Abstract
Background:
Severed donor heart lymphatics are not anastomosed to recipient lymphatics in cardiac transplantation. We evaluated the effects of cellular infiltrates of T cells and macrophages on the morphology of lymphatics in heart grafts.
Methods:
Dark Agouti (DA) hearts were transplanted to Lewis or control DA rats on sub-therapeutic doses of cyclosporin. Transplants were examined by immunohistology and quantitative immunofluorescence microscopy using LYVE-1 as a lymphatic marker and CD8 and CD68 as markers for cellular infiltration at selected intervals from 1 to 8 weeks post-transplantation.
Results:
Allograft inner myocardial lymphatic density decreased by more than 30-fold at 1 week, and recovered to only 15% of the native level at 8 weeks post-transplantation. In contrast, allograft lymphatics in and near the epicardium showed no significant density decline, but increased in size by more than 5-fold at 2 weeks, and sustained about a 3-fold increase at 8 weeks post-transplantation. Lymphatic changes correlated temporally with the extent of T cell and macrophage infiltration in allografts, which peaked at 2-3 weeks post-transplantation. When grafts were retransplanted from allogeneic to isogeneic recipients at 3 weeks post-transplantation, inner lymphatic density returned close to native level within 2 weeks after retransplantation.
Conclusions:
This is the first characterization of regional and morphological effects of immunological responses on heart lymphatics after transplantation. Elimination of alloimmune responses produces rapid restoration of inner lymphatic vessels, suggesting that lymphatics injured during rejection can recover when rejection is reversed during the post-transplantation course.
Keywords: Heart, Lymphatics, Allograft, Alloimmunity, Rejection
INTRODUCTION
Cardiac transplantation is one of the standard treatments for end-stage heart failure. In cardiac transplantation, severed donor lymphatics to the heart are not anastomosed (1). The cardiac lymphatic system plays an important role in interstitial fluid balance and immune responses by providing a significant pathway for lymphocytes, antigen presenting cells, chemokines and interstitial fluid to return to the bloodstream and lymphoid organs (2). Both acute and chronic interruptions of lymphatic flow have been shown to be associated with significant anatomical and functional changes of the heart (3-6).
Restoration of lymphatic drainage from heart grafts after transplantation relies on lymphangiogenesis, a process that has not been well characterized in cardiac transplantation. Recent identification and application of specific lymphatic endothelial markers, such as lymphatic endothelial hyaluronan receptor-1 (LYVE-1), Podoplanin and Prox-1, have led to advances in understanding the molecular regulation of lymphangiogenesis (7,8). A number of signaling pathways have been shown to contribute to lymphangiogenesis in both embryonic development and pathologic processes in experimental models. The most well-studied signaling pathway involves the secreted lymphangiogenic glycoproteins, VEGF-C and VEGF-D, which act directly on the lymphangiogenic receptor, VEGFR-3 (Flt-4) (2,8-10). VEGFR-3 is a receptor expressed predominantly on lymphatic endothelial cells. VEGF-C and VEGF-D have been found to be secreted from dendritic cells and macrophages during inflammation (8-10), as well as viable cardiomyocytes around injured regions in the setting of myocardial infarction (11).
Aberrant lymphangiogenesis has been implicated in several fundamental pathological processes, such as inflammation, tumor metastasis and wound healing (8,12-16). Accordingly, its molecular regulatory pathway has been thought to be a potential therapeutic target for managing these processes (8,12-16). Association of lymphangiogenesis with inflammation and cellular infiltration has also been observed in various transplant lesions (17-19). For example, in chronically rejected human kidney grafts with nodular mononuclear infiltrates, lymphoid neogenesis was observed to develop in conjunction with ectopic germinal centers, which in turn were postulated to be involved in maintaining potentially harmful alloreactive immune responses in kidney grafts (20,21). In human endomyocardial biopsies of transplants, Quilty lesions, which are composed of mononuclear cell aggregations, were found to manifest focal angiogenesis and lymphangiogenesis associated with formation of tertiary lymphoid tissue and lymphatic vessels of recipient origin (22,23). While at present no diagnostic significance has been assigned to Quilty lesions (24), some studies have associated them with both acute and chronic cardiac rejections (22), thus suggesting a potential role of lymphangiogenesis in the dynamics of allograft failure in transplantation.
Based on the association among cardiac lymphatic disruption, lymphangiogenesis, immune cell infiltration and organ pathologies outlined above, we hypothesized that the re-establishment of lymphatics in cardiac allografts after transplantation was influenced by alloimmune responses, which in turn could play a role in allograft pathology. LYVE-1 is constitutively expressed on lymphatic endothelial cells under normal conditions and is a receptor for glycosaminoglycan hyaluronan in the extracellular matrix (2,7,25-27). Given that the extracellular matrix is an important regulator for tissue morphogenesis and lymphangiogenesis, and the interaction with matrix hyaluronan mediates the extravasation and migration of leukocytes during inflammation (2,7,25-27), changes in LYVE-1 expression would reflect direct functional responses of lymphatic endothelial cells to alloimmune reactivity after transplantation.
In the present study, we used LYVE-1 as a lymphatic marker to evaluate and characterize the effects of cellular infiltrates on the lymphatic vessels in rat heart grafts after transplantation using immunohistochemistry and quantitative microscopy.
MATERIALS AND METHODS
Animals
Male DA (RT1a) rats and Lewis (RT1l) rats weighing 200–250 g were used as donors and recipients. All rats were obtained from Harlan (Indianapolis, IN). All animals received humane care in compliance with the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 86–23, revised 1985) and the Public Health Service Policy on Humane Care and Use of Laboratory Animals (1996).
