Abstract
Introduction
With the recent epidemic in childhood obesity, nonalcoholic fatty liver disease (NAFLD) has become an emerging problem and a common cause of chronic liver disease in children.
Methods
In this review, the most recent insights on the pathogenesis, diagnosis, natural history, and treatment of NAFLD in children are discussed.
Keywords: Obesity, Nonalcoholic fatty liver disease (NAFLD), Nonalcoholic steatohepatitis (NASH), Children
Introduction
In children, liver steatosis is a typical finding in a variety of inherited metabolic disorders affecting the liver. Other causes of steatosis are malnutrition, infections (hepatitis C), and drug toxicity (Table 1). Currently, fatty liver disease is often seen in children in the absence of an apparent inherited metabolic defect or a specific cause. The vast majority of these children are found to be obese and insulin resistant. In 1980, Ludwig [1] first described a pattern of liver injury consistent with alcoholic hepatitis in adults but in whom alcohol use was denied: nonalcoholic steatohepatitis (NASH). These patients were found to be largely obese, and they often had hyperlipidemia. Three years later, NASH was first described in obese children [2]. Today, NASH is considered a part of a broader spectrum: nonalcoholic fatty liver disease (NAFLD). This all-embracing term ranges from simple steatosis to NASH and frank cirrhosis. With the recent epidemic in childhood obesity, NAFLD has become an emerging problem and a common cause of chronic liver disease in children. In this review, the most recent insights on the pathogenesis, diagnosis, and treatment of NAFLD in children are discussed.
Table 1.
Differential diagnosis of steatosis
| General/nutritional |
| Acute systemic disease |
| Acute starvation |
| Protein-energy malnutrition |
| Total parenteral nutrition |
| Inflammatory bowel disease |
| Celiac disease |
| Mauriac syndrome |
| Infections |
| Hepatitis C |
| Metabolic |
| Cystic fibrosis |
| Wilson disease |
| α1-Anti-trypsin deficiency |
| Galactosemia |
| Fructosemia |
| Cholesterol ester storage disease (Wolman disease) |
| Glycogen storage disease |
| Mitochondrial and peroxisomal defects of fatty acid oxidation |
| Lipodystrophies |
| Abetalipoproteinemia |
| Weber-Christian disease |
| Schwachman-Diamond syndrome |
| Drug toxicity |
| Amiodarone |
| Methotrexate |
| Prednisolone |
| l-Asparaginase |
| Vitamin A |
| Valproate |
| Tamoxifen |
| Zidovudine and antiretrovirals |
| Ethanol |
Epidemiology and risk factors
The definition of childhood obesity most widely used is body mass index (BMI) of more than 95th percentile, with overweight being defined as BMI between 85th and 95th percentiles, the normal range of BMI varying with age and sex. Age- and sex-specific BMI centile charts are available for different populations. Although BMI does not predict body fat content accurately, these definitions can still be applied adequately for most purposes in clinical practice, public health, and research when the cutoffs used are based on age- and sex-specific national reference data [3].
The prevalence of childhood obesity in the UK is increasing rapidly in the 2000s. Data from the National Study of Health and Growth and the Health Survey for UK show an increase in prevalence of overweight/obesity from 11.3%/1.8% to 22.6%/6% in boys and 9.6%/1.3% to 23.7%/6.6% in girls aged 5–10 years between 1974 and 2002–2003 [4]. Similar results are seen in the USA, where data from the National Health and Nutrition Examination Survey (NHANES) showed a tripling of the prevalence of obesity among adolescents from 5% in 1960 to 17.1% in 2003/2004 [5]. Determining the true prevalence of NAFLD in children is difficult. Liver biopsy is the gold standard for diagnosis, but this is not feasible in large epidemiological studies. Hence, epidemiological studies often use surrogate markers such as serum alanine/aspartate aminotransferases (ALT/AST) or ultrasonographic (US) findings to diagnose NAFLD. However, the sensitivity, specificity, and predictive value of these biochemical and radiological markers are uncertain. Reference ranges of serum transaminases are derived from population samples, including individuals with undiagnosed liver disease such as NAFLD [6, 7]. There is significant variation in the normal ranges of transaminases between different laboratories and studies. Biopsy-proven NAFLD has been found in children with normal transaminases [8, 9]. Data based on these biochemical or radiological findings can therefore only underestimate the real epidemiological burden of pediatric NAFLD. Furthermore, most prevalence studies carry a high selection and referral bias because they are conducted in overweight or obese populations and children/adolescents who come to medical attention. A recent population-based prevalence study in the USA analyzed data of 5,586 adolescents (aged 12–19 years) in the 1999–2004 NHANES. The prevalence of elevated ALT levels (>30 U/L) in the absence of other causes of liver disease was 8% [10]. This result is remarkably higher than the estimated prevalence in the few other large-scale, population-based studies available. Tominaga et al. [11] reported a 2.6% prevalence of NAFLD among 810 Japanese children 4–12-year-old (based on US). Park et al. [12] found a 3.2% prevalence of NAFLD in 1,543 Korean adolescents 10–19-year-old (using ALT > 40 U/L). An autopsy study in San Diego County studied liver histology in 742 children (aged 2–19 years) who died of unnatural death. The estimated population prevalence of NAFLD based on these findings was estimated to be 9.6%, proving that NAFLD is the most common liver abnormality in children aged 2–19 years [13]. The prevalence of NAFLD increases with age and the most important rise coincides with early puberty [13–15]. Multiple studies show a significant male predominance, suggesting that boys are more at risk to develop NAFLD than girls [13, 15, 16].
Major ethnic variations in the prevalence of NAFLD exist. Hispanic children and adolescents have a greater incidence than the white population, whereas black non-Hispanics are significantly less susceptible to NAFLD despite a susceptibility to insulin resistance [13, 15, 17]. Both genetic and environmental factors are likely to be involved in this ethnic distribution. Indeed, familial clustering of the condition, in association with insulin resistance, has been frequently reported [18, 19]. There is evidence that the occurrence of single-nucleotide polymorphisms may be associated with the distribution of NASH. PNPLA31 (the gene for adiponutrin, an insulin-regulated phospholipase) is associated with liver fat content but not with insulin sensitivity or inflammatory change in the liver biopsy [20]. Other polymorphisms described are associated with the inflammatory component of NASH rather than with steatosis per se, for example, polymorphisms in interleukin (IL) 6 (IL-6) (174G/C) [21] and tumor necrosis factor alpha (TNF-α) [22]. Liver fibrosis in NAFLD patients was found to be associated with a splice mutation in the tumor suppressor gene Kruppel-like factor 6 (KLF6) in a genome-wide study [23].
