Abstract
Neutrophils are short-lived granulocytic cells of the innate immune system specialized in the production of reactive oxygen species. S100A8 and S100A9 and their heterocomplex calprotectin play a role in neutrophil recruitment and represent 40% of neutrophil cytosolic protein weight. The present study was designed to test the effect of S100A8 and S100A9 on the rate of neutrophil oxidative metabolism. It is hypothesized that the two S100 proteins inhibit neutrophil associated oxidation. Granulocytes freshly isolated from healthy volunteers were tested for their ability to oxidize dichlorofluorescin-diacetate (DCFH-DA) in-vitro. The data showed that S100A8 and S100A9 inhibited spontaneous and stimulated oxidation of the DCFH-DA probe by neutrophils. The inhibition of neutrophil oxidative metabolism by S100A8 and S100A9 was markedly reduced by the enzymatic activity of adenosine deaminase. Inhibitors of the P1 adenosine receptors also reduced the anti-oxidative effect of S100A8/A9 providing further support for the involvement of adenosine metabolites in S100A8/A9 anti-oxidative effect.
Keywords: Neutrophil, ROS, S100A8, S100A9, calprotectin, adenosine, DCFH-DA
Introduction
The infiltration of damaged tissue by polymorphonuclear neutrophilic leukocytes (neutrophils or PMN) and their subsequent activation is critical for the host defense against microbial threats. Once recruited during acute inflammation, neutrophils produce and release copious amounts of reactive oxygen species (ROS) which target potential bacterial invaders. A failure in sufficient production of ROS leads to infections as observed in chronic granulomatous disease (CGD), a disease prompted by a deficient oxidase system in neutrophils [1]. Conversely, excess ROS production is associated with conditions such as chronic wounds [2] and cardiovascular diseases [3].
Calprotectin, a heterocomplex formed by S100A8 and S100A9, which are two calcium binding proteins, represents 40% of neutrophil cytosolic proteins by weight [4]. High serum levels of calprotectin are associated with an immune deficiency syndrome, growth retardation and arthritis [5–7]. Work done by others and confirmed by us has demonstrated that calprotectin regulates neutrophil migration [8–10]. We have shown that S100A8 and S100A9 repel neutrophils in-vitro and that S100A8 inhibits the recruitment of neutrophils in-vivo [10,11].
Neutrophils isolated from healthy volunteers spontaneously produce and release ROS such as superoxide anion [12] in-vitro. Reports have indicated that exaggerated or reduced rates of spontaneous neutrophil activation are linked to viral [13] or autoimmune conditions [14], implying a physiological function for spontaneous neutrophil activation. Spontaneous and stimulated activation of neutrophil oxidative metabolism has been extensively documented in-vitro [15–18]. The production and release of ROS by neutrophils is dependent on the NADPH oxidase system and it can be accelerated by phorbol 12-myristate 13-acetate (PMA), a molecule which activates protein kinase C (PKC) resulting in the phosphorylation of critical sub-units of the NADPH oxidase complex [19]. Neutrophils oxidative metabolism can also be hastened by bacterial products such as lipopolysaccharides (LPS), whereas adenosine metabolites have been shown to inhibit neutrophil oxidative functions via P1 adenosine receptors [20,21]. P1 receptors are seven-transmembrane purinergic comprising A1, A2A, A2B and A3 receptors. A2A and A3 receptors are functionally expressed in neutrophils and also implicated in chemotaxis [22].
Work done by others has suggested that human and murine S100A9 inhibit the oxidative burst of macrophages contributing to the persistence of inflammatory processes in a mechanism which remains elusive [23]. Whether S100A8 and S100A9 would affect neutrophil oxidative metabolism remains unknown.
Accordingly, in this work, we tested the hypothesis that S100A8 and S100A9 negatively affected spontaneous and stimulated neutrophil oxidative metabolism. We present data supporting this hypothesis and implicating adenosine metabolites in S100A8 and S100A9 anti-oxidative effects.
Materials and methods
Expression and purification of recombinant S100 proteins
Recombinant S100A8 and S100A9 protein were produced and purified based on standard methods as previously described [10,11]. Briefly, both proteins were cloned in a pGEX-2T GST vector (Amersham, Piscataway, NJ). The proteins were expressed in Top-10 F' E-coli as GST fusion proteins. The GST tag was cleaved during the purification process. Protein concentration was assessed through a Bradford protein assay (Pierce, Rockford, IL).
