Abstract
Recent progress on pancreatic stem/progenitor cell research has revealed that the putative multipotent pancreatic stem/progenitor cells and/or more committed beta cell precursors may persist in the pancreatic gland in adult life. The presence of immature pancreatic cells with stem cell-like properties offers the possibility of stimulating their in vivo expansion and differentiation or to use their ex vivo expanded progenies for beta cell replacement-based therapies for type 1 or 2 diabetes mellitus in humans. In addition, the transplantation of either insulin-producing beta cells derived from embryonic, fetal and other tissue-resident adult stem/progenitor cells or genetically modified adult stem/progenitor cells may also constitute alternative promising therapies for treating diabetic patients. The genetic and/or epigenetic alterations in putative pancreatic adult stem/progenitor cells and/or their early progenies may, however, contribute to their acquisition of a dysfunctional behaviour as well as their malignant transformation into pancreatic cancer stem/progenitor cells. More particularly, the activation of distinct tumorigenic signalling cascades, including the hedgehog, epidermal growth factor–epidermal growth factor receptor (EGF–EGFR) system, wingless ligand (Wnt)/β-catenin and/or stromal cell-derived factor-1 (SDF-1)–CXC chemokine receptor 4 (CXCR4) pathways may play a major role in the sustained growth, survival, metastasis and/or drug resistance of pancreatic cancer stem/progenitor cells and their further differentiated progenies. The combination of drugs that target the oncogenic elements in pancreatic cancer stem/progenitor cells and their microenvironment, with the conventional chemotherapeutic regimens, could represent promising therapeutic strategies. These novel targeted therapies should lead to the development of more effective treatments of locally advanced and metastatic pancreatic cancers, which remain incurable with current therapies.
Recent advancements in embryonic, fetal and adult pancreatic stem/progenitor cell research have revealed that these immature cells may provide critical functions for pancreas organogenesis as well as pancreatic regeneration after intense injury in adult life. The presence of multipotent adult pancreatic stem/progenitor cells (PSCs) and/or further differentiated beta cell precursors in the ductal regions and/or within the islet compartment, respectively, is of great therapeutic interest (fig 1).1-13 The in vivo stimulation of these poorly differentiated adult putative PSCs or beta cell precursors within the adult pancreas, or the transplantation of their ex vivo expanded differentiated progenies could represent potential strategies for treating type 1 or 2 diabetes mellitus as well as other pancreatic diseases such as autoimmune and chronic pancreatitis.6 7 10 14 15
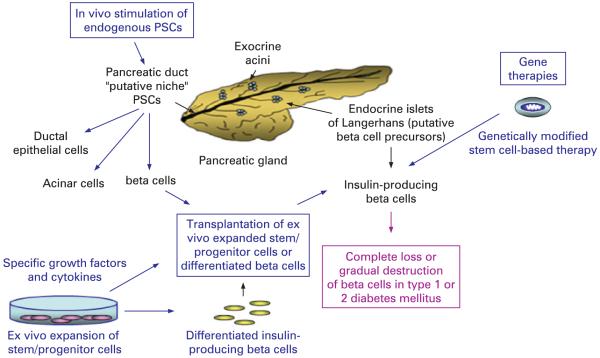
Figure 1.
Schematic representation of the pancreatic gland showing the anatomical localisation of putative pancreatic stem/progenitor cells (PSCs) and beta cell precursors, and stem-cell based therapies for type 1 or 2 diabetes mellitus. The scheme shows the localisation of putative pancreatic stem/progenitor cells within the ductal structure, exocrine acini and putative beta cell precursors found in a region near or within the endocrine islets of Langerhans. The complete loss or gradual destruction of islet beta cells in type 1 or 2 diabetes mellitus, respectively, and stem cell-based therapies for the beta cell replacement are also illustrated.
Diabetes mellitus type 1 and type 2 are associated with a hyperglycaemia due to the absence or reduction of insulin production by pancreatic beta cells.7 15-23 These metabolic disorders may result in the occurrence of severe systemic complications including diabetic retinopathy, nephropathy, heart diseases and stroke during disease progression.7 15-23 More specifically, the complete loss or gradual destruction of beta cells in the islets of Langerhans may lead to type 1 (juvenile or insulin-dependent form) or type 2 (adult or non-insulin-dependent form) diabetes mellitus, respectively.7 15-23 Type 1 diabetes mellitus is a chronic and life-threatening disease that generally results from the autoimmune destruction of pancreatic islet beta cells mediated via T cells, while type 2 is associated with dysfunction of beta cells and the development of insulin resistance in target tissues.
In spite of the possibility of transplantation (either of the whole pancreas or isolated islets of Langerhans) for therapeutic management of diabetic patients, the availability of organ donors suitable for transplantation as well as the immunosuppressive effect of therapeutic regimens used as adjuvant treatments may limit their application in the clinical settings.24 25 The replacement of lost beta cells by the new functional insulin-producing beta cells in the islets of pancreatic gland represents, then, a new alternative therapeutic strategy that could permit improvements in the quality of life and even a cure for patients with diabetes. Among the stem/progenitor cell sources from which mature beta cells could be derived, there are the embryonic, fetal and adult stem/progenitor cells including putative multipotent PSCs endowed with a potential for self-renewal.10 26
Recent progress in the field of normal and malignant stem cells has also revealed the possibility that accumulation of genetic and/or epigenetic alterations occurring in adult stem/progenitor cells including putative PSCs and/or their early progenies, during ageing and severe injuries, such as chronic inflammatory atrophy, may trigger their malignant transformation into cancer stem/progenitor cells.11 27-37 The cancer stem/progenitor cell concepts indicate that these malignant and immature cancer cells and/or their early progenies with stem cell-like properties, which may generate the bulk mass of differentiated cells, may provide critical functions in the initiation and progression of most cancers and disease relapse.28 More specifically, the most common form of pancreatic tumours is pancreatic ductal adenocarcinoma (PDA), which may derive from the malignant transformation of the ductal stem/progenitor cells and/or their early progenies into pancreatic cancer stem/progenitor cells.31-40 PDA is a highly lethal disease with a poor long-term overall 5 year survival rate (less than 5% for patients diagnosed with locally advanced and metastatic disease stages).40-42 Clinically, patients diagnosed with PDA have a poor prognosis. Major reasons for this poor outcome is due to our inability to diagnose PDA at an early stage because of the lack of specific symptoms and diagnostic markers, the inaccessible location of the pancreas, and the early occurrence of metastatic spread.40 41 43 Therefore, in the majority of patients, by the time of diagnosis, pancreatic cancer cells have spread to the surrounding tissues/organs such as bile duct, duodenum (small intestine), stomach, spleen, colon and liver as well as to the regional lymph nodes and more distant metastatic sites including lungs and/or bone through the peripheral circulation (fig 2). In this clinical setting, the treatment of patients with metastatic PDA by surgical resection, radiotherapy and/or chemotherapy is ineffective.44 45 The lack of efficacy of the therapeutic options currently available underlines the critical importance of identifying new targets for diagnosing and treating aggressive and metastatic PDAs. Targeting the pancreatic cancer stem/progenitor cells and/or their early progenies possessing the stem cell-like properties as well as their microenvironment offers great promise in the development of new therapeutic approaches for treating patients diagnosed with locally advanced PDAs or metastatic disease states.28 36 46 47 With regard to this, it has recently been reported that the highly tumorigenic CD133+ PSC cells isolated from PDA were more resistance than the CD133− fraction to gemcitabine treatment, whose chemotherapeutic drug is currently used in clinical settings against aggressive PDAs.37
Figure 2.
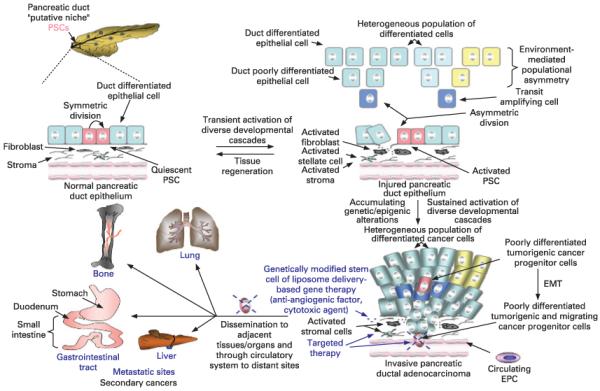
Proposed model of the molecular events associated with the pancreatic duct epithelium regeneration via putative adult pancreatic stem/progenitor cells (PSCs) after tissue injury and cancer initiation and progression through their malignant transformation. This scheme shows the asymmetric division of adult pancreatic duct stem/progenitor cells into transit-amplifying (TA)/intermediate cells expressing the epithelial, acinar and/or endocrine-like markers. In turn, TA cells may regenerate the bulk mass of further differentiated pancreatic epithelial cells within the exocrine compartment during pancreatic tissue repair after severe injury. Moreover, the possibility that TA cells may also give rise through environment-mediated populational asymmetry to the exocrine acinar and/or endocrine cell precursors is also indicated. The malignant transformation of putative pancreatic duct stem/progenitor cells into tumorigenic and migrating pancreatic cancer stem/progenitor cells, which may be induced through the sustained activation of distinct tumorigenic cascades such as hedgehog, epidermal growth factor receptor (EGFR) and wingless ligand (Wnt/β-catenin) during cancer progression, and more particularly during the epithelial–mesenchymal transition (EMT) programme is also illustrated. EPC, endothelial progenitor cell.
Here we review the recent progress in the identification of specific biomarkers and the anatomical location of embryonic, fetal and adult PSCs and their progenies. The extrinsic and intrinsic factors that may be implicated in the regulation of their self-renewal and differentiation abilities during development of the embryonic and fetal pancreas, and the regeneration process of injured pancreatic tissue in adult life, are also described. Importantly, we discuss the accumulating body of evidence suggesting the potential implications of putative PSCs and/or their early progenies in disease development. The emphasis is on the possible functions of their malignant counterpart, pancreatic cancer stem/progenitor cells in PDA initiation and progression. Of clinical interest, we describe the recent progress on the development of novel stem cell-based therapies for restoring the islet insulin-producing beta cell mass and treating type 1 or 2 diabetes mellitus as well as the targeted therapies against the aggressive and recurrent PDA forms.
