1. Introduction
1.1 Introduction to Pd-catalyzed directed C–H functionalization
The development of methods for the direct conversion of carbon–hydrogen bonds into carbon-oxygen, carbon-halogen, carbon-nitrogen, carbon-sulfur, and carbon-carbon bonds remains a critical challenge in organic chemistry. Mild and selective transformations of this type will undoubtedly find widespread application across the chemical field, including in the synthesis of pharmaceuticals, natural products, agrochemicals, polymers, and feedstock commodity chemicals. Traditional approaches for the formation of such functional groups rely on pre-functionalized starting materials for both reactivity and selectivity. However, the requirement for installing a functional group prior to the desired C–O, C–X, C–N, C–S, or C–C bond adds costly chemical steps to the overall construction of a molecule. As such, circumventing this issue will not only improve atom economy but also increase the overall efficiency of multi-step synthetic sequences.
Direct C–H bond functionalization reactions are limited by two fundamental challenges: (i) the inert nature of most carbon-hydrogen bonds and (ii) the requirement to control site selectivity in molecules that contain diverse C–H groups. A multitude of studies have addressed the first challenge by demonstrating that transition metals can react with C–H bonds to produce C–M bonds in a process known as “C–H activation”.1 The resulting C–M bonds are far more reactive than their C–H counterparts, and in many cases they can be converted to new functional groups under mild conditions.
The second major challenge is achieving selective functionalization of a single C–H bond within a complex molecule. While several different strategies have been employed to address this issue, the most common (and the subject of the current review) involves the use of substrates that contain coordinating ligands. These ligands (often termed “directing groups”) bind to the metal center and selectively deliver the catalyst to a proximal C–H bond. Many different transition metals, including Ru, Rh, Pt, and Pd, undergo stoichiometric ligand-directed C–H activation reactions (also known as cyclometalation).2,3 Furthermore, over the past 15 years, a variety of catalytic carbon-carbon bond-forming processes have been developed that involve cyclometalation as a key step.1b–d,4
The current review will focus specifically on ligand-directed C–H functionalization reactions catalyzed by palladium. Palladium complexes are particularly attractive catalysts for such transformations for several reasons. First, ligand-directed C–H functionalization at Pd centers can be used to install many different types of bonds, including carbon-oxygen, carbon-halogen, carbon-nitrogen, carbon-sulfur, and carbon-carbon linkages. Few other catalysts allow such diverse bond constructions,5,6,7 and this versatility is predominantly the result of two key features: (i) the compatibility of many PdII catalysts with oxidants and (ii) the ability to selectively functionalize cyclopalladated intermediates. Second, palladium participates in cyclometalation with a wide variety of directing groups, and, unlike many other transition metals, promotes C–H activation at both sp2 and sp3 C–H sites. Finally, the vast majority of Pd-catalyzed directed C–H functionalization reactions can be performed in the presence of ambient air and moisture, making them exceptionally practical for applications in organic synthesis.
While several accounts have described recent advances, this is the first comprehensive review encompassing the large body of work in this field over the past 5 years (2004–2009). Both synthetic applications and mechanistic aspects of these transformations are discussed where appropriate, and the review is organized on the basis of the type of bond being formed.