Reagents
Dichlorofluorescin diacetate (DCFH-DA) and Bisindolylmaleimide I (GFX 109203X) (GFX for short) were purchased from EMD Calbiochem (San Diego, CA). Phorbol 12-myristate 13-acetate (PMA), lipopolysaccharides (LPS) from escherichia coli, N-(2-Methoxyphenyl)-N′-[2-(3-pyridinyl)-4-quinazolinyl]-urea (VUF5574) (an A3 adenosine antagonist) and Human adenosine deaminase (ADA1), from human erythrocytes were purchased from Sigma-Aldrich (St. Louis, MO). Mouse monoclonal antibody directed against S100A8 or S100A9 were purchased from Novus Biologicals (Littleton, CO).
Isolation of peripheral neutrophils
Human peripheral neutrophils were isolated from heparinized blood donated by healthy volunteers according to a protocol approved by the University of Illinois Institutional Review Board. The cells were isolated using a histopaque gradient Sigma-Aldrich (St. Louis, MO) according to the manufacturer's instructions. Cell viability and identity was confirmed by tryptan blue staining. Live cells and neutrophils represented at least 95% of isolated leukocytes.
Assay for oxidative activation of neutrophils
The method for the measurement of oxidative activation of neutrophils was based on the ROS-dependent oxidation of DCFH-DA to DCF and was adapted from Ciapetti et al. [15]. DCFH-DA crosses the cell membrane and is hydrolysed by non-specific esterases to non-fluorescent DCFH-DA. Its oxidation by ROS results in the generation of highly fluorescent DCF [24]. DCFH-DA is therefore a widely accepted probe for the measurement of an overall index of oxidative activity. DCFH-DA was purchased from Calbiochem (Madison, WI). The assays were run in clear bottom black 96-well plates. ‘Edge effects’ (a higher fluorescence in edge wells) were avoided by using only centre wells. Briefly, 50 μl of Dubelco's Phosphate Buffered Saline (DPBS) containing DCFH- DA was added to each well with the final concentration of 10 μg/ml. S100 proteins or PKC inhibitors were diluted in the wells. Just before a baseline reading 100 000 neutrophils in 50 μl PBS were placed in each well; 96-well plates were incubated at 37°C and 5% CO2 and were read at baseline (immediately after cell addition to the plates) and at indicated time points in a Spectra Max Gemini XS fluorescent plate reader. The excitation wavelength was 485 and the reading was done at 530 nm. Wells with no DCFH-DA were used to measure background fluorescence which was subtracted from each reading. Controls with no cells were also analysed and display no increased fluorescence over time. All assays were conducted in triplicate or quadruplicate wells.
Data analysis
In order to avoid differences between donors, experimental procedures and/or plate-to-plate variation, all experimental conditions and statistical analysis were run within one plate inclusive of all positive and negative controls. Single sample t-tests were calculated to test the S100A8, S100A9 and combined proteins in comparison to the control wells with alpha set to ≤ 0.05 to determine statistical significance. Comparisons between the S100A8, S100A9 and combined proteins were calculated with two sample t-tests using SPSS (SPSS Inc., Chicago, IL) with alpha set to ≤ 0.05 to determine statistical relevance.
Results
S100A8 and S100A9 decrease spontaneous oxidative activation of neutrophils
The experimental conditions in which we tested peripheral neutrophils were such that incubation resulted in the relatively slow oxidation of the DCFH-DA probe in the absence of any further stimulus. This process was followed over time in the presence and absence of different concentrations of S100A8, S100A9 and with a mixture of both (at a 1:1 weight ratio) referred to as calprotectin.
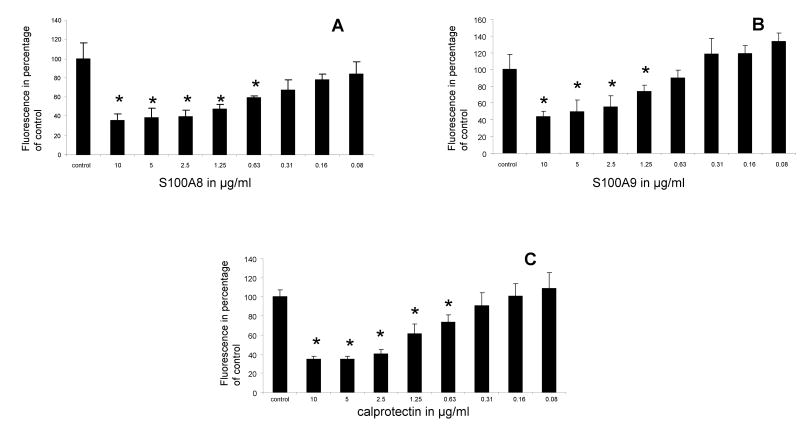
S100A8 and S100A9 together and each alone reduced the rate at which spontaneous oxidation of the DCFH-DA probe occurred in a dose-dependent manner. One hour after their incubation with DCFH-DA, the two proteins reduced the oxidation of the DCFH-DA probe by as much as 75% of control (Figures 1A–C).
Figure 1.
