Abstract
Antiepileptic drugs (AEDs) target ion channels and neurotransmitter systems in the brain; these same targets are responsible for regulation of processes essential for brain development. In this review, experimental findings on adverse effects of AEDs in the developing mammalian brain will be presented, including interference with physiological apoptotic cell death, cell proliferation and migration, neurogenesis, axonal arborization, synaptogenesis, and synaptic plasticity.
Antiepileptic drugs (AEDs) interact with ion channels, metabolic enzymes, and neurotransmitter receptors and transporters in the brain; in addition, they modify bursting properties of neurons, inhibit spread of epileptic activity, and reduce synchronization (1). AEDs are among the most common causes of fetal malformations, such as neural tube defects, congenital heart defects, orofacial clefts, digital anomalies, growth retardation, developmental delay, and microcephaly (2–7). Teratogenic effects have been associated with the use of phenytoin, carbamazepine, valproate, lamotrigine, and phenobarbital. Elevated maternal blood levels of these drugs and combinations of AEDs, as compared with monotherapy, impose increased risks for human infants (3,7–10).
In addition, several studies report that in utero exposure to AEDs increases the risk of cognitive dysfunction later in life (11–15). Morphological changes in the brains of subjects exposed in utero to AEDs have been described. In a group of healthy young adults with prenatal exposure to AEDs and a group of age-matched unexposed healthy controls, MRI and voxel-based morphometry of the brains revealed structural differences between the two groups (16). Among subjects exposed prenatally to AEDs, regional decreases of grey matter volumes were found in the area of the lentiform nucleus, including both the globus pallidus and putamen bilaterally, and in the hypothalamus. The investigators concluded that prenatal exposure to AEDs may cause these subtle morphological changes in grey matter, with the most striking findings consisting of lower grey matter volumes in the basal ganglia and the hypothalamus (16).
Modelling Prenatal Exposure to AEDs in Animal Studies
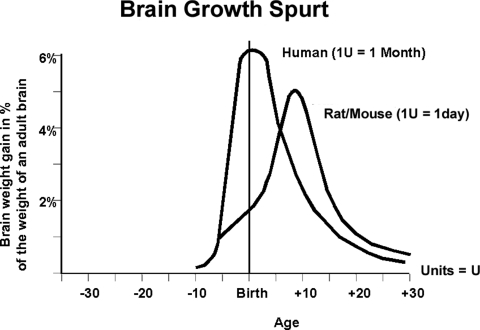
Brain growth varies among mammals, but comparisons of brain development between species are possible (17,18). The developmental ages of human and rat embryos or fetuses are comparable when anatomical features and histological landmarks are similar in appearance between the two species, even though their exact chronological ages are different (17). Dobbing and Sands described the pattern of growth of the mammalian brain as appearing in the shape of a sigmoid trajectory, when weight is plotted against age. The transient phase of rapid growth is known as the “brain growth spurt” period. The brain growth spurt occurs at discrete times, in relation to birth, among different species and, thus, has to be taken into account when extrapolating results obtained from one species and applying them to another (18).
The different mammalian species can be categorized as prenatal, perinatal, or postnatal brain developers. Knowledge of the timing of the brain growth spurt for each species can be used as a marker of developmental age that is a means to identify comparable chronological ages; it is taken into consideration when hypotheses relating to brain vulnerability are formulated. The brain growth spurt of rats and mice occurs postnatally, with peak growth velocity on P7–P10, and ends in the third week (Fig. 1). In humans, the brain growth spurt period starts during the third trimester of pregnancy, with peak growth velocity around the time of birth. Thus, for purposes of modelling human prenatal exposure to AEDs in rodents, exposures ought to occur prior to P10 in rats and mice.
FIGURE 1.
Brain growth spurts in humans and rats, expressed as first-order velocity curves of the increase in weight with age. Reference: Ikonomidou and Turski (55).
Effects of AEDs in Infant Rodents
Perinatal exposure of rats to phenobarbital can reduce brain weight (19) as well as cause a reduction of Purkinje and granule cells in the cerebellum (20) and of pyramidal and granule cells in the hippocampus (21). Studies indicate that administration of phenobarbital to rat pups can result in significant decreases in brain weight and DNA, RNA, protein, and cholesterol concentrations as well as reduced neuronal numbers (22,23). Perinatal exposure of rodents to phenobarbital may cause decrements in various spatial learning tasks (24,25) or may increase aggression and locomotor activity (22,25–27). Prenatal exposure to phenobarbital resulted in deficits in the hippocampal eight-arm maze (23), caused spontaneous alternations in water maze behaviors in adult animals (23), and affected operant conditioning (28,29).
