Abstract
Background
Both alcohol consumption and obesity have been linked with breast cancer morbidity and mortality. An inverse association between alcohol intake and obesity suggests possible confounding between these variables (and perhaps other factors) with breast cancer outcomes.
Methods
Alcohol intake (beer, wine, spirits, and total) was examined in 3088 women previously diagnosed and treated for breast cancer, within an intervention trial that targeted vegetables, fiber, and fat but not alcohol or weight loss. Factors associated with baseline alcohol intake were included in Cox proportional hazards models for recurrence and mortality.
Results
Alcohol intake was significantly associated with higher education and physical activity levels. Neither light alcohol intake nor obesity was significantly associated with breast cancer recurrence, but moderate alcohol intake > 300 g/month was protective against all-cause mortality (HR = 0.69, CI=0.49-0.97) in a proportional hazards model adjusted for obesity. Obese women were 61% more likely to be nondrinkers than drinkers, and 76% more likely to be light drinkers than moderate/heavy drinkers. In non-obese women, alcohol intake > 10 g/month was associated with lower risk of all-cause mortality (HR = 0.68, 95% CI = 0.51-0.91).
Conclusion
Light alcohol intake, regardless of body weight, did not increase the risk of breast cancer recurrence or all-cause mortality in this cohort of middle-aged women previously diagnosed with breast cancer. Alcohol intake was associated with other favorable prognostic indicators that may explain its apparent protective effect in non-obese women.
Keywords: alcohol, breast cancer, obesity, mortality, recurrence
INTRODUCTION
Alcohol consumption has been consistently associated with higher risk of incident breast cancer (1-4). However, the link between alcohol intake and all-cause mortality following diagnosis of invasive breast cancer is less clear, with early studies finding no association (5-8), but two recent studies reporting an inverse association with mortality (9, 10). Journal correspondence noted the lack of any protective association of alcohol in an analysis of Italian breast cancer survivors (11, 12), a null finding consistent with other studies cited in a review article (13). Franceschi (12) suggested that the findings from the United States might be confounded given the high prevalence of minimal drinking in the sample. These conflicting reports suggest a need to investigate the influence of alcohol intake on prognosis in a large cohort of breast cancer survivors and to address the issue of confounding.
Body weight is a prominent potential confounder for studies evaluating associations between alcohol intake and breast cancer outcomes. Obesity at diagnosis has been consistently associated with increased mortality risk (14-17). Consistent evidence also suggests that a woman’s weight may be related to her level of alcohol consumption. Obesity is associated with lower likelihood of initiating alcohol use (18) and with lower odds of both any current drinking (18-20) and current heavy episodic drinking (20, 21). Thus, studies examining the effect of alcohol on health outcomes (including disease-free survival or overall survival) following breast cancer should control for body weight.
In this analysis, we investigate the roles of alcohol intake and obesity as predictors of additional breast cancer events and all-cause mortality in a cohort of 3088 breast cancer survivors who participated in the Women’s Healthy Eating and Living (WHEL) Study. This study was a randomized trial of a dietary intervention that was not associated with breast cancer outcomes or overall mortality (22). The study verified both initial diagnoses and outcomes and had 96% follow-up through a median of 7.3 years. The WHEL Study did not include alcohol intake as a target behavior, and consumption did not change meaningfully in either study arm during the trial.
MATERIALS AND METHODS
Participants and covariates
Women whose first invasive breast cancer was diagnosed between 1991 and 2000 were enrolled in the WHEL Study at 7 sites in the western United States between 1995 and 2000. Eligible participants had stage I (≥ 1 cm), II, or IIIA breast carcinoma within the past 4 years; were aged 18-70 years at diagnosis, had no evidence of recurrent disease or new cancer, and were able to communicate by telephone. Women who were pregnant, had cirrhosis, were scheduled for additional chemotherapy, or were currently taking estrogen replacement therapy were excluded. Further details of eligibility criteria, data collection, and assessment of cancer outcomes have been published elsewhere (23). Briefly, cancer characteristics were obtained by medical record review and confirmed by an oncologist. Weight and height were measured at a clinic visit at study entry, an average of 2 years, and a maximum of 4 years, after diagnosis. Body mass index (BMI) was calculated (kg/m2) and participants were classified as normal, overweight or obese using standard definitions (24). Prior to this baseline visit and at four time points in the study, participants completed a questionnaire on their personal habits (smoking, weight history, alcohol history, current physical activity) that was developed for the Women’s Health Initiative (WHI) (25). The 9-item measure of physical activity was validated for the WHEL Study (26), and responses were converted to metabolic equivalent tasks (MET minutes/week) (27).
