Abstract
Background
Infants (<12 months) who require liver transplantation (LTx) represent a particularly challenging and understudied group of patients.
Methods
This retrospective study aimed to describe a large single center experience of infants who received isolated LTx, illustrate important differences in infants vs. older children, and identify pre-transplant factors which influence survival. Over 25 pre-LTx demographic, laboratory, and operative variables were analyzed using the Log-Rank Test and Cox Proportional Hazards Model.
Results
Between 1984–2006 216 LTx were performed in 186 infants with a mean follow-up time of 62 months. Median age at LTx was 9 months, the majority had cholestatic liver disease, were hospitalized pre-LTx, and received whole grafts. Leading indications for re-LTx (n=30) included vascular complications (43%) and graft nonfunction (40%), while leading causes of death were sepsis and multi-organ failure. 1,5 and 10 year graft and patient survival were 75/72/68 and 79/77/75%. Relative to older pediatric recipients infants had worse overall patient survival (p=0.05). The following were significant univariate predictors of graft loss: age < 6 months, and reduced cadaveric grafts; and of patient loss: age < 6 months, calculated CrCl <90, pre-LTx hospitalization, pre-LTx mechanical ventilation, repeat LTx, infants transplanted for reasons other than cholestatic liver disease, and patients transplanted between 1984–1994.
Conclusions
Long-term outcomes for infants undergoing LTx are excellent and have improved over time. As the largest, single center analysis of LTx in infants this study elucidates a unique set of predictors which can aid in medical decision making.
Keywords: Pediatric, Infant, Liver transplantation, Survival
INTRODUCTION
Over the past twenty-five years liver transplantation (LTx) has become a lifesaving procedure for infants (defined as children < 12 months of age) with end-stage liver disease (1). Despite the fact that children under one year of age represent one out of four pediatric LTx recipients (2), infants with end-stage liver disease remain a challenging and understudied group. While it has been well documented that infants historically have had the highest rates of wait-list mortality amongst all pediatric candidates (3–6), only a limited number of studies exist which investigate the outcomes of these youngest LTx recipients (7–19). To date these prior series have struggled with small sample sizes, reported wide variation in outcomes, and have been unable to clearly identify risk factors of survival for infants undergoing LTx. The specific aims of this study were to examine a large single-center experience of LTx in infants, highlight unique differences in infants vs. older children, and elucidate important pre-LTx predictors of patient and graft survival.
MATERIALS AND METHODS
An IRB-approved, retrospective, single-center cohort study was conducted at the University of California Los Angeles. Inclusion criteria was all children < 1 year of age who received isolated LTx between February1984 – December 2006. Multi-organ recipients were excluded. Data sources consisted of medical center written records and a prospectively maintained LTx electronic database. Over twenty-five variables were collected which were related to recipient and donor demographics, operative characteristics and laboratory values within 24 hours prior to LTx. Specifically for the recipients these included: age, gender, ethnicity, primary diagnosis, height, weight, pre-LTx location, mechanical ventilation, renal replacement therapy, LTx number, year of LTx, graft type, cold and warm ischemia time, albumin, bilirubin, creatinine, and INR. For donors these included: age, height, weight, donor to recipient weight ratio, number of hospital days and number of vasopressors. Calculated variables included body mass index (kg/m2), creatinine clearance (cCrCl) using the Schwartz Formula [cGFR= (k*Height in cm)/Serum Creatinine in mg/dL; in which k= 0.33 for LBW infants <1 yr, and 0.45 for term infants < 1 yr], and calculated PELD score at the time of LTx = {0.436 [age (<1 year)] −0.687 × Loge[albumin g/dL] + 0.480 × Loge [total bilirubin mg/dL] +1.857 × Loge [INR] + 0.667 [growth failure (< 2 SD present)]} × 10 (20, 21).
Our immunosuppression and surveillance protocol has been previously described in detail (22). To summarize, from 1984 to 1987, induction and maintenance therapy consisted of cyclosporine (CsA) and prednisone. Azathioprine was added to this induction regimen between 1987 and 1994 in an attempt to lower steroid and CsA requirements. For children transplanted after 1994, primary immunosuppression consisted of tacrolimus (Tac) and prednisone. Acute rejection episodes during all time periods were treated with high dose intravenous methylprednisolone tapered over 7 days. OKT3 was reserved for steroid resistant rejection, and after 1994 conversion from CsA to Tac was also an option in such cases. Mycophenolate mofetil was used in cases of re-transplantation and post-transplant de-novo autoimmune hepatitis (23). Following LTx, patients were regularly evaluated in clinic and had routine laboratory studies drawn every 1–3 months. Whole grafts were used exclusively in the 1980s, followed by the options of reduced grafts beginning in 1990 (24), and living donation (LRD) beginning in 1992 (25). In situ split liver transplantation was also initiated in 1992 (26). The University of Wisconsin solution has been used for organ preservation since 1990. Prophylactic anticoagulation consisting of heparin and dextran has been used routinely in the initial post-LTx period. Duplex ultrasonography and percutaneous liver biopsies were performed when clinically indicated.