Phenobarbital was found to be an effective anticonvulsant against spontaneous seizures in developing animals that had undergone kainic acid-induced status epilepticus. However, animals receiving anticonvulsant doses of phenobarbital performed worse in the water maze than animals receiving saline (although the drug had been withdrawn during cognitive testing), while saline-treated animals had significantly more spontaneous seizures (26). These results indicate that phenobarbital can have adverse effects on cognition when administered following an acute insult.
Perinatal administration of phenytoin in rodents causes a reduction in brain weight (29,30) and a number of behavioral deficits (seen at subteratogenic doses), including deficits in spatial learning tasks and hyperactivity (31). Furthermore, neural tube defects have been noted in mice administered valproic acid (32); strain differences in susceptibility suggest an underlying genetic predisposition (33). At therapeutically relevant concentrations, valproic acid alters the expression of certain homeobox genes and inhibits histone deacetylase, which is involved in the repression of gene expression (34). Inhibition of histone deacetylase may underlie the ability of valproic acid to reduce proliferation of C6 glioma cells and may correlate to its teratogenicity, as alterations in the normal proliferation rate of these cells can result in an embryo with a neural tube defect (35). Exposure to subteratogenic doses of valproic acid can cause microcephaly and behavioral changes (i.e., deficits in spatial learning tasks and altered locomotor activity) in rodents (30,36). In utero exposure of rats to valproic acid (at peak blood levels of 99–134 mg/mL) causes cerebellar anomalies (36).
In contrast, a study of active avoidance using carbamazepine, administered at low doses, protected against impairment of learning rate caused by repeated application of convulsant shock and had no effect on learned taste aversion (30). In rodents, incidence and severity of teratogenic effects from carbamazepine were less than those observed with other AEDs and occurred mostly at high, therapeutically irrelevant doses (30). Similarly, neonatal rats without seizures exposed to 4 weeks of topiramate did not differ from untreated controls in water maze performance or histologic examination. In addition, long-term administration of high-dose topiramate to normal developing rats does not appear to impair cognitive performance. Experimental data suggest that anticonvulsant doses of topiramate might be neuroprotective in the developing brain in a neonatal hypoxia model of seizures in rats (37).
Mechanisms of Adverse Effects of AEDs in the Developing Brain
Neuronal Apoptosis
Physiological cell death, a process by which cells are deleted from the developing CNS, is a regular phenomenon in the developing brain. Studies have shown that compounds that are used medically as sedatives, anesthetics, or anticonvulsants can trigger widespread apoptotic neurodegeneration throughout the developing brain when administered to immature rodents (38–41). Such compounds include antagonists of NMDA receptors (e.g., ketamine, nitrous oxide), agonists of GABAA receptors (e.g., barbiturates, benzodiazepines, propofol), and AEDs (e.g., sulthiame, phenytoin, vigabatrin, and valproate).
The vulnerable developmental period during which drug-induced neuroapoptosis is observed in rodents spans the first two postnatal weeks of life. The comparable period in humans stretches from the sixth month of gestation to several years after birth. Thus, there is an extended period in prenatal human development, during which immature neurons might be prone to commit suicide if exposed to some AEDs.
Neurotoxic effects of AEDs were systematically studied in infant rodents (38,41,42). It was determined that the majority of AEDs cause apoptotic neurodegeneration in the developing rat brain at doses and plasma concentrations relevant for anticonvulsant treatment.
Neurodegeneration was described within the septum; nucleus accumbens, thalamic, and hypothalamic nuclei; subiculum; amygdala; and the globus pallidus, piriform, entorhinal, frontoparietal, cingulate, and retrosplenial cortices, following treatment with barbiturates, benzodiazepines, valproate, sulthiame, or phenytoin (38). Using electron microscopy, it was determined that the degenerating cells displayed ultrastructural changes similar to those described in neurons undergoing programmed cell death. The threshold dose for triggering an apoptotic response for phenytoin was 20 mg/kg, which resulted in phenytoin plasma concentrations ranging between 10 and 15 μg/mL over 4 hours. When concentrations of phenobarbital were maintained at 25–35 μg/mL over a 12-hour period, significant apoptotic neurodegeneration occurred. Dose-dependent neurodegeneration was also seen following valproate or vigabatrin treatment in infant rats (38). Interestingly, lamotrigine at low doses was devoid of neurotoxic effects in infant rats (42).
