Abstract
Elevated free fatty acids (FFAs) contribute to the development of insulin resistance, type 2 diabetes, and may be atherogenic. We tested the relationship between lipid induced insulin resistance, endothelial dysfunction and monocyte capacity to form foam cells through scavenger receptor A (SRA) and CD36. Ten healthy subjects underwent 24 hour infusion of Intralipid/heparin and saline (0.5ml/min) on two separate occasions followed by brachial artery reactivity testing and a euglycemic hyperinsulinemic (80 mU/(kg *min)) clamp study to determine insulin sensitivity. Isolation of blood monocytes was performed 24 hr following infusion. Surface expression and function of CD36 and SRA to take up oxidized LDL was determined by flow cytometry and quantitative confocal imaging. Lipid infusion resulted in a 2 fold increase in serum FFA levels, reduced whole body glucose disposal by ~20% (P< 0.05), and possibly impaired endothelial dependent vasodilation (P = 0.1). Blood monocytes obtained during lipid infusion demonstrated a ~25% increase in cell surface expression of CD36 (P < 0.05) but no change in SRA expression. Enhanced CD36 expression was associated with a 50% increase in internalization of oxidized LDL (P < 0.05). The increase in CD36 surface expression during lipid infusion correlated inversely with glucose disposal (P < 0.05) and not with FFA levels or brachial artery dilation. These data support a role for FFAs in induction of insulin resistance and provide a link to atherogenic mechanisms mediated by expression of scavenger receptor CD36.
Keywords: FFA, FAT/CD36, euglycemic hyperinsulinemic clamp, monocyte, insulin, oxidized LDL
Introduction
Obesity is an insulin resistant state characterized by increased risk for atherosclerotic cardiovascular disease(1;2). Endothelial dysfunction due to abnormalities of nitric oxide generation or bioavailability is one of the earliest steps in the pathogenesis of atherosclerosis(1;3–6). Abnormalities in lipid metabolism as observed in obesity and type 2 diabetes (T2DM), result in chronic elevations of plasma free fatty acids (FFA) levels (4;6), which have been reported to contribute to the development of insulin resistance, inflammation and endothelial dysfunction in diabetic and non-diabetic individuals (4;7). This suggests that elevated plasma FFA concentration not only contributes to the pathophysiologic development of T2DM, but also to the cardiovascular risk cluster known as the metabolic syndrome. However, the in vivo mechanisms by which sustained elevations of plasma FFA promote endothelial dysfunction and atherogenesis are poorly defined.
Active FFA uptake by diverse cells types, particularly circulating monocytes and tissue macrophages in adipose and muscle tissue(8), has led to a hypothesis that this process may mediate the development of insulin resistance and play a role in atherogenesis(9;10). Recent studies have revealed a role for the multi-ligand cell surface scavenger receptor CD36 in facilitating transport of FFA into cells(11;12). CD36 is an 88 kD transmembrane glycoprotein that was first identified in 1976 as a platelet surface glycoprotein and then in 1987 as a receptor for thrombospondin(13). CD36 was later found to act as a scavenger-receptor for oxidized LDL (oxLDL) and as a cellular transporter of long chain fatty acids (14–16). CD36 is expressed on diverse cell types, including adipocytes, cardiac and skeletal myocytes, retinal pigment epithelial cells, platelets, mononuclear phagocytes, and microvascular endothelium(17;18). Studies with murine models have revealed important functional roles for CD36 in mediating atherosclerosis, inflammation and thrombosis under conditions of oxidant stress via its ability to bind and respond to oxLDL(11;16;17). CD36 signaling in response to its ligands has been shown in several cell types, including macrophages, platelets, endothelial cells and microglia, to involve src and MAP kinases, and inhibition of these pathways has been shown to block oxLDL mediated cellular effects(19).
The role of CD36 in insulin resistance and T2DM is controversial. Genetic mapping of a single kindred from France with inherited T2DM revealed an association with a CD36 mutation (20), and a group in Japan has shown reduced insulin sensitivity in a small number of CD36 deficient subjects(21). Recent studies, however, have reported increased circulating CD36 in insulin resistant states including T2DM, polycystic ovarian disease, and obesity(22;23). Studies with rodents have shown that CD36 deficiency is associated with hyperlipidemia, but data on insulin resistance have not shown a consistent effect(24–26).
CD36 expression in monocytes has been shown to be regulated by PPARγ and certain cytokines(27;28), including IL-4, in a transcriptional manner and by hyperglycemia in a post-transcriptional manner(28), but it is not known if expression can be modulated by circulating levels of FFA or by induction of insulin resistance and subsequent hyperinsulinemia. In this study we investigated the in vivo relationship between FFA-induced insulin resistance and monocyte CD36 expression and function in healthy volunteer subjects, hypothesizing that CD36 may be a link between insulin resistance states and atherosclerosis.
