Abstract
Objective
To determine if the relationship between obesity and usage of colorectal cancer (CRC) screening in women varies when stratifying by race.
Methods
Using nationally representative data from the 2005 National Health Interview Survey, we examined the relationship between obesity and CRC screening for white and African-American women aged 50 and older. Screening usage variables indicated if a woman was up-to-date for any CRC screening test, colonoscopy, or FOBT. We used multivariable logistic regression models that included interaction terms to determine if race moderates the obesity-screening relationship. We also calculated adjusted up-to-date colonoscopy rates using direct standardization to model covariates.
Results
The relationship between obesity and screening differed by race for any CRC screening test (P = 0.04 for interaction) and for colonoscopy (P = 0.01 for interaction), but not for FOBT. Obese white women had a lower adjusted colonoscopy rate (30.2%, 95% CI 25.9–34.8) than non-obese white women (39.1%, 95% CI 36.1–42.2). Obese African-American women, on the other hand, had a higher adjusted colonoscopy rate (41.2%, 95% CI 31.6–51.4) than their non-obese counterparts (35.6%, 95% CI 28.3–43.6). Overall, adjusted colonoscopy rates were lowest among obese white women.
Conclusions
Obesity is associated with lower CRC screening rates in white, but not African-American women.
Keywords: Colorectal neoplasms, Early detection of cancer, Obesity, Women’s health, Minority health
Introduction
Among women in the Unites States, colorectal cancer (CRC) accounts for approximately 10% of all cancers diagnosed annually and was responsible for an estimated 25,700 deaths in 2008, making it the third leading cause of cancer mortality [1]. Regular screening for CRC and adenomatous polyps starting at age 50 has the potential to significantly reduce CRC incidence and mortality [2, 3]. Despite their proven effectiveness, utilization of CRC screening tests remains sub-optimal, especially when compared with screening for other cancers, like breast or cervical cancer [4-6]. Among women under age 65, over 85% are up-to-date with cervical cancer screening recommendations and over 58% with breast cancer screening [7], but only 47.4% are meeting CRC screening recommendations [8]. Many barriers to CRC screening have been identified, including lack of physician recommendation for screening [9], lack of knowledge about testing options and intervals [10], and embarrassment about the testing procedure [11].
In addition, some research indicates that obesity may be a barrier to CRC screening in women [12-15]. Obesity is a prevalent condition in the United States, particularly among older women; it is estimated that more than 31% of women in the United States over age 60 are obese [16]. There is a wide body of research showing a positive relationship between body mass index (BMI) and both colon cancer incidence and mortality. One study found that morbidly obese women (BMI ≥ 40) were 49% more likely to get colon cancer than normal weight women [17]. It is also estimated that obese women (BMI ≥ 30) have a 40–85% greater chance of dying from colon cancer than normal weight individuals (BMI<25) [18]. Because obese women are at high risk for colon cancer, failure to screen for CRC in this population could result in substantial morbidity and mortality.
In order to decrease CRC risk among obese women, it is important to understand if their weight is a barrier to receiving a screening that could potentially prevent them from ever getting CRC. Several studies have investigated the relationship between CRC screening and bodyweight, but the results have been mixed [19]. Two studies that used data from the Behavioral Risk Factor Surveillance Survey (BRFSS) showed that as weight increased the likelihood that an individual was adherent to CRC screening recommendations decreased for women, but not for men [12, 14]. A medical record review of 22 medical practices found that both obese men and women had significantly lower rates of screening than their non-obese counterparts [15]. Research on the Cancer Prevention II Nutrition Cohort, a predominately white (97%) study population, found that, compared to normal weight women, overweight, obese, and morbidly obese women were all significantly less likely to have had a flexible sigmoidoscopy or colonoscopy [20, 21]. However, three other studies have concluded that there was no statistically significant relationship between screening and weight [22-24]. With the exception of Nutrition Cohort Study, the afore-mentioned studies included a similar proportion of African-Americans as is found in the U.S. population (range 9–21%). Despite this none of them stratified by race and gender, which could explain the mixed results. Studies on screening usage for other cancer types suggest that stratifying by race and gender may be important. For example, there is reasonable evidence to suggest that obesity is associated with lower breast and cervical cancer screening rates in white women, but not for other races/ethnic groups [25-27]. A similar pattern may also exist for women with regard to CRC screening.
The goal of this study is to determine whether or not race moderates the relationship between CRC screening and obesity in women. We hypothesize that, as was shown for breast and cervical cancer screening, there may be a previously unrecognized interaction between obesity and race that affects CRC screening rates. If this interaction exists, it could explain why previous research examining the relationship between CRC screening and obesity in women has been inconsistent.
Methods
Study sample
This analysis uses nationally representative data from the 2005 National Health Interview Survey (NHIS), which has one of the most comprehensive screening questionnaires of any national survey [28]. NHIS is a multi-purpose health survey conducted by the Centers for Disease Control and Prevention’s National Center for Health Statistics and is the principal source of information on the health of the civilian, non-institutionalized, household population of the United States. The survey collects a variety of health-related information including socio-demographic characteristics, basic indicators of health status, health insurance coverage, access to and utilization of health care services, medical conditions and history, and health behaviors.
Detailed information on survey and sampling methods for NHIS can be found elsewhere [28]. Briefly, NHIS uses a multistage sample design and one adult per family is randomly selected for an in-person interview by Census interviewers. In 2005, the interviewed sample included 31,428 persons 18 years of age and older. The final response rate for adults was 69.0% [29], which is comparable to other national surveys. For this analysis, individuals were excluded if they were under age 50, reported a race other than white/Caucasian or black/African-American, or if they had missing information on weight, CRC screening, or any relevant covariates.
Measures
Colorectal cancer screening
The primary dependent variable for this analysis was a dichotomous variable Any CRC Screening indicating whether or not a respondent reported being up-to-date with CRC screening. We classified an individual as being up-to-date with CRC screening if he or she reported having a fecal occult blood test (FOBT) within the year prior to the interview, flexible sigmoidoscopy (flex sig) within the past 5 years, or colonoscopy within the past 10 years. In addition to the primary screening variable, we looked separately at whether a respondent reported being up-to-date for each of the following individual screening modalities: colonoscopy, any endoscopy (flex sig or colonoscopy) and FOBT. For example, for the colonoscopy outcome, we only looked at whether or not an individual reported having a colonoscopy in the past 10 years and did not include any other screening modalities.