Fluorescence emission of neutrophils incubated with DCFH-DA probe for 1 h in the presence of various concentrations of S100A8 (A), S100A9 (B) and mixture of both S100 proteins (calprotectin) (C). Each point is the mean ± SD. This is a representative experiment of at least four experiments conducted in triplicates or quadruplicate wells. * p < 0.05 compared to control.
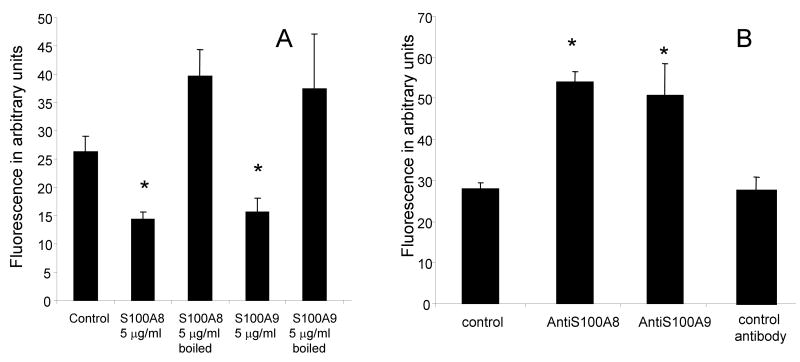
In order to rule out the possibility that S100 proteins reduced the oxidation of the DCFH-DA probe in a non-specific manner, additional control experiments were conducted. First, the S100 proteins were boiled and tested for their anti-oxidative function. Whereas S100A8 or S100A9 at concentrations of 5 μg/ml inhibited the oxidative activation of neutrophils (p < 0.05), the same concentrations of S100A8 or S100A9 did not significantly affect neutrophil oxidative rate when the protein was first boiled (Figure 2A).
Figure 2.
Fluorescence emission of neutrophils incubated with DCFH-DA probe for 3 h in the presence of 5 μg/ml S100A8 or S100A9 which were heat inactivated S100A8 (boiled for 5 min) (A) or antibodies directed against S100A8 or S100A9 (B) at a concentration of 1 μg/ml each. * p< 0.05 compared to control. The data represents the mean ± SD. This is a representative experiment of three experiments conducted in triplicate.
Secondly, because neutrophils produce and release S100A8 and S100A9 [25], we tested the effect of blocking the activity of endogenous S100A8/S100A9 on the oxidative metabolism of neutrophils. The data indicated that antibodies to S100A8 or S100A9 produced an increase in the rate of DCFH-DA oxidation, an effect opposite to the effect observed with the addition of extrinsic S100A8 or S100A9 (Figure 2B). Isotypic control antibodies had no measurable effect on DCFH-DA oxidation. Together those two control experiments supported the hypothesis that S100A8 and S100A9 had a specific inhibitory effect on neutrophil oxidative metabolism and that intrinsic S100A8 and S100A9 had a constitutive activity during the conduct of the DCFH-DA based oxidation assays.
The spontaneous activation of neutrophil oxidative metabolism is inhibited by GFX, a PKC inhibitor and enhanced by PMA a PKC activator
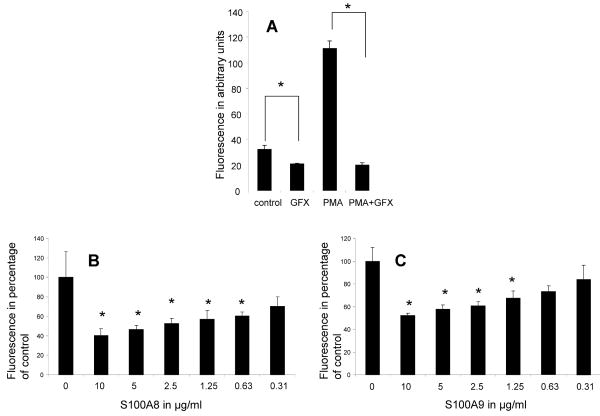
We next tested the effect of phorbol myristate acetate (PMA), a protein kinase C (PKC) activator on neutrophil oxidative activity. Not surprisingly, the data demonstrated that PMA significantly accelerated the rate of DCFH-DA oxidation. GFX (Bisindolylmaleimide I), a broad PKC inhibitor, significantly inhibited both the PMA induced and the spontaneous activation of neutrophils oxidative metabolism (Figure 3A). This would indicate that the spontaneous activation of neutrophils, similarly to the PMA activation, was dependent on PKC activity, supporting the argument that the spontaneous activation was a genuine activation of neutrophil oxidative mechanism.
Figure 3.