Research Design and Methods
Subjects
Ten healthy non-obese subjects (6 male, 4 female) with mean age of 48 ± 4 y and body mass index = 23 ± 3 kg/m2 participated in the study. They were not taking medications known to affect glucose metabolism or endothelial function such as aspirin, anti-hypertensive or cholesterol lowering agents. Normal glucose tolerance was confirmed in all control subjects by a 75-g oral glucose tolerance test using American Diabetes Association criteria. The mean fasting glucose (95 ± 2 mg/dl) and insulin (5 ± 3 uU/ml) levels were normal in all subjects. As expected of healthy subjects, by 2 hours after glucose ingestion levels of glucose and insulin returned to normal (105 ± 8 mg/dl and 17 ± 3 uU/ml, respectively). Mean total cholesterol was 177 ± 4 mg/dl with HDL of 62 ± 3 mg/dl, LDL of 95± 2 and triglyceride levels of 99 ± 8 mg/dl. Both mean systolic and diastolic blood pressure measurements were normal (118 ± 2 and 69 ± 3 mmHg). None of the participants smoked and none of the women were on hormonal replacement therapy or oral contraceptives. The purpose, nature, and potential risks of the study were explained to all subjects, and written consent was obtained before their participation. The protocol was approved by the Institutional Review Board of the Cleveland Clinic Foundation.
Study Design
The initial screening visit consisted of a history and physical examination, routine laboratory studies (CBC, chemistry profile and fasting lipid profile) and oral glucose tolerance test (OGTT) performed in the Clinical Research Unit at Cleveland Clinic Foundation. Following this visit, all subjects were admitted to the Clinical Research Unit on two separate occasions for an infusion of Intralipid/heparin (Abbott Laboratories, Chicago, IL, USA) or saline at 30cc/hr for a total of 27 hours. This length and dose of lipid infusion was chosen to mimic chronic physiologic elevations of plasma FFAs found in conditions of obesity and type 2 diabetes. During hospitalization, blood was collected 3.5, 9 and 24 hours for the determination of glucose, insulin and FFA concentrations. All subjects were given a weight maintaining diet consisting of 50% carbohydrate, 30% protein and 20% fat. Special attention was given to ensure that the timing and caloric distribution of meals were similar for both inpatient visits. At 24 hours following infusion, 20cc of blood was obtained for isolation of peripheral blood monocytes as described previously (27) as well as plasma for arginine metabolites described below. Vascular function assessment was performed using high-resolution duplex ultrasound of the brachial artery to assess endothelial dependent and endothelial independent vasodilation using standardized methods (see below) (29). Following completion of vascular function assessment, a euglycemic hyperinsulinemic clamp (80 mU/(kg*min) study for determination of insulin sensitivity was performed for 120 minutes as previously described(30).
Vascular functional assessment
The brachial artery was imaged above the antecubital fossa using high-resolution B model ultrasound (Seimens Sequoia, 8L/8MHz linear array transducer) with optimization of near and far wall intima. After a rest period of 10 minutes, baseline images of the brachial artery were obtained gated to the R-wave on ECG. To assess endothelial-dependent flow mediated vasodilation in response to ischemia and reactive hyperemia, a blood pressure cuff was positioned on the upper arm and was inflated to 200 mm Hg (or at least 40 mm Hg above baseline systolic blood pressure) for a period of 5 minutes. After rapid release of the cuff, R-wave gated images of the brachial artery were acquired from to 55–65 seconds after cuff release. Pulse Doppler spectra were obtained at baseline and within 10 seconds after cuff release to assess the adequacy of reactive hyperemia. After a rest period of 15 minutes, repeat baseline images were obtained. To assess endothelial-independent vasodilation, one 0.4 mg dose of nitroglycerin spray was administered. Repeat gated brachial artery images were obtained 3 minutes after nitroglycerin administration. All vascular function studies were performed by one of two technologists dedicated to this study. Mean brachial artery diameter at baseline, after reactive hyperemia, at second baseline, and after nitroglycerin were measured by a single investigator (HG) blinded to treatment allocation using specialized edge-detection software (Brachial Analyzer. Vascular Research Tools, version 5.0.4, Medical Imaging Applications, L.L.C., Coralville, Iowa). Baseline brachial artery diameter and mean percent change in vessel diameter in response to reactive hyperemia and nitroglycerin are reported.
Insulin clamp
Prior to the start of the euglycemic insulin clamp study, an antecubital vein was cannulated for infusion of all test substances. A second retrograde catheter was inserted into a vein on the dorsum of the hand and subsequently the hand was placed in a heated pad to obtain arterialized blood samples. A primed continuous infusion of insulin 80 mU/(kg * min) was started and the plasma glucose was measured every 5 minutes with a Glucose Oxidase Analyzer (Beckman Instruments, Fullerton, California, USA). Based upon the negative feedback principle, a variable infusion of 20% glucose was adjusted to maintain the plasma glucose concentration constant at each subject’s fasting glucose level in the saline and lipid infusion conditions.. The insulin infusion was continued for a total of 120 minutes. Whole body glucose disposal (M value) was determined at steady state during the last 40 minutes of insulin infusion.
Hormonal and vascular marker measurement
Plasma glucose concentrations were determined by Beckman instruments (Beckman, Ca). Plasma insulin concentrations were determined by radioimmunoassay (Diagnostic Products, Los Angeles, CA). FFA levels were measured in plasma containing EDTA and lipoprotein lipase inhibitor Paraoxon (diethyl-p-nitrophenyl-phosphate, 0.275 mg/ml blood; Sigma, St. Louis, MO) by colometric assay (Wako, Germany). Plasma concentrations of ultrasensitive c-reactive protein (CRP) was measured with an immunoturbidometric assay (analyzed at Cleveland Clinic autolab). The intra- and interassay coefficients of variation (CV) were 0.8 and 6.7%, respectively. Myeloperoxidase levels were measured by enzyme-linked immunosorbent assay (ELISA) at Southbend Medical Foundation with an intra- and inter-assay coefficients of variation of 0.5 and 8.2%. Endothelin was analyzed by radioimmunoassay at Cleveland Clinic Specialty labs with the intra and interassay CVs of 0.3 and 4.5% respectively. Serum VCAM levels were analyzed by ELISA assay kits (R&D Systems, Minneapolis, MN) with the intra and interassay CVs of 0.9 and 9.2% respectively.
