Abstract
Retinoic acid (RA) exerts diverse biological effects in the control of cell growth in embryogenesis and oncogenesis. These effects of RA are thought to be mediated by the nuclear retinoid receptors. Mannose-6-phosphate (M6P)/insulin-like growth factor-II (IGF-II) receptor is a multifunctional membrane glycoprotein that is known to bind both M6P and IGF-II and function primarily in the binding and trafficking of lysosomal enzymes, the activation of transforming growth factor-β, and the degradation of IGF-II. M6P/IGF-II receptor has recently been implicated in fetal development and carcinogenesis. Despite the functional similarities between RA and the M6P/IGF-II receptor, no direct biochemical link has been established. Here, we show that the M6P/IGF-II receptor also binds RA with high affinity at a site that is distinct from those for M6P and IGF-II, as identified by a photoaffinity labeling technique. We also show that the binding of RA to the M6P/IGF-II receptor enhances the primary functions of this receptor. The biological consequence of the interaction appears to be the suppression of cell proliferation and/or induction of apoptosis. These findings suggest that the M6P/IGF-II receptor mediates a RA response pathway that is important in cell growth regulation. This discovery of the interaction of RA with the M6P/IGF-II receptor may have important implications for our understanding of the roles of RA and the M6P/IGF-II receptor in development, carcinogenesis, and lysosomal enzyme-related diseases.
Retinoic acid (RA) exerts diverse biological effects in the control of cell growth in embryonic development and oncogenesis (1, 2). It is thought that the effects of RA are mediated through two classes of nuclear receptors, the RA receptors and the retinoid X receptors (1, 2). These receptors belong to the steroid/thyroid hormone receptor superfamily, which regulates gene transcription through binding to specific DNA sequence, resulting in an increased or decreased synthesis of specific proteins. Nevertheless, recent evidence (3–5) suggests that other retinoid response pathways that are independent of the nuclear receptors may exist. Thus, despite our knowledge of the nuclear receptors for RA, how RA can exert a great diversity of biological effects is still not fully understood.
The mannose 6-phosphate (M6P)/insulin-like growth factor II receptor (IGF-II) receptor is a multifunctional transmembrane glycoprotein that consists of a 300-kDa single polypeptide chain, with a large extracellular domain, containing 15 repeat regions, and a small cytoplasmic domain (6). The expression of this receptor is developmentally regulated, with the receptor being highly expressed in fetal and neonatal tissues (including plasma and heart) and the expression declining postnatally (7). This receptor is known to bind both M6P and IGF-II at distinct sites (8–10). A major function of the receptor is to bind and transport M6P-bearing glycoproteins (e.g., lysosomal enzymes) from the trans-Golgi network or the cell surface to lysosomes (6). The cell surface M6P/IGF-II receptor also binds and internalizes IGF-II, resulting in the lysosomal degradation of this ligand (11). In this manner, the receptor may serve as a suppressor of IGF-II proliferative actions. In addition, the M6P/IGF-II receptor binds the latent transforming growth factor-β (TGF-β), permitting cleavage into its active form, which is a potent growth inhibitor for most cell types (12). Thus, the M6P/IGF-II receptor plays a critical role in the regulation of cell growth. Indeed, recent findings from transgenic animal experiments, as well as human studies, have shown that M6P/IGF-II receptor is essential for normal fetal development (13–15) and has anti-cancer activity (16–18), similar to those effects reported for RA (1, 2). Despite the functional similarities between RA and M6P/IGF-II receptor, no direct biochemical link has been established.
Photoaffinity labeling has been shown to be a powerful method for identifying new receptor proteins (19). Typically, in a photoaffinity labeling technique, a photoreactive moiety is linked chemically to a receptor’s ligand. Photoactivation of a bound radiolabeled photoaffinity reagent (ligand) results in its covalent attachment to the receptor. The photolabeled receptor can then be identified with standard biochemical techniques from relatively crude preparations by comparing labeling patterns in the presence and absence of saturating concentrations of unlabeled ligand. Because RA has a α,β-unsaturated carbonyl group and a highly conjugated polyene structure, it is photoactivatable (20, 21). Radiolabeled RA (i.e., [3H]RA), therefore, can be used as a photoaffinity reagent without any further modification of its chemical structure (21). This technique has been used previously to identify RA-binding proteins in various tissues and has been proven to be an ideal method for discovery of retinoid-binding proteins (21). In our initial effort to identify the proteins that may be involved in the reported anti-arrhythmic effect of RA (22), we used the photolabeling technique to search for RA-binding proteins in neonatal rat cardiac myocytes and found that RA binds to the M6P/IGF-II receptor with high affinity at a site distinct from those for M6P and IGF-II and that binding of RA to the M6P/IGF-II receptor enhances the primary functions of this receptor.
EXPERIMENTAL PROCEDURES
Cell Isolation and Culture.
Cardiac myocytes were isolated from 1-day-old neonatal Sprague–Dawley rats using the Neonatal Cardiomyocyte Isolation System (Worthington). The isolated cell were cultured on Petri dishes with culture medium (F-10 nutrient mixture/10% horse serum/5% fetal bovine serum/50 μg/ml streptomycin/50 units/ml penicillin G). Cell were used for experiments after 2–6 days of culture.
Photoaffinity Labeling with [3H]RA and Fluorography.
