Abstract
Objectives. We evaluated the relationship between smoking and adenocarcinoma of the prostate.
Methods. We pooled data from 24 cohort studies enrolling 21 579 prostate cancer case participants for a general variance-based meta-analysis. Summary relative risks (RRs) and 95% confidence intervals (CIs) were calculated separately for mortality and incidence studies. We tested the robustness of effect measures and evaluated statistical heterogeneity with sensitivity analyses.
Results. In the pooled data, current smokers had no increased risk of incident prostate cancer (RR = 1.04; 95% CI = 0.87, 1.24), but in data stratified by amount smoked they had statistically significant elevated risk (cigarettes per day or years: RR = 1.22; 95% CI = 1.01, 1.46; pack years of smoking: RR = 1.11; 95% CI = 1.01, 1.22). Former smokers had an increased risk (RR = 1.09; 95% CI = 1.02, 1.16). Current smokers had an increased risk of fatal prostate cancer (RR = 1.14; 95% CI = 1.06, 1.19). The heaviest smokers had a 24% to 30% greater risk of death from prostate cancer than did nonsmokers.
Conclusions. Observational cohort studies show an association of smoking with prostate cancer incidence and mortality. Ill-defined exposure categories in many cohort studies suggest that pooled data underestimate risk.
Prostate cancer is the most common solid tumor diagnosed among men in the United States, with an estimate of more than 186 000 new cases and 28 000 deaths in 2008.1 Unfortunately, few risk factors have been identified, other than advanced age and family history.2 That environmental factors may play a role in its etiology is suggested by data demonstrating wide international variation in incidence. Migrant studies document increased occurrence among those moving from low- to high-incidence countries, as was observed among Japanese men who immigrated to the United States.3 Autopsy studies have documented a consistent prevalence (15%–30%) of histologic or latent prostate cancer across populations.4 These findings suggest that initiating events for prostate cancer may differ from those contributing to progression and the occurrence of clinically evident disease.
Despite the demonstrated links between smoking and several solid tumors, the association between cigarette smoking and prostate cancer remains a matter of debate. Although this disease is not considered to be tobacco related,5 cigarette smoke is known to contain multiple carcinogens, including N-nitroso compounds (recognized animal carcinogens).6,7 An association with smoking could also have a hormonal basis: male smokers were found to have elevated levels of circulating androsterone and testosterone, which may increase prostate cancer risk or contribute to cancer progression.8
Unfortunately, results from human observational studies are inconsistent across study types, with case–control analyses showing particular heterogeneity.5 In addition, epidemiological analyses suggest that the outcomes of studies examining the influence of smoking on prostate cancer incidence may differ from the results in studies of prostate cancer mortality.9 This may further complicate analysis of a causal association. Because this topic had not previously been subjected to meta-analysis and results from epidemiological studies were inconsistent, we pooled data from the available cohort studies to elucidate the possible relationship between smoking and the etiology and progression of adenocarcinoma of the prostate.
METHODS
Our methods are described elsewhere.10,11 Briefly, we designed our meta-analysis to examine the risk of prostate cancer, both incidence and mortality, associated with cigarette smoking. We prospectively determined eligibility criteria for study inclusion and the specific data elements to be extracted from each published report.
We designed a data extraction form for recording relevant information, with 2 researchers performing data extraction. Differences were resolved by consensus. Other data collected but not included in the eligibility criteria were number of patients and location for each study; length of follow-up; cohort description; type of statistical adjustments, if any, to the individual study odds ratios (ORs) or relative risks (RRs); estimates of smoking dose (e.g., number of cigarettes smoked or pack years [packs smoked per day × number of years smoked]).
Literature Search
Our methods of literature retrieval are described elsewhere.10 We conducted a MEDLARS search of English language articles published between January 1966 and February 2007, and a review of CancerLit and the CD-ROM version of Current Contents. We searched the Cochrane database for publications between January 1966 and February 2003. Search terms were smoking and prostatic neoplasms. For series of articles, all data were retrieved from the most recent article. We also performed hand searches of bibliographies of published reports, review articles, and textbooks. Manual searches included review of studies that did not specify smoking as the primary risk factor analyzed.
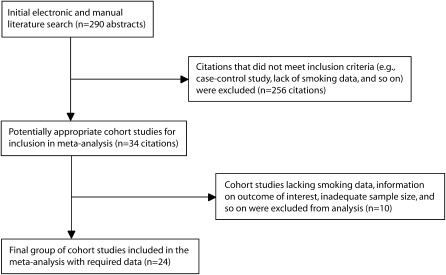
The initial electronic and manual searches yielded 290 abstracts, which were screened by a physician-investigator according to our inclusion criteria. Most excluded articles did not report peer-reviewed prospective cohort studies, reported on studies with a sample of fewer than 50 participants, reported on in vitro or animal studies, or were literature reviews. Eligibility criteria also included publishing an observational study that enrolled participants with histologically proven adenocarcinoma of the prostate, availability of data on cigarette smoking, availability of ORs or RRs with 95% confidence intervals (CIs) for each report or availability of raw data to calculate these values, and availability of data on the outcome of interest, including incident or fatal prostate cancer.
