Abstract
We designed a phase I clinical trial of escalating doses of topotecan with cyclophosphamide and carboplatin in combination with autologous hematopoietic stem cell transplantation (AHSCT) for the treatment of relapsed or persistent platinum sensitive ovarian or primary peritoneal carcinoma. After stem cell collection, sixteen patients received topotecan at 1.5, 2.5, 3.5, 4.5 or 6.0 mg/m2/day combined with cyclophosphamide 1.5 grams/m2/day and carboplatin 200 mg/m2 /day, all by four-day continuous infusion. Steady state pharmacokinetics of topotecan and carboplatin were examined. Pretreatment biopsies were examined for expression of topoisomerase I, Ki67 and Bcl-2 family members by immunohistochemistry. One of six patients at a topotecan dose of 4.5 mg/m2/day and two of three patients at 6.0 mg/m2/day had dose-limiting toxicity of grade 3 stomatitis lasting >2 weeks. There was no treatment-related mortality. Because topotecan clearance was constant over the dose range examined, topotecan steady state plasma concentrations increased with dose. Median progression-free survival and overall survival were 6.5 months and 2.7 years, respectively. Shorter progression-free survival was observed in tumors with low topoisomerase expression (p = 0.04). Topotecan can be safely dose escalated to 4.5 mg/m2 per day in combination with cyclophosphamide, carboplatin and AHSCT. This trial is registered at ClinicalTrials.gov as NCT00652691.
Keywords: topotecan, phase I, autologous hematopoietic stem cell transplantation
Introduction
Ovarian cancer is the second most common female pelvic malignancy1. Despite the ability of treatment to produce states of minimal residual disease, recurrence and death are common; and five-year survival rates for patients with advanced disease are 35%-40%.1,2
Studies in hematologic malignancies have demonstrated that high-dose chemotherapy (HDC) with autologous hematopoietic stem cell transplant (AHSCT) can cure or prolong survival.3,4 Cohort studies of women diagnosed with persistent or relapsed ovarian cancer have reported long-term disease control with HDC-AHSCT.5-8 More recent studies, however, have questioned whether HDC-AHSCT confers any benefit compared to conventional chemotherapy.9,10 Neither of these two studies9,10 utilized topotecan, a topoisomerase (topo) I inhibitor that has been utilized in several prior transplant regimens11-18 and is active in relapsed and refractory ovarian cancer but induces dose-limiting hematological toxicity in the non-transplant setting.19-21
A series of preclinical investigations have suggested that the antiproliferative effects of topotecan and platinating agents such as carboplatin are synergistic in vitro22-24 and in vivo.25 Based on these results as well as the previously demonstrated efficacy of topotecan, carboplatin and cyclophosphamide as single agents in ovarian cancer, the activity of the topotecan/carboplatin and topotecan/cyclophosphamide doublets in clinical studies, and the observation that topotecan can be safely dose escalated, we designed a phase I clinical trial combining escalating doses of topotecan with cyclophosphamide and carboplatin in an attempt to develop a more effective high dose chemotherapy regimen for the treatment of relapsed and persistent ovarian carcinoma. This study was designed to enroll patients who have been shown in previous studies to be most likely to respond to this modality of therapy.6 In conjunction with this study, we examined the steady-state pharmacokinetics of high-dose topotecan and carboplatin as well as pretreatment expression of polypeptides that have been reported to affect the response to topotecan and DNA damaging agents in preclinical models.
MATERIALS AND METHODS
Patients
Women 18–70 years of age with relapsed or persistent platinum sensitive epithelial ovarian cancer, or primary peritoneal cancer were enrolled between September, 1998 and April, 2002. Relapsed platinum-sensitive ovarian carcinoma was defined as histologically documented recurrence with or without rising CA125 levels >6 months after initial platinum-based chemotherapy. Persistent platinum-sensitive ovarian cancer was defined as a positive second-look laparotomy in patients with normalization of CA125 after platinum-based chemotherapy. In patients with relapsed platinum-sensitive cancer the disease was less than 2 cm in greatest dimension at the time of transplant. Some patients underwent a debulking procedure to achieve this. All patients provided written informed consent to this Institutional Review Board - approved study.
Peripheral Blood Stem Cell Collection, High Dose Chemotherapy and Transplant
Therapy was based on the STAMP V HDC-AHSCT regimen for breast cancer26 except that topotecan was substituted for thiotepa. The doses of carboplatin given to the patients in this study were calculated in mg/m2 rather than by the area under the curve (AUC) method, as this is how the dosing was done in the STAMP V regimen. Patients were allowed to receive additional chemotherapy after debulking surgery if there were delays in obtaining insurance approval for HDC-AHSCT. All patients underwent re-staging of their disease within 30 days prior to transplant. For stem cell mobilization, patients received granulocyte-colony stimulating factor (G-CSF) 10 mcg/kg subcutaneously daily until leukapheresis yielded a minimum of 2 ×106 CD34+ cells/kg. Patients who did not mobilize well were allowed to undergo autologous bone marrow harvest. After recovery from harvest, patients received a four-day continuous intravenous infusion of carboplatin, 200 mg/m2/day (total dose 800 mg/m2) and cyclophosphamide, 1500 mg/m2/day (total dose 6000 mg/m2), along with an assigned dose of topotecan. The initial dose level of topotecan was 1.5 mg/m2/day (total dose 6.0 mg/m2) with subsequent planned dose levels of 2.5, 3.5, 4.5, 6.0, 7.5, and 9.0 mg/m2/day in an accelerated titration schedule. Beginning on day +6 post-AHSCT, granulocyte macrophage-colony stimulating factor (GM-CSF) 5 mcg/kg/day was administered until an absolute neutrophil count >0.5 × 109 /L was maintained for 2 consecutive days.
