Abstract
Background/Objective
The purpose of this study was to determine whether FM patients differ from matched healthy controls in clinical tests of balance ability and fall frequency.
Methods
34 FM patients and 32 age matched controls were administered the Balance Evaluation-Systems Test (BESTest), rated their balance confidence with the Activities-Specific Balance Confidence Scale (ABC) and reported the number of falls in the last 6 months. The Fibromyalgia Impact Questionnaire (FIQ) was used to assess FM severity.
Results
FM patients had significantly impaired balance in all components of the BESTest compared to controls. They also scored more poorly on balance confidence. Overall fibromyalgia severity (FIQ) correlated significantly with the BESTest, and the ABC scale. The BESTest and ABC correlated significantly with 6 commonly reported FM symptoms (excluding pain). FM patients reported a total of 37 falls over the last six-months compared to 6 falls in healthy controls.
Conclusion
Fibromyalgia is associated with balance problems and increased fall frequency. Patients were aware of their balance problems. These results suggest that FM may affect peripheral and/or central mechanisms of postural control. Further objective study is needed to identify the relative contributions of neural and musculoskeletal impairments to postural stability in FM, thus providing clinicians with exercise prescriptions that maximize postural stability.
Keywords: Fibromyalgia, balance, falls
BACKGROUND
Fibromyalgia (FM) is a chronic disorder defined by report of chronic widespread pain including axial pain and presence of multiple tender points on physical exam (1). Like most chronic illnesses, however, the symptoms of FM extend far beyond the defining criteria. In addition to pain, many patients also report fatigue, disrupted or non-refreshed sleep, mood disturbances, exercise induced symptom flares and multiple other syndromes (e.g., restless legs, irritable bowel and bladder, cognitive dysfunction and chronic headaches) (2;3). A recent survey of 2,596 persons with self-reported FM reported balance problems as one of the top 10 most debilitating symptoms with the reported prevalence of 45% (4). In another report, 68% of 486 persons with FM reported balance problems (5). Fall frequency has not been reported in FM.
Balance or postural stability is a very complex task that involves the integration of multiple sensory inputs to execute appropriate neuromuscular activity needed to maintain balance (6). Balance control consists of several neural subsystems that may be affected by FM, subsystems that can be differentiated with a new clinical balance assessment tool, the Balance Evaluation Systems Test (7). The BESTest evaluates 5 subcomponents of balance: 1) postural limits of stability, 2) anticipatory postural adjustments, 3) reactive postural responses, 4) sensory orientation during stance, and 5) dynamic stability during gait, as well as providing an overall balance score. Table 1 lists each test by subcomponent. Postural limits of stability evaluates how far patients can lean without loosing balance and whether they can align their trunks with gravity with eyes closed (8). Anticipatory postural adjustments shift body weight prior to rising onto toes, lifting a weight and alternately touching a stair with each foot (9). Reactive postural responses are elicited in response to an isometric hold and release of the shoulders that requires a feet-in-place postural response (7;10). Sensory orientation evaluates ability to use vestibular, somatosensory, and visual inputs for balance by asking patients to stand quietly for 30 minutes with eyes open and closed on a firm and compliant, foam cushion (11). The subsystems of postural control most affected by FM are unknown.
Table 1.
BESTest items and subcomponent tests.
| I. Stability Limits | II. Anticipatory Postural Adjustment | III. Reactive Postural Response | IV. Sensory Orientation | V. Stability in Gait |
|---|---|---|---|---|
| 1. Sitting verticality-R | 8. Sit to stand | 14. In-place forward | 20. Stand EO | 25. Gait-level surface |
| 2. Sitting verticality-L | 9. Rise to toes | 15. In-place backward | 21. Stand EC | 26. Change Speed |
| 3. Lateral lean Right | 10. Stand on R-leg | 16. Stepping forward | 22. Foam EO | 27. Head turns |
| 4. Lateral lean Left | 11. Stand on L-leg | 17. Stepping backward | 23. Foam EC | 28. Pivot turns |
| 5. Stance alignment | 12. Alternate step | 18. Stepping right | 24. Incline EC | 29. Onto foam |
| 6. Reach – forward | 13. Fast arm raise | 19. Stepping left | 30. Timed Get Up and Go | |
| 7. Reach - lateral | 31. Timed Go with dual task |
EO= Eyes open; EC=Eyes closed
We were also evaluated fall incidence and the extent to which FM affects patients’ balance confidence. The purpose of this study was to determine if FM is associated with impaired subsystems for balance control, balance confidence and reported falls.