After the initial screening, we had 34 citations.12–45 Ten reports were excluded because of lack of data on smoking,12–14 small sample size,15–17 lack of specified outcome data (i.e., prostate cancer incidence or mortality),18 or unavailability of 95% CIs or raw data for their calculation.19 One report dealt only with patients treated for benign prostatic hypertrophy or stage A prostatic tumors and was excluded.20 Data for a 1980 study21 were updated in 199134; we used data from the latter report for statistical pooling.
Statistical Analysis
Our data analysis followed meta-analytic procedures described by Greenland.46 For each included study, we derived RRs or ORs reflecting the risk of developing or dying from prostatic adenocarcinoma associated with smoking, followed by calculation of the natural logarithm of the estimated RR for each data set as well as calculation of an estimate of the variance. When both crude and adjusted RRs were provided, we used the most fully adjusted value. We calculated the variance in each study's measure of effect from the 95% CIs. We used ORs or RRs for the highest versus lowest exposure categories. If these measures were missing, we calculated them with standard methods.10 Whenever possible, we used adjusted outcome measures for statistical pooling.
We calculated a weight for each included report as 1 divided by the variance followed by a summation of the weights. We then calculated and summed the product of the study weight and the natural logarithm of the estimated RR. Finally, we calculated a summary RR and 95% CI.
We performed a statistical test for homogeneity (Q). This procedure tests the hypothesis that the effect sizes are equal in all of the included studies.11 If Q exceeds the upper-tail critical value of χ2 (P < .10) at k-1 df, the observed variance in study effect sizes is greater than expected by chance if all studies share a common population effect size. If the studies are not homogeneous, they are not measuring an effect of the same size and calculation of a pooled estimate of effect may be of questionable validity. Explanations for the observed heterogeneity must be sought. Sensitivity analyses or further stratified analyses are then performed according to the magnitude of Q.
We did not examine the potential for publication bias. Publication bias occurs because published studies may not be representative of all studies that have ever been done. The funnel plot method and other statistical tools were constructed to address this issue. Unfortunately, these methods lack firm statistical theoretical support and are not generally recommended for medical applications.11
RESULTS
Our meta-analysis included 24 cohort studies.22–45 Table 1 provides an overview of the entire database. Seventeen reports (71%) were from the United States,25,26,29–37,39–44 3 from Norway,28,38,45 2 from Japan,23,24 and 1 each from Sweden22 and the United Kingdom.27 Prostate cancer cases totaled 21 579.
TABLE 1.
Smoking and Prostate Cancer Risk: Overview of 24 Cohort Studies
| Study (Year Published) | Cohort | Follow-up, y | Prostate Cancer Cases, No. (Endpoint) | Smoking Status | RR (95% CI) | Amount Smoked/Day | RR (95% CI) | Smoking Duration | RR (95% CI) | Adjustments to RR |
| Adami et al.22 (1996) | 135 006 Male construction workers in Sweden | 20 | 2368 (Incident cases) | Ex-smoker | 1.09 (0.98, 1.22) | > 25 Cigarettes (all smokers) | 1.00 (0.72, 1.38) | > 21 y | 1.03 (0.90, 1.19) (Current smokers) | Age, BMI, marital status |
| Current smoker | 1.11 (1.01, 1.23) | |||||||||
| 709 (Fatal cases) | Ex-smoker | 1.03 (0.84, 1.33) | > 25 Cigarettes (all smokers) | 1.05 (0.82, 1.35) | > 41 y | 1.28 (0.99, 1.65) (Current smokers) | ||||
| Current smoker | 1.26 (1.06, 1.50) | |||||||||
| Akiba and Hirayama23 (1990) | 265 000 men and women in Japan, followed between 1966 and 1981 | 15 | 108 (Fatal cases)a | Ever smoker | 1.1 (0.7, 1.5) | > 35 Cigarettes | 3.0 (1.0, 7.1) | … | Residence, age, observation period | |