Photoaffinity labeling was performed according to methods described previously (21), with slight modifications. Briefly, in the dark or under a red light, 7 μCi of all-trans-[11,12-3H]RA (72 Ci/mmol; NEN), with or without 50 μM unlabeled RA (in 10 μl of ethanol), was added to each 1.5-ml microcentrifuge tube. After the ethanol has been evaporated with nitrogen, about 100 μg of proteins of membrane preparations or serum [serum proteins was first concentrated using a Biomax-100K Centrifugal Filter Device (Millipore)] was then added to each tube, and the final volume was adjusted to 100 μl with 50 mM Tris buffer (pH 7.4) containing protease inhibitors (5 mM EDTA/5 mM EGTA/0.1 mM phenylmethylsulfonyl fluoride/0.05% NaN3/20 μg/ml aprotinin/10 μg/ml benzamidine/12.5 μg/ml chymostatin) and 50 μM butylated hydroxytoluene (a radical scavenger, used to minimize the nonspecific labeling), for a final concentration of 1 μM labeled RA. The samples were incubated at room temperature with agitation for 1 h in the dark. The samples were exposed to an intense 365-nm UV light source (model B 100AP; UVP, Upland, CA) for 7 min. (Photodestruction of the starting [3H]RA was completed after 5–6 min of exposure to the UV light, as measured by a spectrophotometer.) To remove the noncovalently bound ligands, the photolabeled samples were extracted with chloroform/methanol (2:1) or washed once by centrifugation (for membrane samples only). The resultant protein pellets were dissolved in SDS sample buffer containing 5% 2-mercaptoethanol and heated at 100°C for 5 min. These samples were then loaded and run on 7% polyacrylamide gels (acrylamide/bisacrylamide, 30:0.2) with standard SDS/PAGE techniques. After electrophoresis, the gels were stained with Coomassie blue, soaked in Amplifier (Amersham Life Science) for 30 min, dried by a vacuum gel dryer, and then exposed to Hyperfilm-TM x-ray films (Amersham Life Science) at −80°C for 1–3 days.
Immunoprecipitation and Immunoblotting.
Neonatal rat serum proteins (500 μg) were immunoprecipitated in 1 ml of 50 mM Hepes buffer (pH 7.4), containing 0.15 M NaCl, 0.05% Triton X-100, and the protease inhibitors, using a rabbit polyclonal antibody against the rat M6P/IGF-II receptor (kindly provided by R. G. MacDonald, University of Nebraska, and C. D. Scott, Kolling Institute of Medical Research, Australia). The protein mixture was incubated overnight at 4°C under constant rotation. The antibody-M6P/IGF-II receptor complexes were adsorbed with 30 μl of protein G-agarose (Santa Cruz Biotechnology). After a further 2-h incubation, with rotation at 4°C, the samples were centrifuged, and the pellets were washed 3 times with ice-cold PBS buffer (pH 7.4). The proteins were finally solubilized from the pellet with SDS/PAGE loading solution by incubation at 100°C for 5 min, and the samples were analyzed by SDS/PAGE and fluorography, as described above. For immunoblotting, proteins were separated by SDS-PAGE under reducing or nonreducing conditions (ref. 23; see Fig. 3) and then electrophoretically transferred onto Immobilon-P Transfer Membrane (Millipore). The blots were blocked with 5% nonfat milk, incubated with anti-IGF-II receptor antibodies, and washed. The immunoreaction was visualized using the peroxidase–chemiluminescence system.
Figure 3.
Comparison of the profiles of neonatal rat serum proteins that were immunoprecipitated and immunoblotted with a polyclonal anti-M6P/IGF-II receptor antibody and an anti-peptide antibody against the cytoplasmic domain (provided by R. G. MacDonald; ref. 23). (A) Immunoprecipitation. Two equivalent aliquots of neonatal rat serum proteins were precipitated with either antibody as described in Methods. The anti-receptor antibody precipitated both a 300- and a 260-kDa protein (lane 1), whereas the anti-peptide antibody precipitated the 300-kDa protein only (lane 2). (B) Western immunoblotting. Aliquots of neonatal rat serum were electrophoresed under nonreducing condition (lanes 1 and 2, both are the same blot) or after reduction with 5% 2-mercaptoethonal (lane 3). Electrophoresed proteins were transferred to nitrocellulose and probed with the anti-receptor antibody (lane 1) or the anti-peptide antibody (lanes 2 and 3). A 300-kDa protein, which did not change with disulfide bond reduction, was recognized by both antibodies, whereas a 220-kDa protein (under nonreducing condition) was recognized by the anti-receptor antibody only.
Purification of the M6P/IGF-II Receptor by M6P-Affinity Chromatography.
To purified serum M6P/IGF-II receptors, phosphomannan was prepared by the method of Bretthauer et al. (24), a phosphomannan-Sepharose affinity column was made according to the method of Sahagian et al. (25), and affinity chromatography was performed in a manner similar to that described by Valenzano et al. (26). Fresh neonatal rat serum was used after being diluted with PBS containing protease inhibitor mixture (see above) and filtered through a 0.2 μM cellulose acetate membrane (Millipore).
Ligand-Binding Assay.