Statistical analysis was performed using JMP 7.0. Standard Kaplan-Meier technique was used to calculate patient and graft survival of infant LTx recipients. Chi-Square Test and Two-tailed Student’s T-test were used to compare proportions of nominal variables and mean values of continuous variables respectively between infants and all other pediatric recipients between 1–18 years of age. Potential univariate predictors of survival were analyzed using the Log-Rank test. Variables with a p-value of < 0.20 at the univariate level were included in a Cox Multivariable Proportional Hazards Model.
RESULTS
Between February 1984– December 2006, 852 LTx were performed in 657 patients ≤ 18 years of age. 216 isolated LTx performed in 186 infants were included in this study. A mean of 9.8 ± 5.8 infant LTx/year were performed; these were normally distributed over time and account for one-quarter of all pediatric LTx. The mean follow-up time was 62 ± 64 months.
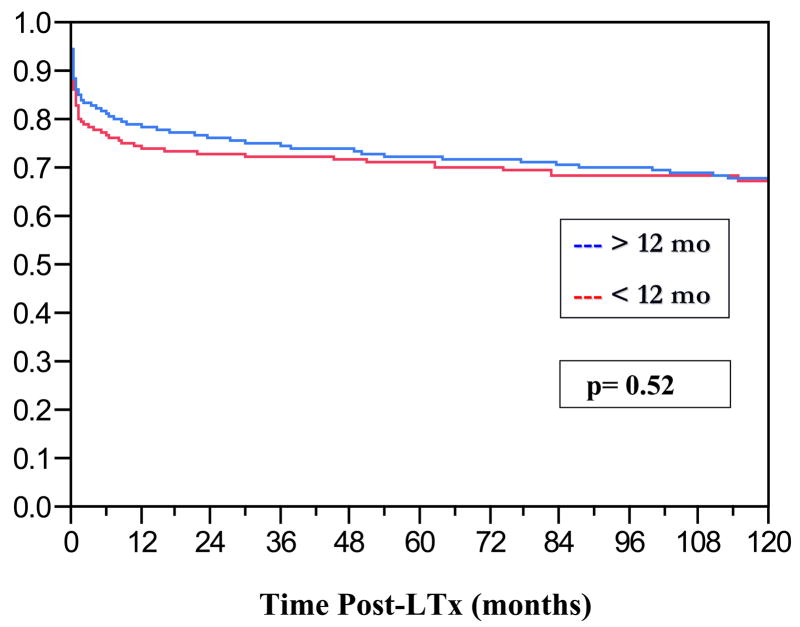
At the time of LTx the mean age was 8.7 ± 7.6 months, 50% of the recipients were female and the ethnic distribution reflected that of our local patient population (Table 1). The leading indications for LTx were cholestatic liver disease (60%), re-LTx (14%) and fulminant hepatic failure (8%). Mean anthropometric data, laboratories 24 hours prior to LTx, cCrCl, PELD scores and ischemia times are displayed in Table 1. Prior to LTx 42% of the infants were in the intensive care unit, 21% required mechanical ventilation and 1% hemodialysis. The graft types which were used were whole (56%), segmental (30%) and living related donor (14%). Graft and patient survival curves for infants vs. older children are shown in Figures 1 and 2. The 1/5/10 year graft and patient survival for infants undergoing LTx were: 75/72/68 and 79/77/75% respectively. While there was no significant difference in graft survival in infants versus older children (p=0.52), overall infants did have worse patient survival relative to older pediatric recipients (p=0.05, Hazard Ratio = 1.6). Other important pre-LTx differences were noted between infants and older pediatric recipients (Table 2). The majority of infant graft and patient loss occurred in the first thirty days post-LTx. Leading causes of infant graft loss were vascular thromboses, primary non-function and immunologic complications. The prevalence of these events leading to re-LTx was: hepatic artery thrombosis= 6.5%, portal vein thrombosis= 4.2%, and primary non-function= 7.4%. The leading causes of death were sepsis and multi-system organ failure.
Table 1.
Baseline Pre-LTx Clinical Characteristics
| VARIABLE | PERCENTAGE |
|---|---|
| Sex | |
| Female | 50 |
| Age at Transplant (months) | |
| 0–3 | 4 |
| 3–6 | 17 |
| 6–9 | 42 |
| 9–12 | 37 |
| Race | |
| African American | 11 |
| Asian | 9 |
| Caucasian | 28 |
| Latino | 49 |
| Middle Eastern | 3 |
| Primary Diagnosis | |
| Autoimmune Liver Disease | 1 |
| Cholestatic Liver Disease | 60 |
| Cryptogenic Cirrhosis | 2 |
| Fulminant Hepatic Failure | 8 |
| Metabolic Liver Disease | 6 |
| Neonatal Hepatitis | 6 |
| Parenteral Nutrition Associated Liver Disease | 1 |
| Re-Transplantation | 14 |
| Tumor | 2 |
| VARIABLE | MEAN ± SD |
| Height (cm) | 64 ± 11 |
| Weight (kg) | 7.2 ± 3.2 |
| Body Mass Index (kg/m2) | 16.9 ± 3.9 |
| Albumin (g/dL) | 3.1 ± 0.7 |
| Bilirubin (mg/dL) | 16.7 ± 13.5 |
| Creatinine (mg/dL) | 0.5 ± 0.5 |
| INR (ratio) | 1.8 ± 1.0 |
| Calculated PELD Score | 20 ± 12 |
| Calculated Creatinine Clearance (ml/min/1.73 m2) | 89 ± 56 |
| Cold Ischemia Time (min) | 357 ± 194 |
| Warm Ischemia Time (min) | 56 ± 22 |
| Donor Body Mass Index (kg/m2) | 20 ± 5.1 |
Figure 1.