Experimental design
The effects of alloimmune responses on lymphatic re-establishment was assessed in a heterotopic heart transplantation group (n=4 per time interval) and corresponding isograft controls (n=4 per time interval). Acute rejection was prevented by injecting the recipients subcutaneously 3 times per week with 5 mg/kg of CsA (cyclosporine injection; Ben Venue Laboratories, Bedford, OH).
In order to study the changes of cardiac lymphatics after termination of alloimmune responses, allografts (n=4 per time interval) with corresponding isograft controls (n=4 per time interval) were explanted 21 days after the initial transplantation when mononuclear cellular infiltrates were at their peak in extent, and were retransplanted to isogeneic recipients.
Heterotopic heart transplantation
Hearts were transplanted to recipients under isoflurane (Abbott Laboratories, North Chicago, IL) inhalational anesthesia. Using a modification of the technique of Ono and Lindsey (28), the donor aorta and pulmonary artery were anastomosed to the abdominal aorta and inferior vena cava of the recipient respectively, in an end-to-side fashion with 8–0 nylon suture (Ethicon, Inc., Somerville, NJ). Cardiac graft function was evaluated by abdominal palpation daily until rejection, which was defined as total cessation of contractions. Rejection was confirmed by direct visualization and histological examination of the recovered grafts.
Tissue recovery
Heart grafts were recovered at serial time points after transplantation. Two full cross sections were obtained for each graft at the time of sacrifice. Native DA hearts were also recovered for control purposes. One section was embedded in O.C.T compound (Miles Inc. Elkhart, IN, USA) for preparing 8 μm-frozen sections. The other section was fixed by immersion in 60% methanol with 10% acetic acid, before embedding in paraffin and sectioning at 7 μm.
Immunohistologic evaluation of heart grafts
Rejection was assessed on sections that were stained with hematoxylin and eosin.
Detection and localization of lymphatic vessels in cardiac grafts
Lymphatic vessels were detected on both paraffin and frozen sections using an antibody to the lymphatic endothelial hyaluronan receptor LYVE-1 (Abcam ab 14917, Cambridge, MA, USA). Specificity for lymphatic vessels was tested by staining one section with LYVE-1 antibody and the sequential section with rabbit polyclonal von Willebrand factor antibody (Dako, A0082, Carpinteria, CA, USA) that is specific for blood endothelial cells.
LYVE-1 detection in paraffin sections: Slides were incubated overnight at 4°C with LYVE-1 antibody 4μg/μL in 1X PBS, rinsed twice in 1X PBS, and then incubated with donkey anti-rabbit secondary antibody. Slides were then stained by a standard immunoperoxidase technique using an avidin-biotinylated enzyme complex.
LYVE-1 detection in frozen sections: Frozen sections were fixed in ice-cold acetone for 45 minutes, and then dried in air at room temperature before being rinsed twice with 1X PBS. Slides were incubated overnight at 4°C with LYVE-1 antibody 4μg/μL in 1X PBS, rinsed twice with 1X PBS, and then incubated with secondary antibodies conjugated with fluorescent probes for two hours at room temperature. Two secondary antibodies were used in this experiment for double-staining and quantification purposes: (i) goat anti-rabbit IgG Alexa-Fluor 488 secondary antibody (Invitrogen 11034, Carlsbad, CA, USA), and (ii) donkey anti-rabbit IgG rhodamine secondary antibody (Jackson, West Grove, PA, USA).
Evaluation of mononuclear infiltrates and expression of Major Histocompatibility Complex (MHC) antigens in cardiac grafts
Paraffin and frozen graft sections were evaluated with the following mouse monoclonal antibodies: (i) ED1 (Serotec Inc. Raleigh, NC, USA) for a lysosomal membrane antigen (CD68) in rat macrophages; (ii) OX8 (BD Pharmingen, San Diego, CA, USA) for rat CD8-positive T cells (iii) CD3 (BD Pharmingen, San Diego, CA, USA) for rat T cells, (iv) OX6 (Abcam Ltd., Cambridge, MA, USA) for a monomorphic determinant of rat MHC class II, and (v) MN4-91-6 (Serotec Inc. Raleigh, NC, USA) for a polymorphic determinant of MHC class I.
On several tissue sections, overlays of double immunofluorescent stains were done for rabbit LYVE-1 antibody paired with mouse monoclonal antibody for CD8, ED1, MHC I or MHC II.
Sections were also scored by an experienced pathologist for relative extent and severity of mononuclear infiltrates (T cells and macrophages) in heart grafts using a scale of minimal (1+), moderate (2+) or extensive (3+) infiltration.
Digitized immunofluorescence microscopy for quantification of lymphatic vessels in heart grafts
All slides were viewed using a Nikon ECLIPSE-TS100 microscope (Nikon Instruments, Inc., Melville, NY) equipped with Plan-fluor lenses and filters for detecting GFP/FITC and RFP/TRITC fluorescence. Regions of interest (ROI) with lymphatic vessels (i.e. LYVE-1-positive structures) were identified on each slide at 20X magnification and digitized using a Nikon Coolpix 5000 digital camera (Nikon Corporation, Tokyo, Japan) for further analysis and off-line morphometric image processing (29).
