagulation[20,21]. In a prospective cohort of 30 children with CSVT, 3/8 non-anticoagulated patients died versus 0/22 in the patients who received anticoagulation[22], most typically heparin, either unfractionated or low molecular weight. There was no hemorrhage in the 12 patients who received low molecular weight heparin and only one clinically silent bleed out of the 10 patients who received heparin. Several other pediatric CSVT studies have also supported the safety of anticoagulation [3,4,7,9]. A recent study from a multicenter European cohort found that recurrent CSVT was more likely in patients who did not receive anticoagulation for their first illness [14].
Additional therapies for CSVT include thrombolysis or surgical thrombectomy. Among the few case reports of thrombolysis for CSVT[23-26], successful recanalization was achieved but with a high risk of intracranial hemorrhage. Current consensus-based guidelines suggest thrombolysis be reserved for patients who fail to improve, or who worsen, after a trial of anticoagulation. Detailed discussion of treatment for childhood CSVT is available in recently published guidelines and are briefly summarized in table 1[10,11].
Table 1.
Antithrombotic treatment guidelines for pediatric CSVT
| American College of Chest Physicians (2008) | American Heart Association Stroke Council (2008) | |
|---|---|---|
| Neonatal CSVT (age <28 days) |
Without ICH:
|
|
| Pediatric CSVT (age >28 days to 18 years) |
Without ICH:
|
|
ICH – intracerebral hemorrhage, UFH – unfractionated heparin, LMWH – low molecular weight heparin, VKA – Vitamin K antagonist
Recurrence
There are limited studies addressing the risk of recurrence in children with CSVT. The single center cohort study of 160 pediatric patients with CSVT from Canada reported 13% recurrent venous thrombosis events, of which 63% were cerebral. Recently, in a large multi-center European cohort study there were 22 recurrent venous thrombotic events in 384 survivors of CSVT (6%) within a median of 6 months from the initial event (range 0.1-85) for a recurrence rate of 21.2/1,000 person-years. Recurrent venous thrombosis occurred only in children whose first CSVT was diagnosed after 2 years of age. There were 2 deaths from CSVT at the time of recurrence. Risk factors for recurrence included age > 2 years, failure to recanalise, the presence of the prothrombin gene mutation G20210A, and no administration of anticoagulation at the initial event.
Outcome
Long term sequelae from pediatric CSVT include chronic pseudotumor cerebri and cognitive and neurological deficits. In a recent European cohort study, 32% (12/37) of the pediatric CSVT survivors had evidence of chronic pseudotumor cerebri[4]. The prevalence of neurologic deficits reported in survivors of pediatric CSVT range from 25-38%[3-5,9,27]. In the Canadian cohort predictors of poor neurological outcome included seizure at presentation in non-neonates and the presence of infarcts in all age groups[3].
Arterial Ischemic Stroke
Case report: a 25-month old male was recovering at home 4 days after uneventful surgery to repair an atrial septal defect, when he developed acute right hemiparesis. MRI disclosed an acute left middle cerebral artery (MCA) infarct with occlusion of distal left MCA branches (Fig. 1, E & F). Echocardiogram showed no intracardiac thrombus or myocardial dysfunction, and laboratory studies revealed no prothrombotic states. He received low molecular weight heparin for 4 weeks, followed by aspirin, as well as intensive rehabilitation services. On follow-up 1 year later his examination and function were normal.
Physiology
Cerebral blood flow normally is tightly regulated to match metabolic activity under widely varying systemic fluctuations in blood pressure, blood glucose, O2 and CO2 content. AIS occurs when flow-limiting arterial stenosis or thromboembolic occlusion cause irreversible tissue injury. The ischemic cascade involves reperfusion and flow redistribution, cellular and subcellular injury and dysfunction, cell death and subsequent reactions to injury including plasticity.
Epidemiology
AIS is estimated to affect 1-13/100,000 children annually in North America and Europe[28,29], with about 30% involving neonates. AIS disproportionately affects males and African-Americans [6,28]. The basis for increased stroke risk related to gender, race/ethnicity, and geography is not understood, though both genetic and environmental factors likely contribute[30].