CD36 Expression and Function
Peripheral blood mononuclear cells were isolated by layering 3 ml of whole blood over Histopaque (Sigma Aldrich, MO) followed by centrifugation. Cells were washed in PBS twice and frozen in a media consisting of 90% FBS with 10% DMSO. CD36, SRA1, and CD14 expression were determined on thawed, washed cells obtained from paired lipid and saline infusion experiments by fluorescence-activated cell sorter (FACS) analysis using fluorescein isothiocyanate (FITC)-conjugated anti-CD36 IgG (BD Sciences), phycoerythrin (PE)-conjugated anti-SRA1 IgG (R&D), and PE-conjugated anti-CD14 IgG (BD Sciences). Briefly, 200μl of mononuclear cell suspension alliquots were stained individually for CD14 and CD36 and then co-stained with both antibodies to detect mean fluorescence intensity of bound CD36 antibody on CD14+ cells. Antibodies were used at 2 μl/ml and were incubated with cell suspensions for 30 min at room temperature in the dark prior to analysis. Functional studies examining oxLDL uptake were determined using a form of LDL oxidized by myeoloperoxidase (MPO) and glucose oxidase. This form of oxLDL which we term NO2(+)LDL (16;19) has been shown to be a highly specific ligand for CD36. Oxidation reactions were terminated after 8 hours by addition of 40 μM butylated hydroxytoluene (BHT; from a 100 mM ethanolic stock) and 300 nM catalase to the reaction mixture. Control LDL for these experiments was prepared by exposing the LDL to all of the same oxidizing conditions except the source of oxygen. LDL preparations were then incubated overnight with the fluorescent probe DiI at 37°C. Freshly thawed washed mononuclear cell suspension were incubated with DiI labeled NO2(+)LDL (50μg/ml) for 30 minutes at 37°C in the dark, washed twice with PBS and analyzed by flow cytometry for DiI fluorescence intensity. A portion of the cells were placed on cover slips with RPMI and 10% fetal bovine serum, treated with DiI labled oxLDL (30 min at 37° in the dark), fixed with 4% paraformaldeyde for 30 min, inverted and sealed for imaging by confocal microscopy(19).
Arginine Metabolites
For measurement of arginine metabolites, including asymmetric dimethylarginine (ADMA), plasma was subjected to cation exchange solid-phase extraction and analyzed by HPLC tandem mass spectroscopy (31). The coefficients of variation for intersample and intrasample variations tested with a pooled plasma sample were less than 3% for all analytes. The detection limit for dimethylarginines was 0.04 μM.
Statistical Methods
All data are presented as the mean ± SE. Differences between saline and lipid infusion were compared using the paired 2-tailed paired t test with statistical significance noted by P < 0.05. Correlation analysis was performed by the Pearson product moment method using Stat View software (version 4.0; SAS Inc., Cary, North Carolina, USA).
Results
Lipid infusion and metabolic parameters
Sustained lipid infusion for 24 hours resulted in a ~2 fold increase in fasting free fatty acid (FFA) levels and ~40% increase in fasting insulin levels with no substantial change in fasting glucose levels (Table 1). Triglyceride levels markedly increased following 24 hour lipid vs. saline infusion (155 ± 5 vs.95 ± 6mg/dl, P < 0.01). Physiologic hyperinsulinemia induced for 2 hours by clamp resulted in an increase in mean insulin levels to 100 ± 11 μU/ml during lipid infusion and were not different from levels during saline infusion. There was an approximate 20% decrease in whole body glucose disposal (M value) associated with lipid infusion (7.9 ± .03 mg/kg*min) compared to saline infusion (9.6 ± 0.03 mg/(kg*min). These values are typical of what we and others have reported in normal subjects.
Table 1.
Mean 24 hr Metabolic and Vascular Determinations During Saline and Lipid Infusion
Mean metabolic and vascular determinations following 24 hour lipid and saline infusion. M value represents whole body glucose disposal rate obtained during steady state (the last 40 minutes) of insulin infusion. Data are present as mean and standard errors.