Assessment of alcohol intake
We assessed alcohol intake using two independent measures: the Arizona Food Frequency questionnaire and a set of four 24-hour recalls. The AFFQ estimated the usual quantity and frequency of beer, wine, spirits, and total alcohol consumed over the previous 3 months. The dietary recalls were conducted by telephone shortly before the clinic visit on random days over a 3-week period, stratified for weekend vs weekdays. Concerns with the validity of estimates of consumption from each of these measures have led to recommendations to use multiple measures (28). To be conservative, we used the higher of the two estimates (AFFQ or set of 24-hour recalls), and converted reported alcohol consumption to grams with 10 g of alcohol equivalent to 10 oz of beer, 3.5 oz of wine, or a 1-oz shot of 80-proof alcohol in a mixed drink(29). Thus, a woman consuming > 300 g/month would have reported an intake, on average, of one alcoholic beverage daily and would be categorized as a moderate/heavy drinker in our analysis. Seven categories of alcohol consumption were used to evaluate trends for mortality and death, but for the remainder of the bivariate and multivariate analyses we collapsed seven categories into three categories. We classified women who consumed no alcohol or less than 10 g per month as “Non- or Minimal drinkers.”
Assessment of study outcomes
Primary study outcomes were invasive breast cancer recurrence or new primary breast cancer and death due to any cause. Throughout the study, participants were contacted every 6 months to obtain information on any hospitalizations or new breast cancer events. Medical records and death certificates were collected for each potential study outcome and these were centrally adjudicated by the study medical director. At the close of the study in June 2006, vital status was known for 96% of participants (see consort diagram in Pierce 2007). During follow-up (median 7.3 years), 518 breast cancer events (69% of which were distal recurrences) and 315 deaths were confirmed, 83% of which were breast cancer related, and only 8% of which were not from any cancer (22).
Statistical analysis
Although the WHEL Study intervention did not attempt to modify alcohol intake, we developed mixed models to explore whether alcohol consumption differed between the intervention and comparison groups, by time period (over 6 years of dietary data collection), or whether a group by time interaction occurred in alcohol consumption. Because no such associations were found, we present baseline alcohol intake in multivariate models, but report 1-year data among the results.
Alcohol intake was analyzed categorically (with chi-square tests against categorical covariates and t-tests against continuous covariates) for bivariate associations with cancer characteristics, demographic and personal characteristics, and physical activity. Because of the highly skewed-to-zero distribution of alcohol intake, median and interquartile ranges for alcohol intake are reported for each category of covariate. Median changes in alcohol intake from baseline to 1 year were tested with a Wilcoxon signed rank test.
We modeled factors related to alcohol intake using binary logistic regression. The first model compared non/minimal alcohol intake (< 10 g/mo) to the two other intake categories; the second model compared light alcohol intake (10-299 g/mo) to moderate/heavy intake (> 300 g/mo). Any covariate associated with alcohol intake at a significance level of p<0.05 in the logistic regression models was included in each of two delayed entry multivariate Cox proportional hazards models evaluating the joint association of alcohol and other covariates with all-cause mortality or additional breast cancer events. A delayed entry Cox model (30) was used because this approach accounts for varying times from diagnosis to study entry. Schoenfeld residuals were plotted to validate conformity with the proportional hazards assumption of the Cox models. Each model included an interaction term for obesity and alcohol intake, which was investigated using the likelihood ratio test. Finally, we computed hazard ratios and 95% confidence intervals for mortality by categories of body mass index and alcohol consumption, controlled for tumor characteristics. All analyses were conducted in SAS version 9.2 (Cary NC).
RESULTS
Details regarding the WHEL Study sample have been published (22, 23). In this sample of 3088 women previously diagnosed and treated for breast cancer, the mean (sd) baseline age was 52(9) years, BMI was 27.3 (6.1) kg/m2; 54% were college graduates, and 85% were Non-Hispanic white.
Characteristics of alcohol consumption
Energy intake from alcohol ranged from 0 to 34% of total energy intake, with the median 0.1% of energy intake and the mean 2.0% of energy intake. Beer consumption accounted for 23% of alcohol intake reported, and wine and spirits comprised 47% and 30% respectively. Among the small group (8%) of women who reported drinking more than 600 g/mo of alcohol, 46% of intake was consumed as spirits and 31% as wine. Non-Hispanic whites constituted 97% of these heavier drinkers. Overall, approximately 37 percent of women were minimal drinkers (< 10 g/mo), 43 percent were light drinkers and 20 percent were moderate/heavy drinkers (> 300 g/mo).
Women with better prognosis (lower cancer stage and grade, estrogen receptor positive [ER+] tumors, no chemotherapy) had higher median alcohol intakes (Table 1). Compared to Non-Hispanic white women in multivariate regression models, African-American and Asian women were more than twice as likely to be non/minimal drinkers and, less likely to be moderate/heavy drinkers. Obese women were more likely to be non/minimal drinkers than light or moderate drinkers (OR=1.61 95% C.I. 1.35-1.93) and to be light compared to moderate/heavy drinkers (OR=1.76 95% C.I: 1.35-2.29). Women who were better educated, physically active, or nulliparous were 22 to 33% less likely to be non/minimal drinkers. Women who had ever smoked were half as likely to be non-drinkers and twice as likely to be moderate/heavy versus light drinkers.
Table 1.