On day 100 post transplant, every 3 months in years 1-2 and then biannually years 2-5 until progression, patients underwent a physical examination that included a complete blood count, blood chemistry profile, CA125 measurement, a tumor assessment and a toxicity assessment. Patients who refused further treatment or discontinued treatment due to toxicity were followed annually for toxicity, progression, and death.
Pharmacokinetic Analyses
Topotecan and unbound platinum were analyzed in blood samples (2 × 7 ml) drawn prior to and at 24, 48, 72, and 96 hours of chemotherapy infusion. Plasma was prepared within 20 min of blood collection and immediately ultrafiltered (for free platinum) or frozen at −70°C until analyzed for topotecan. Total topotecan and ultrafilterable platinum were assayed as described.27 The steady-state plasma concentrations, Css, were calculated as the average of the 24 – 96 hour plasma concentrations. The steady-state clearance, CLss, was calculated by dividing the dose rate by the Css value. CLss values for carboplatin were corrected for the molecular weights of carboplatin (371.25 gm/mol) and platinum (195.1 gm/mol).
Immunohistochemical analysis of pre-transplant tumor samples
Pretreatment biopsies were analyzed for topoisomerase I (topo I), MIB-1, Bcl-2, Bcl-xL, Mcl-1, Bax and p53 by immunohistochemistry (IHC). In brief, a tissue microarray was constructed from the most recent biopsy specimen obtained prior to trial enrollment. Sections (4 □m thick) were deposited on slides; deparaffinized; rehydrated in ethanol; and incubated for 10 min at room temperature in 3% H2O2 in methanol to inactivate endogenous peroxidases. After microwave-induced antigen retrieval (30 min in 0.01 M citric acid, pH 6.0), slides were stained using commercially available antibodies and standard immunoperoxidase methods in the Tissue and Cell Molecular Analysis Shared Resource of the Mayo Clinic Cancer Center. Digital images captured with a using a Bliss “Virtual Microscopy” microscope were visually examined by an investigator who was blinded to the patient outcome; and the intensity of staining of tumor cells was graded as negative (0), weak (1+), moderate (2+) or strong (3+). The percentage of positively stained ovarian cancer cells in each image was also estimated. Values presented are the mean of the three cores on the tissue microarray whenever possible.
Statistical Considerations
The primary objective was to determine the maximum tolerated dose (MTD) of topotecan when used in combination with high dose cyclophosphamide and carboplatin. Dose-limiting toxicities (DLT) were defined to be grade 4 hematologic toxicities lasting >8 weeks, mucositis resulting in aspiration pneumonia or requiring either preventive intubation or parenteral narcotics and/or nutrition for >14 days, and any unexpected grade 3+ non-hematologic toxicity that could not be well controlled or did not improve after two weeks duration. These levels of toxicities were chosen so as not to prematurely terminate the dose escalation schedule of topotecan as a result of a less severe toxicity.
Because topotecan had been successfully dose escalated in a previous transplant study up to the equivalent of 5 mg/m2 per day by continuous infusion,11 an accelerated titration approach was utilized. A minimum of one or a maximum of six patients were to be enrolled onto the first three dose levels and a minimum of two to a maximum of six patients were to be enrolled onto all subsequent dose levels. The serious adverse events that triggered a change in the dose escalation schedule from one patient per dose level to three patients per dose level were grade >2 non-hematologic toxicities except grade 3/4 infection/febrile neutropenia, vomiting, stomatitis, petechiae/purpura or vaginal bleeding and grade 4 bone marrow engraftment or peripheral stem cell toxicity. If no serious adverse events were reported among the patients enrolled on the first three dose levels, then the fourth dose level and all subsequent dose levels would initially treat three patients.
Time to progression was defined as the time from registration to disease progression. Patients who died without documentation of progression were considered to have progressed on the date of their death. Survival was measured from registration to death. Times to event distributions were estimated using the Kaplan-Meier method. Wilcoxon rank sum tests were used to assess whether time to progression or survival time differed with respect to disease site.
RESULTS
Clinical Outcomes
As indicated in Table 1, a total of 16 patients, 10 (62%) with ovarian carcinoma at diagnosis and 6 (38%) with primary peritoneal carcinoma, were enrolled in this study. At initial diagnosis 1 patient had stage II disease, 11 stage IIIC, and 4 stage IV disease. The median age at enrollment was 51 years (range 38-63).
Table 1.
Individual Patient Characteristics
| Patient # | Site of primary disase |
Surgery at initial diagnosis* |
DFI (months) |
Surgery for recurrence |
Disease volume at completion of SLL or debulking |
Cycles of Taxol/ Platinum after surgery & before BMT |
CA 125 (U/mL)^ | Response at 100 days post transplant |
|
|---|---|---|---|---|---|---|---|---|---|
| within 30 days prior to transplant |
100 days post transplant |
||||||||
| 1 | ovary | Optimal debulking (No SLL) |
26 | single mesenteric nodule removed |
microscopic disease |
4 | 15.8 | 24.3 | stable |
| 2 | ovary | Optimal debulking |
0 | SLL positive | macroscopic disease |
1 | 12.0 | 10.7 | CR |
| 3 | ovary | Optimal debulking |
0 | SLL positive | microscopic disease |
1 | 29.7 | 45.0 | stable |
| 4 | ovary | Optimal debulking |
0 | SLL positive | macroscopic disease |
0 | 33.2 | 27.0 | stable |
| 5 | ovary | Optimal debulking |
0 | SLL positive | microscopic disease |
0 | 50.3 | 60.7 | stable |
| 6 | ovary | Optimal debulking |
12 | Debulking | macroscopic disease |
0 | 63.3 | 50.8 | prog |
| 7 | peritoneum | Suboptimal debulking (negative SLL) |
9 | None | < 1 cm on CT scan |
1 | 463.0 | 819.0 | stable |
| 8 | peritoneum | Optimal debulking |
0 | SLL positive | microscopic disease |
0 | 53.9 | 1010.5 | prog |
| 9 | ovary | Suboptimal debulking, 3 cycles of chemo then optimal debulking |
0 | 3rd surgery | microscopic disease |
2 | 3.7 | 6.8 | CR |
| 10 | ovary | Optimal debulking (No SLL) |
6 | debulking | microscopic disease |
0 | 35.0 | 17.2 | CR |
| 11 | peritoneum | Suboptimal debulking |
0 | SLL positive | macroscopic disease |
1 | 57.2 | 321.9 | stable |
| 12 | peritoneum | Suboptimal debulking |
0 | SLL positive | macroscopic disease |
0 | 8.9 | 4.9 | CR |
| 13 | peritoneum | Optimal debulking |
2 | None, rising CA 125 |
No visible disease on CT scan |
0 | 828.0 | 674 | prog |
| 14 | peritoneum | Optimal debulking |
0 | SLL positive | macroscopic disease |
1 | 3.5 | 4.0 | regression |
| 15 | ovary | Optimal debulking |
0 | SLL positive | macroscopic disease |
1 | 12.0 | 11.0 | CR |
| 16 | ovary | Optimal debulking |
0 | SLL positive | macroscopic disease |
3 | 18.0 | 16.0 | prog |
Abbreviations: DFI=Disease-Free Interval, SLL=second look laparotomy, CT scan=computed tomography scan, prog=progression, CR=complete remission, AUC=area under the curve
Optimal debulking – largest diameter of a nodule is < 1cm at the end of surgery.