Kaplan-Meier Graft Survival Curves of Infant LTx Recipients vs. LTx Recipients > 12 months of age
Figure 2.
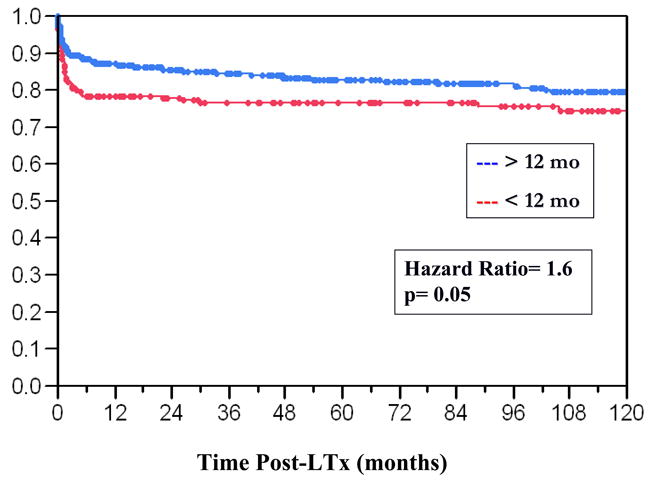
Kaplan-Meier Patient Survival Curves of Infant LTx Recipients vs. LTx Recipients >12 months of age
Table 2.
Significant Differences Between Infants and Older Pediatric Recipients
| Pre-LTx Variable | Infants | Older Children | P-value |
|---|---|---|---|
| Primary Diagnosis (%) | |||
| Cholestatic liver disease | 60 | 32 | <0.0001 |
| Pre-LTx Acuity (%) | |||
| Home/Ward/ICU Location | 38/20/42 | 52/11/37 | 0.001 |
| Mechanical Ventilation | 21 | 15 | 0.11 |
| Renal Replacement Therapy | 1 | 5 | 0.009 |
| Calculated PELD (Mean+/−SD) | 20 ± 12 | 12 ± 13 | <0.0001 |
| Calculated Creatinine Clearance (ml/min/1.73 m2) | 89 ± 56 | 132 ± 85 | <0.0001 |
| Graft Type (%) | |||
| Whole/Segmental/LRD | 56/30/14 | 82/15/3 | <0.0001 |
Chi-Square Test and Two-tailed Student’s T-test were used to compare proportions of nominal variables and mean values of continuous variables respectively between infants and all other pediatric recipients between 1–18 years of age.
Those variables which approached (p<0.20) or reached statistical significance (p<0.05) as univariate predictors of graft and patient survival for infant recipients are displayed along with their respective hazard ratios in Table 3. In multivariate analysis the significant predictor of graft survival was age < 6 months, and for patient survival: pre-LTx mechanical ventilation and re-LTx (Table 4).
Table 3.
Univariate Predictors of Graft and Patient Survival
| Pre-LTx Variable | Category I | Category II | Hazard Ratio | P-value |
|---|---|---|---|---|
| Graft Survival | ||||
| Age | < 6 months | > 6 months | 1.3 | 0.02 |
| Height | < 65 cm | > 65 cm | 1.4 | 0.08 |
| Graft Type | Whole Segmental | LRD | 1.1 3.7 |
0.04 |
| Donor BMI | >20 | <20 | 1.3 | 0.18 |
| Patient Survival | ||||
| Age | < 6 months | > 6 months | 1.9 | 0.05 |
| Calculated CrCl | < 90 | > 90 | 3.7 | 0.02 |
| INR | > 1.5 | < 1.5 | 1.8 | 0.07 |
| PELD | >25 | < 25 | 3.3 | 0.15 |
| Location | ICU Ward | Home | 2.5 1.9 |
0.01 |
| Mechanical Ventilation | Yes | No | 4.7 | 0.004 |
| LTx Number | Repeat | Primary | 6.5 | < 0.001 |
| Cold Ischemia Time | > 600 min | < 600 min | 1.5 | 0.09 |
| Diagnosis | Other | Cholestatic Liver Disease | 2.3 | 0.007 |
| Era | 1984–1994 | 1995–2006 | 2.6 | 0.01 |
p-value > 0.2 was not considered significant, p-value between 0.05 – 0.2 approached significance, and p-value < 0.05 was considered significant. Log-Rank Test was used for categorical variable. Hazard Ratio > 1 implies that patients in Category I have > risk of graft or death than patients in Category II.
Table 4.
Multivariate Predictors of Graft and Patient Survival
| Pre-LTx Variable | Category I | Category II | Hazard Ratio | P-value |
|---|---|---|---|---|
| Graft Survival | ||||
| Age | < 6 months | > 6 months | 2.1 | 0.02 |
| Patient Survival | ||||
| Mechanical Ventilation | Yes | No | 1.7 | 0.05 |
| LTx Number | Repeat | Primary | 2.7 | 0.05 |
The multivariate analyses included all variables from the univariate analysis that were significant or approached significance. Cox proportional hazards model was used. Hazard Ratio > 1 implies that patients in Category I have > risk of graft or patient loss than patients in Category II.