Alcohol intake* at baseline by cancer/demographic characteristics and lifestyle variables in a cohort of US breast cancer survivors followed for a median of 7.3 years (N=3088)
| Median (quartiles) intake g/mo |
% Minimal (<10 g/mo) |
% Light (10-299 g/mo) |
% Moderate/ Heavy (> 300 g/mo) |
P** | ||
|---|---|---|---|---|---|---|
| N | 1079 | 1375 | 634 | |||
| Stage | 0.003 | |||||
| I | 1190 | 50 (3, 276) | 30.5 | 46.1 | 23.5 | |
| II | 1407 | 24 (3, 202) | 38.0 | 44.0 | 18.1 | |
| III | 491 | 33 (3, 237) | 37.1 | 42.4 | 20.6 | |
| Grade | ||||||
| Unspecified | 256 | 48 (3, 336) | 33.2 | 40.6 | 26.2 | 0.01 |
| I | 484 | 52 (3, 284) | 30.4 | 46.3 | 23.4 | |
| II | 1239 | 33 (3, 246) | 34.8 | 44.2 | 21.1 | |
| III | 1109 | 27 (3, 184) | 37.5 | 45.1 | 20.0 | |
| Diagnosis to Randomization | 0.10 | |||||
| < 2 years | 1698 | 31 (3, 222) | 36.6 | 43.7 | 19.7 | |
| 2 – 4 years | 1390 | 45 (3, 249) | 33.0 | 45.5 | 21.5 | |
| Estrogen Receptor Status | 0.02 | |||||
| Negative | 756 | 25 (3, 177) | 38.0 | 44.8 | 17.2 | |
| Positive | 2286 | 39 (3, 249) | 34.1 | 44.3 | 21.7 | |
| Body Mass Index (kg/m2) | <0.0001 | |||||
| Low (< 18.5) | 31 | 3 (0, 87) | 54.8 | 38.7 | 6.5 | |
| Normal (18.5-24.99) | 1299 | 67 (3, 295) | 29.0 | 46.2 | 24.7 | |
| Overweight (25-29.99) | 955 | 39 (3, 246) | 34.2 | 44.0 | 21.8 | |
| Obese (30+) | 803 | 15 (1,117) | 44.6 | 42.6 | 12.8 | |
| Physical Activity | <.0001 | |||||
| < 540 MET*** min/wk | 1380 | 22 (3, 174) | 32.6 | 45.0 | 22.4 | |
| >= 540 MET min/wk | 1605 | 63 (3, 282) | 29.3 | 47.3 | 23.4 | |
| Race/Ethnicity | <.0001 | |||||
| White | 2634 | 49 (3, 258) | 32.6 | 45.0 | 22.4 | |
| African American | 118 | 3 (0, 31) | 56.8 | 34.8 | 8.5 | |
| Hispanic | 165 | 15 (1. 96) | 44.9 | 41.8 | 13.3 | |
| Asian/Pacific Islander | 119 | 6 (3, 17) | 43.7 | 52.9 | 3.4 | |
| Mixed/Other | 52 | 8 (0, 159) | 51.9 | 32.7 | 15.4 | |
| Education | <0.001 | |||||
| Not College Graduate | 1414 | 23 (3, 195) | 39.5 | 41.8 | 18.7 | |
| College Graduate | 1674 | 51 (3, 264) | 31.1 | 46.8 | 22.1 | |
| Parity | 0.0001 | |||||
| Nulliparous | 683 | 67 (3, 330) | 28.6 | 43.9 | 27.5 | |
| Parous, first birth < age35 | 2246 | 31 (3, 207) | 36.8 | 44.8 | 18.4 | |
| Parous, first birth ≥ age35 | 136 | 32 (3, 264) | 34.6 | 42.7 | 22.8 | |
| Smoking Status | <0.0001 | |||||
| Current | 138 | 76 (3, 342) | 34.8 | 37.0 | 28.3 | |
| Former | 1276 | 82 (3. 333) | 29.4 | 44.2 | 27.4 | |
| Never | 1643 | 17 (3, 165) | 39.9 | 45.5 | 14.6 |
10 g of alcohol is equivalent to 10 oz of beer, 3.5 oz of wine, or a 1-oz shot of 80-proof alcohol
P is p for trend in ordinal variables, or p from chi-square for categorical variables
MET = metabolic equivalent tasks
Alcohol intake at either baseline or 1-year follow-up was not related to intervention group assignment. Median alcohol consumption decreased in both groups over 1 year, by 0.9 g/month (p<.05) on the combined measure (AFFQ and recalls), with median decrease even less on each individual instrument.
Association with study outcomes
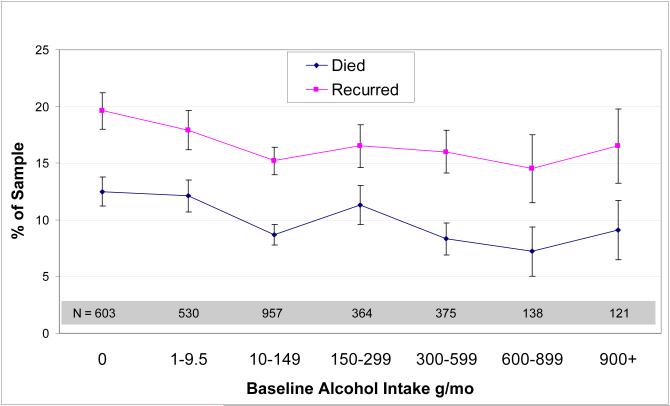
In bivariate analyses, higher alcohol intake was significantly associated with lower all-cause mortality (p=.02), but not with additional breast cancer events, using seven categories of alcohol consumption (Figure 1). Within the simplified three consumption categories, the 634 women in the highest consumption category (>300 g/mo) had a mortality rate of 8.2% compared with 9.4% for those with light alcohol intake (10-299 g/mo) and 12.3% for those with minimal alcohol intake (p=.03). Non/minimal drinkers were also more likely to have an additional breast cancer event than those reporting light or moderate alcohol consumption (18.8% versus 15.7%, p=.03). The type of alcoholic beverage consumed was not related to mortality, but among all drinkers, the unadjusted risk for additional breast cancer events was lower in those who reported drinking spirits (12.8%) compared with those who primarily drank wine (16.3%) or beer (19.5%), p=0.02.