Disease-free interval is time from initial remission to relapse.
Macroscopic disease - largest diameter of a nodule ≤ 3 mm at the end of surgery.
Normal range for CA-125 level is <35 U/mL.
All patients were required to have platinum-sensitive disease. In 11, disease was microscopic or at most a few millimeters in size at second look laparotomy after 6 or more cycles of paclitaxel- and platinum-based intravenous chemotherapy. Four others had relapsed with disease-free intervals (DFIs) of 6, 9, 24, and 26 months after an initial regimen of paclitaxel- and platinum-based treatment. One additional patient was found to be ineligible after enrollment, as her DFI was only two months. Three of the five patients who relapsed had a secondary optimal debulking procedure prior to transplant. Of the 14 patients (11 with persistent disease and three with relapsed disease) who had surgery prior to HDC-AHSCT, no visible disease was left after laparotomy in five and residual nodules of a few millimeters or less were left in nine. The other two patients had no surgery immediately prior to transplant, as one had no measurable disease by clinical exam or CT scan of the abdomen and pelvis and the other had a less than one cm area of abnormality on CT scan.
Eight patients had additional paclitaxel and platinum-based chemotherapy after registration while awaiting insurance approval for transplant and not for purposes of tumor debulking. Of these eight, 5 patients had one cycle and one each had two, three and four cycles.
During administration of the conditioning regimen, carboplatin was administered as 200 mg/m2/day which translated to AUCs ranging from 2.7 to 4.2 (median=3.24) (Table 1)._Topotecan doses were escalated from 1.5 mg/m2/d to 6.0 mg/m2/d. One patient was treated at the 1.5 mg/m2/d dose level without DLT. Because the first patient treated at 2.5 mg/m2/d developed grade 4 emesis, albeit for <14 days, the accelerated dose escalation was terminated. Among two additional patients treated at 2.5 mg/m2/d, three at 3.5 mg/m2/d dose level and six at 4.5 mg/m2/d, there was a single DLT (grade 3 stomatitis lasting >14 days). In contrast, two of three patients at 6.0 mg/m2/d had DLTs consisting of severe stomatitis requiring parenteral nutrition and narcotics for >14 days. Thus, the MTD and recommended phase II dose is topotecan 4.5 mg/m2/d along with carboplatin 200 mg/m2/d and cyclophosphamide 1500 mg/m2/d, all by continuous four-day infusion.
All 16 patients had grade 4 hematologic toxicity and grade 3 febrile neutropenia. Of the 13 patients who developed grade 3 stomatitis, three had cases so severe that they were considered dose limiting. Other serious adverse events are presented in Table 2 and were typical of those seen with AHSCT.
Table 2.
Severe Toxicities Topotecan dose in mg/m2/day
| 1.5 (n=1) |
2.5 (n=3) |
3.5 (n=3) |
4.5 (n=6) |
6.0 (n=3) |
|
|---|---|---|---|---|---|
| Grade 4 Leukopenia-BMT | 1 | 3 | 3 | 6 | 3 |
| Grade 3/4 Neutropenia-BMT | 1 | 3 | 3 | 6 | 3 |
| Grade 3/4 Thrombocytopenia-BMT | 1 | 3 | 3 | 6 | 3 |
| Grade 3/4 Anemia | 1 | 3 | 3 | 4 | 3 |
| Grade 3 Febrile Neutropenia | 1 | 3 | 2 | 6 | 3 |
| Grade 3/4 Infection-ANC | 0 | 0 | 0 | 0 | 2 |
| Grade 3/4 Anorexia | 1 | 3 | 2 | 4 | 3 |
| Grade 3 Stomatitis | 0 | 2 | 2 | 6 | 3 |
| Grade 3 Dehydration | 0 | 1 | 0 | 3 | 0 |
| Grade 3 Epistaxis | 0 | 0 | 0 | 2 | 3 |
| Grade 3 Infection-Catheter | 1 | 2 | 0 | 1 | 0 |
| Grade 3 Hemorrhage-Thrombosis | 0 | 0 | 0 | 2 | 0 |
| Grade 3 Hypokalemia | 0 | 1 | 1 | 2 | 2 |
| Grade 3 Hyponatremia | 0 | 2 | 0 | 1 | 1 |
| Grade 3 Infection-Catheter | 1 | 2 | 0 | 1 | 0 |
| Grade 3 Nausea | 1 | 1 | 1 | 2 | 3 |
| Grade 3 SGOT | 0 | 0 | 0 | 2 | 1 |
| Grade 3 SGPT | 0 | 0 | 0 | 2 | 0 |
| Grade 3 Syncope | 0 | 0 | 0 | 0 | 2 |
The median times to neutrophil engraftment and platelet count >50 × 109/L were 11 days (range: 10-20) and 18 days (range 14-24), respectively. Three patients required a bone marrow harvest because of inadequate peripheral blood stem cell harvest, but all three engrafted well post-transplant.
