Abstract
Specific strains of probiotic, have been identified as beneficial to influence the composition and/or metabolic activity of the endogenous microbiota and some of these strains have been also shown to inhibit the growth of a wide range of enteropathogens. The first aim of using probiotics has been to improve the composition of the intestinal microbiota from a potentially harmful composition towards a composition that would be beneficial to the host.
Beyond their capacity to influence positively the composition of the intestinal microbiota, several lines of evidence suggest that some probiotic bacteria can modulate the immune system both at the local and systemic levels thereby improving immune defense mechanisms and/or downregulate immune disorders such as allergies or intestinal inflammation.
Skin reflects the general health status and aging. Different human trials widely suggest that probiotic supplementation might be useful in the management of atopic dermatitis. Based on these properties it appears that, beyond the gut, probiotics might exert their benefits at the skin level.
In a randomized double blind placebo-controlled clinical trial, we investigated whether the probiotic bacteria Lactobacillus johnsonii NCC 533 (La1) could modulate the cutaneous immune homeostasis altered by solar-simulated UV exposure in humans. After, UV exposure to twice 1.5 MED, we demonstrated that La1 intake facilitated an earlier recovery of Epidermal cells allostimulatory function. Thus, this clinical data strengthen the assumption that certain probiotics can contribute to modulate skin immune system leading to the preservation of the skin homeostasis. Altogether the data affords the possibility of designing new strategies based on a nutritional approach for the prevention of UV-induced damaging effects.
Key words: Lactobacillus johnsonii, skin, immune homeostasis, stress, food supplement, probiotics, photoprotection
Probiotics, Definition and General Health Benefits
The term probiotic, popularized by R. Fuller in 1989, was recently defined by a committee of experts as “living microorganisms, which, when consumed in adequate amounts, confer a health effect on the host.”1,2
Specific strains of probiotic lactic acid bacteria, have been shown to beneficially influence the composition and/or metabolic activity of the endogenous microbiota3–7 and some of these strains have been shown to inhibit the growth of a wide range of enteropathogens.8,9 Competition for essential nutrients, aggregation with pathogenic micro-organisms,10 competition for receptor sites,11 and production of anti-microbial metabolites8,9 have also been reported as probiotics properties.
Probiotics can be consumed in various forms of fermented or non-fermented food products. As a common feature, after ingestion, probiotics become transient constituents of the gut microbiota capable of exerting their biologic effects, thus giving a rationale for their use as a component of functional foods. Weaning, stress, dietary changes, use of antibiotics, and intestinal infections are all conditions that affect the natural balance of the intestinal microbiota for which the application of probiotics might be beneficial.
The most commonly used probiotics in humans and animals are enterococci, lactobacilli and bifidobacteria, which are natural residents of the intestinal tract.
Multiple criteria have been defined for the selection of probiotic strains (reviewed in ref. 12). The most evident criterion is that the selected strains must be safe for use to the host and for the environment. One of the most commonly reported selection criteria is the ability to survive during passage through the gastrointestinal tract (GIT) of the host for which the capacity of a strain to remain unaltered under conditions prevailing in the stomach (acidity) and intestinal tract (bile acids, pancreatic and other digestive enzymes) is crucial. Adhesion to intestinal epithelial cells is considered important for immune modulation, pathogen exclusion and prolonged residence time in the GIT. Metabolic activity may be crucial for the expression of anti-pathogenic activity. There is increasing evidence that bacterial compounds such as DNA (some CpG motifs) or cell-wall fragments and/or inactivated bacteria can elicit certain immune responses.13–16
The first aim of using probiotics has been to improve the composition of the intestinal microbiota from a potentially harmful composition towards a composition that would be beneficial to the host. Indeed, this approach is particularly relevant since the intestinal microbiota is known to play a major role in the physiological balance, the intestinal development and maturation of the host immune system.4–6,17 An adequate balance of the microbiota is crucial to maintain good health conditions. In that sense, a decrease in clostridia and coliforms and an increase in lactobacilli and/or bifidobacteria has often been seen as evidence of healthy gut conditions.18 In contrast, changes of the intestinal microbiota composition are associated with certain types of pathologies, in particular gastro-intestinal infections, inflammations and allergies.12,18
Different studies showed that specific probiotic strains are able to positively influence the microbiota composition.19 In that respect, some probiotic strains have been successfully used to improve the outcome of gastrointestinal diseases, in particular diarrhea or H. pylori infections and related gastritis.20–24
Beyond their capacity to influence positively the composition of the intestinal microbiota, several lines of evidence suggest that some probiotic bacteria can modulate the immune system both at the local and systemic levels5,17 thereby improving immune defense mechanisms and/or downregulate immune disorders such as allergies or intestinal inflammation.5,25–27
Indeed, several strains of lactic acid bacteria were shown to modulate cytokine and growth factor production in vitro and in vivo.28–31 Moreover, results from different pre-clinical and clinical trials have shown the capacity of various probiotic strains to enhance non-specific and specific immunity.32–37
Probiotics and Skin
Skin aggression—Environmental stress.