EMBRYONIC AND FETAL DEVELOPMENT OF THE PANCREAS
In the early stage of human embryonic development, the proliferative pancreatic progenitor cells expressing pancreatic and duodenal homeobox factor 1 (PDX-1, also known as insulin promoter factor 1 (PF1), IDX-1 or STF-1) and cytoplasmic cytokeratin 19 (CK19) are detected during pancreas organogenesis.48 The detection of the ductal CK19 marker in fetal beta cell precursors appears to decrease in early fetal developing human pancreas while PDX1 remains expressed in these cells.48 In mouse pancreas, the results from direct cell lineage tracing through development have also indicated that the PDX-1 expressing progenitor cells can give rise to three pancreatic cell lineages including duct, exocrine and endocrine cells during early embryogenic stage.49 The interplay of several signalling cascades initiated by different growth factors, including the sonic hedgehog ligand (SHH), epidermal growth factor (EGF), Notch ligand and activin, may contribute to the regulation of the proliferation and/or differentiation of pancreatic cell precursors during pancreas development.50-54 With regard to this, several studies revealed that the defects in PDX-1 functions may impair the pancreas development including the generation of morphogenesis of the pancreatic epithelium and differentiation of pancreatic endocrine and exocrine cells.55-59 In humans, mutation in the PDX-1 gene leading to a loss of its functions has been associated with the occurrence of pancreatic agenesis and early-onset type 2 diabetes mellitus (MODY4).55 56 These observations suggest a critical role for the PDX-1 transcription factor during pancreas development. The defects of this gene product, which provides a major function in the regulation of beta target genes, including insulin expression in postnatal mature beta cells, may be associated with the occurrence of human diseases including diabetes mellitus.59 In the late phase of pregnancy, the mature and non-proliferative islet endocrine cells near vasculature, and which are able to independently produce the insulin, glucagon, somatostatin or pancreatic polypeptide (PP), are detected in the human fetal islet of the pancreas.48 This event coincides with the expression of differentiated islet cell-like markers such as prohormone convertase 1/3 (PC1/3), islet amyloid polypeptide, chromogranin A and glucose transporter type 2 (GLUT2).48
In addition, the multipotent pancreatic progenitor cells expressing stem cell-like markers (nestin, ABCG2 and stem cell factor (SCF) receptor (KIT) and neurogenin 3 (NGN-3)), epidermal growth factor receptor (EGFR), hepatocyte growth factor receptor (c-Met) and glucagon-like peptide receptor 1 (GLP-1R) have been isolated from the human fetal pancreas.15 60-62 These immature cells also express several mesenchymal stem cell (MSC)-like markers, including CD44, CD90 and CD147. Importantly, the pancreatic progenitor cells were able to form the islet cell-like clusters (ICCs) when cultured ex vivo and give rise to diverse pancreatic cell lineages, including the insulin-secreting cells and reversed hyperglycaemia in non-obese diabetic and severe combined immunodeficient (NOD-SCID) mice in vivo.15 60-63 The SP cell fraction isolated from ICCs also generated the insulin-producing beta cells in vitro.61 Moreover, analyses of the biomarkers of the ductal epithelial cells with a self-renewal capacity isolated from human fetal ICCs have revealed that these immature cells express nestin, PDX-1, CK7/19, proliferating cell nuclear antigen (PCNA), GLUT-2, vimentin, CD29, CD44 and CD166.24 Hence, the commitment of the embryonic and fetal PSCs along the endocrine cell lineages by using specific morphogenes and differentiation factors may then constitute a promising approach for generating the beta cells for cell replacement therapies for treating type 1 or 2 diabetes mellitus as described below in a more detailed manner.
PANCREAS-RESIDENT ADULT STEM/PROGENITOR CELLS
Accumulating experimental lines of evidence revealed that the putative multipotent PSCs that are capable of giving arise to the pancreatic ductal epithelial cells, acinar cells and/or endocrine beta, alpha and delta cells, also appear to persist in the postnatal stage and the adult mammalian pancreas. In support of this, the results from some studies indicated that the putative ductal PSCs could regenerate all the mature ductal epithelial cells, acinar and endocrine cell types after a partial pancreatectomy in normal rodent models and diabetic animal models in vivo.5 7 64-68 More recently, data from numerous investigations have also revealed that human and rodent mature insulin-producing islet beta cells could arise from adult PSCs.1-13 69 Putative PSCs expressing the markers of ductal epithelial cells (CK19high), exocrine cell lineages (nestin) and endocrine nuclear transcription factor PDX-1 and/or more committed nestin+/PDX-1+/CD19low islet cell precursors appear to be localised in the ductal regions and/or within islet compartment, respectively (fig 1).1-13 69 More specifically, a small subpopulation of human PSCs expressing PDX-1 and nestin and several MSC-like markers including CD29, CD44, CD49, CD50, CD51, CD62E, PDGFR-α, CD73(SH2), CD81 and CD105(SH3) has been isolated in serum-free medium from adult human pancreatic duct by fluorescence-activated cell sorting (FACS).70 These adult PSCs were able to differentiate into insulin-, glucagon- and somatostatin-positive cells in vitro in the presence of specific growth factors.70 More recently, the human CXCR4-positive cells expressing different stem cell-like markers including nestin, NGN-3, Oct-4, Nanog, ABCG2, CD133 and CD117 have been isolated from the islet-depleted pancreatic fraction.71 These immature cells were able to differentiate into the cells (which may correspond to transit amplifying cell progenies) that formed islet-like cell clusters (ILCC) and further acquired a beta cell-like phenotype in vitro.71 Similarly, the mouse ductal epithelial progenitor cells expressing CD133 and c-Met also appear to possess a multilineage potential.72 Additionally, it has been reported that the multipotent precursors from the adult mouse pancreas co-expressing neural and pancreatic precursor markers (nestin and PDX-1), SRY-box containing genes 2 and 3 (Sox2 and Sox3), mammalian achaete schute homolog 1 and 2 (Mash1) and NGN-3low can form clonal colonies. These pancreatic precursors were able to generate distinct populations of neurons and glial cells, pancreatic endocrine beta, alpha and delta cells, and pancreatic exocrine and stellate cells in vitro.2 The beta-like cells derived from these multipotent precursors also displayed a glucose-dependent Ca2+ responsiveness and produced insulin.2 Importantly, a recent study has also provided additional lines of experimental evidence that the multipotent endogenous beta cell progenitors expressing an embryonic islet cell progenitor-like marker, NGN3 exist in ductal lining of adult mouse pancreas.73 In particular, it has been showed that these facultative adult beta cell progenitors can give rise to all mature islet cell types including functional glucose responsive beta cells following partial duct ligation.73
Although together these observations suggests the presence of putative ductal PSCs in adult pancreas, further studies are necessary to more precisely establish the specific stem cell-like biomarkers that characterise the putative ductal PSCs versus their further differentiated progenies and beta cell precursors. The establishment of anatomical localisation of putative PSCs, and more particularly CD133+ PSCs, in the human and rodent pancreatic tissues in vivo also is essential to definite their specialised microenvironment “niche” in exocrine compartment. With respect to this, although some observations in rodent animal models suggest the implication of ductal PSCs in pancreas regeneration after intense injury, the possible differences between the endocrine differentiation programme of the developing pancreas in rodent and human underlines the significance of using the human pancreatic tissues in further investigations. Specifically, it will be important to more precisely ascertain the functional role provided by the putative adult human PSCs versus the more committed progenies and islet beta cell precursors in the maintenance of the beta cell mass as well as the intrinsic and extrinsic signals that may regulate their behaviour in homeostatic conditions and after injuries to the pancreas in vivo. Hence, these future studies should lead to successful strategies for in vivo stimulation or ex vivo expansion of putative human PSCs and/or beta cell precursors and their further differentiation into functional insulin-producing beta cells that could be translated into cell replacement therapies for treating patients with type 1 or 2 diabetes mellitus.
STEM CELL-BASED THERAPIES FOR THE TREATMENT OF TYPE 1 OR 2 DIABETES MELLITUS
PSCs and their progenies
The in vivo stimulation of endogenous PSCs or beta-cell precursors, or the transplantation of ex vivo expanded pancreatic insulin-producing beta cells in the host diseased recipient constitutes a promising therapeutic approach for beta cell mass restoration (fig 1).7 10-12 16-22 67 71 74-76 More specifically, the stimulation of the expression of diverse growth factors and transcription factors that provide critical functions during normal embryonic and fetal pancreas development and beta cell neogenesis in endogenous immature PSCs or beta cell precursors may lead to their differentiation into functional insulin-secreting beta cells in vitro and in vivo.7 10 12 16 22 74 77 78 Among these endocrine differentiation factors there are the PDX-1, NGN-3, paired box protein-4 (PAX-4) and basic helix-loop-helix (bHLH) protein, NeuroD, and morphogenic factors (nestin and clusterin). For instance, a recent study revealed that the occurrence of pancreatic tissue injury may result in the formation of neogenic ductules lined with low nestin-positive epithelial progenitor cells (designated as nestin-positive duct stem “NPDS” cells) that may give rise to pancreatic duct cells and insulin-producing beta cells in culture in vitro.12 The clusterin overexpression in these neogenic NPDS cells also upregulated the PDX-1 and NGN-3 and promoted their differentiation into beta cells in vitro.12 Importantly, the transplantation of purified pancreatic duct cells from islet-depleted human pancreatic tissue plus stromal cell preparation also generated the insulin-producing cells in normoglycaemic NOD/SCID mice.79 Moreover, it has also been reported that the autologous non-myeloablative hematopoietic stem cell (HSC) transplantation in a small group of 15 patients newly diagnosed with type 1 diabetes mellitus improved the beta cell function and induced prolonged insulin independence in the majority of treated patients.19 Altogether, these data indicate that the in vivo stimulation of PSCs and their early progenies or the transplantation of their ex vivo expanded beta cell-like progenies could represent an effective adjuvant treatment for patients with diabetes mellitus and/or other diseases resulting from a severe pancreatic tissue damage.