| N = 10 | Saline | Intralipid/Heparin | P value |
|---|---|---|---|
| FFA (meq/L) | 0.37 ± .05 | 0.73 ± .14 | <0.01 |
| Insulin (μU/mL) | 7 ± 1 | 12 ± 1.2 | <0.05 |
| Glucose (mg/dl) | 96 ± 3 | 99 ± 3 | NS |
| ADMA uM | 0.54 ± 0.01 | 0.58 ± 0.1 | NS |
| Arginine uM | 29 ± 2 | 28.8 ± 1 | NS |
| usCRP (mg/L) | 1.06 ± .05 | 1.02 ± .07 | NS |
| Myeloperoxidase (pmol/L) | 300 ± 25 | 572 ± 50 | <0.02 |
| VCAM (ng/ml) | 825.4 ± 77 | 1070.6 ± 122 | <0.01 |
| Endothelin-1 (pg/mL) | 7.4 ± .8 | 9.2 ± 1 | <0.05 |
| Flow mediated Dilation (%) | 13.9 ± 0.5 | 12.3 ± 0.7 | P = 0.1 |
| M value (mk/kg · min) | 9.6 ± .03 | 7.9 ± .03 | <0.05 |
Lipid infusion and endothelial parameters
There was no difference in baseline brachial artery diameter in the two groups (3.44 ± 0.1 vs. 3.30 ± 0.07 cm, P = NS). Endothelial dependent vasodilation, as measured by percent change in brachial artery diameter due to flow-mediated vasodilation, was reduced by 12% during lipid infusion; however this was not a consistent effect in all subjects and was significant at only P = 0.1 (Table 1). There was no difference in endothelial independent vasodilation between lipid and saline infusion. Circulating makers of endothelial activation, VCAM and endothelin-1, and of oxidant stress (MPO) were markedly increased during lipid infusion. Arginine metabolites including concentrations of asymmetric dimethylarginine (ADMA) were unchanged during lipid and saline infusion (Table 1).
Lipid infusion and monocyte scavenger receptor cell surface expression and function
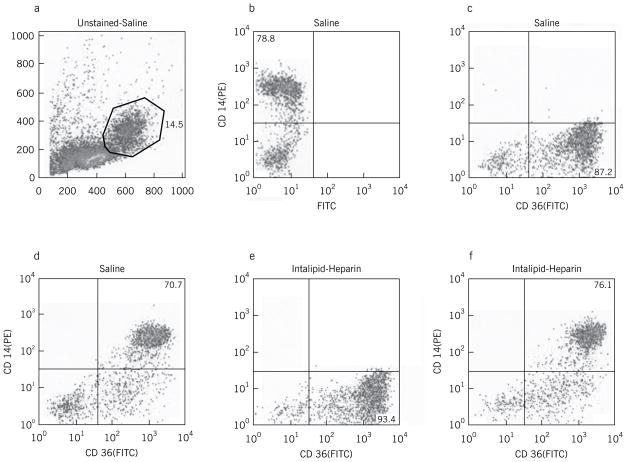
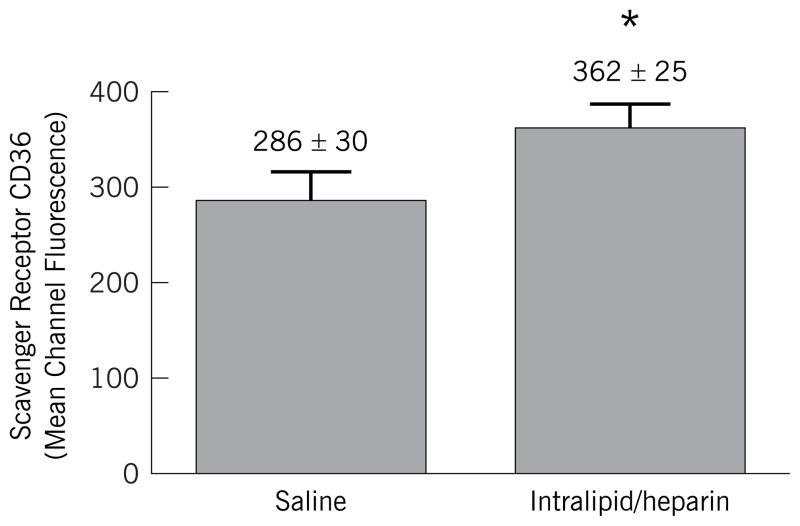
Representative flow cytometric analyses of peripheral blood mononuclear cells obtained following 24 hour lipid or saline infusion are shown in Figure 1. Peripheral blood monocytes were initially analyzed in an unstained sample for 10,000 events by size and scatter characteristics (Fig 1a) to set a monocyte gate. This gate contained ~ 14% of the total population. Single staining with PE-anti-CD14 (Fig 1b) or FITC-anti-CD36 (Fig 1c) revealed that ~ 80–90% of the cells within this gate were monocytes. Co-staining of cells obtained after saline infusion is shown in Figure 1d. Following 24 hour lipid infusion (Fig 1f) there was no change in the CD14 (PE) fluorescence, but a significant increase in CD36 (FITC) mean fluorescence intensity (MFI). The MFI increased by an average of 28% during lipid infusion from a baseline of 286 ± 30 to 362 ± 25, P < 0.05 (Figure 2). In contrast, no change was noted in surface expression of SRA (MFI of 145 ± 33 vs. 122 ± 39, P = NS). In addition to the increase in mean CD36 expression levels, the % of CD36+ monocytes in the peripheral blood mononuclear population increased modestly after lipid infusion from 85% to 93% (compare Fig 1e to 1c).
Figure 1.
(a–f). Representative flow cytometry analyses of peripheral blood mononuclear cells obtained after saline (a–d) or lipid (e,f) infusion. Panel a shows an unstained sample gated for 10,000 events within a forward and side scatter defined window designed to capture monocytes. Approximately 14% of cells were within this gate. Panel b shows PE-anti-CD14 fluorescence of this population and Panel c shows FITC-anti-CD36 fluorescence of this population. Panels d and e show double staining (FITC and PE) of cells after saline infusion (d) and lipid infusion (f).