Different human trials widely suggest that probiotic supplementation might be useful in the management of atopic dermatitis.25,27,38,39 Based on these properties it appears that, beyond the gut, probiotics might exert their benefit at the skin level. Thus, it is assumed that some specific probiotic strains may be useful for the maintenance of cutaneous homeostasis and regulation of the skin immune system.
The skin plays a crucial role in protecting against dehydration and damage or insults from external aggressions, e.g., chemical (pollution, tobacco, xenobiotics), mechanical, physical (ultraviolet exposure (UV), changes in temperature and hygrometry) or infections. It is composed of a stratified epithelium with various cell types, including keratinocytes whose differentiation results in building barrier function and, in a lower proportion, dendritic cells, melanocytes and Langerhans cells. Each of these cell types contributes to skin protection. Moreover, the underlying dermal compartment harbors leukocytes, mastocytes and macrophages that are key actors of cell defense.
Skin reflects the general health status and aging. Although skin ageing is genetically programmed, the health and functions of the skin are also influenced by environmental factors, especially in exposed areas such as the face. Indeed, lifestyle, food, climate conditions, the extent and frequency of UV exposure, free radicals, toxins and allergens, xenobiotics and mechanical damage are all exogenous factors suspected to alter skin health. Furthermore, hormonal status, immunological status, and psychological stress are endogenous factors that can also alter skin quality and biological functions.
In this context, the skin can undergo various changes including immune dysfunction, inflammation, photoaging, dryness, wrinkles, dyschromia and a variety of hyperplasia.40,41
The skin is continuously challenged by diverse environmental stress which can later induce important alterations of the cutaneous homeostasis. Indeed, the skin is known to be an immunecompetent tissue and thus it is important for the protection against infections and the control of cell disorders.42 These skin disorders associated with dysregulation of immunological and/or neurosensitive mechanisms could be modulated or prevented by nutritional support and in particular by the use of certain probiotics.43
UV and photoprotection.
Several epidemiological studies demonstrated that UV exposure induce dramatic changes in immune functions. Among these changes, a decrease in number and morphological modifications of the Langerhans cells as well as an alteration of their capacity to present antigens have been proven.44–47 An increase in immune-suppressive cytokine levels such as IL-10 was also reported.48
These skin disorders associated with dysregulation of immunological and/or neurosensitive mechanisms could be modulated or prevented by nutritional support and in particular by the use of certain probiotics.43
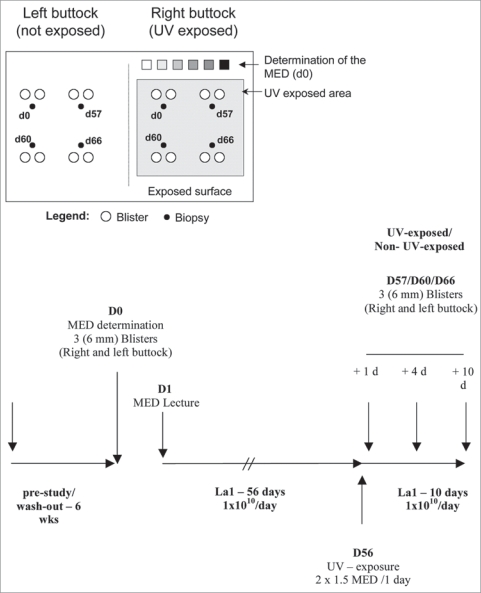
Lactobacillus johnsonii NCC 533 (La1), was isolated from healthy adult microbiota and was shown to have strong antipathogenic activity against a wide range of entero-pathogens.49 In a randomized double blind placebo-controlled clinical trial, we investigated whether the probiotic bacteria La1 could modulate the cutaneous immune homeostasis after solar-simulated UV exposure in humans. For this purpose, 54 volunteers were randomized in two groups (n = 27 per group) taking either La1 or a placebo daily for 8 weeks before UV exposure to 2 × 1.5 MED. Skin biopsies were analyzed to investigate whether this specific probiotic may interfere with allostimulatory function (using the mixed epidermal cell lymphocyte reaction (MECLR)) and in situ activation/maturation phenotypic status of skin dendritic cells after solar-simulated UV exposition (Fig. 1).50
Figure 1.