Embryonic, fetal and adult stem cells from other tissue sources and their progenies
The results from numerous in vitro pre-clinical trials and beta cell-based transplantation studies in animal models in vivo have also revealed the potential of using other sources of stem/progenitor cells for generating insulin-producing beta cells for the treatment of type 1 or 2 diabetes mellitus.10 11 54 76 80-85 In particular, the generation of mature and functional insulin-producing beta cell from human embryonic stem cells (ESCs) has been carried out based on the molecular events that control the cell fate decision through a definitive endoderm and commitment toward an endocrine beta cell-like phenotype during embryonic and fetal development.10 11 54 81-84 86-92 For instance, it has been observed that the induction of NGN-3 expression in mouse ESCs may provide a critical role for their differentiation into pancreatic cell lineages expressing the endocrine terminal differentiation markers as well as the insulin, glucagon and somatostatin.84 Moreover, differentiation of human ESCs into ectodermal insulin-producing beta cell-like progenitors expressing the specific markers related to pancreatic beta cells, including nuclear transcription factors such as Foxa2, PDX-1 and Isl1, has been performed by co-transplantation of ESCs with the dorsal pancreas from mouse embryos.81 More recently, the functional insulin-producing beta cells have also been derived from human ESCs by a treatment consisting of using activin A and all-trans retinoic acid (RA) for their pancreatic differentiation into cells expressing the PDX-1 and HLXB9 followed by their maturation in serum-free medium containing basic fibroblast growth factor (bFGF) and nicotamide.91 The transplantation of these differentiated beta cells in nude mice induced a stable euglycaemia, which was maintained for more than six weeks.91 Similarly, it has been reported that human ESCs differentiation into PDX-1 positive-cells followed by their in vivo transplantation resulted in their further maturation into pancreatic islet cells producing insulin and glucagons.89 This event was also accompanied by reducing the hyperglycaemia and weigh loss in diabetic mice.89
Alternatively, the stem/progenitor cells of other sources expressing the pancreas developing markers including nestin, CK8/18, NGN-3 and/or nuclear transcription factors such as PDX-1, PAX-4, PAX-6 and Isl-1, which are important for beta cell differentiation, might be expanded and transdifferentiated into beta cell-like progenitors under specific culture conditions in vitro. Among them, there are the fetal and umbilical cord blood-derived stem/progenitor cells, placenta derived-multipotent stem cells (PDMSCs), human amniotic epithelial cells (hAECs), and diverse adult stem cells, including bone marrow-resident HSCs and MSCs, hepatic oval cells (HOCs), neural stem cells (NSCs) and adipose tissue-derived stem cells (ADSCs).7 10 11 15 17 20 21 63 74 80 82 93-100 The differentiation of these stem/progenitor cells into pancreatic insulin-producing beta cell-like progenitors has been performed in vitro by using specific growth factors such as bFGF, SCF, EGF, gastrin, nicotinamide, betacellulin, glucagon-like peptide-1, and/or activin A.7 10 11 15 17 20 21 24 63 74 80 82 93-100 Importantly, the insulin-producing cells generated from certain stem/progenitor cells were also effective at reducing the blood glucose levels in diabetic animal models in vivo.15 63 80 96 97 More specifically, the activation of the HOCs by treatment of C57BL/6 mice with a diet containing 0.1% 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) for 4 weeks followed by an induction of hyperglycaemia with streptozotocin (STZ) led to a reversal of hyperglycaemia in this in vivo animal model.100 It has been proposed that the reversal of hyperglycaemia may be due in part to a transdifferentiation of HOCs followed by endogenous beta-cell regeneration in the pancreas.100 Additionally, the transplantation of bone marrow-derived cells plus syngenic or allogenic human MSCs has also been observed to promote pancreatic tissue regeneration and restore the blood glucose and insulin at normal levels in irradiated diabetic mice models in vivo, while the single injection was ineffective.101 On the basis of these observations, it has been suggested that the therapeutic effects associated with this treatment type could be mediated via a stimulation of the regeneration of endogenous insulin-secreting beta cells in the recipient, combined with an inhibition of T cell-mediated immune response against the newly formed beta cells.101 Interestingly, the results from recent studies have revealed that the bone marrow-derived MSCs engineered for expressing PDX-1 could generate the functional insulin-producing beta cells in vitro and induce euglycaemia after transplantation into the diabetic mice model in vivo.97 102 103 In spite of these significant advancements, additional in vivo studies appear to be necessary to establish the beneficial effects of using these stem/progenitor cell types and their further differentiated progenies to treat type 1 or 2 diabetes mellitus or other human pancreatic diseases. In particular, it will be important to establish the effects of insulin-producing beta cell progenitors on the restoration and normalisation of blood sugar levels after long-term treatment in vivo.
FUNCTIONS OF PANCREATIC CANCER STEM/PROGENITOR CELLS IN PDA PROGRESSION
Identification and characterisation of pancreatic cancer stem/progenitor cells in PDA tissues
Some recent investigations revealed that only a few cancer cells, designated as cancer stem/progenitor cells, could drive the tumour growth and metastasis at distant sites in most leukaemias and solid tumours including PDAs.11 26-36 In particular, the presence of stem/progenitor cells expressing stem cell-like markers in normal adult pancreas, suggests that the malignant transformation of these immature cells could contribute to the aetiolgy and progression of certain PDA subtypes as well as the resistance to current therapeutic treatments. In fact, ductal pancreatic cancer stem/progenitor cells as their normal counterpart within the exocrine compartment, could give rise to the transit amplifying (TA)/intermediate cells that in turn could generate a heterogeneous population of further differentiated PC cells expressing the ductal epithelial, acinar and/or endocrine cell-like markers (fig 2). In support of this, a small subpopulation of human pancreatic cancer stem/progenitor cells expressing CD133 stem cell-like marker has recently been identified by immunohistochemical staining in pancreatic adenocarcinoma specimens from patients.37 The isolated CD133+ pancreatic cancer stem/progenitor cells were able to give rise to the bulk mass of further differentiated CK-positive cancer cells in serum-containing medium in vitro, and to reconstitute the patient’s original tumour in athymic mice in vivo while CD133− cell fraction did not induce tumour formation.37 Interestingly, another subpopulation of tumorigenic and migrating CD133+/CXCR4+ pancreatic cancer stem/progenitor cells was also detected by immunostaining in the invasive front of pancreatic tumour samples.37 These tumorigenic and migrating CD133+/CXCR4+ cells may correspond to a more malignant cell subpopulation than tumorigenic CD133+/CXCR4− cells which may have acquired a migratory phenotype during the epithelial–mesenchymal transition (EMT) programme, and thereby be involved in invasion and metastases to distant sites (fig 2).37 Of therapeutic interest, it has also been noticed that the isolated subpopulation of CD133+ pancreatic cancer stem/progenitor cells was more resistant to gemcitabine than the CD133− cell fraction.37 Moreover, another subpopulation of multipotent CD44/CD24/epithelial specific-antigen (ESA)-positive pancreatic cancer stem/progenitor cells endowed with a self-renewal ability has been isolated from a patient’s pancreatic primary tumour.36 These putative pancreatic cancer stem/progenitor cells were more tumorigenic than the CD44/CD24/ESA-negative cell fraction in animal models in vivo and formed the tumours that phenotypically resembled the patients’ original primary neoplasm in vivo.36 With regard to this, it has been noticed that only a proportion of CD44/CD24-positive pancreatic cancer stem/progenitor cells co-expressed CD133 marker suggesting that the CD133+ cell fraction could correspond to a more immature pancreatic cancer stem/progenitor cell subpopulation.37 Additionally, certain studies have also revealed that the specific gene mutations and/or activation of growth factor cascades in pancreatic cancer cells with a ductal and/or acinar cell-like phenotype found within the exocrine compartment could contribute to the formation of pancreatic intraepithelial neoplasias (PanINs).31-35 38 104-106 These PanINs may progress into PDAs constituted by a heterogeneous pancreatic cancer cell population. For instance, it has been reported that the endogenous expression of mutated K-Ras (G12D) in a population of pancreatic exocrine progenitors characterised by the expression of nestin resulted in the formation of PanINs in a mouse model in vivo.31 Moreover, the hedgehog signalling pathway can also cooperate with activated K-Ras to promote PDA development in vivo.33-35
Hence, together these recent observations suggest the possible implication of immature CD133+ pancreatic cancer stem/progenitor cells and/or their early progenies in triggering certain PDA subtypes. Additional studies are necessary, however, in order to establish the other stem cell-like markers and oncogenic signalling elements that are frequently expressed by putative pancreatic cancer stem/progenitor cells versus their further differentiated progenies during PDA aetiolgy and progression. In particular, it will be important to assign the hierarchical cell lineage organisation of pancreatic cancer stem/progenitor cells and their early progenies endowed with the tumorigenic and/or migrating properties as well as the oncogenic events that may contribute to their acquisition of a more malignant behaviour during EMT programme and transition to the invasive and metastatic disease stages.
Identification and characterisation of pancreatic cancer stem/progenitor cells in well-established pancreatic cancer cell lines
The results from FACS analyses and characterisation of biomarkers detected in well-established human pancreatic cancer cell lines have also indicated that certain lines may contain a small cell subpopulation expressing the stem cell-like markers.28 107-109 A side population (SP), which is generally associated with the high expression of ATP-binding cassette (ABC) multidrug transporters such as breast cancer resistant protein-1 (BCRP-1/ABCG2), has notably been detected by Hoechst dye exclusion technique in certain tumorigenic pancreatic cancer epithelial cell lines established from patients’ pancreatic adenocarcinomas. Among these human pancreatic cancer cell lines habouring a SP subpopulation, there are the poorly differentiated CD18 cells derived from HPAF-2 cell line and PANC-1 cell line.28 107-109 The data from RT-PCR and FACS analyses have also indicated that the poorly differentiated PancTu1 and moderately differentiated A818-6 cells co-expressed the high levels of ABCG2 and CD133 markers, while the CD133 surface antigen was undetectable or expressed at a very low level in other pancreatic cancer cell lines such as PANC-1, PANC-89 and Colo-357.109 Moreover, expression of tumorigenic (CD133+/CXCR4−) and migrating (CD133+/CXCR4+) cell marker(s) has also been detected by FACS in diverse pancreatic cancer cells lines including BXPC3, MIA PaCa-2 and highly metastatic L3.6pl cells while ASPC1 cancer cells only expressed significant levels of CD133 marker.37 These observations indicate that these pancreatic cancer cell lines may contain a small cell subpopulation with stem cell-like properties which could be more resistant to chemotherapeutic drug treatment. In support of this assumption, it has been reported that a PANC-1 cell subpopulation overexpressing CD44, lymphocyte antigen 6 complex (LY6E) and tumour-associated calcium signal transducer 1 (TACSTD1) markers could be propagated under form spheres in a clonal colony-forming assay.110 This PANC-1 cell subpopulation also showed the ability to exclude the Hoechst 33342 dye.110 More recently, it has also been reported that the SP cells isolated from a parental PANC-1 cell line, which expressed high levels of ABC multidrug efflux pumps, ABCG2 and ABCB1 were more resistant to gemcitabine treatment than the non-SP cell fraction.111 Moreover, orthotopic implantation of isolated 103 CD133+ L3.6pl cells into the pancreas of mice resulted in the tumour formation in vivo while 106 CD133− L3.6pl cells did not induce tumour development. Importantly, it has also been observed that CD133+ L3.6pl cells were more resistant to gemcitabine treatment as compared to CD133− L3.6pl cells in vitro and an enrichment of the CD133+ L3.6pl cell fraction in the total tumour cell mass also occurred after gemcitabine treatment relative to the control in mice in vivo.37
In addition, the morphological pattern analyses of different human pancreatic cancer cell-derived xenografts have also indicated that they can generate tumours constituted of pancreatic cancer cells expressing distinct stem cell-like and differentiation markers.112-115 More specifically, a decrease of CK7/19 markers concomitant with an increase of CK8/18 and vimentin was observed depending on the cell line phenotype as follows: ductal (YAPC), ductal/solid (DAN-G and CAPAN-1) and solid (PANC-1 and MIA PaCa-2.115 The CK7-positive ductal phenotype (YAPC and DAN-G cell line) was also associated with PDX-1 expression, whereas the CK8-positive solid phenotype was related to the hedgehog signalling element (SHH/hedgehog-patched receptor (PTCH)) expression.115 Moreover, CK20 and nuclear β-catenin expression was mainly linked to a ductal phenotype. Additionally, the PDX-1 transcription factor has also been detected in patients’ adenocarcinoma tissue specimens by immunohistochemical analyses, and it has been proposed that PDX-1-positive pancreatic cancer cells (which may correspond to the putative pancreatic cancer stem/progenitor cells) could possess a higher potential for malignancy compared to PDX-1-negative cells.116 Similarly, it has also been reported that the embryonic stem cell-like marker PAX-6 was expressed in primary tumour specimens from PDA patients and pancreatic cancer cell lines, and its downregulation could be associated with the cancer cell differentiation.117 Importantly, the immunocytochemical analyses of individual CK-positive pancreatic cancer cells disseminated to bone marrow has also indicated that their presence may constitute a predictive factor associated with a reduced overall survival for the patients with PDA.118
Altogether, these investigations suggest that the immature pancreatic cancer stem/progenitor cells and their progenies found in malignant pancreatic tissues and well-established pancreatic cancer cell lines can express the stem cell-like markers (CD133) as well as duct epithelial (CKs), exocrine (nestin) and/or endocrine (PDX-1) cell markers like the putative pancreatic duct stem/progenitor cells found in the exocrine compartment in the normal pancreas. These tumorigenic and/or metastatic pancreatic cancer stem/progenitor cells and their early progenies may then contribute to tumour formation and metastases to distant sites as well as the intratumoral cellular heterogeneity. Based on this assumption, it is likely that the treatment resistance and recurrence of PDA may be due, at least in part, to the persistence of pancreatic cancer stem/progenitor cells with stem cell-like properties including their high expression levels of drug efflux pumps as observed for other aggressive cancer types. Hence, these recent advances emphasise the critical importance of also targeting the tumorigenic and migrating pancreatic cancer stem/progenitor cells and their local microenvironment to eradicate these cancer-initiating cells that may provide critical functions in PDA initiation and progression to the metastatic stages, treatment resistance and disease relapse.