Figure 1.
Unadjusted mortality /additional breast cancer events by baseline alcohol intake, in a cohort of US breast cancer survivors. Values shown are mean ± SEM.
In the multivariate analysis, controlling for stage, grade, time from diagnosis to study entry, ethnicity, education, physical activity, parity, body mass index, and smoking status, (but not including estrogen receptor status, chemotherapy, or tamoxifen use because they did not meet the inclusion criteria), higher alcohol intake was not associated with risk for either additional breast cancer events (Table 2) or additional distal breast cancer events (data not shown). However, compared to non/minimal drinkers, moderate/heavy drinkers had a decreased risk for all-cause mortality (HR= 0.69; 95% C.I. 0.49-0.97), and for breast cancer mortality (HR = 0.70, 95% C.I. 0.48-1.02). Women who had ever smoked were at increased risk for all-cause mortality (HR=1.32 95% C.I. 1.04-1.66) but not for additional breast cancer events. College-educated women had 19% lower probability of an additional breast cancer event and 26% lower risk for death from any cause. In this analysis, obesity was not associated with additional breast cancer events but was marginally associated with all-cause mortality (HR=1.28; 95% CI: 0.97-1.70, p=.09). The interaction term between obesity and alcohol consumption in the model for mortality suggested a weak effect (p=0.11) in the likelihood ratio test.
Table 2.
Multivariate models* for additional breast cancer events and mortality
| N in category |
Additional Breast Cancer Events |
Mortality | |||||
|---|---|---|---|---|---|---|---|
| Variable | N Events |
HR | 95% CI | N Events |
HR | 95% CI | |
| Minimal alcohol intake (< 10 g/mo) | 1133 | 213 | 1.00 | 139 | 1.00 | ||
| Light alcohol intake (10 -290 g/mo) | 1321 | 205 | 0.89 | (0.73, 1.08) | 124 | 0.81 | (0.63-1.04) |
| Moderate/heavy alcohol intake (> 300 g/mo) | 634 | 100 | 0.91 | (0.71, 1.18) | 52 | 0.69 | (0.49, 0.97) |
| Never smoker | 1643 | 273 | 1.00 | 150 | 1.00 | ||
| Ever smoker | 1414 | 237 | 1.01 | (0.84-1.21) | 159 | 1.32 | (1.04-1.66) |
| Not college graduate | 1414 | 260 | 1.00 | 171 | 1.00 | ||
| College graduate | 1674 | 258 | 0.81 | (0.67, 0.97) | 144 | 0.74 | (0.58, 0.94) |
| Parous | 2382 | 386 | 1.00 | 233 | 1.00 | ||
| Nulliparous | 683 | 127 | 1.26 | (1.02, 1.55) | 79 | 1.37 | (1.05, 1.79) |
| Normal weight | 1299 | 212 | 1.00 | 113 | 1.00 | ||
| Overweight | 955 | 148 | 0.91 | (0.74, 1.13) | 92 | 0.98 | (0.74-1.30) |
| Obese | 803 | 151 | 1.10 | (0.88, 1.38) | 105 | 1.28 | (0.97, 1.70) |
Models are controlled for cancer stage, grade, years between diagnosis and study entry, physical activity, and ethnicity in addition to all variables listed in the body of the table.
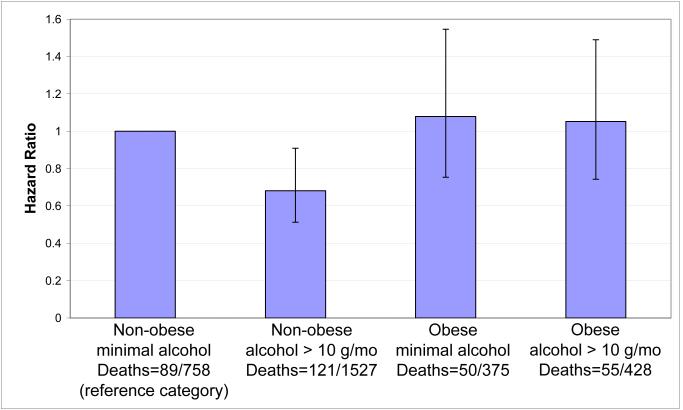
Unadjusted mortality rates were 11.7% of non-obese non-drinkers, 7.9% of non-obese drinkers, 13.3% of obese non-drinkers, and 12.9% of obese drinkers. We present the hazard ratios for all-cause mortality for combinations of binary variables for obesity (obese versus non-obese) and alcohol consumption (minimal drinker versus other), adjusted for cancer stage and grade (Figure 2). Alcohol intake did not significantly alter mortality risk in obese women, but was associated with a 32% lower probability of mortality in non-obese women (HR = 0.68, CI = 0.51-0.91). In this non-obese group, African-American and Asian-American women were three times more likely to report non/minimal alcohol intake compared to other drinking categories (Table 3). Non-obese women who were current or former smokers, physically active, or college educated were 48%, 21%, or 33% less likely, respectively, to be in the non/minimal drinking category.