Dietary chemical composition of fatty acids (FA) may be critical factors in lipotoxicity observed in the setting of insulin resistance. Palmitic acid rather than oleic acid results in lower steatosis but higher cell death and impaired insulin signaling [24]. Fructose is also implicated in pathogenesis [25]. Musso et al. [26] reported a study of 25 adult patients with NASH compared with controls as having higher intake of saturated fat and cholesterol and poorer intake of polyunsaturated fat, fiber, and antioxidant vitamins.
Diagnosis
Children with NAFLD are often asymptomatic but may present with vague nonspecific symptoms such as abdominal pain and/or fatigue. Most children are overweight (gender- and age-specific BMI >85th percentile) or obese (>95th centile) [27]. Hepatomegaly is often present but can be missed at clinical examination. Acanthosis nigricans, a black pigmentation of the skin folds, axillae, and neck, which is often seen in children with insulin resistance, is found in 30–50% of children with NAFLD [8, 17]. Often, these children have a positive family history of NAFLD, insulin resistance, or type 2 diabetes mellitus [16]. Various screening tools, such as serum transaminases and imaging techniques [US, computed tomography (CT), and magnetic resonance imaging (MRI)], are used for the detection of NAFLD. None of these has proven to be reliable and the sensitivity, specificity, and predictive values remain undetermined [6]. A mild-to-moderate elevation in the level of serum transaminases is often seen in NAFLD, but the sensitivity remains poor. Franzese et al. [28] studied the incidence of liver involvement in 72 obese children, using both US and serum transaminases [28]. Fifty-three percent of these children had a US image of bright liver consistent with liver steatosis, whereas only 25% had elevated levels of transaminases. Normal transaminases do not exclude NAFLD or even NASH and abnormal levels of transaminases in overweight or obese children are not necessarily caused by NAFLD. Serum transaminases are not good discriminators of histological severity [9]. Additional biochemical findings in childhood NAFLD are hypertriglyceridemia and low titers of autoantibodies (mainly anti-smooth muscle antibodies). Most children with NAFLD have elevated fasting insulin levels, with normal fasting glucose and homeostatic insulin resistance (HOMA-IR) and QUICKI indices consistent with insulin resistance [17]. Because of the low cost, the absence of radiation exposure, and the wide availability, US is often used in the screening for NAFLD. The accumulation of fat in the liver causes the liver to appear hyperechoic (“bright”) compared with the kidney. This finding, however, is nonspecific and does not differentiate from other chronic liver diseases. When compared with histological findings, the sensitivity of US to detect fat infiltration below 30% of the liver is low [29].
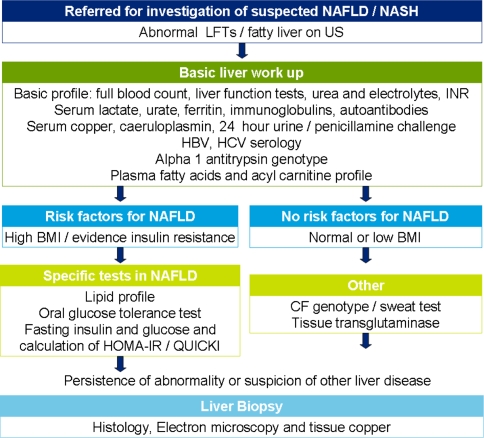
Computed tomography is rarely used for the assessment of NAFLD in children because of its ionizing radiation exposure. Magnetic resonance imaging and spectroscopy are the imaging techniques with the greatest accuracy to determine hepatic fat content [30, 31]. However, aside from liver fat, other features of NASH cannot be assessed. No imaging technique reliably discriminates between simple steatosis and NASH. In the diagnostic workup of NAFLD, alternative causes of chronic liver disease, including chronic hepatitis B and C infection, Wilson disease, α1-antitrypsin deficiency, autoimmune hepatitis, cystic fibrosis, and drug toxicity, should be excluded. Table 1 gives the differential diagnosis of steatosis. In contrast to adults, alcoholic hepatitis is almost nonexistent in children. However, alcohol abuse is rising in the adolescent population, and this should always be questioned. Figure 1 is a flow chart of suggested investigations for suspected NAFLD/NASH. The definite diagnosis of NAFLD requires liver biopsy. This is the only way to assess the histological severity of the disease (degree of steatosis, inflammation, and fibrosis or cirrhosis) and to differentiate between simple steatosis and NASH. Furthermore, it is necessary to exclude these alternative causes of liver disease. However, liver biopsy has important limitations and is not suitable as a screening test. Therefore, simple, reproducible, noninvasive tests are urgently needed that distinguish NAFLD from NASH and determine histological severity. These tests would be useful in diagnosing NAFLD/NASH as well as monitoring disease progression and treatment response [32].
Fig. 1.
Diagnostic flow chart for children with suspected NAFLD/NASH
Histology
NAFLD is defined as macrovesicular steatosis in more than 5% of hepatocytes. NAFLD comprises a histological spectrum ranging from simple steatosis to NASH and frank cirrhosis. Several studies have shown that children with NASH often present different histopathological features compared with adults [8, 9, 33]. In adults, the inflammatory changes typically consist of ballooning degeneration of hepatocytes or focal hepatocyte dropout with a polymorphonuclear infiltrate. Inflammation and fibrosis are most severe in the perivenular zone. Fibrosis is often pericellular, and Mallory’s bodies can be present. This is known as type 1 NASH. In children, the inflammatory infiltrate is often mononuclear and inflammation is periportal. Fibrosis is also periportal and rarely pericellular, whereas Mallory bodies are rare [34]. This is type 2 NASH. Although type 1 NASH is most prevalent in adults and type 2 NASH in children, there is some crossover. A summary of the histopathological features of both subtypes is given in Table 2. Schwimmer et al. [9] reviewed the histologic findings in a cohort of 100 children (aged 2–18 years) with biopsy-proven NAFLD. Type 1 NASH was present in 17%, and type 2 NASH in 51% of the children. Sixteen percent of biopsies had overlapping features of both type 1 and 2 diseases, and the remaining 16% showed simple steatosis. Children with type 2 NASH were younger and had greater severity of obesity than those with type 1 NASH. Boys were more likely to have type 2 NASH than girls. Type 2 NASH was predominant among children of Asian or Native American race and Hispanic ethnicity, whereas type 1 NASH was more common in White children. The mechanism leading to these different phenotypes of NAFLD is not yet understood, but this finding might underline the need to address adult and pediatric NAFLD as two different entities. Care should be taken with extrapolation of data regarding pathogenesis, natural history, and treatment of NAFLD from adults to children.
Table 2.