Clinical Presentation
Acute symptomatic neonatal AIS typically presents with depressed mental status and seizures. Delayed presentation of neonatal AIS (presumed perinatal ischemic stroke, PPIS) appears at 3-6 months of age as impaired motor development and hemiparesis. An excellent summary of the classification and description of clinical features of prenatal and perinatal-onset ischemic stroke is available[31]. AIS in children outside the neonatal period presents as sudden loss of neurologic function in a pattern consistent with an arterial territory. Typical signs and symptoms of anterior circulation stroke (carotid artery and its branches) include: 1)unilateral weakness and/or sensory loss involving face, arm and leg, 2)loss of speech or language comprehension (aphasia), often mistaken for confusion or oppositional behavior, 3)visual field deficit, 4)focal seizures. Posterior circulation stroke (vertebrobasilar arteries) presents with a combination of diffuse signs and focal deficits referable to the brainstem and cerebellum, including acute ataxia, visual field loss, slurred speech, and eye movement deficits. Diffuse non-localizing symptoms, including severe headache, nausea, vomiting and lethargy often dominate the chief complaint. Limited awareness of stroke, the difficulty of performing detailed neurologic examination in acutely ill children, and the need for MRI, which frequently requires sedation, all can delay definitive diagnosis, which on average, occurs approximately 24 hours after symptom onset[32,33].
Etiology
Three major classes of disorders cause childhood AIS: 1) hematologic and coagulation abnormalities, 2) cardiac thromboembolism, and 3)cervical or cerebral vasculopathy [34]. Causes of neonatal AIS include prothrombotic states, including maternal antiphospholipid antibody and placental disorders [35]. Thorough diagnostic evaluation reveals at least one risk factor in 75-80% of children with AIS. About half have a known underlying disease at the time of presentation (symptomatic stroke), including heart disease, hemoglobinopathy (most commonly sickle cell anemia with associated vasculopathy), head and neck trauma, infection, cancer, and autoimmune diseases that are frequently associated with antiphospholipid antibody. Vasculopathies and prothrombotic conditions are the most common risk factors among previously healthy children with first AIS (cryptogenic stroke) [36]. Cerebral and cervical vasculopathies occur in 30-80% of children with AIS[36-38]. Multiple risk factors often coexist.
Prothrombotic abnormalities
Prothrombotic conditions affect 20-63% of newborns and children with AIS[39,40]. Many children have multiple prothrombotic conditions, and many children with a known stroke risk factor such as heart disease also have a prothrombotic condition[41]. In a recent study of neonatal AIS, the combined incidence of prothrombotic conditions in mother and infant was found to be 78%[42]. The spectrum of identified prothrombotic conditions in AIS includes three general categories: 1) Heritable conditions, including deficient protein C, protein S, or antithrombin deficiencies, factor V Leiden, prothrombin gene mutations, folate metabolism defects and lipoprotein (a) elevation; 2) acquired conditions such as circulating prothrombotic antibodies (lupus anticoagulant/antiphospholipid antibody); 3) drug-induced or disease-related prothrombotic defects. In a meta-analysis of ∼3000 cases of childhood AIS and ∼ 9000 controls, Haywood et al found associations between first ischemic stroke and deficiencies in protein C, protein S and antithrombin; elevated plasma homocysteine, factor V Leiden and prothrombin mutations[43]. A detailed discussion of prothrombotic conditions pertinent to childhood AIS can be found in the American Heart Association (AHA) Pediatric Stroke Treatment guidelines and the American College of Chest Physicians' Guidelines[10,11].
Whether to screen for prothrombotic conditions in pediatric CSVT or AIS is controversial, depending on the patient's age and clinical setting. However, identification of specific prothrombotic abnormalities can influence clinical care in numerous ways: 1) severe anticoagulant deficiencies (protein C, S or antithrombin) may be amenable to correction with fresh frozen plasma or recombinant factors 2) the presence of specific abnormalities can influence antithrombotic treatment decisions aimed at secondary stroke prevention, 3) findings may alter management of comorbid conditions such as congenital heart disease, and 4) families may be counseled on the risks for future thrombosis in the patient and their relatives.