DISCUSSION
This study represents the largest single-center long-term analysis of a previously understudied, high-risk group of LTx recipients, children < 12 months old. Our intent was to describe our experience in detail, compare infants and older children, and identify unique risk factors of patient and graft survival. This analysis confirms that while long-term outcomes for infant LTx recipients are excellent and have improved over time, overall patient survival amongst infants is not equivalent to that of older children. We identified a unique set of variables which help explain these results and predict survival for these youngest LTx recipients.
In our twenty-plus year experience infants represent 25%, while neonates, defined as < 3 months old, make up less than 2% of the overall pediatric LTx experience. This distribution reflects nationwide statistics (2, 27, 28). The indications for LTx in infants are unique compared to older children in that a statistically significant greater proportion of infants (60%) relative to older children (32%) are transplanted for cholestatic liver disease. Meanwhile, in our study and prior reports over three-quarters of the LTx performed in the first 3 months of life are accounted for by neonatal hepatitis, metabolic liver disease and fulminant hepatic failure. (12, 27). The importance of pre-LTx diagnosis is that it has been shown in older children to strongly predict outcomes, with cholestatic liver disease consistently demonstrating better results (29–33) and fulminant hepatic failure demonstrating significantly worse post-LTx survival (34–36). These findings also proved true in our univariate analysis of patient survival of infants.
Our series and nationwide data demonstrate that patient survival rates for infants following LTx historically have been inferior to that of older children (28). The first 90 days following LTx is the time during which there is the biggest discrepancy. An explanation of this phenomenon is found by carefully examining pre-LTx characteristics. The pre-LTx medical condition of infants in general is more tenuous than that of older children, as is demonstrated in Table 2 by the fact that a statistically significant greater percentage of infants await LTx in the ICU, require mechanical ventilation, have calculated PELD scores significantly higher and cCrCl significantly lower than older children. All of these variables in turn predict worse patient survival in our univariate analysis. While these findings have been documented in other pediatric studies of older LTx recipients (3, 29, 37,), they represent novel finding in infants. Calculated creatinine clearance as a predictor of survival should be interpreted with caution as renal function changes throughout the first year of life, and cCrCl may often overestimate true GFR in the youngest children (38). Nonetheless, this finding that pre-LTx morbidity affects outcomes in infants lends credit to the idea that timely LTx is particularly important in this population.
Due to their small size and ensuing lack of sufficient number of suitable donors, infants have historically had the highest pediatric waitlist mortality rates, between 4–8 x that of older children (1,5,6). Subsequently, since the early 1990s infants have been considered as candidates for whole, segmental and living-related donor (LRD) grafts (39, 40). Relative to older children, infants in our series and in the Study of Pediatric Liver Transplantation (SPLIT) registry data (41) tend to be transplanted a greater portion of the time using segmental and LRD grafts. These options have improved donor supply for infants and decreased wait-list mortality (38,42,43). In examining our overall experience since 1984, univariate graft survival analysis indicates that segmental LTx from deceased donors had inferior outcomes for infant recipients. OPTN/SRTR and European transplant registry analyses confirm these findings (44–46). However, when the most recent decade is analyzed, graft type no longer plays a role in infant graft survival (Log-Rank Test, p=0.34). This suggests that there seems to be a learning curve involved in LTx of segmental grafts for infant recipients. It is important to note that in our experience this observation applies only to infants, not to older pediatric recipients in which graft survival has not been a significant factor of survival over time (47).
The option of LRD for infant recipients potentially allows for optimal timing of LTx with minimal ischemia time. While LRD poses ethical considerations and potential risks to the donor, the outcomes in our univariate analysis demonstrated that LRD lead to equal or better graft survival than whole or segmental grafts. The Kyoto series which is the largest LRD experience in the world, shows excellent patient and graft survival rates which are comparable to the overall results of cadaveric pediatric LTx (48). Of note, in our multivariate graft survival and in our patient survival analysis LRD did not show a statistical advantage for infants. This mixed picture is echoed in other single center experiences in the literature (49–51).
One cannot discuss pediatric LTx without talking about vascular complications, which account for a large portion of graft loss amongst pediatric recipients (3, 14). Nowhere is this more apparent than in infants. In our series over 10% of all infant primary LTx recipients underwent re-LTx due to HAT or PVT. Along these lines our youngest (< 6 months) and smallest (< 65 cm) recipients sustained worse graft survival in our univariate analysis. In children < 3 months of age long-term graft survival has been reported as low as 50–65% (11–13,18, 52, 53), with higher rates of portal vein and hepatic artery thrombosis reported than in older children (54). Despite technical advances, vascular thromboses remain a serious cause of graft loss with an overall incidence of between 5–26% in infants < 10 kg (10–12, 15, 19, 55–58).
Re-LTx is necessary for 10–30% of pediatric LTx recipients, and relative to primary LTx, re-LTx has been previously shown in older children to carry an increased risk of death (11, 59–61). Infants undergoing re-LTx seem to be particularly vulnerable (Table 4).