Histological features of types 1 and 2 NASH
| Type 1 | Type 2 | |
|---|---|---|
| Ballooning degradation | + | − |
| Perisinusoidal fibrosis | + | − |
| Steatosis | + | + |
| Portal inflammation | − | + |
| Portal fibrosis | − | + |
Adapted from Schwimmer et al. [9]
The Pathology Committee of the NASH Clinical Research Network recently proposed a histological scoring system that could be useful in studies of both pediatric and adult NAFLD. The scoring system comprises the evaluation of steatosis (0–3), lobular inflammation (0–2), hepatocellular ballooning (0–2), and fibrosis (0–4). The NAFLD activity score (NAS) is the unweighted sum of steatosis, lobular inflammation, and hepatocellular ballooning scores. A NAS of 5 or more correlates with the diagnosis of NASH, whereas a NAS less than 3 is defined as “not NASH”. Because this system is typically developed for adult type 1 NASH, the interobserver agreement for pediatric NASH is not as strong as adult type 1 NASH [35]. The issue “if or when to perform a liver biopsy” in children with suspected NAFLD remains controversial. In case of suspected NAFLD, we certainly recommend a biopsy in obese children presenting with raised levels of transaminases when despite attempts at gradual weight loss, enzyme abnormalities persist after 3–6 months. A biopsy should be performed earlier in nonobese children, children with persistently elevated levels of serum transaminases for more than 1–3 months in the absence of another etiology, such as viral hepatitis, in the case of splenomegaly, or when alternative liver disease is suspected.
Natural history
The natural history of NAFLD varies according to the histological pattern of the disease. Simple fatty liver without inflammation or fibrosis seems to have a remarkably benign course, whereas NASH is a potentially serious condition that can progress to cirrhosis. Cirrhosis secondary to NASH has been reported in children as young as 10 years [8]. A recent study by Feldstein et al. [36] describes the long-term outcomes and survival of 66 children with NAFLD followed for up to 20 years. During this period, two children underwent transplantation for decompensated cirrhosis. Of 13 children who underwent follow-up biopsy, four showed progression of fibrosis [36]. In adult studies, the variables most commonly associated with fibrosis are as follows: presence of diabetes, increasing age, increased HOMA-IR, increased AST/ALT ratio, hyaluronic acid, and BMI, and decreased platelet count [37]. Similarly, in children, severity of obesity and insulin resistance seem to be predictors of advanced fibrosis [17].
Pathogenesis
NAFLD is considered as the hepatic manifestation of the metabolic syndrome. This syndrome, also called “syndrome X” or the “insulin resistance syndrome,” links obesity, type 2 diabetes mellitus, hypertension, hyperlipidemia, and NAFLD. Insulin resistance is known to be an almost universal finding in adults with NAFLD. Several studies also show a high prevalence of insulin resistance in obese children and adolescents with NAFLD [17, 38, 39]. The pathogenesis of NAFLD is still incompletely understood. The “two-hit hypothesis” proposed in 1998 [40] consists of a first hit of liver fat accumulation, which is caused by an imbalance in uptake and synthesis of hepatic lipids on one side and export and oxidation on the other side. The steatotic liver becomes then more vulnerable to “second hits” leading to hepatocyte injury, inflammation, and fibrosis. It is widely accepted that insulin resistance and the resulting hyperinsulinemia seem to play a major role in the development of hepatic steatosis and perhaps steatohepatitis. The molecular mechanism leading to insulin resistance is complex and has not yet been fully elucidated. Several molecules appear to interfere with the insulin signaling pathway (TNF-α, PC-1 membrane glycoprotein, leptin, and FA) [41].
Steatosis
Traditionally, steatosis thought to arise from increased hepatic supply of free fatty acids (FFA) as a result of obesity and associated extrahepatic insulin resistance. Normally, the adipocytes of lean, insulin-sensitive people store fat after meals and release fat during fasting. In contrast, fat-laden, insulin-resistant adipocytes of obese people continue to release large amounts of glycerol and FA in the circulation, which gives rise to increased delivery of FFA to the liver. These increased FFA may then induce hepatic insulin resistance [42]. The resulting hyperinsulinemia gives rise to increased hepatic lipogenesis because insulin stimulates lipogenic enzymes via the transcription factors sterol receptor binding protein 1-c (SREBP-1c) and peroxisome proliferator-activated receptor γ (PPARγ) even in the insulin-resistant state [43]. Increased glucose levels also stimulate lipogenesis through the activation of carbohydrate response element binding protein (ChREBP), a transcription factor activating the expression of key enzymes of glycolysis and lipogenesis [44, 45]. Furthermore, the hyperinsulinemic condition results in decreased triglyceride secretion in the form of very low-density lipoprotein because insulin lowers apolipoprotein B synthesis and stability [46, 47]. Hence, hepatic FFA uptake and lipogenesis outweigh FA oxidation and triglyceride secretion leading to hepatic fat accumulation.
Oxidative stress
Because the liver cannot enlarge indefinitely and a new steady state has to be reached, as a result of this accumulation, mitochondrial FA oxidation and ketogenesis are increased. In addition, the augmented pool of FFA activates PPARα, a transcription factor that regulates the expression of different genes encoding enzymes involved in mitochondrial, peroxisomal, and microsomal FA oxidation. However, mitochondrial and peroxisomal oxidations are major sources of reactive oxygen species (ROS) giving rise to oxidative stress. This leads us to the hypothesized “second hit” in the development of NASH. High β-oxidation rates increase electron delivery to the mitochondrial respiratory chain (RC). This RC, however, is put under stress by the release of large amounts of TNF-α by the fat-engorged adipocytes of obese persons that permeabilize hepatic mitochondria and partially release cytochrome c from the mitochondrial intermembrane space. This results in an imbalance between a high input and a restricted flow of electrons over the RC, creating overreduction of RC complexes that can react with oxygen to form ROS [48].
Mitochondrial function is impaired in patients with severe steatosis [49], and NASH patients have ultrastructural abnormalities of mitochondria as well as severe mitochondrial DNA depletion [50, 51]. The overload of the mitochondrial RC, the resulting formation of ROS, and subsequent lipid peroxidation products certainly give rise to mitochondrial damage. On the other hand, mitochondrial abnormalities could also be a preexisting condition enabling the excessive production of ROS in the setting of enhanced FFA β-oxidation [50]. This could explain why for the same amount of obesity, or for the same degree of insulin resistance, certain patients just have steatosis, whereas others develop NASH and cirrhosis. Genetic polymorphisms could also at least partially explain this difference in susceptibility because some of these could favor mitochondrial dysfunction [52]. Enhanced ROS formation in the vulnerable steatotic liver subsequently triggers lipid peroxidation and the formation of reactive aldehydes such as 4-hydroxynonenal and malondialdehyde. These further give rise to mitochondrial damage and ROS formation, resulting in a vicious cycle [48].
Endoplasmic reticulum (ER) stress is also postulated as an important player in the process because this membranous network is the location of folding of proteins [53]. As the work of ER increases with biological stress, transcription factors are activated that coordinate the unfolded protein response, which slows down protein synthesis and promotes protein degradation. An inadequate response leads to protein activation, which gives rise to insulin resistance, apoptosis, inflammation, and mitochondrial dysfunction.
