Abstract
Objective
Aneurysmal rebleeding is a major cause of death and disability. The aim of this study is to investigate the incidence of rebleeding, and the factors related with patient's outcome.
Methods
During a period of 12 years, from September 1995 to August 2007, 492 consecutive patients with aneurysmal subarachnoid hemorrhage (SAH) underwent surgery at our institution. We reviewed the patient's clinical records, radiologic findings, and possible factors inducing rebleeding. Also, we statistically analyzed various factors between favorable outcome group (FG) and unfavorable outcome group (UG) in the rebleeding patients.
Results
Rebleeding occurred in 38 (7.7%) of 492 patients. Male gender, location of aneurysm (anterior communicating artery) were statistically significant between rebleeding group and non-rebleeding group (p = 0.01 and p = 0.04, respectively). Rebleeding occurred in 26 patients (74.3%) within 2 hours from initial attack. There were no statistically significant factors between FG and UG. However, time interval between initial SAH to rebleeding was shorter in the UG compared to FG (FG = 28.71 hrs, UG = 2.9 hrs).
Conclusion
Rebleeding occurs more frequently in the earlier period after initial SAH. Thus, careful management in the earlier period after SAH and early obliteration of aneurysm will be necessary.
Keywords: Aneurysm, Outcome, Rebleeding, Subarachnoid hemorrhage
INTRODUCTION
Aneurysmal rebleeding is a major cause of death and disability in patients with subarachnoid hemorrhage (SAH). However, few risk factors have been identified for rebleeding, and there are many controversies over these risk factors.
Some authors reported that rebleedings occured more often in patients with large aneurysm, however others could not confirm this2,12,16,19). Also, many studies reported that there was highest risk of rebleeding within the first 24 hours, whereas other studies demonstrated after 3 days or no association with time period1,17,20,22).
The aim of this study is to describe the incidence of rebleeding and to investigate the factors related with clinical outcome in the rebleeding patients.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board. During a period of 12 years, from September 1995 to August 2007, 492 consecutive patients with aneurysmal SAH underwent neurosurgical clipping in our institution. We reviewed the patients' clinical records, radiologic findings, and various factors associated with rebleeding.
The inclusion criteria were as follows: 1) rebleeding from ruptured aneurysm; 2) after initial SAH, rebleeding was confirmed by repetitive computed tomographic (CT) scan; 3) the patients underwent surgical clipping.
The investigation had two arms. The first arm included the total number of patients with aneurysmal SAH who underwent surgery within 48 hours after initial event. We divided this population into two groups; rebleeding group (RG) and non-rebleeding group (NG). We compared demographic data (sex, age, and history of diabetes and hypertension), radiologic findings (size, location of aneurysm, and Fisher's grade) and clinical factors [Hunt Hess (H-H) grade, Glasgow Outcome Scale (GOS)] in each group.
The second arm only assessed rebleeding patients. We divided this population into two groups; favorable outcome group (FG; GOS 4, 5) and unfavorable outcome group (UG; GOS 1, 2, 3) after six months from initial SAH. We compared demographic, radiologic, and clinical factors between these two groups. In addition, rebleeding-related factors, including time interval from initial SAH to rebleeding, patient's circumstance of rebleeding, type of rebleeding, initial systolic blood pressure, systolic blood pressure after rebleeding, change in systolic blood pressure, and initial blood glucose.
Comparisons between two groups were analyzed using the independent Student's t-test and chi-square test. All data was presented in mean ± standard deviation. The result less than p = 0.05 was considered statistically significant.
RESULTS
Part I
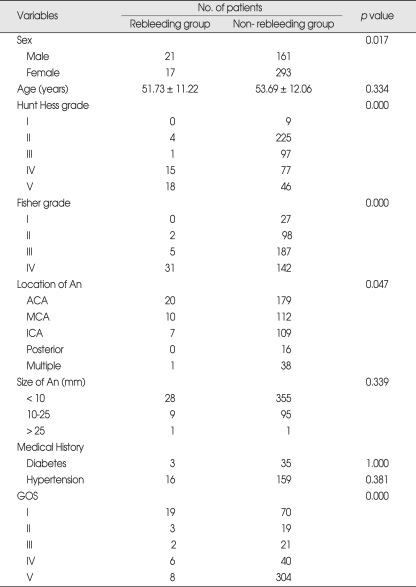
During this study period, 492 patients fulfilled the inclusion criteria. Mean age of the study population was 53.5 ± 12.0 years, 182 were men (37%), and 310 were women (63%). Rebleeding occurred in 38 patients (7.7%). Initial H-H grade, Fisher's grade, male gender, and location of aneurysm (anterior communicating artery) were significantly different (Table 1).
