Abstract
Proteins of the CAS (Crk-associated substrate) family (BCAR1/p130Cas, NEDD9/HEF1/Cas-L, EFS/SIN and CASS4/HEPL) are integral players in normal and pathological cell biology. CAS proteins act as scaffolds to regulate protein complexes controlling migration and chemotaxis, apoptosis, cell cycle, and differentiation, and have more recently been linked to a role in progenitor cell function. Reflecting these complex functions, over-expression of CAS proteins has now been strongly linked to poor prognosis and increased metastasis in cancer, as well as resistance to first-line chemotherapeutics in multiple tumor types including breast and lung cancers, glioblastoma, and melanoma. Further, CAS proteins have also been linked to additional pathological conditions including inflammatory disorders, Alzheimer’s and Parkinson’s disease, as well as developmental defects. This review will explore the roles of the CAS proteins in normal and pathological states in the context of the many mechanistic insights into CAS protein function that have emerged in the past decade.
Keywords: CAS, BCAR1, NEDD9, Scaffold, Cancer, Invasion, Metastasis, Mitosis
Introduction
In the 15 years since identification of the first member of the group, proteins of the CAS protein family (in mammals, p130Cas/BCAR1, HEF1/NEDD9/Cas-L, EFS/SIN and HEPL/CASS4; Table 1) have been recognized as highly connected nodes in cellular signaling networks, which play important modulatory roles in both normal and pathological cell growth regulation. Although to date no catalytic function has been identified for CAS proteins, these proteins contain multiple protein interaction motifs, which allow them to serve as scaffolds for a significant number of partners, regulating their activity in time and space. To date, CAS proteins have been most studied in the context of control of cell migration and invasion in the context of cancer, processes in which they have assumed growing importance in the past 5 years. However, a growing body of work on specific members of the CAS family supports the broader mechanistic role of these proteins in influencing cell cycle, differentiation, survival, and involvement in additional disease-associated states such as stroke and inflammatory response.
Table 1.
The CAS gene family: Summary of gene ID, human chromosomal location, and murine gene knockout phenotype for four human family members
| Name(s) | Gene ID | Year identified | Chromosomal localization | Knockout phenotype in mice | References |
|---|---|---|---|---|---|
| BCAR1/p130Cas/CASS1 | 9564 | 1994 | 16q23.1 | Lethal at embryonic stage 11.5–12.5 due to cardiovascular defects | [1–4] |
| EFS/Sin | 10278 | 1995 | 14q11.2 | Viable, fertile, aging mice incur Intestinal inflammation | [5, 6] |
| NEDD9/HEF1/Cas-L | 4739 | 1996 | 6p24.1 | Viable, fertile, minor defects in maturation of immune system | [7–9] |
| HEPL/CASS4 | 57091 | 2008 | 20q13.31 | n/a | [10] |
This article will review the current state of understanding of CAS protein biology as follows:
-
-
Identification and nomenclature for CAS family members
-
-
Conserved and unique structural features, and intracellular localization
-
-
General abundance and transcriptional regulation of CAS gene products
-
-
Post-transcriptional and post-translational regulation of CAS family members
-
-Biological functions of CAS proteins
-
-Cell attachment, migration, chemotaxis, and invasion
-
-Apoptosis/anoikis
-
-Cell cycle control
-
-Ciliary resorption
-
-Osteoclast reorganization
-
-Microbial pathogenesis
-
-
-
-
CAS proteins in development
-
-
CAS functionality in cancer
-
-
CAS proteins in other pathological states
-
-
Summary and prospects
Identification and nomenclature
The designation CAS, for (Crk-associated substrate), reflects the historical basis for identification of this group of scaffolding proteins. Preliminary studies by the Parsons group had identified an abundant cellular protein migrating at 130 kDa that was hyperphosphorylated in v-Crk (chicken tumor virus no. 10 regulator of kinase) and v-Src-transformed cells [1, 2]. Sakai et al. [3] cloned this founding family member, rat p130Cas, establishing that it bound tightly to both v-Crk and v-Src, and was directly phosphorylated by Src. The official gene name for the ortholog group, BCAR1 (breast cancer resistance 1), reflects the identification of the human p130Cas gene in a retroviral insertion screen for genes that promote resistance to tamoxifen [4]. Alternative rare designations for the protein include CAS1, CASS1, and CRKAS. Human BCAR1 localizes to chromosome 16q22-q23.
The second member of the group to be reported, EFS (embryonal Fyn substrate; also known as CAS3, CASS3, HEFS, SIN), was identified based on interactions with the Src-family kinases Fyn and Yes, in a 1995 expression library screen for proteins with high affinity for Src-family SH3 domains [5], and in a subsequent similar screen, which isolated the protein as SIN, for Src Interacting [6]. Human EFS localizes to chromosome 14q11-q12.
The third CAS protein (known alternatively as HEF1, CASL, CAS-L, NEDD9, CAS2, and CASS2) was first described in 1996. Law et al. [7] identified HEF1 (human enhancer of filamentation 1) from a functional complementation/genetic screen, seeking for human genes that promote filamentous growth in yeast, with the goal of identify genes regulating cell cycle and polarity. The same protein was identified later the same year as CAS-related protein, lymphocyte type (CAS-L), a protein hyperphosphorylated on tyrosines upon integrin β1 stimulation in T-lymphocytes [8]. The official gene name, NEDD9 (neural precursor cell expressed, developmentally down-regulated 9) was assigned after recognition that a 3′ untranslated region of a gene expressed exclusively in early embryonic, but not adult, mouse brain [9] was orthologous to the HEF1/CAS-L mRNAs. Human NEDD9 localizes to chromosome 6p25-p24.
A fourth family member, CASS4 (HEF1-EFS-P130Cas-like)/CAS4/CASS4, was identified only in 2008, based on an extensive analysis of genomic and transcriptome sequences, followed by confirmation that the protein was expressed and acted similarly to other family members in some cell types [10]. Human CASS4 localizes to chromosome 20q13.2-q13.31.
An evolutionary analysis of conservation of the CAS family, reported in [10], has determined that these four family members are conserved from gnathostomes (jawed vertebrates) through mammals. There is only one CAS family member found in lower vertebrates, chordates, and insects including Drosophila melanogaster. No protein strongly homologous to the CAS group can be discerned in Caenorhabditis elegans, Saccharomyces cerevisiae, or other lower eukaryotes. In subsequent discussion, most work describes studies performed on BCAR1 and NEDD9; relatively few publications address the functions of EFS and CASS4.
Conserved and unique structural features, and intracellular localization
The size of the human CAS proteins ranges from 561 amino acids for EFS to 870 amino acids for BCAR1. Characteristically, CAS proteins migrate at a significantly higher molecular weight than predicted from analysis of their sequences: for example, BCAR1, MW 125 kDa, migrates at 130 kDa; NEDD9, MW 93 kDa, migrates as a doublet of 105 and 115 kDa. These apparently higher molecular weights largely reflect the extensive phosphorylation of CAS proteins, discussed below. CAS proteins conserve a similar domain structure, characterized by four main elements (Fig. 1). Each CAS protein has an amino-terminal Src homology 3 (SH3) domain, which confers binding to protein substrates containing polyproline motifs, with the most studied partners being FAK [7] and PYK2/RAFTK kinases [11–18], but also C3G [19], PTP-PEST [20], PTP1B [21], CIZ [22], and FRNK [23]. The structure of the BCAR1 SH3 domain has been solved at 1.1 Å, and compared to the SH3 domain of α-spectrin (PDB ID: 1SHG) [24]. High homology among the CAS proteins in this region (for instance, 75% amino acid identity, 92% similarity with NEDD9) suggests the BCAR1 SH3 domain can be used to model the SH3 binding domains of the other family members.
Fig. 1.
CAS protein structure and interactions. a CAS proteins conserve a common structure marked by four discrete domains: SH3 domain, an unstructured substrate domain, a 4-helix bundle, and an evolutionarily conserved C-terminal domain. Crystal structures have been obtained for the SH3 domain (1wyx) of human BCAR1 and 4-helix bundle (1z23) of rat BCAR1 are shown. Secondary structure predictions for the C-terminal domain are also shown; pattern of α-helices has some features of a focal adhesion-targeting (FAT) domain, but is not exactly the same. Amino acid sequences correspond to those for human BCAR1, and vary slightly for other family members. b For a number of important CAS interacting proteins, exact domains of interaction have been mapped, and are shown. Proteins known to interact with the substrate domain specifically through SH2/phospho-tyrosine interactions are thus indicated. A poly-proline domain common to BCAR1 and EFS is shown (Pro). See text for details
Adjacent to the SH3 domain is a large region often known as the “substrate domain” (SD). The most notable feature of the SD is the presence of a large number of YxxP motifs, which when phosphorylated by Src-family kinases provide canonical binding sites for proteins containing SH2 domains such as Crk, Crk-L, and CRKII [3, 5, 7, 10, 12, 23, 25–27]. The number of motifs ranges between the different family members, from a low of 8 (EFS) to 9–13 in the other family members, with variance in the length of this region accounting for the difference in length between the family members [3, 5, 7, 10, 28]. Extensive biophysical studies by the Sheetz group on BCAR1 suggest that this region is intrinsically unstructured, and that mechanical forces associated with stretching of the protein may induce an opening that allows phosphorylation by regulatory kinases and association with binding partners [29, 30].
Further downstream lies a domain that has relatively little amino acid homology among the four family members, but which is characteristically serine-rich. Structural analysis of this domain on BCAR1 and NEDD9 reveals a four-helix bundle [24, 31]. Computational modeling of this region suggests that this bundle is likely conserved among other family members [10], and provides a docking site for a subset of CAS family partners (e.g., 14-3-3 proteins [32], GRB2 [33]).
The carboxy-terminus of the CAS proteins is extremely well conserved in primary amino acid sequence and predicted secondary structure among the CAS protein group, but has little similarity to proteins outside this protein family. There is no structural data available on this region and efforts to study this domain are hindered by the extreme toxicity of overexpressing this isolated domain in cell culture [34] and the tendency of the isolated purified domain to aggregate (unpublished). NEDD9 has been shown to homodimerize, and to heterodimerize with BCAR1 through this domain [35]. For all family members except CASS4, the C-terminal domain contains a YDYVHL motif [6, 7, 36], which is phosphorylated as an important early event during cell adhesion, as discussed below. A BCAR1 peptide encompassing this site has been co-crystallized with the Src-family member LCK [37]. CAS proteins have been described to interact with a considerable number of other proteins through this region, including BCAR3/AND-34, which contains a GDP-exchange factor (GEF)-like domain, and the BCAR3-related protein CHAT-H [38, 39], as well as a subunit of ubiquitin ligase APC/C, CDH1 and E3 ubiquitin ligase AIP4 [40, 41]. One study established interactions between the NEDD9 carboxy-terminus and a number of proteins with helix-loop-helix (HLH) domains, and identified sequence features within NEDD9 similar to those found in HLH proteins such as ID2 [35]; these predictions have not been rigorously examined at the structural level.
In contrast to these structural features, which are held in common by all the proteins in the CAS family, BCAR1 and EFS also contain polyproline motifs that are bound by SRC-family kinases, and contribute to processive phosphorylation of BCAR1 and EFS by SRC [5, 42]. NEDD9 [34, 43, 44] and BCAR1 [45] contain internal caspase cleavage sites that are used for processing and destruction of these proteins during apoptosis; such motifs have not yet been reported for other family members. Finally, a number of critical phosphorylation sites have been identified that regulate protein association, localization, and function; these are discussed in the sections on cell signaling, below.
Three of the four CAS proteins have been shown to localize to and function at focal adhesions [7, 10, 46]. In addition, the SH3 domain of EFS/SIN binds to the first proline rich-region of FAK, but has not been tested for localization to focal adhesions [47, 48]. Structure–function analysis has shown separate contributions of the SH3 domain and the carboxy-terminal domains of BCAR1 for focal adhesion association, and provided evidence that phosphorylation by SRC contributed to the localization [49]. Although concentrated at focal adhesions, substantial unanchored cytoplasmic pools of CAS proteins also exist, and can be differentiated from the focal adhesion-associated pool based on differential phosphorylation (e.g., [50]); specific adhesion-associated serines have recently been identified [51]. One CAS protein, NEDD9, has uniquely been shown to associate with centrosomes, the mitotic apparatus, and the ciliary basal body (a structure derived from centrosomes) [52, 53]. Structural domains governing these NEDD9 localizations have not been established. Finally, some reports have placed BCAR1 at cell–cell junctions of polarized cells [54], or the caspase-cleaved carboxy-terminal fragment of BCAR1 in the nucleus, acting as a transcriptional repressor [55].
General abundance and transcriptional regulation of CAS gene products
Among the CAS proteins, BCAR1 has been reported as most ubiquitously expressed, whether in cultured cells or in vivo [3, 56, 57]. Some early studies of BCAR1 versus NEDD9 in the hematopoietic system suggested BCAR1 was more highly expressed in differentiated blood cells, while NEDD9 was elevated in more immature precursors [8, 12, 58, 59]. BCAR1 protein levels remain consistent throughout the cell cycle [44]. In contrast to BCAR1, both EFS and CASS4 have been reported to have extremely restricted expression patterns. Expression of Efs/Sin is primarily limited to T-lymphocytes, the thymus, brain, and skeletal tissue [6]. CASS4 expression is highest in lung and spleen with lower levels in many other tissues [10]. Essentially no studies have addressed transcriptional regulation of the BCAR1, EFS, and CASS4 proteins.
In contrast, the abundance of NEDD9 varies significantly in different cell types and tissues, and under different growth conditions. In normal human tissues, highest levels of NEDD9 mRNA and protein are found in the lung and kidney, in tissues rich in immature lymphoid cells, and in the fetal brain (although downregulated in adult brain) [7–9]. In cell lines, NEDD9 is abundant in tumor cells derived from epithelial lineages, in lymphoma cell lines, and in glioblastomas [58, 60]; as discussed below, this reflects the common genomic amplification and/or transcriptional upregulation of NEDD9 during cancer progression [61–66]. NEDD9 transcription was found to be highly upregulated in gastrointestinal stromal tumor (GIST) cells selected for imatinib resistance, although the mechanism of induction is not known [61].
Several distinct transcription factors or growth conditions have been specifically reported as controlling NEDD9 abundance. TGF-β induces transcription of NEDD9 mRNA in human breast epithelial cells and in the T-lymphoblastoid H9 cell line [65]. TGF-β induction of NEDD9 gene expression in human dermal fibroblasts is both dose- and adhesion-dependent, and can result in an increase as high as 16-fold of NEDD9 protein [66]. All-trans retinoic acid (ATRA), a vitamin A derivative, upregulates NEDD9 transcription in human neuroblastoma cells [63, 64]. ATRA induces heterodimerization of the receptors RAR and RXR, which bind an ATRA response element located in the NEDD9 promoter [63, 64]. In addition, progesterone receptor A overexpression upregulates NEDD9 [67], while estrogen treatment or estrogen receptor overexpression downregulates NEDD9 in human breast [68] and osteosarcoma [69] cell lines. In human progenitor cells derived from bone marrow or obtained from umbilical cord blood, hypoxia induces the upregulation of NEDD9 mRNA [70]. Serum stimulation induces NEDD9 accumulation and hyperphosphorylation as cells transition from quiescence to initiation of cell cycle [44, 53]. Given recent papers suggesting NEDD9 expression promotes a progenitor or stem cell phenotype in some cell lineages [71, 72], the observation that the promoter of NEDD9 is bound significantly by the pro-stem cell factor SOX2 is suggestive [73]. Another intriguing recent report indicated that the dioxin signals through the aryl hydrocarbon receptor (AhR) to strongly induce NEDD9 transcription, providing an unexpected connection to growth deregulation induced in response to environmental pollutants [74]. Finally, NEDD9 has been reported to be the target of a microRNA regulatory module alternatively regulated in cancer cells, under the control of miR-145 and miR-125b [75].
In sum, these studies may suggest that BCAR1 supports essential/baseline functions of the CAS protein group, CASS4 and EFS function in limited and specialized circumstances, and NEDD9 is adapted for response to multiple dynamic stimuli.
Post-transcriptional and post-translational regulation
CAS proteins undergo significant and rapid changes in phosphorylation in response to both intrinsic and external cues. These changes in phosphorylation are integral to the ability of the CAS proteins to act as scaffolding proteins, to localize to specific cellular structures, and to regulate the steady state levels of the proteins by influencing rate of proteolysis. The most studied stimuli for phosphorylation of CAS proteins in normal cells are cell attachment and extrinsic forces; treatment with growth factors or hormones; and cell cycle. Some of the CAS proteins (notably NEDD9) are also regulated by targeted cleavage and proteolytic degradation. Specific defects in regulation of these processes relevant to cancer and other pathogenic states are discussed separately, in “CAS proteins in cancer”
Attachment and force
The first identified regulated phosphorylation process involving CAS proteins was in the context of cellular attachment to extracellular matrix, activation of integrins and subsequent spreading [7, 11, 12, 14, 26, 33, 46–48, 76–78]. As shown in Fig. 2, soon after cell attachment, integrin signaling activates FAK (or depending on cell type, the FAK paralog RAFTK/CAK-β/Pyk2) [79]. FAK binds directly to CAS proteins, based in part on interactions between the CAS SH3 domain and FAK polyproline sequences. FAK phosphorylates CAS proteins (BCAR1, NEDD9, EFS) directly on the C-terminal binding site YDYVHL [77]. This creates a high affinity site for binding by the SH2 domain of SRC-family kinases (including SRC, LYN, FYN, and others), contributing to the recruitment of these kinases. SRC kinases then rapidly phosphorylate multiple tyrosine phosphorylation motifs within the CAS SD, creating binding sites for proteins that promote cell spreading, including most notably Crk and Crk-L.
Fig. 2.
Upstream regulators of CAS mRNA and protein expression, and protein activation. Signaling proteins inducing transcription of CAS proteins, or influencing their phosphorylation or dephosphorylation, are indicated. In addition, some stimuli such as hypoxia induce dynamic changes in CAS expression or modification, but specific proteins mediating the responses are unknown. See text for details
While part of the initiation signal is integrin-directed activation of the FAK and SRC kinases, an additional signal for CAS hyperphosphorylation arises from the physical stretching of the protein. Studies by the Sheetz group determined that physical stretching induced force-dependent association of BCAR1 with a membrane-associated cytoskeletal matrix, and stretch-dependent phosphorylation of BCAR1 by the FYN kinase [29, 30]. In this model, association of the SH3 domain of BCAR1 with FAK connects through talin to the actin cytoskeleton at the N-terminus of BCAR1, while the C-terminus binds to SRC-family kinases; extension of the molecule based by forces generated in the extracellular environment promote-force-dependent phosphorylation.
More recently, a second adhesion-dependent signaling process was demonstrated for BCAR1 [51]. BCAR1 and NEDD9 interact with a partner protein BCAR3 to mediate anti-estrogen resistance, and to influence activation of the Rap1 GTPase [39, 80, 81]. Following cell attachment, BCAR3 supports the gradual emergence of a population of BCAR1 phosphorylated on serines 139, 437, and 639 [51]. Appearance of this population correlates with spreading/invasive morphology of cells; significance in terms of association of BCAR1 with specific signaling partners is not yet clear.