At day 100 post-transplant, five patients had a complete response to treatment based on normalization of their CA125 level and/or resolution of CT scan abnormalities, one demonstrated regression of disease, six were stable and four had already progressed.
All 16 patients have died with disease progression. The observed median PFS was 6.5 months, with one-year PFS rate of 31% (95% CI: 15-65%). The median overall survival was 2.7 years. Because of the excellent recovery of blood counts following transplant, patients who relapsed were able to tolerate additional salvage chemotherapy. Of the 15 patients with adequate follow-up post-relapse, the median number of regimens of cytotoxic chemotherapy administered was five (range 0-9).
The observed median PFS and median OS was 7.9 months and 3.3 years, respectively, among the women with ovarian carcinoma (n=10) and 4.8 months and 2.3 years, respectively, among the women with primary peritoneal carcinoma (n=6). As expected, neither PFS (p=0.368) nor OS (p=0.712) were found to differ with respect to primary disease site.
Pharmacokinetic Analysis
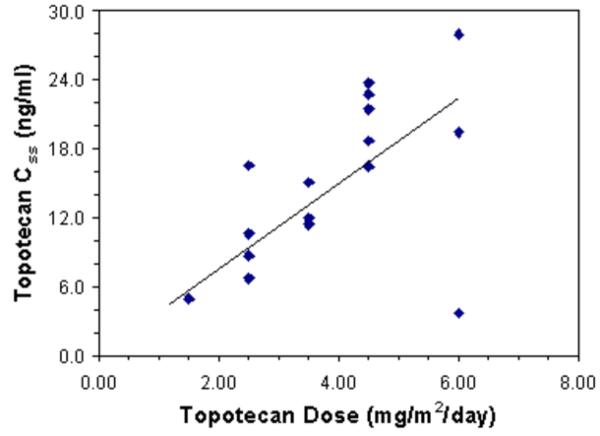
Steady state pharmacokinetics of total topotecan were evaluable for all 16 patients (Table 3). Topotecan CLss was similar over the dose range examined (Spearman's rank correlation coefficient =0.02), with a median value of 184 ml/min/m2 (range: 104-1068 ml/min/m2, Table 4). Accordingly, the topotecan Css tended to increase with topotecan dose (Spearman's rank correlation coefficient =0.57, Fig. 1A).
Table 3.
Topotecan and Carboplatin Pharmacokinetic Data
|
Patient |
Infusion Duration (hours) |
N |
Topotecan |
Carboplatin |
||||
|---|---|---|---|---|---|---|---|---|
| Dose (mg/m2) |
Css (ng/ml) |
CLss (ml/min/m2) |
Dose (mg/m2) |
Css (ng/ml) |
CLss (ml/min/m2) |
|||
|
| ||||||||
| 1 | 99.0 | 4 | 1.5 | 4.91 | 206 | 200 | 1105 | 64.0 |
| 2 | 89.3 | 4 | 2.5 | 6.72 | 278 | 200 | 1010 | 77.7 |
| 3 | 95.5 | 4 | 2.5 | 8.70 | 201 | 200 | 1268 | 57.9 |
| 4 | 96.0 | 4 | 2.5 | 10.59 | 164 | 200 | 1454 | 50.2 |
| 5 | 95.8 | 4 | 3.5 | 11.94 | 204 | 200 | 1222 | 59.9 |
| 6 | 95.5 | 4 | 3.5 | 11.42 | 214 | 200 | 1153 | 63.6 |
| 7 | 87.0 | 4 | 3.5 | 15.07 | 178 | 200 | 1200 | 67.1 |
| 8 | 97.0 | 4 | 2.5 | 16.52 | 104 | 200 | 1211 | 59.6 |
| 9 | 96.4 | 4 | 4.5 | 16.46 | 189 | 200 | 1031 | 70.5 |
| 10 | 96.7 | 4 | 4.5 | 18.67 | 166 | 200 | 1286 | 56.4 |
| 11 | 97.3 | 4 | 4.5 | 21.44 | 144 | 200 | 1405 | 51.2 |
| 12 | 97.5 | 4 | 4.5 | 22.74 | 135 | 200 | 1182 | 60.8 |
| 13 | 81.8 | 3 | 4.5 | 23.71 | 155 | 200 | 1249 | 68.6 |
| 14 | 80.3 | 4 | 6.0 | 27.94 | 178 | 200 | 1222 | 71.4 |
| 15 | 101.4 | 3 | 6.0 | 3.69 | 1068 | 200 | 1156 | 59.8 |
| 16 | 97.2 | 2 | 6.0 | 19.43 | 212 | 200 | 893 | 80.7 |
| Means (SD) |
237 (225) |
1190 (140) |
64 (9) |
|||||
N=number of concentration values used to define Css; Css=steady state plasma concentration; CLss=steady state clearance; SD=standard deviation
Table 4.