Morphometric image analysis of tissue sections for quantification of lymphatic vessels
Automated morphometric analysis of each digitized fluorescent image was done using an in-house program written for ImageJ. Briefly, color images were converted to 8-bit grayscale, normalized, smoothed, binarized, and morphometrically closed. All binarized objects in the final image >5 pixels in size were counted as being LYVE-1-positive features (29). Objects at the image edges were excluded. Two morphometric variables were computed for each digitized image: (i) the average size occupied by LYVE-1-positive structures (μm2), and (ii) the density of LYVE-1-positive structures (number per μm2).
Statistical method
All data were analyzed using NCSS software (NCSS, Kaysville UT). Data were expressed as mean plus and minus standard deviations. The two-tail nonparametric Mann-Whitney test was used to determine if there was any significant difference (α ≤ 0.05) between the morphometric variables in allografts and isografts at different time points after transplantation.
RESULTS
Distribution and morphology of lymphatic vessels in native rat hearts
Lymphatic vessels in native DA rat hearts were specifically identified by LYVE-1 (Fig.1A-B). Subepicardial lymphatics branched along with the blood vascular network into the outer myocardial region. Lymphatics in these regions appeared as flattened vessels under non-inflammatory conditions with density of 0.17 ± 0.04 per μm2 and average size of 2.45 ± 0.45 μm2. As lymphatics progressed into the inner two-thirds of myocardium within the same plane, the density became higher (1.54 ± 0.17 per μm2) and average size decreased (0.84 ± 0.085 μm2) when compared to the outer myocardial and subepicardial lymphatics (p=0.03 for both size and density; Fig. 1C-D). These would be analogous to the myocardial lymphatic plexus and subepicardial lymphatic plexus that have been described in humans (30).
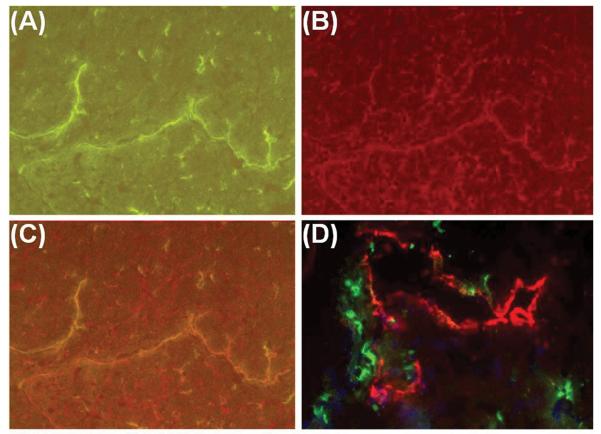
FIGURE 1.
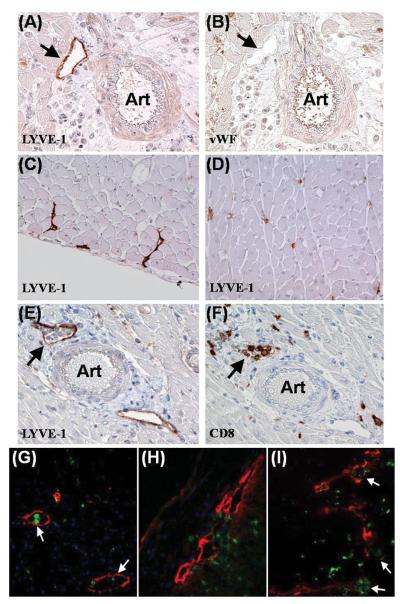
(A-B) Specificity of LYVE-1 for lymphatic endothelium. (A) DA-to-Lewis allograft, post-transplant day 7. LYVE-1-labeled lymphatic vessel (arrow) next to an arteriole (Art) in outer myocardium. The blood vascular endothelium was not stained by LYVE-1. (B) Sequential section of (A) showed the same arteriole being stained for von Willebrand factor (vWF)(brown), a blood vascular endothelial marker. (C-D) Different morphologies of lymphatics across DA native heart. (C) Subepicardium. Lymphatics (brown) appeared as flattened vessels. (D) Inner myocardium on the same plane and orientation. Lymphatics (brown) had a smaller size and higher density compared with the subepicardial lymphatics shown in (C). (E-F) Lymphatics containing T cells. DA-to-Lewis allograft, post-transplant day 14. (E) Lymphatic vessel (arrow) with cellular infiltrates inside the lumen. (F) Sequential section of (E) indicated that some of the infiltrative cells were CD8-positive T cells. (G) Lymphatics containing macrophages. DA-to-Lewis allograft, post-transplant day 14. Double immunofluorescence showed CD68-positive cells (green) inside the lymphatic lumen (arrow). (H-I) Lymphatics as possible targets of T cell-mediated disruption. DA-to-Lewis allografts. (H) Post-transplant day 7. CD8-positive T cells (green) infiltrating into lymphatics (red) with compromised endothelial integrity in the outer myocardium. (I) Post-transplant day 14. Outer lymphatic endothelium was further disrupted (red)(arrows). CD8-positive T cells (green) were seen clustering inside the lymphatic lumen. Magnifications: X100 in A through F. X400 in G through H.
Lymphatic morphology was similar in different strains of native rat hearts including Lewis, PVG.1U and PVG.R8 rats (data not shown).
Spatial correlation between lymphatics and cellular infiltrates
Sequential immunohistological preparations of cardiac allografts showed cellular infiltrates clustering around and inside lymphatics as early as 7 days after transplantation (Fig. 1E-I). Lumens of subepicardial and outer myocardial lymphatics became more dilated after transplantation. As mononuclear cell infiltrates peaked in allografts at 2-3 weeks post-transplantation (Fig. 2), T cells and macrophages were identified inside the lymphatics (Fig. 1E-G). No colocalization of markers for cellular infiltrates and LYVE-1 was detected on cells in serial sections and in double immunofluorescent stains of allografts in inner or outer myocardium after single transplantation and retransplantation. T cell and macrophage infiltration was found to distribute uniformly across the inner and outer myocardium in our experiment.