Over time patient survival in infant recipients has improved significantly. A survival advantage was noted in our analysis when the more recent decade is compared with the prior decade. In our study there was no difference in patient survival rates between infants and older pediatric patients when the analysis is limited to the past ten years (Log-Rank Test p=0.62). As our group and other centers have previously reported, infants have experienced the most improvement in outcomes amongst pediatric recipients in comparing the past two decades (1, 19). This phenomenon is verified by OPTN/SRTR data since 2000 in which the death rate for infant recipients during the first year after LTx has fallen steadily (5). Such trends are likely in part due to continued experience and refinement in surgical techniques, improved pre and post-LTx care, and perhaps the adoption of the PELD system in 2002 which recognized that children < 1 year of age had a higher incidence of death on the waiting list thus requiring timely consideration for LTx (4).
One of the main limitations of this study is that this was a retrospective analysis based on a database which is prospectively maintained, therefore the predictors should be interpreted as associations. The study also spanned over twenty plus years time during which changes have occurred in immunosuppression, surgical technique and experience, graft types and the organ allocation system. Our analysis was not designed to examine in detail important long-term issues for infants including growth and development, post-transplant lymphoproliferative disease and metabolic complications. Nonetheless, this analysis is to date the largest, detailed single-center study of infant LTx. The long-term results indicate excellent outcomes which have improved substantially over time. The identification of these significant predictors, all of which relate to the recipients’ pre-LTx condition, can aid clinicians and surgeons in medical decision making.
Abbreviations
- LTx
Liver Transplantation
- cCrCl
Calculated Creatinine Clearance
- CsA
Cyclosporine
- Tac
Tacrolimus
- LRD
Living Related Donor
Footnotes
Robert S Venick
Participated in research design, Participated in the writing of the paper, Participated in the performance of the research, Participated in data analysis
Dr. Robert Venick’s work is supported by the NIH Loan Repayment Program and the American Society of Transplantation Clinical Faculty Development Award
No conflict of interest
Pediatric Gastroenterology, Hepatology & Nutrition, Mattel Children’s Hospital at UCLA, Los Angeles, CA
Douglas G Farmer
Participated in research design, Participated in the writing of the paper, Participated in the performance of the research, Participated in data analysis No support received for this study; No conflict of interest Surgery, David Geffen School of Medicine at UCLA, Los Angeles, CA
Sue V McDiarmid
Participated in research design, Participated in the writing of the paper, Participated in the performance of the research, Participated in data analysis No support received for this study; No conflict of interest Pediatric Gastroenterology, Hepatology & Nutrition, Mattel Children’s Hospital at UCLA, Los Angeles, CA
John P Duffy
Participated in research design, Participated in the writing of the paper, Participated in data analysis No support received for this study; No conflict of interest Surgery, David Geffen School of Medicine at UCLA, Los Angeles, CA
Sherilyn A Gordon
Participated in research design, Participated in the writing of the paper, Participated in the performance of the research No support received for this study; No conflict of interest Surgery, David Geffen School of Medicine at UCLA, Los Angeles, CA
Hasan Yersiz
Participated in research design, Participated in the writing of the paper, Participated in data analysis No support received for this study; No conflict of interest Surgery, David Geffen School of Medicine at UCLA, Los Angeles, CA
Johnny C Hong
Participated in research design, Participated in the writing of the paper, Participated in data analysis No support received for this study; No conflict of interest Surgery, David Geffen School of Medicine at UCLA, Los Angeles, CA
Jorge H Vargas
Participated in research design, Participated in the writing of the paper, Participated in the performance of the research No support received for this study; No conflict of interest Pediatric Gastroenterology, Hepatology & Nutrition, Mattel Children’s Hospital at UCLA, Los Angeles, CA
Marvin E Ament
Participated in research design, Participated in the writing of the paper, Participated in the performance of the research No support received for this study; No conflict of interest Pediatric Gastroenterology, Hepatology & Nutrition, Mattel Children’s Hospital at UCLA, Los Angeles, CA
Ronald W Busuttil
Participated in research design, Participated in the writing of the paper, Participated in data analysis No support received for this study; No conflict of interest Surgery, David Geffen School of Medicine at UCLA, Los Angeles, CA
References
- 1.McDiarmid SV. Current status of liver transplantation in children. The Pediatric Clinics of North America. 2003;50:1335–1374. doi: 10.1016/s0031-3955(03)00150-0. [DOI] [PubMed] [Google Scholar]
- 2. [Accessed on August 1, 2007];The Organ Procurement and Transplantation Network. Available at: http://www.OPTN.org.
- 3.Farmer DG, Venick RS, McDiarmid SV, et al. Predictors of outocmes after pediatric liver transplantation: an analysis of more than 800 cases performed at a single institution. Journal of the American College of Surgeons. 2007;204:904–914. doi: 10.1016/j.jamcollsurg.2007.01.061. [DOI] [PubMed] [Google Scholar]
- 4.McDiarmid S, Merion R, Dykstra D, et al. Use of a pediatric end-stage liver disease score for deceased donor allocation: The United States experience. Indian Journal of Pediatrics. 2007;74:387–392. doi: 10.1007/s12098-007-0066-2. [DOI] [PubMed] [Google Scholar]
- 5.Horslen S, Barr M, Christensen L, et al. Pediatric Transplantation in the United States, 1996–2005. American Journal of Transplantation. 2007;7(Part 2):1339–1359. doi: 10.1111/j.1600-6143.2007.01780.x. [DOI] [PubMed] [Google Scholar]
- 6.Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients. Transplant Data 1992–2001. :489. HHS/HRSA/DOT, UNOS, URREA. Table 9.3. Available at: http://www.ustransplant.org.