Figure 2.
Hazard ratios, event counts, and 95% confidence intervals for mortality by obesity and alcohol intake categories in a cohort of US breast cancer survivors. Bars compare risk for all-cause mortality by obesity and alcohol intake. The reference category is minimal alcohol intake (< 10 g/mo) and non-obese women. Models are adjusted for cancer stage and grade.
Table 3.
Binary logistic regression model* for minimal alcohol intake (< 10 g/month) in non-obese WHEL women (N=2285)
| Minimal alcohol (< 10 g/mo) vs. light, moderate/heavy |
||
|---|---|---|
| Predictor | Odds | 95% CI |
| Node positive cancer | 1.19 | (0.99-1.43) |
| Time from diagnosis < 2 yrs | 1.26 | (1.04-1.52) |
| Ethnicity | ||
| African American | 3.10 | (1.78-5.37) |
| Asian | 3.01 | (1.89-4.78) |
| Hispanic | 1.26 | (0.83-1.91) |
| College graduate | 0.67 | (0.56-0.82) |
| Physical activity > 540 METs/wk | 0.79 | (0.65-0.96) |
| Nulliparity | 0.78 | (0.62-0.97) |
| Ever smoker | 0.52 | (0.43-0.63) |
Model shows odds of consuming <10 g alcohol/mo, and are controlled for tumor estrogen receptor status and cancer grade. Reference category for ethnicity is non-Hispanic white.
DISCUSSION
In this large cohort of breast cancer survivors, moderate alcohol intake compared to non/minimal drinking was associated with reduced all-cause mortality. However, alcohol consumption among WHEL Study breast cancer survivors was low, with only 21% consuming more than 300g/month (about one alcoholic drink a day). This level is comparable to that reported for other US samples (2, 9) and less than half that reported in the Italian sample (11, 12). Some of this difference may be associated with the more heterogeneous population in the United States which has significant numbers of African-Americans and Asian-Americans. Both of these racial/ethnic groups have traditionally reported lower levels of alcohol consumption (31, 32) than non-Hispanic white women. Another reason for the difference could be that the WHEL sample represents women with more serious breast disease (83% of deaths were from breast cancer) and that U.S. oncologists may be more assertive in recommending reduced alcohol intake for these patients. Previously, we have noted that 41% of the WHEL sample reported reducing their alcohol consumption following breast cancer diagnosis (33). One year alcohol intake, which changed little after baseline in the WHEL Study, suggests that these post-diagnosis decreases in alcohol intake were maintained after study enrollment.
Our finding that light to moderate alcohol intake did not increase mortality after breast cancer was in line with that reported in two recent studies in breast cancer patients (9, 10) as well as a study in the general population (32). The protective hazard ratio in the WHEL Study for daily drinkers was comparable to that reported in the United Kingdom study (10). However, our data did not show a protective association between alcohol consumption and cancer recurrence. Of note, 92% of the WHEL population consumed fewer than 2 small drinks daily, and most “moderate drinkers” appeared to drink approximately 1 drink per day. Alcohol intake in a primarily white, educated population of breast cancer survivors who elected to participate in a dietary intervention trial may be associated with other healthy behaviors, as contrasted with alcohol intake in studies focusing on primary prevention, in which alcohol intake may be associated with less desirable health behaviors.
One of the mechanisms by which alcohol intake may influence risk for primary breast cancer is via effects on estrogen metabolism (34, 35). Alcohol intake has been observed to be directly associated with circulating sex hormones in several studies (36, 37). However, the hormonal milieu is considerably altered following the diagnosis and treatment of breast cancer, and the majority of women are treated with chemotherapy and/or anti-estrogenic agents which further modify reproductive hormonal status. Li et al demonstrated a positive association between retrospectively self-reported alcohol consumption and second primary contralateral breast cancer (2), results which are difficult to compare given differences in sample characteristics including tumor ER status, frequency of chemotherapy use, different rates of new primaries, and greater frequency of metastatic disease in WHEL
Bioactive constituents in beer and wine, such as flavonoids and polyphenols, have been hypothesized to reduce mortality risk after cancer (38). This effect was not observed in the WHEL sample, where higher consumption of spirits was predictive of lower mortality. Indeed, the effect observed in this study may not be related to alcohol consumption per se but rather to correlates of alcohol intake. Further examination demonstrated that the lower risk of death among alcohol consumers was confined to women who were not obese at enrollment in the study. In this subsample of the WHEL population, women with higher education and physical activity levels were more likely to be in the upper two categories of alcohol consumption. Both socioeconomic status/education (32) and physical activity (39, 40) have been associated with improved survival. Additionally, African American women, who have higher mortality following breast cancer diagnosis (41), were three times more likely to be non/minimal drinkers and this association could also partially explain the observed effect.