NOVEL THERAPIES TARGETING PANCREATIC CANCER STEM/PROGENITOR CELLS AND THEIR MICROENVIRONMENT
Although tumour surgical resection, radiotherapy and chemotherapy, alone or in combination, may represent a potential curative treatment for the patients diagnosed with localised PDA at an early stage, the rapid progression to metastatic, recurrent and lethal disease states underlines the urgent need to develop new therapeutic regimen options.40-45 The combination of cytotoxic agents that are able to block distinct tumorigenic cascades activated in pancreatic cancer stem/progenitor cells and their further differentiated progenies during PDA initiation and progression represents a new promising strategy for treating patients who have aggressive PDA. More particularly, these targeted therapies could be used in combination with current clinical chemotherapeutic drug regimens such as gemcitabine and/or 5-flurouracil (5-FU) for overcoming drug resistance and improving the efficacy of treatments for the patients with locally advanced and/or metastatic PDAs (fig 3).41 43 119 120 With regard to this, the intrinsic and acquired resistance of pancreatic cancer cells to gemcitabine treatment has been associated with multiple factors including changes in gemcitabine transport and metabolism as well as the expression and/or activity of distinct signalling elements implicated in the cytotoxic response induced by this chemotherapeutic drug.41 121-134 Among the molecular mechanisms of gemcitabine resistance, there are an enhanced expression of ABC multidrug transporters, tissue transglutaminase (TG2), ribonucleotide reductase M1 and M2 (RRM1 and RRM2) subunit, glutathione-S-transferase, heat shock protein 27 and anti-apoptotic and survival factors such as Bcl-2, Bcl-xL and nuclear factor-kappaB (NF-κB).41 122-124 126 127 130-134 Moreover, a reduced expression of tensin homologue deleted on chromosome 10 (PTEN) concomitant with a phosphatidylinositol-3′ kinase (PI3K)/Akt activation, and downregulating pro-apoptotic effector, caspase 3 may also contribute to resistance of pancreatic cancer cells to gemcitabine treatment in certain PDA subtypes.124 126 135 Therefore, the targeting of these deregulated signalling elements that may contribute to the intrinsic and/or acquired resistance of pancreatic cancer cells including pancreatic cancer stem/progenitor cells to conventional gemcitabine-based clinical treatments is of great therapeutic interest (fig 3). In support of this, numerous investigations have revealed the beneficial therapeutic effect to inhibit the expression and/or activity of Bcl-2, Bcl-xL, TG2, NF-κB and/or focal adhesion kinase (FAK)/PI3K/Akt signalling elements for inducing growth inhibition and apoptotic death of pancreatic cancer cells and improving the response to gemcitabine treatment.41 122 124 127 130-132 134 For instance, it has been reported that the over-expression of TG2 may occur in the majority of PDA tumours and cell lines and contribute to gemcitabine resistance by inhibiting PTEN and inducing a constitutive activation of FAK/PI3K/Akt and NF-κB signalling pathways.124 136 137 Importantly, it has also been observed that the inhibition of endogenous TG2 by small interfering RNA significantly enhanced the tumour growth inhibitory and anti-metastatic effects induced by gemcitabine on orthotopically implanted Panc-226 cells in an animal model in vivo.124 Additionally, reduced expression of gemcitabine transporters including human equilibrative nucleoside transporter (hENT1) and/or deoxycytidine kinase (dCK), which is the enzyme that catalyses the first limiting step of phosphorylation involved in the transformation of the prodrug gemcitabine into its active metabolites, may also influence its inhibitory effect on DNA repair in certain cancer cell types (fig 3).41 121 125 128 129 Thus, the development of new gemcitabine derivatives such as a phospholipid gemcitabine conjugate could represent another potential strategy for overcoming the treatment resistance for certain subtypes of patients with PDA characterised by a decreased expression of nucleoside transporters and/or dCK.41 138
Figure 3.
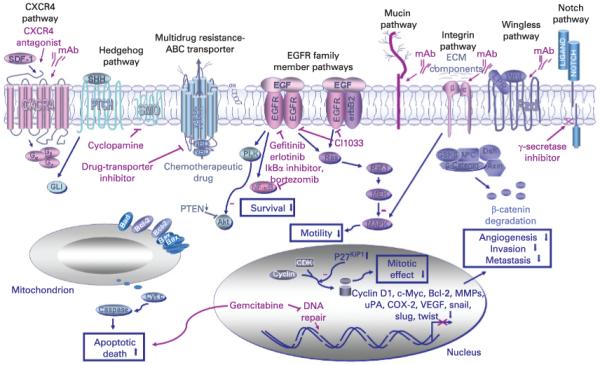
Scheme showing the novel therapeutic strategies against locally advanced and/or metastatic pancreatic ductal adenocarcinomas (PDAs) by targeting different oncogenic cascades signalling elements in pancreatic cancer cells. The cytotoxic agents acting as the potent inhibitors of the tumorigenic cascades including the selective inhibitors of the epidermal growth factor–epidermal growth factor receptor (EGF–EGFR) system (gefitinib, erlotinib and CI1033), smoothened hedgehog signalling element (cyclopamine), Notch (γ-secretase inhibitor), ATP-binding cassette (ABC) multidrug transporter inhibitor, CXC chemokine receptor 4 (CXCR4) antagonist and monoclonal antibody (mAb) directed against CXCR4, mucin, integrin or Wnt ligand are also indicated. Moreover, growth inhibitory and apoptotic effects induced by the current chemotherapeutic drug, gemcitabine, are illustrated. CDK, cyclin-dependent kinase; Cyt c, cytochrome c, Fzd, frizzled receptor; MAPKs, mitogen-activated protein kinases; MEK, extracellular signal-related kinase kinase; MMPs, matrix metalloproteinases; NBD, nucleotide binding domain; SMO, smoothened; uPA, urokinase plasminogen activator; VEGF, vascular epidermal growth factor; Wnt, wingless ligand.
In addition, targeting distinct tumorigenic signalling pathways, which may provide critical roles for the sustained growth, survival, invasion and/or metastasis of pancreatic cancer cells, including pancreatic cancer stem/progenitor cells during the EMT process and PDA progression into metastatic stages as well as disease relapse, also represents a potential therapeutic approach for improving the efficacy of current clinical chemotherapies (fig 3).34 35 41 105 106 119 120 139-150 Among these oncogenic targets there are the hedgehog, EGFR, Wnt/β-catenin, Notch, c-Met, integrin, SDF-1-CXCR4 and mucins 1 and 4 cascades and diverse signalling elements (K-Ras, PI3K/Akt, NF-κB, cyclooxygenase-2 (COX-2) and snail). With regard to this, numerous in vitro and in vivo investigations and clinical trials have revealed the beneficial effects of using the specific inhibitors of hedgehog (cyclopamine) and/or EGFR (erlotinib or gefitinib), alone or in combination therapies with gemcitabine for inducing apoptotic death, inhibiting tumour growth and counteracting the metastases of pancreatic cancer cells.35 41 119 120 Importantly, it has also been noticed that cyclopamine may preferentially reduce pancreatic cancer cells expressing high levels of a stem cell-like marker, aldehyde dehydrogenase, suggesting its potential cytotoxic effect on the immature tumour-initiating cells.35 The inhibition of the SDF-1/CXCR4 axis by using the non-peptidic antagonist of CXCR4, AMD3100, also reduced tumour metastasis established by the migrating CD133+/CXCR4+ L3.6pl cell fraction orthotopically injected into athymic mice.37 This observation indicates that this treatment type could be effective in counteracting the dissemination of pancreatic tumour-initiating cells.
On the other hand, several lines of evidence have also revealed that the acquisition of a more malignant phenotype and migratory potential by cancer progenitor cells including pancreatic cancer-initiating cells, and more particularly during the EMT programme, may also be influenced by the changes occurring in their local microenvironment (fig 2).28 30 41 43 46 115 151-154 Therefore, targeting tumour stromal cells, including activated myofibroblasts, stellate cells and bone marrow-derived endothelial progenitor cells (EPCs) that may promote PDA progression and treatment resistance, may also represent a potential adjuvant strategy for treating the aggressive and metastatic PDAs.28 30 41 151 152 155-157 In support of this, the use of pharmacological agents, including antibodies and specific or dual tyrosine kinase inhibitors targeting the vascular endothelial factor receptor (VEGFR) (SU5416), the VEGFR/EGFR (AEE788, PTK 787 and PKI 166), and/or the platelet-derived growth factor (PDGFR) (STI571 or AX102), alone or in combination therapies with other drugs such as gemcitabine, has been reported to inhibit angiogenesis, tumour growth and/or the metastatic spread of pancreatic cancer cells in animal models in vivo.41 154 156 158-160
Taken together, these observations underline the importance of eradicating the total pancreatic cancer cell mass, including the highly tumorigenic and/or migrating pancreatic cancer stem/progenitor cells, in order to counteract disease progression and relapse, and thereby improve the current therapies against metastatic and recurrent PDA forms.