1.2. Mechanistic manifolds
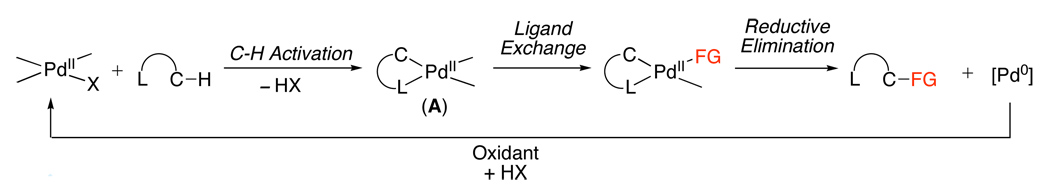
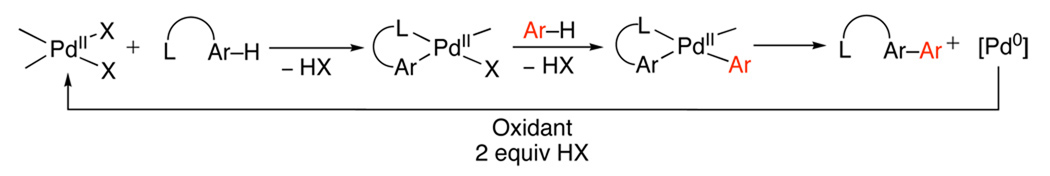
In general, ligand-directed C–H activation takes place at PdII centers to afford cyclopalladated intermediates of general structure A. Complex A can then undergo functionalization by two distinct mechanistic manifolds. The first involves a PdII/0 pathway (Scheme 1), where functionalization occurs via a reductive process (typically reductive elimination or β-hydride elimination/deprotonation) to release the product. The resulting Pd0 intermediate is then oxidized to regenerate the PdII catalyst.
Scheme 1.
Representative PdII/0 Catalytic Cycle for C–H Functionalization
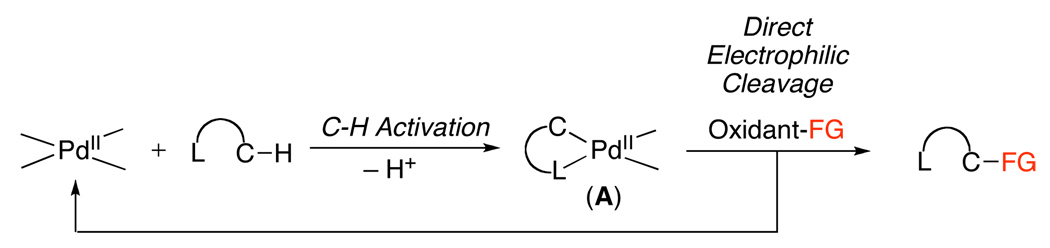
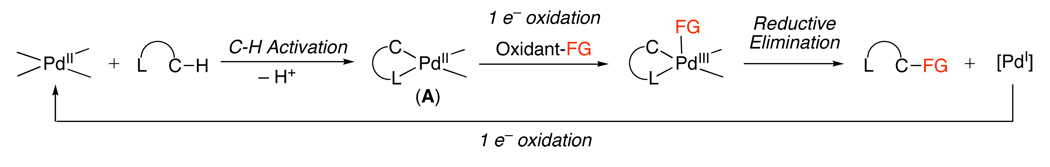
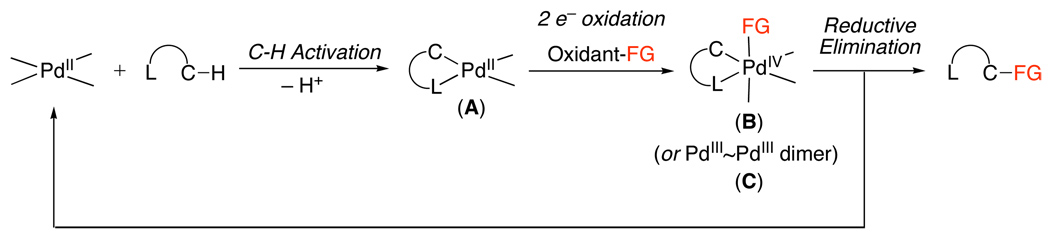
A second pathway involves functionalization of palladacycle A with an electrophilic reagent. Such reagents can react by several distinct mechanisms, including: (i) direct electrophilic cleavage of the Pd–C bond without a change in oxidation state at the metal center (Scheme 2), (ii) one-electron oxidation of the PdII starting material A (Scheme 3), or (iii) two-electron oxidation of the palladacyclic complex (Scheme 4). In the latter case (iii), two electron oxidation could proceed to generate a PdIV intermediate (B, Scheme 4) or a PdIII~PdIII dimer (C, Scheme 4), depending on the nature of the ancillary ligands at the metal center. Notably, the reactions in Scheme 1–Scheme 4 all show delivery of the functional group from an external reagent; however, intramolecular functionalization can also occur in these systems.