The association of alcohol consumption with decreased all-cause mortality may be attributed to other potential confounders. In the WHEL Study, women with more serious disease (node positive, higher grade, ER− tumors, or a history of chemotherapy) were more likely to be minimal drinkers. Further, women who were more highly educated (and presumably had higher socioeconomic status) were less likely to be minimal drinkers. The association between socioeconomic status and improved health outcomes has been well established (32, 42).
A number of strengths as well as limitations of this analysis should be considered. Many of the measures in the WHEL Study were validated, although alcohol consumption was self-reported. The alcohol data in this study were collected shortly after the publication of observational studies that suggested that alcohol increased breast cancer risk. Thus, social desirability may have led some participants to underreport their alcohol intake. However, strengths of our study are our use of two separate instruments to measure alcohol intake and our application of a conservative algorithm for assigning participants to a category of consumption. Further, the WHEL Study assessed alcohol intake five times over the duration of the study and intake demonstrated considerable stability in measurement (data not shown). A major strength in the WHEL Study is the oncologist verification of initial diagnosis and reported outcomes. Nevertheless, the WHEL Study results cannot be generalized to all breast cancer survivors. The WHEL population was comprised of women who elected to participate in a dietary intervention trial, excluded breast cancer survivors with low level disease (e.g. less than 1 cm tumors or carcinoma in situ) and it was limited to early stage disease (through Stage IIIA using the AJCC classification IV edition). Further, the WHEL Study allowed enrollment up to 4 years post-diagnosis and therefore may under-represent women diagnosed with ER− tumors (43).
In summary, light alcohol consumption reported by breast cancer survivors in the United States was not associated with adverse outcomes (either additional breast cancer events or death). A moderate level of alcohol consumption, approximately one alcoholic drink per day, was associated with reduced all-cause mortality in the study, particularly among women who were not obese. However, this study cannot rule out that women at lower risk for death were more likely to be moderate drinkers.
ACKNOWLEDGEMENTS
WHEL Study Coordinating Center: University of California, San Diego, Cancer Prevention and Control Program, Moores UCSD Cancer Center, San Diego, CA (John P. Pierce, PhD; Susan Faerber, BA; Barbara A. Parker, MD; Loki Natarajan, PhD, Cheryl L. Rock, PhD; Vicky A. Newman, MS; Shirley W. Flatt, MS; Sheila Kealey, MPH; Ruth Patterson, PhD, Linda Wasserman, MD; Wayne A. Bardwell, PhD; Lisa Madlensky, PhD.; Wael Al-Delaimy MD
WHEL Study Clinical Sites: Center for Health Research-Portland, Portland, OR (Njeri Karanja, PhD, Mark U. Rarick, MD); Kaiser Permanente Northern California, Oakland, CA (Bette J. Caan, DrPH, Lou Fehrenbacher, MD); Stanford Prevention Research Center, Stanford University, CA (Marcia L. Stefanick, PhD, Robert Carlson, MD); University of Arizona, Tucson & Phoenix, AZ (Cynthia Thomson, PhD, James Warneke, MD); University of California, Davis, Davis, CA (Ellen B. Gold, PhD, Sidney Scudder, MD); University of California, San Diego, Moores UCSD Cancer Center, San Diego, CA (Kathryn A. Hollenbach, PhD, Vicky Jones, MD); University of Texas M.D. Anderson Cancer Center, Houston, TX (Lovell A. Jones, PhD, Richard Hajek, PhD, Richard Theriault, DO)
Financial Support: The Women’s Healthy Eating and Living (WHEL) Study was initiated with the support of the Walton Family Foundation and continued with funding from NCI grant CA 69375. Some of the data were collected from General Clinical Research Centers, NIH grants M01-RR00070, M01-RR00079, and M01-RR00827.
Footnotes
CONFLICT OF INTEREST
All of the authors declare that there are no conflicts of interest for this paper.