| Allen et al.24 (2004) | Life-Span Study, 18 115 men in Japan, followed since 1963 | 33 | 196 (Incident cases) | Ever smoker | 0.80 (0.60, 1.07) | … | … | Age period, city of residence, radiation dose, education | ||
| Cerhan et al.25 (1997) | Rural Health Study, 1 050 men aged ≥ 65 y in Iowa, followed since 1982 | 11 | 71 (Incident cases) | Current smoker | 2.2 (1.2, 4.4) | ≤ 20 Cigarettes | 1.8 (0.7, 4.4) | > 55 Pack yearsb | 2.0 (1.1, 3.8) | Age |
| > 20 Cigarettes | 2.7 (1.2, 6.0) | … | ||||||||
| Ex-smoker | 1.2 (0.7, 2.1) | |||||||||
| Coughlin et al.26 (1996) | Multiple Risk Factor Intervention Trial, 348 874 men in the United States, average age 46 y at initial screening | 16 | 826 (Fatal cases) | Current smoker | 1.31 (1.13, 1.52) | ≤ 25 Cigarettes | 1.21 (1.01, 1.46) | … | Age, race, serum cholesterol, income, diabetes | |
| ≥ 26 Cigarettes | 1.45 (1.19, 1.77) | … | ||||||||
| Doll et al.27 (2005) | 34 439 Male physicians in the United Kingdom, initiated 1951 | 50 | 878 (Fatal cases) | Current smoker | 0.99 (0.87, 1.34) | … | … | Age, study | ||
| Ex-smoker | 0.86 (0.80, 1.12) | |||||||||
| Engeland et al.28 (1996) | Migrant Study, 11 863 men in Norway | 27 | 707 (Incident cases) | Current smoker | 1.1 (0.9, 1.3) | … | … | Age at start of smoking, of cigarette smoked, urban/rural residence, pipe/cigar smoking | ||
| Ex-smoker | 0.9 (0.7, 1.1) | |||||||||
| Giovannucci et al.29 (2007) | Health Professionals Follow-Up Study, 51 529 male health professionals in the United States, initiated in 1986 | 16 | 3544 (Incident cases) | Current smoker | 0.98 (0.89, 1.07) | … | … | Smoking history, race, family history, physical activity, BMI, height, energy consumption, Calcium, tomato sauce, and alpha-linoleic acid intake | ||
| 312 (Fatal cases) | Current smoker | 1.41 (1.04, 1.91) | ||||||||
| Giovannucci et al.30 (1999) | Health Professionals Follow-Up Study, 51 529 male health professionals in the United States, initiated in 1986 | 8 | 1369 (Incident cases) | NA | … | Total lifetime pack years | 1.07 (0.85, 1.36) | Smoking history, race, family history, physical activity, BMI, height, energy consumption, Calcium, tomato sauce, and alpha-linoleic acid intake | ||
| Hammond31 (1958) | American Cancer Society cohort, 187 783 White men aged 50–69 y | 3 | 134 (Fatal cases) | Ever smoker | 1.75 (1.37, 2.19) | … | … | Age | ||
| Hammond et al.32 (1966) | American Cancer Society cohort follow-up | 7 | 319 (Fatal cases) | Ex-smoker | 1.02 (0.81, 1.28) | … | … | Age | ||
| Hiatt et al.33 (1994) | Kaiser Permanente Medical Care Program, 43 432 men in the United States, aged > 30 y, enrolled 1978–1985 | 7 | 238 (Incident cases) | Ex-smoker | 1.1 (0.8, 1.5) | < 1 Pack | 1.0 (0.6, 1.6) | … | Age, race, education, alcohol | |
| > 1 Pack | 1.9 (1.2, 3.1) | |||||||||
| Hsing et al.34 (1991) | Veterans Administration Cohort, 293 916 US veterans who served 1917–1940 | 26 | 4607 (Fatal cases) | Ex-smoker | 1.13 (1.03, 1.24) | > 39 Cigarettes (current smokers) | 1.51 (1.20, 1.90) | > 39 y (Current smokers) | 1.23 (0.97, 1.56) | Age |
| Current smoker | 1.18 (1.09, 1.28) | |||||||||
| Hsing et al.35 (1990) | Lutheran Brotherhood Cohort Study, 17 633 White men in the United States, aged ≥ 35 y, followed 1966–1986 | 20 | 149 (Fatal cases) | Ever smokerc | 1.8 (1.1, 2.9) | 20–29 Cigarettesd | 1.7 (0.8, 3.5) | … | Age | |
| LeMarchand et al.36 (1994) | Hawaii State Department of Health Cohort, 20 316 men of various ethnicities (36% Caucasian), 1975–1989 | 14 | 198 (Incident cases) | … | Highest quartilee | 1.0 (0.6, 1.6) | … | Age, ethnicity, income | ||
| Lotufo et al.37 (2000) | Physicians Health Study, 22 071 men in the United States, aged 40–84 y at baseline (92% White, 49% nonsmokers) | 12.5 | 883 (Incident cases) 113 (fatal cases) | Ex-smoker | 1.14 (1.00, 1.30) | < 20 Cigarettes (current smokers) | 1.10 (0.78, 1.55) | ≥ 40 Pack years (incident cases) | 1.18 (0.95, 1.46) | Age, aspirin/beta carotene assignment, BMI, height, physical activity, alcohol use |