Study protocol: schematic representation of the exposed and non-exposed buttock areas used for MED determination, biopsy and blister collection. D0 is Day 0 of the study before the start of La1 supplementation, D57, D60 and D66 are Day 1, 4 and 10 after UV exposure, respectively.
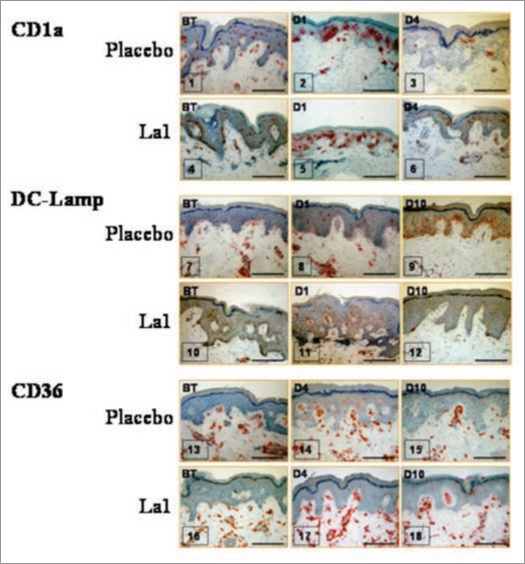
Results show that La1 intake did not prevent the early UV-induced phenotypic activation of LCs. The results confirm previous in vivo studies by Laihia and Jansen,51 by showing that a large number of LCs not only acquire expression of activation markers such as CD86, but also express DC-Lamp as early as Day 1 post UV exposure and most probably reflect a population that matured within the epidermis (Fig. 2). Despite in vivo phenotypic activation/maturation known to favor T cell priming,52 LCs displayed reduced in vitro allostimulatory function on Day 1 post UV exposure (Fig. 3). This is in agreement with many reports53,54 and might be due to rapid in situ LC death following phenotypic maturation or, alternatively, to increased sensitivity to trypsin treatment during isolation procedure and, subsequently, to increased in vitro cell death.
Figure 2.
Representative immunohistochemical staining for three antigens, before treatment (BT) or on different days after UV exposure. Bars = 100 µm.
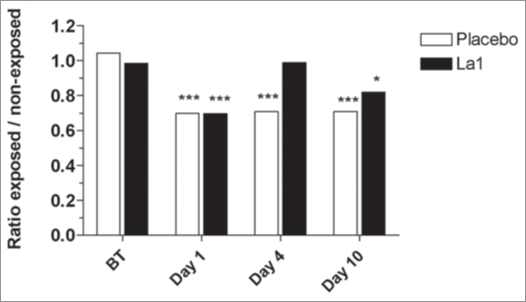
Figure 3.
Results from MECLR: mean cpm ratios from exposed versus non-exposed skin samples. The ratios were calculated from the 54 volunteers (A) or from the UV-sensitive (UVS) and UV-resistant (UVR) subjects (B) distributed among the La1 and placebo groups. *** Statistically significant differences at p < 0.001; ** statistically significant differences at p < 0.01; * statistically significant differences at p < 0.05 between exposed and non-exposed sides. BT: before treatment.