Table 1.
Univariate analysis of demographic and clinical data in 492 patients studied*
*Data are presented as number or mean ± standard deviation. An: aneurysm, ACA: anterior cerebral artery, MCA: middle cerebral artery, ICA: internal cerebral artery, GOS: Glasgow Outcome Scale
Part II
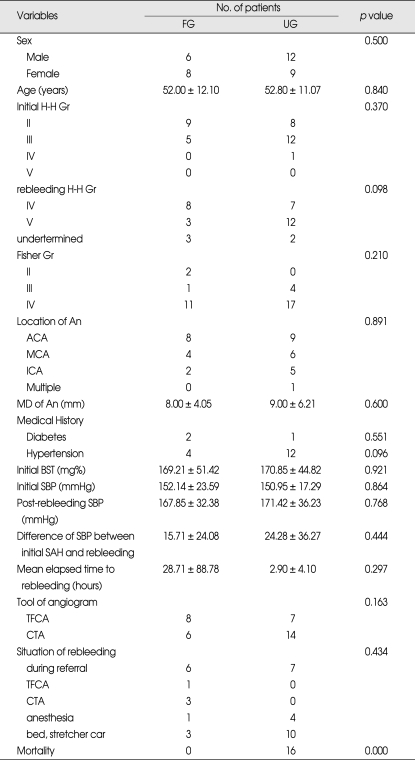
A total of 35 patients fulfilled the inclusion criteria. Three patients were excluded because rebleeding-related factors were unidentified. Mean age was 52.5 ± 11.3 years, 18 were men (51.4%), and 17 were women (48.6%). Among these patients, 14 patients (40%) showed favorable outcome and 21 patients (60%) showed unfavorable outcome after 6 months from initial SAH.
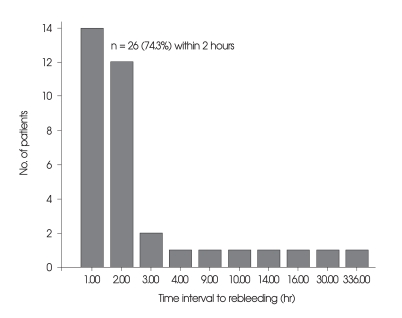
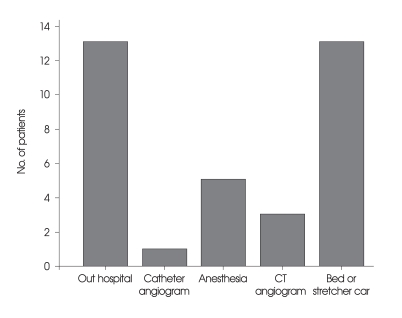
In 26 patients, the time elapsed from initial SAH to rebleeding was within 2 hours (74.3%) (Fig. 1). Mean time interval of rebleeding in FG was 26.7 hours, and 2.9 hours in UG (p = 0.297). The most frequent type of rebleeding was intraventricular hemorrhage (n = 10, 28.6%) followed by intracerebral hemorrhage (n = 9, 25.7%). Rebleeding occurred during transfer (n = 13, 37.1%), anesthesia (n = 5, 14.3%) and angiogram (n = 4, 11.4%), also in bed or stretcher car (n = 13, 37.1%), (Fig. 2). There were no significant differences between FG and UG (Table 2).
Fig. 1.
Time intervals from the initial subarachnoid hemorrhage to rebleeding in the 35 patients. Note that aneurysmal rebleeding occurred within 2 hours after initial subarachnoid hemorrhage in the 26 patients (74.3%).
Fig. 2.
Various conditions that have occurred rebleeding in the 35 patients. Note that in-hospital rebleeding occurred in the 22 patients (62.8%). CT: computed tomography.
Table 2.