Although not studied in the same detail as BCAR1, generally similar mechanisms are likely to apply to NEDD9, EFS, and CASS4 [5, 7, 10, 28, 58], based on the common domain structure and common motifs of these proteins. Of note, the fact that CASS4 lacks a YDYVHL motif but is nevertheless phosphorylated in a SRC-dependent manner following adhesion implies that an initial phosphorylation of this motif by FAK cannot be essential in the attachment-dependent activation process [10]. Some studies have suggested that a preferred partner of NEDD9 is RAFTK rather than FAK [11, 12]. Additional kinases have been described as directly phosphorylating or promoting the phosphorylation of NEDD9 or BCAR1, although not studied in as great detail. These include Abl, binding and phosphorylating NEDD9 [7]; and BMX/ETK, enhancing the phosphorylation of BCAR1 during cell migration [82].
Growth factors and hormones
Over the past decade, a large number of extrinsic signals have been identified as leading to rapid phosphorylation of CAS proteins. Ligand engagement of receptor tyrosine kinases (RTKs) including fibroblast growth factor 2 receptor (FGFR2) in breast cancer cells [83], vascular endothelial growth factor receptor (VEGFR) in brain microvascular endothelial cells [84] and multiple myeloma cells [85], insulin growth factor receptor (IGFR) in aortic smooth muscle cells [86], and macrophage colony stimulating factor (M-CSF) in osteoclasts [87], have each been reported to stimulate BCAR1. Activation of the platelet-derived growth factor receptor (PDGFR) leads to NEDD9 phosphorylation in glioblastoma cell lines [60].
Activation of G-protein coupled receptors (GPCR) also causes hyperphosphorylation of BCAR1 and NEDD9. Neuropeptide GPCR agonists activate BCAR1 in neural and endocrine cells [88]. Activation of the calcitonin GPCR induces tyrosine phosphorylation of NEDD9 in osteoclasts [89, 90]. Activation of the endothelin receptor activates BCAR1 in cardiomyocytes [91]. Stimulation of the muscarinic receptor causes tyrosine phosphorylation of NEDD9 and BCAR1 in neuroblastoma cell lines [92]. Additional non-GPCR upstream activators include TGF-β, which have been shown to dramatically increase tyrosine phosphorylation of NEDD9 [66]; angiotensin II in vascular smooth muscle cells [93], the chemokine CCL20/MIP-3α in intestinal epithelial cells [94], and tamoxifen, in estrogen receptor positive breast cancer epithelial cells [95].
Ischemia also causes hyperphosphorylation of NEDD9 in neurons of the cerebral cortex and hippocampus [96]. Glucose stimulates BCAR1 tyrosine phosphorylation in pancreatic beta cells [97]. For many (although not all) of the initiating signals discussed above, SRC-family kinases have been clearly identified as inducing the tyrosine hyperphosphorylation. Changes in CAS serine or threonine phosphorylation have generally been less investigated. For a number of these signals, an intact actin cytoskeleton and adherent cell status are necessary for the signal to propagate (e.g., calcitonin-dependent NEDD9 phosphorylation [96]); other stimuli act independent of focal adhesion integrity and actin cytoskeleton (e.g., TGF-β).
Cell cycle
Both BCAR1 and NEDD9 are subject to cell cycle-regulated phosphorylation. For BCAR1, serine and threonine phosphorylation as cells enter mitosis results in disruption of the association between BCAR1, SRC, and FAK, and is thought to contribute to the disassembly of focal adhesions as cells round [98, 99]. NEDD9 typically oscillates between a faster migrating (~105 kDa) form in G1/S cells to a slower migrating form in G2/M cells [44]. NEDD9 has been defined as a partner, activator, and substrate for the mitotic Aurora-A kinase [52], with the phosphorylation of NEDD9 by Aurora-A leading to disruption of the Aurora-A/NEDD9 complex. Whether EFS or CASS4 are subject to cell cycle-regulated changes in phosphorylation is not known.
Dephosphorylation
Balancing the action of kinases, a number of phosphatases have been found to target CAS proteins. The transmembrane receptor-like protein tyrosine phosphatase (PTP) LAR influences focal adhesion and actin cytoskeletal dynamics, and induces apoptosis when overexpressed in mammalian cells. LAR dephosphorylates BCAR1, inducing its subsequent cleavage and degradation [100–102]. PTP-PEST, implicated in mitogenic and cell adhesion-induced signaling, and in transformation by a variety of oncogenes, binds the SH3 domain of BCAR1 [20, 24] and dephosphorylates it [103], leading to decreased CAS–CRK interactions [104]. Based on substrate trapping, PTP-PEST also binds to NEDD9 and EFS [105], although these interactions have been less investigated. In PTP-PEST knockout mice, BCAR1 is hyperphosphorylated, indicating physiological relevance as a PTP-PEST substrate [106]. PTP-1B, has also been found to bind to the SH3 domain of BCAR1, and similarly dephosphorylate it [21]. The Src homology 2 (SH2) domain containing PTP, SHP-2, binds NEDD9 at focal adhesions, and inhibits extracellular matrix-induced NEDD9 phosphorylation, limiting NEDD9-dependent cell migration [107]. The stomach cell-associated protein tyrosine phosphatase 1 (SCAP1) dephosphorylates BCAR1 to inhibit cell growth and motility in various transformed cell lines [108].
Protein phosphatase 2A (PP2A) binds and dephosphorylates both BCAR1 [109] and NEDD9 [110, 111]. PP2A negatively regulates the mitotic phosphorylation of BCAR1 [109]. PP2A is required for the de-adhesion-induced conversion from p115 to p105 kDa NEDD9 [110], potentially by targeting the Ser369 phosphorylation associated with this conversion [111]. The PRL (phosphatases of regenerating liver) subfamily of kinases are also linked to positive regulation of tumor growth; overexpression of PRL-1 was found to reduce BCAR1 levels [112], but inhibition of PRL phosphatases with a small molecule, thienopyridone, leads to BCAR1 cleavage [113]. The biological significance of this relationship requires more study. Insulin treatment induces the dephosphorylation of BCAR1 [114], although the relevant phosphatase is not known. Finally, the Yersinia species bacterium encode a PTPase, YopH, that is required for bacterial uptake. YopH targets BCAR1, dephosphorylating it at focal adhesions [115–117], and promoting bacterial virulence, as discussed below (see “Microbial pathogenesis”).
Proteolysis
NEDD9 and BCAR1 are known targets of regulated proteolysis. Most dramatically, both NEDD9 [34, 43, 44] and BCAR1 [45, 118] are cleaved into fragments by caspases following stimuli leading to cell death, including detachment-induced cell death (anoikis). Exact sites of cleavage have been mapped, and include a DLVD site at aa 363 and a DDYD site at aa 630 on NEDD9 [43, 44] and a DVPD site at aa 416 and a DSPD site at aa 746 on BCAR 1 [45], with functional consequences of the cleavage discussed in “Apoptosis/anoikis”.
NEDD9 is also subject to proteasomal degradation. This occurs during the cell cycle [43], with NEDD9 protein levels sharply reduced at the end of mitosis. The more slowly migrating, p115-kDa, serine/threonine phosphorylated form of NEDD9 that accumulates in attached and in G2/M cells has been described as more susceptible to proteolytic degradation [110]. Conversion of p105 to p115 NEDD9 has been reported as triggered by a phosphorylation at aa S369 by a kinase inhibited by Hesperadin, with this phosphorylation promoting proteasomal degradation [111]. NEDD9 is also targeted for proteasomal degradation by induced TGF-β signaling [65, 66]. In this context, the degradation is mediated via direct interactions between NEDD9, SMAD3, APC10, CDH1, and hITCH, which promote ubiquitination and destruction of the protein [40, 41].
Biological functions of CAS proteins
Cell attachment, migration, chemotaxis, and invasion
CAS proteins help mediate cell spreading [10, 57, 119] and play an important role in driving cell migration [6, 119, 120] (Fig. 3). As noted above, CAS proteins are tyrosine-phosphorylated by FAK and SRC during cell attachment to the extracellular matrix [8, 11, 23, 26, 47, 121]. CAS proteins concentrate at focal adhesions, although cytoplasmic pools of the proteins exist; BCAR1 also concentrates at the podosome in Src-transformed cells [49]. CAS proteins are not required for formation of focal adhesions, and indeed, focal adhesions become more prominent in fibroblasts derived from BCAR1 null mice [57]. These results, coupled with extensive data arising from knockdown experiments, suggest CAS proteins function to regulate focal adhesion turnover.
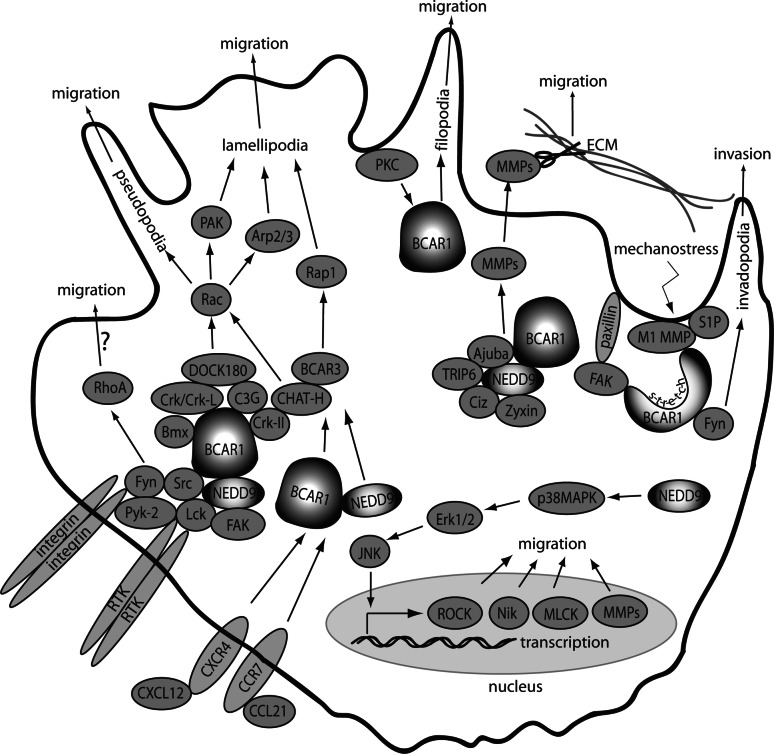
Fig. 3.
CAS proteins in cell migration. Activation or overexpression of CAS proteins activates multiple downstream effectors to promote formation of filopodia, lamellipodia, pseudopodia, invadopodia, and induce additional changes in the cytoskeleton that support migration. See text for details
Extensive studies of integrin-dependent BCAR1 signaling [11, 21, 23, 25, 26, 33, 47, 121–125] and NEDD9 signaling (particularly in lymphoid cells [7, 8, 12–18, 44, 58, 126–128]) have outlined a sequence of events following integrin ligation. Briefly, subsequent to CAS protein phosphorylation by the FAK or PYK22/RAFTK/CAK-β kinases and SRC-family kinases, the multiple tyrosine-phosphorylated YDxP motifs within the CAS substrate domain provide binding sites for the adaptor proteins CRK and CRKL. This complex in turn recruits C3G and DOCK180, a GDP-exchange factor for the small GTPase RAC. Activated RAC induces membrane ruffling and actin cytoskeleton remodeling, and promotes cell migration through ARP2/3 and PAK kinase activation, in a well-defined downstream effector pathway (review in [129]).
Overexpression of the BCAR1 protein is sufficient to trigger the activation of the downstream signaling cascade and promote migration [120]. Overexpression of NEDD9 has variously been reported as pro-migratory [10, 60, 119] and anti-migratory [130], which may reflect a difference in required co-factors for migration, or confounding factors, as discussed below in “CAS proteins in cancer”. Although integrin is the best-defined activator of CAS pro-motility signaling cascade, the hyperphosphorylation of CAS proteins induced by multiple growth factors, hormones, and physiological stimuli such as ischemia discussed in “Post-transcriptional and post-translational regulation”, couple CAS-dependent migration to many additional processes. For example, the urokinase-type plasminogen activator receptor (uPAR) pathway interacts with DOCK180 at integrin-associated complexes to activate Rac and induce of mesynchymal cell morphology and motility [131]. BMX/ETK association with BCAR1 at membrane ruffles enhances BCAR1 phosphorylation and cell motility again triggering association with CRK [82].
Although the CAS-CRK-C3G/DOCK180 pathway was defined first, other CAS-dependent promigratory effector pathways have been established. Both BCAR1 and NEDD9 interact with Zyxin family proteins, including zyxin, Ajuba, and TRIP6, to regulate cell motility [132–135]. Epistasis experiments with Ajuba null cells suggest that Ajuba is upstream of BCAR1 [134]. Zyxin interacts both with BCAR1 and a nucleocytoplasmic transcription factor, CIZ/NMP4/ZNF384 [133], which independently binds BCAR1 to induce the expression of multiple matrix metalloproteinases [22]. It is likely these interactions support migration and invasion. Additionally, NEDD9 overexpression activates a number of genes associated with promotion of cell migration and invasion (including p38MAPK, ERK1/2, and JNK), and transcriptionally upregulates MLCK, ROCK, NIK, matrix metalloproteases, and others [119, 136].
Cellular protrusions are a dynamic part of cellular reaction to ECM-substrate and are important for cellular motility and environmental sensing. BCAR1 mediates filopodia formation out of “growth cones” of neuroblastoma cell lines through interaction with protein kinase C (PKC) [137]. Moreover, BCAR1 and CrkII are essential for filopodia formation mediated by β1 integrin signaling [138], and BCAR1 activation-associated phosphorylation increases invadopodia activity [139]. BCAR1 complex formation with Crk is required for pseudopodia formation, and the subsequent Rac1 recruitment and activation to positively regulate BCAR1/Crk activity drives pseudopodia extension [140]. Lamellipodia formation, which is important for wound healing, is dependent on Rac1, BCAR1, and paxillin; all these proteins are sensitive to shear flow or sparse cell number [141]. NEDD9 also activates the small GTPase RhoA [142], which may contribute to NEDD9-dependent cell migration. Interestingly, inhibition of the RhoA effector Rho kinase in NEDD9-overexpressing cells instead promotes neurite-like extensions and inhibits migration in MCF-7 cells [143]. BCAR1 stimulates ERK1/2 and JNK (but not p38MAPK) activation to promote angiotensin II-mediated migration of vascular smooth muscle cells [144]. In contrast, BCAR1 positively contributes to TGF-β-mediated activation of p38MAPK during TGF-β-dependent migration of tumor cells [145].
One of the important functions of focal adhesions and cellular protrusions is mechano-sensing of the surrounding physical environment. Increasing evidence indicates that BCAR1 acts as mechano-sensor and responds to ECM rigidity, tension, and stretching [30]. Binding of the SH3 domain to FAK, and the carboxy-terminal domain to Src-family kinases, provides anchors for BCAR1 at focal adhesions so that in cells without attachments, BCAR1 is relaxed so that the substrate domain is unstructured and inaccessible to kinases [30]. During “stretching” in the process of integrin-engagement of extracellular matrix, the substrate domain is pulled open and becomes phosphorylated [30]. Rigidity response induced by cell stretching induces Fyn recruitment to the leading edge of the cell and Fyn-dependent phosphorylation of BCAR1 that correlates positively with increasingly rigid substrates such as fibronectin [29]. Invadopodia number and activity both increase with high ECM rigidity through activation of BCAR1 and FAK [139]. The relationship of BCAR1 with ECM rigidity indicates an important role for BCAR1 in mediating ECM degradation. Indeed, membrane-type 1 matrix metalloproteinase (MT1-MMP) binds BCAR1 at the leading edge of endothelial cells stimulated by sphingosine-1 phosphate (S1P) [146]. Because NEDD9 conserves all relevant structural features of BCAR1, NEDD9 may also be important in invadopodia signaling and particular in mechanical sensing of the ECM. The sensing of the ECM is important not only for various migration signaling but also for induction of apoptosis, should the cell become detached from the ECM.
Leukocytes bind loosely to the endothelium where cytokines induce integrin-engagement, allowing cells to subsequently migrate to the underlying tissue [147]. Chemotactic and immune receptor-mediated responses of hematopoietic cells are also an important component of CAS protein family signaling. NEDD9 was identified separately as CAS-L for its critical role in integrin-mediated signaling in T-lymphocytes [8], with NEDD9-induced cell migration active in human lymphoid cells with activated T-cell receptor (TCR/CD3), or following chemokine stimulation. Soon after, NEDD9 and BCAR1 were both shown to regulate integrin-mediated chemotaxis of B-cells [12]. However, BCAR1 expression is limited to only certain B-cells [12] and, possibly, only adherent leukocytes [8]. In lymphocytes, NEDD9 complexes with Lck and Fyn play an important role as an effector of T-cell antigen receptor activation [127]. Integrin-engagement of leukocytes induces rapid phosphorylation of NEDD9 in the H9 T-cell line [14] as well as the formation of a NEDD9-C3G-CRKL complex in B-cells [58] and T-cells [16]. Independently from integrin stimulation, TCR can activate NEDD9, inducing its interaction with two complexes: BCAR3/SHEP-2/SH3D3D and CHAT-H/SHEP-1/SH2D3C. CHAT-H association with NEDD9 at the leukocyte membrane directs CXCL12- or CCL21-induced T-cell migration by activating the GTPase RAP1 [148]. BCAR3 and CHAT-H, which also interact with BCAR1, have subsequently been found to collaborate with the CAS proteins to regulate additional small GTPases beyond RAP1, including RAC [38, 39, 81, 149], and are currently a topic of intense interest.
Activation-associated phosphorylation of both BCAR1 and NEDD9 has been shown to control various aspects of chemokine-induced chemotaxis in both B- and T-cells. Indeed, NEDD9 phosphorylation is not only high in the T-cells isolated from the synovial fluid of rheumatoid arthritis patients, but CCL5/RANTES stimulation enhances NEDD9 phosphorylation leading to enhanced and aberrant chemotaxis [150]. BCAR1, FAK, and paxillin phosphorylation is induced in response to gastrin binding CCK2 receptors on colon cells [151]. The chemokine, stromal cell-derived factor 1 alpha (SDF-1α, also CXCL12) causes rapid and transient phosphorylation of BCAR1, paxillin, and PYK2/RAFTK that can be sustained by co-treatment with the chemokine stem cell factor/kit ligand (SCF/KL), thereby resulting in enhanced chemotaxis [152]. The stimulatory effect of SDF-1α on BCAR1 and other focal adhesion components is dependent on protein kinase c (PKC), PI3 K [153], as well as Janus kinase 2 (JAK2) signaling [154]. Interaction of SDF-1α/CXCL12 and its receptor CXCR4 is also partially mediated by the tyrosine phosphatase CD45 via CD45-dependent effects on BCAR1, FAK, and paxillin phosphorylation [155]. B- and T-cells in mice lacking NEDD9 have specific defects in CXCL12-, CXCL13-, and CCL21-dependent chemotaxis, leading to a failure of B-cells to colonize the marginal zone during immune system maturation, and other immune defects [156].
Although the best studied, NEDD9 and BCAR1 are not the only CAS proteins important in immune cell chemotaxis. Deletion of EFS/SIN in mice causes extreme over-activation of T-cell-mediated inflammation response and induces a phenotype of severe inflamed regions of the small intestines similar to the phenotype of patients suffering from Crohn’s disease [157]. Indeed, overexpression of a truncated form of EFS/SIN that is constitutively phosphorylated by the SRC-family kinase FYN prevents thymocyte maturation to CD4 and CD8 positive T-cells. The role of CASS4 has not yet been addressed, although it is has been reported to be particularly abundant in immune cells [10].