Although UV-induced LC depletion has been widely reported, little is known about the kinetics of reconstitution of skin immune function. In the placebo group UV exposure to twice 1.5 MED, still induces significant inhibition of EC allostimulatory function 4 days post-UV-exposure (p < 0.001) and this inhibition correlates with significant decrease in CD1a+ cells in the epidermis (Fig. 3). In agreement with previous studies by Cooper et al.54 concomitant epidermal infiltration with CD36+ macrophages was observed in both groups, beginning on Day 1 and still visible on Day 4 post-UV-exposure. The important result is that La1 intake facilitated an earlier recovery of EC allostimulatory function, a process that correlated with recovery of basal CD1a+ cell staining within the exposed epidermis. The origin of these CD1a+ cells remains an open question. It is likely that activated LCs had disappeared from epidermis either by apoptosis or migration, a process that might be facilitated by La1 supplementation. Accordingly, the recovered EC allostimulatory function in the La1 supplemented group might be related to repopulating cells, most probably to CD1a+ cells derived from precursor cells. These might be local proliferative precursor cells as previously described.55 Alternatively, we show here that CD36+ monocytic cells that colonize the exposed epidermis completely disappeared on Day 10 in the La1 supplemented group, whereas CD36+ cells could still be observed in biopsies from the placebo group (Fig. 2). This might suggest that the monocytic cells could have served as potential LC precursors and that La1 intake favors their differentiation. Indeed, there is now evidence in murine models that LCs arise from monocytes following severe skin injury.56
It can be speculated that La1 is somehow able to modulate local or systemic cytokine levels.54 Whether La1 favor the production of TGFβ, known to promote LC differentiation,57 or some chemokines that favor the homing of skin LC precursors require further investigations.
In conclusion, in this randomized double blind clinical study, we show that the intake of L. johnsonii La1 contributes to reinforce cutaneous immune homeostasis following UV exposure in humans and may thus represent a new strategy for photoprotection.
Mechanisms.
The maintenance and protection of the gastrointestinal tract contributes to the overall host equilibrium. Although a direct relationship between probiotics and the bioavailability of nutrients has not yet been established, probiotics nonetheless positively influence the gastrointestinal homeostasis which contributes to promote the absorption of dietary nutrients at the intestinal mucosal level. This may help to provide essential nutrients for cell metabolism and the synthesis of the various functional and structural components of the skin.
The mechanisms whereby probiotics may play a role on skin physiology are not fully elucidated. However, it is proposed that, as shown for other commensal bacteria, probiotics could be directly sampled in the lumen by mucosal dendritic cells, which express tight junction proteins and penetrate gut epithelial monolayer (reviewed in ref. 58). It is postulated that upon interaction of the probiotic bacteria (or their components) with the intestinal epithelium and/or direct interaction with dendritic cells, other immune cells, such as B and T lymphocytes may be activated and immune mediators, including cytokines may subsequently be released. These cytokines, bacterial fractions and primed immune cells may be transported via the blood to other organs, including the skin, where they could modulate the immune status.
In addition, the capacity of certain probiotics to modulate the production of regulating cytokines and growth factors may play a role in the rebalancing skin immune system and in damping inflammatory reactions.
Conclusions and Perspectives
In conclusion, this clinical data strengthen the assumption that certain probiotic strains exert their effects beyond the gut confer benefits at the skin level. There are indeed emerging evidences that such probiotics can contribute to modulate skin immune system leading to the preservation of the skin homeostasis.
Altogether the data affords the possibility of designing new strategies based on a nutritional approach for the prevention of UV-induced damaging effects.
Footnotes
Previously published online: www.landesbioscience.com/journals/dermatoendocrinology/article/9849
References
- 1.Fuller R. Probiotics in man and animals. J App Bacteriol. 1989;66:365–378. [PubMed] [Google Scholar]
- 2.FAO/WHO, authors. Joint expert consultation on evaluation of health and nutritional properties of probiotics in food including powdered milk with live lactic acid bacteria. 2001.