The screening variables were created based upon responses to the following survey questions about CRC screening test completion (1) “The following questions are about the blood stool or occult blood test, a test to determine whether you have blood in your stool or bowel movement. The blood stool test can be done at home using a kit. You smear a small amount of stool on cards at home and send the cards back to the doctor or lab. Have you EVER HAD a blood stool test, using a HOME test kit?” (2) “Have you EVER HAD a sigmoidoscopy, colonoscopy, or proctoscopy? These are exams in which a health care professional inserts a tube into the rectum to look for signs of cancer or other problems.” For participants who answered yes to one or both of these questions, follow-up questions ascertained exactly which test the respondent had and the approximate date of their last test. Data on a particular test was coded as missing if the individual answered “don’t know” or refused the question. If data on either endoscopy or FOBT was missing for a respondent, then the data was coded as missing for primary CRC screening variable. Screening data were available for 86% of female respondents aged 50 and older.
Data were only collected for the respondent’s most recent endoscopic test (colonoscopy or flexible sigmoidoscopy) and her most recent FOBT. For these tests, respondents were asked if the test was “part of a routine exam,” “because of a problem,” or for an “other reason.” We completed analyses both including and excluding tests that were not reported to be part of a routine examine (i.e., diagnostic vs. screening). Although screening rates across the board were slightly higher when diagnostic tests were included, the trends remained the same. For the results presented in this paper, diagnostic tests are included in the screening rate estimates, since a test administered for diagnostic purposes still “counts” toward whether or not an individual is considered up-to-date with CRC screening guidelines.
Obesity
The primary independent variable for this analysis was the dichotomous variable obesity status (i.e., obese vs. non-obese). We calculated each respondent’s BMI as weight in kilograms divided by height in meters squared based on responses to self-reported questions on height and weight. For the dichotomous obesity status variable, we classified individuals as either obese (BMI ≥ 30) or non-obese (BMI 18.5–29.9). Because it was not clear whether the relationship between BMI and CRC screening would exhibit a threshold or graded pattern, we also examined results for trends using a BMI group variable. For the BMI group variable, individuals were classified as underweight (BMI<18.5), normal weight (BMI 18.5–24.9), overweight (BMI 25.0–29.9), obese I (BMI 30.0–34.9), obese II (BMI 35.0–59.9) or obese III (BMI ≥ 40).
Race/ethnicity
The original NHIS race variable included 6 possible categories: White only, Black/African-American, American Indian/Alaskan Native, Asian only, race group not releasable, and multiple races. We converted race to a dichotomous variable, using only the first two racial groups (White and African-American); all other categories were excluded from the analysis. These two racial sub-groups were examined because they have the highest CRC incidence rates of any racial sub-group [30]. We coded ethnicity as a dichotomous variable (Hispanic vs. Non-Hispanic) based on participants’ responses to the question “[Do you] consider [yourself] Hispanic/Latino?” Since there were no differences in the proportions of Hispanics in each of the race and obesity categories, we did not stratify analyses by ethnicity. Additionally, separate analyses completed excluding Hispanics showed similar trends, thus we retained them in the final analyses.
Other covariates
Several potential demographic, behavioral, and healthcare-related confounders were included in the analyses because previous literature indicated that they might be associated with CRC screening. Sociodemographic variables examined included age, education, and marital status. Due to the high percentage of missing data for personal income (64%), NHIS created an imputed income variable using a variety of sociodemographic indicators [31]. The original imputed income variable had 11 categories, however, for this analysis we collapsed it into 4 categories ($0–$24,999, $25,000–$54,999, $55,000–$74,999, and $75,000 and up). Behavioral variables included smoking status (daily smoker, occasional smoker, former smoker or never a smoker), alcohol usage (never, former drinker, light drinker, or moderate/heavy drinker) and recreational physical activity. Physical activity was expressed as MET (metabolic equivalents) minutes per week. This figure was calculated as (3 METs × moderate/light minutes/week) + (7 METS × vigorous minutes/week). Recreational activity was then categorized as no activity/unable to exercise (0 METS), some activity (1–<675 MET minutes/week) or meets/exceeds the surgeon general’s recommendation (≥675 MET minutes/week) [32]. We coded both reported physician recommendation for screening (either FOBT or any endoscopic test) and insurance coverage as dichotomous (yes/no) variables. Number of visits to a health care provider was ascertained using the question “During the past 12 months, how many times have you seen a doctor or other health care professional about your own health at a doctor’s office, clinic or some other place? Do not include times that you were hospitalized overnight, visits to hospital emergency rooms, home visits, dental visits or telephone calls.” A past-year visits variable was then created using the following categories: 0, 1, 2–3, 4–5, 6 or more. Participants were also asked about co-morbidities: “Have you EVER been told by a doctor or other health professional that you had [condition]?” We used a sum of all yes answers for the following conditions to determine the number of co-morbidities: hypertension, myocardial infarction, coronary heart disease, other heart disease, emphysema, stroke, asthma, any kind of cancer, ulcer, diabetes or arthritis. Number of co-morbidities was then categorized as 0, 1, 2–3, 4–5 and 6 or more.
Statistical analyses
All analyses were conducted using SAS v9.1.3 to account for the multistage sampling structure used by NHIS (Procedures: SURVEYFREQ, SURVEYMEAN, SURVEYLOGISTIC). We limited all analyses to women aged 50 and older, since this is the age at which screening is first recommended for normal risk individuals. Additionally, we used NHIS sampling weights for all analyses to create U.S. population estimates. We used Rao-Scott chi-square [33] to test for relationships between any CRC screening (up-to-date or not) and each of the covariates for both races and then separately for white and African-American women. Rao-Scott chi-square tests were also used to examine the relationship between obesity status (obese vs. non-obese) and all covariates.
All covariates found to be associated (P<0.1) with either screening or obesity status were entered into a multivariable logistic regression model with screening as the dependent variable. We created four separate regression models for each of the screening variables (Any CRC screening, colonoscopy, endoscopy and FOBT) to test the significance of the interaction term (obesity status × race) while controlling for possible confounders. A step-wise elimination procedure was used to create a logistic regression model of the relationship between each screening outcome variable and the interaction term while holding the race and obesity status variables constant in the model. We eliminated potential confounders if they had a P-value>0.1 and did not change the estimate of the interaction term by more than 10%. The regression model was also run using the BMI group variable and results were found to be similar, thus for simplicity further analyses were performed using the obesity variable.