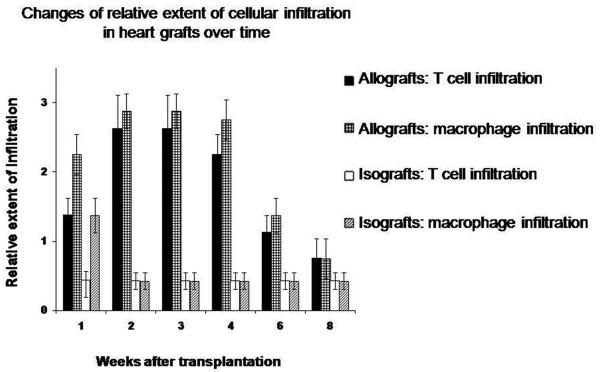
FIGURE 2. Extent of T cell and macrophage infiltration after transplantation.
T cell infiltrates (identified as CD3-positive cells and CD8-positive cells) and macrophage infiltrates (identified as CD68-positive cells) were scored based on relative extent of infiltration in cardiac graft sections (minimal (1+), moderate (2+) or extensive (3+). Infiltrates were found to peak at week 2-3 post-transplantation and regress afterwards. Mononuclear cellular infiltrates were found to have diffuse distribution across the transverse heart section.
It was also noted that the lymphatic endothelial lining became disrupted as cellular infiltrates collected around the lymphatics in the subepicardial and outer myocardial regions at week 1 post-transplantation (Fig. 1H). As cellular infiltration increased in extent during week 2 to 3 after transplantation (Fig. 2), the disruption of lymphatic endothelium became more severe (Fig. 1I). Inner myocardial lymphatics decreased significantly as early as week 1 after transplantation.
Quantitative morphological changes of lymphatic vessels in cardiac grafts from week 1 to week 8 post-transplantation
Due to different native morphologies observed between lymphatics in the outer one-third (subepicardial and outer myocardial lymphatics) and inner two-thirds (inner myocardium) of transverse heart sections on the same plane, quantification of morphologic changes in these two regions were done separately with quantitative immunofluorescent microscopy. Differential responses in densities and sizes were observed in inner and outer lymphatics after transplantation.
Lymphatic density changes
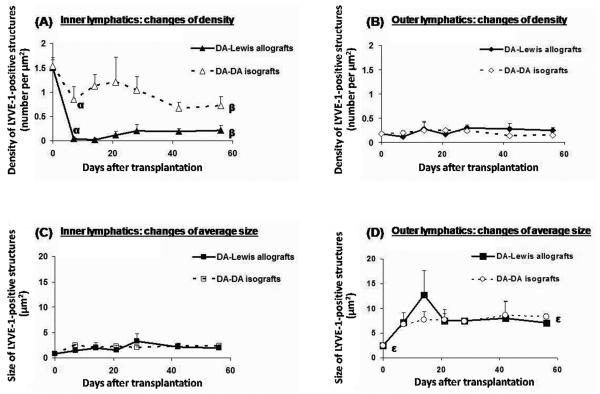
Allograft inner lymphatic density decreased 38.6-fold at week 1 post-transplantation (p=0.03; Fig. 3A). Lymphatic density gradually recovered to 14.29% of the level in DA native hearts at 8 weeks post-transplantation (p=0.02). In isograft controls, density decreased less (53.25% of native level at 8 weeks post-transplantation; p=0.02) with no statistically significant variation throughout the post-transplantation course (Fig. 3A). In contrast, allografts and isografts had very limited changes in lymphatic density in the outer myocardium and subepicardium, which showed no statistically significant difference from that in native hearts throughout the post-transplantation course (Fig. 3B).
FIGURE 3. Morphological changes of lymphatics after transplantation (n=4 per time point).
(A) Lymphatic density in the inner myocardium (inner 2/3 of transverse heart section). Allograft inner lymphatic density decreased 38.6-fold at week 1 post-transplantation and recovered to 14.29% of the level in DA native hearts at 8 weeks post-transplantation. There was a significantly greater decrease in inner lymphatic density in allografts compared to isografts. αp=0.02. βp=0.02. (B) Lymphatic density in the outer myocardium and subepicardium (outer 1/3 of transverse heart section). No significant difference was seen among allografts, isografts and native hearts. (C) Lymphatic size in the inner myocardium (inner 2/3 of transverse heart section). Allografts and isografts had similar increase (2.3-fold) at 8 weeks post-transplantation when compared to native hearts (p=0.02). (D) Lymphatic size in the outer myocardium and subepicardium (outer 1/3 of transverse heart section). Allografts and isografts had similar increases in size and showed no significant difference throughout the post-transplantation course. Outer lymphatic size stayed at about 2.9 times that of native hearts at the time of transplantation from week 3 through week 8 after transplantation in isografts and allografts (εp=0.03).
Lymphatic size changes
The size of outer lymphatics increased significantly in allografts by 5.2-fold (p=0.03) within 2 weeks, then decreased and stayed at about 2.9 times that of native level from week 3 through week 8 after transplantation (p=0.02). No significant difference was found in size of outer lymphatics between isografts and allografts (Fig. 3D). For inner lymphatics, both allografts and isografts demonstrated similar increases of 2.3-fold of native level (p=0.03) at 8 weeks post-transplantation (Fig. 3C).