| > 20 Cigarettes (current smokers) | 1.10 (0.84, 1.44) | ≥ 40 Pack years (fatal cases) | 0.91 (0.47, 1.75) | |||||||
| Lund Nilsen et al.38 (2000) | National Health Screening Service, 22 895 men in Norway, aged ≥ 40 y, enrolled 1984–1986 | 9.3 | 644 (Incident cases) | Current smoker | 0.96 (0.78, 1.19) | > 15 Cigarettes | 1.27 (0.91, 1.76) | 25 Pack years | 1.22 (0.93, 1.60) | Age, cigarettes/d, alcohol consumption, leisure time physical activity index, marital status, occupation |
| Ex- smoker | 0.98 (0.80, 1.19) | |||||||||
| Mills et al.39 (1989) | Adventist Health Study, 14 000 men in the United States, aged ≥ 25 y, enrolled in 1976 | 6.0 | 180 (Incident cases) | Ex-smokerf | 1.24 (0.91, 1.67) | … | … | Age | ||
| Putnam et al.40 (2000) | Retrospective cohort study, 1572 men in Iowa, 1986–1989 | Up to 9.0 | 56 (Incident cases) | Ex-smokerg | 1.4 (0.9, 2.3) | … | … | Age | ||
| Rodriguez et al.41 (1997) | Cancer Prevention Study II, 450 279 men in the United States, aged ≥ 30 y, enrolled in 1982 | 9.0 | 1748 (Fatal cases) | Ever smoker | 1.02 (0.92, 1.14) | … | … | Age, race, education, family history, vasectomy, exercise, BMI, alcohol use, vegetable/meat intake | ||
| Ex-smoker | 0.99 (0.87, 1.12) | |||||||||
| Current smoker | 1.34 (1.16, 1.56) | > 20 Cigarettes | 1.25 (1.00, 1.57) | > 45 y | 1.26 (1.04, 1.53) | |||||
| Rohrmann et al.42 (2007) | 102 Men from 2 private censuses in Washington County, MD, 1963 and 1975 | 15 and 19 | 498 (Incident cases) | Current smoker (1963 cohort) | 1.00 (0.63, 1.59) | ≥ 20 Cigarettes (1963 cohort) | 1.38 (0.75, 2.54) | … | Age | |
| Ex-smoker (1963 cohort) | 1.33 (0.85, 2.10) | |||||||||
| Current smoker (1975 cohort) | 0.98 (0.73, 1.33) | ≥ 20 Cigarettes (1975 cohort) | 1.01 (0.65, 1.57) | … | ||||||
| Ex-smoker (1975 cohort) | 1.04 (0.80, 1.36) | |||||||||
| 424 (Fatal cases) | Current smoker (1963 cohort) | 0.93 (0.67, 1.29) | ≥ 20 Cigarettes (1963 cohort) | 0.95 (0.62, 1.47) | ||||||
| Ex-smoker (1963 cohort) | 1.01 (0.70, 1.46) | |||||||||
| Current smoker (1975 cohort) | 1.25 (0.84, 1.87) | ≥ 20 Cigarettes (1975 cohort) | 1.58 (0.94, 2.64) | |||||||
| Ex-smoker (1975 cohort) | 1.02 (0.69, 1.50) | |||||||||
| Severson et al.43 (1989) | 7999 Men of Japanese ancestry in Hawaii, born 1900–1919, enrolled 1965–1968 | 21 | 174 (Incident cases) | Current smoker | 0.87 (0.61, 1.23) | … | … | Age | ||
| Ex-smoker | 0.89 (0.61, 1.29) | |||||||||
| Thompson et al.44 (1989) | Lipid Research Clinics Prevalence Study, 6 110 adults from Rancho Bernardo, CA, 1972–1974 | 14 | 54 (Incident cases) | Current smoker | 1.06 (0.62, 1.80) | … | … | None | ||
| Veierod et al.45 (1997) | 25 708 Men in Norway, aged 16–56 y, enrolled 1977–1983 via a health screening | 15 | 72 (Incident cases) | Ex-smokerh | 0.6 (0.3, 1.1) | ≤ 10 Cigarettesi (current smoker) | 0.5 (0.3, 1.1) | … | Age | |
| ≥ 11 Cigarettesj (current smoker) | 0.6 (0.3, 1.2) |
Note. RR = relative risk; CI = confidence interval; BMI = body mass index; NA = not applicable. Ellipses indicate data that were not included in study.
This study had 12 357 fatal cases from all cancers.
Update published in 2007 for same cohort; 1999 study was included for total pack years risk data.
Classified smokers as “ever used tobacco.”
Highest intake category of tobacco by cigarettes per day had only 3 prostate cancer deaths, making risk estimates questionable. RRs shown are for second highest intake, 20 to 29 cigarettes per day.
Explicit definition of quartiles of cigarettes per day not specified in article; data are presented for highest quartile.
Only data on former smokers was used because there were only 3 cases of prostate cancer among current smokers.
Number of cases for current smokers of fewer than 20 cigarettes per day and 20 or more cigarettes per day were both fewer than 10 participants; therefore only data on former smokers were included in the meta-analysis.
Based on data from 20 patients.