CONCLUSIONS AND FUTURE RESEARCH
Recent progress in research on normal and malignant pancreatic stem/progenitor cells indicates their potential implications in the maintenance of tissue homeostatic state under physiological conditions as well as in the development of pancreatic disorders such as aggressive and recurrent PDAs. These recent advancements support the feasibility of stimulating these immature cells or more committed beta cell precursors in vivo or to transplant their further differentiated progenies after their ex vivo expansion for the beta cell replacement therapies for treating type 1 or 2 diabetes mellitus. Moreover, embryonic, fetal, placental and adult stem/cell progenitors resident in other adult tissues may also constitute alternative sources of beta cells for treating patients who have diabetes. In addition, the molecular targeting of the pancreatic cancer stem/progenitor cells and/or their early progenies endowed with a self-renewal potential and their local microenvironment may also constitute a promising approach for improving the current clinical treatments against aggressive and recurrent PDAs.
Further research is necessary to more precisely establish the gene expression patterns of normal and malignant pancreatic stem/progenitor cell progenies and putative beta cell precursors versus their differentiated progenies in order to identify the specific biomarker patterns as well as the molecular mechanisms that may regulate their biological behaviour in vivo and/or after their ex vivo expansion. The identification of the specific intrinsic factors that govern the decision between the self-renewal versus differentiation of normal and malignant pancreatic stem/progenitor cells as well as the influence of the extracellular signals from their local microenvironment “niche” on their behaviour is notably of immense interest for the design of new therapeutic strategies. These future studies should lead to the identification of specific growth factors, cytokines and/or chemokines and host cells that control the expansion and commitment of these immature cells into the specific differentiated pancreatic cell lineages in physiological and pathological conditions. Further characterisation of cancer cells with stem cell-like properties isolated from PDA tissue specimens of patients at different early and late stages or established pancreatic cell lines is also necessary to establish new in vitro and in vivo cell models more relevant to pancreas carcinogenesis and disease progression. These new cell models could be used to estimate the cytotoxic effects of drugs such as hedgehog and EGFR inhibitors on the SP cell fraction or pancreatic cancer stem/progenitor cells with the stem cell-like properties isolated from PDA specimens or well-established pancreatic cancer cell lines is also of therapeutic interest. Hence, the establishment of the specific properties of normal versus malignant pancreatic stem/progenitor cells is essential for the successful formulation of stem cellbased therapeutic approaches that could be translated into treatment and even a cure for diabetes patients as well as the patients with locally advanced and metastatic PDAs, which remain incurable with the current conventional therapies in the clinics.
Acknowledgements
We thank Ms K L Berger, Eppley Institute, UNMC, for editing the manuscript.
Funding: MM and SKB are supported by grants from the U.S. Department of Defense (PC04502, OC04110) and the National Institutes of Health (CA78590, CA111294, CA131944 and CA1133774).
Footnotes
Competing interests: None.
REFERENCES
- 1.Waguri M, Yamamoto K, Miyagawa JI, et al. Demonstration of two different processes of beta-cell regeneration in a new diabetic mouse model induced by selective perfusion of alloxan. Diabetes. 1997;46:1281–90. doi: 10.2337/diab.46.8.1281. [DOI] [PubMed] [Google Scholar]
- 2.Seaberg RM, Smukler SR, Kieffer TJ, et al. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat Biotechnol. 2004;22:1115–24. doi: 10.1038/nbt1004. [DOI] [PubMed] [Google Scholar]
- 3.Taguchi M, Otsuki M. Co-localization of nestin and PDX-1 in small evaginations of the main pancreatic duct in adult rats. J Mol Histol. 2004;35:785–9. doi: 10.1007/s10735-004-0948-9. [DOI] [PubMed] [Google Scholar]
- 4.Wang GS, Rosenberg L, Scott FW. Tubular complexes as a source for islet neogenesis in the pancreas of diabetes-prone BB rats. Lab Invest. 2005;85:675–88. doi: 10.1038/labinvest.3700259. [DOI] [PubMed] [Google Scholar]
- 5.Bouwens L, Rooman I. Regulation of pancreatic beta-cell mass. Physiol Rev. 2005;85:1255–70. doi: 10.1152/physrev.00025.2004. [DOI] [PubMed] [Google Scholar]
- 6.Bonner-Weir S, Toschi E, Inada A, et al. The pancreatic ductal epithelium serves as a potential pool of progenitor cells. Pediatr Diabetes. 2004;5:16–22. doi: 10.1111/j.1399-543X.2004.00075.x. [DOI] [PubMed] [Google Scholar]
- 7.Bonner-Weir S, Weir GC. New sources of pancreatic beta-cells. Nat Biotechnol. 2005;23:857–61. doi: 10.1038/nbt1115. [DOI] [PubMed] [Google Scholar]
- 8.Zulewski H, Abraham EJ, Gerlach MJ, et al. Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vitro into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes. 2001;50:521–33. doi: 10.2337/diabetes.50.3.521. [DOI] [PubMed] [Google Scholar]
- 9.Yang C, Wang JM, Du CY, et al. Expression of stem cell markers CK-19 and PDX-1 mRNA in pancreatic islet samples of different purity from rats. Hepatobiliary Pancreat Dis Int. 2007;6:544–8. [PubMed] [Google Scholar]
- 10.Mimeault M, Batra SK. Recent advances on the significance of stem cells in tissue regeneration and cancer therapies. Stem Cells. 2006;24:2319–45. doi: 10.1634/stemcells.2006-0066. [DOI] [PubMed] [Google Scholar]
- 11.Mimeault M, Hauke R, Batra SK. Stem cells – A revolution in therapeutics – Recent advances on the stem cell biology and their therapeutic applications in regenerative medicine and cancer therapies. Clin Pharmacol Ther. 2007;82:252–64. doi: 10.1038/sj.clpt.6100301. [DOI] [PubMed] [Google Scholar]
- 12.Kim SY, Lee S, Min BH, et al. Functional association of the morphogenic factors with the clusterin for the pancreatic beta-cell differentiation. Diabetes Res Clin Pract. 2007;77:S122–6. doi: 10.1016/j.diabres.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 13.D’Alessandro JS, Lu K, Fung BP, et al. Rapid and efficient in vitro generation of pancreatic islet progenitor cells from nonendocrine epithelial cells in the adult human pancreas. Stem Cells Dev. 2007;16:75–89. doi: 10.1089/scd.2006.0073. [DOI] [PubMed] [Google Scholar]
- 14.Halban PA. Cellular sources of new pancreatic beta cells and therapeutic implications for regenerative medicine. Nat Cell Biol. 2004;6:1021–5. doi: 10.1038/ncb1104-1021. [DOI] [PubMed] [Google Scholar]
- 15.Suen PM, Leung PS. Pancreatic stem cells: a glimmer of hope for diabetes? J Pancreas (online) 2005;6:422–4. [PubMed] [Google Scholar]
- 16.Fellous TG, Guppy NJ, Brittan M, et al. Cellular pathways to beta-cell replacement. Diabetes Metab Res Rev. 2007;23:87–99. doi: 10.1002/dmrr.692. [DOI] [PubMed] [Google Scholar]
- 17.Gangaram-Panday ST, Faas MM, de Vos P. Towards stem-cell therapy in the endocrine pancreas. Trends Mol Med. 2007;13:164–73. doi: 10.1016/j.molmed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Lees JG, Tuch BE. Conversion of embryonic stem cells into pancreatic beta-cell surrogates guided by ontogeny. Regen Med. 2006;1:327–36. doi: 10.2217/17460751.1.3.327. [DOI] [PubMed] [Google Scholar]
- 19.Voltarelli JC, Couri CE, Stracieri AB, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2007;297:1568–76. doi: 10.1001/jama.297.14.1568. [DOI] [PubMed] [Google Scholar]
- 20.Schaffler A, Buchler C. Concise review: adipose tissue-derived stromal cells – basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818–27. doi: 10.1634/stemcells.2006-0589. [DOI] [PubMed] [Google Scholar]
- 21.Lu P, Liu F, Yan L, et al. Stem cells therapy for type 1 diabetes. Diabetes Res Clin Pract. 2007;78:1–7. doi: 10.1016/j.diabres.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Noguchi H, Xu G, Matsumoto S, et al. Induction of pancreatic stem/progenitor cells into insulin-producing cells by adenoviral-mediated gene transfer technology. Cell Transplant. 2006;15:929–38. doi: 10.3727/000000006783981431. [DOI] [PubMed] [Google Scholar]
- 23.Krishna KA, Rao GV, Rao KS. Stem cell-based therapy for the treatment of Type 1 diabetes mellitus. Regen Med. 2007;2:171–7. doi: 10.2217/17460751.2.2.171. [DOI] [PubMed] [Google Scholar]
- 24.Qiao H, Zhao T, Wang Y, et al. Isolation, purification and identification of epithelial cells derived from fetal islet-like cell clusters. Sheng Wu Gong Cheng Xue Bao. 2007;23:246–51. doi: 10.1016/s1872-2075(07)60022-3. [DOI] [PubMed] [Google Scholar]
- 25.Truong W, Lakey JR, Ryan EA, et al. Clinical islet transplantation at the University of Alberta – the Edmonton experience. Clin Transpl. 2005:153–72. [PubMed] [Google Scholar]
- 26.Mimeault M, Batra SK. Recent progress on tissue-resident adult stem cell biology and their therapeutic implications. Stem Cell Rev. 2008;4:27–49. doi: 10.1007/s12015-008-9008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mimeault M, Hauke R, Batra SK. Recent advances on the molecular mechanisms involved in drug-resistance of cancer cells and novel targeting therapies. Clin Pharmacol Ther. 2008;83:673–91. doi: 10.1038/sj.clpt.6100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mimeault M, Hauke R, Mehta PP, et al. Recent advances on cancer stem/progenitor cell research: therapeutic implications for overcoming resistance to the most aggressive cancers. J Mol Cell Med. 2007;11:981–1011. doi: 10.1111/j.1582-4934.2007.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mimeault M, Batra SK. Interplay of distinct growth factors during epithelial-mesenchymal transition of cancer progenitor cells and molecular targeting as novel cancer therapies. Ann Oncol. 2007;18:1605–19. doi: 10.1093/annonc/mdm070. [DOI] [PubMed] [Google Scholar]