Fluorescence emission of neutrophils incubated with DCFH-DA probe in the presence of 1 μM PMA; 5 μM GFX caused a significant decrease in the spontaneous and the PMA induced oxidation of the DCFH-DA probe (A). S100A8 (B) and S100A9 (C) produce a dose sensitive inhibition of PMA (1 μM) induced oxidative activation. * p < 0.05 compared to control. The data represents the mean ± SD. This is a representative experiment of at least three experiments conducted in triplicate or quadruplicate.
S100A8 and S100A9 inhibited the stimulated activation of neutrophils oxidative metabolism
In the next set of experiments, we tested the effect of S100A8 and S100A9 on PMA stimulation of neutrophil oxidative metabolism. The data indicated that S100A8 and S100A9 produced a dose-dependent inhibition of PMA activation of neutrophils (Figures 3B and C) similar to what was observed above with the inhibition of the spontaneous oxidative activation.
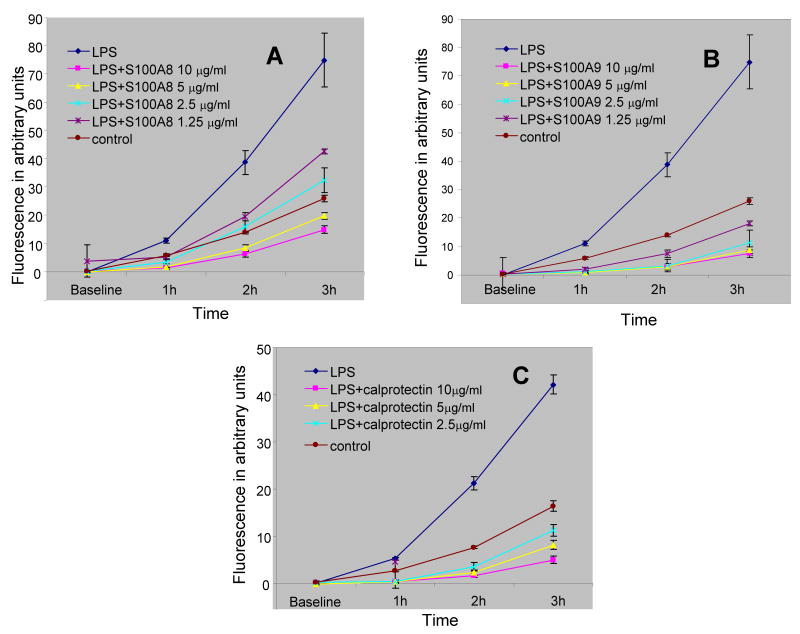
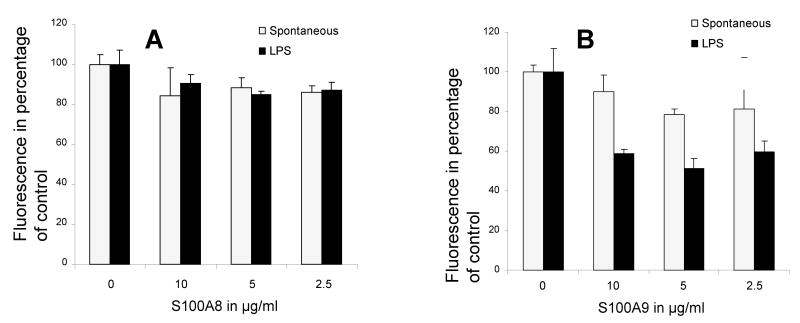
The effect of S100A8 and S100A9 together and each alone was further tested on the rate at which LPS stimulated neutrophil. The data indicated that at concentrations ranging from 2.5–10 μg/ml of protein S100A8 and S100A9 strongly inhibited LPS stimulation of neutrophil oxidative activity (Figures 4A–C), further indicating that S100A8 and S100A9 anti-oxidative effects were not restricted to the spontaneous activation of neutrophil but were also relevant to controlled stimulated oxidative activation.
Figure 4.
Time course of fluorescence emission of neutrophils incubated with DCFH-DA probe and 10 μg/ml LPS alone or with various concentrations of S100A8 (A), S100A9 (B) and calprotectin (C). Each point is the mean ± SD. S100A8, S100A9 and calprotectin produced a significant reduction to LPS response at all time points and all concentrations ≥ 2.5 μg/ml with a p < 0.05 when compared to LPS control. Each point is the mean ± SD. This is a representative experiment of at least three experiments conducted in quadruplicate.