MATERIALS AND METHODS
Study sample and setting
We recruited patients who met 1990 ACR criteria for diagnosis of FM (1) from our large database of patients referred for clinical care. We mailed letters of invitation to 75 randomly selected FM patients who met the selected inclusion and exclusion criteria (i.e., they had a confirmed diagnosis of FM and were 18 years or older and lived within an 11 mile radius of the OHSU University campus, free from dementia, diabetes, or vestibular disorder). Of the recruited patients, 52 (69%) responded and were screened. Of the 52 screened, 46 were eligible to participate and underwent balance testing. The six individuals who were screened but did not participate were either not fully ambulatory (e.g., intermittently in a wheelchair) or did not want to participate in the study. Five FM patients self-reported lower limb sensations that were not possible to distinguish from neuropathy in this study. Thus, they were not included in group analyses. They were instead compared to the FM group who did not report possible neuropathy. Healthy controls were recruited via IRB approved flier at the University’s School of Nursing. Testing occurred at the School of Nursing in an aerobics studio with mirrored walls and a wooden floor. Of the 34 healthy volunteers, all completed testing and only one patient’s data was not used due to a history of chemotherapy, which may impair balance due to peripheral changes in sensation.
Measures
Prior to balance testing, patients completed an investigator-designed demographics and clinical form including years with FM symptoms and diagnoses, a list of medical comorbidities and current medications. Patients also completed the Fibromyalgia Impact Questionnaire (FIQ), a subjective Activities of Balance Confidence (ABC) and reported the number of falls that had occurred in the last six months.
The Fibromyalgia Impact Questionnaire (FIQ)
FM symptoms and disease severity were measured with the FIQ, a 10-item instrument that measures physical impairment, severity of specific symptoms, including pain, fatigue, morning fatigue, stiffness, depression and anxiety, and disability and overall well-being during the past week. The severity of each symptom was measured on a scale from 0 (absence of symptom) to 10 (very severe). Total FIQ scores range from 0–100 with higher numbers indicating greater impairment. A FIQ score over 50 generally indicates moderately high negative impairment (12). The FIQ has been validated (13), and shows sensitivity to change in a wide variety of FM intervention studies (14–16). A reliability analysis was conducted for the current study yielding high internal consistency (Cronbach’s Alpha = .96).
The Activities-Specific Balance Confidence Scale (ABC)
The ABC is a 16-item validated questionnaire designed to identify fear of falling by assessing the degree of confidence with which participants can perform specific tasks without losing balance (17). Confidence is measured from 0 to 100% with higher scores indicating more balance confidence. Scores less than 67% have been demonstrated to be both sensitive and specific in predicting falls and significantly reduced independence in ADLs in community-dwelling elders(18) while well edlers generally score 90–100%. Internal consistency for the ABC Scale in the current study was α =.97.
Balance Evaluation-Systems Test (BESTest)
Patients completed a novel clinical test designed by one of the authors (FH) to identify the subsystems underlying balance deficits (7). This test identified five subsystems of balance: Stability Limits, Anticipatory Postural Adjustments, Reactive Postural Responses, Sensory Orientation, and Stability in Gait (Table 1). Each of the 31 tasks administered in this study are rated on a 4-level ordinal scale from 0-unable to perform to 3-normal performance, using stop-watches and other performance criteria. An inter-rater reliability study with 9 therapists and 10 patients who had a variety of balance disorders due to Parkinson’s Disease, vestibular disorders and peripheral neuropathy showed excellent interclass correlation coefficients for all 5 sections of the BESTest, ranging from .79–.98. Each subsystem category comprises 20% of the total balance score. The BESTest total score is a sum of all the individual items with a maximum of 100, with higher numbers indicating better balance. A single examiner trained by the first two authors completed all BESTests evaluations. The test averaged 17 minutes to complete with a range of 9–26 minutes. Current internal consistency and reliability for the total BESTest based on the 25 items for 4 of the scales (not including reactive postural response) was 0.88. Internal consistency and reliability for the 4 scales ranged from 0.66 to 0.83.
Falls
Patients were asked to recall how many falls they had in the last week, month and 6-month period. Falls were defined as an unintentionally coming to rest on the floor or low surface (bed, chair, etc).
Statistical Analyses
Descriptive statistics were used to describe and compare the FM patient population and healthy controls. Prior to testing differences between FM patients and controls on balance scores, these two groups were examined for differences in age and BMI. Independent sample t-tests revealed that there was no significant difference in age (p=.74), but there was a significant difference in mean BMI score between the two groups (p<.01). Since BMI can influence some balance components (19), BMI was controlled for in subsequent analyses.
Analysis of covariance test (ANCOVAs) controlling for BMI was conducted to determine if there were mean differences on the total BESTest score, each of the five subcomponents of the BESTest, and the ABC measure between FM patients and controls. For FM patients only, correlations between the FIQ total score and individual items (i.e., symptom severity), and the BESTest total were examined. Lastly, chi square analysis was employed to determine if FM patients reported more frequent falls in the past six months than healthy controls.