We calculated adjusted odds ratios of the relationship between obesity and colonoscopy, stratified by race, using multivariable logistic regression to control for confounders. Both unadjusted and adjusted screening rates, including 95% confidence intervals, are reported for selected sub-groups. Adjusted colonoscopy rates for each weight and race group were calculated by direct standardization to the demographic characteristics of the study population using the coefficients from the multivariable model [34].
Results
Descriptive statistics
Since NHIS is designed to be nationally representative, demographic characteristics of this population should reflect those of White and African-American women aged 50 and older in the United States (Table 1). The average age of this population is 64.5 (95% CI 64.2–64.8), and the mean BMI is 32.0 (95% CI 31.5–32.5). Overall, 28.3% (95% CI 27.1–29.6) of women were obese, and 51.7% (95% CI 50.1–52.9) were up-to-date with any CRC screening. Obesity was more prevalent in African-American women than in white women (33.0% vs. 24.9%, P<0.0001). All measures capturing screening rates were significantly higher for white women (Table 1). Using combined data from all women, a statistically significant non-linear relationship was seen between the primary CRC screening variable and the following variables: age, marital status, alcohol usage, and smoking status (Table 2). Women aged 70–79, married women, light drinkers and former smokers had the highest screening rates in their respective categories. There was also significant positive association between screening and the following variables: reported physician recommendation for screening, past-year healthcare visits, number of co-morbidities and recreational physical activity. We did not find any difference in reported rate of physician recommendation for screening between obese and non-obese women overall or when stratifying by race (data not shown). Additionally, there were no statistically significant differences in CRC screening rates between insured and uninsured women (50.8% vs. 51.8%, P = 0.6).
Table 1.
Characteristics of White and African-American women aged 50 and older in the U.S
| White and African-American (n = 7,469) (%) |
White only (n = 6,459) (%) |
African-American only (n = 1,010) (%) |
P-value* | |
|---|---|---|---|---|
| Screening | ||||
| Up-to-date for Any CRC Screening |
51.7 | 52.5 | 44.8 | 0.0004 |
| Up-to-date Colonoscopy | 42.3 | 43.0 | 36.1 | 0.002 |
| Up-to-date Endoscopy | 45.3 | 46.0 | 39.2 | 0.002 |
| Up-to-date FOBT | 15.4 | 15.7 | 12.7 | 0.046 |
| Hispanic/Latino origin | 23.9 | 23.8 | 25.4 | 0.34 |
| Age | ||||
| 50–59 | 40.9 | 40.3 | 46.4 | 0.001 |
| 60–69 | 26.9 | 26.9 | 26.9 | |
| 70–79 | 19.2 | 19.4 | 17.5 | |
| 80+ | 13.0 | 13.5 | 9.2 | |
| BMI Group | ||||
| Underweight | 2.1 | 2.3 | 1.1 | <0.0001 |
| Normal weight | 37.3 | 39.1 | 21.7 | |
| Overweight | 32.8 | 32.3 | 37.4 | |
| Obese I | 16.6 | 16.1 | 21.0 | |
| Obese II | 6.7 | 6.3 | 10.1 | |
| Obese III | 4.4 | 3.9 | 8.6 | |
| Marital status | ||||
| Married/living w/partner | 56.4 | 59.0 | 34.0 | <0.0001 |
| Divorced/separated | 15.3 | 13.9 | 26.8 | |
| Never married | 4.7 | 3.9 | 10.9 | |
| Widowed | 23.7 | 23.1 | 28.2 | |
| Education | ||||
| Less than high school | 37.4 | 36.9 | 38.5 | 0.46 |
| High school/GED | 22.2 | 22.5 | 20.1 | |
| Some college/associates | 22.2 | 22.1 | 22.8 | |
| College degree | 12.8 | 12.7 | 13.9 | |
| Graduate/professional | 5.7 | 5.8 | 4.7 | |
| Personal income | ||||
| $0–$24,999 | 46.5 | 46.7 | 44.4 | 0.26 |
| $25,000–$54,999 | 33.9 | 33.5 | 37.3 | |
| $55,000–$74,999 | 9.2 | 9.0 | 10.1 | |
| $75,000 and up | 10.4 | 10.7 | 8.1 | |
| Health insurance coverage | 83.7 | 83.6 | 84.5 | 0.52 |
| Past year medical visits | ||||
| 0 | 7.9 | 7.8 | 8.0 | 0.62 |
| 1 | 11.6 | 11.8 | 10.1 | |
| 2–3 | 24.8 | 24.7 | 25.2 | |
| 4–5 | 18.4 | 18.2 | 19.9 | |
| 6 or more | 37.4 | 37.5 | 36.7 | |
| Physician CRC screening recommendation |
56.4 | 58.6 | 50.4 | 0.0002 |
| Self-reported health status | ||||
| Excellent | 33.9 | 33.9 | 33.7 | 0.93 |
| Very good | 29.3 | 29.4 | 28.7 | |
| Good | 26.3 | 26.3 | 26.6 | |
| Fair | 7.6 | 7.6 | 7.5 | |
| Poor | 2.9 | 2.8 | 3.4 | |
| Co-morbidities | ||||
| 0 | 21.9 | 22.4 | 17.4 | 0.002 |
| 1 | 27.3 | 27.6 | 24.7 | |
| 2–3 | 36.1 | 35.5 | 40.6 | |
| 4–5 | 11.6 | 11.4 | 13.8 | |
| 6 or more | 3.1 | 3.0 | 3.6 | |
| Alcohol usage | ||||
| Never | 32.2 | 30.8 | 44.1 | <0.0001 |
| Former | 20.0 | 19.2 | 26.5 | |
| Light | 36.6 | 37.9 | 25.7 | |
| Moderate/heavy | 11.3 | 12.1 | 4.0 | |
| Smoking status | ||||
| Daily | 11.4 | 11.3 | 12.5 | <0.0001 |
| Occasional | 2.2 | 2.1 | 3.8 | |
| Former | 26.5 | 27.1 | 21.8 | |
| Never | 59.8 | 59.5 | 61.9 | |
| Recreational physical activity (MET minutes/week) | ||||
| None/unable to exercise | 47.5 | 45.6 | 63.6 | <0.0001 |
| <675 | 30.0 | 30.7 | 24.8 | |
| ≥675 | 22.5 | 23.7 | 11.7 | |
P value calculated using Rao-Scoot Chi-Square test of the relationship between race and the selected variable
Table 2.