Scheme 2.
Mechanism Involving Direct Electrophilic Functionalization of Complex A
Scheme 3.
Mechanism Involving One Electron Oxidation of Complex A
Scheme 4.
Mechanism Involving Two Electron Oxidation of Complex A
2. Carbon-Oxygen Bond Formation
A variety of different oxidants have been used to effect Pd-catalyzed ortho-oxygenation reactions. This section details the current state of the art in terms of reaction scope and mechanism for each class of oxygenation reagent.
2.1. Iodine(III) oxidants
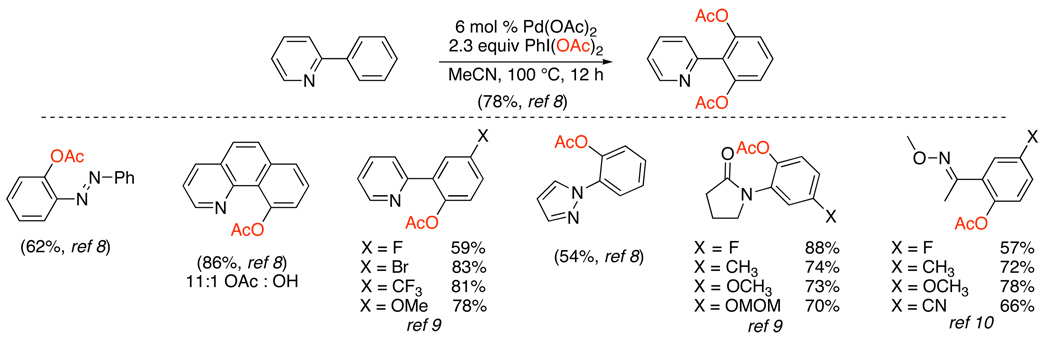
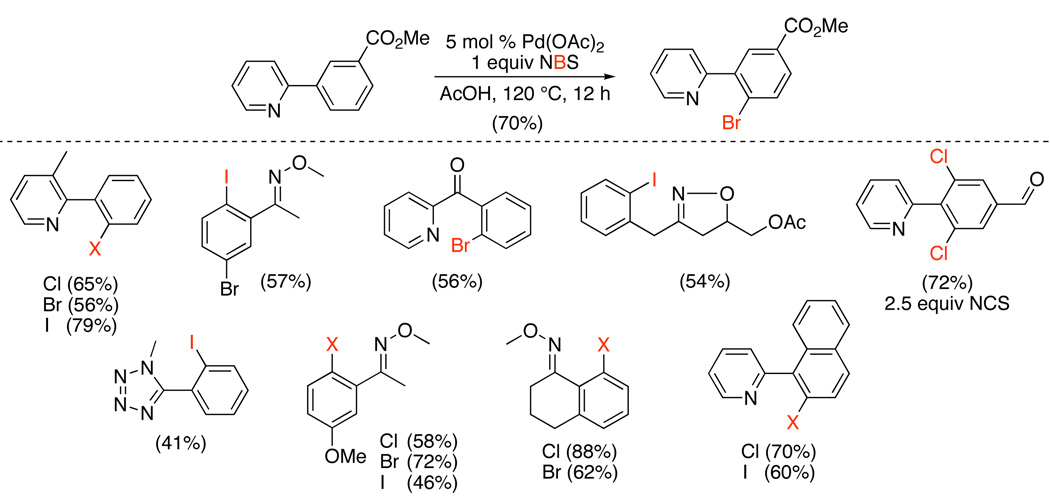
The first examples of Pd-catalyzed ligand-directed sp2 C–H bond oxygenation used PhI(OAc)2 as a stoichiometric oxidant in conjunction with catalytic Pd(OAc)2.8 A variety of pyridine derivatives served as excellent directing groups, and the reactions produced ortho-acetoxylated products in yields ranging from 52–88% (Scheme 5). Other nitrogen-based directing groups, including imines, oxime ethers, azobenzene derivatives, and nitrogen heterocycles (e.g., pyrazoles and isoxazolines) were also effective; furthermore, cyclic amides, which contain relatively basic oxygen atoms, could be used to direct these reactions.9,10 In contrast, simple ketones and aldehydes did not undergo ortho-acetoxylation, presumably because these are relatively poor ligands for PdII. This is a limitation of the vast majority of Pd-catalyzed reactions discussed in this review.11
Scheme 5.