Cytokines and inflammation
Free fatty acids can directly activate the nuclear factor kappa B (NFκB) pathway. Increased production of inflammatory cytokines by hepatocytes resulting from NFκB activation leads to Kupffer cell activation with subsequent inflammatory mediator release and hepatic and systemic insulin resistance [54]. ROS also increases the expression of several cytokines (TNF-α, Fas ligand, TGF-β, IL-8) which are involved in the different lesions of NASH such as cell death, inflammation, and fibrosis [48]. Visceral adipose tissue is also a source of many inflammatory mediators such as leptin, adiponectin, TNF-α, and IL-6. These adipokines directly target the liver through the portal vein [55] and are generally proinflammatory and profibrogenic. Adiponectin has an anti-inflammatory role and levels are decreased with increasing severity of disease. Serum levels of these factors correlate well with the severity of disease in NASH [56].
Fibrosis
Development and progression of fibrosis are associated with inflammation, oxidative stress, and hepatocellular damage. Inflammatory cytokines (TNF-α, IL-6, and TGF-β) and oxidative stress-related molecules induce hepatic stellate cell (HSC) activation to myofibroblast. The myofibroblast is contractile and proliferative and produces cytokines and matrix components. Apoptotic hepatocytes also have a direct effect on HSC [57]. HSC also express pattern recognition receptor (e.g., Toll-like receptor 4) activation (by lipopolysaccharide, for example), which leads to the expression of proinflammatory cytokines and amplification of profibrogenic stimulus [58].
The exact sequence, however, of development of obesity, fatty liver, and NAFLD remains unclear and whether insulin resistance causes hepatic steatosis or whether the accumulation of fat in the liver is the primary event leading to hepatic and peripheral insulin resistance is uncertain [59].
Noninvasive biomarkers
On the basis of the current understanding of the pathogenesis of NAFLD, investigators are trying to identify novel biomarkers that could be used as noninvasive screening tools with the aim of identifying those with advanced disease or risk of progression and those with simple steatosis not requiring follow-up. Many markers of inflammation, hepatocyte apoptosis, fibrosis, and oxidative stress are under investigation. Promising new approaches that use proteomics, metabolomics, and genomics may help in the identification of these new biomarkers [32].
Markers of inflammation, including adipocytokines and cytokines, correlate well with disease [56]. In particular, high serum levels of TNF-α and low levels of adiponectin are associated with greater degree of liver damage [60]. Markers of apoptosis/cell death are very useful in differentiating simple steatosis from NASH. Activation of caspase 3 results in cleavage of cytokeratin 18 (CK18), which is a major intermediary filament in hepatocytes. CK18 fragments have recently been shown by a number of studies to correlate well with severity of NASH [61, 62].
Noninvasive markers of fibrosis may consist of simple bedside tests or indices that have been studied in large cohorts of patients, for example, the AST to platelet ratio index [63] and the Forn index [64]. Others measure extracellular matrix turnover [65, 66]. Combinations of both include the Fibrotest [67, 68] and the Hepascore [69]. A promising combination of markers is the European Fibrosis Score, which combines hyaluronic acid, matrix metalloproteinase, and tissue inhibitory of metalloproteinase-1 (TIMP1). This demonstrated AUROC of 0.92, 0.98, and 0.99 in distinguishing any, significant, and advanced fibrosis in 112 children with NAFLD [70]. Other panels of markers specific for NAFLD include NAFLD fibrosis score (incorporating age, glucose, AST, ALT, BMI, platelets, and albumin), which gives an AUROC of 0.88 for advanced fibrosis [71], and the BARD score (BMI, AST/ALT ratio, and diabetes), which was found to be useful in excluding patients without advanced disease [72]. Other such tools include the HAIR score (hypertension, ALT, and insulin resistance) [73], the NASH test (weight, triglycerides, glucose, alpha 2 macroglobulin, and apolipoprotein A [74], and a tool proposed by Palekar et al. [75], which composites the score of age, sex, BMI, AST/ALT ratio, and hyaluronic acid. These panels of markers have not been evaluated in children, however, and are not likely to give the same predictive power in this population. These biomarkers perform best at extremes of the spectrum and are not useful in distinguishing those with intermediate stages of disease.
Fibroscan (transient elastography) is a useful method to detect fibrosis, using ultrasound and low-frequency (50 Hz) elastic waves with a propagation velocity directly related to the stiffness of the liver. A recent study has shown fibrosis in 52 children with NAFLD with an AUROC of 0.977, 0.992, and 1 for distinguishing any, significant, and severe fibrosis [76]. However, reproducibility of transient elastography may be affected in the setting of a BMI of more than 30 [77]. A combination both serum markers and transient elastography may give optimal predictive power for the detection or exclusion of fibrosis.
Treatment
Treatment of childhood NAFLD remains a largely unsolved question. Proposed strategies consist of lifestyle modifications and pharmacological treatment. Weight reduction through diet and physical exercise is the only effective treatment of childhood NASH currently known. Weight loss should be gradual because rapid, extensive weight loss may exacerbate liver disease. Several case series and uncontrolled trials have demonstrated the effect of weight loss on improvement of transaminase levels or ultrasound abnormalities [28, 78, 79]. A recent prospective study carried out in 84 children (aged 3–18.8 years) who had elevated levels of transaminases and histologically proven NAFLD demonstrated a significant decrease in BMI, levels of fasting glucose, insulin, lipids, liver enzymes, and liver echogenicity on US after a 12-month program of lifestyle advice consisting of diet and physical exercise [80]. Up to now, there are no published data on the effect of diet and exercise on liver histology in children. Determination of the optimal dietary intervention and the optimal rate and degree of weight reduction should be subject for further research [6]. Only very few randomized, double-blinded, controlled trials are available for drugs used in the treatment of childhood NAFLD. On the basis of the current understanding of the pathogenesis of NAFLD, the main treatment options used in children are insulin-sensitizing agents and antioxidant therapy. A small, uncontrolled, open-label pilot study with vitamin E (400–1,200 IU/day) in 11 children with NASH showed improvement of serum transaminase levels despite no major changes in BMI or US appearance of the liver [81]. Other studies with vitamin E in children did not confirm these results [82, 83]. Ursodeoxycholic acid (UDCA) has been studied in both adult and pediatric NAFLD. A small study that evaluated the efficacy of UDCA in 31 obese children with abnormal transaminases [84] did not show any effect of UDCA treatment. Further studies, however, are needed to determine the efficacy of UDCA. On the basis of almost universal finding of insulin resistance in NAFLD patients, insulin-sensitizing agents have been used in the treatment of NAFLD. In children, an open-label pilot study of metformin (500 mg twice daily for 24 weeks) was conducted in ten nondiabetic children with biopsy-proven NASH and elevated ALT levels [85]. Significant improvement was observed in serum ALT levels and hepatic steatosis as assessed by MRI. A subsequent study conducted in children did not show any benefit of metformin compared with lifestyle advice [83]. A third study of metformin in insulin-resistant adolescents resulted in lower severity scores of fatty liver on US and a decrease in prevalence of fatty liver disease in the metformin group [86]. Treatment of adults with thiazoladinediones seems promising, but caution with the use of this drug in children is warranted in view of the lack of safety data in children with liver disease. Table 3 gives an overview of the currently available clinical trials (both lifestyle changes and pharmacological interventions) in the field of pediatric NAFLD. Currently, a large, randomized, double-blind, placebo-controlled trial is under investigation by the Clinical Research Network in NASH, in which both vitamin E and metformin are used. The purpose of this study was to determine whether therapeutic modification of insulin resistance or oxidative stress leads to improvement in serum or histological indicators of liver injury and quality of life (TONIC trial) [6].