Morphological changes after retransplantation of allografts to isogeneic recipients
To determine whether the changes in lymphatic vessels were reversible, hearts that had been transplanted to allogeneic recipients were removed at week 3 post-transplantation when mononuclear cellular infiltrates were at their peak in extent, and were retransplanted to isogeneic recipients.
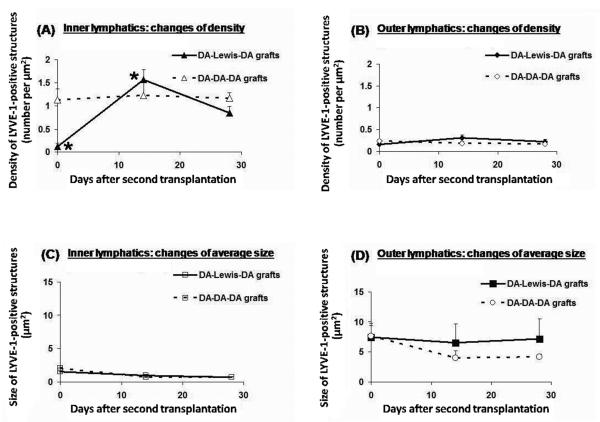
Inner lymphatic density and outer lymphatic size, which were the most altered parameters of lymphatics in cardiac allografts, responded differently to the termination of alloimmune responses. As shown in figure 4, inner lymphatic density recovered rapidly to a level (1.56 ± 0.21 per μm2) similar to that in native DA hearts (1.54 ± 0.17 per μm2) by 2 weeks, and gradually decreased to about 55.00% of the native level at 4 weeks after retransplantation, which was similar to isografts at 8 weeks after single transplantation (Fig. 3A, 4A). In isograft retransplants, inner lymphatic density showed no significant variation and reached 75.25% of the native level at 4 weeks after retransplantation, which was statistically higher than that in isografts without retransplantation (46.75% of native level) at 8 weeks after single transplantation (p=0.049) (Fig. 3A, 4A).
FIGURE 4. Morphological changes after retransplantation of allografts to isogeneic recipients at week 3 post-transplantation when mononuclear cellular infiltrates were at their peak in extent (n=4 per time point). (A) Inner lymphatic density changes.
Two weeks after retransplantation, inner lymphatic density rapidly increased by 13.4-fold (*p=0.05) to a level comparable to that in native DA hearts. By 4 weeks post-transplantation, the inner myocardial lymphatic density decreased to about 55.00% of the native level. In isograft controls, inner lymphatic density showed no significant variation and reached 75.25% of the native level at 4 weeks after retransplantation. (B) Outer lymphatic density changes. No significant variation was seen in grafts or controls. (C) Inner lymphatic size changes. Retransplanted grafts and controls showed similar mild decrease (about 15%) in size compared with native level at week 4 post-transplantation. (D) Outer lymphatic size changes after second transplantation. No significant difference was seen between retransplanted grafts and controls in the post-transplantation course.
In contrast, the outer lymphatics remained persistently dilated in both allograft and isograft retransplants without significant density changes (Fig. 4B, 4D). There was no significant change in the size of the outer lymphatics between retransplants and single transplants at the endpoints of this experiment (Fig. 3C, 3D, 4C, 4D). At week 4 after retransplantation, size of inner lymphatics in the allograft retransplants (85.18% of native level) and isograft retransplants (85.00% of native level) showed similar mild decrease when compared to native level (p=0.05; Fig. 4C).
MHC antigen expression in lymphatics
Allograft tissues were immunofluorescently stained for MHC I and MHC II antigens to investigate the potential antigenic targets on lymphatics for T cells. MHC I expression was found to colocalize with the lymphatic endothelial lining (Fig. 5A-C), but no significant MHC II expression was detected on lymphatic endothelium in allografts (Fig. 5D).
FIGURE 5. Expression of MHC antigens on lymphatic endothelium. DA-to-Lewis allograft, post-transplant day 7.
(A) Lymphatic vessels labeled with green immunofluorescence (LYVE-1 and Alexa-488). (B) Lymphatic vessels in (A) doubly labeled with red immunofluorescence for MN4-91-6, a polymorphic determinant of MHC I. (C) Overlay of (A) and (B) demonstrated co-expression of LYVE-1 and MHC I on the lymphatic endothelium. (D) Immunofluorescent label of MHC II with OX6 (green), a monomorphic determinant of rat MHC class II, showed that MHC II expression did not overlap with LYVE-1-positive lymphatic endothelial cells (red). Magnifications: X200 in A through C. X400 in D.
DISCUSSION
These experiments were designed to investigate the effects of acute alloimmune responses on lymphatic regeneration following cardiac transplantation. Cardiac transplants in rats offered several experimental advantages. The size of the transplants permitted full cross sections of the heart to be evaluated in order to assess regional differences in lymphatic changes relative to alloimmune responses. Inbred rat strains allowed findings in allografts to be compared with genetically identical isografts in order to distinguish between the effects mediated by antigen-specific alloimmune responses versus inflammatory or physiological alterations. Finally, the alloimmune responses could be experimentally terminated in rats by retransplanting the hearts from allogeneic recipients to isogeneic recipients.