An assay of [3H]RA binding to M6P/IGF-II receptor was performed as described (27), with some modifications. Briefly, partially purified M6P/IGF-II receptors from neonatal rat serum [by a fast protein liquid chromatography system using a Sepharose-12 column (Pharmacia)] were diluted in binding buffer [20 mM Tris⋅HCl (pH 8.0)/150 mM NaCl/1 mM DTT/protease inhibitors] at a concentration of 50 μg/ml. Various amounts of tritiated and unlabeled RA in ethanol were added to incubation tubes and evaporated under nitrogen, and 200 μl of diluted protein solution was immediately added. Incubation was carried out at 22°C for 2 h in the dark. After the incubation, 0.2 ml of chilled charcoal–dextran suspension (charcoal/dextran/glycerol, 0.5:0.05:20) was added to remove the unbound ligand. The bound [3H]RA in the supernatant was counted for radioactivity. Nonspecific binding was usually measured in the presence of a 200-fold excess of nonradioactive RA.
Assay of β-Glucuronidase Binding and Endocytosis.
For binding assay, cells were permeabilized with saponin, incubated with β-glucuronidase (20,000 units/ml), with or without 1 μM RA, for 3 h on ice, washed, solubilized, and assayed for β-glucuronidase, as described previously (28). Specific enzyme binding was calculated by subtracting nonspecific binding (in the presence of 10 mM M6P) from total binding. Under these conditions (pH 7.5), the cation-dependent 46-kDa receptor does not contribute to enzyme binding (28). Endocytosis of extracellular β-glucuronidase was determined as described (29). Cultured neonatal rat cardiac myocytes were washed three times with MEM and incubated for 2 h at 37°C with 1 ml of MEM containing 10,000 units of human β-glucuronidase, with or without 5 mM mannose 6-phosphate, in the presence or absence of 2 μM RA. Following the incubation, the cells were washed five times with ice-cold PBS (pH 7.4) and solubilized in 0.5% sodium deoxycholate. Detergent-solubilized extract is assayed for human β-glucuronidase activity as described above.
RESULTS AND DISCUSSION
Photoaffinity Labeling of Membrane and Serum Proteins with All-trans-[3H]RA.
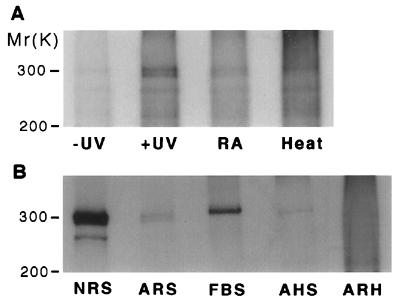
Incubation of the membrane preparations of neonatal rat cardiac myocytes with [3H]RA, followed by photolysis, led to the labeling of a doublet protein at 300 kDa (Fig. 1A). The protein was not labeled when the sample, plus [3H]RA, was not irradiated and when the [3H]RA was first photolyzed before being mixed with the proteins. Denaturation of the proteins by boiling the membrane sample resulted in loss of the labeling. Incubation with a 50-fold molar excess of nonradioactive all-trans RA before photolysis almost completely abolished the labeling of the 300-kDa protein (Fig. 1A). In contrast, other hydrophobic compounds, such as eicosapentaenoic acid (C20:5n-3), which has molecular weight and structural similarities to RA, could not be cross-linked to the 300-kDa protein nor could it displace [3H]RA cross-linking to the protein (data not shown). These results indicate that [3H]RA cross-linking to the 300-kDa protein is specific. Subsequently, we surveyed other tissues using the photolabeling technique and observed that a protein with the same mass (300 kDa), photolabeled by [3H]RA, is abundant in neonatal rat serum and fetal bovine serum but sparse in adult rat serum, adult horse serum, and adult rat heart (Fig. 1B), indicating that the photolabeled protein is highly expressed during fetal and neonatal periods.
Figure 1.
Autoradiographies showing photoaffinity labeling of a 300-kDa protein by [3H]RA in neonatal rat heart cells (A) and other different tissues (B). One hundred micrograms membrane or serum proteins was incubated with 7 μCi of all-trans-[3H]RA (1 μM) in the dark for 1 h at 23°C, photolyzed for 7 min under an intensive UV (365 nm) light source, and then analyzed by SDS/PAGE and fluorography (16). (A) Lane −UV, sample unexposed to UV light; lane +UV, sample exposed to UV for 7 min; lane RA, protein sample was incubated with [3H]RA in the presence of 50 μM unlabeled RA before light exposure; lane Heat, protein sample was boiled for 10 min prior to incubation with [3H]RA. (B) Lane NRS, neonatal rat serum; lane ARS, adult rat serum; lane FBS, fetal bovine serum; lane AHS, adult horse serum; lane ARH, adult rat heart.
Identification of the Photolabeled RA-Binding Protein.