S100A8/A9 anti-oxidative effect is mediated by adenosine metabolites
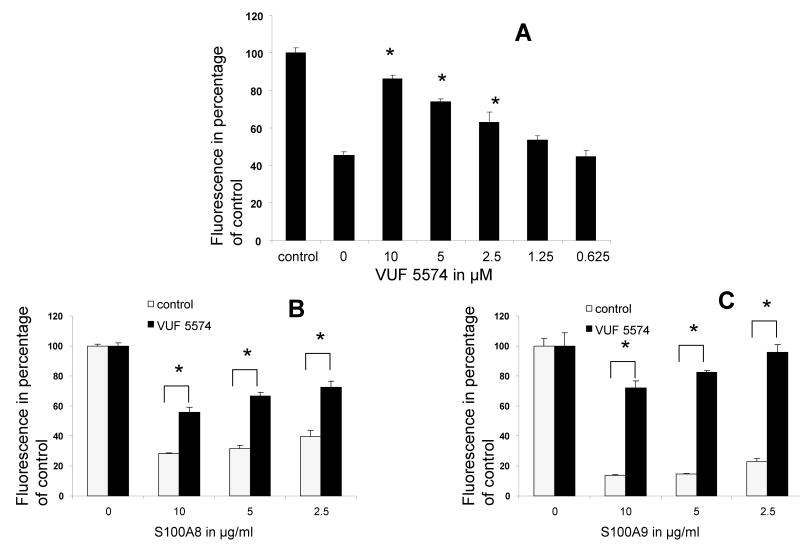
To test whether S100A8/A9 activity was mediated through adenosine metabolites, we next examined their effect on PMN oxidative metabolism in the presence of adenosine deaminase (ADA1), an enzyme which deaminates adenosine to inosine and inhibits its anti-oxidative functions in humans [26]. The data indicated that whereas S100A8 and S100A9 inhibited both the spontaneous and LPS stimulated oxidative metabolism at concentration ranging from 2.5–10 μg/ml (Figures 1 and 4), this effect was partially or fully abrogated in the presence of ADA1 (Figures 5A and B). PMNs express several purinergic receptors including A2A and A3 receptors [22]. Those two receptors have been implicated in the inhibition leukocytes oxidative metabolism [20,21,27]. Accordingly, we next tested the inhibition of A2A and A3 adenosine receptors on S100A8/A9 anti-oxidative effect. The data indicated that inhibitors of A3 receptors abrogated the anti-oxidative effects of S100A8 and S100A9 (Figures 6A and B) on LPS stimulated and on the constitutive oxidative metabolism of neutrophils. Inhibitors of A2A had a more modest effect, whereas inhibitors of A1 and A2B P1 adenosine or P2 purinergic receptors had no measurable effect (data not shown)
Figure 5.
Fluorescence emission of neutrophils incubated with DCFH-DA probe during 1 h alone (spontaneous) or in the presence of LPS 10 μg/ml. The addition of 0.01 mU/ml of human adenosine deaminase 1 (ADA1) caused a substantial reduction in S100A8 (A) and S100A9 (B) anti-oxidative effect when compared to data presented earlier in Figures 1 and 4. Each point is the mean ± SD. This is a representative experiment of at least three experiments conducted in quadruplicate.
Figure 6.
Fluorescence emission of neutrophils incubated with DCFH-DA probe during 1 h alone, LPS, S100A8, S100A9 and/or VUF5574 (an A3 adenosine antagonist). (A) Neutrophils were treated with 2.5 μg/ml S100A8 except for control. VUF 5574 produces a dose-sensitive inhibition the anti-oxidative effect of S100A8 on neutrophil spontaneous oxidative metabolism. S100A8 (B) and S100A9 (C) inhibited the oxidative metabolism of neutrophils exposed to 10 μg/ml LPS. This anti-oxidative effect was countered by 10 μM, VUF 5574. * p < 0.05 compared to control treated with S100A8 alone (A) or as indicated. Each point is the mean ± SD. This is a representative experiment of at least three experiments conducted in quadruplicate.
Discussion
One of the most important functions of neutrophils is the production of oxidative metabolites in order to kill invading micro-organisms. The same oxidative metabolites may cause serious injury to host tissues. Their production must therefore be tightly regulated. The data included in this report indicate that S100A8 and S100A9 inhibit the spontaneous and stimulated oxidative metabolism of neutrophils in-vitro.
In further support for the anti-oxidative effect of S100A8 and S100A9, the data indicated that blocking the intrinsic S100A8 or S100A9 with specific monoclonal antibodies resulted in an acceleration of the rate of DCFH-DA oxidation, an effect opposite to what was observed with the addition of extrinsic S100 proteins. This finding indicates that S100A8/A9 may play a biologically relevant anti-oxidative function regulating neutrophil activation.
While we have referred to the oxidative activity of neutrophils as spontaneous, it is important to note that this activity is likely the result of cell manipulation and derived from the experimental procedures. Nevertheless, the data showed that spontaneous oxidative metabolism of neutrophils had the hallmarks of a genuine controlled activation of neutrophil oxidative burst. The spontaneous activation was dependent on PKC activity [19], as demonstrated by the effect of GFX. Consequently, whereas it may be difficult to interpret the biological relevancy of the so-called spontaneous activation of neutrophils, it presents the signalling hallmarks of a genuine neutrophil oxidative activation suitable for mechanistic signalling studies.