Diagnosis/Imaging
Evaluation of infants and children with suspected AIS rests on an accurate diagnosis, which requires adequate neuroimaging. Head CT is fast and widely available, but lacks sensitivity and specificity for ischemic injury. Brain MRI provides more reliable diagnosis of AIS in children, distinguishing it from stroke mimics such as demyelinating disease and tumor. MR angiography (MRA) of the head and neck should be obtained at the same time as MRI in any child with clinically suspected stroke, including newborns. CT angiogram is an alternative to MRA in situations where MR is inaccessible, but has the major disadvantage of high radiation exposure. Comprehensive evaluation is recommended for all infants and children with acute AIS, including: 1)vascular – imaging of the head and neck; 2) cardiac – EKG and echocardiogram, possibly including bubble contrast; 3) hematologic – basic hematology profile, coagulation screens, and extended thrombophilia evaluation. The ideal thrombophilia evaluation is controversial and continually evolving as new information appears regarding the nature and significance of specific defects. Testing recommendations reflecting current evidence are summarized in the AHA Pediatric Stroke and Chest guidelines [10,11].
Therapy
Therapy for AIS in childhood is guided by three principles (Table 2): 1) limit the extent of injury acutely, 2) promote recovery, 3) prevent recurrence There have been no clinical trials in childhood AIS other than the use of chronic transfusion to prevent stroke in children with sickle cell anemia. A key decision concerns the use of antithrombotic treatment, chosen from antiplatelet agents such as aspirin, systemic anticoagulation with a heparinoid, and thrombolytics. The use of thrombolysis (recombinant tissue plasminogen activator, tPA) is highly controversial in childhood AIS. There is strong consensus that tPA should not be used in neonatal stroke due to uncertainty regarding stroke onset. In older children and adolescents there is very limited data on safety and efficacy, and to date there is limited consensus among experts. The current AHA Guidelines suggest that tPA be used in children (pre-adolescents) only in the context of a clinical trial, and if considered for adolescents its use should adhere strictly to adult guidelines. Current practices with respect to antiplatelet and anticoagulation treatments vary widely among established pediatric stroke centers, reflecting uncertainty and lack of evidence-based guidelines[44]. Comprehensive reviews of this topic and consensus-based treatment recommendations are outlined in the AHA and Chest guidelines[10,11].
Table 2.
Treatment in Pediatric Arterial Ischemic Stroke (AIS)*
| Perfusion: restore & maintain cerebral circulation & energy substrate delivery (glucose, oxygen) |
|
| Neuroprotection: limit the extent of infarction, salvage the penumbra |
|
| Rehabilitation: promote recovery & plasticity |
|
| Secondary prevention: prevent recurrence* |
|
Use of antithrombotics in neonatal AIS is not usually recommended due to very low recurrence risk. Exception would be to consider systemic anticoagulation where intracardiac or systemic thrombosis is identified, or a major prothrombotic defect is identified.
Recurrence and Outcome
Long-term sequelae affect 60% of children with AIS[35,45-47]. Neurocognitive and psychosocial morbidities are the most significant. Predictors of long-term sequelae are incompletely understood, and are influenced by infarct size and location, age of onset, and underlying etiology. Reports of recurrence vary widely, ranging from 15-60% at 1-5 years. Children with chronic and progressive vasculopathies are at the highest risk of recurrence[48-50]. Prothrombotic risk factors increase recurrence risk[43,51]. Current research efforts are focused on better understanding how systemic thrombophilias and arteriopathies interact biologically to influence AIS recurrence and long-term functional outcome[47,52].
Conclusion
Important advances have been made in the understanding of childhood AIS and CSVT as a result of large well-investigated prospective cohort studies. These studies show that risk factors in children differ significantly from adults, and include prothrombotic disorders and cerebral vasculopathies. Continued progress in caring for children with AIS and CSVT will require greatly improved awareness of cerebrovascular disease among primary providers who are most often the first point of contact. In particular, improved care will depend on more rapid and specific diagnosis using appropriate advanced neuroimaging technologies, comprehensive hematologic evaluation for inherited and acquired thrombophilias, and multidisciplinary approaches to treatment. Additional large cohort studies and clinical trials are greatly needed to develop sound evidence-based management guidelines.