RESULTS
The FM patients’ were mostly female (88%) and Caucasian (100%), with an average age of 47.4 years. Their average FIQ score was 59.3 indicating a moderately high negative impact of FM. The average FM patient was on 4.7 medications with a range from 1 to 12. Healthy controls averaged 1.13 medications with a range from 0 to 5. The most common medications among FM patients were short acting opiates (44%, n=15), muscle relaxants (47%, n=16), and anti-depressants (e.g., SSRIs, SNRIs, tricyclics; 74%, n=25). The most common medications among healthy controls were non-fibromyalgia prescription medications (e.g., birth control, hyperlipidemia and thyroid medication; 50%, n=16), NSAIDS and over-the-counter pain meds (12%, n=4), and supplements (9%, n=3). Demographics for this sample are presented in Table 2.
Table 2.
Demographics and Clinical Variables for FM and Healthy Controls
| FM Patients N=34 M (SD) |
Controls N=32 M (SD) |
|
|---|---|---|
| Age | 47.44 (10.88) | 46.53 (10.96) |
| BMI | 30.4 (6.08) | 25.57 (5.52) |
| Gender | 88% female | 100% female |
| Race: White | 85% | 91% |
| Years with FM Symptoms | 14 (13.23) | n/a |
| FIQ Total Score | 59.26 (17.85) | 6.16 (8.11) |
| FIQ Item: Interference with work | 6.69 (2.21) | .03 (.19) |
| FIQ Item: Pain | 6.81 (2.36) | .19 (.54) |
| FIQ Item: Fatigue | 7.28 (2.34) | 1.32 (2.33) |
| FIQ Item: Awaking tired | 7.85 (1.97) | 1.71 (2.21) |
| FIQ Item: Stiffness | 7.00 (2.29) | .55 (1.03) |
| FIQ Item: Anxiety | 4.04 (3.06) | .58 (.96) |
| FIQ Item: Depression | 3.88 (3.09) | .39 (.84) |
| ABC Total | 73.23 (24.02) | 98.53 (2.24) |
| BESTest Total | 84.13 (11.87) | 95.59 (2.94) |
| BESTest Sub-Components: | ||
| I. Stability Limits | 83.33 (11.79) | 92.82 (4.58) |
| II. Anticipatory Postural Adjustment | 81.67 (15.79) | 94.09 (7.16) |
| III Reactive Postural Response | 85.00 (26.39) | 96.77 (6.69) |
| IV. Sensory Orientation | 88.69 (15.80) | 98.81 (3.17) |
| V. Stability in Gait | 81.04 (11.62) | 95.39 (6.21) |
BMI= Body Mass Index; FIQ= Fibromyalgia Impact Questionnaire; ABC= Activities-specific Balance Competence
FM patients scored significantly worse on the BESTest total balance score compared to healthy controls, 85.37 for FM versus 94.27 for controls, F(1)=10.62, p=.002. The five subsystem categories of the BESTest were each significantly impaired in FM patients compared to controls: Stability Limits, F(1)=9.27, p=.004, Anticipatory Postural Adjustments, F(1)=8.40, p=.005, Reactive, F(1)=5.42, p=.02, Sensory Orientation, F(1)=6.73, p=.01, and Stability in Gait, F(1)=25.51, p=.000. Fifty percent of the FM patients had total balance scores below 90%, compared to only 4% of the age-matched controls (X2(1)=14.53, p=.000).
FM patients were also significantly different from controls in ABC self-report balance confidence, F(1)=28.21, p<.001 (also shown in Table 3). Sixty-nine percent of the FM patients reported balance confidence below 90% compared to 0% of the age-matched controls (X2(1)=32.75, p=.000).
Table 3.
Results of ANCOVAs comparing FM to healthy controls on objective and subjective balance scores.