REFERENCES
- 1.Allen NE, Beral V, Casabonne D, et al. Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst. 2009;101:296–305. doi: 10.1093/jnci/djn514. [DOI] [PubMed] [Google Scholar]
- 2.Li CI, Daling JR, Porter PL, Tang MT, Malone KE. Relationship Between Potentially Modifiable Lifestyle Factors and Risk of Second Primary Contralateral Breast Cancer Among Women Diagnosed With Estrogen Receptor-Positive Invasive Breast Cancer. J Clin Oncol. 2009 doi: 10.1200/JCO.2009.23.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahoney MC, Bevers T, Linos E, Willett WC. Opportunities and strategies for breast cancer prevention through risk reduction. CA Cancer J Clin. 2008;58:347–71. doi: 10.3322/CA.2008.0016. [DOI] [PubMed] [Google Scholar]
- 4.Tjonneland A, Christensen J, Olsen A, et al. Alcohol intake and breast cancer risk: the European Prospective Investigation into Cancer and Nutrition (EPIC) Cancer Causes Control. 2007;18:361–73. doi: 10.1007/s10552-006-0112-9. [DOI] [PubMed] [Google Scholar]
- 5.Ewertz M, Gillanders S, Meyer L, Zedeler K. Survival of breast cancer patients in relation to factors which affect the risk of developing breast cancer. Int J Cancer. 1991;49:526–30. doi: 10.1002/ijc.2910490409. [DOI] [PubMed] [Google Scholar]
- 6.Holm LE, Nordevang E, Hjalmar ML, Lidbrink E, Callmer E, Nilsson B. Treatment failure and dietary habits in women with breast cancer. J Natl Cancer Inst. 1993;85:32–6. doi: 10.1093/jnci/85.1.32. [DOI] [PubMed] [Google Scholar]
- 7.Holmes MD, Stampfer MJ, Colditz GA, Rosner B, Hunter DJ, Willett WC. Dietary factors and the survival of women with breast carcinoma. Cancer. 1999;86:826–35. doi: 10.1002/(sici)1097-0142(19990901)86:5<826::aid-cncr19>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 8.Rohan TE, Hiller JE, McMichael AJ. Dietary factors and survival from breast cancer. Nutr Cancer. 1993;20:167–77. doi: 10.1080/01635589309514283. [DOI] [PubMed] [Google Scholar]
- 9.Reding KW, Daling JR, Doody DR, O’Brien CA, Porter PL, Malone KE. Effect of prediagnostic alcohol consumption on survival after breast cancer in young women. Cancer Epidemiol Biomarkers Prev. 2008;17:1988–96. doi: 10.1158/1055-9965.EPI-07-2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnett GC, Shah M, Redman K, Easton DF, Ponder BA, Pharoah PD. Risk factors for the incidence of breast cancer: do they affect survival from the disease? J Clin Oncol. 2008;26:3310–6. doi: 10.1200/JCO.2006.10.3168. [DOI] [PubMed] [Google Scholar]
- 11.Dal Maso L, Zucchetto A, Talamini R, et al. Effect of obesity and other lifestyle factors on mortality in women with breast cancer. Int J Cancer. 2008;123:2188–94. doi: 10.1002/ijc.23747. [DOI] [PubMed] [Google Scholar]
- 12.Franceschi S, Dal Maso L, Zucchetto A, Talamini R. Alcohol consumption and survival after breast cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:1011–2. doi: 10.1158/1055-9965.EPI-08-0904. author reply 2-3. [DOI] [PubMed] [Google Scholar]
- 13.Rock CL, Demark-Wahnefried W. Nutrition and survival after the diagnosis of breast cancer: a review of the evidence. J Clin Oncol. 2002;20:3302–16. doi: 10.1200/JCO.2002.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Majed B, Moreau T, Senouci K, Salmon RJ, Fourquet A, Asselain B. Is obesity an independent prognosis factor in woman breast cancer? Breast Cancer Res Treat. 2008;111:329–42. doi: 10.1007/s10549-007-9785-3. [DOI] [PubMed] [Google Scholar]
- 15.Caan BJ, Kwan ML, Hartzell G, et al. Pre-diagnosis body mass index, post-diagnosis weight change, and prognosis among women with early stage breast cancer. Cancer Causes Control. 2008;19:1319–28. doi: 10.1007/s10552-008-9203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carmichael AR. Obesity and prognosis of breast cancer. Obes Rev. 2006;7:333–40. doi: 10.1111/j.1467-789X.2006.00261.x. [DOI] [PubMed] [Google Scholar]
- 17.Abrahamson PE, Gammon MD, Lund MJ, et al. General and abdominal obesity and survival among young women with breast cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:1871–7. doi: 10.1158/1055-9965.EPI-06-0356. [DOI] [PubMed] [Google Scholar]
- 18.Duncan AE, Grant JD, Bucholz KK, Madden PA, Heath AC. Relationship between body mass index, alcohol use, and alcohol misuse in a young adult female twin sample. J Stud Alcohol Drugs. 2009;70:458–66. doi: 10.15288/jsad.2009.70.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kruger J, Ham SA, Prohaska TR. Behavioral risk factors associated with overweight and obesity among older adults: the 2005 National Health Interview Survey. Prev Chronic Dis. 2009;6:A14. [PMC free article] [PubMed] [Google Scholar]
- 20.Wilsgaard T, Jacobsen BK, Arnesen E. Determining lifestyle correlates of body mass index using multilevel analyses: the Tromso Study, 1979-2001. Am J Epidemiol. 2005;162:1179–88. doi: 10.1093/aje/kwi328. [DOI] [PubMed] [Google Scholar]
- 21.Breslow RA, Smothers BA. Drinking patterns and body mass index in never smokers: National Health Interview Survey, 1997-2001. Am J Epidemiol. 2005;161:368–76. doi: 10.1093/aje/kwi061. [DOI] [PubMed] [Google Scholar]
- 22.Pierce JP, Natarajan L, Caan BJ, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial. Jama. 2007;298:289–98. doi: 10.1001/jama.298.3.289. PMCID: 2083253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pierce JP, Faerber S, Wright FA, et al. A randomized trial of the effect of a plant-based dietary pattern on additional breast cancer events and survival: the Women’s Healthy Eating and Living (WHEL) Study. Control Clin Trials. 2002;23:728–56. doi: 10.1016/s0197-2456(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 24.Kuczmarski RJ, Flegal KM. Criteria for definition of overweight in transition: background and recommendations for the United States. Am J Clin Nutr. 2000;72:1074–81. doi: 10.1093/ajcn/72.5.1074. [DOI] [PubMed] [Google Scholar]
- 25.WHI [Accessed September 2, 2009];WHI Personal Habits Questionnaire. Women’s Health Initiative. http://www.whiscience.org/data/forms/F34v2.pdf.