Immunohistochemical analysis of pre-transplant tumor specimens for topo-1 and other polypeptides implicated in topotecan resistance
| Pt. # | TTP (months) |
Topo I intensity |
MIB-1 (% +)a |
p53 intensity |
Bcl-2 intensity |
Mcl-1 intensity |
Bcl-xL intensity |
Bax intensity |
|---|---|---|---|---|---|---|---|---|
| 1 | 18.1 | 2 | 8* | 0 | 0 | 2 | 3 | 3 |
| 2 | 28.5 | 3 | 13 | 3 | 3 | 1 | 3 | 3 |
| 3 | 5.3 | 2 | 33 | 3 | 1 | 1 | 3 | 3 |
| 4 | 8.7 | 3 | 6 | 2.5 | 0 | 1 | 3 | 3 |
| 5 | 5.9 | 3 | 45 | 3 | 0 | 1 | 2 | 3 |
| 8 | 4.3 | 0 | 40 | 3 | 0 | 1.5 | 3 | 3 |
| 9 | 7.1 | 0 | 5 | 0 | 1 | 1 | 3 | 3 |
| 10 | 11.6 | 0 | 25 | 3 | 0 | 1 | 3 | 3 |
| 11 | 5.0 | 2 | 0 | 3 | 0 | 1 | 3 | 3 |
| 12 | 21.4 | 2 | 45 | 3 | 1 | 3 | 3 | 3 |
| 13 | 2.2 | 0.5 | 0 | 0 | 1.5 | 3 | 3 | 3 |
| 14 | 13.2 | 2.5 | 90 | 2.5 | 0 | 0 | 3 | 3 |
| 15 | 13.4 | 2.5 | 43 | 3 | 0 | 1 | 3 | 3 |
| 16 | 4.3 | 1 | 0 | 0 | 0 | 1.5 | 3 | 3 |
Estimated percentage of cells staining positive with MIB-1 antibody. All other values represent mean staining intensity in tumor cells on a scale of 0 to 3++, with values representing the mean of intensity scores from 3 or, in some cases, 2 separate cores on the same tissue microarray.
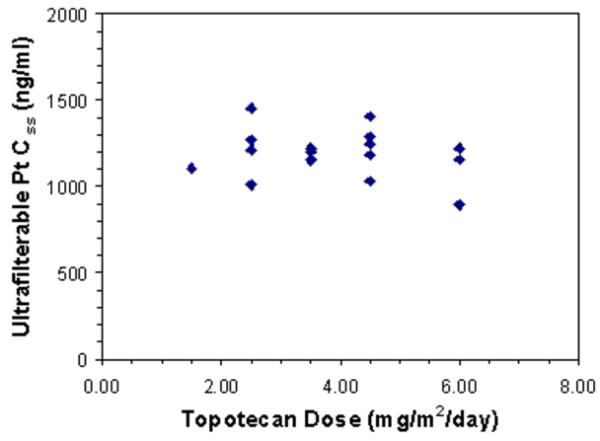
Figure 1.
A) Graph of topotecan steady-state plasma concentration (CSS) versus topotecan dose. B) Graph of ultrafilterable platinum (Pt) steady-state plasma concentration (CSS) versus topotecan dose.
The carboplatin CLss likewise was similar over the topotecan dose range examined (Spearman's rank correlation coefficient = −0.07, Fig. 1B), with a median value of 62 ml/min/m2 (range: 50-81 ml/min/m2). These pharmacokinetic parameters were similar to those reported in other clinical trials.27-29 There was no evidence of a significant association between steady-state free platinum levels and PFS (Spearman's rank correlation coefficient = −0.16).
Analysis of pre-transplant tumor specimens for topo I and other polypeptides implicated in topotecan resistance
Previous studies have identified a number of factors that can affect topotecan sensitivity in preclinical models. These include cell cycle progression30,31 and levels of the target enzyme topo I.31,32 In addition, recent studies have demonstrated that platinated DNA acts like a topo I poison to trap covalent topo -DNA complexes,33 leading to the suggestion that decreased topo I content might diminish sensitivity to cisplatin and topotecan. Moreover, expression of antiapoptotic Bcl-2 family members such as Bcl-2 and Bcl-xL has been reported to affect sensitivity to topotecan and DNA cross-linking agents.34,35 Accordingly, we analyzed Ki67 staining (a marker of proliferation) with the antibody MIB-1 and topo I staining as well as expression of the Bcl-2 family members Bcl-2, Bcl-xL, Mcl-1 and Bax by immunohistochemistry. Results of this analysis are summarized in Table 4.
Fourteen of 16 enrolled patients had a pre-registration biopsy specimen available for analysis. We observed that tumors with weak (1+) or undetectable topo I had a median time to progression (TTP) of 4.3 months (n = 5), whereas tumors with readily detectable topo I staining (2+ or 3+ intensity) had a median TTP of 13.2 months (n=9, Wilcoxon rank sum test p=0.042). There was also a modest positive correlation between the percentage of Mib-1 positive cells and progression-free survival time (Spearman rho=0.47, p = 0.09). Strong staining intensity was seen with Bax in all 14 pretreatment biopsies and with Bcl-xL in 13 (93%). Undetectable or weak staining was seen with Bcl-2 in 13 (93%) samples and with Mcl-1 in 9 (65%).
DISCUSSION
In this study, we were able to successfully dose escalate topotecan to 4.5 mg/m2/day (total dose 18 mg/m2) for four days in combination with cyclophosphamide and carboplatin. The vast majority of patients had undergone optimal debulking surgery of their tumor prior to transplant. As anticipated from the results of studies of topotecan in leukemia patients, the DLT was severe mucositis requiring parenteral nutrition and narcotics for more than two weeks. Based on this DLT at 6.0 mg/m2 per day, the MTD is 4.5 mg/m2/day. The regimen was otherwise generally well tolerated. All patients achieved neutrophil and platelet engraftment in a timely fashion, despite the fact that three patients required a bone marrow harvest. Although all patients have subsequently had disease progression, four patients survived more than four years after transplant. With the excellent recovery of blood counts following transplant, many patients were able to tolerate and respond to salvage chemotherapy as has been shown by others.36
Topotecan CLss was similar over the dose range administered. As a result, topotecan Css increased as the topotecan dose increased. Carboplatin CLss was also similar at all dose levels. In contrast to cisplatin, which diminishes topotecan clearance,37 we did not detect a significant pharmacokinetic interaction between topotecan and carboplatin. These results are consistent with a recent report that carboplatin does not affect topotecan clearance in leukemia patients.27
Examination of a variety of parameters in pretreatment ovarian cancer specimens indicated that low or absent staining for topo I, the target of topotecan, was associated with rapid progression. This correlation is consistent with the known relationship between topo I content and topotecan sensitivity observed in model systems.31,32 While topotecan was only one of three agents administered in this trial, it is important to note that platinated DNA33 and other DNA lesions38 have been reported to trap topo I-DNA covalent complexes. For topotecan and certain DNA damaging agents, diminished topo I expression has been associated with resistance in preclinical models, providing a potential explanation for the shortened disease-free survival in the present patients with tumors that expressed little or no topo I. Although two previous studies failed to observe any relationship between topo I expression and response of ovarian cancer patients to cisplatin-based therapy,39,40 the present study differed from the earlier ones by examining topo I polypeptide levels rather than mRNA and by specifically focusing on cancer cells rather than tumor samples containing both neoplastic epithelium and stroma. Further study is required to determine whether diminished topo I content correlates with resistance to other topotecan- or platinum-based ovarian cancer regimens.