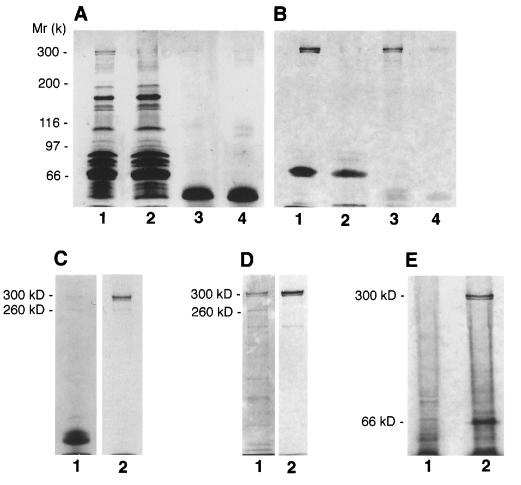
On the basis of the molecular weight of the [3H]RA-labeled protein and its abundance in fetal and neonatal tissues, we suspected that it is the M6P/IGF-II receptor. To verify this prediction, we immunoprecipitated the proteins of neonatal rat serum before or after [3H]RA photolabeling (irradiation) with a polyclonal antibody to rat M6P/IGF-II receptor. As shown in Fig. 2 A and B, the 300-kDa labeled doublet band almost completely disappeared in the sample immunodepleted of the M6P/IGF-II receptor (lane 2), whereas a single 300-kDa strongly labeled doublet band appeared in the sample immunoprecipitated by the anti-M6P/IGF-II receptor antibody (lane 3). In contrast, the 66-kDa albumin, which is known to bind RA (30), could also be photolabeled by [3H]RA, but its labeling was not significantly affected by the immunoprecipitation with anti-M6P/IGF-II receptor antibody (lane 2). When unlabeled RA was added, in an excessive molarity (50 μM), to the immunoprecipitated protein, together with [3H]RA, before irradiation, the radiolabel at the 300-kDa protein was lost (lane 4). Alternatively, immunoprecipitation of [3H]RA-photolabeled protein mixtures of neonatal rat serum with the polyclonal anti-M6P/IGF-II receptor antibody also yielded a single 300-kDa radiolabeled protein (Fig. 2C). This protein was not precipitated by nonimmune rabbit serum or purified IgG (data not shown), indicating that the immunoprecipitation was not nonspecific. To investigate further the identity of the 300-kDa labeled protein, we purified the M6P/IGF-II receptors from neonatal rat serum by pentamannosyl-6-phosphate affinity chromatography and then subjected the preparations to affinity-photolabeling with [3H]RA. Results showed that a purified 300-kDa doublet protein (identical to that precipitated by the anti-IGF-II receptor antibody) was intensively photolabeled by [3H]RA (Fig. 2D). In addition, we have performed photolabeling experiments using cultured mouse P388D1 cells, which are known to lack the M6P/IGF-II receptor (31). As shown in Fig. 2E, the 300-kDa [3H]RA-labeled protein band, which we readily detected in the membrane preparation of neonatal rat cardiac myocytes, was totally absent in P388D1 cells. Thus, these data collectively demonstrate that the 300-kDa photolabeled protein is, indeed, the M6P/IGF-II receptor.
Figure 2.
Identification of the photolabeled RA-binding protein. (A and B) [3H]RA photolabeling of serum proteins following immunoprecipitation. Neonatal rat serum proteins (500 μg) were immunoprecipitated with a rabbit polyclonal antibody against the rat M6P/IGF-II receptor. Both the immunoprecipitated sample and the supernatant immunodepleted of the M6P/IGF-II receptor were photolabeled by [3H]RA and analyzed by SDS/PAGE (7% polyacrylamide gel), and radiolabeled protein bands were visualized by fluorography. (A) Protein profile (Coomassie blue stain) of the photolabeled protein samples. Lane 1, control sample (a complex mixture of neonatal rat serum proteins prior to immnoprecipitation); lane 2, protein sample (the supernatant) immunodepleted of the M6P/IGF-II receptor; lane 3, protein sample precipitated by the anti-M6P/IGF-II receptor antibody; lane 4, same precipitated sample as that in lane 3, but the proteins were incubated and photolabeled with [3H]RA in the presence of excessive molar (50 μM) unlabeled RA. Note that the lower protein bands around the 50-kDa region in lanes 3 and 4 are the residual protein G and the immunoglobulin in the immunoprecipitates. (B) Autoradiogram of the protein samples shown in A. Note that the 66-kDa labeled bands in lanes 1 and 2 represent the serum albumin. (C) Serum proteins were photolabeled by [3H]RA and then immunoprecipitaed with the anti-M6P/IGF-II receptor antibody. Lane 1, Coomassie stain of the precipitated proteins; lane 2, autoradiography of the proteins shown in lane 1. (D), [3H]RA photolabeling of purified M6P/IGF-II receptors. Lane 1, Coomassie stain of the proteins obtained by M6P affinity chromatography; lane 2, autoradiography of the proteins shown in lane 1. (E) Comparison of [3H]RA photolabeling of cell extracts from receptor-negative mouse P388D1 cells (lane 1) and from cultured neonatal rat cardiac myocytes (lane 2).
It is interesting to note that another protein at 260 kDa from neonatal rat serum was also immunoprecipitated by the anti-M6P/IGF-II receptor antibody (Fig. 2C) and was enriched by M6P affinity (Fig. 2D), but it was not affinity-photolabeled by [3H]RA. It is likely that the 260-kDa protein is a truncated form (probably missing the C-terminal region) of the M6P/IGF-II receptor that lacks the RA-binding site. To investigate this possibility, we immunoprecipitated and immunoblotted the neonatal rat serum with two different antibodies: the polyclonal anti-receptor antibody and an anti-peptide antibody that specifically recognizes a 22-aa peptide located 32 residues C-terminal to the transmembrane domain of the M6P/IGF-II receptor (kindly provided by R. G. MacDonald; ref. 23). As shown in Fig. 3, the 260-kDa protein was recognized only by the anti-receptor antibody but not by the anti-peptide antibody, whereas the 300-kDa protein was recognized by both of these antibodies. Interestingly, the 300-kDa band did not change with disulfide bond reduction, consistent with the results previously reported for the M6P/IGF-II receptor (32). In addition, we observed that the 300-kDa labeled protein in the cellular extracts or conditioned (serum-free) medium of cultured neonatal rat cardiac myocytes was specifically recognized by these two antibodies and also exhibited no change to disulfide bond reduction (data not shown). These results demonstrate that both intact (300-kDa) and truncated (260-kDa) forms of M6P/IGF-II receptor exist in the serum. Because only the intact (300-kDa) form is able to bind RA, it is suggested that the 40-kDa C-terminal region, which is missing from the 300-kDa receptor and contains the cytoplasmic domain needed for rapid endocytosis and efficient lysosomal enzyme sorting (6), is essential for RA binding.