Apoptosis/anoikis
Cell death is associated with significant morphological changes in cells. Cells induced to die by a variety of physiological stimuli (both intrinsic and extrinsic) typically round up and lose their contacts with substrate, with this severing of contacts thought to contribute to the removal of dead cells from organized tissues [158]. Separately, loss of epithelial cell contact with substrate, and concurrent failure to ligate integrins, independently triggers a cell death program termed anoikis in non-transformed epithelial cells [159, 160]. Anoikis is thought to serve as a protective mechanism to limit the dispersal of cells through the adult organism, and typically must be subverted during cell transformation [160]. As CAS proteins play important roles in assembling and contributing to the integrity of focal adhesions, elimination of CAS protein function contributes to the rounding that occurs in both apoptosis and anoikis (Fig. 4).
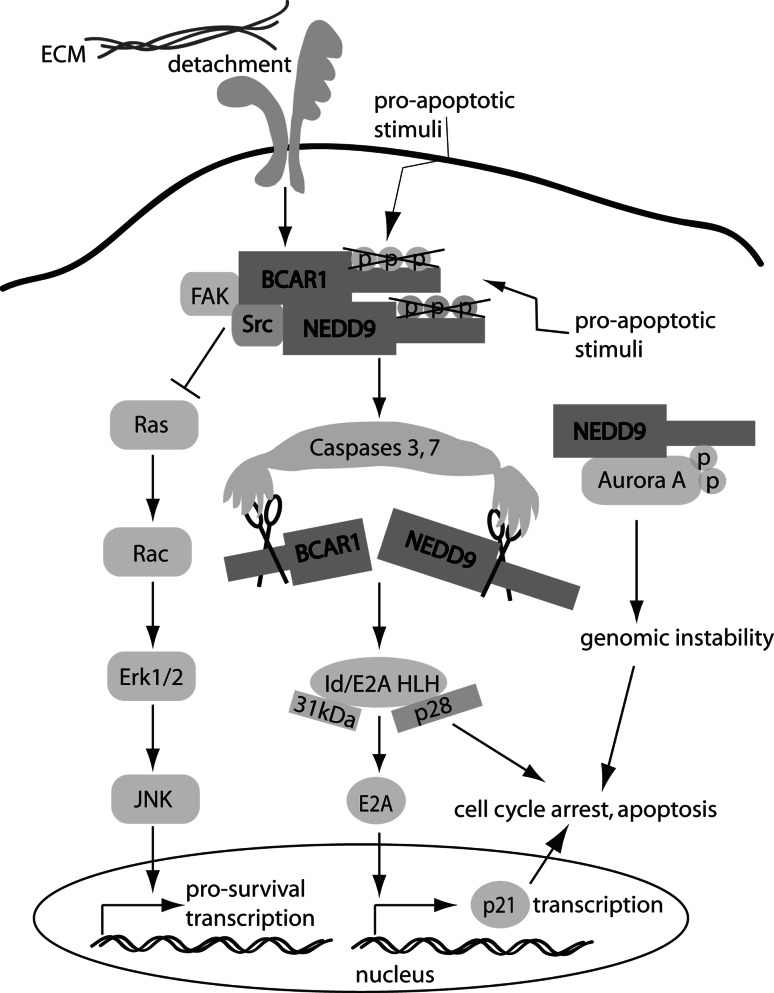
Fig. 4.
CAS proteins in apoptosis. Pro-apoptotic stimuli trigger dephosphorylation and cleavage of CAS proteins, leading to direct dominant-negative mediated focal adhesion disassembly, altering the transcriptional balance between CAS-dependent pro- and anti-survival factors. Overexpression of NEDD9 can induce cell cycle checkpoints that trigger apoptosis through additional routes. See text for details
The most direct functional connection between CAS proteins and apoptosis is in the generation of dominant negative fragments that actively interfere with focal adhesion integrity and survival-associated cell signaling. In apoptosis induced by cell detachment or other pro-apoptotic stimuli, NEDD9 is rapidly cleaved by caspases 3 and/or 7 at a DDYD (627-630aa) site, producing a C-terminal p28 fragment [43]. Importantly, p28 overexpressed in MCF-7 and HeLa cells causes rapid and complete disassembly of focal adhesions, cell detachment, and apoptosis [34]. This suggests NEDD9 may itself contribute to pro-apoptotic behavior of cells by disrupting survival signaling. A similar apoptotic cleavage product of BCAR1 releases a 31-kDa fragment that has similarly been found to be independently pro-apoptotic [55, 161]. It is possible that these fragments have additional pro-apoptotic activities. Law et al. [35] first identified that the p28 fragment encodes a capacity to bind a subset of Id/E2A HLH proteins that regulate differentiation and cell survival, and O’Neill and Golemis [34] determined that disruption of these motifs abrogated the pro-apoptotic function of p28. In studies of the BCAR1 p31 protein, Kim et al. [55] showed that this fragment is able to repress E2A transcription to regulate p21 and induce cell cycle arrest, with subsequent work supporting the relevance of CAS-HLH protein interactions to apoptosis [162]. As an additional complication, some work has shown that factors triggering cell death also lead to very rapid dephosphorylation of CAS proteins [34, 163]. This dephosphorylation may contribute to the targeting of the CAS proteins by caspases, although the mechanism remains to be elucidated in detail. Interestingly, overexpression of SDF-1α/CXCL12 causes anoikis through BCAR1 cleavage in colon carcinoma cells that have used epigenetic silencing mechanisms to limit endogenous SDF-1α/CXCL12 expression [164].
Overexpression of intact BCAR1 has been shown to be antiapoptotic in multiple studies [4, 95, 159, 163, 165–169]. Mechanisms proposed to support this anti-apoptotic activity include BCAR1-dependent hyperactivation of FAK and SRC, and laterally of the EGFR pathway signaling proteins (EGFR, Ras, Rac, JNK, and ERK1/2) [124, 170, 171], and at least in breast cells, there is evidence for additional although poorly defined signaling activities [172]. Overexpression of intact NEDD9 has yielded more complicated results, with overexpression in at least some cell lines leading to apoptosis [34, 43], and NEDD9 being unable to support the anti-estrogenic resistance of BCAR1 in breast cells [166]. NEDD9 has been shown to act similarly to BCAR1 in supporting activation of FAK, SRC, and EGFR-dependent signaling [119, 173]. One potential reason for the pro-apoptotic action of NEDD9 in some cell lines may lie in the secondary activity of this protein in inducing cell cycle abnormalities and hence mitotic checkpoints that trigger apoptosis, as discussed below. There is no work regarding CASS4 or EFS/SIN and regulation of apoptosis or survival to date.
Cell cycle control
Cell cycle regulation by CAS proteins has been explored in two contexts. The first context, most addressed for BCAR1, addresses the role of this protein as a scaffold for proteins necessary to support ERK1/2 activation, and thereby generally support transition through G1 and cell proliferation. BCAR1 scaffolding of complexes involving c-Src activates ERKs [170]. As further evidence of BCAR1 coupling to the EGFR pathway, BCAR1 associates with GRB2 and SHC to mediate integrin-mediated adhesion [33] and serum responses [170]. While no direct evidence has so far demonstrated NEDD9 binding to GRB2, NEDD9 does bind SHC, positioning it to mediate growth factor-induced signals [173]. BCAR1 is also important for transmitting the non-genomic proliferation signals initiating from the liganded estrogen receptor [174]. In this context, BCAR1 binds estrogen receptor (ER) to promote ER-dependent activation of not only SRC and ERK activation but also the cell cycle regulating protein cyclin D1 to induce G1 transit [174]. Further, BCAR1 is required for TGF-β regulation of SMAD3-dependent growth arrest; integrin-stimulated phosphorylation of BCAR1 allows BCAR1 association with Smad3 and consequent reduction of Smad3 phosphorylation and inhibition of p21 and p15 cell cycle inhibitory proteins [175]. It is likely that NEDD9 and the other CAS proteins conserve at least some of these activities, but this has not been addressed in detail.
The second context is in the regulation of mitotic entry and exit. BCAR1 levels are relatively consistent throughout the cell cycle; however, hyperphosphorylation of BCAR1 on serines and threonines at mitotic entry uncouples CAS-FAK interactions, and contributes to the transient loss of focal adhesions during M phase [98, 99]. The regulation of BCAR1 and its association with FAK and paxillin during mitosis is primarily through protein phosphatase 2a (PP2A) [109].
In contrast, NEDD9 has a very different role in mitotic regulation. Most quiescent cells contain minimal quantities of NEDD9. In non-quiescent cells, NEDD9 levels fluctuate significantly throughout cell cycle, rising during S phase, maximal at G2/M and decreasing as the cell undergoes mitosis and enters G1 [52]. Uniquely among the CAS proteins, NEDD9 associates with centrosomes, with this association most detectable in G2 and M phase; and subsequently translocates to the mitotic spindle, then midzone/midbody. The NEDD9 role at centrosomes and the mitotic apparatus is functional rather than passive. Overexpression of NEDD9 in cells induces appearance of a multipolar spindle, amplification of centrosomes and cytokinetic defects [52], and failure to resolve cell–cell bridges at the final stage of cytokinesis [142]. In contrast, depletion of NEDD9 with siRNA induces premature centrosome separation and disrupts microtubular organization in mitosis, which causes asymmetric and/or monopolar spindle formation [52]. siRNA-mediated knockdown of NEDD9 reduces the number of cells rounding up for mitosis, and leads to cleavage furrow regression and multinucleation [52, 142].
Two mechanisms by which NEDD9 influences M-phase have been identified. In one mechanism, NEDD9 interacts with and activates Aurora A, a kinase that phosphorylates substrates associated with spindle assembly and other processes required for mitotic entry [52]; for example, timed activation and degradation of Aurora A in mitosis is essential for control over appropriate chromosome separation [176]. Aurora-A is normally degraded at the end of mitosis [177]. Sustained overexpression and hyperactivation of Aurora-A is associated with cytokinetic failure and acquisition of supernumerary centrosomes [178], and NEDD9 may promote both these processes through Aurora-A. Reciprocally, mitotically activated AURKA phosphorylates NEDD9 at Ser296 to induce the disassociation of NEDD9 from AURKA in late M phase. This phosphorylation potentially enhances the ability of a pool of NEDD9 to return to focal adhesions at the end of mitosis, contributing to focal adhesion reassembly and the generation of traction forces necessary for cytokinesis [52, 179]. As a second mechanism, tightly controlled activation and inactivation of RhoA is important for successful mitosis, as RhoA coordinates cortical actin contractility during cell rounding up [180], and is highly regulated during cleavage furrow ingression [181]. NEDD9 immunoprecipitates with ECT2, a RhoA GDP-exchange factor, to positively regulate mitotic RhoA [142]; NEDD9 signaling through RhoA is also likely to impact mitosis.
Ciliary resorption
An unexpected, novel activity of NEDD9 was recently identified as regulation of ciliary disassembly [53]. Most normal mammalian cells have single primary, non-motile cilia that act as “antenna”, displaying growth factor receptors and receiving diffusible and mechanical cues from the extracellular environment [182]. Defects in cilia-related signaling have been linked to many different diseases, including notably polycystic kidney disease, Bardet-Biedl syndrome, Kartagener’s syndrome, and others [182]. Most transformed cells lack cilia, but whether this is cause or consequence of transformation is not known. In normal cells, cilia are resorbed in two waves, prior to G1/S transition, and prior to M phase, but are re-established in G0/G1 cells. It is not clear whether this cycle actively contributes to cell cycle control regulation, with arguments pro and con this position [183]. This resorption process has been found to be dependent on transient serum-dependent induction of NEDD9, and NEDD9-dependent activation of Aurora A kinase immediately prior to ciliary resorption, at each of the two waves of resorption. Aurora-A in turn phosphorylates and activates a tubulin deacetylase, HDAC6, which de-acetylates microtubules of the ciliary axoneme and potentially other substrates, triggering disassembly of the cilium [53]. As in cell cycle, the role of NEDD9 appears to be to support the activation of Aurora-A.
Osteoclast function
Osteoclasts are highly differentiated cells that regulate bone remodeling and resorption. When osteoclasts adhere to extracellular matrix in the marrow microenvironment, integrin-engagement triggers downstream signals that induce cytoskeletal reorganization, polarization, and formation of a “sealing zone” that links the osteoclast to the bone [184]. BCAR1 is a critical component of osteoclast adhesion signaling, first being shown to be hyperphosphorylated and important in actin restructuring [123]. As in other cell systems, BCAR1 activity is significantly associated with PYK2/CAK-β/RAFTK2 activation [185, 186]. PYK2/RAFTK2 forms a complex with BCAR1, c-SRC, and the TNFR-associated factor 6 (TRAF-6) for adhesion-initiated signaling in the osteoclast in response to bone matrix contact and interleukin-1 (IL-1) stimulation [187]. PYK2 and BCAR1 are hyperphosphorylated and localized to podosome structures integral to the formation of the sealing zone that connects osteoclasts to the bone matrix [123]. Activity and phosphorylation of BCAR1 in these cells is dependent on PYK2, as well as c-Src activation [187, 188]. PYK2-BCAR1 activation in osteoclasts is potentiated by macrophage colony stimulating factor (M-CSF) and gelosin [87, 189].
Calcitonin, a known inhibitor of osteoclast activity, regulates BCAR1 and NEDD9 to control cytoskeleton integrity in HEK-293 cells [89, 90]. Building on this result, Zhang et al. [190] looked at calcitonin regulation of NEDD9 in osteoclasts and found that, while calcitonin dephosphorylates PYK2, BCAR1 may be more important as a target than NEDD9 in these cells. Therefore, NEDD9 and BCAR1 are important for osteoclast adhesion to the bone matrix required for bone remodeling, but the two CAS proteins may have different responses to various regulators of bone integrity such as calcitonin. Finally, ECM rigidity increases BCAR1 phosphorylation and activity, thereby leading to enhanced ECM-degradation, consistent with the action of BCAR1 as a force-sensing protein [29, 30, 139]. Osteoclast functionality and bone integrity are sensitive to level and duration of applied force [184]. It is possible that force-generated signals may prove to be particularly relevant in the regulation of CAS proteins in controlling bone integrity.
Microbial pathogenesis
For BCAR1, a major theme of research has been in control of bacterial infection. When bacteria infect a host, macrophages, neutrophils, and some target cells perform phagocytosis to engulf and destroy the invading pathogens. Phagocytosis requires the action of focal adhesion-associated proteins, including BCAR1, which activates Rac to reorganize the actin cytoskeleton during the process. A number of elegant studies have shown that Yersinia species bacteria encode YopH to dephosphorylate and hence inactivate BCAR1 as part of the pathogenic process, protecting the pool of circulating virus [117, 191]. Conversely, Salmonella typhimurium infects the host through an organized phagocytosis-based uptake process in the epithelial lining of the intestine. Work by Shi and Casanova demonstrates that in this case, activity of BCAR1 supports the invasion process [192].
Recent studies of Leishmania, a parasitic trypanosome, have addressed the mechanism of its invasion of macrophages and fibroblasts. This work has established the parasitic protease GP63 degrades BCAR1 and other proteins influencing cytoskeletal dynamics; an exact mechanism by which BCAR1 status may contribute to this process is not yet known [193]. Respiratory syncytial virus (RSV) infection of the lung can produce life-threatening infections in vulnerable (immunocompromised or infant) hosts. RSV signals through RhoA to reorganize the actin skeleton and enhance viral entry; this has been associated with hyperphosphorylation of BCAR1 [194]. In addition, internalization of adenovirus is not only mediated by integrins but requires activation of BCAR1 and PI3 K [195]. Whether BCAR1 contributes actively to the infection process has not been determined. None of the other CAS proteins have yet been investigated for activity in the sphere of infection.
Cas proteins in development
As of 2009, knockout mice have been developed for three of the CAS proteins: BCAR1, NEDD9, and EFS. Despite the similarity of BCAR1 and NEDD9 in their structure and certain cellular functions, the phenotypes of Bcar1 -/- and Nedd9 -/- animals are very different, as Bcar1 -/- animals die between embryonic days 11.5 and 12.5, while Nedd9 -/- and Efs -/- animals are viable and fertile [57, 156] [157]. The inability of other CAS family members to compensate for BCAR1 loss indicates that BCAR1 has a unique function in embryonal development [57]. Proximal factors leading to the embryonic lethality of Bcar1-/- mice are likely an insufficient cytoskeletal framework of cardiomyocytes that prevents developing BCAR1-/- embryos from beginning productive heart pumping, as electron microscopic analysis demonstrated disorganization of myofibrils and disruption of Z-disks within the dissected heart, and gross morphological organization indicated systemic congestion and slower overall embryonal growth [57]. Interestingly, the lack of sufficiently long actin filaments in fibroblasts from BCAR1-/- is similar to the phenotype observed in FAK-/- animals [196]. BCAR1, FAK, and paxillin all localize at the sarcomeric Z-lines of cardiomyocyte, and disruption of BCAR1 causes aberrant sarcomeric organization and cardiomyopathy [91, 197].
In contrast, Nedd9 -/- mice have defects in the development of the immune system [156]. Nedd9 -/- knockout lymphocytes are characterized by poor chemotactic responses and impaired cell adhesion. In young Nedd9 -/- mice, the marginal zone B-cell population (MZB) of spleen is almost absent. This was due to insufficient migration of B-cells into the marginal zone (MZ), rather than defects in B-cell development and differentiation. This migration deficiency could result from failure of B-cells to adhere and/or migrate to MZ, which could be due to a decreased response of Nedd9 -/- B-cells to chemokines and/or their reduced ability to adhere to marginal zone adhesion molecules ICAM-1 and VCAM-1. Cell populations of spleen other than MZB cells remained largely unaffected; however, the number of lymphocytes in bone marrow and thymus of NEDD9 knockout mice was also decreased [156]. Analysis of aged Nedd9 -/- mice showed a generalized hyperplasia involving B- and T-cells and macrophages, suggesting an inflammatory state [173]. Detailed pathological analysis of a number of tissues including mammary pads, liver, kidney, lung, and others in the same study detected no apparent difference from wild-type littermates [173]. However, a number of reports have indicated that NEDD9 undergoes dynamic expression changes during neuronal differentiation and development [9, 71], actively contributes to neural crest cell migration [71], and regulates the fate of cortical and hippocampal progenitor cells [72]. These observations support the possibility that Nedd9 -/- mice may have some degree of cognitive impairment, but this has not been directly assessed. Finally, the chromosomal localization of human NEDD9 at 6p24-p25 corresponds to a reported locus for orofacial dysplasia (OFD1), and one group has noted NEDD9 as a candidate locus for this developmental disorder [198]; as OFD syndromes have been linked to defects in ciliogenesis [199], and NEDD9 plays a role in ciliary integrity [53], a connection is plausible, but requires investigation.
Animals lacking EFS also have immune system abnormalities. These mice have hyperactive T-lymphocytes leading to excessive T-cell-dependent antibody and cytokine production, and B- and T-cell organ infiltration, particularly in the small intestine. In aged mice, this results in chronic inflammation of the intestinal mucosa similar to that seen in models of Crohn’s disease [157], marked by crypt cell enlargement, and damage to the villi.