Acknowledgments
Dr. Witmer is supported by a grant from the NIH; Dr. Ichord is supported by grants from the NIH/NINDS and NHLBI.
References
- 1.Chan AK, deVeber G, Monagle P, et al. Venous thrombosis in children. J Thromb Haemost. 2003;1:1443–1455. doi: 10.1046/j.1538-7836.2003.00308.x. [DOI] [PubMed] [Google Scholar]
- 2.Barron TF, Gusnard DA, Zimmerman RA, et al. Cerebral venous thrombosis in neonates and children. Pediatr Neurol. 1992;8:112–116. doi: 10.1016/0887-8994(92)90030-3. [DOI] [PubMed] [Google Scholar]
- 3.deVeber G, Andrew M. Cerebral sinovenous thrombosis in children. N Engl J Med. 2001;345:417–423. doi: 10.1056/NEJM200108093450604. [DOI] [PubMed] [Google Scholar]
- 4.Sebire G, Tabarki B, Saunders DE, et al. Cerebral venous sinus thrombosis in children: risk factors, presentation, diagnosis and outcome. Brain. 2005;128:477–489. doi: 10.1093/brain/awh412. [DOI] [PubMed] [Google Scholar]
- 5.Carvalho KS, Bodensteiner JB, Connolly PJ, et al. Cerebral venous thrombosis in children. J Child Neurol. 2001;16:574–580. doi: 10.1177/088307380101600807. [DOI] [PubMed] [Google Scholar]
- *6.Golomb MR, Fullerton HJ, Nowak-Gottl U, et al. Male predominance in childhood ischemic stroke: findings from the international pediatric stroke study. Stroke. 2009;40:52–57. doi: 10.1161/STROKEAHA.108.521203. [DOI] [PubMed] [Google Scholar]; *This paper describes the methods and demographic characteristics of the largest multicenter cohort study to date in childhood AIS from the International Pediatric Stroke Study
- 7.Heller C, Heinecke A, Junker R, et al. Cerebral venous thrombosis in children: a multifactorial origin. Circulation. 2003;108:1362–1367. doi: 10.1161/01.CIR.0000087598.05977.45. [DOI] [PubMed] [Google Scholar]
- 8.Wasay M, Dai AI, Ansari M, et al. Cerebral venous sinus thrombosis in children: a multicenter cohort from the United States. J Child Neurol. 2008;23:26–31. doi: 10.1177/0883073807307976. [DOI] [PubMed] [Google Scholar]
- 9.Kenet G, Waldman D, Lubetsky A, et al. Paediatric cerebral sinus vein thrombosis. A multi-center, case-controlled study. Thromb Haemost. 2004;92:713–718. doi: 10.1160/TH04-03-0182. [DOI] [PubMed] [Google Scholar]
- **10.Roach ES, Golomb MR, Adams R, et al. Management of stroke in infants and children: a scientific statement from a Special Writing Group of the American Heart Association Stroke Council and the Council on Cardiovascular Disease in the Young. Stroke. 2008;39:2644–2691. doi: 10.1161/STROKEAHA.108.189696. [DOI] [PubMed] [Google Scholar]; **This is the first American Heart Association-sponsored Scientific Statement providing management guidelines for pediatric stroke. It includes detailed review of existing literature on arterial ischemic stroke and cerebral sinovenous thrombosis. Most recommendations are consensus-based as the existing literature includes only case series and cohort studies.
- **11.Monagle P, Chalmers E, Chan A, et al. Antithrombotic therapy in neonates and children: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. (8th) 2008;133:887S–968S. doi: 10.1378/chest.08-0762. [DOI] [PubMed] [Google Scholar]; ** This monograph provides a comprehensive review of the literature and evidence-based guidelines on antithrombotic treatment in children. It includes detailed sections on arterial ischemic stroke and cerebral sinovenous thrombosis. It overlaps partially and is complimentary to the AHA Scientific Statement.