RA Binds to the M6P/IGF-II Receptor with High Affinity.
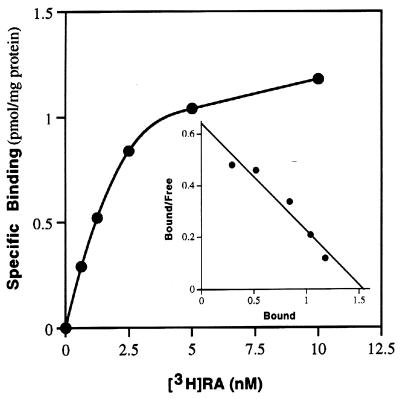
To determine the affinity of the binding of [3H]RA to M6P/IGF-II receptor, we performed equilibrium binding of [3H]RA to partially purified rat M6P/IGF-II receptor proteins using the dextran–charcoal absorption technique (27). Scatchard analysis of the binding data revealed a single class of high-affinity binding sites for RA, with a KD of 2.5 ± 0.3 nM (n = 3; Fig. 4).
Figure 4.
RA binds to the M6P/IGF-II receptor with high affinity. Partially purified M6P/IGF-II receptor from neonatal rat serum was incubated with increasing concentrations of [3H]RA in the absence (total binding) or presence (nonspecific binding) of 200-fold excess unlabeled RA. Nonspecific binding was subtracted from total binding and plotted as specific binding. (Inset) Specific RA binding to M6P/IGF-II receptor was transformed by Scatchard analysis and plotted. Linear regression yielded a KD of 2.4 nM (r = 0.97).
Interaction Between RA and M6P or IGF-II in M6P/IGF-II Receptor Binding.
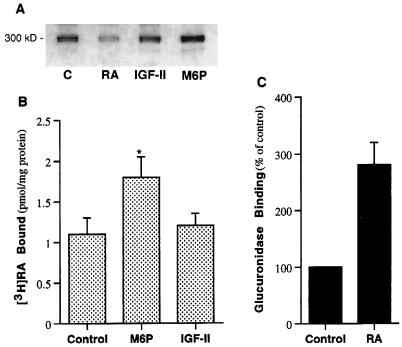
It is unique that a single receptor binds three different classes of ligands: M6P (a carbohydrate), IGF-II (a protein), and RA (a lipid). The receptor should now be named M6P/IGF-II/RA or “MIR” receptor. It is of interest to determine how these ligands interact on each other within the same receptor. Therefore, we examined the effects of M6P and IGF-II on the [3H]RA affinity-photolabeling of the M6P/IGF-II receptor. As shown in Fig. 5A, neither M6P nor IGF-II inhibited [3H]RA binding to the M6P/IGF-II receptor, suggesting that they do not bind to the same site. However, M6P but not IGF-II significantly enhanced the cross-linking of [3H]RA to M6P/IGF-II receptor. The [3H]RA labeling of M6P/IGF-II receptor was 2 times higher in the presence of 5 mM M6P than it was in the absence of M6P, as measured by NIH Image, n = 3 (Fig. 5A). Similar results were obtained by measurement of the binding of [3H]RA to the partially purified receptors (Fig. 5B). The increased binding of RA to the receptor in the presence of M6P appeared to be the result of increased binding affinity. (Dissociation constants for M6P-treated receptor and control were 1.2 ± 0.4 nM and 2.5 ± 0.3 nM, respectively.) The lysosomal enzyme, β-glucuronidase (2,500 units/ml), was also shown to increase [3H]RA binding to the receptor (data not shown). Conversely, examination of the effect of RA on M6P binding to M6P/IGF-II receptor in saponin-permeabilized neonatal rat cardiac myocytes using the M6P-containing lysosomal enzyme, β-glucuronidase, as a probe showed that the M6P-inhibitable binding of the lysosomal enzyme to the cells was 2–3 times higher in the presence of RA (1 μM) than it was in the absence of RA (Fig. 5C). These results suggest a positive cooperativity between M6P and RA binding to M6P/IGF-II receptor.
Figure 5.
Interaction between RA with M6P or IGF-II in binding to the M6P/IGF-II receptor. (A) Photolabeling of M6P/IGF-II receptor by [3H]RA (1 μM) in the absence (C) or the presence of 50 μM unlabeled all-trans-RA (RA), 20 μM IGF-II, or 5 mM M6P. (B) Effects of M6P (5 mM) and IGF-II (20 μM) on [3H]RA binding to partially purified M6P/IGF-II receptors. ∗, P < 0.05 (n = 4). (C) Effect of RA (1 μM) on β-glucuronidase binding to permeabilized neonatal rat cardiac myocytes (n = 3).