Univariate analysis of demographic and clinical variables of 35 rebleeding patients associated with GOS*
*Data are presented as number or mean ± standard deviation. GOS: Glasgow outcome scale, FG: favorable outcome group (GOS IV,V), UG: unfavorable outcome group (GOS I,II,III), H-H: Hunt-Hess, Gr: grade, An: aneurysm, ACA: anterior cerebral artery, MCA: middle cerebral artery, ICA: internal cerebral artery, MD: maximal diameter, BST: blood sugar test, SBP: systolic blood pressure, SAH: subarachnoid hemorrhage, TFCA: transfemoral cerebral angiogram, CTA, computed tomographic angiogram.
DISCUSSION
In this study, the incidence of aneurysmal rebleeding was 7.7%. Although previous studies3,6,21) have reported the range of incidence of aneurysmal rebleeding was between 17.3% to 19.9%, our result corresponds with the results of recent studies2,16,23), ranging from 4.0% to 9.7%. This discrepancy could be explained by the development of endovascular and surgical therapies, and advances in intensive care management.
Some investigators have reported the timing of rebleeding after initial SAH. In a series of 273 patients, Ohkuma et al.17) reported the peak time of rebleeding was within 2 hours (77%) after the initial SAH. In 2007, Tanno et al.23) reported that overall rebleeding occurred in 88 out of 181 patients within 6 hours (48.6%). Consistent with these reports, we found that the most of rebleeding occurred within 2 hours (74.3%) after ictus in our series. Accordingly, these findings suggest aneurysmal rebleeding occurs more frequently in the earlier period after the initial SAH than previous reports14,22).
In the analysis of factors associated with aneurysmal rebleeding by Naidech et al.16), H-H grade on admission and maximal aneurysm diameter were independent predictors of rebleeding. On the other hand, Ohkuma et al.17) reported the significant differences in H-H grade, rate of intracerebral hematoma, intranventricular hematoma, subdural hematoma on CT scan at the time of admission, operability and prognosis between RG and NG. Also, systolic arterial pressure ≥ 160 mmHg was a possible risk factor of rebleeding. In 2006, Pleizier et al.13) suggested that the hazard ratio of rebleeding in large versus small aneurysms was 1.6 [95% confidence interval (CI) 1.0-2.6].
In this study, we found that initial H-H grades, Fisher's grades, and GOS were significant rebleeding factors. Another interesting finding of this study was that male gender and location of aneurysm (anterior communicating artery) were significant different factors between RG and NG. Previous reports suggested that in male gender, ruptured aneurysms were more common in the anterior cerebral artery7,18). Although there was no significant difference, a recent retrospective study showed that aneurysm was located in the anterior communicating artery (30.4%) and the internal carotid artery (26.0%) in the rebleeding patients23). Hemodynamic factors are more important in aneurysm formation in male gender due to size of the anterior cerebral artery and its complexities8,9,13). The relationship between aneurysmal rebleeding and hemodynamic factor can explain why rebleeding in male gender and anterior communicating artery is more common. Also, it seems reasonable to speculate that gender-related predisposition to rebleeding might reflect prevalent location of aneurysm and anatomical relationship between aneurysm and surrounding structures. Further investigation for epidemiology of the rebleeding patients will be necessary.
Multiple clinical factors were evaluated for their clinical significance to predict the outcome in our series. Although there were no significant clinical factors, the time interval between initial SAH to rebleeding was longer in FG. This suggests that careful management and early intervention for obliteration of aneurysm could yield to favorable outcome in the rebleeding patients.
Some previous reports have mentioned the prevalent circumstances of rebleeding. In large series of 559 patients, Sasaki et al.21) reported that 19.9% of rebleeding from the ruptured aneurysm had occurred during transfer. In 2006, Ohkuma et al.17) reported that 13.6% of patients suffered from rebleeding in the ambulance or at the referring hospital before admission. We found that out-hospitalization rebleeding rate was 37.1%. Our results were somewhat higher than the studies above. This discrepancy may be due to the difference of emergency medical delivery system. Consistent with previous reports3-6,10,11,15), we experienced rebleeding during angiogram (4 cases) and anesthesia (5 cases). Thus, more careful attention will be necessary while performing these procedures.