Figure 2. Mean Channel Fluorescence for CD36 Expression in CD14 Positive Cells from Peripheral Blood Mononuclear Cells during Saline and Lipid Infusion.
Mean fluorescence intensity of FITC-conjugated anti-CD36 binding to CD14+ cells. Cells were co-incubated with FITC-conjugated andti-CD36 and PE-conjugated anti-CD14 or isotype matched controls and analyzed by flow cytometry as in Figure 1. * = P < 0.05
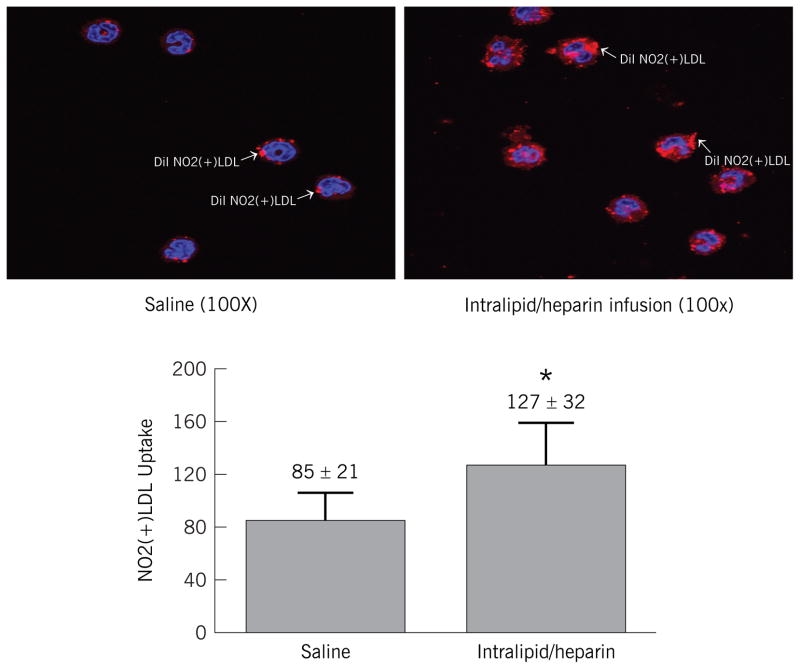
To determine if the change in CD36 expression level was associated with an increase in functional capacity to internalize DiI labeled NO2(+)LDL, we exposed peripheral blood mononuclear cells to fluorescently labeled NO2(+)LDL. Peripheral blood mononuclear cells obtained during lipid infusion had significantly increased uptake of NO2(+)LDL compared to cells obtained during saline infusion (Figure 3). Uptake of NO2(−)LDL or native LDL was unchanged between lipid and saline (P = NS). These data suggest that lipid infusion increased ability of monocytes to form foam cells.
Figure 3. DiI Labeled NO2(+)LDL Uptake in CD14 Positive Cells During Saline and Lipid Infusion.
Uptake of DiI labeled NO2(+)LDL by monocytes obtained after lipid or saline infusion. Cells were plated on cover slips, incubated with DiI labeled NO2(+)LDL for 30 minutes at 37 degrees. Cells on cover slips were fixed, inverted and sealed on slides and analyzed by quantitative confocal imaging at 100X. * = P < 0.05
Relationships between Metabolic and Vascular Variables
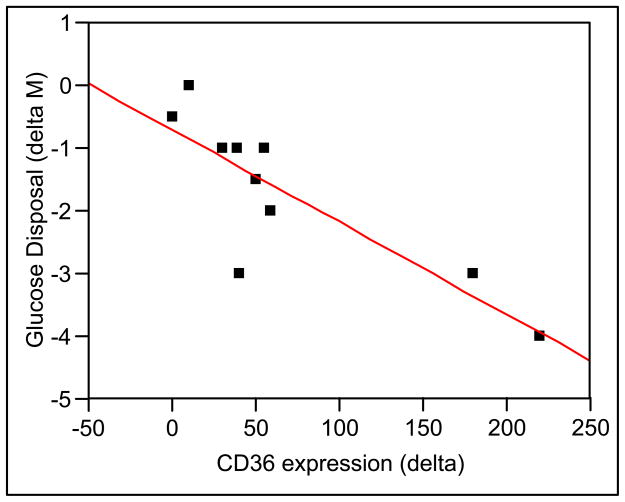
The increase in monocyte CD36 expression observed during lipid infusion correlated linearly with the reduction in whole body glucose disposal (M value) (R2 = 0.68, P < 0.05) (Figure 4). No association was noted between CD36 and levels of FFA (R2 = 0.18, P = NS), insulin (R2 = −0.15, P = NS), or glucose (R2 = − 0.15, P = NS) during saline or lipid infusion. Endothelial dependent vasodilation during lipid infusion did not relate to VCAM, endothelin or MPO levels. MPO levels related to concentrations of FFAs linearly (R = 0.53, P < 0.01). Vascular markers of endothelial dysfunction did not associated with metabolic markers (insulin, glucose, or FFA) levels during lipid infusion.
Figure 4.
Correlation analysis between decrease in whole body glucose disposal (M) during lipid infusion and change in CD36 expression determined by flow cytometry.