Taking advantage of these attributes of transplants in inbred rats, we made three novel findings: (i) There are regional differences in lymphatic changes as manifested by a persistent increase in size but no significant change in density of subepicaridal and outer myocardial lymphatics (outer lymphatics), versus a dramatic decrease in density and relatively milder increase in size of inner myocardial lymphatics (inner lymphatics) in allografts. (ii) Restoration of cardiac lymphatics is influenced by alloimmune responses as indicated by the different responses seen in allografts versus isografts. (iii) The inner myocardial lymphatics in allografts can recover when alloimmune responses are terminated by retransplantation.
The lymphatic size changes observed after transplantation in this study were likely affected more by physiological factors (31), such as myocardial edema and compensatory responses related to decreased cardiac preload in heterotopic setting (32-35), instead of alloimmunity because there was a persistent size increase in both the inner and outer lymphatics that was similar in allografts and isografts.
In contrast, the significant difference in density between inner myocardial lymphatics in allografts and isografts is most likely related to alloimmune responses. This observation is consistent with the decrease in LYVE-1 positive lymphatics seen in human endomyocardial biopsies in the first year after cardiac transplantation (36). Although this difference of density was quantitatively greater in inner lymphatics than that in outer lymphatics, CD8-positive cells were readily detected in clusters around the larger outer lymphatics in rat cardiac allografts. Furthermore, double immunofluorescent stains showed that the CD8-positive T cells were located at sites where the LYVE-1-positive endothelium was disrupted. The demonstration that T cells are associated with disrupted LYVE-1-expressing vessels in the outer lymphatics suggests that the same process occurred in the smaller inner lymphatics before the subsequent decreased detection of LYVE-1 in the inner myocardial region. MHC antigens are the primary target of rejection in allografts, and the expression of MHC class I antigens on lymphatic endothelium provides a ligand for cytotoxic T cells that express CD8. The finding of compromised lymphatic endothelium after cardiac transplantation is consistent with report of decreased expression of 5′-nucleotidase in lymphatic endothelium made by Demetris et al in the early course of acute cardiac rejection in rats (37). Although the complete functional properties of lymphatic endothelium in different organs are not fully known on a molecular level, lymphatics are vital to immune cell trafficking. For example, common lymphatic endothelial and vascular endothelial receptor-1 (CLEVER-1) is expressed in lymphatic vessels and mediates lymphocyte adhesion to lymphatic vessels (38). Certain subsets of normally functioning lymphatic endothelial cells have been shown to express the chemokine receptor D6, which limits inflammatory responses by clearance of inflammatory chemokine (39). Loss of intact lymphatic endothelium during transplantation and rejection thus has direct relevance to lymphatic dysfunction in transplant rejection.
Although CD8 T cells are only required for the rejection of hearts transplanted to a heterotopic location in some strain combinations (40), this may in part be a reflection of the fact that these hearts have a very limited workload and myocardial dysfunction caused by disruption of lymphatics is not critical. In fact, many vigorously beating heterotpically transplanted hearts develop fibrosis and different subsets of CD8 T cells have been shown to cause arteritis and interstitial fibrosis (41). The complete effects of lymphatic compromise on cardiac transplants are probably best studied in orthotopic transplants with a full workload, in which myocardial dysfunction related to lymphatic disruption would be critical to survival.
The loss of inner myocardial lymphatics in allografts was rapidly reversed by eliminating alloimmune responses when allografts were retransplanted to isogeneic recipients. Potential recipient lymphatic progenitor cells, such as monocytes (42-43), have been reported to be delivered by the circulation and incorporate into growing lymphatic vessels in the presence of lymphangiogenic factors, e.g. VEGF-C, which are secreted from dendritic cells and macrophages in inflammatory infiltrates (42-43). Although we demonstrated in our study an abundance of CD68-positive macrophages in the hearts at the time of retransplantation, these cells did not co-express LYVE-1. The significantly smaller size of LYVE-1-positive structures observed in the retransplanted allografts compared with allografts without retransplantation is more consistent with newly organized or recovered inner lymphatics than cells which have transdifferentiated into potential endothelial progenitor cells with lymphatic marker expression, e.g. CD11b+ LYVE-1+ macrophages. As demonstrated by Maruyama et al (44), such endothelial progenitor cells can join existing lymphatic vessels in corneal transplants. The subsequent progressive decrease of inner lymphatic density in the retransplanted allografts in our study mimicked the isograft response seen after single transplantation. The decrease seen in the retransplanted allografts in the late post-transplantation course might be related to the cessation of inflammation and lymphangiogenic cytokines secreted by dendritic cells and macrophages.
Besides actual loss or growth of lymphatic capillaries, the changes of LYVE-1 detection in inner myocardium might also indicate potential phenotypic and functional variation of lymphatic endothelium in allografts over time in relation to alloimmune responses. LYVE-1, the molecular lymphatic marker used in our present study, is constantly exposed to the luminal and abluminal surfaces of lymphatic endothelium, and it has been suggested to shuttle across the lymphatic endothelium to transport hyaluronan from tissue to lymph (25-27). Hyaluronan is a key mediator of cell migration (25-27). Changes of endothelial LYVE-1 expression may therefore reflect alteration of molecular functional properties because changes in interactions with matrix hyaluronan on or beneath the surface of inflamed endothelium affects both the extravasation and the subsequent migration of activated leukocytes during inflammation (25-27).
In summary, our study is the first to demonstrate regional changes of cardiac lymphatics after transplantation. Inner myocardial lymphatics in allografts underwent a dramatic and persistent decrease in density while the outer myocardial and subepicardial lymphatics increased in size but not in density at the end-point of our experiment. Elimination of alloimmune responses produced rapid restoration of inner lymphatic vessels, suggesting that lymphatics injured during rejection can recover when rejection is reversed.