Table 3.
Overview of treatment trials in pediatric NAFLD
| Intervention | No. of patients | Design | Outcome |
|---|---|---|---|
| Diet and lifestyle | |||
| Vajro et al. [79] | 9 | Open prospective single arm | Biochemical and US improvement |
| Franzese et al. [28] | 75 | Open prospective single arm | Biochemical and US improvement |
| Tock et al. [87] | 73 | Open prospective single arm | Reduction in visceral adiposity and US improvement |
| Nobili et al. [80] | 84 | Open prospective single arm | Decrease in BMI, fasting glucose, insulin, lipids, liver enzymes, liver echogenicity |
| Wang et al. [88] | 38 no intervention | Open randomized | Both lifestyle and vitamin E decrease in BMI, lipids, liver enzymes, HOMA-IR, but lifestyle is more effective |
| 19 lifestyle | |||
| 19 oral vitamin E | |||
| Reinehr et al. [89] | 43 no intervention | Open longitudinal study not randomized | Improvement in BMI and liver enzymes (persists 1 year after intervention) |
| 109 lifestyle | |||
| Antioxidant therapy | |||
| Lavine et al. [81] | 11 oral vitamin E | Open prospective single arm | Biochemical improvement independent of weight loss |
| Vajro et al. [82] | 14 diet + placebo | Blinded randomized placebo controlled | Decrease in ALT with both vitamin E and diet only, US improvement associated with weight loss only |
| 14 diet + vitamin E | |||
| Nobili et al. [83] | 43 diet + placebo | Blinded randomized placebo controlled | No beneficial effect from antioxidant therapy versus diet only on ALT and HOMA-IR |
| 45 diet + vitamin E + vitamin C | |||
| Metformin | |||
| Schwimmer et al. [85] | 10 | Open prospective single arm | Improvement in ALT, liver fat, insulin sensitivity, and quality of life |
| Nobili et al. [90] | 30 lifestyle + placebo | Open placebo controlled | No effect on transaminases, steatosis, histology |
| 30 lifestyle + metformin | |||
| Nadeau et al. [86] | 13 lifestyle + placebo | Double blind placebo controlled | Lower fasting insulin, fatty liver score on US and prevalence of steatosis |
| 37 lifestyle + metformin | |||
| UDCA | |||
| Vajro et al. [84] | 13 diet only | Open randomized | No benefit compared with diet only |
| 7 UDCA only | |||
| 7 UDCA + diet 6 control | |||
ALT alanine aminotransferase; BMI body mass index; HOMA-IR homeostatic insulin resistance; US ultrasonography
Prevention
Since treatment options are limited, prevention is the best strategy in the management of NAFLD. The environment (family, peers, neighborhood, and school) plays an extremely important role in the development of eating behavior and lifestyle. To change diet and lifestyle habits, intervention at different levels of society is indicated. Increasing public awareness, incorporating preventive measures in the school curriculum, and eradication of child poverty are important strategies to tackle this growing epidemiological problem.
Future research
The many unsolved questions regarding the diagnosis, pathogenesis, natural history, and management of pediatric NAFLD are topics for future research. There is a need to determine sensitive and specific surrogate noninvasive markers of NAFLD/NASH, allowing the screening of large, at-risk populations. Research should also focus on the further unraveling of the pathogenesis of NAFLD (role of environmental factors, identification of candidate genes, or polymorphisms involved in NAFLD). The differences in pathogenesis, natural history, and treatment of the two distinct subtypes of pediatric NASH (types 1 and 2) should be further investigated. Longitudinal follow-up studies are needed to better understand the natural history and outcome of NAFLD in children. Future clinical studies should help determine the optimal dietary intervention and pharmacological treatment of children with NAFLD.
Conclusion
With the recent epidemic of obesity, NAFLD is an emerging problem. The exact prevalence of NAFLD/NASH in the pediatric population is still unknown because current noninvasive screening methods lack sensitivity and specificity and do not discriminate between steatosis and steatohepatitis. Liver biopsy remains the gold standard for diagnosis and staging of NAFLD. Because this is not feasible for screening large populations, there is a need for development of noninvasive surrogate markers. To date, the pathogenesis of NAFLD is incompletely understood, but insulin resistance seems to play a major role. Moreover, recent data suggest that pediatric NAFLD is a different entity from adult NAFLD, so vigilance is warranted with extrapolation of results. Weight reduction seems the only effective treatment. Large, multicenter, randomized, double-blind controlled trials are needed to evaluate the efficacy of pharmacological therapies (under way), but the most important intervention in childhood NAFLD should be the prevention of childhood obesity.
Contributor Information
Ruth M. L. De Bruyne, Email: Ruth.debruyne@ugent.be
Emer Fitzpatrick, Email: Emer.fitzpatrick@kcl.ac.uk.
Anil Dhawan, Email: Anil.dhawan@kcl.ac.uk.