Drosophila melanogaster has a single CAS family member, known as Dcas (or CG1212). In 2007, Huang et al. [200] showed that Dcas is highly expressed in the central and peripheral nervous system of Drosophila. The DcasP1 strain contains a transposable p-element inserted in the coding region of Dcas, which dramatically lowers Dcas expression, was used to analyze the integrity of neurons in development. A DcasP1 genotype induced axonal guidance defects in CNS and motor neurons, by regulating defasciculation (debundling). This phenotype was found to be dependent on expression of integrins, which also control axonal guidance and the cycle of fasciculation–defasciculation in Drosophila embryos. A transgenic Dcas overexpression strain induced a similar phenotype, suggesting the importance of controlling Dcas expression within a narrow range. It is likely that further investigation of the Dcas genetic interactions will yield relevant insights for studies of the mammalian CAS family.
CAS proteins in cancer
Given the numerous connections of CAS family proteins with key cellular functions such as adhesion, migration, chemotaxis, survival, and cell cycle, it is not surprising that there is a significant link between aberrant expression and activation of these proteins with the progression and metastasis of several cancer types. Indeed, three of the CAS proteins are identified as either binding partners to known oncogenes or as controlling morphology associated with oncogenic transformation [5–8, 11, 121, 201]. In cancer cell lines, upregulation of FAK, SRC, or CRK can durably mimic normally transient integrin ligation, leading to hyperphosphorylation of BCAR1 [3, 27, 122, 202–204] or NEDD9 [66, 77]. For a growing number of cancers, specific CAS proteins are known to be upregulated by amplification, transcriptional upregulation, or changes in stability. The sustained presence of these hyperphosphorylated proteins drives cell migration and invasion, and also promotes cell survival and drug resistance [4, 61, 149, 167, 169, 172, 205–210]. This section summarizes the current data linking changes in the expression or functionality of CAS proteins in different cancer types.
Breast cancer
The linkage between CAS protein functionality and breast cancer is extensive. Using estrogen-dependent cell lines, a retroviral-insertion mutagenesis screen identified BCAR1 as principal gene responsible for tamoxifen resistance [4, 206]. Patients with primary tumors expressing high BCAR1 levels are more likely to experience relapse and an intrinsic reduced response to tamoxifen treatment; however, BCAR1 expression changes do not appear to occur in acquired resistance to tamoxifen, but rather higher expression of BCAR1 is an intrinsic resistance factor [167, 172, 205, 206]. In independent studies of human breast tumors, BCAR1 levels positively correlate with HER2/Neu expression levels and enhanced proliferation [165]. Pleural effusions taken from breast cancer patients (likely to be enriched in cells transitioning to metastatic capability) show higher levels of BCAR1 than do the primary tumors [211]. Studies in feline and canine models of mammary tumors also indicate that increased BCAR1 activation and expression levels correlate with increased malignancy [212].
HER2/Neu induces BCAR1 and c-CRKII complex formation leading to downstream ERK1/2 activation to mediate cellular invasion [213]. Analysis of a transgenic mouse overexpressing BCAR1 under the control of the murine mammary tumor virus (MMTV) promoter [165] indicated that BCAR1 overexpression did not transform mammary epithelial cells, but did lead to extensive hyperplasia during mammary gland development and pregnancy, as well as delays in involution post-pregnancy [165]. However, MMTV-BCAR1 mice crossed to MMTV-HER2/Neu mice exhibited decreased latency prior to the appearance of tumors, and cancer cell growth dependent on BCAR1 expression [165].
One mechanism by which BCAR1 may confer anti-estrogen resistance in mammary cells is through binding and regulation of BCAR3 activity [80, 149]. BCAR3 (also known as NSP2 and AND-34) emerged from the same screen for tamoxifen resistance that identified BCAR1. BCAR3 was originally identified as having GDF-exchange factor (GEF) activity [81] and structural analysis of BCAR3 reveals a GEF-like structure [31], but GEF activity of BCAR3 towards reported substrates such as Rap1 may be indirect [149]. BCAR3/BCAR1 complex is highest in estrogen receptor-negative breast cancer cells, and the expression of BCAR3 is required for induction of cyclin D1 expression-linked anti-estrogen resistance [207]. This complex is also important for regulating BCAR1 phosphorylation at key adhesion-dependent serine residues 139, 437, and 639 [51]. The complex of BCAR3 and BCAR1 synergistically activates SRC-dependent migration [214]. In turn, breast cancer cells treated with the SRC inhibitor bosutinib have decreased FAK and BCAR1 phosphorylation and consequent inhibition of migration and invasion [215].
Changes in NEDD9 expression were initially proposed to influence breast cancer metastasis, although the literature reports conflicting results. Minn et al. [216] used an invasive TGFβ-stimulated MDA-MB-231 cell line and a high-throughput Affymetrix assay to analyze mRNA expression and establish a gene signature associated with increased breast cancer metastasis to lung. This study indicated a 3-fold downregulation of Nedd9 was part of the metastatic signature. However, analyzing genes and proteins expressed in MCF-7 breast adenocarcinoma cells upon activation of ErbB receptor with its ligand heregulin, NEDD9 emerged as one of only five hits upregulated and hyperphosphorylated in both transcriptome and proteome datasets [217]. Separately, a study aimed to proteins up- or downregulated in estrogen-resistant tumors, used an antibody, which detects both BCAR1 and NEDD9, and found a positive correlation between CAS protein expression and loss of estrogen-receptor in 2,593 tumor samples, indicating that elevated BCAR1, NEDD9, or both proteins might be a prognostic marker for breast cancer [206]. These latter reports suggested a positive function. A recent study of breast cancer metastasis identified a TGF-β-dependent transcriptional signature that includes upregulation of Nedd9 induces a shift from cohesive (or multi-cellular) motility to single cell motility, which allows the isolated cells to invade blood vessels and undergo blood-borne metastasis, rather than remaining restricted to invasion of the lymphatic system [218]. This study highlights important distinctions between the metastatic niche of tumor cells in vivo and the requirement for heterogenic TGF-β signaling patterns [218]. Therefore, it is possible that selective manipulation of NEDD9 expression or phosphorylation may be important for determination of cancer propagation via distinct dissemination routes, reconciling some of the conflicting transcriptional data. BCAR1 may also act as a rheostat for TGF-β signals, as BCAR1 overexpression in mammary tumor cells blocks TGF-β-induced Smad 2/3 induction and promotes TGF-β-induced activation of p38MAPK, while BCAR1 inhibition leads to the restoration of TGF-β-Smad 2/3-dependent tumor inhibition and abrogation of TGF-β-MAPK tumor progression [145]. Together, BCAR1 and NEDD9 are emerging as integral determinants of the tumor suppressive and promoting activities of TGF-β in cancer signaling.
Distinct from metastasis, a recent analysis of the MMTV-polyoma virus middle T antigen (PyVT) mouse model for mammary tumorigenesis crossed to Nedd9 null versus wild-type animals revealed that NEDD9 positively contributes to the early stages of mammary tumor growth [173]. Nedd9 -/- status significantly increased latency of MMTV-PyVT-induced tumor formation, and reduced total tumor burden and total number of tumors arising [173]. No differences in immune system cell infiltration and vascularization were detected in tumors of PyVT Nedd9 +/+ and Nedd9 -/- mice, implying the tumor growth delay was tumor cell-intrinsic. This study explored the signaling consequences of null NEDD9 status in tumor development, and found that most of the mammary tumors excised from MMTV-PyVT; Nedd9 -/- mice had reduced activation of AKT, FAK, SRC, and ERK1/2, versus Nedd9 wild-type littermates [173]. These data strongly supported a positive role for NEDD9 in breast cancer development. Interestingly, this study found no evidence for absence of Nedd9 conditioning the metastasis of the primary tumors, with differences appearing from the time of initial tumor appearance.
As noted above, studies analyzing the function of NEDD9 by overexpression in the MCF7 breast adenocarcinoma cell line and other models found that overexpressed NEDD9 promoted cell migration and invasion in some contexts [119], but reduced it in others [130], increased the mRNA and protein levels of ErbB2 and matrix metalloproteases [119], and like BCAR1, formed a signaling complex with BCAR3 [39], but also activated the oncogenic Aurora A kinase to increase cytokinetic failure and centrosomal amplification, and could trigger apoptosis [34, 43, 52]. Hence, the disagreement regarding the function in cancer may reflect NEDD9 involvement in different stages of breast cancer tumorigenesis; a cancer cell may upregulate NEDD9 during initiation of metastasis, or in the context of additional lesions that inactivate mitotic checkpoints, but downregulate it when metastasis is formed and attachment is needed.
Glioblastomas
In glioblastoma cells, FAK overexpression or PDGF treatment specifically phosphorylates and activates NEDD9, but not BCAR1 or EFS, to confer a metastatic and promigratory phenotype [60]. A separate study of BCAR1 in cells overexpressing the PTEN tumor suppressor indicated that BCAR1 overexpression could override PTEN suppression of cell spreading and migration, although not cell proliferation, and did not induce FAK phosphorylation [219]. Similarly, a study of the non-tyrosine kinase VEGF receptor neuropilin 1 (NRP1) suggested a role for BCAR1 in supporting mesenchymal invasive movement in glioblastoma cells [220]. More work is clearly required to determine the relative contribution of the CAS proteins in this tumor type.
Hematopoietic malignancies
Extensive evidence links changes in function of CAS proteins to lymphomas and leukemias. This topic has been reviewed in detail in [221], and undoubtedly reflects the important roles of CAS proteins as mediators of T- and B-cell receptor-dependent signaling, and integrin-mediated co-stimulation [8, 11, 12, 16, 58, 222]. BCR-ABL leukemogenesis, which leads to development of chronic myelogenous leukemia (CML) and acute lymphoplastic leukemia (ALL), induces hyperphosphorylation of BCAR1 and NEDD9, driving their phosphorylation with CRK-L and C3G, and promoting cell migration through a Rap1 GTPase effector pathway [27, 223, 224]. BCAR3–BCAR1 interactions, important in breast cancer cell migration, are activated by inflammatory cytokines to mediate adhesion response of thymic cells [80], and may be relevant to lymphomagenesis. NEDD9 also binds BCAR3; this complex regulates CDC42-mediated adhesion and motility of B-cells [39], but the relevance of the interaction has not been explored in lymphomas.
Lymphomas arising from translocations activating anaplastic lymphoma kinase (ALK) are dependent on BCAR1, and ALK has been found to bind directly to BCAR1 [225]. In mast cell leukemias, inhibiting signaling from the mutationally activated c-Kit kinases downregulates phosphorylation of BCAR1 [226]; moreover, acute myelogenous leukemia (AML) cell lines with c-Kit or FML-3 mutations treated with either celecoxib or E7123 undergo cell death due to the inhibition of BCAR1 and FAK-dependent adhesion signaling pathways [168]. T-cell leukemias have typically elevated expression of hyperphosphorylated NEDD9; in leukemias arising from human T-lymphotropic virus type 1 (HTLV-1) infection, NEDD9 phosphorylation is enhanced, and NEDD9 binds to the viral Tax protein, inhibiting Tax-dependent activation of NF-κB [13].
Melanomas
A large comparative genome hybridization (CGH) study strongly implicated upregulation of NEDD9 levels as the driver of melanoma metastasis in both Ras and Met mouse models [62]. NEDD9 overexpression in nontransformed melanocytes was not sufficient to enhance invasion or metastasis, but signaled through FAK to dramatically enhance metastatic potential in combination with oncogenic H-RASV12G or B-RAFV600E expression [62]. Importantly, immunohistochemical analysis of human melanoma microarrays revealed that 35% of metastatic melanomas have significantly elevated NEDD9 expression [62]. Analysis of copy number alterations by FISH and confirmed by aCGH in human metastatic melanoma tissues reveals that 57% of such cases have gains of chromosome 6p24 containing NEDD9 [227].
NEDD9 appears to be more important than BCAR1 in the melanoma models. Kim et al. [62] showed that knockdown of BCAR1 had little effect on cell growth, but NEDD9 inhibition induced S-phase arrest. Mesenchymal movement of Rac-activated melanoma cells is dependent on NEDD9 and its interaction with DOCK3, but not on BCAR1 [228]. Nevertheless, several cell surface antigens implicated in melanoma progression through integrin-mediated interaction with the ECM (melanoma chondroitin sulphate proteoglycan, MCSP, high molecular weight melanoma associated antigen, HMW-MAA, and the ganglioside GD3), are also associated with BCAR1 phosphorylation and activation-mediated invasion and spreading of melanoma cells [229–233]. Further, overexpression of the lipid raft-associated tumor suppressor caveolin 1 (Cav-1) in human melanoma cells prevented GD3-dependent activation and membrane localization of BCAR1 and paxillin [234]. Hence, a role for BCAR1 cannot be excluded.
Other cancer types
CAS proteins have also been linked to oncogenic signaling in other cancer types. For example, BCAR1 expression is higher in metastatic prostate cancer as compared to localized lesions and expression correlates with EGFR expression [235]. Also, the metastasis suppressor KAI1/CD82 inhibits expression of BCAR1, limiting the formation of BCAR1-Crk complexes to inhibit the motility and invasiveness of DU145 prostate cancer cells [236]. Hepatocellular carcinoma cell invasion and poor prognosis correlates with high BCAR1 levels and aberrant expression of E-cadherin and β-catenin [237]. In human lung adenocarcinomas, NEDD9 expression is upregulated upon loss of the tumor suppressor STK11/LKB1, with this expression reversed upon re-expression of LKB1 [238]. Nasal angiofibroma and nasal polyps are also associated with significant upregulation of BCAR1 and TGF-β protein expression [239]. Treatment of colon cancer cells with the COX-2 inhibitor celecoxib induces BCAR1 proteolysis and release of p31, which contributes to apoptosis [240]; exogenous overexpression of BCAR1 in colon cancer cells leads to resistance to celecoxib-induced cell death in a pathway independent of pro-survival signaling from FAK and AKT [240]. Given the fact that aberrant activity and expression of BCAR1 is linked to the resistance of tumor cells to chemotherapeutics, it is also interesting to note that radiation is strongly induces expression of NEDD9 [241] and BCAR1 [242], and that cellular survival after ionizing radiation is dependent on integrin-mediated BCAR1 phosphorylation [243].
Cas proteins in other pathological states
Although most of the research on CAS proteins in disease has emphasized their roles in cancer, a number of other studies have raised the possibility that they play active roles in other pathological conditions. Besides the functions of BCAR1 in “Microbial pathogenesis” discussed above, functions in NEDD9 have been proposed in arthritis [244], ischemia (stroke) [70, 96], and neurodegenerative disease [245, 246].
For arthritis and ischemia, results are predominantly correlative. NEDD9 expression and phosphorylation are enhanced in lymphocytes migrating to inflamed joints, with this correlated with the increased phosphorylation of the SRC-family kinases FYN and LCK [244]. Martin-Rendon and coworkers established that NEDD9 levels were strongly increased by short exposure to hypoxia in umbilical cord blood and bone marrow-derived stem cells [70]. In vivo, following induction of ischemia (stroke) in rats, NEDD9 expression and activation were both strongly enhanced over the subsequent 2 weeks [96]. Given the paired observation that NEDD9 promotes neurite outgrowth [96, 143], and neural progenitor populations [71, 72], it is likely that NEDD9 activity may support recovery from this insult. In this context, it is extremely interesting that naturally occurring polymorphisms in the promoter region of NEDD9 have been linked to susceptibility to Alzheimer’s and Parkinson’s disease [246]. This linkage remains controversial, with one follow-up study failing to find a linkage [245]. However, the rich biology linking NEDD9 to neural development, homeostasis, and cancer makes a conditioning role for NEDD9 in neurodegeneration syndromes an attractive hypothesis demanding further research. Intriguingly, a TREM2 loss-of-function mutation linked with dementia may lead to significant downregulation of NEDD9 mRNA [247].
Summary and prospects
The CAS proteins were last cumulatively reviewed as a group in 2000 [128], and individually in 2006 and 2007 [56, 248]. Since then, the surge in the number of publications addressing this group of proteins makes it clear that stimuli regulating many aspects of organismal growth and homeostasis involve filtering of signals through CAS proteins. Their carefully calibrated expression levels and dynamic localization changes, coupled with their ability to assemble multiple different classes of signaling factors, imply that CAS proteins may help coordinate the synchronous changes in growth, movement, and death that are requisite for development. Reciprocally, these similar properties make aberrant function of CAS proteins a likely target for generally enhancing cell transformation and tumor growth. Given the links now being discovered between individual CAS proteins in organ-specific functions, the increased validation of defective CAS protein activity as drivers in other pathologies seems likely. For example, BCAR1 appears to be a critical nexus governing cardiomyocyte integrity in development, and may play a role in heart disease. EFS/SIN appears critical for proper immune cell infiltration of the intestines, and may be relevant in not only Crohn’s disease but also other inflammatory syndromes affecting the gut, such as celiac or ulcerative colitis [157]. Although the non-catalytic nature of the CAS proteins does not make them convenient targets for drug development, over the next decade it is possible that either select protein–protein interaction inhibitors or conformational inhibitors might find use in regulating their function. At present, the most obvious use of available inhibitors would be to limit the function of overexpressed NEDD9 in advanced cancers. However, the complexity of CAS protein biology already known indicates there is much more to be discovered regarding their respective roles in human development and disease.
Acknowledgments
We are grateful to Dr. Mark Andrake of the Fox Chase Cancer Center Molecular Modeling Facility for help in producing Fig. 1a. N.T. and E.A.G. were supported by NIH R01 CA63366 and R01 CA113342, and by Pennsylvania Tobacco Settlement funding (to E.A.G.); J.L.L. was supported by NIH T32 CA009035; and all authors by NIH core grant CA06927 and a gift from the Pew Charitable Trust (to Fox Chase Cancer Center).