| Group | |||||||
|---|---|---|---|---|---|---|---|
| Healthy controls | FM | ||||||
| Outcome variable | N | Adjusted Mean | SE | N | Adjusted Mean | SE | F-statistic and p-value |
| 1. BESTest: Total Score | 26 | 94.27 | 1.82 | 26 | 85.37 | 1.82 | F(1)=10.62, p=.002 |
| 2. Best I: Stability Limits/Verticality | 29 | 92.05 | 1.77 | 30 | 84.08 | 1.74 | F(1)=9.27, p=.004 |
| 3. Best II: Transitions/Anticipatory | 31 | 92.64 | 2.20 | 30 | 83.16 | 2.24 | F(1)=8.40, p=.005 |
| 4. Best III: Reactive | 31 | 97.11 | 3.61 | 30 | 84.65 | 3.67 | F(1)=5.42, p=.023 |
| 5. Best IV: Sensory Orientation | 28 | 98.07 | 2.25 | 28 | 89.44 | 2.25 | F(1)=6.73, p=.012 |
| 6. Best V: Stability in Gait | 31 | 94.65 | 1.68 | 28 | 81.86 | 1.78 | F(1)25.51, p=.000 |
| 7. ABC: Activities-Specific Balance Confidence Scale | 31 | 98.24 | 2.71 | 28 | 76.60 | 2.86 | F(1)=28.21, p=.000 |
Means adjusted for BMI
The most difficult individual tests (BESTest subcomponent line items) for FM patients were and ability to quickly perform the Get Up and Go test with a secondary cognitive task (subtracting by 7s backward from 100 while walking three meters) (FM time 10 seconds; control time 6 seconds, p<.000), reaching forward while keeping their heels on the floor (FM reach 11 inches; control reach 15 inches, p<.000) and maintaining balance and gait speed while walking over a foam block. In contrast, FM patients tended to score normally in some seated tests, such as leaning laterally and then returning to vertical posture with eyes closed. They also scored similarly to health controls in their ability to rise once from a seated position without swaying or using their hands or arms.
The 5 FM patients with self-reported sensory problems in their feet (e.g., potential neuropathy) scored significantly differently than the FM patients without neuropathy on the BESTest total score (p=.03) and trended toward significance on the ABC p=.06.
The BESTest total score correlated with the total FIQ severity score (r = −.48, p<.05). Moreover, two BESTest subcomponents were also significantly correlated with the total FIQ severity score (Stability Limits/Verticality: r =−.59, p<.001; Sensory Orientation: r=−.41, p<.05). The other three subcomponents of the BESTest were not significantly correlated with total FIQ severity. The total BESTest correlated specifically with fatigue (r = −.50, p<.01), stiffness (r = −.45, p<.05) anxiety (r=−.55, p<.01) and depression (r-−.55, p<.01) as reported by the FIQ, such that poorer balance was related to more fatigue, stiffness and mood symptoms. Pain did not correlate significantly with Total BESTest scores. However, pain did correlate significantly with the Stability Limits/Verticality subscale of the BESTest (r =−.43, p <.05). Moreoever, all FM symptoms correlated significantly with Stability Limits/Verticality (See Table 4).
Table 4.
Correlations between FIQ total score, and individual symptom severity, with balance measures: ABC, BESTest Total and Bestest Subcomponents I–V.
| ABC (subjective balance) | BESTest Total (objective balance) | I. Stability Limits | II. Anticipatory Postural Adjustment | III. Reactive Postural Response | IV. Sensory Orientation | V. Stability in Gait | |
|---|---|---|---|---|---|---|---|
| FIQ total score | −.64** | −.48* | −.57** | −.29 ns | −.28 ns | −.41* | −.25 ns |
| Fatigue | −.60** | −.50** | −.57** | −.27 ns | −.41* | −.36† | −.23 ns |
| Stiffness | −.40* | −.45* | −.47* | −.15 ns | −.42* | −.36† | −.18 ns |
| Pain | −.56** | −.30 | −.43* | −.33† | −.13 ns | −.21 ns | −.16 ns |
| Sleep | −.16 | −.11 | −.39* | .21 ns | −.11 ns | −.28 ns | .06 ns |
| Anxiety | −.40* | −.55** | −.54** | −.37† | −.44* | −.29 ns | −.24 ns |
| Feeling depressed | −.55** | −.55** | −.56** | −.42* | −.35† | −.36† | −.27 ns |
p<.05;
p<.01;
p=.06 ABC and BESTest were correlated at .60**.
FIQ= Fibromyalgia Impact Questionnaire
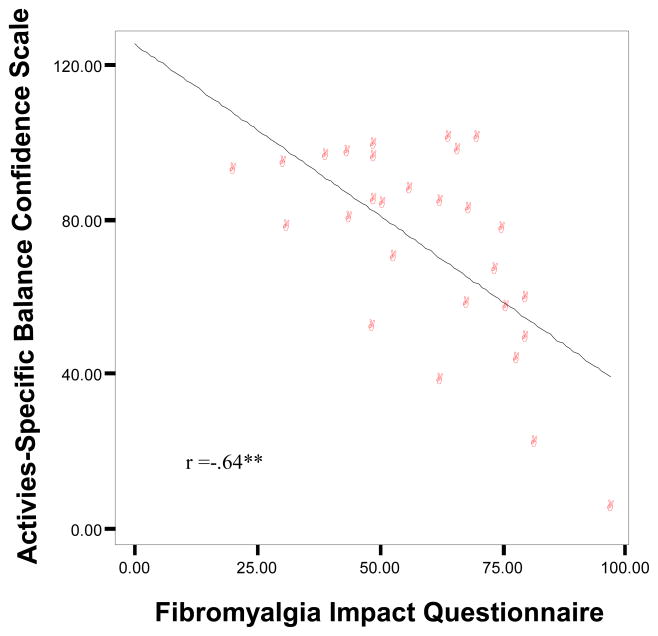
Activities-specific balance confidence (ABC) correlated significantly the total FIQ severity (r = −.64, p<.001; Fig. 1). The ABC also correlated the best with fatigue (r = −.60, p<.01), stiffness (r = −.40, p<.05), physical impairment (r = −.71, p<.001), and pain (r = −.56, p<.01), anxiety (r = −.40, p<.05) and depression (r = −.55, p<.01) such that poorer balance confidence was related to worse symptoms (Table 4). The correlation between the ABC and BESTest scores was significant (r = .60, p<.01).