- 12.Deschiens MA, Conard J, Horellou MH, et al. Coagulation studies, factor V Leiden, and anticardiolipin antibodies in 40 cases of cerebral venous thrombosis [see comments] Stroke. 1996;27:1724–1730. doi: 10.1161/01.str.27.10.1724. [DOI] [PubMed] [Google Scholar]
- 13.Wysokinska EM, Wysokinski WE, Brown RD, et al. Thrombophilia differences in cerebral venous sinus and lower extremity deep venous thrombosis. Neurology. 2008;70:627–633. doi: 10.1212/01.wnl.0000297195.97325.a8. [DOI] [PubMed] [Google Scholar]
- 14.Kenet G, Kirkham F, Niederstadt T, et al. Risk factors for recurrent venous thromboembolism in the European collaborative paediatric database on cerebral venous thrombosis: a multicentre cohort study. Lancet Neurol. 2007;6:595–603. doi: 10.1016/S1474-4422(07)70131-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Connor SE, Jarosz JM. Magnetic resonance imaging of cerebral venous sinus thrombosis. Clin Radiol. 2002;57:449–461. doi: 10.1053/crad.2001.0880. [DOI] [PubMed] [Google Scholar]
- 16.Linn J, Pfefferkorn T, Ivanicova K, et al. Noncontrast CT in deep cerebral venous thrombosis and sinus thrombosis: comparison of its diagnostic value for both entities. AJNR Am J Neuroradiol. 2009;30:728–735. doi: 10.3174/ajnr.A1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bianchi D, Maeder P, Bogousslavsky J, et al. Diagnosis of cerebral venous thrombosis with routine magnetic resonance: an update. Eur Neurol. 1998;40:179–190. doi: 10.1159/000007978. [DOI] [PubMed] [Google Scholar]
- 18.Dormont D, Anxionnat R, Evrard S, et al. MRI in cerebral venous thrombosis. J Neuroradiol. 1994;21:81–99. [PubMed] [Google Scholar]
- 19.Nwosu ME, Williams LS, Edwards-Brown M, et al. Neonatal sinovenous thrombosis: presentation and association with imaging. Pediatr Neurol. 2008;39:155–161. doi: 10.1016/j.pediatrneurol.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Einhaupl KM, Villringer A, Meister W, et al. Heparin treatment in sinus venous thrombosis. Lancet. 1991;338:597–600. doi: 10.1016/0140-6736(91)90607-q. [DOI] [PubMed] [Google Scholar]
- 21.de Bruijn SF, Stam J. Randomized, placebo-controlled trial of anticoagulant treatment with low-molecular-weight heparin for cerebral sinus thrombosis. Stroke. 1999;30:484–488. doi: 10.1161/01.str.30.3.484. [DOI] [PubMed] [Google Scholar]
- 22.deVeber G, Chan A, Monagle P, et al. Anticoagulation therapy in pediatric patients with sinovenous thrombosis: a cohort study. Arch Neurol. 1998;55:1533–1537. doi: 10.1001/archneur.55.12.1533. [DOI] [PubMed] [Google Scholar]
- 23.Wasay M, Bakshi R, Kojan S, et al. Nonrandomized comparison of local urokinase thrombolysis versus systemic heparin anticoagulation for superior sagittal sinus thrombosis. Stroke. 2001;32:2310–2317. doi: 10.1161/hs1001.096192. [DOI] [PubMed] [Google Scholar]
- 24.Griesemer DA, Theodorou AA, Berg RA, et al. Local fibrinolysis in cerebral venous thrombosis. Pediatr Neurol. 1994;10:78–80. doi: 10.1016/0887-8994(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 25.Liebetrau M, Mayer TE, Bruning R, et al. Intra-arterial thrombolysis of complete deep cerebral venous thrombosis. Neurology. 2004;63:2444–2445. doi: 10.1212/01.wnl.0000148607.40764.df. [DOI] [PubMed] [Google Scholar]