Multiple phase I trials have reported the administration of escalating doses of topotecan in combination with etoposide, ifosfamide, carboplatin, paclitaxel, and/or thiotepa, demonstrating the feasibility of this approach with acceptable toxicity and modest response rates.11-18 The present study extends these observations to a topotecan/carboplatin/cyclophosphamide regimen that contains three agents known to have activity in ovarian cancer.
The role of autologous transplant in the treatment of ovarian carcinoma remains uncertain. A pilot study of three cycles of high dose carboplatin and paclitaxel followed by high-dose melphalan with AHSCT demonstrated only one pathologic complete response out of nine patients treated.41 A trial of 110 patients with advanced ovarian carcinoma (9 stage III and 101 stage IV) randomized after second-look laparotomy to receive either high-dose carboplatin and cyclophosphamide with AHSCT or conventional maintenance chemotherapy with carboplatin and cyclophosphamide9 demonstrated median disease-free and overall survival of 12 and 43 months in the conventional chemotherapy arm and 18 (p=0.22) and 50 (p=0.43) months in the high-dose chemotherapy arm. Another trial that randomized 149 patients following optimal debulking surgery to 6 cycles of carboplatin + paclitaxel versus two cycles of paclitaxel + cyclophosphamide followed by three cycles of high dose-carboplatin and paclitaxel with melphalan added to the third cycle demonstrated overall survival of 63 months in the standard chemotherapy arm compared to 54 months in the high dose arm.10 In view of the activity of topotecan, carboplatin and cyclophosphamide in ovarian cancer, we had hoped that the current regimen would yield longer PFS. The lack of long-term PFS, even at the highest doses, was disappointing. Accordingly, while we have defined an MTD for this regimen, this lack of long-term PFS, along with the lack of benefit of AHSCT in the randomized trials cited above, have prompted us to defer pursuing a phase II trial of this regimen at this time and further indicates that there are little or no data to support the use of AHSCT in the treatment of ovarian cancer outside of clinical trials. Nevertheless, we have demonstrated the feasibility of dose-escalating topotecan in the setting of AHSCT in combination with carboplatin and cyclophosphamide and this may have applicability in other tumor settings.
ACKNOWLEDGEMENTS
The authors thank the Mayo Clinic Tissue and Cell Molecular Analysis Shared Resources for assistance with the immunohistochemical analyses of biopsy samples and gratefully acknowledge the contributions of Margo Marzolf for data support, Kim Kalli for sample acquisition and Denise Chase for manuscript preparation. M. Litzow received research funding support from GlaxoSmithKline to conduct this research.: Glaxo SmithKline, R01 CA73709, P30 CA15083
Footnotes
There is no conflict of interest to declare.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.McGuire WP. Treatment of advanced epithelial ovarian cancer. Clin Oncol. 2002;5:1–8. [Google Scholar]
- 3.Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med. 1995;333:1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 4.Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 5.Stiff PJ, Bayer R, Kerger C, Potkul RK, Malhotra D, Peace DJ, et al. High-dose chemotherapy with autologous transplantation for persistent/relapsed ovarian cancer: a multivariate analysis of survival for 100 consecutively treated patients. J Clin Oncol. 1997;15:1309–1317. doi: 10.1200/JCO.1997.15.4.1309. [DOI] [PubMed] [Google Scholar]
- 6.Stiff PJ, Veum-Stone J, Lazarus HM, Ayash L, Edwards JR, Keating A, et al. High-dose chemotherapy and autologous stem-cell transplantation for ovarian cancer: an autologous blood and marrow transplant registry report. Ann Intern Med. 2000;133:504–515. doi: 10.7326/0003-4819-133-7-200010030-00009. [DOI] [PubMed] [Google Scholar]
- 7.Donato ML, Aleman A, Champlin RE, Saliba RM, Wharton JR, Burke TW, et al. Analysis of 96 patients with advanced ovarian carcinoma treated with high-dose chemotherapy and autologous stem cell transplantation. Bone Marrow Transplantation. 2004;33:1219–1224. doi: 10.1038/sj.bmt.1704473. [DOI] [PubMed] [Google Scholar]
- 8.Bengala C, Guarneri V, Ledermann J, Rosti G, Wandt H, Lotz JP, et al. High-dose chemotherapy with autologous haemopoietic support for advanced ovarian cancer in first complete remission: retrospective analysis from the solid tumour registry of the Europeon group for blood and marrow transplantation (EBMT) Bone Marrow Transplantation. 2005;36:25–31. doi: 10.1038/sj.bmt.1705007. [DOI] [PubMed] [Google Scholar]
- 9.Cure H, Battista C, Guastalla J, Fabbro M, Tubiana N, Bourgeois H, et al. Phase III randomized trial of high-dose chemotherapy (HDC) and peripheral blood stem cell (PBSC) support as consolidation in patients (pts) with responsive low-burden advanced ovarian cancer (AOC): 5 year follow-up of a GINECO/FNCLCC/SFGM-TC Study. Proc of ASCO. 2004;22:450s. abstract 5006. [Google Scholar]