These transformations are typically conducted without any special purification of solvents or reagents, and all of the ortho-oxygenation reactions in this section were similarly tolerant of ambient air and moisture. Many common functional groups are stable under the reaction conditions, including aryl halides, ethers, benzylic hydrogens, nitro groups, and enolizable ketones, oximes, and amides. A systematic study of meta-substituted arylpyridines and arylpyrrolidinones revealed that Pd-catalyzed sp2 C–H acetoxylation occurs selectively at the less hindered ortho-site, regardless of the electronic character or potential chelating ability of the meta-substituent.9 This selectivity appears to be general for most Pd-catalyzed C–H functionalization reactions of meta-substituted arenes (vide infra for examples).
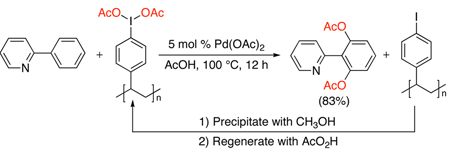
Polymer-immobilized ArI(OAc)2 has also been used for the Pd-catalyzed acetoxylation of many of the substrates shown in Scheme 5, and afforded the products in comparable yields to PhI(OAc)2.12 This oxidant offers the advantage that it can be readily recovered from the reaction mixture and recycled (eq. 1).
 |
(1) |
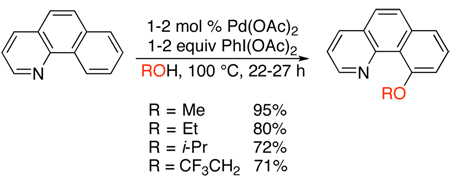
When the solvent for these reactions was changed from AcOH to an alcohol (ROH), aryl ether products were formed in high yields.8 For example, the Pd-catalyzed reaction of benzo[h]quinoline with PhI(OAc)2 in ROH could be used to prepare methyl, ethyl, iso-propyl, and trifluoroethyl ethers (eq. 2). The in situ reaction of the alcohol solvent with PhI(OAc)2 to afford PhI(OR)213 is believed to be a key step in these transformations.