Effect of RA on the Functions of the M6P/IGF-II Receptor.
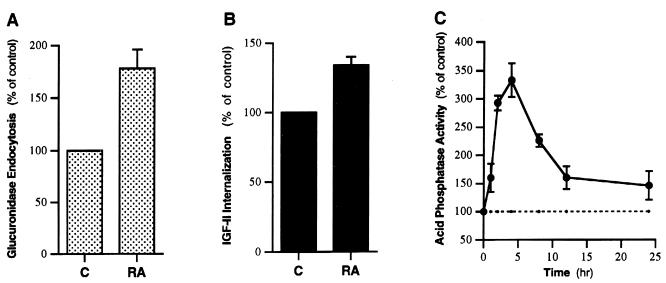
Because the primary functions of the Man-6-P/IGF-II receptor are to mediate endocytosis of extracellular M6P-containing proteins (e.g., lysosomal enzymes) and IGF-II and to sort newly synthesized lysosomal enzymes, an important question raised by the direct interaction of RA with M6P/IGF-II receptor is whether RA influences these functions of this receptor. To answer this question, we examined the effect of RA on endocytosis of exogenous β-glucuronidase and 125I-labeled IGF-II and intracellular activity of acid phosphatase in cultured neonatal rat cardiac myocytes, which express high levels of the M6P/IGF-II receptor (7). RA, at a concentration of 2 μM, almost doubled the M6P-inhibitable endocytosis of β-glucuronidase, increased the internalization of IGF-II by 30–50%, and induced a transient cellular accumulation of acid phosphatase (which indicates an increased sorting of lysosomal enzymes; Fig. 6), whereas the structurally similar lipid, eicosapentaenoic acid (C20:5n-3), at 10 μM, had no such effects (data not shown). These effects of RA were not altered in the presence of a protein synthesis inhibitor (0.5 mM cyclohexemide) and were not observed in the receptor-negative P388D1 cells (data not shown), supporting a direct interaction of RA with the M6P/IGF-II receptor. These data suggest that RA, upon binding to the M6P/IGF-II receptor, acts as a functional regulator to induce rapid endocytosis and efficient lysosomal enzyme sorting.
Figure 6.
Effect of RA on the functions of the M6P/IGF-II receptor in neonatal rat cardiac myocytes. (A) Effect on the endocytosis of exogenous β-glucuronidase. Myocytes were incubated for 2 h at 37°C with 1 ml of MEM containing 10,000 units of human β-glucuronidase, with or without 5 mM M6P, in the presence or absence of 2 μM RA. Following the incubation, the internalized human β-glucuronidase was determined as described in Methods. (B) Effect on the internalization of 125I-labeled IGF-II. Assay was performed by incubating cells at 37°C for 2 h in medium [150 mM NaCl/5 mM KCl/1.2 mM MgSO4/8 mM glucose/100 mM Hepes (pH 7.6)] or F-10 culture medium containing 125I-labeled IGF-II (0.5 ng/ml), with or without excessive unlabeled IGF-II (2 μg/ml), in the presence or absence of 2 μM RA. Following the incubation, the cells were washed three times with ice-cold PBS, and cell-associated radioactivity was determined by a γ counter. (C) Effect on cellular acid phosphatase activity. Cultured neonatal rat cardiac myocytes were treated with 2 μM RA at 37°C for various times, washed, and harvested. Cellular proteins were then solubilized by sonication, and acid phosphatase activity was assayed using a Diagnostics Acid Phosphatase kit (Sigma). C, control cells; RA, RA-treated cells (n = 4 for each).
The direct biochemical link between RA and the M6P/IGF-II receptor reported here is consistent with numerous previous observations on the functional connection and similarity between RA and the M6P/IGF-II receptor. For example, RA was found to enhance the activation of TGF-β (33), a process that requires the binding of TGF-β latent complex to the M6P/IGF-II receptor through the M6P-binding sites (12). RA was shown to be highly effective in modulating the release of lysosomal enzymes (34, 35). Both retinoids and the M6P/IGF-II receptor were also shown to be involved in extracellular matrix degradation (34–36). An increasing number of studies have indicated that the M6P/IGF-II receptor, like RA, is essential for normal fetal development (13–15) and has an anti-cancer activity (16–18).
Potential Biological Consequence of the Interaction.