Beyond the spontaneous activation, the data clearly indicated that S100A8 and S100A9 inhibited the PMA and the biologically relevant LPS stimulation of neutrophil oxidative metabolism. We therefore contend that increased plasma, salivary or faecal levels of S100A8/A9 observed in association with inflammation [28–30] are aimed in part at diminishing the oxidative activation of neutrophils and protect the organism against exaggerated immune response.
DCFH-DA is cleaved by intracellular esterases to the non-fluorescent DCF-DA. It is then believed to be trapped in the cell due to its polarity [15]. The measurements made with the method used in this study are therefore that of intracellular oxidation which likely may also reflect an extracellular oxidative state.
The concentrations at which S100A8 and S100A9 inhibited neutrophil oxidative metabolism are within the range of concentrations recorded during acute inflammation [31–33] and in a syndrome of hypercalprotectinaemia [7] associated with opportunistic infections and auto-immune conditions. It is therefore possible that S100A8/A9 may reach biologically relevant anti-oxidative concentration in-vivo.
Whereas the exact mechanism(s) by which S100A8 and S100A9 exert their anti-oxidative effect remains elusive, the data indicated that adenosine metabolites were implicated. Deamination of adenosine resulted in a loss of S100A8/A9 activity. Moreover, pharmaceutical agents targeting adenosine receptors also partially or fully reversed the anti-oxidative effect of the S100A8 and S100A9. The contribution of adenosine metabolites in the regulation of neutrophils or monocytes oxidative metabolism as well as their association with redox-dysregulation associated conditions has been widely reported [20,21,34]. Targeting adenosine signalling to reduce oxidation related damage has also been successfully pursued [35–37]. The implication of adenosine metabolites in S100A8/A9 produced anti-oxidative effect is, however, novel and potentially valuable in the pursuit of adenosine-related therapeutic interventions in conditions associated with deleterious inflammation and redox imbalance.
In conclusion, the anti-oxidative and anti-inflammatory effect of S100A8/A9 may represent a threshold to overcome in the induction of acute inflammation. This model is supported by our previous work in which we presented data indicating that S100A8 and S100A9 repel neutrophils and inhibit their chemotaxis toward pro-inflammatory molecules [10,11]. In this previous work, we reported that the oxidation of S100A8 and S100A9 regulated its anti-chemotactic effect. The complex interactions between neutrophil oxidative metabolism and S100A8/A9 may therefore represent one more layer of regulation by which feedbacks mechanisms cross-regulate the redox state of S100A8/A9 and neutrophil recruitment and activation.
Acknowledgments
This work was funded by a NIH grant number K22 DE017161.
Abbreviations
- ROS
reactive oxygen species
- PKC
Protein Kinase C
- DCFH-DA
dichlorofluorescin-diacetate
- CGD
Chronic Granulomatous Disease
- LPS
Lipopolysaccharide
References
- 1.Eckert JW, Abramson SL, Starke J, Brandt ML. The surgical implications of chronic granulomatous disease. Am J Surg. 1995;169:320–323. doi: 10.1016/S0002-9610(99)80167-6. [DOI] [PubMed] [Google Scholar]
- 2.Gordillo GM, Sen CK. Revisiting the essential role of oxygen in wound healing. Am J Surg. 2003;186:259–263. doi: 10.1016/s0002-9610(03)00211-3. [DOI] [PubMed] [Google Scholar]
- 3.Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, Shah AM. NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal. 2006;8:691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- 4.Edgeworth J, Gorman M, Bennett R, Freemont P, Hogg N. Identification of p8,14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. J Biol Chem. 1991;266:7706–7713. [PubMed] [Google Scholar]
- 5.Saito Y, Saito K, Hirano Y, Ikeya K, Suzuki H, Shishikura K, Manno S, Takakuwa Y, Nakagawa K, Iwasa A, Fujikawa S, Moriya M, Mizoguchi N, Golden BE, Osawa M. Hyperzincemia with systemic inflammation: a heritable disorder of calprotectin metabolism with rheumatic manifestations? J Pediatr. 2002;140:267–269. doi: 10.1067/mpd.2002.121699. [DOI] [PubMed] [Google Scholar]