- 3.Langhendries JP, Detry J, Van Hees J, Lamboray JM, Darimont J, Mozin MJ, et al. Effect of fermented infant formula containing viable bifidobacteria on the fecal flora composition and pH of healthy full-term infants. J Pediatr Gastroenterol Nutr. 1995;21:177–181. doi: 10.1097/00005176-199508000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Macfarlane GT, Gibson GR. The human colonic microbiota. In: Gibson GR, editor. Colonic microbiota, Nutrition and Health. Kluwer Academic Publisher; 1999. pp. 1–25. [Google Scholar]
- 5.Isolauri E, Sutas Y, Kankaanpaa P, Arvilommi H, Salminen S. Probiotics: effects on immunity. Am J Clin Nutr. 2001;73:444–450. doi: 10.1093/ajcn/73.2.444s. [DOI] [PubMed] [Google Scholar]
- 6.Isolauri E. Probiotics in the prevention and treatment of allergic disease. Pediatr Allergy Immunol. 2001;12:56–59. doi: 10.1034/j.1399-3038.2001.121413.x. [DOI] [PubMed] [Google Scholar]
- 7.Mohan R, Koebnick C, Schildt J, Schmidt S, Mueller M, Possner M, et al. Effect of Bifidobacterium lactis Bb12 supplementation on intestinal microbiota of preterm infants. J Clin Microbiol. 2006;44:4025–4031. doi: 10.1128/JCM.00767-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernet-Camard MF, Lievin V, Brassart D, Neeser JR, Servin AL, Hudault H. The human Lactobacillus acidophilus strain LA1 secretes a nonbacteriocin antibacterial substance(s) active in vitro and in vivo. Appl Environ Microbiol. 1997;63:2747–2753. doi: 10.1128/aem.63.7.2747-2753.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coconnier MH, Lievin V, Hemery E, Servin AL. Antagonistic activity against Helicobacter infection in vitro and in vivo by the human Lactobacillus acidophilus strain LB. Appl Environ Microbiol. 1998;64:4573–4580. doi: 10.1128/aem.64.11.4573-4580.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rolfe RD. The role of probiotic cultures in the control of gastrointestinal health. J Nutr. 2000;130:396–402. doi: 10.1093/jn/130.2.396S. [DOI] [PubMed] [Google Scholar]
- 11.Coconnier MH, Bernet MF, Kerneis S, Chauviere G, Fourniat J, Servin AL. Inhibition of adhesion of entero-invasive pathogens to human intestinal Caco-2 cells by Lactobacillus acidophilus strain LB decreases bacterial invasion. FEMS Microbiol Lett. 1993;110:299–2305. doi: 10.1111/j.1574-6968.1993.tb06339.x. [DOI] [PubMed] [Google Scholar]
- 12.Ouwehand AC, Salminen S, Isolauri E. Probiotics: an overview of beneficial effects. Antonie Van Leeuwenhoek. 2002;82:279–289. [PubMed] [Google Scholar]
- 13.Lammers KM, Brigidi P, Vitali B, Gionchetti P, Rizello F, Caramelli E, et al. Immunomodulatory effects of probiotics bacteria DNA: IL-1 and IL-10 response in human peripheral blood mononuclear cells. FEMS Immunol Med Microbiol. 2003;38:165–172. doi: 10.1016/S0928-8244(03)00144-5. [DOI] [PubMed] [Google Scholar]
- 14.Watson JL, McKay DM. The immunophysiological impact of bacterial CpG DNA on the gut. Clin Chim Acta. 2006;364:1–11. doi: 10.1016/j.cca.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 15.Tejada-Simon MV, Pestka JJ. Proinflammatory cytokine and nitric oxide induction in murine macrophages by cell wall and cytoplasmic extracts of lactic acid bacteria. J Food Prot. 1999;62:1435–1444. doi: 10.4315/0362-028x-62.12.1435. [DOI] [PubMed] [Google Scholar]
- 16.Matsuzaki T, Yamazaki R, Hashimoto S, Yokokura T. The effect of oral feeding of Lactobacillus casei strain Shirota on immunoglobulin E production in mice. J Dairy Sci. 1998;81:48–53. doi: 10.3168/jds.S0022-0302(98)75549-3. [DOI] [PubMed] [Google Scholar]