What might be the biological significance of up-regulation of the functions of the M6P/IGF-II receptor? This receptor seems to exert primarily a significant growth-suppressive effect because it binds and stimulates the activation of the potent growth inhibitor, TGF-β, and also mediates the internalization and degradation of the growth-stimulatory factor, IGF-II. In addition, the function of the M6P/IGF-II receptor in sorting and trafficking lysosomal enzymes may also have an impact on regulation of cell death because it could regulate the bioavailability and activity of lysosomal enzymes, including certain cell death-related proteins, such as cathepsin B and D (proteases), which are known to involve the induction of apoptosis (37–39). Thus, the M6P/IGF-II receptor normally plays a role in suppression of cell growth. This is supported by recent animal experiments in mutant mice showing that loss of the M6P/IGF-II receptor results in fetal overgrowth and perinatal lethality as a consequence of major cardiac abnormalities (13–15) and by human studies showing that loss or mutation of M6P/IGF-II receptor gene is associated with the etiology of both human breast and liver cancer (16, 17). On the basis of these observations, it would be predicted that enhanced functional activity (i.e., higher affinity binding, rapid endocytosis, and efficient lysosomal enzyme sorting) of M6P/IGF-II receptor, upon binding of RA, may result in a decreased concentration of IGF-II, increased levels of activated TGF-β, and, perhaps, other apoptosis-related lysosomal enzymes, leading to decreased cellular proliferation and/or increased apoptosis. This is supported by our preliminary experiments showing that overexpression of M6P/IGF-II receptor in the human promyelocytic leukemia cell line HL-60R, a RA-resistant cancer cell line in which the retinoid nuclear receptor (RA receptor/retinoid X receptor) function has been voided due to a mutant RA receptor α and its trans-dominant negative activity (40, 41), resulted in a marked reduction in cell growth rate and a significant increase in the susceptibility of the cells to apoptosis in response to RA. Thus, binding of RA to the M6P/IGF-II receptor, leading to potentiation of the functional activity of this receptor, provides a mechanism for the action of RA on regulation of cell growth and a possible explanation for the recent observations that RA could influence cell death during embryogenesis (3) and cancer therapy (4, 5), independent of retinoid nuclear receptors.
Our data demonstrate a direct, functional interaction of RA with M6P/IGF-II receptor that may be important in regulation of cell growth and death. However, much more remains to be investigated to formulate a coherent picture of how they function together to regulate cell death. It would be particularly interesting to know where the RA-binding site is located on the M6P/IGF-II receptor protein, how RA binding could modulate the function of this receptor, and what is the biological role of this interaction and its relationship to the RA receptors/retinoid X receptors system in regulation of cellular growth during embryogenesis and carcinogenesis. In addition, the efficacies of interaction of various RA analogues with the M6P/IGF-II receptor also need to be tested. The knowledge derived from these studies will lead to a better understanding of how retinoids can elicit a diversity of biological responses.
Acknowledgments
We thank R. G. MacDonald and C. D. Scott for the anti-M6P/IGF-II receptor antibodies; S. J. Collins for HL-60R cells; W. Sly for the M6P/IGF-II receptor cDNA; and P. Lobel for purified bovine serum M6P/IGF-II receptor. We also thank Jennifer Bell for her technical assistance. This work was supported by NIH Grant DK-38165 (to A.L.) and American Cancer Society Grant IRG-173H (to J.X.K.).
ABBREVIATIONS
- RA
retinoic acid
- M6P
mannose-6-phosphate
- IGF-II
insulin-like growth factor II
- TGF-β
transforming growth factor-β
References
- 1.De Luca M L. FASEB J. 1991;5:2924–2933. [PubMed] [Google Scholar]
- 2.Sporn M B, Roberts A B, Goodman O S. The Retinoids: Biology, Chemistry and Medicine. 2nd. Ed. New York: Raven Press; 1994. [Google Scholar]
- 3.Ahuja H S, James W, Zakeri Z. Dev Dyn. 1997;208:466–481. doi: 10.1002/(SICI)1097-0177(199704)208:4<466::AID-AJA3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 4.O’Connell J M, Chua R, Hoyos B, Buck J, Chen Y, Derguini F, Hammerling U. J Exp Med. 1996;184:549–555. doi: 10.1084/jem.184.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delia D, Aiello A, Lombardi L, Pelicci P G, Griguini F, Formelli F, Minard S, Costa A, Veronesi U, Pierotti M A. Cancer Res. 1993;53:6036–6041. [PubMed] [Google Scholar]
- 6.Kornfeld S. Annu Rev Biochem. 1992;61:307–330. doi: 10.1146/annurev.bi.61.070192.001515. [DOI] [PubMed] [Google Scholar]