- 30.Mimeault M, Batra SK. Functions of tumorigenic and migrating cancer progenitor cells in cancer progression and metastasis and their therapeutic implications. Cancer Metastasis Rev. 2007;26:203–14. doi: 10.1007/s10555-007-9052-4. [DOI] [PubMed] [Google Scholar]
- 31.Carriere C, Seeley ES, Goetze T, et al. The Nestin progenitor lineage is the compartment of origin for pancreatic intraepithelial neoplasia. Proc Natl Acad Sci U S A. 2007;104:4437–42. doi: 10.1073/pnas.0701117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu L, Shi G, Schmidt CM, et al. Acinar cells contribute to the molecular heterogeneity of pancreatic intraepithelial neoplasia. Am J Pathol. 2007;171:263–73. doi: 10.2353/ajpath.2007.061176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pasca di MM, Sekine S, Ermilov A, et al. Hedgehog/Ras interactions regulate early stages of pancreatic cancer. Genes Dev. 2006;20:3161–73. doi: 10.1101/gad.1470806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morton JP, Mongeau ME, Klimstra DS, et al. Sonic hedgehog acts at multiple stages during pancreatic tumorigenesis. Proc Natl Acad Sci U S A. 2007;104:5103–8. doi: 10.1073/pnas.0701158104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feldmann G, Dhara S, Fendrich V, et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res. 2007;67:2187–96. doi: 10.1158/0008-5472.CAN-06-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–7. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 37.Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cells. 2007;1:313–23. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Hruban RH, Adsay NV, bores-Saavedra J, et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579–86. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Esni F, Miyamoto Y, Leach SD, et al. Primary explant cultures of adult and embryonic pancreas. Methods Mol Med. 2005;103:259–71. doi: 10.1385/1-59259-780-7:259. [DOI] [PubMed] [Google Scholar]
- 40.Brand RE, Lerch MM, Rubinstein WS, et al. Advances in counselling and surveillance of patients at risk for pancreatic cancer. Gut. 2007;56:1460–9. doi: 10.1136/gut.2006.108456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mimeault M, Brand RE, Sasson AA, et al. Recent advances on the molecular mechanisms involved in pancreatic cancer progression and therapies. Pancreas. 2005;31:301–16. doi: 10.1097/01.mpa.0000175893.04660.1b. [DOI] [PubMed] [Google Scholar]
- 42.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 43.el-Kamar FG, Grossbard ML, Kozuch PS. Metastatic pancreatic cancer: emerging strategies in chemotherapy and palliative care. Oncologist. 2003;8:18–34. doi: 10.1634/theoncologist.8-1-18. [DOI] [PubMed] [Google Scholar]
- 44.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–10. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 45.Carpelan-Holmstrom M, Nordling S, Pukkala E, et al. Does anyone survive pancreatic ductal adenocarcinoma? A nationwide study re-evaluating the data of the Finnish Cancer Registry. Gut. 2005;54:385–7. doi: 10.1136/gut.2004.047191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Omary MB, Lugea A, Lowe AW, et al. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest. 2007;117:50–9. doi: 10.1172/JCI30082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kleger A, Busch T, Liebau S, et al. The bioactive lipid sphingosylphosphorylcholine induces differentiation of mouse embryonic stem cells and human promyelocytic leukaemia cells. Cell Signal. 2007;19:367–77. doi: 10.1016/j.cellsig.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 48.Piper K, Brickwood S, Turnpenny LW, et al. Beta cell differentiation during early human pancreas development. J Endocrinol. 2004;181:11–23. doi: 10.1677/joe.0.1810011. [DOI] [PubMed] [Google Scholar]
- 49.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–57. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 50.Hebrok M, Kim SK, St Jacques B, et al. Regulation of pancreas development by hedgehog signaling. Development. 2000;127:4905–13. doi: 10.1242/dev.127.22.4905. [DOI] [PubMed] [Google Scholar]
- 51.Kawahira H, Scheel DW, Smith SB, et al. Hedgehog signaling regulates expansion of pancreatic epithelial cells. Dev Biol. 2005;280:111–21. doi: 10.1016/j.ydbio.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 52.Mfopou JK, Willems E, Leyns L, et al. Expression of regulatory genes for pancreas development during murine embryonic stem cell differentiation. Int J Dev Biol. 2005;49:915–22. doi: 10.1387/ijdb.052004jm. [DOI] [PubMed] [Google Scholar]
- 53.Mfopou JK, De Groote V, Xu X, et al. Sonic hedgehog and other soluble factors from differentiating embryoid bodies inhibit pancreas development. Stem Cells. 2007;25:1156–65. doi: 10.1634/stemcells.2006-0720. [DOI] [PubMed] [Google Scholar]
- 54.McLean AB, D’Amour KA, Jones KL, et al. Activin a efficiently specifies definitive endoderm from human embryonic stem cells only when phosphatidylinositol 3-kinase signaling is suppressed. Stem Cells. 2007;25:29–38. doi: 10.1634/stemcells.2006-0219. [DOI] [PubMed] [Google Scholar]
- 55.Stoffers DA, Zinkin NT, Stanojevic V, et al. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat Genet. 1997;15:106–10. doi: 10.1038/ng0197-106. [DOI] [PubMed] [Google Scholar]
- 56.Stoffers DA, Ferrer J, Clarke WL, et al. Early-onset type-II diabetes mellitus (MODY4) linked to IPF1. Nat Genet. 1997;17:138–9. doi: 10.1038/ng1097-138. [DOI] [PubMed] [Google Scholar]
- 57.Ahlgren U, Jonsson J, Jonsson L, et al. beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. 1998;12:1763–8. doi: 10.1101/gad.12.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li H, Edlund H. Persistent expression of Hlxb9 in the pancreatic epithelium impairs pancreatic development. Dev Biol. 2001;240:247–53. doi: 10.1006/dbio.2001.0440. [DOI] [PubMed] [Google Scholar]
- 59.Edlund H. Pancreatic organogenesis – developmental mechanisms and implications for therapy. Nat Rev Genet. 2002;3:524–32. doi: 10.1038/nrg841. [DOI] [PubMed] [Google Scholar]
- 60.Huang H, Tang X. Phenotypic determination and characterization of nestin-positive precursors derived from human fetal pancreas. Lab Invest. 2003;83:539–47. doi: 10.1097/01.lab.0000062890.40534.1c. [DOI] [PubMed] [Google Scholar]
- 61.Zhang L, Hu J, Hong TP, et al. Monoclonal side population progenitors isolated from human fetal pancreas. Biochem Biophys Res Commun. 2005;333:603–8. doi: 10.1016/j.bbrc.2005.05.111. [DOI] [PubMed] [Google Scholar]
- 62.Zhang L, Hong TP, Hu J, et al. Nestin-positive progenitor cells isolated from human fetal pancreas have phenotypic markers identical to mesenchymal stem cells. World J Gastroenterol. 2005;11:2906–11. doi: 10.3748/wjg.v11.i19.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suen PM, Li K, Chan JC, et al. In vivo treatment with glucagon-like peptide 1 promotes the graft function of fetal islet-like cell clusters in transplanted mice. Int J Biochem Cell Biol. 2006;38:951–60. doi: 10.1016/j.biocel.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 64.Bonner-Weir S, Baxter LA, Schuppin GT, et al. A second pathway for regeneration of adult exocrine and endocrine pancreas. A possible recapitulation of embryonic development. Diabetes. 1993;42:1715–20. doi: 10.2337/diab.42.12.1715. [DOI] [PubMed] [Google Scholar]
- 65.Plachot C, Portha B. Impaired pancreatic duct-cell growth in focal areas of regeneration after partial pancreatectomy in the adult Goto–Kakizaki rat, a spontaneous model of non-insulin dependent diabetes mellitus. Histochem J. 2001;33:141–7. doi: 10.1023/a:1017935808074. [DOI] [PubMed] [Google Scholar]
- 66.Plachot C, Movassat J, Portha B. Impaired beta-cell regeneration after partial pancreatectomy in the adult Goto–Kakizaki rat, a spontaneous model of type II diabetes. Histochem Cell Biol. 2001;116:131–9. doi: 10.1007/s004180100302. [DOI] [PubMed] [Google Scholar]
- 67.Liu T, Wang C, Wan C, et al. Proliferation and differentiation of duct epithelial cells after partial pancreatectomy in rats. J Huazhong Univ Sci Technolog Med Sci. 2006;26:567–9. doi: 10.1007/s11596-006-0522-7. [DOI] [PubMed] [Google Scholar]
- 68.Liu T, Wang CY, Gou SM, et al. PDX-1 expression and proliferation of duct epithelial cells after partial pancreatectomy in rats. Hepatobiliary Pancreat Dis Int. 2007;6:424–9. [PubMed] [Google Scholar]
- 69.Delacour A, Nepote V, Trumpp A, et al. Nestin expression in pancreatic exocrine cell lineages. Mech Dev. 2004;121:3–14. doi: 10.1016/j.mod.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 70.Lin HT, Chiou SH, Kao CL, et al. Characterization of pancreatic stem cells derived from adult human pancreas ducts by fluorescence activated cell sorting. World J Gastroenterol. 2006;12:4529–35. doi: 10.3748/wjg.v12.i28.4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koblas T, Zacharovova K, Berkova Z, et al. Isolation and characterization of human CXCR4-positive pancreatic cells. Folia Biol (Praha) 2007;53:13–22. [PubMed] [Google Scholar]
- 72.Oshima Y, Suzuki A, Kawashimo K, et al. Isolation of mouse pancreatic ductal progenitor cells expressing CD133 and c-Met by flow cytometric cell sorting. Gastroenterology. 2007;132:720–32. doi: 10.1053/j.gastro.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 73.Xu X, D’Hoker J, Stange G, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 74.Banerjee M, Kanitkar M, Bhonde RR. Approaches towards endogenous pancreatic regeneration. Rev Diabet Stud. 2005;2:165–76. doi: 10.1900/RDS.2005.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Santana A, Ensenat-Waser R, Arribas MI, et al. Insulin-producing cells derived from stem cells: recent progress and future directions. J Cell Mol Med. 2006;10:866–83. doi: 10.1111/j.1582-4934.2006.tb00531.x. [DOI] [PubMed] [Google Scholar]