- 17.Cebra JJ. Influences of microbiota on intestinal immune system development. Am J Clin Nutr. 1999;69:1046–1051. doi: 10.1093/ajcn/69.5.1046s. [DOI] [PubMed] [Google Scholar]
- 18.Roberfroid MB, Bornet F, Bouley C, Cummings JH. Colonic microflora: nutrition and health. Summary and conclusions of an International Life Sciences Institute (ILSI) [Europe] workshop held in Barcelona, Spain. Nutr Rev. 1995;53:127–130. doi: 10.1111/j.1753-4887.1995.tb01535.x. [DOI] [PubMed] [Google Scholar]
- 19.Benno Y, He F, Hosoda M, Hashimoto H, Kojima T, Yamazaki K, et al. Effects of Lactobacillus GG yogurt on human intestinal microecology in Japanese subjects. Nutrition Today. 1996;31:9–11. [Google Scholar]
- 20.Szajewska H, Mrukowicz JZ. Probiotics in the treatment and prevention of acute infectious diarrhea in infants and children: a systematic review of published randomized, double-blind, placebo-controlled trials. J Pediatr Gastroenterol Nutr. 2001;33:17–25. doi: 10.1097/00005176-200110002-00004. [DOI] [PubMed] [Google Scholar]
- 21.Sarker SA, Sultana S, Fuchs GJ, Alam NH, Azim T, Brussow H, Hammarstrom L. Lactobacillus paracasei ST11 has no effect on rotavirus but ameliorates the outcome of nonrotavirus diarrhea in children from Bangladesh. Pediatrics. 2005;116:221–228. doi: 10.1542/peds.2004-2334. [DOI] [PubMed] [Google Scholar]
- 22.Midolo PD, Lambert JR, Hull R, Luo F, Grayson ML. In vitro inhibition of Helicobacter pylori NCTC11637 by organic acids and lactic acid bacteria. J Appl Bacteriol. 1995;79:475–479. doi: 10.1111/j.1365-2672.1995.tb03164.x. [DOI] [PubMed] [Google Scholar]
- 23.Bergonzelli GE, Blum S, Brussow H, Corthezy-Theulaz IE. Probiotics as a treatment strategy for gastrointestinal diseases? Digestion. 2005;72:57–68. doi: 10.1159/000087638. [DOI] [PubMed] [Google Scholar]
- 24.Pochapin M. The effects of probiotics on Clostridium difficile diarrhea. Am J Gastroenterol. 2000;95:11–13. doi: 10.1016/s0002-9270(99)00809-6. [DOI] [PubMed] [Google Scholar]
- 25.Kalliomäki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 2. 2001;357:1057–1059. doi: 10.1016/S0140-6736(00)04259-8. [DOI] [PubMed] [Google Scholar]
- 26.Isolauri E, Arvola T, Sutas Y, Moilanen E, Salminen S. Probiotics in the management of atopic eczema. Clin Exp Allergy. 2000;30:1604–1610. doi: 10.1046/j.1365-2222.2000.00943.x. [DOI] [PubMed] [Google Scholar]
- 27.Rautava S, Isolauri E. The development of gut immune responses and gut microbiota: effects of probiotics in prevention and treatment of allergic disease. Curr Issues Intest Microbiol. 2002;3:15–22. [PubMed] [Google Scholar]
- 28.Miettinen M, Vuopio-Varkila J, Varkila K. Production of human tumor necrosis factor, interleukin 6 and interleukin 10 is induced by lactic acid bacteria. Infect Immun. 1996;64:5403–5405. doi: 10.1128/iai.64.12.5403-5405.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borruel N, Casellas F, Antolin M, Llopis M, Carol M, Espiin E, et al. Effects of nonpathogenic bacteria on cytokine secretion by human intestinal mucosa. Am J Gastroenterol. 2003;98:865–870. doi: 10.1111/j.1572-0241.2003.07384.x. [DOI] [PubMed] [Google Scholar]