- 26.Soleau SW, Schmidt R, Stevens S, et al. Extensive experience with dural sinus thrombosis. Neurosurgery. 2003;52:534–544. doi: 10.1227/01.neu.0000047815.21786.c1. [DOI] [PubMed] [Google Scholar]
- 27.De Schryver EL, Blom I, Braun KP, et al. Long-term prognosis of cerebral venous sinus thrombosis in childhood. Dev Med Child Neurol. 2004;46:514–519. doi: 10.1017/s0012162204000866. [DOI] [PubMed] [Google Scholar]
- 28.Fullerton HJ, Wu YW, Zhao S, et al. Risk of stroke in children: ethnic and gender disparities. Neurology. 2003;61:189–194. doi: 10.1212/01.wnl.0000078894.79866.95. [DOI] [PubMed] [Google Scholar]
- 29.Giroud M, Lemesle M, Gouyon JB, et al. Cerebrovascular disease in children under 16 years of age in the city of Dijon, France: a study of incidence and clinical features from 1985 to 1993. J Clin Epidemiol. 1995;48:1343–1348. doi: 10.1016/0895-4356(95)00039-9. [DOI] [PubMed] [Google Scholar]
- *30.Nowak-Gottl U, Langer C, Bergs S, et al. Genetics of hemostasis: differential effects of heritability and household components influencing lipid concentrations and clotting factor levels in 282 pediatric stroke families. Environ Health Perspect. 2008;116:839–843. doi: 10.1289/ehp.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This paper is a unique contribution, well-designed and rigorously carried out, providing important insights into sources of variability in the thrombotic risk factors of pediatric arterial ischemic stroke.
- 31.Raju TN, Nelson KB, Ferriero D, et al. Ischemic perinatal stroke: summary of a workshop sponsored by the National Institute of Child Health and Human Development and the National Institute of Neurological Disorders and Stroke. Pediatrics. 2007;120:609–616. doi: 10.1542/peds.2007-0336. [DOI] [PubMed] [Google Scholar]
- 32.Rafay MF, Pontigon AM, Chiang J, et al. Delay to diagnosis in acute pediatric arterial ischemic stroke. Stroke. 2009;40:58–64. doi: 10.1161/STROKEAHA.108.519066. [DOI] [PubMed] [Google Scholar]
- 33.Srinivasan J, Miller SP, Phan TG, et al. Delayed recognition of initial stroke in children: need for increased awareness. Pediatrics. 2009;124:e227–234. doi: 10.1542/peds.2008-3544. [DOI] [PubMed] [Google Scholar]
- 34.deVeber G. Stroke and the child's brain: an overview of epidemiology, syndromes and risk factors. Curr Opin Neurol. 2002;15:133–138. doi: 10.1097/00019052-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Nelson KB, Lynch JK. Stroke in newborn infants. Lancet Neurol. 2004;3:150–158. doi: 10.1016/S1474-4422(04)00679-9. [DOI] [PubMed] [Google Scholar]
- 36.Ganesan V, Prengler M, McShane MA, et al. Investigation of risk factors in children with arterial ischemic stroke. Ann Neurol. 2003;53:167–173. doi: 10.1002/ana.10423. [DOI] [PubMed] [Google Scholar]
- *37.Amlie-Lefond C, Bernard TJ, Sebire G, et al. Predictors of cerebral arteriopathy in children with arterial ischemic stroke: results of the International Pediatric Stroke Study. Circulation. 2009;119:1417–1423. doi: 10.1161/CIRCULATIONAHA.108.806307. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This paper presents the analysis of the role of arteriopathy from the largest single multicenter cohort study of pediatric arterial ischemic stroke to date, from the IPSS investigators.