Additional experiments were performed to determine how the apoptotic response to AEDs might differ as a function of developmental age. These experiments revealed that there is a time window between P0 and P14 when various neuronal populations in the rat forebrain show transient sensitivity to the proapoptotic effects of AEDs (38). One study reported that neonatal exposure of mice to phenytoin leads to cerebellar damage, characterized by apoptotic death, delayed migration of granule cells, and altered development of Purkinje cells (43). In vitro experiments have confirmed that phenytoin induces apoptotic cell death of cultured cerebellar granule cells, degeneration of Purkinje cells, and toxicity in cerebral cortical cell cultures (44). Interestingly, topiramate did not elicit a neurotoxic effect in infant rat brain until a dose of 50 mg/kg, which is higher than the effective anticonvulsant doses in infant rodent seizure models (45). Therefore, the investigators concluded that topiramate has a rather beneficial profile, since it shows no detectable toxicity at anticonvulsant doses. Interestingly, levetiracetam exhibited no neurotoxicity in the infant rat brain (46).
The proapoptotic action of AEDs in the developing brain is partially due to reduction in synthesis of neurotrophins, including brain-derived neurotrophic factor (BDNF) and neurotrophins 3 and 4, as well as to reduced levels of the active phosphorylated forms of extracellular signal regulated kinase (ERK1/2) and protein kinase B (AKT). These kinases are key players in two major survival-promoting pathways, the MEK-ERK1/2 and the PI3 kinase-AKT pathways, both of which are activated by tyrosine kinase receptors upon binding of growth factors (38). Such changes reflect an imbalance between neuroprotective and neurodestructive mechanisms in the brain that will likely promote apoptotic death. Interestingly, 17β-estradiol counteracted inactivation of the ERK1/2 and AKT pathways and, in doing so, conferred protection against apoptotic neuronal deletion following treatment with some AEDs (47).
Cell Proliferation, Differentiation, and Migration
In addition to their proapoptotic effects in the developing brain, AEDs may also impair cell proliferation and differentiation, synaptogenesis, synaptic plasticity, cell migration, and axonal arborization. It is possible that a disruption of these developmental processes could account for the neurological deficits seen in humans exposed to AEDs prenatally; however, this theory remains unsubstantiated, because, unfortunately, the effects of AEDs in the developing brain have not been systematically analyzed.
Glutamate and GABA neurotransmitter systems are implicated in neuronal proliferation and migration during CNS development. The application of a single dose of diazepam (5 mg/kg) at P11 induced a significant reduction of mitotic activity in rodent cerebral cortex and anterior pituitary gland (48). Valproate, in contrast, promoted neuronal differentiation in human fetal forebrain stem cell cultures (49), while increased numbers of immature granule cells in the dentate gyrus resulted from treatment of mice with phenytoin from P5 to P14 (50).
In a recent study, the question of whether or not drugs that induce neuroapoptosis in the developing rodent brain also impair neurogenesis was addressed. The NMDA antagonist, MK801, and the GABAA agonists, phenobarbital and diazepam, were administered to infant rats; cell proliferation and neurogenesis were studied in the brain, using BrdU and doublecortin immunohistochemistry, and stereology (51). Neurogenesis was quantified in the dentate gyrus on P15, following treatment with MK801 or with phenobarbital on P6–P10. MK801, phenobarbital, and diazepam reduced numbers of newly born cells in the brain. In the dentate gyrus, many of the newly formed cells differentiated toward a neuronal phenotype. Phenobarbital and MK801 significantly reduced numbers of new neurons in that structure. At the age of 6 months, phenobarbital-treated rats had fewer neurons in the dentate gyrus and performed worse than saline-treated littermates in water maze learning and memory task. These findings show that blockade of NMDA-receptor mediated excitation, as well as enhancement of GABAA-receptor activation, impair cell proliferation, and inhibit neurogenesis in the immature rat brain (51).