- 30.Von der Weid T, Bulliard C, Schiffrin EJ. Induction by a lactic acid bacterium of a population of CD4(+) T cells with low proliferative capacity that produce transforming growth factor beta and interleukin-10. Clin Diagn Lab Immunol. 2001;8:695–701. doi: 10.1128/CDLI.8.4.695-701.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christensen HR, Frokiaer H, Pestka JJ. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J Immunol. 2002;168:171–178. doi: 10.4049/jimmunol.168.1.171. [DOI] [PubMed] [Google Scholar]
- 32.Perdigón G, de Macias ME, Alvarez S, Oliver G, Ruiz Holgado AP. Systemic augmentation of the immune response in mice by feeding fermented milks with Lactobacillus casei and Lactobacillus acidophilus. Immunology. 1998;63:17–23. [PMC free article] [PubMed] [Google Scholar]
- 33.Schiffrin EJ, Rochat F, Link-Amster H, Aeschlimann JM, Donnet-Hughes A. Immunomodulation of human blood cells following the ingestion of Lactic acid bacteria. J Dairy Sci. 1995;78:491–497. doi: 10.3168/jds.S0022-0302(95)76659-0. [DOI] [PubMed] [Google Scholar]
- 34.Haller D, Bode C, Hammes WP, Pfeifer AM, Schiffrin EJ, Blum S. Non-pathogenic bacteria elicit a differential cytokine response by intestinal epithelial cell/leucocyte co-cultures. Gut. 2000;47:79–87. doi: 10.1136/gut.47.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meydani SN, Ha WK. Immunologic effects of yogurt. Am J Clin Nutr. 2000;71:861–872. doi: 10.1093/ajcn/71.4.861. [DOI] [PubMed] [Google Scholar]
- 36.Kaila M, Isolauri E, Soppi E, Virtanen E, Laine E, Laine S, Arvilommi H. Enhancement of the circulating antibody secreting cell response in human diarrhea by a human Lactobacillus strain. Pediatr Res. 1992;32:141–144. doi: 10.1203/00006450-199208000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Link-Amster H, Rochat F, Saudan KY, Mignot O, Aeschlimann JM. Modulation of a specific humoral immune response and changes in intestinal flora mediated through fermented milk intake. FEMS Immunol Med Microbiol. 1994;10:55–63. doi: 10.1111/j.1574-695X.1994.tb00011.x. [DOI] [PubMed] [Google Scholar]
- 38.Kalliomäki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet 2. 2003;361:1869–1871. doi: 10.1016/S0140-6736(03)13490-3. [DOI] [PubMed] [Google Scholar]
- 39.Kalliomäki M, Salminen S, Poussa T, Isolauri E. Probiotic during the first 7 years of life : a cumulative risk reduction of eczema in a randomized, placebo-controlled trial. J Allergy Clin Immunol 2. 2007;119:1019–1021. doi: 10.1016/j.jaci.2006.12.608. [DOI] [PubMed] [Google Scholar]
- 40.Krutmann J, Ahrens C, Roza L, Arlett CF. The role of DNA damage and repair in ultraviolet B radiation—induced immunomodulation: relevance for human photocarcinogenesis. Photochem photobiol. 1996;63:394–396. doi: 10.1111/j.1751-1097.1996.tb03053.x. [DOI] [PubMed] [Google Scholar]
- 41.Scharffetter-Kochanek K, Brenneisen P, Wenk J, Herrmann G, Ma W, Kuhr L, et al. Photoaging of the skin from phenotype to mechanisms. Exp Gerontol. 2000;35:307–316. doi: 10.1016/s0531-5565(00)00098-x. [DOI] [PubMed] [Google Scholar]
- 42.Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Eng J Med. 2003;348:1681–1691. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- 43.Salminen SJ, Gueimonde M, Isolauri E. Probiotics that modify disease risk. J Nutr. 2005;135:1294–1298. doi: 10.1093/jn/135.5.1294. [DOI] [PubMed] [Google Scholar]
- 44.Cooper KD, Neises G, Katz SI. Effects of ultraviolet radiation on human epidermal cell alloantigen presentation: initial depression of Langerhans cell-dependent function is followed by the appearance of T6-DR+ cells that enhance alloantigen presentation. J Immunol. 1985;134:129–137. [PubMed] [Google Scholar]