Discussion
In this study, induction of insulin resistance in healthy subjects was associated with an increase in surface expression of scavenger receptor CD36 in peripheral blood monocytes. Moreover, uptake of NO2(+)LDL, a specific oxLDL ligand for CD36, by monocytes obtained during lipid infusion was markedly increased. The increase in CD36 expression correlated only with decrease in whole body glucose disposal, rather than increase in serum free fatty acid levels. This suggests that expression of CD36 is associated with induction of insulin resistance and may be one link that contributes to atherogenesis in insulin resistant states.
In our small cohort of healthy subjects, sustained elevations of plasma FFA had a more robust effect on reduction of whole body glucose disposal (~20%, P < 0.05)) than impairment of endothelial dependent brachial artery dilation to transient ischemia (~12%, P = 0.10) (Table 1). Two subjects in this cohort, despite induction of adequate hyperemia stimulus, experienced no change in impairment of flow mediated dilation during lipid infusion and one subject experienced an increase in hyperemia-induced dilation. Thus, there was no relationship between reductions of whole body glucose disposal with impairment of endothelial function, as measured by brachial artery reactivity. Previous studies infusing lipids have either focused on glucose metabolism (32)or endothelial function outcomes(6;33), but no studies to our knowledge have measured both metabolic and vascular effects of sustained lipid infusion concurrently. These results suggest that FFAs may modulate insulin mediated glucose metabolism and endothelial function via different mechanisms.
Sustained lipid infusion was not associated with changes in plasma concentrations of asymmetric dimethylarginine (ADMA), or arginine concentrations, suggesting that these factors associated with reduced nitric oxide generation, are not associated with elevations in plasma FFA levels. However, in vitro data (34)supports a role of FFA to increase oxidative stress that may attenuate the function of nitric oxide generated. Other markers of endothelial activation such as soluble vascular cellular adhesion molecule and endothelin 1 were increased in our study, have been implicated in obese and insulin resistant subjects(23;35), and may promote monocyte infiltration into activated endothelium. Increases in endothelial adhesion markers did not correspond to decrease in whole body glucose disposal (M value). This suggests that FFAs have independent effects on endothelial activation and glucose disposal.
Our study reports an increase in surface expression of CD36 in CD14 positive peripheral blood monocytes during lipid infusion and thus supports a novel link between elevated free fatty acids as seen in obesity and diabetes and atherogenesis. Intralipid/heparin infusion in rodents was associated with increased transcription of CD36 in rat skeletal muscle and this increase was associated with reduction in muscle glucose uptake(36). In humans, Intralipid/heparin infusion has been associated with increase in CD36 mRNA expression in subcutaneous fat tissue(37). These studies implicate a role for CD36 in lipid uptake for fuel metabolism and suggest dysregulation of CD36 expression in induction of insulin resistance in these tissues. Since lipogenic regulatory pathways including PPARγ(28), LXR, and oxLDL(19), have been associated with transcriptional regulation of CD36, it is tempting to speculate that FFA directly or indirectly modulate these factors that in turn upregulate CD36. Since insulin resistance occurs prior to hyperglycemia, and atherogenesis is thought to parallel emergence of insulin resistance(5;6), our study supports the effect of physiologic increases in plasma FFAs and up-regulation of CD36. The other possibility is that hyperinsulinemia that accompanies induction of resistance may up-regulate CD36, since in our study no association between FFA levels and CD36 expression was noted. Monocyte activation and tissue infiltration in rodents models fed a high fat diet is associated with occurrence of hyperinsulinemia(38), it is possible that insulin may also modulate CD36 expression monocyte/macrophages(25). Acute hyperinsulinemia in skeletal muscle has been noted to increase CD36 (39) and linked to the accumulation of intramuscular triglyceride. In a variety of insulin resistant states, insulin signaling of MAP (src/ERK/JNK) kinases is known to be intact (7, 9) and may represent a cellular pathway to regulate CD36.
We have demonstrated a unique effect of elevated circulating FFAs to increase both CD36 expression and internalization of a specific ligand of CD36, NO2(+)LDL in human monocytes. Previous work in rodent models have identified CD36 to be a major receptor on macrophages for binding and internalizing oxLDL (11;16;18;25). CD36 null mice take up 70% less copper oxidized LDL than do wild type cells and 100% less LDL oxidized by myeloperoxidase system, which is an important marker for development of ischemic heart disease. Increased CD36 mediated uptake of copper oxidized LDL has been demonstrated in type 2 diabetic subjects and corresponded with severity of hyperglycemia(28). Generation of NO2(+)LDL involves modification by MPO. Circulating levels of MPO were markedly increased by Intralipid/hearin and is consistent with previous reports of MPO release with heparin(40). However, obesity is associated with increase in inflammation and oxidative stress(41), suggesting that FFA may activate this leukocyte enzyme aside from the known effects of heparin to alter circulating MPO levels.
All these data taken together suggest that lipid induced insulin resistance or lipotoxicity is associated with atherogenesis and inflammation associated with scavenger receptor CD36 expression. Selective downregulation of mononuclear/macrophage CD36 expression and/or inhibition of CD36 function pharmacologically may be an effective therapeutic strategy to retard atherosclerosis accelerated by lipid induced metabolic diseases.