 |
(2) |
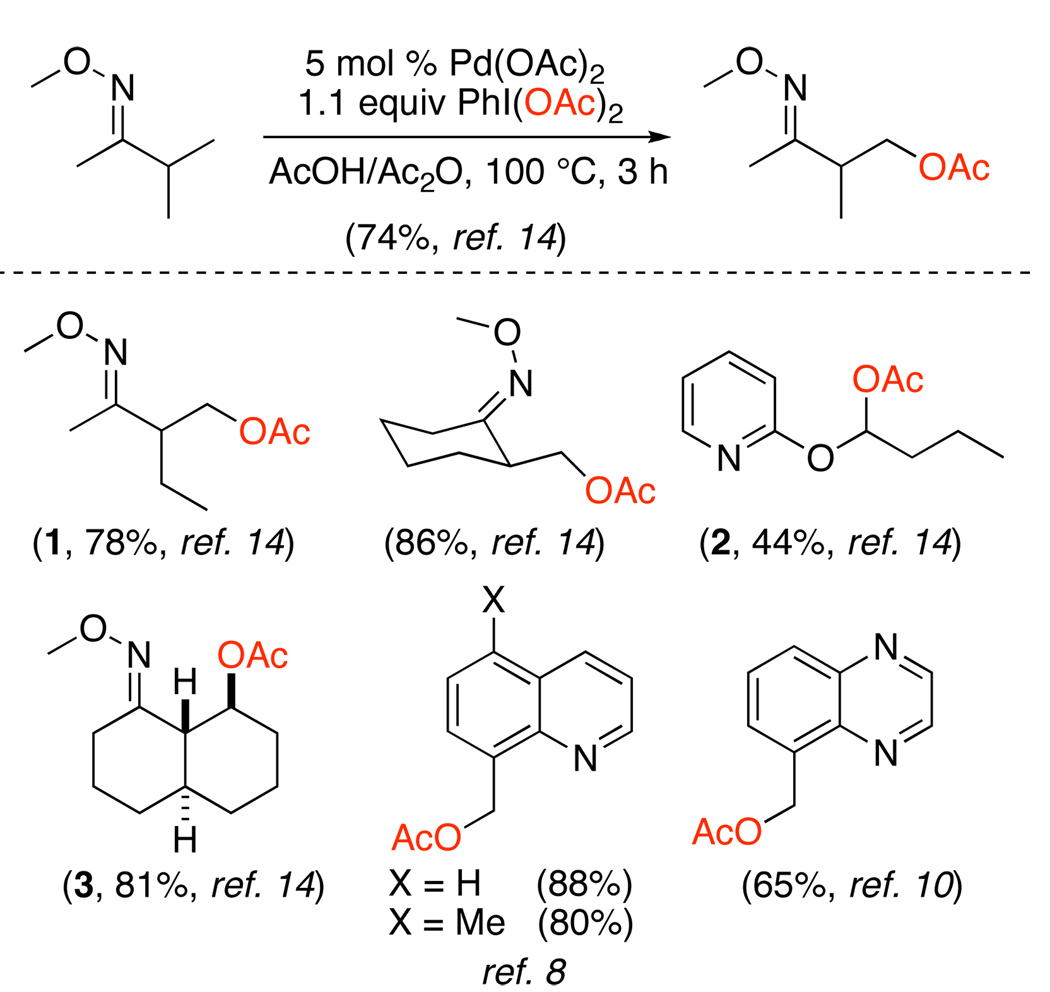
The first report of Pd-catalyzed ligand-directed sp3 C–H bond oxygenation also used PhI(OAc)2 as the terminal oxidant. As summarized in Scheme 6, both benzylic8 and unactivated sp3 C–H bonds14 were readily converted to OAc groups. Oxime ether and pyridine directing groups could be utilized for these transformations, and no products of β-hydride elimination were observed, presumably due to the rigidity of the palladacyclic intermediates.
Scheme 6.
Pd(OAc)2-Catalyzed sp3 C–H Acetoxylation with PhI(OAc)28,10,14
In general, sp3 C–H acetoxylation proceeds with very high selectivity for functionalization of 1° (versus 2°) C–H bonds and for the formation of 5-membered (versus 6-membered) palladacycles. A good example of this selectivity can be seen in the synthesis of product 1 (Scheme 6), which was formed with high (>95%) selectivity in the presence of numerous different types of proximal C–H bonds. While this high selectivity is advantageous for many applications, a current limitation of this chemistry (as well as of many related Pd-catalyzed ligand-directed C–H functionalization reactions) is that the activation of 2° sp3 C–H bonds remains challenging. Acetoxylation at 2° sites with PhI(OAc)2 has only been achieved in substrates containing adjacent activating groups (for example, the oxygen in 2) or in highly rigid molecules such as 3.