Footnotes
N. Tikhmyanova and J. L. Little have contributed equally.
References
- 1.Kanner SB, Reynolds AB, Parsons JT. Tyrosine phosphorylation of a 120-kilodalton pp60src substrate upon epidermal growth factor and platelet-derived growth factor receptor stimulation and in polyomavirus middle-T-antigen-transformed cells. Mol Cell Biol. 1991;11:713–720. doi: 10.1128/mcb.11.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reynolds AB, Roesel DJ, Kanner SB, Parsons JT. Transformation-specific tyrosine phosphorylation of a novel cellular protein in chicken cells expressing oncogenic variants of the avian cellular src gene. Mol Cell Biol. 1989;9:629–638. doi: 10.1128/mcb.9.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakai R, Iwamatsu A, Hirano N, Ogawa S, Tanaka T, Mano H, Yazaki Y, Hirai H. A novel signaling molecule, p130, forms stable complexes in vivo with v-Crk and v-Src in a tyrosine phosphorylation-dependent manner. EMBO J. 1994;13:3748–3756. doi: 10.1002/j.1460-2075.1994.tb06684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinkman A, van der Flier S, Kok EM, Dorssers LC. BCAR1, a human homologue of the adapter protein p130Cas, and antiestrogen resistance in breast cancer cells. J Natl Cancer Inst. 2000;92:112–120. doi: 10.1093/jnci/92.2.112. [DOI] [PubMed] [Google Scholar]
- 5.Ishino M, Ohba T, Sasaki H, Sasaki T. Molecular cloning of a cDNA encoding a phosphoprotein, Efs, which contains a Src homology 3 domain and associates with Fyn. Oncogene. 1995;11:2331–2338. [PubMed] [Google Scholar]
- 6.Alexandropoulos K, Baltimore D. Coordinate activation of c-Src by SH3- and SH2-binding sites on a novel p130Cas-related protein, Sin. Genes Dev. 1996;10:1341–1355. doi: 10.1101/gad.10.11.1341. [DOI] [PubMed] [Google Scholar]
- 7.Law SF, Estojak J, Wang B, Mysliwiec T, Kruh G, Golemis EA. Human enhancer of filamentation 1, a novel p130cas-like docking protein, associates with focal adhesion kinase and induces pseudohyphal growth in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:3327–3337. doi: 10.1128/mcb.16.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minegishi M, Tachibana K, Sato T, Iwata S, Nojima Y, Morimoto C. Structure and function of Cas-L, a 105-kD Crk-associated substrate-related protein that is involved in beta 1 integrin-mediated signaling in lymphocytes. J Exp Med. 1996;184:1365–1375. doi: 10.1084/jem.184.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar S, Tomooka Y, Noda M. Identification of a set of genes with developmentally down-regulated expression in the mouse brain. Biochem Biophys Res Commun. 1992;185:1155–1161. doi: 10.1016/0006-291x(92)91747-e. [DOI] [PubMed] [Google Scholar]
- 10.Singh MK, Dadke D, Nicolas E, Serebriiskii IG, Apostolou S, Canutescu A, Egleston BL, Golemis EA. A novel Cas family member, HEPL, regulates FAK and cell spreading. Mol Biol Cell. 2008;19:1627–1636. doi: 10.1091/mbc.E07-09-0953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Astier A, Manie SN, Avraham H, Hirai H, Law SF, Zhang Y, Golemis EA, Fu Y, Druker BJ, Haghayeghi N, Freedman AS, Avraham S. The related adhesion focal tyrosine kinase differentially phosphorylates p130Cas and the Cas-like protein, p105HEF1. J Biol Chem. 1997;272:19719–19724. doi: 10.1074/jbc.272.32.19719. [DOI] [PubMed] [Google Scholar]
- 12.Manie SN, Beck AR, Astier A, Law SF, Canty T, Hirai H, Druker BJ, Avraham H, Haghayeghi N, Sattler M, Salgia R, Griffin JD, Golemis EA, Freedman AS. Involvement of p130(Cas) and p105(HEF1), a novel Cas-like docking protein, in a cytoskeleton-dependent signaling pathway initiated by ligation of integrin or antigen receptor on human B cells. J Biol Chem. 1997;272:4230–4236. doi: 10.1074/jbc.272.7.4230. [DOI] [PubMed] [Google Scholar]
- 13.Iwata S, Souta-Kuribara A, Yamakawa A, Sasaki T, Shimizu T, Hosono O, Kawasaki H, Tanaka H, Dang NH, Watanabe T, Arima N, Morimoto C. HTLV-I Tax induces and associates with Crk-associated substrate lymphocyte type (Cas-L) Oncogene. 2005;24:1262–1271. doi: 10.1038/sj.onc.1208261. [DOI] [PubMed] [Google Scholar]
- 14.Hunter AJ, Shimizu Y. Alpha 4 beta 1 integrin-mediated tyrosine phosphorylation in human T cells: characterization of Crk- and Fyn-associated substrates (pp105, pp115, and human enhancer of filamentation-1) and integrin-dependent activation of p59fyn1. J Immunol. 1997;159:4806–4814. [PubMed] [Google Scholar]
- 15.Kamiguchi K, Tachibana K, Iwata S, Ohashi Y, Morimoto C. Cas-L is required for beta 1 integrin-mediated costimulation in human Tcells. J Immunol. 1999;163:563–568. [PubMed] [Google Scholar]
- 16.Ohashi Y, Iwata S, Kamiguchi K, Morimoto C. Tyrosine phosphorylation of Crk-associated substrate lymphocyte-type Is a critical element in TCR- and {beta}1 integrin-induced T lymphocyte migration Migration. J Immunol. 1999;163:3727–3734. [PubMed] [Google Scholar]
- 17.van Seventer GA, Salmen HJ, Law SF, O’Neill GM, Mullen MM, Franz AM, Kanner SB, Golemis EA, van Seventer JM. Focal adhesion kinase regulates beta1 integrin-dependent T cell migration through an HEF1 effector pathway. Eur J Immunol. 2001;31:1417–1427. doi: 10.1002/1521-4141(200105)31:5<1417::AID-IMMU1417>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 18.Sattler M, Salgia R, Shrikhande G, Verma S, Uemura N, Law SF, Golemis EA, Griffin JD. Differential signaling after beta1 integrin ligation is mediated through binding of CRKL to p120(CBL) and p110(HEF1) J Biol Chem. 1997;272:14320–14326. doi: 10.1074/jbc.272.22.14320. [DOI] [PubMed] [Google Scholar]
- 19.Kirsch KH, Georgescu MM, Hanafusa H. Direct binding of p130(Cas) to the guanine nucleotide exchange factor C3G. J Biol Chem. 1998;273:25673–25679. doi: 10.1074/jbc.273.40.25673. [DOI] [PubMed] [Google Scholar]
- 20.Garton AJ, Burnham MR, Bouton AH, Tonks NK. Association of PTP-PEST with the SH3 domain of p130cas; a novel mechanism of protein tyrosine phosphatase substrate recognition. Oncogene. 1997;15:877–885. doi: 10.1038/sj.onc.1201279. [DOI] [PubMed] [Google Scholar]
- 21.Liu F, Sells MA, Chernoff J. Protein tyrosine phosphatase 1B negatively regulates integrin signaling. Curr Biol. 1998;8:173–176. doi: 10.1016/s0960-9822(98)70066-1. [DOI] [PubMed] [Google Scholar]
- 22.Nakamoto T, Yamagata T, Sakai R, Ogawa S, Honda H, Ueno H, Hirano N, Yazaki Y, Hirai H. CIZ, a zinc finger protein that interacts with p130(cas) and activates the expression of matrix metalloproteinases. Mol Cell Biol. 2000;20:1649–1658. doi: 10.1128/mcb.20.5.1649-1658.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harte MT, Hildebrand JD, Burnham MR, Bouton AH, Parsons JT. p130Cas, a substrate associated with v-Src and v-Crk, localizes to focal adhesions and binds to focal adhesion kinase. J Biol Chem. 1996;271:13649–13655. doi: 10.1074/jbc.271.23.13649. [DOI] [PubMed] [Google Scholar]
- 24.Wisniewska M, Bossenmaier B, Georges G, Hesse F, Dangl M, Kunkele KP, Ioannidis I, Huber R, Engh RA. The 1.1 A resolution crystal structure of the p130cas SH3 domain and ramifications for ligand selectivity. J Mol Biol. 2005;347:1005–1014. doi: 10.1016/j.jmb.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 25.Petruzzelli L, Takami M, Herrera R. Adhesion through the interaction of lymphocyte function-associated antigen-1 with intracellular adhesion molecule-1 induces tyrosine phosphorylation of p130cas and its association with c-CrkII. J Biol Chem. 1996;271:7796–7801. doi: 10.1074/jbc.271.13.7796. [DOI] [PubMed] [Google Scholar]
- 26.Nojima Y, Morino N, Mimura T, Hamasaki K, Furuya H, Sakai R, Sato T, Tachibana K, Morimoto C, Yazaki Y, et al. Integrin-mediated cell adhesion promotes tyrosine phosphorylation of p130Cas, a Src homology 3-containing molecule having multiple Src homology 2-binding motifs. J Biol Chem. 1995;270:15398–15402. doi: 10.1074/jbc.270.25.15398. [DOI] [PubMed] [Google Scholar]
- 27.Salgia R, Pisick E, Sattler M, Li JL, Uemura N, Wong WK, Burky SA, Hirai H, Chen LB, Griffin JD. p130CAS forms a signaling complex with the adapter protein CRKL in hematopoietic cells transformed by the BCR/ABL oncogene. J Biol Chem. 1996;271:25198–25203. doi: 10.1074/jbc.271.41.25198. [DOI] [PubMed] [Google Scholar]
- 28.Ishino M, Ohba T, Inazawa J, Sasaki H, Ariyama Y, Sasaki T. Identification of an Efs isoform that lacks the SH3 domain and chromosomal mapping of human Efs. Oncogene. 1997;15:1741–1745. doi: 10.1038/sj.onc.1201346. [DOI] [PubMed] [Google Scholar]
- 29.Kostic A, Sheetz MP. Fibronectin rigidity response through Fyn and p130Cas recruitment to the leading edge. Mol Biol Cell. 2006;17:2684–2695. doi: 10.1091/mbc.E05-12-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garron ML, Arsenieva D, Zhong J, Bloom AB, Lerner A, O’Neill GM, Arold ST. Structural insights into the association between BCAR3 and Cas family members, an atypical complex implicated in anti-oestrogen resistance. J Mol Biol. 2009;386:190–203. doi: 10.1016/j.jmb.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Briknarova K, Nasertorabi F, Havert ML, Eggleston E, Hoyt DW, Li C, Olson AJ, Vuori K, Ely KR. The serine-rich domain from Crk-associated substrate (p130cas) is a four-helix bundle. J Biol Chem. 2005;280:21908–21914. doi: 10.1074/jbc.M501258200. [DOI] [PubMed] [Google Scholar]
- 33.Vuori K, Hirai H, Aizawa S, Ruoslahti E. Introduction of p130cas signaling complex formation upon integrin-mediated cell adhesion: a role for Src family kinases. Mol Cell Biol. 1996;16:2606–2613. doi: 10.1128/mcb.16.6.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Neill GM, Golemis EA. Proteolysis of the docking protein HEF1 and implications for focal adhesion dynamics. Mol Cell Biol. 2001;21:5094–5108. doi: 10.1128/MCB.21.15.5094-5108.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Law SF, Zhang YZ, Fashena SJ, Toby G, Estojak J, Golemis EA. Dimerization of the docking/adaptor protein HEF1 via a carboxy-terminal helix-loop-helix domain. Exp Cell Res. 1999;252:224–235. doi: 10.1006/excr.1999.4609. [DOI] [PubMed] [Google Scholar]
- 36.Nakamoto T, Sakai R, Ozawa K, Yazaki Y, Hirai H. Direct binding of C-terminal region of p130Cas to SH2 and SH3 domains of Src kinase. J Biol Chem. 1996;271:8959–8965. doi: 10.1074/jbc.271.15.8959. [DOI] [PubMed] [Google Scholar]
- 37.Nasertorabi F, Tars K, Becherer K, Kodandapani R, Liljas L, Vuori K, Ely KR. Molecular basis for regulation of Src by the docking protein p130Cas. J Mol Recognit. 2006;19:30–38. doi: 10.1002/jmr.755. [DOI] [PubMed] [Google Scholar]
- 38.Sakakibara A, Ohba Y, Kurokawa K, Matsuda M, Hattori S. Novel function of Chat in controlling cell adhesion via Cas-Crk-C3G-pathway-mediated Rap1 activation. J Cell Sci. 2002;115:4915–4924. doi: 10.1242/jcs.00207. [DOI] [PubMed] [Google Scholar]
- 39.Cai D, Felekkis KN, Near RI, O’Neill GM, van Seventer JM, Golemis EA, Lerner A. The GDP exchange factor AND-34 is expressed in B cells, associates with HEF1, and activates Cdc42. J Immunol. 2003;170:969–978. doi: 10.4049/jimmunol.170.2.969. [DOI] [PubMed] [Google Scholar]
- 40.Feng L, Guedes S, Wang T. Atrophin-1-interacting protein 4/human Itch is a ubiquitin E3 ligase for human enhancer of filamentation 1 in transforming growth factor-beta signaling pathways. J Biol Chem. 2004;279:29681–29690. doi: 10.1074/jbc.M403221200. [DOI] [PubMed] [Google Scholar]
- 41.Nourry C, Maksumova L, Pang M, Liu X, Wang T. Direct interaction between Smad3, APC10, CDH1 and HEF1 in proteasomal degradation of HEF1. BMC Cell Biol. 2004;5:20. doi: 10.1186/1471-2121-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pellicena P, Miller WT. Processive phosphorylation of p130Cas by Src depends on SH3-polyproline interactions. J Biol Chem. 2001;276:28190–28196. doi: 10.1074/jbc.M100055200. [DOI] [PubMed] [Google Scholar]
- 43.Law SF, O’Neill GM, Fashena SJ, Einarson MB, Golemis EA. The docking protein HEF1 is an apoptotic mediator at focal adhesion sites. Mol Cell Biol. 2000;20:5184–5195. doi: 10.1128/mcb.20.14.5184-5195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Law SF, Zhang YZ, Klein-Szanto AJ, Golemis EA. Cell cycle-regulated processing of HEF1 to multiple protein forms differentially targeted to multiple subcellular compartments. Mol Cell Biol. 1998;18:3540–3551. doi: 10.1128/mcb.18.6.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kook S, Shim SR, Choi SJ, Ahnn J, Kim JI, Eom SH, Jung YK, Paik SG, Song WK. Caspase-mediated cleavage of p130cas in etoposide-induced apoptotic Rat-1 cells. Mol Biol Cell. 2000;11:929–939. doi: 10.1091/mbc.11.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petch LA, Bockholt SM, Bouton A, Parsons JT, Burridge K. Adhesion-induced tyrosine phosphorylation of the p130 src substrate. J Cell Sci. 1995;108:1371–1379. doi: 10.1242/jcs.108.4.1371. [DOI] [PubMed] [Google Scholar]
- 47.Polte TR, Hanks SK. Interaction between focal adhesion kinase and Crk-associated tyrosine kinase substrate p130Cas. Proc Natl Acad Sci USA. 1995;92:10678–10682. doi: 10.1073/pnas.92.23.10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohba T, Ishino M, Aoto H, Sasaki T. Interaction of two proline-rich sequences of cell adhesion kinase beta with SH3 domains of p130Cas-related proteins and a GTPase-activating protein, Graf. Biochem J. 1998;330(Pt 3):1249–1254. doi: 10.1042/bj3301249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakamoto T, Sakai R, Honda H, Ogawa S, Ueno H, Suzuki T, Aizawa S, Yazaki Y, Hirai H. Requirements for localization of p130cas to focal adhesions. Mol Cell Biol. 1997;17:3884–3897. doi: 10.1128/mcb.17.7.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Polte TR, Hanks SK. Complexes of focal adhesion kinase (FAK) and Crk-associated substrate [p130(Cas)] are elevated in cytoskeleton-associated fractions following adhesion and Src transformation. Requirements for Src kinase activity and FAK proline-rich motifs. J Biol Chem. 1997;272:5501–5509. doi: 10.1074/jbc.272.9.5501. [DOI] [PubMed] [Google Scholar]
- 51.Makkinje A, Near RI, Infusini G, Vanden Borre P, Bloom A, Cai D, Costello CE, Lerner A. AND-34/BCAR3 regulates adhesion-dependent p130Cas serine phosphorylation and breast cancer cell growth pattern. Cell Signal. 2009;21:1423–1435. doi: 10.1016/j.cellsig.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pugacheva EN, Golemis EA. The focal adhesion scaffolding protein HEF1 regulates activation of the Aurora-A and Nek2 kinases at the centrosome. Nat Cell Biol. 2005;7:937–946. doi: 10.1038/ncb1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–1363. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Donaldson JC, Dempsey PJ, Reddy S, Bouton AH, Coffey RJ, Hanks SK. Crk-associated substrate p130Cas interacts with nephrocystin and both proteins localize to cell–cell contacts of polarized epithelial cells. Exp Cell Res. 2000;256:168–178. doi: 10.1006/excr.2000.4822. [DOI] [PubMed] [Google Scholar]
- 55.Kim W, Kook S, Kim DJ, Teodorof C, Song WK. The 31-kDa caspase-generated cleavage product of p130cas functions as a transcriptional repressor of E2A in apoptotic cells. J Biol Chem. 2004;279:8333–8342. doi: 10.1074/jbc.M312026200. [DOI] [PubMed] [Google Scholar]
- 56.Defilippi P, Di Stefano P, Cabodi S. p130Cas: a versatile scaffold in signaling networks. Trends Cell Biol. 2006;16:257–263. doi: 10.1016/j.tcb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 57.Honda H, Oda H, Nakamoto T, Honda Z, Sakai R, Suzuki T, Saito T, Nakamura K, Nakao K, Ishikawa T, Katsuki M, Yazaki Y, Hirai H. Cardiovascular anomaly, impaired actin bundling and resistance to Src-induced transformation in mice lacking p130Cas. Nat Genet. 1998;19:361–365. doi: 10.1038/1246. [DOI] [PubMed] [Google Scholar]
- 58.Astier A, Manie SN, Law SF, Canty T, Haghayghi N, Druker BJ, Salgia R, Golemis EA, Freedman AS. Association of the Cas-like molecule HEF1 with CrkL following integrin and antigen receptor signaling in human B-cells: potential relevance to neoplastic lymphohematopoietic cells. Leuk Lymphoma. 1997;28:65–72. doi: 10.3109/10428199709058332. [DOI] [PubMed] [Google Scholar]
- 59.Kanda H, Mimura T, Morino N, Hamasaki K, Nakamoto T, Hirai H, Morimoto C, Yazaki Y, Nojima Y. Ligation of the T cell antigen receptor induces tyrosine phosphorylation of p105CasL, a member of the p130Cas-related docking protein family, and its subsequent binding to the Src homology 2 domain of c-Crk. Eur J Immunol. 1997;27:2113–2117. doi: 10.1002/eji.1830270840. [DOI] [PubMed] [Google Scholar]
- 60.Natarajan M, Stewart JE, Golemis EA, Pugacheva EN, Alexandropoulos K, Cox BD, Wang W, Grammer JR, Gladson CL. HEF1 is a necessary and specific downstream effector of FAK that promotes the migration of glioblastoma cells. Oncogene. 2006;25:1721–1732. doi: 10.1038/sj.onc.1209199. [DOI] [PubMed] [Google Scholar]
- 61.Thao le B, Vu HA, Yasuda K, Taniguchi S, Yagasaki F, Taguchi T, Watanabe T, Sato Y. Cas-L was overexpressed in imatinib-resistant gastrointestinal stromal tumor cells. Cancer Biol Ther. 2009;8:683–688. doi: 10.4161/cbt.8.8.7779. [DOI] [PubMed] [Google Scholar]