References
- 1.Ludwig R. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed. Mayo Clin Proc. 1980;55:434–438. [PubMed] [Google Scholar]
- 2.Moran JR, Ghishan FK, Halter SA, Greene HL. Steatohepatitis in obese children: a cause of chronic liver dysfunction. Am J Gastroenterol. 1983;78:374–377. [PubMed] [Google Scholar]
- 3.Reilly JJ. Diagnostic accuracy of the BMI for age in paediatrics. Int J Obes. 2006;30:595–597. doi: 10.1038/sj.ijo.0803301. [DOI] [PubMed] [Google Scholar]
- 4.Stamatakis E, Primatesta P, Chinn S, Rona R, Falascheti E. Overweight and obesity trends from 1974 to 2003 in English children: what is the role of socioeconomic factors? Arch Dis Child. 2005;90:999–1004. doi: 10.1136/adc.2004.068932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 6.Patton HM, Sirlin C, Behling C, Middleton M, Schwimmer JB, Lavine JE. Pediatric nonalcoholic fatty liver disease: a critical appraisal of current data and implications for future research. J Pediatr Gastroenterol Nutr. 2006;43:413–427. doi: 10.1097/01.mpg.0000239995.58388.56. [DOI] [PubMed] [Google Scholar]
- 7.Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 8.Rashid M, Roberts EA. Nonalcoholic steatohepatitis in children. J Pediatr Gastroenterol Nutr. 2000;30:48–53. doi: 10.1097/00005176-200001000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Schwimmer JB, Behling C, Newbury R, Deutsch R, Nievergelt C, Schork NJ, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005;42:641–649. doi: 10.1002/hep.20842. [DOI] [PubMed] [Google Scholar]
- 10.Fraser A, Longnecker MP, Lawlor DA. Prevalence of elevated alanine aminotransferase among US adolescents and associated factors: NHANES 1999–2004. Gastroenterology. 2007;133:1814–1820. doi: 10.1053/j.gastro.2007.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tominaga K, Kurata JH, Chen YK, Fujimoto E, Miyagawa S, Abe I, et al. Prevalence of fatty liver in Japanese children and relationship to obesity. An epidemiological ultrasonographic survey. Dig Dis Sci. 1995;40:2002–2009. doi: 10.1007/BF02208670. [DOI] [PubMed] [Google Scholar]
- 12.Park HS, Han JH, Choi KM, Kim SM. Relation between elevated serum alanine aminotransferase and metabolic syndrome in Korean adolescents. Am J Clin Nutr. 2005;82:1046–1051. doi: 10.1093/ajcn/82.5.1046. [DOI] [PubMed] [Google Scholar]
- 13.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 14.Strauss RS, Barlow SE, Dietz WH. Prevalence of abnormal serum aminotransferase values in overweight and obese adolescents. J Pediatr. 2000;136:727–733. doi: 10.1067/mpd.2000.102940. [DOI] [PubMed] [Google Scholar]
- 15.Quiros-Tejeira RE, Rivera CA, Ziba TT, Mehta N, Smith CW, Butte NF. Risk for nonalcoholic fatty liver disease in Hispanic youth with BMI > or =95th percentile. J Pediatr Gastroenterol Nutr. 2007;44:228–236. doi: 10.1097/MPG.0b013e31802d4acc. [DOI] [PubMed] [Google Scholar]
- 16.Schwimmer JB, McGreal N, Deutsch R, Finegold MJ, Lavine JE. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics. 2005;115:e561–e565. doi: 10.1542/peds.2004-1832. [DOI] [PubMed] [Google Scholar]
- 17.Schwimmer JB, Deutsch R, Rauch JB, Behling C, Newbury R, Lavine JE. Obesity, insulin resistance, and other clinicopathological correlates of pediatric nonalcoholic fatty liver disease. J Pediatr. 2003;143:500–505. doi: 10.1067/S0022-3476(03)00325-1. [DOI] [PubMed] [Google Scholar]
- 18.Willner IR, Waters B, Patil SR, Reuben A, Morelli J, Riely CA. Ninety patients with nonalcoholic steatohepatitis: insulin resistance, familial tendency, and severity of disease. Am J Gastroenterol. 2001;96:2957–2961. doi: 10.1111/j.1572-0241.2001.04667.x. [DOI] [PubMed] [Google Scholar]
- 19.Carter-Kent C, Feldstein AE. Non-alcoholic steatohepatitis over multiple generations. Dig Dis Sci 2009 (in press) [DOI] [PubMed]
- 20.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carulli L, Canedi I, Rondinella S, Lombardini S, Ganazzi D, Fargion S, et al. Genetic polymorphisms in non-alcoholic fatty liver disease: interleukin-6-174G/C polymorphism is associated with non-alcoholic steatohepatitis. Dig Liver Dis. 2009;41:823–828. doi: 10.1016/j.dld.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Tokushige K, Takakura M, Tsuchiya-Matsushita N, Taniai M, Hashimoto E, Shiratori K. Influence of TNF gene polymorphisms in Japanese patients with NASH and simple steatosis. J Hepatol. 2007;46:1104–1110. doi: 10.1016/j.jhep.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 23.Miele L, Beale G, Patman G, Nobili V, Leathart J, Grieco A, et al. The Kruppel-like factor 6 genotype is associated with fibrosis in nonalcoholic fatty liver disease. Gastroenterology. 2008;135:282–291. doi: 10.1053/j.gastro.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ricchi M, Odoardi MR, Carulli L, Anzivino C, Ballestri S, Pinetti A, et al. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J Gastroenterol Hepatol. 2009;24:830–840. doi: 10.1111/j.1440-1746.2008.05733.x. [DOI] [PubMed] [Google Scholar]
- 25.Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, et al. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol. 2008;48:993–999. doi: 10.1016/j.jhep.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Musso G, Gambino R, Michieli F, Cassader M, Rizzetto M, Durazzo M, et al. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology. 2003;37:909–916. doi: 10.1053/jhep.2003.50132. [DOI] [PubMed] [Google Scholar]
- 27.Manco M, Marcellini M, Devito R, Comparcola D, Sartorelli MR, Nobili V. Metabolic syndrome and liver histology in paediatric non-alcoholic steatohepatitis. Int J Obes. 2008;32:381–387. doi: 10.1038/sj.ijo.0803711. [DOI] [PubMed] [Google Scholar]
- 28.Franzese A, Vajro P, Argenziano A, Puzziello A, Iannucci MP, MC Saviano, et al. Liver involvement in obese children. Ultrasonography and liver enzyme levels at diagnosis and during follow-up in an Italian population. Dig Dis Sci. 1997;42:1428–1432. doi: 10.1023/A:1018850223495. [DOI] [PubMed] [Google Scholar]
- 29.Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 30.Fishbein M, Castro F, Cheruku S, Jain S, Webb B, Gleason T, et al. Hepatic MRI for fat quantitation: its relationship to fat morphology, diagnosis, and ultrasound. J Clin Gastroenterol. 2005;39:619–625. doi: 10.1097/00004836-200508000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Radetti G, Kleon W, Stuefer J, Pittschieler K. Non-alcoholic fatty liver disease in obese children evaluated by magnetic resonance imaging. Acta Paediatr. 2006;95:833–837. doi: 10.1080/08035250500449890. [DOI] [PubMed] [Google Scholar]