- 10.Möbus V, Wandt H, Frickhofen N, Bengala C, Champion K, Kimmig R, et al. Phase III trial of high-dose sequential chemotherapy with peripheral blood stem cell support compared with standard dose chemotherapy for first-line treatment of advanced ovarian cancer: Intergroup trial of the AGO-Ovar/AIO and EBMT. J Clin Oncol. 2007;25:4187–4193. doi: 10.1200/JCO.2006.09.7527. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan D, Partyka J, Fields K, Goldstein S, Field T, et al. A phase I study of high-dose topotecan, ifosfamide/MESNA and etoposide (TIME) followed by autologous stem cell rescue in refractory malignancies. Exp Hem. 2000;28((7):Suppl 1):110. abstract. [Google Scholar]
- 12.Schilder RJ, Gallo JM, Millenson MM, Bookman MA, Weiner LM, Rogatko A, et al. Phase I trial of multiple cycles of high-dose carboplatin, paclitaxel, and topotecan with peripheral-blood stem-cell support as front-line therapy. J Clin Oncol. 2001;19:1183–1194. doi: 10.1200/JCO.2001.19.4.1183. [DOI] [PubMed] [Google Scholar]
- 13.Park JR, Slattery J, Gooley T, Hawkins D, Lindsley K, Villablanca JG, et al. Phase I topotecan preparative regimen for high-risk neuroblastoma, high-grade glioma, and refractory/recurrent pediatric solid tumors. Med Pediatr Oncol. 2000;35:719–723. doi: 10.1002/1096-911x(20001201)35:6<719::aid-mpo52>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 14.Tiersten A, Selleck M, Smith D, Wertheim I, Kaufman E, Hershman D, et al. Phase I/II study of tandem cycles of high-dose chemotherapy followed by autologous hematopoietic stem cell support in women with advanced ovarian cancer. Int J Gynecol Cancer. 2006;16:57–64. doi: 10.1111/j.1525-1438.2006.00278.x. [DOI] [PubMed] [Google Scholar]
- 15.Lotz J-P, Pautier P, Selle F, Viens P, Fabbro M, et al. Phase I study of high-dose topotecan with haematopoietic stem cell support in the treatment of ovarian carcinomas: the ITOV 01 protocol. Bone Marrow Transplantation. 2006;37:669–675. doi: 10.1038/sj.bmt.1705310. [DOI] [PubMed] [Google Scholar]
- 16.Perez-Martinez A, Lassaletta A, Gonzalez-Vicent M, Sevilla J, Angel Diaz M, et al. High-dose chemotherapy with autologous stem cell rescue for children with high risk and recurrent medulloblastoma and supratentorial primitive neuroectodermal tumors. Journal Neuro-Onc. 2005;71:33–38. doi: 10.1007/s11060-004-4527-4. [DOI] [PubMed] [Google Scholar]
- 17.Donato ML, Aleman A, Champlin RE, Weber D, Alexanian R, et al. High-dose topotecan, melphalan, cyclophosphamide (TMC) with stem cell support: a new regimen for the treatment of multiple myeloma. Leukemia & Lymphoma. 2004;45:755–759. doi: 10.1080/10428190310001603957. [DOI] [PubMed] [Google Scholar]
- 18.Bernbeck B, Bahci S, Meisel R, Troeger A, Schonberger S, et al. Serial intense chemotherapy combining topotecan, etoposide, carboplatin and cyclophosphamide (TECC) followed by autologous hematopoietic stem cell support in patients with high risk soft tissue sarcoma (STS) Klin Padiatr. 219:318–322. doi: 10.1055/s-2007-985896. [DOI] [PubMed] [Google Scholar]
- 19.Bookman MA, Malmstrom H, Bolis G, Gordon A, Lassoni A, Krebs JB, et al. Topotecan for the treatment of advanced epithelial ovarian cancer: an open-label phase II study in patients treated after prior chemotherapy that contained cisplatin or carboplatin and paclitaxel. J Clin Oncol. 1998;16:3345–3352. doi: 10.1200/JCO.1998.16.10.3345. [DOI] [PubMed] [Google Scholar]
- 20.McGuire WP, Blessing JA, Bookman MA, Lentz SS, Dunton CJ. Topotecan has substantial antitumor activity as first-line salvage therapy in platinum-sensitive epithelial ovarian carcinoma: A Gynecologic Oncology Group Study. J Clin Oncol. 2000;18:1062–1067. doi: 10.1200/JCO.2000.18.5.1062. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong D. Topotecan dosing guidelines in ovarian cancer: reduction and management of hematologic toxicity. Oncologist. 2004;9:33–42. doi: 10.1634/theoncologist.9-1-33. [DOI] [PubMed] [Google Scholar]
- 22.Kaufmann S, Peereboom D, Buckwalter C, Svingen PA, Grochow LB, Donehower RC, et al. Cyototoxic effects of topotecan combined with various anticancer agents in human cancer cell lines. J Natl Cancer Inst. 1996;88:699–700. doi: 10.1093/jnci/88.11.734. [DOI] [PubMed] [Google Scholar]
- 23.Jonsson E, Fridborg H, Nygren P, Larsson R. Synergistic interactions of combinations of topotecan with standard drugs in primary cultures of human tumor cells from patients. Eur J Clin Pharmacol. 1998;54:509–514. doi: 10.1007/s002280050505. [DOI] [PubMed] [Google Scholar]
- 24.Ma J, Maliepaard M, Nooter K, Boersma AW, Verweij J, Stoter G, et al. Synergistic cytotoxicity of cisplatin and topotecan or SN-38 in a panel of eight solid-tumor cell lines in vitro. Cancer Chemother Pharmacol. 1998;41:307–316. doi: 10.1007/s002800050744. [DOI] [PubMed] [Google Scholar]