Based on data from 11 patients.
Based on data from 14 patients.
Each study used 1 of 2 endpoints: incident prostate cancer or prostate cancer mortality. Smoking status was categorized by a binary measure, current versus ever or former, or by a quantitative measure, such as number of cigarettes per day or pack years of smoking (Table 1).
Pooling homogeneous data (P = .131) from 8 cohorts in studies examining the risk of incident prostate cancer among current smokers22,25,28,29,38,42–44 gave a summary RR of 1.04 (95% CI = 0.87, 1.24; Figure 1).
FIGURE 1.
Results of literature search: overview of 24 cohort studies.
Because use of the broad exposure categorization of current smoker could mask a dose-dependent effect, if one exists, we then calculated summary RRs for several subgroups of studies that quantified smoking history. Seven cohorts contained information on number of cigarettes smoked per day versus years or pack years of smoking and risk of incident prostate cancer among current smokers.22,25,30,36–38,42 Comparing the highest to the lowest exposure category yielded an RR of 1.13 (95% CI = 1.03, 1.23), consistent with a 13% greater risk of prostate cancer among the heaviest smokers. Combining data only from cohorts that were stratified specifically by number of cigarettes smoked per day25,37,38,42 (i.e., 4 of the 7 studies that collected these data) showed a 22% increased risk of incident prostate cancer (RR = 1.22; 95% CI = 1.01, 1.46).
Homogeneous data (P = .15) on incident cancer risk among current smokers measured in years or pack years of smoking (highest versus lowest) from the 5 cohort studies containing such information22,25,30,37,38 were also consistent with a small but significant increase in prostate cancer risk (RR = 1.11; 95% CI = 1.01, 1.22). As shown in Table 1, these 5 studies used varying cutoff points for their highest smoking exposure categories, ranging from more than 21 years of smoking22 to more than 55 pack years.25 These differences could contribute to attenuation of the resultant RRs.
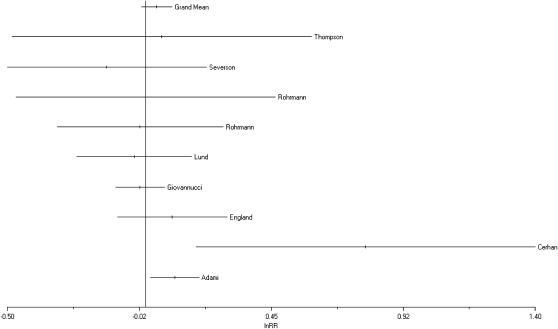
Ten cohort studies also provided data on risk of incident prostate cancer among ex-smokers.22,25,28,33,37–40,42,43 The RR was consistent with our pooled estimates derived from current smokers: 1.09 (95% CI = 1.02, 1.16; Figure 2). Excluding from our analysis a study that analyzed incident and fatal cases together37 slightly attenuated the outcome (RR = 1.07; 95% CI = 1.01, 1.14). None of these 10 studies provided quantitative estimates of amount smoked.
FIGURE 2.
Forest plot of pooled results reflecting risk of incident prostate cancer among subjects stratified as current smokers: overview of 24 cohort studies.
Among reports in which prostate cancer mortality was the outcome of interest,22,26,27,29,31,32,34,35 current smokers had a 17% greater risk of death from prostate cancer than did nonsmokers (RR = 1.17; 95% CI = 1.10, 1.23). The pooled data were heterogeneous (P = .007), with 1 study accounting for more than half of the observed heterogeneity (data not shown).31 That study was among the oldest in the database, dating from 1958, when the demographic characteristics of smokers in the United States differed from those after 1989, the period during which 22 of our 24 cohort studies were published. Excluding the data from that older study from the pooled analysis eliminated the statistical heterogeneity (RR = 1.14; 95% CI = 1.06, 1.19; P = .15).
These findings were further supported by summary RRs derived by pooling data from mortality studies that quantified smoking by cigarettes per day (RR = 1.30; 95% CI = 1.16, 1.46; P = .20)22,23,26,34,35,37,41,42 or pack years (RR = 1.24; 95% CI = 1.09, 1.40; P = .83)22,34,37,41; these data were homogeneous. The available data on ex-smokers and prostate cancer mortality were heterogeneous (P < .001) and could not be statistically pooled.
DISCUSSION
Pooled data from 24 cohort studies enrolling more than 26 000 participants with prostate cancer showed a modest, although consistent, 9% to 30% increase in both incident and fatal prostate cancer associated with smoking (Table 2). Former smokers had the smallest increase in prostate cancer risk: their risk of incident tumors was 9% higher than that of nonsmokers. Because former or ex-smoker is such a broad, nonquantitative measure of exposure, it is reasonable to assume that this RR represents an underestimate of the strength of the true underlying association. This may also be true of the summary estimates of effect reflecting the effect of current smoking on incident and fatal prostate tumors. The higher RRs calculated from incidence or mortality data for current smokers in studies that used quantitative measures of exposure support this contention.