CRC screening rates for White and African-American women aged 50 and older: percent up-to-date for screening by selected demographic and health characteristics
| White and African-American (n = 6,412) (%) |
White (n = 5,566) (%) |
African-American (n = 846) (%) |
|
|---|---|---|---|
| Ethnicity | |||
| Hispanic | 52.4 | 52.9 | 44.7 |
| Non-Hispanic | 51.5 | 52.1 | 44.5 |
| P = 0.58 | P = 0.66 | P = 0.96 | |
| Age | |||
| 50–59 | 42.5 | 43.0 | 37.7 |
| 60–69 | 58.2 | 58.2 | 56.1 |
| 70–79 | 60.4 | 61.3 | 49.8 |
| 80+ | 55.7 | 56.6 | 39.7 |
| P<0.0001 | P<0.0001 | P = 0.0009 | |
| BMI Group | |||
| Underweight | 50.5 | 52.4 | 18.8 |
| Normal weight | 51.9 | 52.8 | 37.5 |
| Overweight | 54.0 | 54.8 | 48.0 |
| Obese I | 50.4 | 50.7 | 48.3 |
| Obese II | 52.5 | 53.6 | 45.9 |
| Obese III | 48.7 | 49.2 | 46.8 |
| P = 0.52 | P = 0.54 | P = 0.27 | |
| Marital status | |||
| Married/living with partner | 52.9 | 53.3 | 46.9 |
| Divorced/separated | 48.6 | 48.6 | 48.8 |
| Never married | 44.4 | 47.4 | 35.8 |
| Widowed | 52.4 | 53.9 | 42.1 |
| P = 0.01 | P = 0.04 | P = 0.23 | |
| Education | |||
| Less than high school | 51.7 | 52.6 | 44.3 |
| High school/GED | 49.1 | 50.0 | 40.1 |
| Some college/associates | 52.5 | 52.7 | 50.6 |
| College degree | 55.8 | 58.0 | 39.6 |
| Graduate/professional | 46.6 | 45.9 | 53.3 |
| P = 0.06 | P = 0.02 | P = 0.37 | |
| Personal income | |||
| $0–$24,999 | 49.3 | 49.8 | 45.1 |
| $25,000–$54,999 | 51.2 | 52.3 | 42.3 |
| $55,000–$74,999 | 54.4 | 54.1 | 56.4 |
| $75,000 and up | 56.1 | 56.9 | 45.5 |
| P = 0.20 | P = 0.22 | P = 0.61 | |
| Health insurance | |||
| Covered | 50.8 | 51.7 | 43.0 |
| Not covered | 51.8 | 52.7 | 44.5 |
| P = 0.60 | P = 0.62 | P = 0.78 | |
| Past year medical visits | |||
| 0 | 18.0 | 18.1 | 17.5 |
| 1 | 39.3 | 40.5 | 27.2 |
| 2–3 | 49.7 | 51.1 | 37.2 |
| 4–5 | 58.0 | 59.6 | 46.7 |
| 6 or more | 61.4 | 61.4 | 61.2 |
| P<0.0001 | P<0.0001 | P<0.0001 | |
| Physician CRC screening recommendation | |||
| No | 12.5 | 12.9 | 9.2 |
| Yes | 87.5 | 86.2 | 85.8 |
| P<0.0001 | P<0.0001 | P<0.0001 | |
| Self-reported health status | |||
| Excellent | 51.9 | 52.7 | 44.7 |
| Very good | 52.0 | 52.6 | 47.2 |
| Good | 51.0 | 51.9 | 42.9 |
| Fair | 49.8 | 51.2 | 38.1 |
| Poor | 59.2 | 59.3 | 58.7 |
| P = 0.42 | P = 0.63 | P = 0.55 | |
| Co-morbidities | |||
| 0 | 36.9 | 37.4 | 31.3 |
| 1 | 50.6 | 52.0 | 36.5 |
| 2–3 | 57.3 | 58.3 | 49.5 |
| 4–5 | 62.3 | 63.1 | 56.4 |
| 6 or more | 58.1 | 59.1 | 50.4 |
| P<0.0001 | P<0.0001 | P = 0.002 | |
| Alcohol usage | |||
| Never | 44.9 | 46.5 | 35.4 |
| Former | 52.4 | 52.3 | 52.8 |
| Light | 56.1 | 56.4 | 52.2 |
| Moderate/heavy | 55.2 | 55.4 | 49.8 |
| P<0.0001 | P<0.0001 | P = 0.0004 | |
| Smoking status | |||
| Daily | 36.3 | 36.0 | 38.1 |
| Occasional | 43.7 | 49.1 | 18.7 |
| Former | 59.0 | 59.1 | 57.4 |
| Never | 51.8 | 52.8 | 43.2 |
| P<0.0001 | P<0.0001 | P = 0.004 | |
| Recreational physical activity (MET minutes/week) | |||
| None/unable to exercise | 44.3 | 45.3 | 38.4 |
| <675 | 57.9 | 58.3 | 53.8 |
| ≥675 | 58.8 | 58.5 | 64.1 |
| P<0.0001 | P<0.0001 | P<0.0001 | |
Unadjusted screening rates
Unadjusted screening rates, stratified by obesity status and race, are shown in Table 3. When white and African-American women were combined, there was little overall difference in the proportion of women up-to-date with any CRC screening by obesity status. We did identify a difference in the percent of obese women who were up-to-date for colonoscopy (P = 0.02), compared with the percent of non-obese individuals who were up-to-date. Colonoscopy rates for obese women were 3.9 percentage points lower than those for non-obese women (95% CI 0.7, 7.2). In the unadjusted analysis, the relationship between obesity status and CRC screening differed according to race, but again only for the colonoscopy variable. Among whites, obese women were less likely to be up-to-date for colonoscopy compared with non-obese women (40.3% vs. 44.7%, P = 0.01). Conversely, among African-American women, there was no statistically significant relationship between obesity and colonoscopy usage (35.3% for non-obese women vs. 38.1% for obese women, P = 0.51).
Table 3.