- 38.Sebire G, Fullerton H, Riou E, et al. Toward the definition of cerebral arteriopathies of childhood. Curr Opin Pediatr. 2004;16:617–622. doi: 10.1097/01.mop.0000144441.29899.20. [DOI] [PubMed] [Google Scholar]
- 39.Barnes C, Deveber G. Prothrombotic abnormalities in childhood ischaemic stroke. Thromb Res. 2006;118:67–74. doi: 10.1016/j.thromres.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 40.Lynch JK, Han CJ, Nee LE, et al. Prothrombotic factors in children with stroke or porencephaly. Pediatrics. 2005;116:447–453. doi: 10.1542/peds.2004-1905. [DOI] [PubMed] [Google Scholar]
- 41.Strater R, Vielhaber H, Kassenbohmer R, et al. Genetic risk factors of thrombophilia in ischaemic childhood stroke of cardiac origin. A prospective ESPED survey. Eur J Pediatr. 1999;158 3:S122–125. doi: 10.1007/pl00014336. [DOI] [PubMed] [Google Scholar]
- 42.Simchen MJ, Goldstein G, Lubetsky A, et al. Factor v Leiden and antiphospholipid antibodies in either mothers or infants increase the risk for perinatal arterial ischemic stroke. Stroke. 2009;40:65–70. doi: 10.1161/STROKEAHA.108.527283. [DOI] [PubMed] [Google Scholar]
- *43.Haywood S, Liesner R, Pindora S, et al. Thrombophilia and first arterial ischaemic stroke: a systematic review. Arch Dis Child. 2005;90:402–405. doi: 10.1136/adc.2004.049163. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This paper is a companion work to the Amlie-Lefond 2009 paper, and describes current treatment practices and short-term outcomes in the largest multicenter cohort study to date of pediatric arterial ischemic stroke, from the IPSS investigators.
- 44.Goldenberg NA, Bernard TJ, Fullerton HJ, et al. Antithrombotic treatments, outcomes, and prognostic factors in acute childhood-onset arterial ischaemic stroke: a multicentre, observational, cohort study. Lancet Neurol. 2009 doi: 10.1016/S1474-4422(09)70241-8. [DOI] [PubMed] [Google Scholar]
- 45.Ganesan V, Hogan A, Shack N, et al. Outcome after ischaemic stroke in childhood. Dev Med Child Neurol. 2000;42:455–461. doi: 10.1017/s0012162200000852. [DOI] [PubMed] [Google Scholar]
- 46.deVeber GA, MacGregor D, Curtis R, et al. Neurologic outcome in survivors of childhood arterial ischemic stroke and sinovenous thrombosis. J Child Neurol. 2000;15:316–324. doi: 10.1177/088307380001500508. [DOI] [PubMed] [Google Scholar]
- 47.Lynch JK, Han CJ. Pediatric stroke: what do we know and what do we need to know? Semin Neurol. 2005;25:410–423. doi: 10.1055/s-2005-923535. [DOI] [PubMed] [Google Scholar]
- 48.Ganesan V, Prengler M, Wade A, et al. Clinical and radiological recurrence after childhood arterial ischemic stroke. Circulation. 2006;114:2170–2177. doi: 10.1161/CIRCULATIONAHA.105.583690. [DOI] [PubMed] [Google Scholar]
- 49.Fullerton HJ, Wu YW, Sidney S, et al. Risk of recurrent childhood arterial ischemic stroke in a population-based cohort: the importance of cerebrovascular imaging. Pediatrics. 2007;119:495–501. doi: 10.1542/peds.2006-2791. [DOI] [PubMed] [Google Scholar]
- 50.Bernard TJ, Goldenberg NA, Tripputi M, et al. Anticoagulation in childhood-onset arterial ischemic stroke with non-moyamoya arteriopathy: findings from the Colorado and German (COAG) collaboration. Stroke. 2009;40:2869–2871. doi: 10.1161/STROKEAHA.109.550699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strater R, Becker S, von Eckardstein A, et al. Prospective assessment of risk factors for recurrent stroke during childhood--a 5-year follow-up study. Lancet. 2002;360:1540–1545. doi: 10.1016/S0140-6736(02)11520-0. [DOI] [PubMed] [Google Scholar]
- 52.Sofronas M, Ichord RN, Fullerton HJ, et al. Pediatric stroke initiatives and preliminary studies: What is known and what is needed? Pediatr Neurol. 2006;34:439–445. doi: 10.1016/j.pediatrneurol.2005.10.016. [DOI] [PubMed] [Google Scholar]