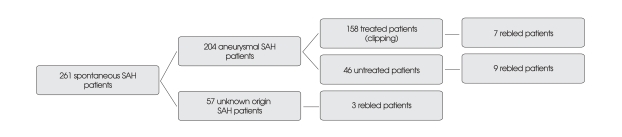
Limitations of this study need to be mentioned. First, we only addressed the patients who underwent neurosurgical clipping. From January 2004 to December 2007, we investigated 261 patients with spontaneous SAH whom were admitted to our institution. Definitive treatment (clipping) of SAH was provided in 158 patients and 103 were untreated. Rebleeding was identified in 19 patients (7.3%), 7 in the clipping group and 12 in the untreated group (Fig. 3). However, we could not completely identify rebleeding, because 35 among untreated aneurysmal SAH patients were suddenly expired or transferred to other hospitals. Also, we could not perform follow up brain CT scan to confirm the rebleeding in all SAH patients due to financial difficulties, refusal from relatives, and moribund status. Therefore, the true incidence of aneurysmal rebleeding was somewhat higher than that of this study and further prospective investigation will be needed. Another limitation was the retrospective design of this study.
Fig. 3.
Flow diagram showing the rebled patients among 261 spontaneous subarachnoid hemorrhage (SAH) patients during a 4-year period from January 2004 to December 2007. Among 57 patients with unknown origin SAH, 25 were verified by angiography and 32 were not verified by angiography.
CONCLUSION
Rebleeding occurs more frequently in the earlier period after initial SAH. We should consider the possibility of rebleeding during early angiogram and anesthesia. Early obliteration of aneurysm would be mandatory.
References
- 1.Bae HG, Do JW, Lee KS, Yun IG, Byun BJ. Clinical significance of rebleeding and risk factors affecting rebleeding in patients with spontaneous subarachnoid hemorrhages. J Korean Neurosurg Soc. 1996;25:1856–1861. [Google Scholar]
- 2.Beck J, Raabe A, Szelenyi A, Berkefeld J, Gerlach R, Setzer M, et al. Sentinel headache and the risk of rebleeding after aneurysmal aubarachnoid hemorrhage. Stroke. 2006;37:2733–2737. doi: 10.1161/01.STR.0000244762.51326.e7. [DOI] [PubMed] [Google Scholar]
- 3.Fujii Y, Takeuchi S, Sasaki O, Minakawa T, Koike T, Tanaka R. Ultra-early rebleeding in spontaneous subarachnoid hemorrhage. J Neurosurg. 1996;84:35–42. doi: 10.3171/jns.1996.84.1.0035. [DOI] [PubMed] [Google Scholar]
- 4.Graf BM, Böhrer H. A fatal hypertensive reaction during anesthesia in a patient with acute subarachnoid hemorrhage. Anaesthesist. 1990;39:434–438. [PubMed] [Google Scholar]
- 5.Hashiguchi A, Mimata C, Ichimura A, Morioka M, Kuratsu J. Rebleeding of ruptured aneurysms during three-dimensional computed tomographic angiography: report of two cases and literature review. Neurosurg Rev. 2007;30:151–154. doi: 10.1007/s10143-007-0068-6. [DOI] [PubMed] [Google Scholar]
- 6.Herrick IA, Gelb AW. Aensthesia for intracranial aneurysm surgery. J Clin Anesth. 1992;4:73–85. doi: 10.1016/0952-8180(92)90124-j. [DOI] [PubMed] [Google Scholar]
- 7.Kassell NF, Drake CG. Timing of aneurysm surgery. Neurosurgery. 1982;10:514–519. doi: 10.1227/00006123-198204000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Kayembe KN, Sasahara M, Hazama F. Cerebral aneurysms and variations in the circle of Willis. Stroke. 1984;15:846–850. doi: 10.1161/01.str.15.5.846. [DOI] [PubMed] [Google Scholar]
- 9.Kongable GL, Lanzino G, Germanson TP, Truskowski LL, Alves WM, Tomer JC, et al. Gender-related differences in aneurysmal subarachnoid hemorrhage. J Neurosurg. 1996;84:43–48. doi: 10.3171/jns.1996.84.1.0043. [DOI] [PubMed] [Google Scholar]