TABLE 2.
Meta-Analysis Overview: 24 Cohort Studies
| Risk Category | Studies, No. | RR (95% CI) |
| Incident prostate cancer | ||
| Current smoker | 8 | 1.04 (0.87, 1.24) |
| Current smokera | 4 | 1.22 (1.01, 1.46) |
| Current smokerb | 5 | 1.11 (1.01, 1.22) |
| Ex-smoker | 10 | 1.09 (1.02, 1.16) |
| Fatal prostate cancer | ||
| Current smoker | 7 | 1.14 (1.06, 1.19) |
| Current smokera | 8 | 1.30 (1.16, 1.46) |
| Current smokerc | 4 | 1.24 (1.09, 1.40) |
| Ex-smokerd | 6 | … |
Note. RRs = summary relative risk; CI = confidence interval. Ellipsis indicates RR not calculated.
Cigarettes per day, highest versus lowest.
Years, pack years, highest versus lowest.
Pack years, highest versus lowest.
Only study that was not homogeneous.
Previous published data suggested no clear evidence of a causal relationship between smoking and prostate cancer development.5 Studies that used cancer incidence as the endpoint had inconsistent outcomes. Mortality studies also provided little evidence of an association.5 By contrast, our meta-analysis revealed a statistically significant and consistent increase in prostate cancer incidence as well as an increased risk of death from this disease with increased smoking.
It is possible that the sample sizes in many previous studies were too small to detect an effect. By contrast, our meta-analysis combined information on more than 21 000 prostate cancer cases. The validity of our findings was further supported by the elevated risk of prostate cancer we observed among former smokers, which was lower than for current smokers and higher than for nonsmokers. It is likely that the use of ill-defined smoking categories, as well as failure to update smoking status over time,28,36 contributed to attenuating, and therefore masked the modest association we observed in our pooled data.
The studies we analyzed varied in their outcome measures, so it is important to interpret their results in context. For example, cancer incidence and mortality address somewhat different issues. Incidence data likely reflect an effect of smoking on disease etiology or initiation; observational studies that take prostate cancer mortality as an endpoint only indirectly provide insight into this relationship.
Mortality studies may reflect the effect of smoking on tumor progression by various proposed mechanisms, for example, smoking may increase serum estrogen metabolites that have been postulated to induce a more aggressive tumor phenotype and thereby increase prostate cancer death.42 Other investigators suggest that smoking may cause mutation of the p53 tumor suppressor gene, creating another pathway to an aggressive tumor phenotype and increased mortality.30 Whether smokers differ from nonsmokers in the type of therapy received, which in turn could influence survival, is also unknown.9
We found that former smokers have increased risk for prostate cancer, which does not support the suggestion that smoking is related to poorer survival during treatment.34 The increasing gradient in risk across higher exposure categories suggests a biological relationship. Although the exact mechanism underlying the positive association between smoking and prostate cancer death is unknown, it is clear that data from incidence and mortality studies should be considered separately.
Despite these caveats, the results of our analysis of pooled data were quite consistent across endpoints. Although the calculated summary estimates of effect showed a modest (weak) effect of smoking on prostate cancer, it is likely that several factors contributed to attenuating the association. These included ill-defined smoking status, lack of repeat assessments of smoking status in many cohorts, and the possibility of a screening effect. The effects of screening for prostate cancer could attenuate an association: previous work suggested lower rates of such screening among smokers than nonsmokers.47,48 Although stage stratification could help address this issue, we did not find such data in our literature search.
As suggested by Hickey et al.,9 future studies should collect data on stage and grade of tumor and on smoking history, including quantity smoked and details of smoking cessation. Although additional work is needed to clarify the relationship between smoking and prostate cancer, our results support a causal association.
Acknowledgements
Funding was provided by the Meta-Analysis Research Group.
Human Participant Protection
No protocol approval was necessary because data were obtained from secondary sources.