Synaptogenesis and Synaptic Plasticity
Concerns have been expressed that AEDs may disrupt synaptogenesis and synapse remodelling as a result of inhibition of excitatory neurotransmission (52). Immature dendritic spines of Purkinje cells were found in immature mice following administration of 35 mg/kg/day of phenytoin from P5 to P14 (50). Phenytoin, added to the culture medium, resulted in cell loss, decreased numbers of neurites, rarefied branching, and decreased levels of the cytoskeletal protein, microtubule-associated protein 2 (MAP2), in cultured mouse cerebellar granule cells—an effect that has been associated with decreased neurite formation (53). In addition, Manet et al. reported that fetal exposure to GABA-acting AEDs can induce hippocampal and cortical dysplasias (54).
Conclusions
The experimental data presented in this brief review demonstrate that many AEDs can alter normal brain development by influencing cell proliferation, neurogenesis, migration, programmed cell death, synaptogenesis, and synaptic plasticity. Various adverse effects can be elicited when exposure occurs prenatally. As discussed, a critical period, characterized by high vulnerability of the brain to AEDs, is the brain growth spurt period, during which widespread neuronal apoptosis is induced by many AEDs. There is substantial clinical evidence to suggest that prenatal exposure to AEDs has negative functional consequences in humans (55).
The findings reviewed here raise concerns regarding the current clinical practice of employing AEDs for seizure control in pregnant women and call for the design of novel AEDs and adjunctive neuroprotective therapies as well as for the generation of new data, by means of well-designed clinical trials, to guide medical practice (55). The finding that β-estradiol ameliorated phenobarbital neurotoxicity in experimental animal models suggests that maternal estrogens may be protecting the fetal human brain against AED-induced neurotoxicity. Preterm infants, who are prematurely deprived of maternal β-estradiol and are frequently treated with AEDs (especially phenobarbital), are expected to be at high risk for AED neurotoxicity.
Experimental studies demonstrated a generally favourable profile for carbamazepine and lamotrigine, whereas topiramate had a favourable therapeutic index in that there is a distinct separation between the anticonvulsant and the neurotoxic- or proapoptotic-dose ranges in the developing rat brain (43). Even more encouraging was the finding that levetiracetam does not demonstrate a proapoptotic effect (44). Thus, by choosing the appropriate antiepileptic therapies, it may be possible to avoid or minimize neurotoxic side effects on the developing human fetus.
References
- 1.Rogawski MA, Gryder D, Castaneda D, Yonekawa W, Banks MK, Lia H. GluR5 kainate receptors, seizures, and the amygdala. Ann NY Acad Sci. 2003;985:150–162. doi: 10.1111/j.1749-6632.2003.tb07079.x. [DOI] [PubMed] [Google Scholar]
- 2.Buehler BA, Rao V, Finnell RH. Biochemical and molecular teratology of fetal hydantoin syndrome. Neurol Clin. 1994;12:741–748. [PubMed] [Google Scholar]
- 3.Holmes LB, Harvey EA, Coull BA, Huntington KB, Khoshbin S, Hayes AM, Ryan LM. The teratogenicity of anticonvulsant drugs. N Engl J Med. 2001;344:1132–1138. doi: 10.1056/NEJM200104123441504. [DOI] [PubMed] [Google Scholar]
- 4.Jones KL, Lacro RV, Johnson KA, Adams J. Pattern of malformations in the children of women treated with carbamazepine during pregnancy. N Engl J Med. 1989;320:1661–1666. doi: 10.1056/NEJM198906223202505. [DOI] [PubMed] [Google Scholar]
- 5.Meador KJ, Baker GA, Finnelli RH. In utero antiepileptic drug exposureFetal death and malformations. Neurology. 2006;67:407–412. doi: 10.1212/01.wnl.0000227919.81208.b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Speidel BD, Meador SR. Maternal epilepsy and abnormalities of the fetus and newborn. Lancet. 1972;308:839–843. doi: 10.1016/s0140-6736(72)92209-x. [DOI] [PubMed] [Google Scholar]