Race stratified unadjusted CRC screening rates for women aged 50 and older by obesity status
| White and African-American Women |
White Women |
African-American Women |
||||
|---|---|---|---|---|---|---|
| Non-obese | Obese | Non-obese | Obese | Non-obese | Obese | |
| Up-to-date for Any CRC Screening, % (95% CI) | 52.9 (51.1–54.6) |
50.6 (47.9–53.3) |
53.7 (51.8–55.5) |
51.2 (48.1–54.2) |
44.1 (38.6–49.5) |
47.4 (41.1–53.7) |
| P = 0.17 | P = 0.16 | P = 0.44 | ||||
| Up-to-date Colonoscopy, % (95% CI) | 43.9 (42.1–45.7) |
40.0 (37.3–42.7) |
44.7 (42.8–46.5) |
40.3 (37.3–43.2) |
35.3 (29.8–40.9) |
38.1 (31.9–44.3) |
| P = 0.02 | P = 0.01 | P = 0.51 | ||||
| Up-to-date Endoscopy, % (95% CI) | 46.7 (45.0–48.5) |
43.6 (40.9–46.3) |
47.5 (45.6–49.3) |
44.0 (41.0–47.0) |
38.8 (33.4–44.3) |
41.0 (34.8–47.2) |
| P = 0.06 | P = 0.06 | P = 0.61 | ||||
| Up-to-date Fecal Occult Blood Test, % (95% CI) | 15.2 (14.0–16.4) |
15.9 (14.0–17.8) |
15.4 (14.2–16.7) |
16.5 (14.4–18.7) |
12.5 (9.2–15.8) |
12.4 (8.4–16.4) |
| P = 0.53 | P = 0.39 | P = 0.96 | ||||
Note Non-obese = BMI 18.5–29.9, Obese = BMI 30+
Obesity-race interaction
Multivariable logistic regression models indicated that race moderates the relationship between up-to-date CRC screening and obesity for all screening tests except FOBT alone. There was a statistically significant association (P = 0.04) between the interaction term (obesity status × race) and the primary CRC screening variable (any up-to-date screening) when controlling for reported physician recommendation, past-year medical visits, number of co-morbidities, education, smoking status, physical activity, and age. The interaction term was also significant in the models which had up-to-date colonoscopy (P = 0.01) and up-to-date endoscopy (P = 0.02) as their dependent variables. When past-year FOBT was used as an outcome variable, neither race, obesity, nor the interaction term were found to be significantly related to screening, after controlling for potential confounders.
Race-stratified regression analyses
Multivariable logistic regression models were created to look at the relationship between colonoscopy usage and the selected covariates in white and African-American women, separately (Table 4). The models were similar for the three screening outcomes which were associated with the interaction term (any CRC screening, colonoscopy, and endoscopy); however, only race-stratified adjusted odds ratios for up-to-date colonoscopy are reported, since this is the screening test with usage most strongly associated with obesity status. Even after controlling for potential confounders, obesity was still significantly related to colonoscopy usage in white women (P = 0.001). Among white women, the odds of being up-to-date for colonoscopy was 33% lower for obese women compared to non-obese women (OR = 0.67, 95% CI 0.53–0.86). There was a non-significant trend among African-American women indicating that obesity actually increased the odds that a woman was up-to-date with a colonoscopy.
Table 4.
Adjusted odds ratios for up-to-date Colonoscopy
| Predictor variable | White (n = 4,430) |
African-American (n = 690) |
||
|---|---|---|---|---|
| Odds ratio (95% CI) | P-value* | Odds ratio (95% CI) | P-value* | |
| Obesity | ||||
| Non-obese | 1.00 | 0.001 | 1.00 | 0.16 |
| Obese | 0.66 (0.50–0.85) | 1.30 (0.83–2.96) | ||
| Age | ||||
| 50–60 | 1.00 | <0.0001 | 1.00 | 0.08 |
| 60–70 | 2.00 (1.51–2.65) | 1.97 (0.90–4.30) | ||
| 70–80 | 2.84 (1.80–3.53) | 2.52 (0.95–6.72) | ||
| 80+ | 2.52 (1.80–3.53) | 2.39 (0.91–6.29) | ||
| Physician CRC screening recommendation | ||||
| No | 1.00 | <0.0001 | 1.00 | <0.001 |
| Yes | 59.72 (47.10–75.72) | 145.58 (75.69–283.86) | ||
| Past year medical visits | ||||
| 0 | 1.00 | 0.001 | 1.00 | 0.01 |
| 1 | 1.01 (0.57–1.78) | 1.60 (0.45–5.69) | ||
| 2–3 | 1.20 (0.71–2.04) | 1.28 (0.11–3.97) | ||
| 4–5 | 1.82 (1.05–3.17) | 4.73 (1.40–15.96) | ||
| 6 or more | 1.92 (1.12–3.30) | 4.37 (1.29–14.80) | ||
| Co-morbidities | ||||
| 0 | 1.00 | 0.03 | 1.00 | 0.50 |
| 1 | 1.00 (0.71–1.40) | 0.51 (0.20–1.28) | ||
| 2–3 | 1.31 (0.91–1.88) | 0.56 (0.22–1.41) | ||
| 4–5 | 1.29 (0.82–2.04) | 0.52 (0.19–1.44) | ||
| 6 or more | 2.26 (1.23–4.16) | 0.89 (0.27–2.95) | ||
| Education | ||||
| Less than high school | 1.00 | 0.05 | 1.00 | 0.23 |
| High school/GED | 0.92 (0.70–1.21) | 0.40 (0.16–0.95) | ||
| Some college/associates | 1.21 (0.90–1.63) | 0.97 (0.40–2.30) | ||
| College degree | 1.49 (1.06–2.08) | 1.03 (0.42–2.56) | ||
| Graduate/professional | 0.82 (0.48–1.40) | 1.34 (0.59–5.32) | ||
| Recreational physical activity | ||||
| None/unable to exercise | 1.00 | 0.06 | 1.00 | 0.06 |
| <675 | 1.26 (0.97–1.63) | 2.41 (1.12–5.17) | ||
| ≥675 | 1.35 (1.02–1.78) | 1.77 (0.59–5.32) | ||
Adjusted P-value based on Wald Chi-Square test
Physician recommendation for screening was the most significant predictor of colonoscopy for both races. White women who reported receiving a recommendation for screening from their physician had a much higher odds of having an up-to-date colonoscopy than those who did not receive a recommendation (OR = 59.72, 95% CI 47.10–75.72). The association was even greater for African-American women (OR = 145.58, 95% CI 75.69–283.86). Among whites, having an up-to-date colonoscopy was also significantly (P<0.05) associated with older age, a greater number of co-morbidities, and a greater number of medical visits. Among African-American women, colonoscopy was significantly associated with a higher number of past-year medical visits (P = 0.01).