ABBREVIATION
- LYVE-1
lymphatic endothelial hyaluronan receptor-1
Footnotes
AUTHOR DISCLOSURE STATEMENT
No competing financial interests exist for all the authors listed for this study.
REFERENCES
- 1.Kong XQ, Wang LX, Kong DG. Cardiac lymphatic interruption is a major cause for allograft failure after cardiac transplantation. Lymphat Res Biol. 2007;5:45–7. doi: 10.1089/lrb.2007.5108. [DOI] [PubMed] [Google Scholar]
- 2.Ji RC. Characteristics of lymphatic endothelial cells in physiological and pathological conditions. Histol Histopathol. 2005;20:155–75. doi: 10.14670/HH-20.155. [DOI] [PubMed] [Google Scholar]
- 3.Symbas PN, Cooper T, Gantner GE, Jr, Willman VL. Lymphatics of the heart: anatomic effects following interruption of the drainage of the cardiac lymph. Arch Path. 1966;81:573–575. [PubMed] [Google Scholar]
- 4.Symbas PN, Schlant RC, Gravanis MB, Shepherd RL. Pathologic and functional effects on the heart following interruption of the cardiac lymph drainage. J Thorac Cardiovasc Surg. 1969;57:577–584. [PubMed] [Google Scholar]
- 5.Jellinek H, Gabor G, Solti F, Veress B. The problem of the coronary changes due to disturbance of vascular wall permeability. Angiology. 1967;18:179–187. doi: 10.1177/000331976701800306. [DOI] [PubMed] [Google Scholar]
- 6.Kline IK, Miller AJ, Pick R, Katz LN. The relationship between human endocardial fibroelastosis and obstruction of the cardiac lymphatics. Circulation. 1964;30:728–735. doi: 10.1161/01.cir.30.5.728. [DOI] [PubMed] [Google Scholar]
- 7.Ji RC. Lymphatic endothelial cells, lymphangiogenesis, and extracellular matrix. Lymphat Res Biol. 2006;4(2):83–100. doi: 10.1089/lrb.2006.4.83. [DOI] [PubMed] [Google Scholar]
- 8.Stacker SA, Hughes RA, Willams RA, Achen MG. Current strategies for modulating lymphangiogenesis signaling pathways in human disease. Curr Med Chem. 2006;13:783–92. doi: 10.2174/092986706776055625. [DOI] [PubMed] [Google Scholar]
- 9.Stacker SA, Caesar C, Baldwin ME, et al. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7(2):186–91. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- 10.Joukov V, Pajusola K, Kaipainen A, et al. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:1751. [PMC free article] [PubMed] [Google Scholar]
- 11.Ishikawa Y, Akishima-Fukasawa Y, Ito K, et al. Lymphangiogenesis in myocardial remodeling after infarction. Histopathology. 2007;51:345–53. doi: 10.1111/j.1365-2559.2007.02785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liersch R, Detmar M. Lymphangiogenesis in development and disease. Thromb Haemost. 2007;98:304–10. [PubMed] [Google Scholar]
- 13.Veikkola T, Lohela M, Ikenberg K, et al. Intrinsic versus microenvironmental regulation of lymphatic endothelial cell phenotype and function. FASEB J. 2003;17:2006–2013. doi: 10.1096/fj.03-0179com. [DOI] [PubMed] [Google Scholar]
- 14.Stacker SA, Hughes RA, Achen MG. Molecular targeting of lymphatics for therapy. Curr Pharm Des. 2004;10:65–74. doi: 10.2174/1381612043453513. [DOI] [PubMed] [Google Scholar]
- 15.Szuba A, Skobe M, Karkkainen MJ, et al. Therapeutic lymphangiogenesis with human recombinant VEGF-C. FASEB J. 2002;16:1985–7. doi: 10.1096/fj.02-0401fje. [DOI] [PubMed] [Google Scholar]
- 16.Kim H, Dumont DJ. Molecular mechanisms in lymphangiogenesis: model systems and implications in human disease. Clin Genet. 2003;64:282–92. doi: 10.1034/j.1399-0004.2003.00152.x. [DOI] [PubMed] [Google Scholar]
- 17.Cursiefen C, Chen L, Dana MR, Streilein JW. Corneal lymphangiogenesis: evidence, mechanisms, and implications for corneal transplant immunology. Cornea. 2003;22:273–81. doi: 10.1097/00003226-200304000-00021. [DOI] [PubMed] [Google Scholar]
- 18.Cursiefen C, Chen L, Borges LP, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kerjaschki D, Regele HM, Moosberger I, et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol. 2004;15:603–12. doi: 10.1097/01.asn.0000113316.52371.2e. [DOI] [PubMed] [Google Scholar]
- 20.Thaunat O, Patney N, Morelon E, Michel JB, Nicoletti A. Lymphoid neogenesis in chronic rejection: the murderer is in the house. Curr Opin Immunol. 2006;18:576–9. doi: 10.1016/j.coi.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Stuht S, Gwinner W, Franz I, et al. Lymphatic neoangiogenesis in human renal allografts: results from sequential protocol biopsies. Am J Transplant. 2007;7:377–84. doi: 10.1111/j.1600-6143.2006.01638.x. [DOI] [PubMed] [Google Scholar]
- 22.Di Carlo E, D'Antuono T, Continto S, Di Nicola M, Ballone E, Sorrentino C. Quilty effect has the features of lymphoid neogenesis and shares CXCL13-CXCR5 pathway with recurrent acute cardiac rejections. Am J Transplant. 2007;7:201–10. doi: 10.1111/j.1600-6143.2006.01584.x. [DOI] [PubMed] [Google Scholar]