References
- 1.American Cancer Society Cancer Facts and Figures. Available at: http://www.cancer.org/docroot/CRI/content/CRI_2_2_1X_How_many_men_get_prostate_cancer_36.asp?sitearea=tp. Accessed April 6, 2008
- 2.Chan JM, Jou RM, Carroll PR. The relative impact and future burden of prostate cancer in the United States. J Urol 2004;172:S13–S16 [PubMed] [Google Scholar]
- 3.Haenszel W, Kurihara M. Studies of Japanese migrants. I. Mortality from cancer and other diseases among Japanese in the United States. J Natl Cancer Inst 1968;40:43–68 [PubMed] [Google Scholar]
- 4.Pienta KJ, Esper PS. Risk factors for prostate cancer. Ann Intern Med 1993;118:793–803 [DOI] [PubMed] [Google Scholar]
- 5.Colditz G. Consensus conference: smoking and prostate cancer. Cancer Causes Control 1996;7:560–562 [DOI] [PubMed] [Google Scholar]
- 6.Tobacco Smoking Lyon, France: International Agency for Research on Cancer Press; 1986:199–298 IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; vol 38 [Google Scholar]
- 7.Pour PM. A new prostatic cancer mode: systemic induction of prostatic cancer in rats by nitrosamine. Cancer Lett 1981;13:303–308 [DOI] [PubMed] [Google Scholar]
- 8.Dai WS, Gutai JP, Kuller LH, Cauley JA. Cigarette smoking and serum sex hormones in men. Am J Epidemiol 1988;128:796–805 [DOI] [PubMed] [Google Scholar]
- 9.Hickey K, Do KA, Green A. Smoking and prostate cancer. Epidemiol Rev 2001;23(1):115–125 [DOI] [PubMed] [Google Scholar]
- 10.Cooper H, Hedges LV. The Handbook of Research Synthesis New York, NY: Russell Sage; 1994:286–298 [Google Scholar]
- 11.Petitti DB. Meta-Analysis, Decision Analysis and Cost Effectiveness Analysis. Methods for Quantitative Synthesis in Medicine 2nd ed New York, NY: Oxford University Press; 2000:133–135 [Google Scholar]
- 12.Dosemeci M, Hayes RB, Vetter R, et al. Occupational physical activity, socioeconomic status, and risk of 15 cancer sites in Turkey. Cancer Causes Control 1993;4:313–321 [DOI] [PubMed] [Google Scholar]
- 13.Hirayama T. Epidemiology of prostate cancer with special reference to the role of diet. Natl Cancer Inst Monogr 1979;53:149–155 [PubMed] [Google Scholar]
- 14.Nilsen TI, Romundstad PR, Vatten LJ. Recreational physical activity and risk of prostate cancer: a prospective population-based study in Norway (the HUNT study). Int J Cancer 2006;119:2943–2947 [DOI] [PubMed] [Google Scholar]
- 15.Eichholzer M, Bernasconi F, Jordan P, Stahelin HB. Body mass index and the risk of male cancer mortality of various sites: 17-year follow-up of the Basel Cohort Study. Swiss Med Wkly 2005;135:27–33 [DOI] [PubMed] [Google Scholar]
- 16.Eichholzer M, Stahelin HB, Ludin E, Bernasconi F. Smoking, plasma vitamins C, E, retinol and carotene and fatal prostate cancer: seventeen-year follow-up of the Prospective Basel Study. Prostate 1999;38:189–198 [DOI] [PubMed] [Google Scholar]
- 17.Tverdal A, Thelle D, Stensvold I, Leren P, Bjartveit K. Mortality in relation to smoking history: 13 years follow-up of 68,000 Norwegian men and women 35–46 years. J Clin Epidemiol 1993;46(5):475–487 [DOI] [PubMed] [Google Scholar]
- 18.Carstensen JM, Pershagen G, Eklund G. Mortality in relation to cigarette and pipe smoking: 16 years observation of 25,000 Swedish men. J Epidemiol Community Health 1987;41:166–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weir JM, Dunn JE. Smoking and mortality: a prospective study. Cancer 1970;25:105–112 [DOI] [PubMed] [Google Scholar]
- 20.Daniell HW. More stage A prostatic cancers, less surgery for benign hypertrophy in smokers. J Urol 1993;149:68–72 [DOI] [PubMed] [Google Scholar]
- 21.Rogot E, Murray JL. Smoking and causes of death among US veterans: 16 years of observation. Public Health Rep 1980;95:213–222 [PMC free article] [PubMed] [Google Scholar]
- 22.Adami HO, Bergström R, Engholm G, et al. A prospective study of smoking and risk of prostate cancer. Int J Cancer 1996;67:764–768 [DOI] [PubMed] [Google Scholar]
- 23.Akiba S, Hirayama T. Cigarette smoking and cancer mortality risk in Japanese men and women—results from reanalysis of the six-prefecture cohort study data. Environ Health Perspect 1990;87:19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen NE, Sauvaget C, Roddman AW, et al. A prospective study of diet and prostate cancer in Japanese men. Cancer Causes Control 2004;15:911–920 [DOI] [PubMed] [Google Scholar]
- 25.Cerhan JR, Torner JC, Lynch CF, et al. Association of smoking, body mass, and physical activity with risk of prostate cancer in the Iowa 65+ Rural Health Study (United States). Cancer Causes Control 1997;8:229–238 [DOI] [PubMed] [Google Scholar]