- 7.Nissley P, Kiess W, Sklar M. Mol Reprod Dev. 1993;35:408–413. doi: 10.1002/mrd.1080350415. [DOI] [PubMed] [Google Scholar]
- 8.MacDonald R G, Pfeffer S R, Coussens L, Tepper M A, Brocklebank C M, Mole J E, Anderson J K, Chen E, Czech M P, Ullrich A. Science. 1988;239:1134–1137. doi: 10.1126/science.2964083. [DOI] [PubMed] [Google Scholar]
- 9.Morgan D O, Edmoan J C, Standring D N, Fried V A, Smith M C, Roth R A, Rutter W J. Nature. 1987;329:301–307. doi: 10.1038/329301a0. [DOI] [PubMed] [Google Scholar]
- 10.Kiess W, Blickenstaff G D, Sklar M M, Thomas C L, Nissley S P, Sahagian G G. J Biol Chem. 1988;263:9339–9344. [PubMed] [Google Scholar]
- 11.Oka Y, Rozek L M, Czech M P. J Biol Chem. 1985;260:9435–9442. [PubMed] [Google Scholar]
- 12.Dennis P A, Rifkin D B. Proc Natl Acad Sci USA. 1991;88:580–584. doi: 10.1073/pnas.88.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z Q, Fung M R, Bariow D P, Wagner E F. Nature. 1994;372:2585–2588. doi: 10.1038/372464a0. [DOI] [PubMed] [Google Scholar]
- 14.Lau M M H, Stewart C E H, Liu Z, Bhatt H, Rotwein P, Stewart C L. Genes Dev. 1994;8:2953–2963. doi: 10.1101/gad.8.24.2953. [DOI] [PubMed] [Google Scholar]
- 15.Ludwig T, Eggenschwiler J, Fisher P, D’Ercol A J, Davenport M L, Efstratiadis A. Dev Biol. 1996;177:517–535. doi: 10.1006/dbio.1996.0182. [DOI] [PubMed] [Google Scholar]
- 16.Hankins R G, Souza A T D, Bentley R C, Patel M R, Marks J R, Iglehart J D, Jirtle R L. Oncogene. 1996;12:2003–2009. [PubMed] [Google Scholar]
- 17.Souza A T D, Hankins G R, Washington M K, Orton T C, Jirtle R L. Nat Genet. 1995;11:447–449. doi: 10.1038/ng1295-447. [DOI] [PubMed] [Google Scholar]
- 18.Wang S, Souza R F, Kong D, Yin J, Smolinski K N, Zou T-T, Frank T, Young J, Flanders K C, Sugimura H, Abraham J M, Meltzer S J. Cancer Res. 1997;57:2543–2546. [PubMed] [Google Scholar]
- 19.Eberle A N, deGraan P N E. Methods Enzymol. 1985;109:129–156. [Google Scholar]
- 20.Bayley H, Knowles J R. Methods Enzymol. 1977;46:69–114. doi: 10.1016/s0076-6879(77)46012-9. [DOI] [PubMed] [Google Scholar]
- 21.Bernstein P S, Choi S Y, Ho Y C, Rando R R. Proc Natl Acad Sci USA. 1995;92:654–658. doi: 10.1073/pnas.92.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang J X, Leaf A. Circulation. 1996;94:1774–1780. doi: 10.1161/01.cir.94.7.1774. [DOI] [PubMed] [Google Scholar]
- 23.MacDonald R G, Tepper M A, Clairmont K B, Perregaux S B, Czech M P. J Biol Chem. 1989;264:3256–3261. [PubMed] [Google Scholar]
- 24.Bretthauer R K, Kaczorowski G J, Weise M J. Biochemistry. 1973;12:1251–1256. doi: 10.1021/bi00731a002. [DOI] [PubMed] [Google Scholar]
- 25.Sahagian G G, Distler J J, Jourdian G W. Methods Enzymol. 1982;83:392–396. doi: 10.1016/0076-6879(82)83036-x. [DOI] [PubMed] [Google Scholar]
- 26.Valenzano K J, Remmler J, Lobel P. J Biol Chem. 1995;270:16441–16448. doi: 10.1074/jbc.270.27.16441. [DOI] [PubMed] [Google Scholar]
- 27.Sablonniere B, Dallery N, Grillier I, Formstecher P, Dautrevaux M. Anal Biochem. 1994;217:110–118. doi: 10.1006/abio.1994.1090. [DOI] [PubMed] [Google Scholar]
- 28.Miniti C P. J Biol Chem. 1992;267:9000–9004. [PubMed] [Google Scholar]
- 29.Kyle J W, Nolan C M, Oshima A, Sly W S. J Biol Chem. 1988;263:16230–16235. [PubMed] [Google Scholar]
- 30.Goodman D S. N Engl J Med. 1984;310:1023–1031. doi: 10.1056/NEJM198404193101605. [DOI] [PubMed] [Google Scholar]
- 31.Gabel C A, Goldberg D E, Kornfeld S. Proc Natl Acad Sci USA. 1983;80:775–779. doi: 10.1073/pnas.80.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee P D K, Hodges D, Hintz R L, Wyche J H, Rosenfeld R G. Biochem Biophys Res Commun. 1986;134:595–600. doi: 10.1016/s0006-291x(86)80461-2. [DOI] [PubMed] [Google Scholar]
- 33.Glick A B, Flanders K C, Danielpour D, Yuspa S H, Sporn M B. Cell Regul. 1989;1:87–97. doi: 10.1091/mbc.1.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fell H B, Dingle J T. Biochem J. 1963;87:403–408. doi: 10.1042/bj0870403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodman D W S, Smith J E, Hembry R M, Dingle J T. J Lipid Res. 1974;15:406–414. [PubMed] [Google Scholar]
- 36.Roff C F, Wozniak R W, Blenis J, Wang J L. Exp Cell Res. 1983;144:333–344. doi: 10.1016/0014-4827(83)90412-3. [DOI] [PubMed] [Google Scholar]
- 37.Deiss L P, Galinka H, Berissi H, Cohen O, Kimchi A. EMBO J. 1996;15:3861–3870. [PMC free article] [PubMed] [Google Scholar]
- 38.Narvaez C J, Vanweelden K, Byrne I, Welsh J. Endocrinology. 1996;137:400–409. doi: 10.1210/endo.137.2.8593782. [DOI] [PubMed] [Google Scholar]
- 39.Moallem S A, Hales B F. Teratology. 1995;52:3014. doi: 10.1002/tera.1420520103. [DOI] [PubMed] [Google Scholar]
- 40.Roberston R A, Emami B, Collins S J. Blood. 1992;80:1885–1889. [PubMed] [Google Scholar]
- 41.Nagy L, Thomazy V A, Shipley G L, Fesus L, Lamph W, Heyman R A. Mol Cell Biol. 1995;15:3540–3551. doi: 10.1128/mcb.15.7.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]