- 26.Johnson-Kozlow M, Rock CL, Gilpin EA, Hollenbach KA, Pierce JP. Validation of the WHI brief physical activity questionnaire among women diagnosed with breast cancer. Am J Health Behav. 2007;31:193–202. doi: 10.5555/ajhb.2007.31.2.193. [DOI] [PubMed] [Google Scholar]
- 27.Hong S, Bardwell WA, Natarajan L, et al. Correlates of physical activity level in breast cancer survivors participating in the Women’s Healthy Eating and Living (WHEL) Study. Breast Cancer Res Treat. 2007;101:225–32. doi: 10.1007/s10549-006-9284-y. [DOI] [PubMed] [Google Scholar]
- 28.Natarajan L, Flatt SW, Sun X, et al. Validity and systematic error in measuring carotenoid consumption with dietary self-report instruments. Am J Epidemiol. 2006;163:770–8. doi: 10.1093/aje/kwj082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pennington JAT. Bowes & Church’s Food Values of Portions Commonly Used. Lippincott Williams & Wilkins; Philadelphia: 2004. [Google Scholar]
- 30.Therneau TM, Grambsch PM. Statistics for Biology and Health. Springer-Verlag; New York: 2000. Modeling Survival Data. Extending the Cox Model. [Google Scholar]
- 31.Juarbe TC, Kaplan CP, Somkin CP, Pasick R, Gildengorin G, Perez-Stable EJ. Are risk factors for breast cancer associated with follow-up procedures in diverse women with abnormal mammography? Cancer Causes Control. 2005;16:245–53. doi: 10.1007/s10552-004-4028-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee SJ, Sudore RL, Williams BA, Lindquist K, Chen HL, Covinsky KE. Functional limitations, socioeconomic status, and all-cause mortality in moderate alcohol drinkers. J Am Geriatr Soc. 2009;57:955–62. doi: 10.1111/j.1532-5415.2009.02184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomson CA, Flatt SW, Rock CL, Ritenbaugh C, Newman V, Pierce JP. Increased fruit, vegetable and fiber intake and lower fat intake reported among women previously treated for invasive breast cancer. J Am Diet Assoc. 2002;102:801–8. doi: 10.1016/s0002-8223(02)90180-x. [DOI] [PubMed] [Google Scholar]
- 34.Singletary KW, Gapstur SM. Alcohol and breast cancer: review of epidemiologic and experimental evidence and potential mechanisms. Jama. 2001;286:2143–51. doi: 10.1001/jama.286.17.2143. [DOI] [PubMed] [Google Scholar]
- 35.Dumitrescu RG, Shields PG. The etiology of alcohol-induced breast cancer. Alcohol. 2005;35:213–25. doi: 10.1016/j.alcohol.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Maskarinec G, Morimoto Y, Takata Y, Murphy SP, Stanczyk FZ. Alcohol and dietary fibre intakes affect circulating sex hormones among premenopausal women. Public Health Nutr. 2006;9:875–81. doi: 10.1017/phn2005923. [DOI] [PubMed] [Google Scholar]
- 37.Dorgan JF, Baer DJ, Albert PS, et al. Serum hormones and the alcohol-breast cancer association in postmenopausal women. J Natl Cancer Inst. 2001;93:710–5. doi: 10.1093/jnci/93.9.710. [DOI] [PubMed] [Google Scholar]
- 38.Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am J Clin Nutr. 2005;81:243S–55S. doi: 10.1093/ajcn/81.1.243S. [DOI] [PubMed] [Google Scholar]
- 39.Pierce JP, Stefanick ML, Flatt SW, et al. Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity. J Clin Oncol. 2007;25:2345–51. doi: 10.1200/JCO.2006.08.6819. PMCID: 2274898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. Jama. 2005;293:2479–86. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 41.Menashe I, Anderson WF, Jatoi I, Rosenberg PS. Underlying causes of the black-white racial disparity in breast cancer mortality: a population-based analysis. J Natl Cancer Inst. 2009;101:993–1000. doi: 10.1093/jnci/djp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marmot M, Shipley M, Brunner E, Hemingway H. Relative contribution of early life and adult socioeconomic factors to adult morbidity in the Whitehall II study. J Epidemiol Community Health. 2001;55:301–7. doi: 10.1136/jech.55.5.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Natarajan L, Pu M, Parker BA, et al. Time-varying effects of prognostic factors associated with disease-free survival in breast cancer. Am J Epidemiol. 2009;169:1463–70. doi: 10.1093/aje/kwp077. [DOI] [PMC free article] [PubMed] [Google Scholar]