Figure 1.
Subjective Balance Confidence is Significantly Negatively Correlated with Fibromyalgia Impact Questionnaire for the Fibromyalgia Group
p<.001
The FM patients reported a total of 37 falls over the last six months compared to 6 falls in the healthy controls (Χ2(1)=17.4, p <.001). The number of falls ranged from 0 to 5 falls per FM patient in the last six months (Table 5).
Table 5.
Fall frequency in past 6 months in FM patients compared to controls
| Number of falls | 0 | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| FM | 16 | 6 | 4 | 6 | 0 | 1 |
| Controls | 25 | 4 | 1 | 0 | 0 | 0 |
Χ2(4)=10.83, p<.05.
DISCUSSION
This study adds three new findings to the FM fall and balance literature: 1. objective balance scores, 2. subjective balance confidence, and 3. fall frequency. All were impaired in FM compared to age-matched healthy controls. All five subcomponents of the objective BESTest were abnormal in FM, and significantly different from controls, indicating that no single component of balance was responsible for the poor total balance scores. These findings are consistent with the hypothesis that FM affects several subsystems responsible for postural control. Furthermore, the patients were well aware of their balance deficits reflected in their low subjective balance confidence. In this FM sample, particularly striking was their impaired forward and backwards limits of stability, instability when using anticipatory postural adjustments associated with rising onto toes or onto one foot and inability to maintain gait speed during cognitive distraction.
Furthermore, it is notable that the standard deviations for balance in FM patients, as measured by the BESTest (total and subcomponents), were three to four times those of the healthy controls, suggesting a high degree of variability in balance impairment in this FM sample. Based on our data, FM patients with concurrent neuropathic-type pain may represent such a subgroup.
We also explored whether total FIQ scores or individual FM symptoms correlated with any of the five subcomponents of the BESTest. Interestingly, all individual FM symptoms were significantly correlated with the Stability Limits subcomponent. This indicates that symptoms of fatigue, stiffness, pain, sleep, anxiety, and feeling depressed were associated with difficulty in knowing how far one can lean from a seated position, accurately attaining vertical realignment, maintaining balance while standing with eyes closed, and reaching laterally or forward while keeping heels on the ground. Importantly, our data suggest that it is the constellation of FM symptoms rather than pain alone that may be responsible for the poor balance demonstrated in this study.
Significant slowing of walking when dividing attention to a secondary cognitive task, such as mental arithmetic, is thought to represent increased attentional resources on balance and gait, which are normally automatically controlled. Balance and postural stability during gait requires more attention with increasing constraints on motor performance due to impaired sensory systems, musculoskeletal systems, or central processing, all which could be affected by FM. A reduced attentional capacity due to cognitive deficits or pain may also result in slowing of gait during a cognitive task, a response exhibited by our FM patients but not by healthy controls. In elderly patients, combining the Timed-up-and-Go test (TUG) with a cognitive task, as in our study, increases the time to complete the TUG the longest in those with a history of multiple falls (11). The ability to dual process motor and cognitive tasks is better in younger compared to older individuals (20), suggesting that the increased difficulty of performing dual task walking in FM patients could be similar to what occurs with aging.
Weakness, reduced flexibility, and high BMI could also contribute to poor balance control but this cannot likely explain the poor balance in the patients with FM in this study. We controlled for high BMI in our analysis and all patients were strong enough to walk independently and had strong enough ankle muscles stand on their toes or heels while holding onto the tester. They had enough trunk flexibility to lean sideways in sitting although they had small forward and backward limits of stability when attempting to lean forward and backwards, perhaps in part due to axial pain present in all FM patients. Plantar fasciitis and ankle tendonitis could further impair balance in this population, as pain in the feet due to arthritis is one of the highest risk factor for falls (21–23). As people with FM age, we expect that their risk of falling would increase even faster than for people without FM. However, our data revealed no correlation between falls and age in FM patients, r =.04, p=.84.