- 32.Wieckowska A, McCullough AJ, Feldstein AE. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: present and future. Hepatology. 2007;46:582–589. doi: 10.1002/hep.21768. [DOI] [PubMed] [Google Scholar]
- 33.Baldridge AD, Perez-Atayde AR, Graeme-Cook F, Higgins L, Lavine JE. Idiopathic steatohepatitis in childhood: a multicenter retrospective study. J Pediatr. 1995;127:700–704. doi: 10.1016/S0022-3476(95)70156-7. [DOI] [PubMed] [Google Scholar]
- 34.Roberts EA. Non-alcoholic steatohepatitis in children. Clin Liver Dis. 2007;11:155–172. doi: 10.1016/j.cld.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Kleiner DE, Brunt EM, Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 36.Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders FB, Angulo P. The natural history of nonalcoholic fatty liver disease in children: a follow-up study for up to 20-years. Gut 2009 (in press) [DOI] [PMC free article] [PubMed]
- 37.Guha IN, Parkes J, Roderick PR, Harris S, Rosenberg WM. Non-invasive markers associated with liver fibrosis in non-alcoholic fatty liver disease. Gut. 2006;55:1650–1660. doi: 10.1136/gut.2006.091454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ciba I, Widhalm K. The association between non-alcoholic fatty liver disease and insulin resistance in 20 obese children and adolescents. Acta Paediatr. 2007;96:109–112. doi: 10.1111/j.1651-2227.2007.00031.x. [DOI] [PubMed] [Google Scholar]
- 39.Chan DF, Li AM, Chu WC, Chan MH, Wong EM, Liu EK, et al. Hepatic steatosis in obese Chinese children. Int J Obes Relat Metab Disord. 2004;28:1257–1263. doi: 10.1038/sj.ijo.0802734. [DOI] [PubMed] [Google Scholar]
- 40.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/S0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 41.Duvnjak M, Lerotic I, Barsic N, Tomasic V, Virovic Jukic L, Velagic V. Pathogenesis and management issues for non-alcoholic fatty liver disease. World J Gastroenterol. 2007;13:4539–4550. doi: 10.3748/wjg.v13.i34.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lam TK, Werve G, Giacca A. Free fatty acids increase basal hepatic glucose production and induce hepatic insulin resistance at different sites. Am J Physiol Endocrinol Metab. 2003;284:E281–E290. doi: 10.1152/ajpendo.00332.2002. [DOI] [PubMed] [Google Scholar]
- 43.Tamura S, Shimomura I. Contribution of adipose tissue and de novo lipogenesis to nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1139–1142. doi: 10.1172/JCI200524930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dentin R, Pegorier JP, Benhamed F, Foufelle F, Ferre P, Fauveau V, et al. Hepatic glucokinase is required for the synergistic action of ChREBP and SREBP-1c on glycolytic and lipogenic gene expression. J Biol Chem. 2004;279:20314–20326. doi: 10.1074/jbc.M312475200. [DOI] [PubMed] [Google Scholar]
- 45.Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc Natl Acad Sci USA. 2004;101:7281–7286. doi: 10.1073/pnas.0401516101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taghibiglou C, Carpentier A, Iderstine SC, Chen B, Rudy D, Aiton A, et al. Mechanisms of hepatic very low density lipoprotein overproduction in insulin resistance. Evidence for enhanced lipoprotein assembly, reduced intracellular ApoB degradation, and increased microsomal triglyceride transfer protein in a fructose-fed hamster model. J Biol Chem. 2000;275:8416–8425. doi: 10.1074/jbc.275.12.8416. [DOI] [PubMed] [Google Scholar]
- 47.Charlton M, Sreekumar R, Rasmussen D, Lindor K, Nair KS. Apolipoprotein synthesis in nonalcoholic steatohepatitis. Hepatology. 2002;35:898–904. doi: 10.1053/jhep.2002.32527. [DOI] [PubMed] [Google Scholar]
- 48.Begriche K, Igoudjil A, Pessayre D, Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6:1–28. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 49.Quash G, Fournet G, Reichert U. Anaplerotic reactions in tumour proliferation and apoptosis. Biochem Pharmacol. 2003;66:365–370. doi: 10.1016/S0006-2952(03)00106-0. [DOI] [PubMed] [Google Scholar]
- 50.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 51.Caldwell SH, Swerdlow RH, Khan EM, Iezzoni JC, Hespenheide EE, Parks JK, et al. Mitochondrial abnormalities in non-alcoholic steatohepatitis. J Hepatol. 1999;31:430–434. doi: 10.1016/S0168-8278(99)80033-6. [DOI] [PubMed] [Google Scholar]
- 52.Fromenty B, Robin MA, Igoudjil A, Mansouri A, Pessayre D. The ins and outs of mitochondrial dysfunction in NASH. Diabetes Metab. 2004;30:121–138. doi: 10.1016/S1262-3636(07)70098-8. [DOI] [PubMed] [Google Scholar]
- 53.Kapoor A, Sanyal AJ. Endoplasmic reticulum stress and the unfolded protein response. Clin Liver Dis. 2009;13:581–590. doi: 10.1016/j.cld.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 54.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marra F, Bertolani C. Adipokines in liver diseases. Hepatology. 2009;50:957–969. doi: 10.1002/hep.23046. [DOI] [PubMed] [Google Scholar]
- 56.Jarrar MH, Baranova A, Collantes R, Ranard B, Stepanova M, Bennett C, et al. Adipokines and cytokines in non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2008;27:412–421. doi: 10.1111/j.1365-2036.2007.03586.x. [DOI] [PubMed] [Google Scholar]
- 57.Watanabe A, Hashmi A, Gomes DA, Town T, Badou A, Flavell RA, et al. Apoptotic hepatocyte DNA inhibits hepatic stellate cell chemotaxis via toll-like receptor 9. Hepatology. 2007;46:1509–1518. doi: 10.1002/hep.21867. [DOI] [PubMed] [Google Scholar]
- 58.Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, Brenner DA. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003;37:1043–1055. doi: 10.1053/jhep.2003.50182. [DOI] [PubMed] [Google Scholar]
- 59.Utzschneider KM, Kahn SE. Review: the role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006;91:4753–4761. doi: 10.1210/jc.2006-0587. [DOI] [PubMed] [Google Scholar]
- 60.Manco M, Marcellini M, Giannone G, Nobili V. Correlation of serum TNF-alpha levels and histologic liver injury scores in pediatric nonalcoholic fatty liver disease. Am J Clin Pathol. 2007;127:954–960. doi: 10.1309/6VJ4DWGYDU0XYJ8Q. [DOI] [PubMed] [Google Scholar]
- 61.Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44:27–33. doi: 10.1002/hep.21223. [DOI] [PubMed] [Google Scholar]
- 62.Diab DL, Yerian L, Schauer P, Kashyap SR, Lopez R, Hazen SL, et al. Cytokeratin 18 fragment levels as a noninvasive biomarker for nonalcoholic steatohepatitis in bariatric surgery patients. Clin Gastroenterol Hepatol. 2008;6:1249–1254. doi: 10.1016/j.cgh.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 64.Forns X, Ampurdanes S, Llovet JM, Aponte J, Quinto L, Martinez-Bauer E, et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986–992. doi: 10.1053/jhep.2002.36128. [DOI] [PubMed] [Google Scholar]