Acknowledgments
We acknowledge the skilled assistance of the Clinical Research Unit nurses and laboratory personnel, our research coordinator, Jackie Payne, and the technicians from the Flow Cytometry Core at Lerner Research Institute. This work was supported by the National Institutes of Health, National Center for Research Resources (CTSA 1UL1RR024989), Multidisciplinary Clinical Research Career Development Programs Grant 5K12RR023264 (SRK), National Heart Lung and Blood Institute HL087018 (RLS) and Endocrine Fellows Foundation (AGI).
Abbreviations
- FFA
free fatty acids
- SRA
scavenger receptor A
- T2DM
type 2 diabetes mellitus
- MPO
myeloperoxidase
- BART
brachial artery reactivity test
- FPI
fasting plasma insulin
- FPG
fasting plasma glucose
- MFI
mean fluorescence intensity
Reference List
- 1.Defronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 2004;88(4):787–835. ix. doi: 10.1016/j.mcna.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Reaven GM. Insulin resistance, the insulin resistance syndrome, and cardiovascular disease. Panminerva Med. 2005;47(4):201–210. [PubMed] [Google Scholar]
- 3.Libby P, Aikawa M, Jain MK. Vascular endothelium and atherosclerosis. Handb Exp Pharmacol. 2006;(176 Pt 2):285–306. doi: 10.1007/3-540-36028-x_9. [DOI] [PubMed] [Google Scholar]
- 4.Defronzo RA. Dysfunctional fat cells, lipotoxicity and type 2 diabetes. Int J Clin Pract Suppl. 2004;(143):9–21. doi: 10.1111/j.1368-504x.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- 5.Defronzo RA. Is insulin resistance atherogenic? Possible mechanisms Atheroscler Suppl. 2006;7(4):11–15. doi: 10.1016/j.atherosclerosissup.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Baron AD. Insulin resistance and vascular function. J Diabetes Complications. 2002;16(1):92–102. doi: 10.1016/s1056-8727(01)00209-4. [DOI] [PubMed] [Google Scholar]
- 7.Kashyap SR, Defronzo RA. The insulin resistance syndrome: physiological considerations. Diab Vasc Dis Res. 2007;4(1):13–19. doi: 10.3132/dvdr.2007.001. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Veledo S, Nieto-Vazquez I, de CJ, et al. Hyperinsulinemia induces insulin resistance on glucose and lipid metabolism in a human adipocytic cell line: paracrine interaction with myocytes. J Clin Endocrinol Metab. 2008;93(7):2866–2876. doi: 10.1210/jc.2007-2472. [DOI] [PubMed] [Google Scholar]
- 9.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51(7):2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 10.Tripathy D, Mohanty P, Dhindsa S, et al. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes. 2003;52(12):2882–2887. doi: 10.2337/diabetes.52.12.2882. [DOI] [PubMed] [Google Scholar]
- 11.Febbraio M, Abumrad NA, Hajjar DP, et al. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem. 1999;274(27):19055–19062. doi: 10.1074/jbc.274.27.19055. [DOI] [PubMed] [Google Scholar]
- 12.Febbraio M, Guy E, Coburn C, et al. The impact of overexpression and deficiency of fatty acid translocase (FAT)/CD36. Mol Cell Biochem. 2002;239(1–2):193–197. [PubMed] [Google Scholar]
- 13.Silverstein RL, Febbraio M. CD36 and atherosclerosis. Curr Opin Lipidol. 2000;11(5):483–491. doi: 10.1097/00041433-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Kunjathoor VV, Febbraio M, Podrez EA, et al. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002;277(51):49982–49988. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- 15.Martin CA, Longman E, Wooding C, et al. Cd36, a class B scavenger receptor, functions as a monomer to bind acetylated and oxidized low-density lipoproteins. Protein Sci. 2007;16(11):2531–2541. doi: 10.1110/ps.073007207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Podrez EA, Poliakov E, Shen Z, et al. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J Biol Chem. 2002;277(41):38517–38523. doi: 10.1074/jbc.M205924200. [DOI] [PubMed] [Google Scholar]
- 17.Febbraio M, Hajjar DP, Silverstein RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest. 2001;108(6):785–791. doi: 10.1172/JCI14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Febbraio M, Guy E, Silverstein RL. Stem cell transplantation reveals that absence of macrophage CD36 is protective against atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24(12):2333–2338. doi: 10.1161/01.ATV.0000148007.06370.68. [DOI] [PubMed] [Google Scholar]
- 19.Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RL. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 2006;4(3):211–221. doi: 10.1016/j.cmet.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lepretre F, Linton KJ, Lacquemant C, et al. Genetic study of the CD36 gene in a French diabetic population. Diabetes Metab. 2004;30(5):459–463. doi: 10.1016/s1262-3636(07)70143-x. [DOI] [PubMed] [Google Scholar]
- 21.Kashiwagi H, Tomiyama Y, Nozaki S, et al. Analyses of genetic abnormalities in type I CD36 deficiency in Japan: identification and cell biological characterization of two novel mutations that cause CD36 deficiency in man. Hum Genet. 2001;108(6):459–466. doi: 10.1007/s004390100525. [DOI] [PubMed] [Google Scholar]