- 62.Kim M, Gans JD, Nogueira C, Wang A, Paik JH, Feng B, Brennan C, Hahn WC, Cordon-Cardo C, Wagner SN, Flotte TJ, Duncan LM, Granter SR, Chin L. Comparative oncogenomics identifies NEDD9 as a melanoma metastasis gene. Cell. 2006;125:1269–1281. doi: 10.1016/j.cell.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 63.Merrill RA, See AW, Wertheim ML, Clagett-Dame M. Crk-associated substrate (Cas) family member, NEDD9, is regulated in human neuroblastoma cells and in the embryonic hindbrain by all-trans retinoic acid. Dev Dyn. 2004;231:564–575. doi: 10.1002/dvdy.20159. [DOI] [PubMed] [Google Scholar]
- 64.Merrill RA, Ahrens JM, Kaiser ME, Federhart KS, Poon VY, Clagett-Dame M. All-trans retinoic acid-responsive genes identified in the human SH-SY5Y neuroblastoma cell line and their regulated expression in the nervous system of early embryos. Biol Chem. 2004;385:605–614. doi: 10.1515/BC.2004.075. [DOI] [PubMed] [Google Scholar]
- 65.Liu X, Elia AE, Law SF, Golemis EA, Farley J, Wang T. A novel ability of Smad3 to regulate proteasomal degradation of a Cas family member HEF1. EMBO J. 2000;19:6759–6769. doi: 10.1093/emboj/19.24.6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng M, McKeown-Longo PJ. Regulation of HEF1 expression and phosphorylation by TGF-beta 1 and cell adhesion. J Biol Chem. 2002;277:39599–39608. doi: 10.1074/jbc.M202263200. [DOI] [PubMed] [Google Scholar]
- 67.Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem. 2002;277:5209–5218. doi: 10.1074/jbc.M110090200. [DOI] [PubMed] [Google Scholar]
- 68.Buterin T, Koch C, Naegeli H. Convergent transcriptional profiles induced by endogenous estrogen and distinct xenoestrogens in breast cancer cells. Carcinogenesis. 2006;27:1567–1578. doi: 10.1093/carcin/bgi339. [DOI] [PubMed] [Google Scholar]
- 69.Monroe DG, Getz BJ, Johnsen SA, Riggs BL, Khosla S, Spelsberg TC. Estrogen receptor isoform-specific regulation of endogenous gene expression in human osteoblastic cell lines expressing either ERalpha or ERbeta. J Cell Biochem. 2003;90:315–326. doi: 10.1002/jcb.10633. [DOI] [PubMed] [Google Scholar]
- 70.Martin-Rendon E, Hale SJ, Ryan D, Baban D, Forde SP, Roubelakis M, Sweeney D, Moukayed M, Harris AL, Davies K, Watt SM. Transcriptional profiling of human cord blood CD133 + and cultured bone marrow mesenchymal stem cells in response to hypoxia. Stem Cells. 2007;25:1003–1012. doi: 10.1634/stemcells.2006-0398. [DOI] [PubMed] [Google Scholar]
- 71.Aquino JB, Lallemend F, Marmigere F, Adameyko II, Golemis EA, Ernfors P. The retinoic acid inducible Cas-family signaling protein Nedd9 regulates neural crest cell migration by modulating adhesion and actin dynamics. Neuroscience. 2009;162:1106–1119. doi: 10.1016/j.neuroscience.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vogel T, Ahrens S, Buttner N, Krieglstein K (2009) Transforming growth factor {beta} promotes neuronal cell fate of mouse cortical and hippocampal progenitors in vitro and in vivo: identification of Nedd9 as an essential signaling component. Cereb Cortex (in press) [DOI] [PMC free article] [PubMed]
- 73.Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bui LC, Tomkiewicz C, Chevallier A, Pierre S, Bats AS, Mota S, Raingeaud J, Pierre J, Diry M, Transy C, Garlatti M, Barouki R, Coumoul X (2009) Nedd9/Hef1/Cas-L mediates the effects of environmental pollutants on cell migration and plasticity. Oncogene (in press) [DOI] [PubMed]
- 75.Tran DH, Satou K, Ho TB. Finding microRNA regulatory modules in human genome using rule induction. BMC Bioinformatics. 2008;9(Suppl 12):S5. doi: 10.1186/1471-2105-9-S12-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schlaepfer DD, Broome MA, Hunter T. Fibronectin-stimulated signaling from a focal adhesion kinase-c-Src complex: involvement of the Grb2, p130cas, and Nck adaptor proteins. Mol Cell Biol. 1997;17:1702–1713. doi: 10.1128/mcb.17.3.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tachibana K, Urano T, Fujita H, Ohashi Y, Kamiguchi K, Iwata S, Hirai H, Morimoto C. Tyrosine phosphorylation of Crk-associated substrates by focal adhesion kinase. A putative mechanism for the integrin-mediated tyrosine phosphorylation of Crk-associated substrates. J Biol Chem. 1997;272:29083–29090. doi: 10.1074/jbc.272.46.29083. [DOI] [PubMed] [Google Scholar]
- 78.Sanders MA, Basson MD. p130Cas but not paxillin is essential for Caco-2 intestinal epithelial cell spreading and migration on collagen IV. J Biol Chem. 2005;280:23516–23522. doi: 10.1074/jbc.M413165200. [DOI] [PubMed] [Google Scholar]
- 79.Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog Biophys Mol Biol. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- 80.Cai D, Clayton LK, Smolyar A, Lerner A. AND-34, a novel p130Cas-binding thymic stromal cell protein regulated by adhesion and inflammatory cytokines. J Immunol. 1999;163:2104–2112. [PubMed] [Google Scholar]
- 81.Cai D, Iyer A, Felekkis KN, Near RI, Luo Z, Chernoff J, Albanese C, Pestell RG, Lerner A. AND-34/BCAR3, a GDP exchange factor whose overexpression confers antiestrogen resistance, activates Rac, PAK1, and the cyclin D1 promoter. Cancer Res. 2003;63:6802–6808. [PubMed] [Google Scholar]
- 82.Abassi YA, Rehn M, Ekman N, Alitalo K, Vuori K. p130Cas Couples the tyrosine kinase Bmx/Etk with regulation of the actin cytoskeleton and cell migration. J Biol Chem. 2003;278:35636–35643. doi: 10.1074/jbc.M306438200. [DOI] [PubMed] [Google Scholar]
- 83.Korah R, Choi L, Barrios J, Wieder R. Expression of FGF-2 alters focal adhesion dynamics in migration-restricted MDA-MB-231 breast cancer cells. Breast Cancer Res Treat. 2004;88:17–28. doi: 10.1007/s10459-004-6006-2. [DOI] [PubMed] [Google Scholar]
- 84.Avraham HK, Lee TH, Koh Y, Kim TA, Jiang S, Sussman M, Samarel AM, Avraham S. Vascular endothelial growth factor regulates focal adhesion assembly in human brain microvascular endothelial cells through activation of the focal adhesion kinase and related adhesion focal tyrosine kinase. J Biol Chem. 2003;278:36661–36668. doi: 10.1074/jbc.M301253200. [DOI] [PubMed] [Google Scholar]
- 85.Podar K, Shringarpure R, Tai YT, Simoncini M, Sattler M, Ishitsuka K, Richardson PG, Hideshima T, Chauhan D, Anderson KC. Caveolin-1 is required for vascular endothelial growth factor-triggered multiple myeloma cell migration and is targeted by bortezomib. Cancer Res. 2004;64:7500–7506. doi: 10.1158/0008-5472.CAN-04-0124. [DOI] [PubMed] [Google Scholar]
- 86.Ceacareanu AC, Ceacareanu B, Zhuang D, Chang Y, Ray RM, Desai L, Chapman KE, Waters CM, Hassid A. Nitric oxide attenuates IGF-I-induced aortic smooth muscle cell motility by decreasing Rac1 activity: essential role of PTP-PEST and p130cas. Am J Physiol Cell Physiol. 2006;290:C1263–C1270. doi: 10.1152/ajpcell.00241.2005. [DOI] [PubMed] [Google Scholar]
- 87.Nakamura I, Rodan GA, Duong le T. Distinct roles of p130Cas and c-Cbl in adhesion-induced or macrophage colony-stimulating factor-mediated signaling pathways in prefusion osteoclasts. Endocrinology. 2003;144:4739–4741. doi: 10.1210/en.2003-0615. [DOI] [PubMed] [Google Scholar]
- 88.Rozengurt E. Signal transduction pathways in the mitogenic response to G protein-coupled neuropeptide receptor agonists. J Cell Physiol. 1998;177:507–517. doi: 10.1002/(SICI)1097-4652(199812)177:4<507::AID-JCP2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 89.Zhang Z, Baron R, Horne WC. Integrin engagement, the actin cytoskeleton, and c-Src are required for the calcitonin-induced tyrosine phosphorylation of paxillin and HEF1, but not for calcitonin-induced Erk1/2 phosphorylation. J Biol Chem. 2000;275:37219–37223. doi: 10.1074/jbc.M001818200. [DOI] [PubMed] [Google Scholar]
- 90.Zhang Z, Hernandez-Lagunas L, Horne WC, Baron R. Cytoskeleton-dependent tyrosine phosphorylation of the p130(Cas) family member HEF1 downstream of the G protein-coupled calcitonin receptor. Calcitonin induces the association of HEF1, paxillin, and focal adhesion kinase. J Biol Chem. 1999;274:25093–25098. doi: 10.1074/jbc.274.35.25093. [DOI] [PubMed] [Google Scholar]
- 91.Kovacic-Milivojevic B, Roediger F, Almeida EA, Damsky CH, Gardner DG, Ilic D. Focal adhesion kinase and p130Cas mediate both sarcomeric organization and activation of genes associated with cardiac myocyte hypertrophy. Mol Biol Cell. 2001;12:2290–2307. doi: 10.1091/mbc.12.8.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jope RS, Song L, Grimes CA, Zhang L. Oxidative stress oppositely modulates protein tyrosine phosphorylation stimulated by muscarinic G protein-coupled and epidermal growth factor receptors. J Neurosci Res. 1999;55:329–340. doi: 10.1002/(SICI)1097-4547(19990201)55:3<329::AID-JNR8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 93.Takahashi T, Kawahara Y, Taniguchi T, Yokoyama M. Tyrosine phosphorylation and association of p130Cas and c-Crk II by ANG II in vascular smooth muscle cells. Am J Physiol. 1998;274:H1059–H1065. doi: 10.1152/ajpheart.1998.274.4.H1059. [DOI] [PubMed] [Google Scholar]
- 94.Yang CC, Ogawa H, Dwinell MB, McCole DF, Eckmann L, Kagnoff MF. Chemokine receptor CCR6 transduces signals that activate p130Cas and alter cAMP-stimulated ion transport in human intestinal epithelial cells. Am J Physiol Cell Physiol. 2005;288:C321–C328. doi: 10.1152/ajpcell.00171.2004. [DOI] [PubMed] [Google Scholar]
- 95.Cowell LN, Graham JD, Bouton AH, Clarke CL, O’Neill GM. Tamoxifen treatment promotes phosphorylation of the adhesion molecules, p130Cas/BCAR1, FAK and Src, via an adhesion-dependent pathway. Oncogene. 2006;25:7597–7607. doi: 10.1038/sj.onc.1209747. [DOI] [PubMed] [Google Scholar]
- 96.Sasaki T, Iwata S, Okano HJ, Urasaki Y, Hamada J, Tanaka H, Dang NH, Okano H, Morimoto C. Nedd9 protein, a Cas-L homologue, is upregulated after transient global ischemia in rats: possible involvement of Nedd9 in the differentiation of neurons after ischemia. Stroke. 2005;36:2457–2462. doi: 10.1161/01.STR.0000185672.10390.30. [DOI] [PubMed] [Google Scholar]
- 97.Lee TN, Gold G, Workman R, Cook CA, Konrad RJ. Glucose stimulates the association of Crk with p130Cas in pancreatic beta cells. Pancreas. 2004;29:e100–e105. doi: 10.1097/00006676-200411000-00163. [DOI] [PubMed] [Google Scholar]
- 98.Ma A, Richardson A, Schaefer EM, Parsons JT. Serine phosphorylation of focal adhesion kinase in interphase and mitosis: a possible role in modulating binding to p130Cas. Mol Biol Cell. 2001;12:1–12. doi: 10.1091/mbc.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yamakita Y, Totsukawa G, Yamashiro S, Fry D, Zhang X, Hanks SK, Matsumura F. Dissociation of FAK/p130CAS/c-Src complex during mitosis: role of mitosis-specific serine phosphorylation of FAK. J Cell Biol. 1999;144:315–324. doi: 10.1083/jcb.144.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hoon Kim D, Jeon Choi S, Kook S, Kim W, Keun Song W. Phosphorylation-dependent cleavage of p130cas in apoptotic rat-1 cells. Biochem Biophys Res Commun. 2003;300:141–148. doi: 10.1016/s0006-291x(02)02786-9. [DOI] [PubMed] [Google Scholar]
- 101.Wang X, Weng LP, Yu Q. Specific inhibition of FGF-induced MAPK activation by the receptor-like protein tyrosine phosphatase LAR. Oncogene. 2000;19:2346–2353. doi: 10.1038/sj.onc.1203558. [DOI] [PubMed] [Google Scholar]
- 102.Weng LP, Wang X, Yu Q. Transmembrane tyrosine phosphatase LAR induces apoptosis by dephosphorylating and destabilizing p130Cas. Genes Cells. 1999;4:185–196. doi: 10.1046/j.1365-2443.1999.00251.x. [DOI] [PubMed] [Google Scholar]
- 103.Garton AJ, Flint AJ, Tonks NK. Identification of p130(cas) as a substrate for the cytosolic protein tyrosine phosphatase PTP-PEST. Mol Cell Biol. 1996;16:6408–6418. doi: 10.1128/mcb.16.11.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Garton AJ, Tonks NK. Regulation of fibroblast motility by the protein tyrosine phosphatase PTP-PEST. J Biol Chem. 1999;274:3811–3818. doi: 10.1074/jbc.274.6.3811. [DOI] [PubMed] [Google Scholar]
- 105.Cote JF, Charest A, Wagner J, Tremblay ML. Combination of gene targeting and substrate trapping to identify substrates of protein tyrosine phosphatases using PTP-PEST as a model. Biochemistry. 1998;37:13128–13137. doi: 10.1021/bi981259l. [DOI] [PubMed] [Google Scholar]
- 106.Sirois J, Cote JF, Charest A, Uetani N, Bourdeau A, Duncan SA, Daniels E, Tremblay ML. Essential function of PTP-PEST during mouse embryonic vascularization, mesenchyme formation, neurogenesis and early liver development. Mech Dev. 2006;123:869–880. doi: 10.1016/j.mod.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yo K, Iwata S, Hashizume Y, Kondo S, Nomura S, Hosono O, Kawasaki H, Tanaka H, Dang NH, Morimoto C. SHP-2 inhibits tyrosine phosphorylation of Cas-L and regulates cell migration. Biochem Biophys Res Commun. 2009;382:210–214. doi: 10.1016/j.bbrc.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 108.Noguchi T, Tsuda M, Takeda H, Takada T, Inagaki K, Yamao T, Fukunaga K, Matozaki T, Kasuga M. Inhibition of cell growth and spreading by stomach cancer-associated protein-tyrosine phosphatase-1 (SAP-1) through dephosphorylation of p130cas. J Biol Chem. 2001;276:15216–15224. doi: 10.1074/jbc.M007208200. [DOI] [PubMed] [Google Scholar]
- 109.Yokoyama N, Miller WT. Protein phosphatase 2A interacts with the Src kinase substrate p130(CAS) Oncogene. 2001;20:6057–6065. doi: 10.1038/sj.onc.1204735. [DOI] [PubMed] [Google Scholar]
- 110.Zheng M, McKeown-Longo PJ. Cell adhesion regulates Ser/Thr phosphorylation and proteasomal degradation of HEF1. J Cell Sci. 2006;119:96–103. doi: 10.1242/jcs.02712. [DOI] [PubMed] [Google Scholar]
- 111.Hivert V, Pierre J, Raingeaud J. Phosphorylation of human enhancer of filamentation (HEF1) on serine 369 induces its proteasomal degradation. Biochem Pharmacol. 2009;78:1017–1025. doi: 10.1016/j.bcp.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 112.Achiwa H, Lazo JS. PRL-1 tyrosine phosphatase regulates c-Src levels, adherence, and invasion in human lung cancer cells. Cancer Res. 2007;67:643–650. doi: 10.1158/0008-5472.CAN-06-2436. [DOI] [PubMed] [Google Scholar]
- 113.Daouti S, Li WH, Qian H, Huang KS, Holmgren J, Levin W, Reik L, McGady DL, Gillespie P, Perrotta A, Bian H, Reidhaar-Olson JF, Bliss SA, Olivier AR, Sergi JA, Fry D, Danho W, Ritland S, Fotouhi N, Heimbrook D, Niu H. A selective phosphatase of regenerating liver phosphatase inhibitor suppresses tumor cell anchorage-independent growth by a novel mechanism involving p130Cas cleavage. Cancer Res. 2008;68:1162–1169. doi: 10.1158/0008-5472.CAN-07-2349. [DOI] [PubMed] [Google Scholar]
- 114.Sorokin A, Reed E. Insulin stimulates the tyrosine dephosphorylation of docking protein p130cas (Crk-associated substrate), promoting the switch of the adaptor protein crk from p130cas to newly phosphorylated insulin receptor substrate-1. Biochem J. 1998;334:595–600. doi: 10.1042/bj3340595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Black DS, Bliska JB. Identification of p130Cas as a substrate of Yersinia YopH (Yop51), a bacterial protein tyrosine phosphatase that translocates into mammalian cells and targets focal adhesions. EMBO J. 1997;16:2730–2744. doi: 10.1093/emboj/16.10.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Persson C, Carballeira N, Wolf-Watz H, Fallman M. The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130Cas and FAK, and the associated accumulation of these proteins in peripheral focal adhesions. EMBO J. 1997;16:2307–2318. doi: 10.1093/emboj/16.9.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bruce-Staskal PJ, Weidow CL, Gibson JJ, Bouton AH. Cas, Fak and Pyk2 function in diverse signaling cascades to promote Yersinia uptake. J Cell Sci. 2002;115:2689–2700. doi: 10.1242/jcs.115.13.2689. [DOI] [PubMed] [Google Scholar]
- 118.Bannerman DD, Sathyamoorthy M, Goldblum SE. Bacterial lipopolysaccharide disrupts endothelial monolayer integrity and survival signaling events through caspase cleavage of adherens junction proteins. J Biol Chem. 1998;273:35371–35380. doi: 10.1074/jbc.273.52.35371. [DOI] [PubMed] [Google Scholar]
- 119.Fashena SJ, Einarson MB, O’Neill GM, Patriotis C, Golemis EA. Dissection of HEF1-dependent functions in motility and transcriptional regulation. J Cell Sci. 2002;115:99–111. doi: 10.1242/jcs.115.1.99. [DOI] [PubMed] [Google Scholar]
- 120.Klemke RL, Leng J, Molander R, Brooks PC, Vuori K, Cheresh DA. CAS/Crk coupling serves as a “molecular switch” for induction of cell migration. J Cell Biol. 1998;140:961–972. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vuori K, Ruoslahti E. Tyrosine phosphorylation of p130Cas and cortactin accompanies integrin-mediated cell adhesion to extracellular matrix. J Biol Chem. 1995;270:22259–22262. doi: 10.1074/jbc.270.38.22259. [DOI] [PubMed] [Google Scholar]
- 122.Cary LA, Han DC, Polte TR, Hanks SK, Guan JL. Identification of p130Cas as a mediator of focal adhesion kinase-promoted cell migration. J Cell Biol. 1998;140:211–221. doi: 10.1083/jcb.140.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nakamura I, Jimi E, Duong LT, Sasaki T, Takahashi N, Rodan GA, Suda T. Tyrosine phosphorylation of p130Cas is involved in actin organization in osteoclasts. J Biol Chem. 1998;273:11144–11149. doi: 10.1074/jbc.273.18.11144. [DOI] [PubMed] [Google Scholar]