- 7.Esquivel CO, Koneru B, Karrer F, et al. Liver transplantation before 1 year of age. Journal of Pediatrics. 1987;110:545. doi: 10.1016/s0022-3476(87)80545-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sokal EM, Veyckemans F, deVille de Goyet J, et al. Liver transplantation in children less than 1 year of age. Journal of Pediatrics. 1990;117:205. doi: 10.1016/s0022-3476(05)80531-1. [DOI] [PubMed] [Google Scholar]
- 9.Beath SV, Brook GD, Kelly DA, et al. Successful liver transplantation in babies under 1 year. BMJ. 1993;307:825. doi: 10.1136/bmj.307.6908.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colombani PM, Cigarroa FG, Schwarz K, et al. Liver transplantation in infants younger than 1 year of age. Annals of Surgery. 1996;223:658. doi: 10.1097/00000658-199606000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cacciarelli TV, Esquivel CO, Moore DH, et al. Factors affecting survival after orthotopic liver transplantation in infants. Transplantation. 1997;64:242–248. doi: 10.1097/00007890-199707270-00011. [DOI] [PubMed] [Google Scholar]
- 12.Woodle ES, Millis JM, So SKS, et al. Liver transplantation in the first 3 months of life. Transplantation. 1998;66:606–609. doi: 10.1097/00007890-199809150-00010. [DOI] [PubMed] [Google Scholar]
- 13.Noujaim HM, Mayer DA, Buckles JA, et al. Techniques for and outcomes of liver transplantation in neonates and infants weighing up to 5 kilograms. Journal of Pediatric Surgery. 2002;37:159–164. doi: 10.1053/jpsu.2002.30242. [DOI] [PubMed] [Google Scholar]
- 14.Sundaram S, Alonso EM, Whitington PF. Liver transplantation in neonates. Liver Transplantation. 2003;9:783. doi: 10.1053/jlts.2003.50104. [DOI] [PubMed] [Google Scholar]
- 15.Grabhorn E, Schulz A, Helmke K, et al. Short and long-term results of liver transplantation in infants aged less than 6 months. Transplantation. 2004;78:235–241. doi: 10.1097/01.tp.0000128189.54868.18. [DOI] [PubMed] [Google Scholar]
- 16.Lucianetti A, Guizzetti M, Bertani A, et al. Liver transplantation in children weighing less than 6 kg: the Bergamo experience. Transplantation Proceedings. 2005;37:1143–1145. doi: 10.1016/j.transproceed.2004.12.307. [DOI] [PubMed] [Google Scholar]
- 17.Tiao G, Alonso M, Bezerra J, et al. Liver transplantation in children younger than 1 year- the Cincinnati experience. Journal of Pediatric Surgery. 2005;40:269–273. doi: 10.1016/j.jpedsurg.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 18.Mekeel K, Langham M, Gonzalez-Peralta R, et al. Liver Transplantation in very small infants. Pediatric Transplantation. 2007;11:66–72. doi: 10.1111/j.1399-3046.2006.00610.x. [DOI] [PubMed] [Google Scholar]
- 19.Neto J, Caroner V, Pugliese V, et al. Living donor liver transplantation for children in Brazil weighing less than 10 kilograms. Liver Transplantation. 2007;13:1153–1158. doi: 10.1002/lt.21206. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz GJ, Haycock GB, Edelmann CM, Jr, et al. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–263. [PubMed] [Google Scholar]
- 21.Freeman RB, Jr, Wiesner RH, Harper A, et al. UNOS/OPTN Liver Disease Severity Score, UNOS/OPTN Liver and Intestine, and UNOS/OPTN Pediatric Transplantation Committees. The new liver allocation system: moving toward evidence based transplantation policy. Liver Transplantation. 2002;8:851–858. doi: 10.1053/jlts.2002.35927. [DOI] [PubMed] [Google Scholar]
- 22.McDiarmid SV, Farmer DA, Goldstein LI, et al. A randomized prospective trial of steroid withdrawal after liver transplantation. Transplantation. 1995;60:1443–1450. doi: 10.1097/00007890-199560120-00013. [DOI] [PubMed] [Google Scholar]
- 23.Venick RS, McDiarmid SV, Farmer DG, et al. Rejection and Steroid Dependence: Unique Risk Factors in the Development of Pediatric Posttransplant De Novo Autoimmune Hepatitis. American Journal of Transplantation. 2007;7(4):955–63. doi: 10.1111/j.1600-6143.2006.01717.x. [DOI] [PubMed] [Google Scholar]
- 24.Jurim O, Csete M, Gelabert HA, et al. Reduced-size grafts--the solution for hepatic artery thrombosis after pediatric liver transplantation? J Pediatr Surg. 1995 Jan;30(1):53–5. doi: 10.1016/0022-3468(95)90609-6. [DOI] [PubMed] [Google Scholar]
- 25.Busuttil RW, Goss JA. Split liver transplantation. Annals of Surgery. 1999;229:313–321. doi: 10.1097/00000658-199903000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goss JA, Yersiz H, Shackleton CR, et al. In situ splitting of the cadaveric liver for transplantation. Transplantation. 1997;64:871–7. doi: 10.1097/00007890-199709270-00014. [DOI] [PubMed] [Google Scholar]