- 26.Coughlin SS, Neaton JD, Sengupta A. Cigarette smoking as a predictor of death from prostate cancer in 348,874 men screened for the Multiple Risk Factor Intervention Trial. Am J Epidemiol 1996;143:1002–1006 [DOI] [PubMed] [Google Scholar]
- 27.Doll R, Peto R, Boreham J, Sutherland I. Mortality from cancer in relation to smoking: 50 years observations on British doctors. Br J Cancer 2005;92(3):426–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engeland A, Andersen A, Haldorsen T, Tretli S. Smoking habits and risk of cancers other than lung cancer: 28 years' follow-up of 26,000 Norwegian men and women. Cancer Causes Control 1996;7:497–506 [DOI] [PubMed] [Google Scholar]
- 29.Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. Risk factors for prostate cancer incidence and progression in the Health Professionals follow-up study. Int J Cancer 2007;121:1571–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giovannucci E, Rimm EB, Ascherio A, et al. Smoking and risk of total and fatal prostate cancer in the United States health professionals. Cancer Epidemiol Biomarkers Prev 1999;8:277–282 [PubMed] [Google Scholar]
- 31.Hammond EC, Horn D. Smoking and death rates—report on forty-four months of follow-up of 187,783 men. II. Death rates by cause. JAMA 1958;166:1294–1308 [DOI] [PubMed] [Google Scholar]
- 32.Hammond EC. Smoking in relation to the death rates of one million men and women. Natl Cancer Inst Monogr 1966;19:127–204 [PubMed] [Google Scholar]
- 33.Hiatt RA, Armstrong MA, Klatsky AL, Sidney S. Alcohol consumption, smoking and other risk factors and prostate cancer in a large health plan cohort in California (United States). Cancer Causes Control 1994;5:66–72 [DOI] [PubMed] [Google Scholar]
- 34.Hsing AW, McLaughlin JK, Hrubec Z, Blot WJ, Fraumeni JF. Tobacco use and prostate cancer: 26-year follow-up of US veterans. Am J Epidemiol 1991;133:437–441 [DOI] [PubMed] [Google Scholar]
- 35.Hsing AW, McLaughliln JK, Shuman LM, et al. Diet, tobacco use and fatal prostate cancer: results from the Lutheran Brotherhood cohort study. Cancer Res 1990;50:6836–6840 [PubMed] [Google Scholar]
- 36.Le Marchand L, Kolonel LN, Wilkens LR, Myers BC, Hiohata T. Animal fat consumption and prostate cancer: a prospective study in Hawaii. Epidemiology 1994;5:276–282 [DOI] [PubMed] [Google Scholar]
- 37.Lotufo PA, Lee IM, Ajani UA, Hennekens CH, Manson JE. Cigarette smoking and risk of prostate cancer in the Physicians Health Study (United States). Int J Cancer 2000;87:141–144 [DOI] [PubMed] [Google Scholar]
- 38.Lund Nilsen TI, Johnsen R, Vatten LJ. Socio-economic and life-style factors associated with the risk of prostate cancer. Br J Cancer 2000;82:1358–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mills PK, Beeson WL, Phillips RL, Fraser GE. Cohort study of diet, lifestyle and prostate cancer in Adventist men. Cancer 1989;64:598–604 [DOI] [PubMed] [Google Scholar]
- 40.Putnam SD, Cerhan JR, Parker AS, et al. Lifestyle and anthropometric risk factors for prostate cancer in a cohort of Iowa men. Ann Epidemiol 2000;10:361–369 [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez C, Tatham LM, Thun MJ, Calle EE, Heath CW., Jr Smoking and fatal prostate cancer in a large cohort of adult men. Am J Epidemiol 1997;145:466–475 [DOI] [PubMed] [Google Scholar]
- 42.Rohrmann S, Genkinger JM, Burke A, et al. Smoking and risk of fatal prostate cancer in a prospective study. Urology 2007;69:721–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Severson RK, Nomura AM, Grove JS, Stemmermann GN. A prospective study of demographics, diet and prostate cancer among men of Japanese ancestry in Hawaii. Cancer Res 1989;49:1857–1860 [PubMed] [Google Scholar]
- 44.Thompson MM, Garland C, Barett-Connor E, Khaw KT, Friedlander NJ, Wingard DL. Heart disease risk factors, diabetes, and prostatic cancer in an adult community. Am J Epidemiol 1989;129:511–517 [DOI] [PubMed] [Google Scholar]
- 45.Veierod MB, Laake P, Thelle DS. Dietary fat intake and risk of total and fatal prostate cancer: a prospective study of 25,708 Norwegian men. Int J Cancer 1997;73:634–638 [DOI] [PubMed] [Google Scholar]
- 46.Greenland S. Quantitative methods in the review of epidemiological literature. Epidemiol Rev 1987;9:1–30 [DOI] [PubMed] [Google Scholar]
- 47.Close DR, Kristal AR, Li S, Patterson RE, White E. Associations of demographic and health-related characteristics with prostate cancer screening in Washington State. Cancer Epidemiol Biomarkers Prev 1998;7:627–630 [PubMed] [Google Scholar]
- 48.Chui BC, Anderson JR, Corbin D. Predictors of prostate cancer screening among health fair participants. Public Health 2005;119:686–693 [DOI] [PubMed] [Google Scholar]