Poor postural stability during gait in the BESTest is consistent with recently reported gait disturbances in FM patients (24–26). Auvinet examined relaxed walking in 14 women with FM compared to 14 controls matched for sex, age, height, and body weight. Their data demonstrate that walking speed was significantly diminished (P<0.001) as a result of reductions in stride length (P<0.001) and cycle frequency (P<0.001). In another gait-mat study, Pierrynowski tested 22 women with FM and 11 healthy controls (HCs). In contrast to Auvinet, these data suggest that FM and HCs walk with externally similar stride lengths, times, and velocities, and joint angles and ground reaction forces. The patients differed from controls, however, in their muscle recruitment patterns. Specifically, FM patients preferentially power their gait using their hip flexors instead of their ankle plantar flexors. Graven-Nielsen has assessed resting, static, and dynamic muscle activity’s response to hypertonic saline injection into the vastus medialis muscle with electromyography (EMG) activity and anterior tibialis muscle contraction force in FM patients. At rest, no evidence of EMG hyperactivity was found during experimental muscle pain but contraction endurance time was significantly decreased (p < 0.043). Furthermore, during dynamic contractions, EMG activity increased in the muscle antagonistic to the painful muscle, suggesting a functional adaptation of muscle co-ordination in order to limit movements. These data support the notion that balance and gait tasks are very dependent on somatosensory inputs from muscles, and may be disrupted by muscle pain. The importance of muscle training in improving balance was highlighted in recent 6 month exercise intervention program in which a statistically significant improvement in one-legged stand time was reported in the exercise group (27). Moreover, a 12 week aquatic exercise program in FM recently demonstrated that exercise not only improved blinded one-legged stance time, but after 4 months of detraining, scores declined, returning baseline (28).
Future research will need to determine whether FM is associated with deficits in vestibular function, proprioception, spatio-visual orientation, muscle strength, postural reflexes, orthostatic blood pressure dysfunction or attentional deficits. Although we excluded patients with dizziness, prior head injuries and a diagnosis of vestibular problems or peripheral neuropathy, it is possible that deficits in these systems were present. We did not measure blood pressure in this study. A few studies have documented otological disturbances in FM patients that could result in abnormal vestibular function. A sensorineural hearing loss was reported in 15% of FM patients and (29) in another study, dizziness was the most common complaint followed by tinnitus, hearing loss and vertigo. The Dix-Halpike maneuver was positive for rotary vertigo in 21% of patients, consistent with peripheral positional vertigo, without signs of vestibular loss with bithermal caloric testing (30). Cortical, P300 auditory event related potentials (ERP’s) are also significantly lower in amplitude in FM patients than controls. After treatment with sertraline, the P300 auditory ERPs amplitudes of the FM group erewas almost the same as the control group. It was hypothesized that the lower amplitude ERP’s in FM were a result of higher order cognitive dysfunction, and that this could be reversed by treatment with sertraline.
Auditory brainstem responses (ABR) were also found to be abnormal in 30–31% of FM patients (31). Unlike the P 300 event related potentials, which measure conscious awareness of sound, an abnormal brainstem response is more suggestive of a brainstem, neurophysiological deficit. Disordered brainstem function in FM was also supported by an investigation using occulomotor testing in 36 fibromyalgia patients compared to 71 healthy controls. Saccadic eye movements were abnormal in 42% and the smooth pursuit eye movements were abnormal in 18. 9% of FM patients (32). The brainstem is important site not only for control of eye movement and auditory processing, but also for multisensory integration and muscle synergy circuitry for postural control (6;10;31).
The current study is limited by small sample size and lack of normal distribution of findings in healthy control patients, who were skewed toward perfect balance scores. Nonetheless, larger sample sizes are expected to increase power and further differentiate FM balance problems from those in healthy controls. Future trials could consider comparing FM patients to healthy older controls. This design was developed by Glass and colleagues who demonstrated that memory and cognition in FM were similar to disease free but otherwise matched healthy controls who were 20 years older (33). Generalizability of the results is limited by the tertiary care sample. The cross-sectional design of this study limits our ability to make inferences about the causal relationship between FM and balance perturbations. However, these results are strongly supportive of the notion that FM patients have multiple objective balance problems that are related to falls.
The fall history conducted in this study is potentially limited by retrospective recall bias. Follow-up trials show consider real time fall reporting such as use of an electronic diary or phone calls to inquire bout falls each week. Fall reporting should include not only “unintentionally coming to rest on the floor or lower surface as in this study, ” but also “near falls” in which patients catch themselves on furniture or other surfaces after a loss of equilibrium. Data should also be collected regarding circumstances surrounding the fall.