- 27.Alonso EM, Besedovsky A, Emerick K, et al. General criteria for pediatric transplantation. In: Busuttill RW, Klintmalm GB, editors. Transplantation of the Liver. 2. Philadelphia: Elseveir Saunders; 2005. pp. 287–302. [Google Scholar]
- 28.Colombani PM, Dunn SP, Harmon WE, et al. Pediatric Transplantation. American Journal of Transplantation. 2003;3 (Suppl 4):53–63. doi: 10.1034/j.1600-6143.3.s4.6.x. [DOI] [PubMed] [Google Scholar]
- 29.Fouquet V, Alves A, Branchereau S, et al. Long-term outcome of pediatric liver transplantation for biliary atresia: a 10-year follow-up in a single center. Liver Transplantation. 2005;11:152–160. doi: 10.1002/lt.20358. [DOI] [PubMed] [Google Scholar]
- 30.Goss JA, Shackleton C, Swenson K, et al. Orthotopic liver transplantation for congenital biliary atresia. An 11-year, single center experience. Annals of Surgery. 1996;224:276–284. doi: 10.1097/00000658-199609000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diem HV, Evrard V, Vinh HT, et al. Pediatric liver transplantation for biliary atresia: results of primary grafts in 328 recipients. Transplantation. 2003;75:1692–1697. doi: 10.1097/01.TP.0000062570.83203.A3. [DOI] [PubMed] [Google Scholar]
- 32.Utterson E, Shepherd R, Sokol R, et al. Biliary atresia: clinical profiles, risk factors, and outcomes of 755 patients listed for liver transplantation. Journal of Pediatrics. 2005;147:180–185. doi: 10.1016/j.jpeds.2005.04.073. [DOI] [PubMed] [Google Scholar]
- 33.Barshes N, Lee T, Balkrishnan R, et al. Orthotopic liver transplantation for biliary atresia: the US experience. Liver Transplantation. 2005;11:1193–1200. doi: 10.1002/lt.20509. [DOI] [PubMed] [Google Scholar]
- 34.Ueda M, Oike F, Ogura Y, et al. Long-term outcomes of 600 living donor liver transplants for pediatric patients at a single center. Liver Transplantation. 2006;12:1326–1336. doi: 10.1002/lt.20826. [DOI] [PubMed] [Google Scholar]
- 35.Martin S, Atkison P, Anand R, et al. SPLIT Research Group: Studies of pediatric liver transplantation 2002: patient and graft survival and rejection in pediatric recipients of a first liver transplant in the United States and Canada. Pediatric Transplantation. 2004;8:273–283. doi: 10.1111/j.1399-3046.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- 36.Baliga P, Alvarez S, Lindblad A, et al. Posttransplant survival in pediatric fulminant hepatic failure: the SPLIT experience. Liver Transplantation. 2004;10:1364–1371. doi: 10.1002/lt.20252. [DOI] [PubMed] [Google Scholar]
- 37.Barshes NR, Lee TC, Udell IW, et al. The pediatric end-stage liver disease (PELD) model as a predictor of survival benefit and posttransplant survival in pediatric liver transplant recipients. Liver Transplantation. 2006;12:475–480. doi: 10.1002/lt.20703. [DOI] [PubMed] [Google Scholar]
- 38.van Rossum L, Mathot R, Cransberg K, et al. Estimation of the glomerular filtration rate in children: which algorithm should be used? Pediatr Nephrol. 2005;20:1769–1775. doi: 10.1007/s00467-005-2001-y. [DOI] [PubMed] [Google Scholar]
- 39.Broelsch CE, Emond JC, Thistlethwaite JR, et al. Liver transplantation, including the concept of reduced-size liver transplants in children. Annals of Surgery. 1988;208:410–20. doi: 10.1097/00000658-198810000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Broelsch CE, Whitington PF, Emond JC, et al. Liver transplantation in children from living related donors: surgical techniques and results. Annals of Surgery. 1991;214:428–37. doi: 10.1097/00000658-199110000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDiarmid SV, Anand R, Linblad AS SPLIT Research Group. Studies of Pediatric Liver Transplantation: 2002 update. An overview of demographics, indications, timing, and immunosuppressive practices in pediatric liver transplantation in the United States and Canada Pediatric. Transplantation. 2004;8:284–94. doi: 10.1111/j.1399-3046.2004.00153.x. [DOI] [PubMed] [Google Scholar]
- 42.Otte JB, de Ville de Goyet J, Reding R, et al. Pediatric liver transplantation: from the full-size liver graft to reduced, split, and living related liver transplantation. Pediatr Surg Int. 1998;13:308–18. doi: 10.1007/s003830050328. [DOI] [PubMed] [Google Scholar]