- 7.Zahn CA. Neurologic care of pregnant women with epilepsy. Epilepsia. 1998;39(Suppl 8):S26–S31. doi: 10.1111/j.1528-1157.1998.tb02604.x. [DOI] [PubMed] [Google Scholar]
- 8.Shor S, Koren G, Nulman I. Teratogenicity of lamotrigine. Can Fam Phys. 2007;53:1007–1009. [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez-Diaz S, Smith CR, Wyszynski DP, Holmes LB. Malformations among infants exposed to carbamazepine during pregnancy. Birth Def. Res. (Part A) Clin Mol Teratol. 2007;79:357. [Google Scholar]
- 10.Morrow JI, Russell A, Gutherie E, Parsons L, Robertson I, Waddell R, Irwin B, Morrison P, McGivern CR, Craig J. Malformation risks of anti-epileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry. 2006;77:193–198. doi: 10.1136/jnnp.2005.074203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marsh ED, Brooks-Kayal AR, Ported BE. Seizures and antiepileptic drugs: does exposure alter normal brain development? Epilepsia. 2006;47:1999–2010. doi: 10.1111/j.1528-1167.2006.00894.x. [DOI] [PubMed] [Google Scholar]
- 12.Meador KL, Baker G, Cohen MJ, Gaily E, Westerveld M. Cognitive/behavioral teratogenic effects of antiepileptic drugs. Epilepsy Behav. 2007;11:292–302. doi: 10.1016/j.yebeh.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meador KJ, Baker GA, Browning N, Clayton-Smith J, Combs-Cantrell DT, Cohen M, Kalayjian LA, Kanner A, Liporace JD, Pennell PB, Privitera M, Loring DW, NEAD Study Group Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med. 2009;360:1597–1605. doi: 10.1056/NEJMoa0803531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicolai J, Vles JSH, Aldenkamp AP. Neurodevelopmental delay in children exposed to antiepileptic drugs in utero: a critical review directed at structural study-bias. J Neurol Sci. 2008;271:1–14. doi: 10.1016/j.jns.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Vinten J, Adab N, Kini U. Neuropsychological effects of exposure to anticonvulsant medication in utero. Neurology. 2005;64:949–954. doi: 10.1212/01.WNL.0000154514.82948.69. [DOI] [PubMed] [Google Scholar]
- 16.Ikonomidou C, Scheer J, Wilhelm T, Juengling F, Titze K, Stöver U, Lehmkuhl U, Koch S, Kassubek J. Brain morphology alterations following prenatal exposure to antiepileptic drugs. Eur J Ped Neurol. 2007;11:297–301. doi: 10.1016/j.ejpn.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- 18.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Human Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 19.Diaz J, Schain RJ. Phenobarbital: effects of long-term administration on behavior and brain of artificially reared rats. Science. 1978;199:90–91. doi: 10.1126/science.199.4324.90. [DOI] [PubMed] [Google Scholar]
- 20.Yanai J, Fares F, Gavish M, Greenfeld Z, Katz Y, Marcovici G. Neural and behavioral alterations after early exposure to phenobarbital. Neurotoxicology. 1989;10:543–554. [PubMed] [Google Scholar]
- 21.Pick CG, Yanai J. Long-term reduction in eight arm maze performance after early exposure to phenobarbital. Int J Dev Neurosci. 1985;3:223–227. doi: 10.1016/0736-5748(85)90027-9. [DOI] [PubMed] [Google Scholar]
- 22.File SE, Wilks LJ. Changes in seizure threshold and aggression during chronic treatment with three anticonvulsants and on drug withdrawal. Psychopharmacology (Berl) 1990;100:237–242. doi: 10.1007/BF02244413. [DOI] [PubMed] [Google Scholar]
- 23.Picker M, Thomas J, Koch C, Poling A. Effects of phenytoin, phenobarbital, and valproic acid, alone and in selected combinations, on schedule-controlled behavior of rats. Pharmacol Biochem Behav. 1985;22:389–393. doi: 10.1016/0091-3057(85)90037-1. [DOI] [PubMed] [Google Scholar]