Adjusted colonoscopy rates were created to examine differences between obese and non-obese white and African-American women after accounting for possible confounders included in the regression model for up-to-date colonoscopy (Fig. 1). Obese white women had an adjusted colonoscopy rate of 30.2% (95% CI 25.9–34.8), which was significantly lower than the 39.1% (95% CI 36.1–42.2) colonoscopy rate seen in non-obese white women (P = 0.001). Obese African-American women, on the other hand, had a higher adjusted colonoscopy rate (41.2%, 95% CI 31.6–51.4) than their non-obese counterparts (35.6%, 95% CI 28.3–43.6), but these differences were not statistically significant (P = 0.16). Overall, adjusted colonoscopy rates were lowest among obese white women.
Fig. 1.
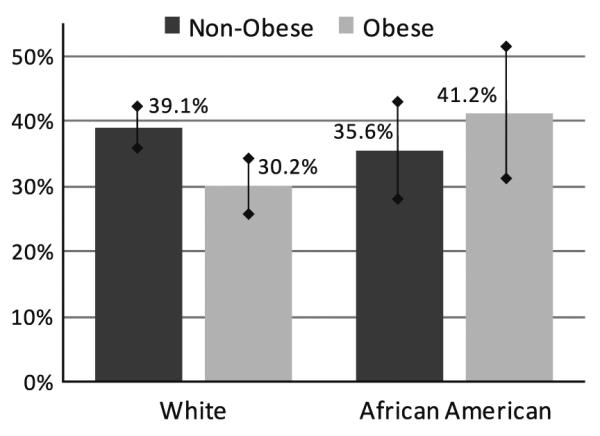
Race stratified adjusted colonoscopy rates for women aged 50 and older by obesity status. Note Adjusted colonoscopy rates for each weight and race group were calculated by direct standardization to the demographic characteristics of the study population using the coefficients from the multivariable model [34]
Discussion
Our analysis found that race moderates the relationship between obesity and colon cancer screening usage. Among white women, being obese reduced the chances that a woman was up-to-date with CRC screening. The opposite, however, was true for African-American women, for whom obesity was associated with higher screening rates. This screening disparity appears to be driven by the lower rates of colonoscopy seen among obese white women, since the interaction between obesity and race affected whether or not a woman was up-to-date for any type of screening test and colonoscopy or flexible sigmoidoscopy, but was not related to past-year FOBT usage. Similar to past research on screening correlates, reported physician recommendation had the largest affect on reported screening rates; however, obesity was still related to screening even after controlling for physician recommendation and other factors. The relationship between CRC screening and health insurance has been inconsistent across studies [35]. In this analysis, whether or not a woman reported having health insurance was not related to being up-to-date with CRC screening.
Previous studies which have examined the relationship between screening and weight have shown mixed results. Three of these studies were limited to a specific geographic area and/or did not include high numbers of African-Americans [15, 20, 22]. Even studies that used more nationally representative data from BRFSS, were not able to differentiate between colonoscopy and flexible sigmoidoscopy, which have different screening timeframes and thus were not accurately able to classify women as up-to-date for colonoscopy or not [12, 14, 36]. One advantage of using data from NHIS is that it is sampled and weighted to be representative of the U.S. population. It also has one of the most comprehensive screening questionnaires of any nationally representative survey. Unlike BRFSS and other surveys, NHIS is able to differentiate between flexible sigmoidoscopy and colonoscopy. Additionally, NHIS is interviewer administered and allows respondents to answer in multiple formats to indicate when they had their last screening, which improves data accuracy. NHIS also included questions about many covariates including doctor recommendation for screening.
Only two previous studies have looked at the relationship between weight and screening using NHIS data, both of these used data from 2000. While both studies controlled for relevant confounders, they did not stratify their results by gender or race. Seeff et al. found no differences between normal, overweight or obese individuals for rates of past year-FOBT or endoscopy in the previous 10 years. A study by Wee et al. found a statistically significant relationship between screening and BMI, similar to our results, but this relationship was attenuated in the adjusted model. The present study improved upon previous research on CRC screening and weight by using a comprehensive nationally representative data set, examining each recommended screening modality within its recommended time frame, and stratifying results by race and gender. The literature on how weight affects breast and cervical cancer screening rates in women indicate that stratifying by race is appropriate and necessary. Similar to our findings with colonoscopy, previous research has shown that obese white women have lower rates of mammography and pap smears than non-obese white women, but there was no relationship between these screening tests and obesity in African-American women [25-27].
Despites its many advantages, using NHIS data did present a few limitations. First, because it is meant to be representative of the U.S. population, it has much smaller number of African-Americans and other minority groups compared to whites. Small numbers in the African-American group limit the power of the analysis when compared to that done with white women. Another limitation of NHIS is that it does not include questions on Barium Enema, a recommended screening test, so some women who have had this test may be improperly classified. We do not believe this will appreciably affect the data, since barium enema has steeply declined over the last decade and is now rarely used to screen for colon cancer; approximately 0.05% of the Medicare population received a barium enema in 2005 [37].
Future research is needed to better understand why obese white women are less likely to get screened than their non-obese counterparts. Findings from the breast and cervical cancer screening literature provide some insights. In one study investigating barriers to gynecological screening, more than half of morbidly obese women reported that they delayed seeking health care because of their weight and over 70% reported that their weight was a barrier to receiving appropriate health care [25, 38]. In a recent qualitative study examining mammography usage, obese women reported additional barriers to screening, including previous bad experiences, discomfort, fear, poor treatment by providers, and low perceived susceptibility to cancer. They also stated that having a female doctor would increase their likelihood of having a screening.1 It has been suggested that obese individuals may have lower screening rates because they have more co-morbidities or acute needs that may be prioritized over cancer screening tests [39, 40]. Conversely, our data supports previous studies which have actually shown a positive relationship between screening and number of reported medical conditions [41]. Nevertheless, these theories do not fully explain why weight-related screening disparities may differ by race.
It is possible that racial differences in screening are related to differences in body image and body esteem. Previous research has indicated that obese women may delay preventive care because they are embarrassed or fear disrespectful treatment because of their weight [42]. Many obese women also are deterred from going to the doctor because they do not want to receive unsolicited weight loss advice, and they are made uncomfortable by small gowns and equipment that may not be appropriate for their size. These issues may be particularly salient for women who have poor body image or body esteem. This could explain why obese African-American women, who tend to be more accepting of a larger body size than their white counterparts [43], do not have lower screening rates than non-obese African-American women.