- 76.Lock LT, Tzanakakis ES. Stem/progenitor cell sources of insulin-producing cells for the treatment of diabetes. Tissue Eng. 2007;13:1399–412. doi: 10.1089/ten.2007.0047. [DOI] [PubMed] [Google Scholar]
- 77.Jensen J, Heller RS, Funder-Nielsen T, et al. Independent development of pancreatic alpha- and beta-cells from neurogenin3-expressing precursors: a role for the notch pathway in repression of premature differentiation. Diabetes. 2000;49:163–76. doi: 10.2337/diabetes.49.2.163. [DOI] [PubMed] [Google Scholar]
- 78.Liu T, Wang CY, Yu F, et al. In vitro pancreas duodenal homeobox-1 enhances the differentiation of pancreatic ductal epithelial cells into insulin-producing cells. World J Gastroenterol. 2007;13:5232–7. doi: 10.3748/wjg.v13.i39.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yatoh S, Dodge R, Akashi T, et al. Differentiation of affinity-purified human pancreatic duct cells to beta-cells. Diabetes. 2007;56:1802–9. doi: 10.2337/db06-1670. [DOI] [PubMed] [Google Scholar]
- 80.Ianus A, Holz GG, Theise ND, et al. In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J Clin Invest. 2003;111:843–50. doi: 10.1172/JCI16502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brolen GK, Heins N, Edsbagge J, et al. Signals from the embryonic mouse pancreas induce differentiation of human embryonic stem cells into insulin-producing beta-cell-like cells. Diabetes. 2005;54:2867–74. doi: 10.2337/diabetes.54.10.2867. [DOI] [PubMed] [Google Scholar]
- 82.Trounson A. The production and directed differentiation of human embryonic stem cells. Endocr Rev. 2006;27:208–19. doi: 10.1210/er.2005-0016. [DOI] [PubMed] [Google Scholar]
- 83.Liu H, Dalton S, Xu Y. Transcriptional profiling of definitive endoderm derived from human embryonic stem cells. Comput Syst Bioinformatics Conf. 2007;6:79–82. [PubMed] [Google Scholar]
- 84.Serafimidis I, Rakatzi I, Episkopou V, et al. Novel effectors of directed and ngn3 mediated differentiation of mouse embryonic stem cells into endocrine pancreas progenitors. Stem Cells. 2008;26:3–16. doi: 10.1634/stemcells.2007-0194. [DOI] [PubMed] [Google Scholar]
- 85.Wu DC, Byod AS, Wood KJ. Embryonic stem cell transplantation: potential applicability in cell replacement therapy and regenerative medicine. Front Biosci. 2007;12:4525–35. doi: 10.2741/2407. [DOI] [PubMed] [Google Scholar]
- 86.Gradwohl G, Dierich A, LeMeur M, et al. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci U S A. 2000;97:1607–11. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yao S, Chen S, Clark J, et al. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc Natl Acad Sci U S A. 2006;103:6907–12. doi: 10.1073/pnas.0602280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mellitzer G, Bonne S, Luco RF, et al. IA1 is NGN3-dependent and essential for differentiation of the endocrine pancreas. EMBO J. 2006;25:1344–52. doi: 10.1038/sj.emboj.7601011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shim JH, Kim SE, Woo DH, et al. Directed differentiation of human embryonic stem cells towards a pancreatic cell fate. Diabetologia. 2007;50:1228–38. doi: 10.1007/s00125-007-0634-z. [DOI] [PubMed] [Google Scholar]
- 90.Jiang J, Au M, Lu K, et al. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells. 2007;25:1940–53. doi: 10.1634/stemcells.2006-0761. [DOI] [PubMed] [Google Scholar]
- 91.Jiang W, Shi Y, Zhao D, et al. In vitro derivation of functional insulin-producing cells from human embryonic stem cells. Cell Res. 2007;17:333–44. doi: 10.1038/cr.2007.28. [DOI] [PubMed] [Google Scholar]
- 92.Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–52. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 93.Zulewski H. Stem cells with potential to generate insulin-producing cells in man. Swiss Med Wkly. 2007;137:60S–7S. [PubMed] [Google Scholar]
- 94.Koblas T, Harman SM, Saudek F. The application of umbilical cord blood cells in the treatment of diabetes mellitus. Rev Diabet Stud. 2005;2:228–34. doi: 10.1900/RDS.2005.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ilancheran S, Michalska A, Peh G, et al. Stem cells derived from human fetal membranes display multi-lineage differentiation potential. Biol Reprod. 2007;77:577–88. doi: 10.1095/biolreprod.106.055244. [DOI] [PubMed] [Google Scholar]
- 96.Chang CM, Kao CL, Chang YL, et al. Placenta-derived multipotent stem cells induced to differentiate into insulin-positive cells. Biochem Biophys Res Commun. 2007;357:414–20. doi: 10.1016/j.bbrc.2007.03.157. [DOI] [PubMed] [Google Scholar]
- 97.Li Y, Zhang R, Qiao H, et al. Generation of insulin-producing cells from PDX-1 gene-modified human mesenchymal stem cells. J Cell Physiol. 2007;211:36–44. doi: 10.1002/jcp.20897. [DOI] [PubMed] [Google Scholar]
- 98.Seeberger KL, Dufour JM, Shapiro AM, et al. Expansion of mesenchymal stem cells from human pancreatic ductal epithelium. Lab Invest. 2006;86:141–53. doi: 10.1038/labinvest.3700377. [DOI] [PubMed] [Google Scholar]
- 99.Sun Y, Chen L, Hou XG, et al. Differentiation of bone marrow-derived mesenchymal stem cells from diabetic patients into insulin-producing cells in vitro. Chin Med J (Engl) 2007;120:771–6. [PubMed] [Google Scholar]
- 100.Kim S, Shin JS, Kim HJ, et al. Streptozotocin-induced diabetes can be reversed by hepatic oval cell activation through hepatic transdifferentiation and pancreatic islet regeneration. Lab Invest. 2007;87:702–12. doi: 10.1038/labinvest.3700561. [DOI] [PubMed] [Google Scholar]
- 101.Urban VS, Kiss J, Kovacs J, et al. Mesenchymal stem cells cooperate with bone marrow cells in therapy of diabetes. Stem Cells. 2008;26:244–53. doi: 10.1634/stemcells.2007-0267. [DOI] [PubMed] [Google Scholar]
- 102.Sun J, Yang Y, Wang X, et al. Expression of Pdx-1 in bone marrow mesenchymal stem cells promotes differentiation of islet-like cells in vitro. Sci China C Life Sci. 2006;49:480–9. doi: 10.1007/s11427-006-2016-z. [DOI] [PubMed] [Google Scholar]
- 103.Karnieli O, Izhar-Prato Y, Bulvik S, et al. Generation of insulin-producing cells from human bone marrow mesenchymal stem cells by genetic manipulation. Stem Cells. 2007;25:2837–44. doi: 10.1634/stemcells.2007-0164. [DOI] [PubMed] [Google Scholar]
- 104.Schreiber FS, Deramaudt TB, Brunner TB, et al. Successful growth and characterization of mouse pancreatic ductal cells: functional properties of the Ki-RAS(G12V) oncogene. Gastroenterology. 2004;127:250–60. doi: 10.1053/j.gastro.2004.03.058. [DOI] [PubMed] [Google Scholar]
- 105.Hingorani SR, Wang L, Multani AS, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–83. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 106.Deramaudt T, Rustgi AK. Mutant KRAS in the initiation of pancreatic cancer. Biochim Biophys Acta. 2005;1756:97–101. doi: 10.1016/j.bbcan.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 107.Lieber M, Mazzetta J, Nelson-Rees W, et al. Establishment of a continuous tumor-cell line (panc-1) from a human carcinoma of the exocrine pancreas. Int J Cancer. 1975;15:741–7. doi: 10.1002/ijc.2910150505. [DOI] [PubMed] [Google Scholar]
- 108.Sipos B, Moser S, Kalthoff H, et al. A comprehensive characterization of pancreatic ductal carcinoma cell lines: towards the establishment of an in vitro research platform. Virchows Arch. 2003;442:444–52. doi: 10.1007/s00428-003-0784-4. [DOI] [PubMed] [Google Scholar]
- 109.Olempska M, Eisenach PA, Ammerpohl O, et al. Detection of tumor stem cell markers in pancreatic carcinoma cell lines. Hepatobiliary Pancreat Dis Int. 2007;6:92–7. [PubMed] [Google Scholar]
- 110.Gou S, Liu T, Wang C, et al. Establishment of clonal colony-forming assay for propagation of pancreatic cancer cells with stem cell properties. Pancreas. 2007;34:429–35. doi: 10.1097/MPA.0b013e318033f9f4. [DOI] [PubMed] [Google Scholar]
- 111.Zhou J, Wang CY, Liu T, et al. Persistence of side population cells with high drug efflux capacity in pancreatic cancer. World J Gastroenterol. 2008;14:925–30. doi: 10.3748/wjg.14.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim YW, Kern HF, Mullins TD, et al. Characterization of clones of a human pancreatic adenocarcinoma cell line representing different stages of differentiation. Pancreas. 1989;4:353–62. doi: 10.1097/00006676-198906000-00013. [DOI] [PubMed] [Google Scholar]
- 113.Sun C, Yamato T, Furukawa T, et al. Characterization of the mutations of the K-ras, p53, p16, and SMAD4 genes in 15 human pancreatic cancer cell lines. Oncol Rep. 2001;8:89–92. doi: 10.3892/or.8.1.89. [DOI] [PubMed] [Google Scholar]
- 114.Sipos B, Moser S, Kalthoff H, et al. A comprehensive characterization of pancreatic ductal carcinoma cell lines: towards the establishment of an in vitro research platform. Virchows Arch. 2003;442:444–52. doi: 10.1007/s00428-003-0784-4. [DOI] [PubMed] [Google Scholar]
- 115.Neureiter D, Zopf S, Dimmler A, et al. Different capabilities of morphological pattern formation and its association with the expression of differentiation markers in a xenograft model of human pancreatic cancer cell lines. Pancreatology. 2005;5:387–97. doi: 10.1159/000086539. [DOI] [PubMed] [Google Scholar]
- 116.Liu T, Gou SM, Wang CY, et al. Pancreas duodenal homeobox-1 expression and significance in pancreatic cancer. World J Gastroenterol. 2007;13:2615–8. doi: 10.3748/wjg.v13.i18.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lang D, Mascarenhas JB, Powell SK, et al. PAX6 is expressed in pancreatic adenocarcinoma and is downregulated during induction of terminal differentiation. Mol Carcinog. 2008;47:148–56. doi: 10.1002/mc.20375. [DOI] [PubMed] [Google Scholar]
- 118.Roder JD, Thorban S, Pantel K, et al. Micrometastases in bone marrow: prognostic indicators for pancreatic cancer. World J Surg. 1999;23:888–91. doi: 10.1007/s002689900594. [DOI] [PubMed] [Google Scholar]