- 6.Fessatou S, Fagerhol MK, Roth J, Stamoulakatou A, Kitra V, Hadarean M, Paleologos G, Chandrinou H, Sampson B, Papassotiriou I. Severe anemia and neutropenia associated with hyperzincemia and hypercalprotectinemia. J Pediatr Hematol Oncol. 2005;27:477–480. doi: 10.1097/01.mph.0000179958.19524.9c. [DOI] [PubMed] [Google Scholar]
- 7.Sampson B, Fagerhol MK, Sunderkotter C, Golden BE, Richmond P, Klein N, Kovar IZ, Beattie JH, Wolska-Kusnierz B, Saito Y, Roth J. Hyperzincaemia and hypercalprotectinaemia: a new disorder of zinc metabolism. Lancet. 2002;360:1742–1745. doi: 10.1016/S0140-6736(02)11683-7. [DOI] [PubMed] [Google Scholar]
- 8.Cornish CJ, Devery JM, Poronnik P, Lackmann M, Cook DI, Geczy CL. S100 protein CP-10 stimulates myeloid cell chemotaxis without activation. J Cell Physiol. 1996;166:427–437. doi: 10.1002/(SICI)1097-4652(199602)166:2<427::AID-JCP21>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 9.Ryckman C, Vandal K, Rouleau P, Talbot M, Tessier PA. Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J Immunol. 2003;170:3233–3242. doi: 10.4049/jimmunol.170.6.3233. [DOI] [PubMed] [Google Scholar]
- 10.Sroussi HY, Berline J, Palefsky JM. Oxidation of methionine 63 and 83 regulates the effect of S100A9 on the migration of neutrophils in vitro. J Leukoc Biol. 2007;81:818–824. doi: 10.1189/jlb.0706433. Epub 2006 Nov 2030. [DOI] [PubMed] [Google Scholar]
- 11.Sroussi HY, Berline J, Dazin P, Green P, Palefsky JM. S100A8 triggers oxidation-sensitive repulsion of neutrophils. J Dent Res. 2006;85:829–833. doi: 10.1177/154405910608500910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koethe SM, Kuhnmuench JR, Becker CG. Neutrophil priming by cigarette smoke condensate and a tobacco anti-idiotypic antibody. Am J Pathol. 2000;157:1735–1743. doi: 10.1016/S0002-9440(10)64810-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guerreiro JB, Porto MA, Santos SB, Lacerda L, Ho JL, Carvalho EM. Spontaneous neutrophil activation in HTLV-1 infected patients. Braz J Infect Dis. 2005;9:510–514. doi: 10.1590/s1413-86702005000600010. Epub 2006 Jan 2009. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh SC, Tsai CY, Sun KH, Yu HS, Tsai ST, Wang JC, Tsai YY, Han SH, Yu CL. Decreased spontaneous and lipopolysaccharide stimulated production of interleukin 8 by polymorphonuclear neutrophils of patients with active systemic lupus erythematosus. Clin Exp Rheumatol. 1994;12:627–633. [PubMed] [Google Scholar]
- 15.Ciapetti G, Granchi D, Verri E, Savarino L, Cenni E, Savioli F, Pizzoferrato A. Fluorescent microplate assay for respiratory burst of PMNs challenged in vitro with orthopedic metals. J Biomed Mater Res. 1998;41:455–460. doi: 10.1002/(sici)1097-4636(19980905)41:3<455::aid-jbm15>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 16.Farhadi A, Keshavarzian A, Fitzpatrick LR, Mutlu E, Zhang Y, Banan A. Modulatory effects of plasma and colonic milieu of patients with ulcerative colitis on neutrophil reactive oxygen species production in presence of a novel antioxidant, rebamipide. Dig Dis Sci. 2002;47:1342–1348. doi: 10.1023/a:1015382800434. [DOI] [PubMed] [Google Scholar]
- 17.Tan AS, Berridge MV. Superoxide produced by activated neutrophils efficiently reduces the tetrazolium salt, WST-1 to produce a soluble formazan: a simple colorimetric assay for measuring respiratory burst activation and for screening anti-inflammatory agents. J Immunol Methods. 2000;238:59–68. doi: 10.1016/s0022-1759(00)00156-3. [DOI] [PubMed] [Google Scholar]
- 18.Mohanty JG, Jaffe JS, Schulman ES, Raible DG. A highly sensitive fluorescent micro-assay of H2O2 release from activated human leukocytes using a dihydroxyphenoxazine derivative. J Immunol Methods. 1997;202:133–141. doi: 10.1016/s0022-1759(96)00244-x. [DOI] [PubMed] [Google Scholar]