- 22.Glintborg D, Hojlund K, Andersen M, Henriksen JE, Beck-Nielsen H, Handberg A. Soluble CD36 and risk markers of insulin resistance and atherosclerosis are elevated in polycystic ovary syndrome and significantly reduced during pioglitazone treatment. Diabetes Care. 2008;31(2):328–334. doi: 10.2337/dc07-1424. [DOI] [PubMed] [Google Scholar]
- 23.Handberg A, Levin K, Hojlund K, Beck-Nielsen H. Identification of the oxidized low-density lipoprotein scavenger receptor CD36 in plasma: a novel marker of insulin resistance. Circulation. 2006;114(11):1169–1176. doi: 10.1161/CIRCULATIONAHA.106.626135. [DOI] [PubMed] [Google Scholar]
- 24.Glatz JF, Bonen A, Luiken JJ. Exercise and insulin increase muscle fatty acid uptake by recruiting putative fatty acid transporters to the sarcolemma. Curr Opin Clin Nutr Metab Care. 2002;5(4):365–370. doi: 10.1097/00075197-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Smith AC, Mullen KL, Junkin KA, et al. Metformin and exercise reduce muscle FAT/CD36 and lipid accumulation and blunt the progression of high-fat diet-induced hyperglycemia. Am J Physiol Endocrinol Metab. 2007;293(1):E172–E181. doi: 10.1152/ajpendo.00677.2006. [DOI] [PubMed] [Google Scholar]
- 26.Todd MK, Watt MJ, Le J, Hevener AL, Turcotte LP. Thiazolidinediones enhance skeletal muscle triacylglycerol synthesis while protecting against fatty acid-induced inflammation and insulin resistance. Am J Physiol Endocrinol Metab. 2007;292(2):E485–E493. doi: 10.1152/ajpendo.00080.2006. [DOI] [PubMed] [Google Scholar]
- 27.Cipolletta C, Ryan KE, Hanna EV, Trimble ER. Activation of peripheral blood CD14+ monocytes occurs in diabetes. Diabetes. 2005;54(9):2779–2786. doi: 10.2337/diabetes.54.9.2779. [DOI] [PubMed] [Google Scholar]
- 28.Griffin E, Re A, Hamel N, et al. A link between diabetes and atherosclerosis: Glucose regulates expression of CD36 at the level of translation. Nat Med. 2001;7(7):840–846. doi: 10.1038/89969. [DOI] [PubMed] [Google Scholar]
- 29.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 30.Kashyap S, Belfort R, Gastaldelli A, et al. A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes. 2003;52(10):2461–2474. doi: 10.2337/diabetes.52.10.2461. [DOI] [PubMed] [Google Scholar]
- 31.Nicholls SJ, Wang Z, Koeth R, et al. Metabolic profiling of arginine and nitric oxide pathways predicts hemodynamic abnormalities and mortality in patients with cardiogenic shock after acute myocardial infarction. Circulation. 2007;116(20):2315–2324. doi: 10.1161/CIRCULATIONAHA.107.693986. [DOI] [PubMed] [Google Scholar]
- 32.Roden M, Price TB, Perseghin G, et al. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996;97(12):2859–2865. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tripathy D, Mohanty P, Dhindsa S, et al. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes. 2003;52(12):2882–2887. doi: 10.2337/diabetes.52.12.2882. [DOI] [PubMed] [Google Scholar]
- 34.Wright MM, Schopfer FJ, Baker PR, et al. Fatty acid transduction of nitric oxide signaling: nitrolinoleic acid potently activates endothelial heme oxygenase 1 expression. Proc Natl Acad Sci U S A. 2006;103(11):4299–4304. doi: 10.1073/pnas.0506541103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tripathy D, Mohanty P, Dhindsa S, et al. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes. 2003;52(12):2882–2887. doi: 10.2337/diabetes.52.12.2882. [DOI] [PubMed] [Google Scholar]
- 36.Vettor R, Fabris R, Serra R, et al. Changes in FAT/CD36, UCP2, UCP3 and GLUT4 gene expression during lipid infusion in rat skeletal and heart muscle. Int J Obes Relat Metab Disord. 2002;26(6):838–847. doi: 10.1038/sj.ijo.0802005. [DOI] [PubMed] [Google Scholar]
- 37.Nisoli E, Carruba MO, Tonello C, Macor C, Federspil G, Vettor R. Induction of fatty acid translocase/CD36, peroxisome proliferator-activated receptor-gamma2, leptin, uncoupling proteins 2 and 3, and tumor necrosis factor-alpha gene expression in human subcutaneous fat by lipid infusion. Diabetes. 2000;49(3):319–324. doi: 10.2337/diabetes.49.3.319. [DOI] [PubMed] [Google Scholar]
- 38.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112(12):1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corpeleijn E, Pelsers MM, Soenen S, et al. Insulin acutely upregulates protein expression of the fatty acid transporter CD36 in human skeletal muscle in vivo. J Physiol Pharmacol. 2008;59(1):77–83. [PubMed] [Google Scholar]
- 40.Li G, Keenan AC, Young JC, et al. Effects of unfractionated heparin and glycoprotein IIb/IIIa antagonists versus bivalirdin on myeloperoxidase release from neutrophils. Arterioscler Thromb Vasc Biol. 2007;27(8):1850–1856. doi: 10.1161/ATVBAHA.107.144576. [DOI] [PubMed] [Google Scholar]
- 41.Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114(12):1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]