- 23.Jonigk D, Lehmann U, Stuht S, et al. Recipient-derived neoangiogenesis of arterioles and lymphatics in quilty lesions of cardiac allografts. Transplantation. 2007;84(10):1335–42. doi: 10.1097/01.tp.0000287458.72440.75. [DOI] [PubMed] [Google Scholar]
- 24.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–20. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 25.Banerji S, Ni J, Wang SX, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144(4):789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson DG. The lymphatics revisited: new perspectives from the hyaluronan receptor LYVE-1. Trends Cardiovasc Med. 2003;13(1):1–7. doi: 10.1016/s1050-1738(02)00189-5. [DOI] [PubMed] [Google Scholar]
- 27.Jackson DG. Biology of the lymphatic marker LYVE-1 and applications in research into lymphatic trafficking and lymphangiogenesis. APMIS. 2004;112(78):526–38. doi: 10.1111/j.1600-0463.2004.apm11207-0811.x. [DOI] [PubMed] [Google Scholar]
- 28.Ono K, Kindsey ES. Improved technique of heart transplantation in rats. J Thorac Cardiovasc Surg. 1969;57:225–9. [PubMed] [Google Scholar]
- 29.Pathak AP, Artemov D, Neeman M, Bhujwalla ZM. Lymph node metastasis in breast cancer xenografts is associated with increased regions of extravascular drain, lymphatic vessel area, and invasive phenotype. Cancer Res. 2006;66:5151–8. doi: 10.1158/0008-5472.CAN-05-1788. [DOI] [PubMed] [Google Scholar]
- 30.Sacchi G, Weber E, Aglianò M, Cavina N, Comparini L. Lymphatic vessels of the human heart: precollectors and collecting vessels. A morpho-structural study. J Submicrosc Cytol Pathol. 1999;31(4):515–25. [PubMed] [Google Scholar]
- 31.Davis MJ, Davis AM, Ku CW, Gashev AA. Myogenic constriction and dilation of isolated lymphatic vessels. Am J Physiol Heart Circ Physiol. 2009;296(2):H293–302. doi: 10.1152/ajpheart.01040.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji RC. Lymphatic endothelial cells, inflammatory lymphangiogenesis, and prospective players. Curr Med Chem. 2007;14:2359–68. doi: 10.2174/092986707781745541. [DOI] [PubMed] [Google Scholar]
- 33.Laine GA, Granger HJ. Microvascular, interstitial, and lymphatic interactions in the normal heart. Am J Physiol. 1985;249:H834–H842. doi: 10.1152/ajpheart.1985.249.4.H834. [DOI] [PubMed] [Google Scholar]
- 34.Malek P, Vrubel J. Lymphatic system and organ transplantation. Lymphology. 1968;1:4–22. [PubMed] [Google Scholar]
- 35.Starling EH. On the absorption of fluids from the connective tissue spaces. J Physiol Lond. 1896;19:312–326. doi: 10.1113/jphysiol.1896.sp000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geissler HJ, Dashkevich A, Fischer UM, et al. First year changes of myocardial lymphatic endothelial markers in heart transplant recipients. Eur J Cardiothorac Surg. 2006;29:767–71. doi: 10.1016/j.ejcts.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 37.Demetris AJ, Murase N, Ye Q, et al. Analysis of chronic rejection and obliterative arteriopathy. Possible contributions of donor antigen-presenting cells and lymphatic disruption. Am J Pathol. 1997;150:563–78. [PMC free article] [PubMed] [Google Scholar]
- 38.Salmi M, Koskinen K, Henttinen T, Elima K, Jalkanen S. CLEVER-1 mediates lymphocyte transmigration through vascular and lymphatic endothelium. Blood. 2004;104(13):3849–57. doi: 10.1182/blood-2004-01-0222. [DOI] [PubMed] [Google Scholar]
- 39.Nibbs RJ, Kriehuber E, Ponath PD, et al. The beta-chemokine receptor D6 is expressed by lymphatic endothelium and a subset of vascular tumors. Am J Pathol. 2001;158(3):867–77. doi: 10.1016/s0002-9440(10)64035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ilano AL, McConnell MV, Gurley KE, Spinelli A, Pearce NW, Hall BM. Cellular basis of allograft rejection in vivo. V. Examination of the mechanisms responsible for the differing efficacy of monoclonal antibody to CD4+ T cell subsets in low- and high-responder rat strains. J Immunol. 1989;143(9):2828. [PubMed] [Google Scholar]
- 41.Delfs MW, Furukawa Y, Mitchell RN, Lichtman AH. CD8+ T cell subsets TC1 and TC2 cause different histopathologic forms of murine cardiac allograft rejection. Transplantation. 2001;71(5):606. doi: 10.1097/00007890-200103150-00005. [DOI] [PubMed] [Google Scholar]
- 42.Kerjaschki D. The crucial role of macrophages in lymphangiogenesis. J Clin Invest. 2005 Sep;115(9):2316–9. doi: 10.1172/JCI26354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kerjaschki D, Huttery N, Raab I, et al. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat Med. 2006;12:230–4. doi: 10.1038/nm1340. [DOI] [PubMed] [Google Scholar]
- 44.Maruyama K, Ii M, Cursiefen C, et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest. 2005;115:2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]