- 10.Kusumi M, Yamada M, Kitahara T, Endo M, Kan S, Iida H, et al. Rerupture of cerebral aneurysms during angiography? A retrospective study of 13 patients with subarachnoid hemorrhage. Acta Neurochir (Wien) 2005;147:831–837. doi: 10.1007/s00701-005-0541-3. [DOI] [PubMed] [Google Scholar]
- 11.Levati A, Roverato S, Solaini C, Boselli L. Anesthesia in early surgery and endovascular therapy for aneurysmic subarachnoid heomrrahge. Minerva Anesthesiol. 1998;64:185–187. [PubMed] [Google Scholar]
- 12.Machiel Pleizier C, Algra A, Velthuis BK, Rinkel GJ. Relation between size of aneurysms and risk of rebleeding in patients with subarachnoid hemorrhage. Acta Neurochir (Wien) 2006;148:1277–1279. doi: 10.1007/s00701-006-0911-5. discussion 1279-1280. [DOI] [PubMed] [Google Scholar]
- 13.Mackenzie JM. The anatomy of aneurysm-bearing circles of Willis. Clin Neuropathol. 1991;10:187–189. [PubMed] [Google Scholar]
- 14.Maurice-Williams RS. Ruptured intracranial aneurysms: has the incidence of early rebleeding been over-estimated? J Neurol Neurosurg Psychiatry. 1982;45:774–779. doi: 10.1136/jnnp.45.9.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagai M, Koizumi Y, Tsukue J, Watanabe E. A case of extravasation from a cerebral aneurysm during 3-dimensional computed tomography angiography. Surg Neurol. 2008;69:411–413. doi: 10.1016/j.surneu.2007.02.048. [DOI] [PubMed] [Google Scholar]
- 16.Naidech AM, Janjua N, Kreiter KT, Ostapkovich ND, Fitzsimmons BF, Parra A, et al. Predictors and impact of aneurysm rebleeding after subarachnoid hemorrhage. Arch Neurol. 2005;62:410–416. doi: 10.1001/archneur.62.3.410. [DOI] [PubMed] [Google Scholar]
- 17.Ohkuma H, Tsurutani H, Suzuki S. Incidence and significance of early aneurysmal rebleeding before neurosurgical or neurological management. Stroke. 2001;32:1176–1180. doi: 10.1161/01.str.32.5.1176. [DOI] [PubMed] [Google Scholar]
- 18.Park SK, Kim JM, Kim JH, Cheong JH, Bak KH, Kim CH. Aneurysmal subarachnoid hemorrhage in young adults: a gender comparison study. J Clin Neurosci. 2008;15:389–392. doi: 10.1016/j.jocn.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Roos EJ, Rinkel GJ, Velthuis BK, Algra A. The relation between aneurysm size and outcome in patients with subarachnoid hemorrhage. Neurology. 2000;54:2334–2336. doi: 10.1212/wnl.54.12.2334. [DOI] [PubMed] [Google Scholar]
- 20.Ross N, Hutchinson PJ, Seeley H, Kirkpatrick PJ. Timing of surgery for supratentorial aneurysmal subarachnoid hemorrhage: report of a prospective study. J Neurol Neurosurg Psychiatry. 2002;72:480–484. doi: 10.1136/jnnp.72.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakaki T, Morimoto T, Hoshida T, Kawaguchi S, Nakase H, Fukuzumi A. Rebleeding during transport of patients with a ruptured intracranial aneurysm. J Stroke Cerebrovasc Dis. 1999;8:38–41. doi: 10.1016/s1052-3057(99)80038-x. [DOI] [PubMed] [Google Scholar]
- 22.Steiger HJ, Fritschi J, Seiler RW. Current pattern of in-hospital aneurismal rebleeds. Analysis of a series treated with individually timed surgery and intravenous nimpdipine. Acta Neurochir (Wien) 1994;127:21–26. doi: 10.1007/BF01808541. [DOI] [PubMed] [Google Scholar]
- 23.Tanno Y, Homma M, Oinuma M, Kodama N, Ymamoto T. Rebleeding from ruptured intracranial aneurysms in North Eastern Province of Japan. A cooperative study. J Neurol Sci. 2007;258:11–16. doi: 10.1016/j.jns.2007.01.074. [DOI] [PubMed] [Google Scholar]