More information is needed on FM medication side effects and their potential contribution to falls and balance impairment since multiple medications are known to increase fall risk (21). Many medications to relieve pain for FM such as opiates, muscle relaxants and anti-depressants may impair balance (34) and 44–74% of our recruited FM patients were taking these medications. At a minimum, future trials could require that patients be on a stable regimen of medications for whatever minimum amount of time is required to physiologically adjust to the drugs. We did not differentiate new from established medications in this trial.
In summary, balance is compromised in FM based on both objective and subjective data. Further objective study is needed to identify the relative contribution of neural and muscular impairments to postural stability in patients with FM. These findings will be critical to future exercise and fall-prevention interventions aimed at reducing falls and improving balance in patients with FM.
Acknowledgments
Grant Support: 2 R37 AG006457–19 NIH/NIA (Horak, PI) and 5R01 NR8150–4 NIH/NINR (Jones, PI)
Reference List
- 1.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33(2):160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 2.Bennett RM. Fibromyalgia and Chronic Fatigue Syndrome. In: Goldman L, Ausiello D, editors. Cecil: Textbook of Medicine. Philadelphia: Saunders; 2007. pp. 2078–2083. [Google Scholar]
- 3.Mease P, Arnold LM, Bennett R, Boonen A, Buskila D, Carville S, et al. Fibromyalgia syndrome. J Rheumatol. 2007;34(6):1415–1425. [PubMed] [Google Scholar]
- 4.Bennett RM, Jones J, Turk DC, Russell IJ, Matallana L. An internet survey of 2,596 people with fibromyalgia. BMC Musculoskelet Disord. 2007;8:27. doi: 10.1186/1471-2474-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katz R, Ferbert S, Leavitt F. Fibromyalgia patients report many symptoms other than pain and fatigue. Arthritis Rheum. 2007;56(9S):1532. [Google Scholar]
- 6.Horak FB. Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing. 2006;35(Suppl 2):ii7–ii11. doi: 10.1093/ageing/afl077. [DOI] [PubMed] [Google Scholar]
- 7.Horak FB, Frank JS, Meyer L, Tompkins T, Wrisley D. The balance evaluation-system test: reliability, concurrent validity, and internal consistency. APTA Cobmined Section Meetings; Nashville, TN. 2004; Ref Type: Abstract. [Google Scholar]
- 8.Duncan PW, Weiner DK, Chandler J, Studenski S. Functional reach: a new clinical measure of balance. J Gerontol. 1990;45(6):M192–M197. doi: 10.1093/geronj/45.6.m192. [DOI] [PubMed] [Google Scholar]
- 9.Rocchi L, Chiari L, Mancini M, Carlson-Kuhta P, Gross A, Horak F. Step initiation in Parkinson’sdisease: influence of initial stance conditions. Neuroscience Letters. 2006;406(1–2):128–132. doi: 10.1016/j.neulet.2006.07.027. Ref Type: Abstract. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs JV, Horak FB, Van Tran K, Nutt JG. An alternative clinical postural stability test for patients with Parkinson’s disease. J Neurol. 2006;253(11):1404–1413. doi: 10.1007/s00415-006-0224-x. [DOI] [PubMed] [Google Scholar]
- 11.Shumway-Cook A, Horak FB. Assessing the influence of sensory interaction on balance. Physical Therapy. 1986;66:1548–1550. doi: 10.1093/ptj/66.10.1548. [DOI] [PubMed] [Google Scholar]
- 12.Bennett R. The Fibromyalgia Impact Questionnaire (FIQ): a review of its development, current version, operating characteristics and uses. Clin Exp Rheumatol. 2005;23(5 Suppl 39):S154–S162. [PubMed] [Google Scholar]
- 13.Burckhardt CS, Clark SR, Bennett RM. The fibromyalgia impact questionnaire: development and validation. J Rheumatol. 1991;18(5):728–33. [PubMed] [Google Scholar]
- 14.Bennett RM, Kamin M, Karim R, Rosenthal N. Tramadol and acetaminophen combination tablets in the treatment of fibromyalgia pain: a double-blind, randomized, placebo-controlled study. Am J Med. 2003;114(7):537–545. doi: 10.1016/s0002-9343(03)00116-5. [DOI] [PubMed] [Google Scholar]
- 15.Arnold LM, Pritchett YL, D’Souza DN, Kajdasz DK, Iyengar S, Wernicke JF. Duloxetine for the treatment of Fibromyalgia in women: Pooled results from two randomized, placebo-controlled clinical trials. Journal of Womens Health. 2007;16(8):1145–1156. doi: 10.1089/jwh.2006.0213. [DOI] [PubMed] [Google Scholar]