- 124.Moro L, Dolce L, Cabodi S, Bergatto E, Boeri Erba E, Smeriglio M, Turco E, Retta SF, Giuffrida MG, Venturino M, Godovac-Zimmermann J, Conti A, Schaefer E, Beguinot L, Tacchetti C, Gaggini P, Silengo L, Tarone G, Defilippi P. Integrin-induced epidermal growth factor (EGF) receptor activation requires c-Src and p130Cas and leads to phosphorylation of specific EGF receptor tyrosines. J Biol Chem. 2002;277:9405–9414. doi: 10.1074/jbc.M109101200. [DOI] [PubMed] [Google Scholar]
- 125.Kiyokawa E, Hashimoto Y, Kobayashi S, Sugimura H, Kurata T, Matsuda M. Activation of Rac1 by a Crk SH3-binding protein, DOCK180. Genes Dev. 1998;12:3331–3336. doi: 10.1101/gad.12.21.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Feller SM, Posern G, Voss J, Kardinal C, Sakkab D, Zheng J, Knudsen BS. Physiological signals and oncogenesis mediated through Crk family adapter proteins. J Cell Physiol. 1998;177:535–552. doi: 10.1002/(SICI)1097-4652(199812)177:4<535::AID-JCP5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 127.Kanda H, Mimura T, Hamasaki K, Yamamoto K, Yazaki Y, Hirai H, Nojima Y. Fyn and Lck tyrosine kinases regulate tyrosine phosphorylation of p105CasL, a member of the p130Cas docking protein family, in T-cell receptor-mediated signalling. Immunology. 1999;97:56–61. doi: 10.1046/j.1365-2567.1999.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.O’Neill GM, Fashena SJ, Golemis EA. Integrin signalling: a new Cas(t) of characters enters the stage. Trends Cell Biol. 2000;10:111–119. doi: 10.1016/s0962-8924(99)01714-6. [DOI] [PubMed] [Google Scholar]
- 129.Ridley AJ. Rho GTPases and cell migration. J Cell Sci. 2001;114:2713–2722. doi: 10.1242/jcs.114.15.2713. [DOI] [PubMed] [Google Scholar]
- 130.Simpson KJ, Selfors LM, Bui J, Reynolds A, Leake D, Khvorova A, Brugge JS. Identification of genes that regulate epithelial cell migration using an siRNA screening approach. Nat Cell Biol. 2008;10:1027–1038. doi: 10.1038/ncb1762. [DOI] [PubMed] [Google Scholar]
- 131.Smith HW, Marra P, Marshall CJ. uPAR promotes formation of the p130Cas-Crk complex to activate Rac through DOCK180. J Cell Biol. 2008;182:777–790. doi: 10.1083/jcb.200712050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Furuya K, Nakamoto T, Shen ZJ, Tsuji K, Nifuji A, Hirai H, Noda M. Overexpression of Cas-interacting zinc finger protein (CIZ) suppresses proliferation and enhances expression of type I collagen gene in osteoblast-like MC3T3E1 cells. Exp Cell Res. 2000;261:329–335. doi: 10.1006/excr.2000.5051. [DOI] [PubMed] [Google Scholar]
- 133.Janssen H, Marynen P. Interaction partners for human ZNF384/CIZ/NMP4–zyxin as a mediator for p130CAS signaling? Exp Cell Res. 2006;312:1194–1204. doi: 10.1016/j.yexcr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 134.Pratt SJ, Epple H, Ward M, Feng Y, Braga VM, Longmore GD. The LIM protein Ajuba influences p130Cas localization and Rac1 activity during cell migration. J Cell Biol. 2005;168:813–824. doi: 10.1083/jcb.200406083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yi J, Kloeker S, Jensen CC, Bockholt S, Honda H, Hirai H, Beckerle MC. Members of the Zyxin family of LIM proteins interact with members of the p130Cas family of signal transducers. J Biol Chem. 2002;277:9580–9589. doi: 10.1074/jbc.M106922200. [DOI] [PubMed] [Google Scholar]
- 136.Dolfi F, Garcia-Guzman M, Ojaniemi M, Nakamura H, Matsuda M, Vuori K. The adaptor protein Crk connects multiple cellular stimuli to the JNK signaling pathway. Proc Natl Acad Sci USA. 1998;95:15394–15399. doi: 10.1073/pnas.95.26.15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fagerstrom S, Pahlman S, Nanberg E. Protein kinase C-dependent tyrosine phosphorylation of p130cas in differentiating neuroblastoma cells. J Biol Chem. 1998;273:2336–2343. doi: 10.1074/jbc.273.4.2336. [DOI] [PubMed] [Google Scholar]
- 138.Gustavsson A, Yuan M, Fallman M. Temporal dissection of beta1-integrin signaling indicates a role for p130Cas-Crk in filopodia formation. J Biol Chem. 2004;279:22893–22901. doi: 10.1074/jbc.M309693200. [DOI] [PubMed] [Google Scholar]
- 139.Alexander NR, Branch KM, Parekh A, Clark ES, Iwueke IC, Guelcher SA, Weaver AM. Extracellular matrix rigidity promotes invadopodia activity. Curr Biol. 2008;18:1295–1299. doi: 10.1016/j.cub.2008.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cho SY, Klemke RL. Purification of pseudopodia from polarized cells reveals redistribution and activation of Rac through assembly of a CAS/Crk scaffold. J Cell Biol. 2002;156:725–736. doi: 10.1083/jcb.200111032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zaidel-Bar R, Kam Z, Geiger B. Polarized downregulation of the paxillin-p130CAS-Rac1 pathway induced by shear flow. J Cell Sci. 2005;118:3997–4007. doi: 10.1242/jcs.02523. [DOI] [PubMed] [Google Scholar]
- 142.Dadke D, Jarnik M, Pugacheva EN, Singh MK, Golemis EA. Deregulation of HEF1 impairs M-phase progression by disrupting the RhoA activation cycle. Mol Biol Cell. 2006;17:1204–1217. doi: 10.1091/mbc.E05-03-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bargon SD, Gunning PW, O’Neill GM. The Cas family docking protein, HEF1, promotes the formation of neurite-like membrane extensions. Biochim Biophys Acta. 2005;1746:143–154. doi: 10.1016/j.bbamcr.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 144.Kyaw M, Yoshizumi M, Tsuchiya K, Kagami S, Izawa Y, Fujita Y, Ali N, Kanematsu Y, Toida K, Ishimura K, Tamaki T. Src and Cas are essentially but differentially involved in angiotensin II-stimulated migration of vascular smooth muscle cells via extracellular signal-regulated kinase 1/2 and c-Jun NH2-terminal kinase activation. Mol Pharmacol. 2004;65:832–841. doi: 10.1124/mol.65.4.832. [DOI] [PubMed] [Google Scholar]
- 145.Wendt MK, Smith JA, Schiemann WP (2009) p130Cas is required for mammary tumor growth and transforming growth factor-{beta} (TGF-{beta})-mediated metastasis through regulation of Smad2/3 activity. J Biol Chem (in press) [DOI] [PMC free article] [PubMed]
- 146.Gingras D, Michaud M, Di Tomasso G, Beliveau E, Nyalendo C, Beliveau R. Sphingosine-1-phosphate induces the association of membrane-type 1 matrix metalloproteinase with p130Cas in endothelial cells. FEBS Lett. 2008;582:399–404. doi: 10.1016/j.febslet.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 147.Imhof BA, Aurrand-Lions M. Adhesion mechanisms regulating the migration of monocytes. Nat Rev Immunol. 2004;4:432–444. doi: 10.1038/nri1375. [DOI] [PubMed] [Google Scholar]
- 148.Regelmann AG, Danzl NM, Wanjalla C, Alexandropoulos K. The hematopoietic isoform of Cas-Hef1-associated signal transducer regulates chemokine-induced inside-out signaling and T cell trafficking. Immunity. 2006;25:907–918. doi: 10.1016/j.immuni.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 149.Gotoh T, Cai D, Tian X, Feig LA, Lerner A. p130Cas regulates the activity of AND-34, a novel Ral, Rap1, and R-Ras guanine nucleotide exchange factor. J Biol Chem. 2000;275:30118–30123. doi: 10.1074/jbc.M003074200. [DOI] [PubMed] [Google Scholar]
- 150.Hisakawa N, Tanaka H, Hosono O, Nishijima R, Ohashi Y, Saito S, Nishiya K, Hashimoto K, Morimoto C. Aberrant responsiveness to RANTES in synovial fluid T cells from patients with rheumatoid arthritis. J Rheumatol. 2002;29:1124–1134. [PubMed] [Google Scholar]
- 151.Yu HG, Schrader H, Otte JM, Schmidt WE, Schmitz F. Rapid tyrosine phosphorylation of focal adhesion kinase, paxillin, and p130Cas by gastrin in human colon cancer cells. Biochem Pharmacol. 2004;67:135–146. doi: 10.1016/j.bcp.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 152.Dutt P, Wang JF, Groopman JE. Stromal cell-derived factor-1 alpha and stem cell factor/kit ligand share signaling pathways in hemopoietic progenitors: a potential mechanism for cooperative induction of chemotaxis. J Immunol. 1998;161:3652–3658. [PubMed] [Google Scholar]
- 153.Wang JF, Park IW, Groopman JE. Stromal cell-derived factor-1alpha stimulates tyrosine phosphorylation of multiple focal adhesion proteins and induces migration of hematopoietic progenitor cells: roles of phosphoinositide-3 kinase and protein kinase C. Blood. 2000;95:2505–2513. [PubMed] [Google Scholar]
- 154.Zhang XF, Wang JF, Matczak E, Proper JA, Groopman JE. Janus kinase 2 is involved in stromal cell-derived factor-1alpha-induced tyrosine phosphorylation of focal adhesion proteins and migration of hematopoietic progenitor cells. Blood. 2001;97:3342–3348. doi: 10.1182/blood.v97.11.3342. [DOI] [PubMed] [Google Scholar]
- 155.Fernandis AZ, Cherla RP, Ganju RK. Differential regulation of CXCR4-mediated T-cell chemotaxis and mitogen-activated protein kinase activation by the membrane tyrosine phosphatase, CD45. J Biol Chem. 2003;278:9536–9543. doi: 10.1074/jbc.M211803200. [DOI] [PubMed] [Google Scholar]
- 156.Seo S, Asai T, Saito T, Suzuki T, Morishita Y, Nakamoto T, Ichikawa M, Yamamoto G, Kawazu M, Yamagata T, Sakai R, Mitani K, Ogawa S, Kurokawa M, Chiba S, Hirai H. Crk-associated substrate lymphocyte type is required for lymphocyte trafficking and marginal zone B cell maintenance. J Immunol. 2005;175:3492–3501. doi: 10.4049/jimmunol.175.6.3492. [DOI] [PubMed] [Google Scholar]
- 157.Donlin LT, Danzl NM, Wanjalla C, Alexandropoulos K. Deficiency in expression of the signaling protein sin/efs leads to t-lymphocyte activation and mucosal inflammation. Mol Cell Biol. 2005;25:11035–11046. doi: 10.1128/MCB.25.24.11035-11046.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rosenblatt J, Raff MC, Cramer LP. An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr Biol. 2001;11:1847–1857. doi: 10.1016/s0960-9822(01)00587-5. [DOI] [PubMed] [Google Scholar]
- 159.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 161.Wei L, Yang Y, Zhang X, Yu Q. Cleavage of p130Cas in anoikis. J Cell Biochem. 2004;91:325–335. doi: 10.1002/jcb.10760. [DOI] [PubMed] [Google Scholar]
- 162.Bhattacharya S, Guo H, Ray RM, Johnson LR. Basic helix-loop-helix protein E47-mediated p21Waf1/Cip1 gene expression regulates apoptosis of intestinal epithelial cells. Biochem J. 2007;407:243–254. doi: 10.1042/BJ20070293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wei L, Yang Y, Zhang X, Yu Q. Anchorage-independent phosphorylation of p130(Cas) protects lung adenocarcinoma cells from anoikis. J Cell Biochem. 2002;87:439–449. doi: 10.1002/jcb.10322. [DOI] [PubMed] [Google Scholar]
- 164.Wendt MK, Drury LJ, Vongsa RA, Dwinell MB. Constitutive CXCL12 expression induces anoikis in colorectal carcinoma cells. Gastroenterology. 2008;135:508–517. doi: 10.1053/j.gastro.2008.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cabodi S, Tinnirello A, Di Stefano P, Bisaro B, Ambrosino E, Castellano I, Sapino A, Arisio R, Cavallo F, Forni G, Glukhova M, Silengo L, Altruda F, Turco E, Tarone G, Defilippi P. p130Cas as a new regulator of mammary epithelial cell proliferation, survival, and HER2-neu oncogene-dependent breast tumorigenesis. Cancer Res. 2006;66:4672–4680. doi: 10.1158/0008-5472.CAN-05-2909. [DOI] [PubMed] [Google Scholar]
- 166.Brinkman A, de Jong D, Tuinman S, Azaouagh N, van Agthoven T, Dorssers LC (2009) The substrate domain of BCAR1 is essential for anti-estrogen-resistant proliferation of human breast cancer cells. Breast Cancer Res Treat (in press) [DOI] [PubMed]
- 167.van der Flier S, Brinkman A, Look MP, Kok EM, Meijer-van Gelder ME, Klijn JG, Dorssers LC, Foekens JA. Bcar1/p130Cas protein and primary breast cancer: prognosis and response to tamoxifen treatment. J Natl Cancer Inst. 2000;92:120–127. doi: 10.1093/jnci/92.2.120. [DOI] [PubMed] [Google Scholar]
- 168.Casanova I, Bosch R, Lasa A, Parreno M, Cespedes MV, Brunet S, Nomdedeu JF, Mangues MA, Sierra J, Mangues R. A celecoxib derivative inhibits focal adhesion signaling and induces caspase-8-dependent apoptosis in human acute myeloid leukemia cells. Int J Cancer. 2008;123:217–226. doi: 10.1002/ijc.23516. [DOI] [PubMed] [Google Scholar]
- 169.Ta HQ, Thomas KS, Schrecengost RS, Bouton AH. A novel association between p130Cas and resistance to the chemotherapeutic drug adriamycin in human breast cancer cells. Cancer Res. 2008;68:8796–8804. doi: 10.1158/0008-5472.CAN-08-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hakak Y, Martin GS. Cas mediates transcriptional activation of the serum response element by Src. Mol Cell Biol. 1999;19:6953–6962. doi: 10.1128/mcb.19.10.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pylayeva Y, Gillen KM, Gerald W, Beggs HE, Reichardt LF, Giancotti FG. Ras- and PI3 K-dependent breast tumorigenesis in mice and humans requires focal adhesion kinase signaling. J Clin Invest. 2009;119:252–266. doi: 10.1172/JCI37160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dorssers LC, van Agthoven T, Brinkman A, Veldscholte J, Smid M, Dechering KJ. Breast cancer oestrogen independence mediated by BCAR1 or BCAR3 genes is transmitted through mechanisms distinct from the oestrogen receptor signalling pathway or the epidermal growth factor receptor signalling pathway. Breast Cancer Res. 2005;7:R82–R92. doi: 10.1186/bcr954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Izumchenko E, Singh MK, Plotnikova OV, Tikhmyanova N, Little JL, Serebriiskii IG, Seo S, Kurokawa M, Egleston BL, Klein-Szanto A, Pugacheva EN, Hardy RR, Wolfson M, Connolly DC, Golemis EA. NEDD9 promotes oncogenic signaling in mammary tumor development. Cancer Res. 2009;69:7198–7206. doi: 10.1158/0008-5472.CAN-09-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cabodi S, Moro L, Baj G, Smeriglio M, Di Stefano P, Gippone S, Surico N, Silengo L, Turco E, Tarone G, Defilippi P. p130Cas interacts with estrogen receptor alpha and modulates non-genomic estrogen signaling in breast cancer cells. J Cell Sci. 2004;117:1603–1611. doi: 10.1242/jcs.01025. [DOI] [PubMed] [Google Scholar]
- 175.Kim W, Seok Kang Y, Soo Kim J, Shin NY, Hanks SK, Song WK. The integrin-coupled signaling adaptor p130Cas suppresses Smad3 function in transforming growth factor-beta signaling. Mol Biol Cell. 2008;19:2135–2146. doi: 10.1091/mbc.E07-10-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Marumoto T, Zhang D, Saya H. Aurora-A - a guardian of poles. Nat Rev Cancer. 2005;5:42–50. doi: 10.1038/nrc1526. [DOI] [PubMed] [Google Scholar]
- 177.Honda K, Mihara H, Kato Y, Yamaguchi A, Tanaka H, Yasuda H, Furukawa K, Urano T. Degradation of human Aurora2 protein kinase by the anaphase-promoting complex-ubiquitin-proteasome pathway. Oncogene. 2000;19:2812–2819. doi: 10.1038/sj.onc.1203609. [DOI] [PubMed] [Google Scholar]
- 178.Zhou H, Kuang J, Zhong L, Kuo WL, Gray JW, Sahin A, Brinkley BR, Sen S. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat Genet. 1998;20:189–193. doi: 10.1038/2496. [DOI] [PubMed] [Google Scholar]
- 179.Pugacheva EN, Golemis EA. HEF1-aurora A interactions: points of dialog between the cell cycle and cell attachment signaling networks. Cell Cycle. 2006;5:384–391. doi: 10.4161/cc.5.4.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fritz G, Kaina B. Rho GTPases: promising cellular targets for novel anticancer drugs. Curr Cancer Drug Targets. 2006;6:1–14. [PubMed] [Google Scholar]
- 181.Glotzer M. Cytokinesis: progress on all fronts. Curr Opin Cell Biol. 2003;15:684–690. doi: 10.1016/j.ceb.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 182.Singla V, Reiter JF. The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science. 2006;313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- 183.Plotnikova OV, Golemis EA, Pugacheva EN. Cell cycle-dependent ciliogenesis and cancer. Cancer Res. 2008;68:2058–2061. doi: 10.1158/0008-5472.CAN-07-5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Roodman GD. Advances in bone biology: the osteoclast. Endocr Rev. 1996;17:308–332. doi: 10.1210/edrv-17-4-308. [DOI] [PubMed] [Google Scholar]
- 185.Duong LT, Lakkakorpi PT, Nakamura I, Machwate M, Nagy RM, Rodan GA. PYK2 in osteoclasts is an adhesion kinase, localized in the sealing zone, activated by ligation of alpha(v)beta3 integrin, and phosphorylated by src kinase. J Clin Invest. 1998;102:881–892. doi: 10.1172/JCI3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lakkakorpi PT, Nakamura I, Nagy RM, Parsons JT, Rodan GA, Duong LT. Stable association of PYK2 and p130(Cas) in osteoclasts and their co-localization in the sealing zone. J Biol Chem. 1999;274:4900–4907. doi: 10.1074/jbc.274.8.4900. [DOI] [PubMed] [Google Scholar]
- 187.Nakamura I, Kadono Y, Takayanagi H, Jimi E, Miyazaki T, Oda H, Nakamura K, Tanaka S, Rodan GA, Duong le T. IL-1 regulates cytoskeletal organization in osteoclasts via TNF receptor-associated factor 6/c-Src complex. J Immunol. 2002;168:5103–5109. doi: 10.4049/jimmunol.168.10.5103. [DOI] [PubMed] [Google Scholar]
- 188.Duong LT, Nakamura I, Lakkakorpi PT, Lipfert L, Bett AJ, Rodan GA. Inhibition of osteoclast function by adenovirus expressing antisense protein-tyrosine kinase 2. J Biol Chem. 2001;276:7484–7492. doi: 10.1074/jbc.M008368200. [DOI] [PubMed] [Google Scholar]
- 189.Chellaiah MA, Biswas RS, Yuen D, Alvarez UM, Hruska KA. Phosphatidylinositol 3, 4, 5-trisphosphate directs association of Src homology 2-containing signaling proteins with gelsolin. J Biol Chem. 2001;276:47434–47444. doi: 10.1074/jbc.M107494200. [DOI] [PubMed] [Google Scholar]