- 119.Hu WG, Liu T, Xiong JX, et al. Blockade of sonic hedgehog signal pathway enhances antiproliferative effect of EGFR inhibitor in pancreatic cancer cells. Acta Pharmacol Sin. 2007;28:1224–30. doi: 10.1111/j.1745-7254.2007.00620.x. [DOI] [PubMed] [Google Scholar]
- 120.Hu WG, Wang CY, Liu T, et al. Expression of sonic hedgehog, EGFR and PCNA proteins in pancreatic cancer and their correlations to cell proliferation. Ai Zheng. 2007;26:947–51. [PubMed] [Google Scholar]
- 121.Gregoire V, Rosier JF, De BM, et al. Role of deoxycytidine kinase (dCK) activity in gemcitabine’s radioenhancement in mice and human cell lines in vitro. Radiother Oncol. 2002;63:329–38. doi: 10.1016/s0167-8140(02)00106-8. [DOI] [PubMed] [Google Scholar]
- 122.Schniewind B, Christgen M, Kurdow R, et al. Resistance of pancreatic cancer to gemcitabine treatment is dependent on mitochondria-mediated apoptosis. Int J Cancer. 2004;109:182–8. doi: 10.1002/ijc.11679. [DOI] [PubMed] [Google Scholar]
- 123.Akar U, Ozpolat B, Mehta K, et al. Tissue transglutaminase inhibits autophagy in pancreatic cancer cells. Mol Cancer Res. 2007;5:241–9. doi: 10.1158/1541-7786.MCR-06-0229. [DOI] [PubMed] [Google Scholar]
- 124.Verma A, Guha S, Diagaradjane P, et al. Therapeutic significance of elevated tissue transglutaminase expression in pancreatic cancer. Clin Cancer Res. 2008;14:2476–83. doi: 10.1158/1078-0432.CCR-07-4529. [DOI] [PubMed] [Google Scholar]
- 125.Bergman AM, Pinedo HM, Peters GJ. Determinants of resistance to 2′,2′-difluorodeoxycytidine (gemcitabine) Drug Resist Updat. 2002;5:19–33. doi: 10.1016/s1368-7646(02)00002-x. [DOI] [PubMed] [Google Scholar]
- 126.Bai J, Sata N, Nagai H. Gene expression analysis for predicting gemcitabine sensitivity in pancreatic cancer patients. HPB (Oxford) 2007;9:150–5. doi: 10.1080/13651820601175918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tang ZY, Wu YL, Gao SL, et al. Effects of the proteasome inhibitor bortezomib on gene expression profiles of pancreatic cancer cells. J Surg Res. 2008;145:111–23. doi: 10.1016/j.jss.2007.03.061. [DOI] [PubMed] [Google Scholar]
- 128.Giovannetti E, Del TM, Mey V, et al. Transcription analysis of human equilibrative nucleoside transporter-1 predicts survival in pancreas cancer patients treated with gemcitabine. Cancer Res. 2006;66:3928–35. doi: 10.1158/0008-5472.CAN-05-4203. [DOI] [PubMed] [Google Scholar]
- 129.Nakano Y, Tanno S, Koizumi K, et al. Gemcitabine chemoresistance and molecular markers associated with gemcitabine transport and metabolism in human pancreatic cancer cells. Br J Cancer. 2007;96:457–63. doi: 10.1038/sj.bjc.6603559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nakahira S, Nakamori S, Tsujie M, et al. Involvement of ribonucleotide reductase M1 subunit overexpression in gemcitabine resistance of human pancreatic cancer. Int J Cancer. 2007;120:1355–63. doi: 10.1002/ijc.22390. [DOI] [PubMed] [Google Scholar]
- 131.Mori-Iwamoto S, Kuramitsu Y, Ryozawa S, et al. Proteomics finding heat shock protein 27 as a biomarker for resistance of pancreatic cancer cells to gemcitabine. Int J Oncol. 2007;31:1345–50. [PubMed] [Google Scholar]
- 132.Hering J, Garrean S, Dekoj TR, et al. Inhibition of proliferation by omega-3 fatty acids in chemoresistant pancreatic cancer cells. Ann Surg Oncol. 2007;14:3620–8. doi: 10.1245/s10434-007-9556-8. [DOI] [PubMed] [Google Scholar]
- 133.Itoi T, Sofuni A, Fukushima N, et al. Ribonucleotide reductase subunit M2 mRNA expression in pretreatment biopsies obtained from unresectable pancreatic carcinomas. J Gastroenterol. 2007;42:389–94. doi: 10.1007/s00535-007-2017-0. [DOI] [PubMed] [Google Scholar]
- 134.Okamoto K, Ocker M, Neureiter D, et al. bcl-2-specific siRNAs restore gemcitabine sensitivity in human pancreatic cancer cells. J Cell Mol Med. 2007;11:349–61. doi: 10.1111/j.1582-4934.2007.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chow JY, Quach KT, Cabrera BL, et al. RAS/ERK modulates TGFbeta-regulated PTEN expression in human pancreatic adenocarcinoma cells. Carcinogenesis. 2007;28:2321–7. doi: 10.1093/carcin/bgm159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mann AP, Verma A, Sethi G, et al. Overexpression of tissue transglutaminase leads to constitutive activation of nuclear factor-kappaB in cancer cells: delineation of a novel pathway. Cancer Res. 2006;66:8788–95. doi: 10.1158/0008-5472.CAN-06-1457. [DOI] [PubMed] [Google Scholar]
- 137.Verma A, Wang H, Manavathi B, et al. Increased expression of tissue transglutaminase in pancreatic ductal adenocarcinoma and its implications in drug resistance and metastasis. Cancer Res. 2006;66:10525–33. doi: 10.1158/0008-5472.CAN-06-2387. [DOI] [PubMed] [Google Scholar]
- 138.Alexander RL, Greene BT, Torti SV, et al. A novel phospholipid gemcitabine conjugate is able to bypass three drug-resistance mechanisms. Cancer Chemother Pharmacol. 2005;56:15–21. doi: 10.1007/s00280-004-0949-0. [DOI] [PubMed] [Google Scholar]
- 139.Thayer SP, di Magliano MP, Heiser PW, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–6. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nakashima H, Nakamura M, Yamaguchi H, et al. Nuclear factor-kappaB contributes to hedgehog signaling pathway activation through sonic hedgehog induction in pancreatic cancer. Cancer Res. 2006;66:7041–9. doi: 10.1158/0008-5472.CAN-05-4588. [DOI] [PubMed] [Google Scholar]
- 141.Chaturvedi P, Singh AP, Moniaux N, et al. MUC4 mucin potentiates pancreatic tumor cell proliferation, survival, and invasive properties and interferes with its interaction to extracellular matrix proteins. Mol Cancer Res. 2007;5:309–20. doi: 10.1158/1541-7786.MCR-06-0353. [DOI] [PubMed] [Google Scholar]
- 142.Moniaux N, Chaturvedi P, Varshney GC, et al. Human MUC4 mucin induces ultra-structural changes and tumorigenicity in pancreatic cancer cells. Br J Cancer. 2007;97:345–57. doi: 10.1038/sj.bjc.6603868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chaturvedi P, Singh AP, Batra SK. Structure, evolution, and biology of the MUC4 mucin. FASEB J. 2008;22:966–81. doi: 10.1096/fj.07-9673rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pasca di MM, Biankin AV, Heiser PW, et al. Common activation of canonical wnt signaling in pancreatic adenocarcinoma. PLoS ONE. 2007;2:e1155. doi: 10.1371/journal.pone.0001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wehler T, Wolfert F, Schimanski CC, et al. Strong expression of chemokine receptor CXCR4 by pancreatic cancer correlates with advanced disease. Oncol Rep. 2006;16:1159–64. [PubMed] [Google Scholar]
- 146.Grzesiak JJ, Smith KC, Burton DW, et al. Integrin-mediated laminin-1 adhesion upregulates CXCR4 and IL-8 expression in pancreatic cancer cells. Surgery. 2007;141:804–14. doi: 10.1016/j.surg.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hurtado M, Lozano JJ, Castellanos E, et al. Activation of the epidermal growth factor signalling pathway by tissue plasminogen activator in pancreas cancer cells. Gut. 2007;56:1266–74. doi: 10.1136/gut.2006.097188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stoll V, Calleja V, Vassaux G, et al. Dominant negative inhibitors of signalling through the phosphoinositol 3-kinase pathway for gene therapy of pancreatic cancer. Gut. 2005;54:109–16. doi: 10.1136/gut.2004.046706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yin T, Wang C, Liu T, et al. Expression of snail in pancreatic cancer promotes metastasis and chemoresistance. J Surg Res. 2007;141:196–203. doi: 10.1016/j.jss.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 150.Shah AN, Summy JM, Zhang J, et al. Development and characterization of gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol. 2007;14:3629–37. doi: 10.1245/s10434-007-9583-5. [DOI] [PubMed] [Google Scholar]
- 151.Rafii S, Lyden D, Benezra R, et al. Vascular and haematopoietic stem cells: novel targets for anti-angiogenesis therapy? Nat Rev Cancer. 2002;2:826–35. doi: 10.1038/nrc925. [DOI] [PubMed] [Google Scholar]
- 152.Peters BA, Diaz LA, Polyak K, et al. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nat Med. 2005;11:261–2. doi: 10.1038/nm1200. [DOI] [PubMed] [Google Scholar]
- 153.Orimo A, Weinberg RA. Stromal fibroblasts in cancer: A novel tumor-promoting cell type. Cell Cycle. 2006;5:1597–601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- 154.Kleeff J, Beckhove P, Esposito I, et al. Pancreatic cancer microenvironment. Int J Cancer. 2007;121:699–705. doi: 10.1002/ijc.22871. [DOI] [PubMed] [Google Scholar]
- 155.Okada T, Ozawa K. Vector-producing tumor-tracking multipotent mesenchymal stromal cells for suicide cancer gene therapy. Front Biosci. 2008;13:1887–91. doi: 10.2741/2808. [DOI] [PubMed] [Google Scholar]
- 156.Sennino B, Falcon BL, McCauley D, et al. Sequential loss of tumor vessel pericytes and endothelial cells after inhibition of platelet-derived growth factor B by selective aptamer AX102. Cancer Res. 2007;67:7358–67. doi: 10.1158/0008-5472.CAN-07-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hwang RF, Moore T, Arumugam T, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918–26. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Baker CH, Solorzano CC, Fidler IJ. Blockade of vascular endothelial growth factor receptor and epidermal growth factor receptor signaling for therapy of metastatic human pancreatic cancer. Cancer Res. 2002;62:1996–2003. [PubMed] [Google Scholar]
- 159.Abdollahi A, Lipson KE, Sckell A, et al. Combined therapy with direct and indirect angiogenesis inhibition results in enhanced antiangiogenic and antitumor effects. Cancer Res. 2003;63:8890–8. [PubMed] [Google Scholar]
- 160.Yokoi K, Sasaki T, Bucana CD, et al. Simultaneous inhibition of EGFR, VEGFR, and platelet-derived growth factor receptor signaling combined with gemcitabine produces therapy of human pancreatic carcinoma and prolongs survival in an orthotopic nude mouse model. Cancer Res. 2005;65:10371–80. doi: 10.1158/0008-5472.CAN-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]