- 16.Arnold LM, Goldenberg DL, Stanford SB, Lalonde JK, Sandhu HS, Keck PE, et al. Gabapentin in the treatment of fibromyalgia - A randomized, double-blind, placebo-controlled, multicenter trial. Arthritis and Rheumatism. 2007;56(4):1336–1344. doi: 10.1002/art.22457. [DOI] [PubMed] [Google Scholar]
- 17.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci. 1995;50A(1):M28–M34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- 18.Lajoie Y, Gallagher SP. Predicting falls within the elderly community: comparison of postural sway, reaction time, the Berg balance scale and the Activities-specific Balance Confidence (ABC) scale for comparing fallers and non-fallers. Arch Gerontol Geriatr. 2004;38(1):11–26. doi: 10.1016/s0167-4943(03)00082-7. [DOI] [PubMed] [Google Scholar]
- 19.Paquette C, Teasdale N, Prud’homme D, Tremblay A. Weight reduction yields a more stable postural behavior in obese men. Arch Physiol Biochem. 2008;108:223. [Google Scholar]
- 20.Hollman JH, Kovash FM, Kubik JJ, Linbo RA. Age-related differences in spatiotemporal markers of gait stability during dual task walking. Gait Posture. 2007;26(1):113–119. doi: 10.1016/j.gaitpost.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Tinetti ME, Williams TF, Mayewski R. Fall risk index for elderly patients based on number of chronic disabilities. Am J Med. 1986;80(3):429–434. doi: 10.1016/0002-9343(86)90717-5. [DOI] [PubMed] [Google Scholar]
- 22.Tinetti ME, Allore H, Araujo KL, Seeman T. Modifiable impairments predict progressive disability among older persons. J Aging Health. 2005;17(2):239–256. doi: 10.1177/0898264305275176. [DOI] [PubMed] [Google Scholar]
- 23.Tinetti ME, Gordon C, Sogolow E, Lapin P, Bradley EH. Fall-risk evaluation and management: challenges in adopting geriatric care practices. Gerontologist. 2006;46(6):717–725. doi: 10.1093/geront/46.6.717. [DOI] [PubMed] [Google Scholar]
- 24.Auvinet B, Bileckot R, Alix AS, Chaleil D, Barrey E. Gait disorders in patients with fibromyalgia. Joint Bone Spine. 2006;73(5):543–546. doi: 10.1016/j.jbspin.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 25.Graven-Nielsen T, Svensson P, Arendt-Nielsen L. Effects of experimental muscle pain on muscle activity and co-ordination during static and dynamic motor function. Electroencephalogr Clin Neurophysiol. 1997;105(2):156–164. doi: 10.1016/s0924-980x(96)96554-6. [DOI] [PubMed] [Google Scholar]
- 26.Pierrynowski MR, Tiidus PM, Galea V. Women with fibromyalgia walk with an altered muscle synergy. Gait Posture. 2005;22(3):210–218. doi: 10.1016/j.gaitpost.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Jones KD, Burckhardt CS, Deodhar AA, Perrin NA, Hanson GC, Bennett RM. A six-month randomized controlled trial of exercise and pyridostigmine in the treatment of fibromyalgia. Arthritis Rheum. 2008;58(2):612–622. doi: 10.1002/art.23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomas-Carus P, Hakkinen A, Gusi N, Leal A, Hakkinen K, Ortega-Alonso A. Aquatic training and detraining on fitness and quality of life in fibromyalgia. Med Sci Sports Exerc. 2007;39(7):1044–1050. doi: 10.1249/01.mss.0b0138059aec4. [DOI] [PubMed] [Google Scholar]
- 29.Rosenhall U, Johansson G, Orndahl G. Otoneurologic and audiologic findings in fibromyalgia. Scand J Rehabil Med. 1996;28(4):225–232. [PubMed] [Google Scholar]
- 30.Bayazit YA, Gursoy S, Ozer E, Karakurum G, Madenci E. Neurotologic manifestations of the fibromyalgia syndrome. J Neurol Sci. 2002;196(1–2):77–80. doi: 10.1016/s0022-510x(02)00032-1. [DOI] [PubMed] [Google Scholar]
- 31.Rosenhall U, Johansson G, Orndahl G. Neuroaudiological findings in chronic primary fibromyalgia with dysesthesia. Scand J Rehabil Med. 1987;19(4):147–152. [PubMed] [Google Scholar]
- 32.Rosenhall U, Johansson G, Orndahl G. Eye motility dysfunction in chronic primary fibromyalgia with dysesthesia. Scand J Rehabil Med. 1987;19(4):139–145. [PubMed] [Google Scholar]
- 33.Park DC, Glass JM, Minear M, Crofford LJ. Cognitive function in fibromyalgia patients. Arthritis Rheum. 2001;44(9):2125–33. doi: 10.1002/1529-0131(200109)44:9<2125::AID-ART365>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 34.Chou R, Huffman LH. Medications for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147(7):505–514. doi: 10.7326/0003-4819-147-7-200710020-00008. [DOI] [PubMed] [Google Scholar]