Body image-related issues may also explain why obesity appeared to affect rates of endoscopic tests, particularly colonoscopy, but not of FOBTs. The invasive nature of the colonoscopy and the fact that it must be performed in a clinical setting (as opposed to at home which is the case with FOBT) may explain why screening rates are lower for obese women but not men. One focus group study found that men and women had similar views about FOBT testing, but noted gender differences in attitudes toward colonoscopy [11]. Women expressed more anxiety about being unclothed and exposed during endoscopic procedures. These feelings could be enhanced in higher weight women, especially if they have poor body image.
While the present study provides further evidence that obesity affects screening behavior in women, especially for more invasive tests like colonoscopy, it is still unclear why these disparities exist. Qualitative research is needed to better understand how weight affects a woman’s decision to get a cancer screening. Once the source of weight-related screening disparities are better understood, we can improve screening promotion and education to better meet the needs of women with the lowest screening rates.
Acknowledgments
Ms. Leone is supported by the Lineberger Comprehensive Cancer Center’s Cancer Control and Education Program Pre-Doctoral Fellowship (NCI CA057726-16), the University of North Carolina at Chapel Hill Graduate School’s Dr. Thomas S. and Caroline H. Royster Fellowship, and the Triangle Community Foundation’s George H. Hitchings New Investigator Award in Health Research and Training. Dr. Pignone is supported by a National Cancer Institute Career Development Award (K05 CA129166). Special thanks to Rachel Tabak, May May Leung, Lisa Lowenstein and Dr. Dianne Ward for their help with editing this manuscript and to Chris Wiesen at the Odum Institute for the Social Sciences for his statistical assistance.
Footnotes
Personal communication with Jeanne Ferrante (New Jersey-Robert Wood Johnson Medical School) regarding unpublished focus group data.
Contributor Information
Lucia A. Leone, Department of Nutrition, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, 1700 Martin Luther King Jr. Blvd., CB#7294, Chapel Hill, NC 27599-7294, USA
Marci K. Campbell, Department of Nutrition, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, 1700 Martin Luther King Jr. Blvd., CB#7294, Chapel Hill, NC 27599-7294, USA
Jessie A. Satia, Department of Nutrition, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, 1700 Martin Luther King Jr. Blvd., CB#7294, Chapel Hill, NC 27599-7294, USA
J. Michael Bowling, Department of Health Behavior and Health Education, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Michael P. Pignone, Department of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, 1700 Martin Luther King Jr. Blvd., CB#7294, Chapel Hill, NC 27599-7294, USA
References
- 1.American Cancer Society, editor. Cancer facts & Figs 2008. American Cancer Society; Atlanta: 2008. [Google Scholar]
- 2.Mandel JS, Church TR, Bond JH, Ederer F, Geisser MS, Mongin SJ, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000;343(22):1603–1607. doi: 10.1056/NEJM200011303432203. [DOI] [PubMed] [Google Scholar]
- 3.Frazier AL, Colditz GA, Fuchs CS, Kuntz KM. Cost-effectiveness of screening for colorectal cancer in the general population. JAMA. 2000;284(15):1954–1961. doi: 10.1001/jama.284.15.1954. [DOI] [PubMed] [Google Scholar]
- 4.Anderson LM, May DS. Has the use of cervical, breast, and colorectal cancer screening increased in the United States? Am J Public Health. 1995;85(6):840–842. doi: 10.2105/ajph.85.6.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruffin MT, Gorenflo DW, Woodman B. Predictors of screening for breast, cervical, colorectal, and prostatic cancer among community-based primary care practices. J Am Board Fam Pract. 2000;13(1):1–10. doi: 10.3122/jabfm.13.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Seeff LC, Shapiro JA, Nadel MR. Are we doing enough to screen for colorectal cancer? Findings from the 1999 Behavioral Risk Factor Surveillance System. J Fam Pract. 2002;51(9):761–766. [PubMed] [Google Scholar]
- 7.Smith RA, Cokkinides V, Eyre HJ. Cancer screening in the United States, 2007: a review of current guidelines, practices, and prospects. CA Cancer J Clin. 2007;57(2):90–104. doi: 10.3322/canjclin.57.2.90. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, Georgia: Behavioral risk factor surveillance system survey data. 2004
- 9.Brawarsky P, Brooks DR, Mucci LA, Wood PA. Effect of physician recommendation and patient adherence on rates of colorectal cancer testing. Cancer Detect Prev. 2004;28(4):260–268. doi: 10.1016/j.cdp.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Tessaro I, Mangone C, Parkar I, Pawar V. Knowledge, barriers, and predictors of colorectal cancer screening in an Appalachian church population. Prev Chronic Dis. 2006;3(4):A123. [PMC free article] [PubMed] [Google Scholar]
- 11.Friedemann-Sanchez G, Griffin JM, Partin MR. Gender differences in colorectal cancer screening barriers and information needs. Health Expect. 2007;10(2):148–160. doi: 10.1111/j.1369-7625.2006.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosen AB, Schneider EC. Colorectal cancer screening disparities related to obesity and gender. J Gen Intern Med. 2004;19(4):332–338. doi: 10.1111/j.1525-1497.2004.30339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James AS, Leone L, Katz ML, McNeill LH, Campbell MK. Multiple health behaviors among overweight, class I obese, and class II obese persons. Ethn. Dis. 2008 Spring;18(2):157–162. [PubMed] [Google Scholar]
- 14.Heo M, Allison DB, Fontaine KR. Overweight, obesity, and colorectal cancer screening: disparity between men and women. BMC Public Health. 2004;4:53. doi: 10.1186/1471-2458-4-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrante JM, Ohman-Strickland P, Hudson SV, Hahn KA, Scott JG, Crabtree BF. Colorectal cancer screening among obese versus non-obese patients in primary care practices. Cancer Detect Prev. 2006;30(5):459–465. doi: 10.1016/j.cdp.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 17.Adams KF, Leitzmann MF, Albanes D, Kipnis V, Mouw T, Hollenbeck A, et al. Body mass and colorectal cancer risk in the NIH-AARP cohort. Am J Epidemiol. 2007;166(1):36–45. doi: 10.1093/aje/kwm049. [DOI] [PubMed] [Google Scholar]