- 24.Krafft K, Lyon DO, Poling A. Effects of phenytoin on schedule-controlled performance of rats. Psychopharmacology. 1982;78:93–95. doi: 10.1007/BF00470597. [DOI] [PubMed] [Google Scholar]
- 25.Mikati MA, Holmes GL, Chronopoulos A, Hyde P, Thurber S, Gatt A. Phenobarbital modifies seizure-related brain injury in the developing brain. Ann Neurol. 1994;36:425–433. doi: 10.1002/ana.410360314. [DOI] [PubMed] [Google Scholar]
- 26.Renfrey G, Schlinger H, Jakubow J, Poling A. Effects of phenytoin and phenobarbital on schedule-controlled responding and seizure activity in the amygdala-kindled rat. J Pharmacol Exp Ther. 1989;248:967–973. [PubMed] [Google Scholar]
- 27.Rostock A, Hoffmann W, Siegemund C, Bartsch R. Effects of carbamazepine, valproate calcium, clonazepam and piracetam on behavioral test methods for evaluation of memory-enhancing drugs. Methods Find Exp Clin Pharmacol. 1989;11:547–553. [PubMed] [Google Scholar]
- 28.Smith DF. Lithium and carbamazepine: effects on learned taste aversion and open field behavior in rats. Pharmacol Biochem Behav. 1983;18:483–488. doi: 10.1016/0091-3057(83)90268-x. [DOI] [PubMed] [Google Scholar]
- 29.Hatta T, Ohmori H, Murakami T, Takano M, Yamashita K, Yasuda M. Neurotoxic effects of phenytoin on postnatal mouse brain development following neonatal administration. Neurotoxicol Teratol. 1999;21:21–28. doi: 10.1016/s0892-0362(98)00028-2. [DOI] [PubMed] [Google Scholar]
- 30.Vorhees CV. Fetal hydanthoin syndrome in rats: dose-effect relationships of prenatal phenytoin on postnatal development and behavior. Teratology. 1987;35:287–303. doi: 10.1002/tera.1420350302. [DOI] [PubMed] [Google Scholar]
- 31.Schilling MA, Inman SL, Morford LL, Moran MS, Vorhees CV. Prenatal phenytoin exposure and spatial navigation in offspring: effects on reference and working memory and on discrimination learning. Neurotoxicol Teratol. 1999;21:567–578. doi: 10.1016/s0892-0362(99)00019-7. [DOI] [PubMed] [Google Scholar]
- 32.Paulson RB, Sucheston ME, Hayes TG, Paulson GW. Teratogenic effects of valproate in the CD-1 mouse fetus. Arch Neurol. 1985;42:980–983. doi: 10.1001/archneur.1985.04060090062015. [DOI] [PubMed] [Google Scholar]
- 33.Faiella A, Wernig M, Consalez GG, Hostick U, Hoffman C, Hustert E. A mouse model for valproate teratogenicity: parental effects, homeotic transformation, and altered HOX expression. Hum Mol Genet. 2000;9:227–236. doi: 10.1093/hmg/9.2.227. [DOI] [PubMed] [Google Scholar]
- 34.Finnell RH, Gelineau-van Waes J, Eudy JD, Rosenquist TH. Molecular basis of environmentally induced birth defects. Annu Rev Pharmacol Toxicol. 2002;42:181–208. doi: 10.1146/annurev.pharmtox.42.083001.110955. [DOI] [PubMed] [Google Scholar]
- 35.Martin ML, Regan CM. The anticonvulsant valproate teratogen restricts the glial cell cycle at a defined point in the mid G1 phase. Brain Res. 1991;554:223–228. doi: 10.1016/0006-8993(91)90193-y. [DOI] [PubMed] [Google Scholar]
- 36.Ingram JL, Peckham SM, Tisdale B, Rodier PM. Prenatal exposure of rats to valproic acid reproduces the cerebellar anomalies associated with autism. Neurotoxicol Teratol. 2000;22:319–324. doi: 10.1016/s0892-0362(99)00083-5. [DOI] [PubMed] [Google Scholar]
- 37.Jensen FE, Blume H, Alvarado S, Firkusny I, Geary C. NBQX blocks acute and late epileptogenic effects of perinatal hypoxia. Epilepsia. 1995;36:966–972. doi: 10.1111/j.1528-1157.1995.tb00954.x. [DOI] [PubMed] [Google Scholar]
- 38.Bittigau P, Sifringer M, Genz K, Reith E, Pospischil D, Govindarajalu S, Dzietko M, Pesditschek S, Mai I, Dikranian K, Olney JW, Ikonomidou C. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci USA. 2002;99:15089–15094. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vöckler J, Dikranian Tenkova T, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 40.Ikonomidou C, Bittigau P, Ishimaru MJ, Koch C, Genz K, Price MT, Stefovska V, Hörster F, Tenkova T, Dikranian K, Olney JW. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- 41.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim J, Kondratyev A, Gale K. Antiepileptic drug-induced neuronal cell death in the immature brain: effects of carbamazepine, topiramate, and levetiracetam as monotherapy versus polytherapy. J Pharmacol Exp Ther. 2007;323:165–173. doi: 10.1124/jpet.107.126250. [DOI] [PubMed] [Google Scholar]