- 43.de Ville de Goyet J, Hausleithner V, Reding R, et al. Impact of innovative techniques on the waiting list and results in pediatric liver transplantation. Transplantation. 1993;56:1130–6. doi: 10.1097/00007890-199311000-00016. [DOI] [PubMed] [Google Scholar]
- 44.Roberts JP, Hulbert-Shearon TE, Merion RM, et al. Influence of Graft Type on Outcomes after Pediatric Liver Transplantation. American Journal of Transplantation. 2004;4:373–377. doi: 10.1111/j.1600-6143.2004.00359.x. [DOI] [PubMed] [Google Scholar]
- 45.Sindhi R, Rosendale J, Mundy D, et al. Impact of segmental grafts on pediatric liver transplantation: a review of the United Network for Organ Sharing Scientific Registry data (1990–1996) Journal of Pediatric Surgery. 1999;34(1):107–10. doi: 10.1016/s0022-3468(99)90238-5. [DOI] [PubMed] [Google Scholar]
- 46.Wiesenhaan-Stellingwerff G, Smits J, de Boer J, et al. Pediatric liver transplantation:10 years Eurotransplant experience [abstract] Pediatric Transplantation. 2000;4(2):124. [Google Scholar]
- 47.Ghobrial R, Yersiz H, Farmer D, et al. Predictors of Survival after in vivo split liver transplantation: analysis of 110 consecutive patients. Annals of Surgery. 2000;232(3):312–323. doi: 10.1097/00000658-200009000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kiuchi T, Ueomoto S, Egawa H, et al. Living donor liver transplantation in Kyoto, 2001. Clin Transplant. 2001:195–201. [PubMed] [Google Scholar]
- 49.Deshpande RR, Bowles MJ, Vilca-Melendez H, et al. Results of split liver transplantation in children. Annals of Surgery. 2002;236:248–53. doi: 10.1097/00000658-200208000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wallot MA, Mathot M, Janssen M, et al. Long-term survival and late graft loss in pediatric liver transplant recipients: a 15-year single-center experience. Liver Transplantation. 2002;8:615–22. doi: 10.1053/jlts.2002.34149. [DOI] [PubMed] [Google Scholar]
- 51.Spada M, Gridelli B, Colledan M, et al. Extensive use of split liver for pediatric liver transplantation: a single-center experience. Liver Transplantation. 2000;6:415–28. doi: 10.1053/jlts.2000.7570. [DOI] [PubMed] [Google Scholar]
- 52.Cox K, Berquist W, Castillo R. Pediatric liver transplantation: Indications, timing and medical complications. J Gastroenterol Hepatol. 1999;14(Suppl):S61–S66. doi: 10.1046/j.1440-1746.1999.01904.x. [DOI] [PubMed] [Google Scholar]
- 53.Bonatti H, Muiesan P, Connelly S, et al. Hepatic transplantation in children under 3 months of age: A single centre’s experience. J Pediatr Surg. 1997;32:486–488. doi: 10.1016/s0022-3468(97)90612-6. [DOI] [PubMed] [Google Scholar]
- 54.Van der Werf WJ, D’Alessandro AM, Knechtle SJ, et al. Infant pediatric liver transplantation results equal those for older pediatric patients. J Pediatr Surg. 1998;33:20–23. doi: 10.1016/s0022-3468(98)90353-0. [DOI] [PubMed] [Google Scholar]
- 55.Tzakis AG, Gordon RD, Shaw BWJ, et al. Clinical presentation of hepatic artery thrombosis after liver transplantation in the cyclosporine era. Transplantation. 1985;40:667–71. doi: 10.1097/00007890-198512000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mazzaferro V, Esquivel CO, Makowka L, et al. Factors responsible for hepatic artery thrombosis after pediatric liver transplantation. Transplant Proceedings. 1989;21:2466–7. [PMC free article] [PubMed] [Google Scholar]
- 57.Saing H, Fan ST, Chan KL, et al. Liver transplantation in infants. J Pediatr Surg. 1999;34:1721–1724. doi: 10.1016/s0022-3468(99)90653-x. [DOI] [PubMed] [Google Scholar]
- 58.Iglesias J, Lopez JA, Ortega J, et al. Liver transplantation in infants weighing under 7 kilograms: management and outcome of PICU. Pediatric Transplant. 2004;8:228–232. doi: 10.1111/j.1399-3046.2004.00128.x. [DOI] [PubMed] [Google Scholar]
- 59.Newell KA, Millis JM, Bruce DS, et al. An analysis of hepatic retransplantation in children. Transplantation. 1998;65:1172. doi: 10.1097/00007890-199805150-00005. [DOI] [PubMed] [Google Scholar]
- 60.McDiarmid SV. Liver Transplantation: The Pediatric Challenge. Clinic in Liver Disease. 2000;4:879–927. doi: 10.1016/s1089-3261(05)70146-x. [DOI] [PubMed] [Google Scholar]
- 61.Esquivel CO. Results: Survival and Quality of Life After Orthotopic Liver Transplantation in Children. In: Busuttill RW, Klintmalm GB, editors. Transplantation of the Liver. 2. Philadelphia: Elseveir Saunders; 2005. pp. 1335–1354. [Google Scholar]