- 18.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. New Engl J Med. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 19.Cohen SS, Palmieri RT, Nyante SJ, Koralek DO, Kim S, Bradshaw P, et al. Obesity and screening for breast, cervical, and colorectal cancer in women: a review. Cancer. 2008;112(9):1892–1904. doi: 10.1002/cncr.23408. [DOI] [PubMed] [Google Scholar]
- 20.Chao A, Connell CJ, Cokkinides V, Jacobs EJ, Calle EE, Thun MJ. Underuse of screening sigmoidoscopy and colonoscopy in a large cohort of US adults. Am J Public Health. 2004;94(10):1775–1781. doi: 10.2105/ajph.94.10.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calle EE, Rodriguez C, Jacobs EJ, Almon ML, Chao A, McCullough ML, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort: rationale, study design, and baseline characteristics. Cancer. 2002;94(2):500–511. doi: 10.1002/cncr.10197. [DOI] [PubMed] [Google Scholar]
- 22.Menis M, Kozlovsky B, Langenberg P, Zhan M, Dwyer DM, Israel E, et al. Body mass index and up-to-date colorectal cancer screening among Marylanders aged 50 years and older. Prev Chronic Dis. 2006;3(3):A88. [PMC free article] [PubMed] [Google Scholar]
- 23.Slattery ML, Kinney AY, Levin TR. Factors associated with colorectal cancer screening in a population-based study: the impact of gender, health care source and time. Prev Med. 2004;38:276–283. doi: 10.1016/j.ypmed.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Wee CC, McCarthy EP, Phillips RS. Factors associated with colon cancer screening: the role of patient factors and physician counseling. Prev Med. 2005;41(1):23–29. doi: 10.1016/j.ypmed.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Wee CC, McCarthy EP, Davis RB, Phillips RS. Obesity and breast cancer screening. J Gen Intern Med. 2004;19(4):324–331. doi: 10.1111/j.1525-1497.2004.30354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wee CC, McCarthy EP, Davis RB, Phillips RS. Screening for cervical and breast cancer: is obesity an unrecognized barrier to preventive care? Ann Intern Med. 2000;132(9):697–704. doi: 10.7326/0003-4819-132-9-200005020-00003. [DOI] [PubMed] [Google Scholar]
- 27.Fontaine KR, Heo M, Allison DB. Body weight and cancer screening among women. J Womens Health Gend Based Med. 2001;10(5):463–470. doi: 10.1089/152460901300233939. [DOI] [PubMed] [Google Scholar]
- 28.National Center for Health Statistics Data File Documentation . National Health Interview Survey, 2005 (machine readable data file and documentation). Data File Documentation, National Health Interview Survey, 2005 (machine readable data file and documentation) National Center for Health, Centers for Disease Control and Prevention; Hyattsville, Maryland: 2006. [Google Scholar]
- 29.Division of Health Interview Statistics, National Center for Health Statistics, editor. Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; Hyattsville, Maryland: NHIS Survey Description. 2006
- 30.Ries LA, Melbert D, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2005. National Cancer Institute; Bethesda, Marlyland: 2007. [Google Scholar]
- 31.Schenkera N, Raghunathanb TE, Chiua P, Makuca DM, Zhangb G, Cohena AJ. Multiple imputation of family income and personal earnings in the national health interview survey: methods and examples. 2008. 2008 August 28.
- 32.Seeff LC, Nadel MR, Klabunde CN, Thompson T, Shapiro JA, Vernon SW, et al. Patterns and predictors of colorectal cancer test use in the adult U.S. population. Cancer. 2004;100(10):2093–2103. doi: 10.1002/cncr.20276. [DOI] [PubMed] [Google Scholar]
- 33.Rao JNK, Scott AJ. Th analysis of categorical data from complex sample surveys: chi-squared tests for goodnes of fit and independence in two-way tables. J Am Stat. 1981;76:221–230. [Google Scholar]
- 34.Flanders WD, Rhodes PH. Large sample confidence intervals for regression standardized risks, risk ratios, and risk differences. J Chronic Dis. 1987;40(7):697–704. doi: 10.1016/0021-9681(87)90106-8. [DOI] [PubMed] [Google Scholar]
- 35.Beydoun HA, Beydoun MA. Predictors of colorectal cancer screening behaviors among average-risk older adults in the United States. Cancer Causes Control. 2008;19(4):339–359. doi: 10.1007/s10552-007-9100-y. [DOI] [PubMed] [Google Scholar]
- 36.Chao A, Connell CJ, Jacobs EJ, McCullough ML, Patel AV, Calle EE, et al. Amount, type, and timing of recreational physical activity in relation to colon and rectal cancer in older adults: the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2187–2195. [PubMed] [Google Scholar]
- 37.Schenck AP, Peacock SC, Klabunde CN, Lapin P, Coan JF, Brown ML. Trends in colorectal cancer test use in the medicare population, 1998-2005. Am J Prev Med. 2009;37(1):1–7. doi: 10.1016/j.amepre.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Amy NK, Aalborg A, Lyons P, Keranen L. Barriers to routine gynecological cancer screening for White and African-American obese women. Int J Obes (Lond) 2006;30(1):147–155. doi: 10.1038/sj.ijo.0803105. [DOI] [PubMed] [Google Scholar]
- 39.Kiefe CI, Funkhouser E, Fouad MN, May DS. Chronic disease as a barrier to breast and cervical cancer screening. J Gen Intern Med. 1998;13(6):357–365. doi: 10.1046/j.1525-1497.1998.00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patterson RE, Frank LL, Kristal AR, White E. A comprehensive examination of health conditions associated with obesity in older adults. Am J Prev Med. 2004;27(5):385–390. doi: 10.1016/j.amepre.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Levy BT, Dawson J, Hartz AJ, James PA. Colorectal cancer testing among patients cared for by Iowa family physicians. Am J Prev Med. 2006;31(3):193–201. doi: 10.1016/j.amepre.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 42.Ferrante JM, Chen PH, Jacobs A. Breast and cervical cancer screening in obese minority women. J Womens Health (Larchmt) 2006;15(5):531–541. doi: 10.1089/jwh.2006.15.531. [DOI] [PubMed] [Google Scholar]
- 43.Cash TE, Henry PE. Women’s body images: The results of a national survey in the USA. Sex Roles. 1995;33(1/2):19–28. [Google Scholar]