- 25.Johnson R, McCabe FL, Yu Y. Combination regimens with topotecan in animal models. Ann Oncol. 1992;3(Suppl 1):85. abstract. [Google Scholar]
- 26.Antman K, Ayash L, Elias A, Wheeler C, Hunt M, Eder JP, et al. A phase II study of high-dose cyclophosphamide, thiotepa, and carboplatin with autologous marrow support in women with measurable advanced breast cancer responding to standard-dose therapy. J Clin Oncol. 1992;10:102–110. doi: 10.1200/JCO.1992.10.1.102. [DOI] [PubMed] [Google Scholar]
- 27.Kaufmann SH, Karp JE, Letendre L, Kottke TJ, Safgren SL, Greer J, et al. Phase I and Pharmacological Study of Infusional topotecan and Carboplatin in Relapsed and Refractory Acute Leukemia. Clin Cancer Res. 2005;11:6641–6649. doi: 10.1158/1078-0432.CCR-05-0817. [DOI] [PubMed] [Google Scholar]
- 28.Creemers GJ, Gerrits CJ, Schellens JH, Planting AS, van der Burg ME, van Beurden VM, et al. Phase II and pharmacologic study of topotecan administered as a 21-day continuous infusion to patients with colorectal cancer. J Clin Oncol. 1996;14:2540–2545. doi: 10.1200/JCO.1996.14.9.2540. [DOI] [PubMed] [Google Scholar]
- 29.Herben VM, ten Bokkel Huinink WW, Schot ME, Hudson I, Beijnen JH. Continuous infusion of low-dose topotecan: pharmacokinetics and pharmacodynamics during a phase II study in patients with small cell lung cancer. Anticancer Drugs. 1998;9:411–418. doi: 10.1097/00001813-199806000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Pizao P, Smitskamp-Wilms E, Van Ark-Otte J, Beijnen JH, Peters GJ, Pinedo HM, et al. Antiproliferative activity of the topoisomerase I inhibitors topotecan and camptothecin, on sub- and postconfluent tumor cell cultures. Biochemical Pharmacol. 1994;48:1145–1154. doi: 10.1016/0006-2952(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 31.Li T, Liu L. Tumor cell death induced by topoisomerase-targeting drugs. Ann Rev Pharmacol and Toxicol. 2001;41:53–77. doi: 10.1146/annurev.pharmtox.41.1.53. [DOI] [PubMed] [Google Scholar]
- 32.Nitiss J, Rose A, Sykes K, Harris J, Zhou J. Using yeast to understand drugs that target topoisomerases. Ann NY Acad Sci. 1996;803:32–43. doi: 10.1111/j.1749-6632.1996.tb26374.x. [DOI] [PubMed] [Google Scholar]
- 33.van Waardenburg R, de Jong L, van Eijndhoven MAJ, Verseyden C, Pluim D, Jansen LET, et al. Platinated DNA adducts enhance poisoning of DNA topoisomerase I by camptothecin. J Biol Chem. 2004;279:54502–9. doi: 10.1074/jbc.M410103200. [DOI] [PubMed] [Google Scholar]
- 34.Walton M, Whysong D, O'Connor P, Hockenbery D, Korsmeyer SJ, Kohn KW. Constitutive expression of human bcl-2 modulates nitrogen mustard and camptothecin induced apoptosis. Cancer Res. 1993;53:1583–1861. [PubMed] [Google Scholar]
- 35.Williams J, Lucas P, Griffith K, Choi M, Fogoros S, Hu YY, et al. Expression of bcl-xL in ovarian carcinoma is associated with chemoresistance and recurrent disease. Gynecol Oncol. 2005;96:287–295. doi: 10.1016/j.ygyno.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 36.Cook S, Penson R, Duska L, Nikrui N, Goodman A, Fuller A, et al. Efficacy and hematologic toxicity of salvage chemotherapy following stem cell-supported high-dose chemotherapy in women with recurrent ovarian cancer. Gynecol Oncol. 2000;77:48–54. doi: 10.1006/gyno.1999.5710. [DOI] [PubMed] [Google Scholar]
- 37.Rowinsky E, Kaufmann S, Baker S, Grochow LB, Chen TL, Peereboom D, et al. Sequences of topotecan and cisplatin: phase I, pharmacologic, and in vitro studies to examine sequence dependence. J Clin Oncol. 1996;14:3074–84. doi: 10.1200/JCO.1996.14.12.3074. [DOI] [PubMed] [Google Scholar]
- 38.Pourquier P, Waltman J, Urasaki Y, Loktionova NA, Pegg AE, Nitiss JL, et al. Topoisomerase I-mediated cytotoxicity of N-methyl-N′-nitro-N-nitrosoguanidine: trapping of topoisomerase I by the O6-methylguanine. Cancer Res. 2001;61:53–8. [PubMed] [Google Scholar]
- 39.Cornaratti M, Capranico G, Bohm S, Oriana S, Spatti GB, Mariani L, et al. Gene expression of DNA topoisomerases I, II alpha and II beta and response to cisplatin-based chemotherapy in advanced ovarian carcinoma. Int J Cancer. 1996;67:479–84. doi: 10.1002/(SICI)1097-0215(19960807)67:4<479::AID-IJC3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 40.Materna V, Pleger J, Hoffmann U, Lage H. RNA expression of MDR1/P-glycoprotein, DNA-topoisomerase I, and MRP2 in ovarian carcinoma patients: correlation with chemotherapeutic response. Gynecol Oncol. 2004;94:152–60. doi: 10.1016/j.ygyno.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 41.Schilder RJ, Brady MF, Spriggs D, Shea T. Pilot evaluation of high-dose carboplatin and paclitaxel followed by high-dose melphalan supported by peripheral blood stem cells in previously untreated advanced ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2003;88:3–8. doi: 10.1006/gyno.2003.6882. [DOI] [PubMed] [Google Scholar]