- 43.Ohmori H, Ogura H, Yasuda M, Nakamura S, Hatta T, Kawano K. Developmental neurotoxicity of phenytoin on granule cells and Purkinje cells inmouse cerebellum. J Neurochem. 1999;72:1497–1506. doi: 10.1046/j.1471-4159.1999.721497.x. [DOI] [PubMed] [Google Scholar]
- 44.Blank NK, Nishimura RN, Seil FJ. Phenytoin neurotoxicity in developing mouse cerebellum in tissue culture. J Neurol Sci. 1982;55:91–97. doi: 10.1016/0022-510x(82)90172-1. [DOI] [PubMed] [Google Scholar]
- 45.Glier C, Dzietko M, Bittigau P, Jarosz B, Korobowicz E, Ikonomidou C. Therapeutic doses of topiramate are not toxic to the developing brain. Exp Neurol. 2004;185:403–409. doi: 10.1016/j.expneurol.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 46.Manthey D, Asimiadou S, Stefovska V, Kaindl AM, Fassbender J, Ikonomidou C, Bittigau P. Sulthiame but not levetiracetam exerts neurotoxic effect in the developing rat brain. Exp Neurol. 2005;193:497–503. doi: 10.1016/j.expneurol.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 47.Asimiadou S, Bittigau P, Felderhoff-Mueser U, Manthey D, Sifringer M, Pesditschek S, Dzietko M, Kaindl AM, Pytel M, Studniarczyk D, Mozrzymas JW, Ikonomidou C. Protection with estradiol in developmental models of apoptotic neurodegeneration. Ann Neurol. 2005;58:266–276. doi: 10.1002/ana.20553. [DOI] [PubMed] [Google Scholar]
- 48.Pawlikowski M, Stępien H, Mróz-Wasilewska Z, Pawlikowska A. Effects of diazepam on cell proliferation in cerebral cortex, anterior pituitary and thymus of developing rats. Life Sci. 1987;40:1131–1135. doi: 10.1016/0024-3205(87)90577-7. [DOI] [PubMed] [Google Scholar]
- 49.Laeng P, Pitts RL, Lemire AL, Drabik CE, Weiner A, Tang H. The mood stabilizer valproic acid stimulates GABA neurogenesis from rat forebrain stem cells. J Neurochem. 2004;91:238–251. doi: 10.1111/j.1471-4159.2004.02725.x. [DOI] [PubMed] [Google Scholar]
- 50.Ogura H, Yasuda M, Nakamura S, Yamashita H, Mikoshiba K, Ohmori H. Neurotoxic damage of granule cells in the dentate gyrus and the cerebellum and cognitive deficit following neonatal administration of phenytoin in mice. J Neuropathol Exp Neurol. 2002;61:956–967. doi: 10.1093/jnen/61.11.956. [DOI] [PubMed] [Google Scholar]
- 51.Stefovska V, Czuczwar M, Smitka M, Czuczwar P, Kis J, Kaindl AM, Turski L, Turski WA, Ikonomidou C. Sedative and anticonvulsant drugs suppress postnatal neurogenesis. Ann Neurol. 2008;64:434–445. doi: 10.1002/ana.21463. [DOI] [PubMed] [Google Scholar]
- 52.Wong WT, Wong RO. Changing specificity of neurotransmitter regulation of rapid dendritic remodeling during synaptogenesis. Nat Neurosci. 2001;4:351–352. doi: 10.1038/85987. [DOI] [PubMed] [Google Scholar]
- 53.Kempermann G, Volk B. Phenytoin inhibits expression of microtubule-associated protein 2 and influences cell-viability and neurite growth of cultured cerebellar granule cells. Brain Res. 1995;687:194–198. doi: 10.1016/0006-8993(95)00469-7. [DOI] [PubMed] [Google Scholar]
- 54.Manent JB, Jorquera I, Mazzucchelli I, Depaulis A, Perucca E, Ben-Ari Y, Represa A. Fetal exposure to GABA-acting antiepileptic drugs generates hippocampal and cortical dysplasias. Epilepsia. 2007;48:684–693. doi: 10.1111/j.1528-1167.2007.01056.x. [DOI] [PubMed] [Google Scholar]
- 55.Ikonomidou C, Turski L. Antiepileptic drugs and brain development. Epilepsy Res. 2009 doi: 10.1016/j.eplepsyres.2009.09.019. Epub ahead of print. [DOI] [PubMed] [Google Scholar]