- 45.Ullrich SE. Mechanisms underlying UV-induced immune suppression. Mutat Res. 2005;571:185–205. doi: 10.1016/j.mrfmmm.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 46.Ullrich SE. Photoimmune suppression and photocarcinogenesis. Frontiers in Bioscience. 2002;7:684–703. doi: 10.2741/A804. [DOI] [PubMed] [Google Scholar]
- 47.Seite S, Zucchi H, Moyal D, Tison S, Compan D, Christiaens F, et al. Alterations in human epidermal Langerhans cells by ultraviolet radiation: quantitative and morphological study. Br J Dermatol. 2003;148:291–299. doi: 10.1046/j.1365-2133.2003.05112.x. [DOI] [PubMed] [Google Scholar]
- 48.Vink AA, Faith M, Strickland FM, Bucana C, Cox PA, Roza L, et al. Localization of DNA damage and its role in altered antigen-presenting function in ultra-violet-irradiated mice. J Exp Med. 1996;183:1491–1500. doi: 10.1084/jem.183.4.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cruchet S, Obregon MC, Salazar G, et al. Effect of the ingestion of a dietary product containing Lactobacillus johnsonii La1 on Helicobacter pylori colonization in children. Nutrition. 2003;19:716–721. doi: 10.1016/s0899-9007(03)00109-6. [DOI] [PubMed] [Google Scholar]
- 50.Peguet-Navarro J, Dezutter-Dambuyant C, Buetler T, Leclaire J, Smola H, Blum S, et al. Supplementation with oral probiotic bacteria protects human cutaneous immune homeostasis after UV exposure-double-blind, randomized placebo controlled clinical trial. Eur J Dermatol. 2008;18:504–511. doi: 10.1684/ejd.2008.0496. [DOI] [PubMed] [Google Scholar]
- 51.Laihia JK, Jansen CT. Upregulation of human epidermal Langerhans’ cell B7-1 and B7-2 co-stimulatory molecules in vivo by solar-simulating irradiation. Eur J Immunol. 1997;27:984–989. doi: 10.1002/eji.1830270427. [DOI] [PubMed] [Google Scholar]
- 52.Valladeau J, Saeland S. Cutaneous dendritic cells. Semin Immunol. 2005;17:273–283. doi: 10.1016/j.smim.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 53.Barr RM, Walker SL, Tsang W, et al. Suppressed alloantigen presentation, increased TNFalpha, IL-1, IL-1Ra, IL-10, and modulation of TNF-R in UV-irradiated human skin. J Invest Dermatol. 1999;112:692–698. doi: 10.1046/j.1523-1747.1999.00570.x. [DOI] [PubMed] [Google Scholar]
- 54.Ullrich SE. The role of epidermal cytokines in the generation of cutaneous immune reactions and ultra-violet radiation-induced immune suppression. Photochem Photobiol. 1995;62:389. doi: 10.1111/j.1751-1097.1995.tb02359.x. [DOI] [PubMed] [Google Scholar]
- 55.Merad M, Manz MG, Karsunky H, et al. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol. 2002;3:1135–1141. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ginhoux F, Tacke F, Angeli V, et al. Langerhans cells arise from monocytes in vivo. Nat Immunol. 2006;7:265–273. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Borkowski TA, Letterio JJ, Farr AG, Udey MC. A role for endogenous transforming growth factor beta1 in Langerhans cell biology: the skin of transforming growth factor beta1 null mice is devoid of epidermal Langerhans cells. J Exp Med. 1996;184:2417–2422. doi: 10.1084/jem.184.6.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Uhlig HH, Powrie F. Dendritic cells and the intestinal bacterial flora: a role for localized mucosal immune responses. J Clin Invest. 2003;112:648–651. doi: 10.1172/JCI19545. [DOI] [PMC free article] [PubMed] [Google Scholar]