- 19.Decoursey TE, Ligeti E. Regulation and termination of NADPH oxidase activity. Cell Mol Life Sci. 2005;62:2173–2193. doi: 10.1007/s00018-005-5177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Hoeven D, Wan TC, Auchampach JA. Activation of the A(3) adenosine receptor suppresses superoxide production and chemotaxis of mouse bone marrow neutrophils. Mol Pharmacol. 2008;74:685–696. doi: 10.1124/mol.108.048066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun WC, Moore JN, Hurley DJ, Vandenplas ML, Linden J, Cao Z, Murray TF. Adenosine A2A receptor agonists inhibit lipopolysaccharide-induced production of tumor necrosis factor-alpha by equine monocytes. Vet Immunol Immunopathol. 2008;121:91–100. doi: 10.1016/j.vetimm.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 23.Aguiar-Passeti T, Postol E, Sorg C, Mariano M. Epithelioid cells from foreign-body granuloma selectively express the calcium-binding protein MRP-14, a novel down-regulatory molecule of macrophage activation. J Leukoc Biol. 1997;62:852–858. doi: 10.1002/jlb.62.6.852. [DOI] [PubMed] [Google Scholar]
- 24.LeBel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol. 1992;5:227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- 25.Ryckman C, Gilbert C, De Medicis R, Lussier A, Vandal K, Tessier PA. Monosodium urate monohydrate crystals induce the release of the proinflammatory protein S100A8/A9 from neutrophils. J Leukoc Biol. 2004 doi: 10.1189/jlb.0603294. [DOI] [PubMed] [Google Scholar]
- 26.Levy O, Coughlin M, Cronstein BN, Roy RM, Desai A, Wessels MR. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J Immunol. 2006;177:1956–1966. doi: 10.4049/jimmunol.177.3.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Broussas M, Cornillet-Lefebvre P, Potron G, Nguyen P. Adenosine inhibits tissue factor expression by LPS-stimulated human monocytes: involvement of the A3 adenosine receptor. Thromb Haemost. 2002;88:123–130. [PubMed] [Google Scholar]
- 28.Golden BE, Clohessy PA, Russell G, Fagerhol MK. Calprotectin as a marker of inflammation in cystic fibrosis. Arch Dis Child. 1996;74:136–139. doi: 10.1136/adc.74.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tibble JA, Bjarnason I. Fecal calprotectin as an index of intestinal inflammation. Drugs Today (Barc) 2001;37:85–96. doi: 10.1358/dot.2001.37.2.614846. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura T, Kido J, Kido R, Ohishi K, Yamauchi N, Kataoka M, Nagata T. The association of calprotectin level in gingival crevicular fluid with gingival index and the activities of collagenase and aspartate aminotransferase in adult periodontitis patients. J Periodontol. 2000;71:361–367. doi: 10.1902/jop.2000.71.3.361. [DOI] [PubMed] [Google Scholar]
- 31.Dunlop O, Bruun JN, Myrvang B, Fagerhol MK. Calprotectin in cerebrospinal fluid of the HIV infected: a diagnostic marker of opportunistic central nervous system infection? Scand J Infect Dis. 1991;23:687–689. doi: 10.3109/00365549109024294. [DOI] [PubMed] [Google Scholar]
- 32.Muller F, Froland SS, Aukrust P, Fagerhol MK. Elevated serum calprotectin levels in HIV-infected patients: the calprotectin response during ZDV treatment is associated with clinical events. J Acquir Immune Defic Syndr. 1994;7:931–939. [PubMed] [Google Scholar]
- 33.Brun JG, Haga HJ, Boe E, Kallay I, Lekven C, Berntzen HB, Fagerhol MK. Calprotectin in patients with rheumatoid arthritis: relation to clinical and laboratory variables of disease activity. J Rheumatol. 1992;19:859–862. [PubMed] [Google Scholar]
- 34.Daley JM, Reichner JS, Mahoney EJ, Manfield L, Henry WL, Jr, Mastrofrancesco B, Albina JE. Modulation of macrophage phenotype by soluble product(s) released from neutrophils. J Immunol. 2005;174:2265–2272. doi: 10.4049/jimmunol.174.4.2265. [DOI] [PubMed] [Google Scholar]
- 35.Halle JN, Kasper CE, Gidday JM, Koos BJ. Enhancing adenosine A1 receptor binding reduces hypoxic-ischemic brain injury in newborn rats. Brain Res. 1997;759:309–312. doi: 10.1016/s0006-8993(97)00364-8. [DOI] [PubMed] [Google Scholar]
- 36.Chen GJ, Harvey BK, Shen H, Chou J, Victor A, Wang Y. Activation of adenosine A3 receptors reduces ischemic brain injury in rodents. J Neurosci Res. 2006;84:1848–1855. doi: 10.1002/jnr.21071. [DOI] [PubMed] [Google Scholar]
- 37.Reece TB, Okonkwo DO, Ellman PI, Maxey TS, Tache-Leon C, Warren PS, Laurent JJ, Linden J, Kron IL, Tribble CG, Kern JA. Comparison of systemic and retrograde delivery of adenosine A2A agonist for attenuation of spinal cord injury after thoracic aortic cross-clamping. Ann Thorac Surg. 2006;81:902–909. doi: 10.1016/j.athoracsur.2005.09.023. [DOI] [PubMed] [Google Scholar]