- 65.Guechot J, Laudat A, Loria A, Serfaty L, Poupon R, Giboudeau J. Diagnostic accuracy of hyaluronan and type III procollagen amino-terminal peptide serum assays as markers of liver fibrosis in chronic viral hepatitis C evaluated by ROC curve analysis. Clin Chem. 1996;42:558–563. [PubMed] [Google Scholar]
- 66.Hartley JL, Brown RM, Tybulewicz A, Hayes P, Wilson DC, Gillett P, et al. Hyaluronic acid predicts hepatic fibrosis in children with hepatic disease. J Pediatr Gastroenterol Nutr. 2006;43:217–221. doi: 10.1097/01.mpg.0000228121.44606.9f. [DOI] [PubMed] [Google Scholar]
- 67.Cales P, Oberti F, Michalak S, Hubert-Fouchard I, Rousselet MC, Konate A, et al. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology. 2005;42:1373–1381. doi: 10.1002/hep.20935. [DOI] [PubMed] [Google Scholar]
- 68.Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069–1075. doi: 10.1016/S0140-6736(00)04258-6. [DOI] [PubMed] [Google Scholar]
- 69.Adams LA, Bulsara M, Rossi E, DeBoer B, Speers D, George J, et al. Hepascore: an accurate validated predictor of liver fibrosis in chronic hepatitis C infection. Clin Chem. 2005;51:1867–1873. doi: 10.1373/clinchem.2005.048389. [DOI] [PubMed] [Google Scholar]
- 70.Nobili V, Parkes J, Bottazzo G, Marcellini M, Cross R, Newman D, et al. Performance of ELF serum markers in predicting fibrosis stage in pediatric non-alcoholic fatty liver disease. Gastroenterology. 2009;136:160–167. doi: 10.1053/j.gastro.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 71.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 72.Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57:1441–1447. doi: 10.1136/gut.2007.146019. [DOI] [PubMed] [Google Scholar]
- 73.Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 74.Poynard T, Ratziu V, Charlotte F, Messous D, Munteanu M, Imbert-Bismut F, et al. Diagnostic value of biochemical markers (NashTest) for the prediction of non alcoholo steato hepatitis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol 2006;6:34 [DOI] [PMC free article] [PubMed]
- 75.Palekar NA, Naus R, Larson SP, Ward J, Harrison SA. Clinical model for distinguishing nonalcoholic steatohepatitis from simple steatosis in patients with nonalcoholic fatty liver disease. Liver Int. 2006;26:151–156. doi: 10.1111/j.1478-3231.2005.01209.x. [DOI] [PubMed] [Google Scholar]
- 76.Nobili V, Vizzutti F, Arena U, Abraldes JG, Marra F, Pietrobattista A, et al. Accuracy and reproducibility of transient elastography for the diagnosis of fibrosis in pediatric nonalcoholic steatohepatitis. Hepatology. 2008;48:442–448. doi: 10.1002/hep.22376. [DOI] [PubMed] [Google Scholar]
- 77.Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, et al. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968–973. doi: 10.1136/gut.2006.111302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Manton ND, Lipsett J, Moore DJ, Davidson GP, Bourne AJ, Couper RT. Non-alcoholic steatohepatitis in children and adolescents. Med J Aust. 2000;173:476–479. doi: 10.5694/j.1326-5377.2000.tb139299.x. [DOI] [PubMed] [Google Scholar]
- 79.Vajro P, Fontanella A, Perna C, Orso G, Tedesco M, Vincenzo A. Persistent hyperaminotransferasemia resolving after weight reduction in obese children. J Pediatr. 1994;125:239–241. doi: 10.1016/S0022-3476(94)70202-0. [DOI] [PubMed] [Google Scholar]
- 80.Nobili V, Marcellini M, Devito R, Ciampalini P, Piemonte F, Comparcola D, et al. NAFLD in children: a prospective clinical-pathological study and effect of lifestyle advice. Hepatology. 2006;44:458–465. doi: 10.1002/hep.21262. [DOI] [PubMed] [Google Scholar]
- 81.Lavine JE. Vitamin E treatment of nonalcoholic steatohepatitis in children: a pilot study. J Pediatr. 2000;136:734–738. doi: 10.1067/mpd.2000.106566. [DOI] [PubMed] [Google Scholar]
- 82.Vajro P, Mandato C, Franzese A, Ciccimarra E, Lucariello S, Savoia M, et al. Vitamin E treatment in pediatric obesity-related liver disease: a randomized study. J Pediatr Gastroenterol Nutr. 2004;38:48–55. doi: 10.1097/00005176-200401000-00012. [DOI] [PubMed] [Google Scholar]
- 83.Nobili V, Manco M, Devito R, Ciampalini P, Piemonte F, Marcellini M. Effect of vitamin E on aminotransferase levels and insulin resistance in children with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2006;24:1553–1561. doi: 10.1111/j.1365-2036.2006.03161.x. [DOI] [PubMed] [Google Scholar]
- 84.Vajro P, Franzese A, Valerio G, Iannucci MP, Aragione N. Lack of efficacy of ursodeoxycholic acid for the treatment of liver abnormalities in obese children. J Pediatr. 2000;136:739–743. doi: 10.1067/mpd.2000.106565. [DOI] [PubMed] [Google Scholar]
- 85.Schwimmer JB, Middleton MS, Deutsch R, Lavine JE. A phase 2 clinical trial of metformin as a treatment for non-diabetic paediatric non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2005;21:871–879. doi: 10.1111/j.1365-2036.2005.02420.x. [DOI] [PubMed] [Google Scholar]
- 86.Nadeau KJ, Ehlers LB, Zeitler PS, Love-Osborne K. Treatment of non-alcoholic fatty liver disease with metformin versus lifestyle intervention in insulin-resistant adolescents. Pediatr Diabetes. 2009;10:5–13. doi: 10.1111/j.1399-5448.2008.00450.x. [DOI] [PubMed] [Google Scholar]
- 87.Tock L, Prado WL, Caranti DA, Cristofalo DM, Lederman H, Fisberg M, et al. Nonalcoholic fatty liver disease decrease in obese adolescents after multidisciplinary therapy. Eur J Gastroenterol Hepatol. 2006;18:1241–1245. doi: 10.1097/01.meg.0000243872.86949.95. [DOI] [PubMed] [Google Scholar]
- 88.Wang CL, Liang L, Fu JF, Zou CC, Hong F, Xue JZ, Lu JR, et al. Effect of lifestyle intervention on non-alcoholic fatty liver disease in Chinese obese children. World J Gastroenterol. 2008;14:1598–1602. doi: 10.3748/wjg.14.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reinehr T, Schmidt C, Toschke AM, Andler W. Lifestyle intervention in obese children with non-alcoholic fatty liver disease: 2-year follow-up study. Arch Dis Child. 2009;94:437–442. doi: 10.1136/adc.2008.143594. [DOI] [PubMed] [Google Scholar]
- 90.Nobili V, Manco M, Ciampalini P, Alisi A, Devito R, Bugianesi E, et al. Metformin use in children with nonalcoholic fatty liver disease: an open-label, 24-month, observational pilot study. Clin Ther. 2008;30:1168–1176. doi: 10.1016/j.clinthera.2008.06.012. [DOI] [PubMed] [Google Scholar]