- 190.Zhang Z, Neff L, Bothwell AL, Baron R, Horne WC. Calcitonin induces dephosphorylation of Pyk2 and phosphorylation of focal adhesion kinase in osteoclasts. Bone. 2002;31:359–365. doi: 10.1016/s8756-3282(02)00834-7. [DOI] [PubMed] [Google Scholar]
- 191.Weidow CL, Black DS, Bliska JB, Bouton AH. CAS/Crk signalling mediates uptake of Yersinia into human epithelial cells. Cell Microbiol. 2000;2:549–560. doi: 10.1046/j.1462-5822.2000.00079.x. [DOI] [PubMed] [Google Scholar]
- 192.Shi J, Casanova JE. Invasion of host cells by Salmonella typhimurium requires focal adhesion kinase and p130Cas. Mol Biol Cell. 2006;17:4698–4708. doi: 10.1091/mbc.E06-06-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Halle M, Gomez MA, Stuible M, Shimizu H, McMaster WR, Olivier M, Tremblay ML. The Leishmania surface protease GP63 cleaves multiple intracellular proteins and actively participates in p38 mitogen-activated protein kinase inactivation. J Biol Chem. 2009;284:6893–6908. doi: 10.1074/jbc.M805861200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gower TL, Peeples ME, Collins PL, Graham BS. RhoA is activated during respiratory syncytial virus infection. Virology. 2001;283:188–196. doi: 10.1006/viro.2001.0891. [DOI] [PubMed] [Google Scholar]
- 195.Li E, Stupack DG, Brown SL, Klemke R, Schlaepfer DD, Nemerow GR. Association of p130CAS with phosphatidylinositol-3-OH kinase mediates adenovirus cell entry. J Biol Chem. 2000;275:14729–14735. doi: 10.1074/jbc.275.19.14729. [DOI] [PubMed] [Google Scholar]
- 196.Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 197.Kovacic-Milivojevic B, Damsky CC, Gardner DG, Ilic D. Requirements for the localization of p130 Cas to Z-lines in cardiac myocytes. Cell Mol Biol Lett. 2002;7:323–329. [PubMed] [Google Scholar]
- 198.Beaty TH, Fallin MD, Hetmanski JB, McIntosh I, Chong SS, Ingersoll R, Sheng X, Chakraborty R, Scott AF. Haplotype diversity in 11 candidate genes across four populations. Genetics. 2005;171:259–267. doi: 10.1534/genetics.105.043075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Toriello HV. Are the oral-facial-digital syndromes ciliopathies? Am J Med Genet A. 2009;149A:1089–1095. doi: 10.1002/ajmg.a.32799. [DOI] [PubMed] [Google Scholar]
- 200.Huang Z, Yazdani U, Thompson-Peer KL, Kolodkin AL, Terman JR. Crk-associated substrate (Cas) signaling protein functions with integrins to specify axon guidance during development. Development. 2007;134:2337–2347. doi: 10.1242/dev.004242. [DOI] [PubMed] [Google Scholar]
- 201.Burnham MR, Harte MT, Richardson A, Parsons JT, Bouton AH. The identification of p130cas-binding proteins and their role in cellular transformation. Oncogene. 1996;12:2467–2472. [PubMed] [Google Scholar]
- 202.Iwahara T, Akagi T, Fujitsuka Y, Hanafusa H. CrkII regulates focal adhesion kinase activation by making a complex with Crk-associated substrate, p130Cas. Proc Natl Acad Sci USA. 2004;101:17693–17698. doi: 10.1073/pnas.0408413102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sakai R, Nakamoto T, Ozawa K, Aizawa S, Hirai H. Characterization of the kinase activity essential for tyrosine phosphorylation of p130Cas in fibroblasts. Oncogene. 1997;14:1419–1426. doi: 10.1038/sj.onc.1200954. [DOI] [PubMed] [Google Scholar]
- 204.Auvinen M, Paasinen-Sohns A, Hirai H, Andersson LC, Holtta E. Ornithine decarboxylase- and ras-induced cell transformations: reversal by protein tyrosine kinase inhibitors and role of pp130CAS. Mol Cell Biol. 1995;15:6513–6525. doi: 10.1128/mcb.15.12.6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.de Cremoux P, Tran-Perennou C, Brockdorff BL, Boudou E, Brunner N, Magdelenat H, Lykkesfeldt AE. Validation of real-time RT-PCR for analysis of human breast cancer cell lines resistant or sensitive to treatment with antiestrogens. Endocr Relat Cancer. 2003;10:409–418. doi: 10.1677/erc.0.0100409. [DOI] [PubMed] [Google Scholar]
- 206.Dorssers LC, Grebenchtchikov N, Brinkman A, Look MP, van Broekhoven SP, de Jong D, Peters HA, Portengen H, Meijer-van Gelder ME, Klijn JG, van Tienoven DT, Geurts-Moespot A, Span PN, Foekens JA, Sweep FC. The prognostic value of BCAR1 in patients with primary breast cancer. Clin Cancer Res. 2004;10:6194–6202. doi: 10.1158/1078-0432.CCR-04-0444. [DOI] [PubMed] [Google Scholar]
- 207.Near RI, Zhang Y, Makkinje A, Vanden Borre P, Lerner A. AND-34/BCAR3 differs from other NSP homologs in induction of anti-estrogen resistance, cyclin D1 promoter activation and altered breast cancer cell morphology. J Cell Physiol. 2007;212:655–665. doi: 10.1002/jcp.21059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Schrecengost RS, Riggins RB, Thomas KS, Guerrero MS, Bouton AH. Breast cancer antiestrogen resistance-3 expression regulates breast cancer cell migration through promotion of p130Cas membrane localization and membrane ruffling. Cancer Res. 2007;67:6174–6182. doi: 10.1158/0008-5472.CAN-06-3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Soni S, Lin BT, August A, Nicholson RI, Kirsch KH. Expression of a phosphorylated p130(Cas) substrate domain attenuates the phosphatidylinositol 3-kinase/Akt survival pathway in tamoxifen resistant breast cancer cells. J Cell Biochem. 2009;107:364–375. doi: 10.1002/jcb.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.van Agthoven T, Sieuwerts AM, Meijer-van Gelder ME, Look MP, Smid M, Veldscholte J, Sleijfer S, Foekens JA, Dorssers LC. Relevance of breast cancer antiestrogen resistance genes in human breast cancer progression and tamoxifen resistance. J Clin Oncol. 2009;27:542–549. doi: 10.1200/JCO.2008.17.1462. [DOI] [PubMed] [Google Scholar]
- 211.Konstantinovsky S, Smith Y, Zilber S, Tuft Stavnes H, Becker AM, Nesland JM, Reich R, Davidson B. Breast carcinoma cells in primary tumors and effusions have different gene array profiles. J Oncol. 2010;2010:969084. doi: 10.1155/2010/969084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Scibelli A, d’Angelo D, Pelagalli A, Tafuri S, Avallone L, Della Morte R, Staiano N. Expression levels of the focal adhesion-associated proteins paxillin and p130CAS in canine and feline mammary tumors. Vet Res. 2003;34:193–202. doi: 10.1051/vetres:2002066. [DOI] [PubMed] [Google Scholar]
- 213.Spencer KS, Graus-Porta D, Leng J, Hynes NE, Klemke RL. ErbB2 is necessary for induction of carcinoma cell invasion by ErbB family receptor tyrosine kinases. J Cell Biol. 2000;148:385–397. doi: 10.1083/jcb.148.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Riggins RB, Quilliam LA, Bouton AH. Synergistic promotion of c-Src activation and cell migration by Cas and AND-34/BCAR3. J Biol Chem. 2003;278:28264–28273. doi: 10.1074/jbc.M303535200. [DOI] [PubMed] [Google Scholar]
- 215.Vultur A, Buettner R, Kowolik C, Liang W, Smith D, Boschelli F, Jove R. SKI-606 (bosutinib), a novel Src kinase inhibitor, suppresses migration and invasion of human breast cancer cells. Mol Cancer Ther. 2008;7:1185–1194. doi: 10.1158/1535-7163.MCT-08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massague J. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nagashima T, Oyama M, Kozuka-Hata H, Yumoto N, Sakaki Y, Hatakeyama M. Phosphoproteome and transcriptome analyses of ErbB ligand-stimulated MCF-7 cells. Cancer Genomics Proteomics. 2008;5:161–168. [PubMed] [Google Scholar]
- 218.Giampieri S, Manning C, Hooper S, Jones L, Hill CS, Sahai E (2009) Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol (in press) [DOI] [PMC free article] [PubMed]
- 219.Tamura M, Gu J, Takino T, Yamada KM. Tumor suppressor PTEN inhibition of cell invasion, migration, and growth: differential involvement of focal adhesion kinase and p130Cas. Cancer Res. 1999;59:442–449. [PubMed] [Google Scholar]
- 220.Frankel P, Pellet-Many C, Lehtolainen P, D’Abaco GM, Tickner ML, Cheng L, Zachary IC. Chondroitin sulphate-modified neuropilin 1 is expressed in human tumour cells and modulates 3D invasion in the U87MG human glioblastoma cell line through a p130Cas-mediated pathway. EMBO Rep. 2008;9:983–989. doi: 10.1038/embor.2008.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Seo S, Ichikawa M, Kurokawa M. Structure and function of cas-L and integrin-mediated signaling. Crit Rev Immunol. 2006;26:391–406. doi: 10.1615/critrevimmunol.v26.i5.20. [DOI] [PubMed] [Google Scholar]
- 222.Iwata S, Kobayashi H, Miyake-Nishijima R, Sasaki T, Souta-Kuribara A, Nori M, Hosono O, Kawasaki H, Tanaka H, Morimoto C. Distinctive signaling pathways through CD82 and beta1 integrins in human T cells. Eur J Immunol. 2002;32:1328–1337. doi: 10.1002/1521-4141(200205)32:5<1328::AID-IMMU1328>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 223.de Jong R, van Wijk A, Haataja L, Heisterkamp N, Groffen J. BCR/ABL-induced leukemogenesis causes phosphorylation of Hef1 and its association with Crkl. J Biol Chem. 1997;272:32649–32655. doi: 10.1074/jbc.272.51.32649. [DOI] [PubMed] [Google Scholar]
- 224.Cho YJ, Hemmeryckx B, Groffen J, Heisterkamp N. Interaction of Bcr/Abl with C3G, an exchange factor for the small GTPase Rap1, through the adapter protein Crkl. Biochem Biophys Res Commun. 2005;333:1276–1283. doi: 10.1016/j.bbrc.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 225.Ambrogio C, Voena C, Manazza AD, Piva R, Riera L, Barberis L, Costa C, Tarone G, Defilippi P, Hirsch E, Boeri Erba E, Mohammed S, Jensen ON, Palestro G, Inghirami G, Chiarle R. p130Cas mediates the transforming properties of the anaplastic lymphoma kinase. Blood. 2005;106:3907–3916. doi: 10.1182/blood-2005-03-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Petti F, Thelemann A, Kahler J, McCormack S, Castaldo L, Hunt T, Nuwaysir L, Zeiske L, Haack H, Sullivan L, Garton A, Haley JD. Temporal quantitation of mutant Kit tyrosine kinase signaling attenuated by a novel thiophene kinase inhibitor OSI-930. Mol Cancer Ther. 2005;4:1186–1197. doi: 10.1158/1535-7163.MCT-05-0114. [DOI] [PubMed] [Google Scholar]
- 227.Moore SR, Persons DL, Sosman JA, Bobadilla D, Bedell V, Smith DD, Wolman SR, Tuthill RJ, Moon J, Sondak VK, Slovak ML. Detection of copy number alterations in metastatic melanoma by a DNA fluorescence in situ hybridization probe panel and array comparative genomic hybridization: a southwest oncology group study (S9431) Clin Cancer Res. 2008;14:2927–2935. doi: 10.1158/1078-0432.CCR-07-4068. [DOI] [PubMed] [Google Scholar]
- 228.Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S, Sahai E, Marshall CJ. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135:510–523. doi: 10.1016/j.cell.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 229.Eisenmann KM, McCarthy JB, Simpson MA, Keely PJ, Guan JL, Tachibana K, Lim L, Manser E, Furcht LT, Iida J. Melanoma chondroitin sulphate proteoglycan regulates cell spreading through Cdc42, Ack-1 and p130cas. Nat Cell Biol. 1999;1:507–513. doi: 10.1038/70302. [DOI] [PubMed] [Google Scholar]
- 230.Chang CC, Campoli M, Luo W, Zhao W, Zaenker KS, Ferrone S. Immunotherapy of melanoma targeting human high molecular weight melanoma-associated antigen: potential role of nonimmunological mechanisms. Ann N Y Acad Sci. 2004;1028:340–350. doi: 10.1196/annals.1322.040. [DOI] [PubMed] [Google Scholar]
- 231.Hamamura K, Furukawa K, Hayashi T, Hattori T, Nakano J, Nakashima H, Okuda T, Mizutani H, Hattori H, Ueda M, Urano T, Lloyd KO. Ganglioside GD3 promotes cell growth and invasion through p130Cas and paxillin in malignant melanoma cells. Proc Natl Acad Sci USA. 2005;102:11041–11046. doi: 10.1073/pnas.0503658102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hamamura K, Tsuji M, Ohkawa Y, Nakashima H, Miyazaki S, Urano T, Yamamoto N, Ueda M, Furukawa K. Focal adhesion kinase as well as p130Cas and paxillin is crucially involved in the enhanced malignant properties under expression of ganglioside GD3 in melanoma cells. Biochim Biophys Acta. 2008;1780:513–519. doi: 10.1016/j.bbagen.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 233.Ohkawa Y, Miyazaki S, Miyata M, Hamamura K, Furukawa K. Essential roles of integrin-mediated signaling for the enhancement of malignant properties of melanomas based on the expression of GD3. Biochem Biophys Res Commun. 2008;373:14–19. doi: 10.1016/j.bbrc.2008.05.149. [DOI] [PubMed] [Google Scholar]
- 234.Nakashima H, Hamamura K, Houjou T, Taguchi R, Yamamoto N, Mitsudo K, Tohnai I, Ueda M, Urano T, Furukawa K. Overexpression of caveolin-1 in a human melanoma cell line results in dispersion of ganglioside GD3 from lipid rafts and alteration of leading edges, leading to attenuation of malignant properties. Cancer Sci. 2007;98:512–520. doi: 10.1111/j.1349-7006.2007.00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Fromont G, Vallancien G, Validire P, Levillain P, Cussenot O. BCAR1 expression in prostate cancer: association with 16q23 LOH status, tumor progression and EGFR/KAI1 staining. Prostate. 2007;67:268–273. doi: 10.1002/pros.20516. [DOI] [PubMed] [Google Scholar]
- 236.Zhang XA, He B, Zhou B, Liu L. Requirement of the p130CAS-Crk coupling for metastasis suppressor KAI1/CD82-mediated inhibition of cell migration. J Biol Chem. 2003;278:27319–27328. doi: 10.1074/jbc.M303039200. [DOI] [PubMed] [Google Scholar]
- 237.Guo C, Liu QG, Yang W, Zhang ZL, Yao YM. Relation among p130Cas, E-cadherin and beta-catenin expression, clinicopathologic significance and prognosis in human hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2008;7:490–496. [PubMed] [Google Scholar]
- 238.Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, Kozlowski P, Torrice C, Wu MC, Shimamura T, Perera SA, Liang MC, Cai D, Naumov GN, Bao L, Contreras CM, Li D, Chen L, Krishnamurthy J, Koivunen J, Chirieac LR, Padera RF, Bronson RT, Lindeman NI, Christiani DC, Lin X, Shapiro GI, Janne PA, Johnson BE, Meyerson M, Kwiatkowski DJ, Castrillon DH, Bardeesy N, Sharpless NE, Wong KK. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 239.Zhang PJ, Weber R, Liang HH, Pasha TL, LiVolsi VA. Growth factors and receptors in juvenile nasopharyngeal angiofibroma and nasal polyps: an immunohistochemical study. Arch Pathol Lab Med. 2003;127:1480–1484. doi: 10.5858/2003-127-1480-GFARIJ. [DOI] [PubMed] [Google Scholar]
- 240.Casanova I, Parreno M, Farre L, Guerrero S, Cespedes MV, Pavon MA, Sancho FJ, Marcuello E, Trias M, Mangues R. Celecoxib induces anoikis in human colon carcinoma cells associated with the deregulation of focal adhesions and nuclear translocation of p130Cas. Int J Cancer. 2006;118:2381–2389. doi: 10.1002/ijc.21662. [DOI] [PubMed] [Google Scholar]
- 241.Seidl C, Port M, Apostolidis C, Bruchertseifer F, Schwaiger M, Senekowitsch-Schmidtke R, Abend M (2009) Differential gene expression triggered by highly cytotoxic alpha-Emitter-immunoconjugates in gastric cancer cells. Invest New Drugs (in press) [DOI] [PubMed]
- 242.Beinke C, Van Beuningen D, Cordes N. Ionizing radiation modules of the expression and tyrosine phosphorylation of the focal adhesion-associated proteins focal adhesion kinase (FAK) and its substrates p130cas and paxillin in A549 human lung carcinoma cells in vitro. Int J Radiat Biol. 2003;79:721–731. doi: 10.1080/09553000310001610231. [DOI] [PubMed] [Google Scholar]
- 243.Seidler J, Durzok R, Brakebusch C, Cordes N. Interactions of the integrin subunit beta1A with protein kinase B/Akt, p130Cas and paxillin contribute to regulation of radiation survival. Radiother Oncol. 2005;76:129–134. doi: 10.1016/j.radonc.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 244.Miyake-Nishijima R, Iwata S, Saijo S, Kobayashi H, Kobayashi S, Souta-Kuribara A, Hosono O, Kawasaki H, Tanaka H, Ikeda E, Okada Y, Iwakura Y, Morimoto C. Role of Crk-associated substrate lymphocyte type in the pathophysiology of rheumatoid arthritis in tax transgenic mice and in humans. Arthritis Rheum. 2003;48:1890–1900. doi: 10.1002/art.11047. [DOI] [PubMed] [Google Scholar]
- 245.Chapuis J, Moisan F, Mellick G, Elbaz A, Silburn P, Pasquier F, Hannequin D, Lendon C, Campion D, Amouyel P, Lambert JC. Association study of the NEDD9 gene with the risk of developing Alzheimer’s and Parkinson’s disease. Hum Mol Genet. 2008;17:2863–2867. doi: 10.1093/hmg/ddn183. [DOI] [PubMed] [Google Scholar]
- 246.Li Y, Grupe A, Rowland C, Holmans P, Segurado R, Abraham R, Jones L, Catanese J, Ross D, Mayo K, Martinez M, Hollingworth P, Goate A, Cairns NJ, Racette BA, Perlmutter JS, O’Donovan MC, Morris JC, Brayne C, Rubinsztein DC, Lovestone S, Thal LJ, Owen MJ, Williams J. Evidence that common variation in NEDD9 is associated with susceptibility to late-onset Alzheimer’s and Parkinson’s disease. Hum Mol Genet. 2008;17:759–767. doi: 10.1093/hmg/ddm348. [DOI] [PubMed] [Google Scholar]
- 247.Chouery E, Delague V, Bergougnoux A, Koussa S, Serre JL, Megarbane A. Mutations in TREM2 lead to pure early-onset dementia without bone cysts. Hum Mutat. 2008;29:E194–E204. doi: 10.1002/humu.20836. [DOI] [PubMed] [Google Scholar]
- 248.Singh M, Cowell L, Seo S, O’Neill G, Golemis E. Molecular basis for HEF1/NEDD9/Cas-L action as a multifunctional co-ordinator of invasion, apoptosis and cell cycle. Cell Biochem Biophys. 2007;48:54–72. doi: 10.1007/s12013-007-0036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]