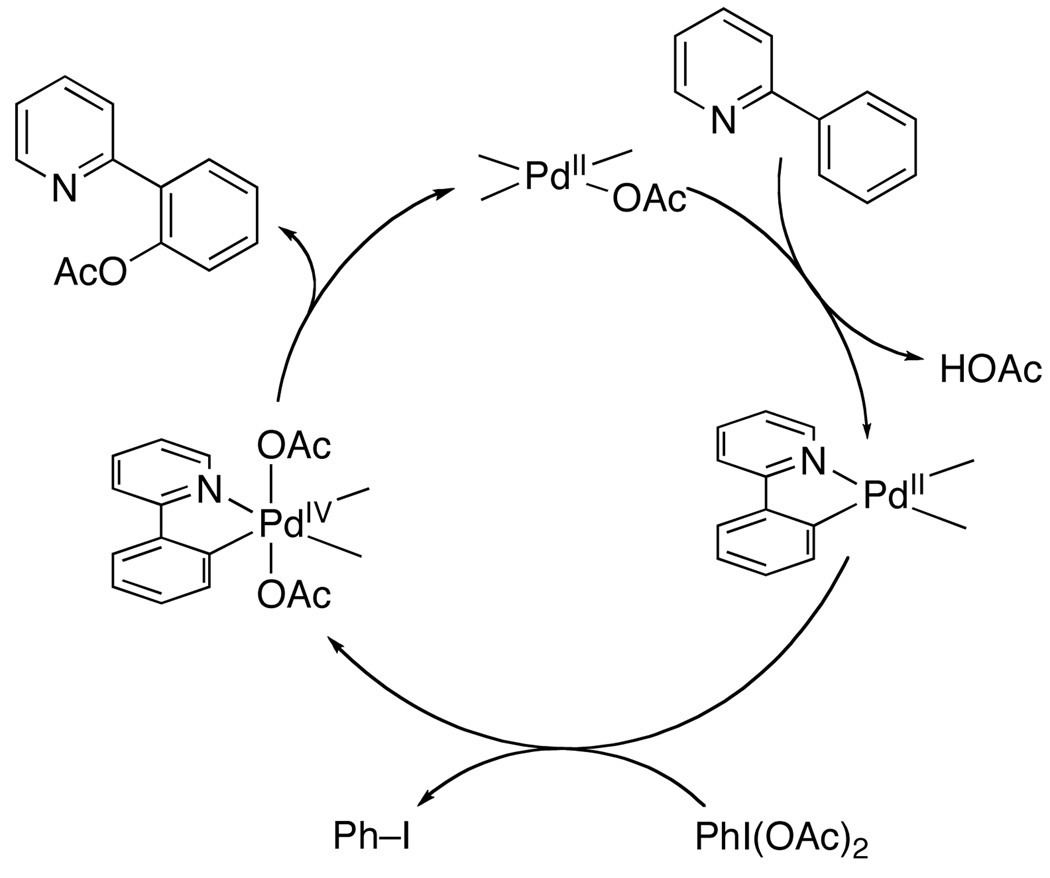
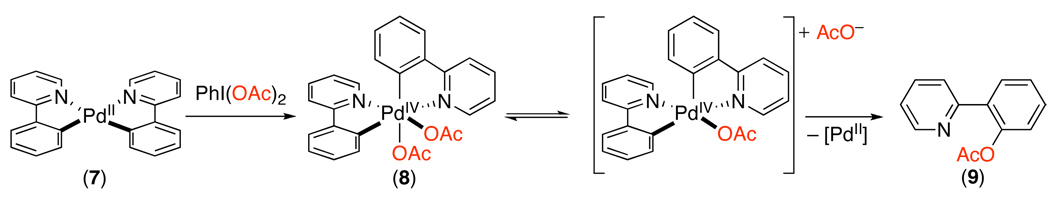
The mechanism of Pd-catalyzed C–H oxygenation with PhI(OAc)2 has been explored in some detail. Initial work showed that these reactions proceed with similar rates using either Pd(OAc)2 or palladacycle 4 (eq. 3) as the catalyst.8 In addition, 4 was shown to react directly with PhI(OAc)2 to afford acetoxylated product 5 (eq. 3). The former result suggests that 4 is a kinetically competent intermediate, while the latter provides evidence against a PdII/0 process. On this basis, the catalytic cycle in Scheme 7 was proposed for this transformation (shown with 2-phenylpyridine as a representative substrate). This cycle involves the following steps: (i) ligand-directed C–H activation to generate a cyclopalladated intermediate, (ii) two electron oxidation of the palladcycle to generate a PdIV species, and finally (iii) C–O bond-forming reductive elimination to release the product.8,15 Notably, the other ancillary ligands at Pd during the catalytic cycle are not currently known and are represented as sticks in Scheme 7. More recent studies have suggested the possibility of a closely related two electron mechanism involving dimeric PdIII~PdIII intermediates in this or related transformations.16,17,18
Scheme 7.
Proposed Mechanism for Pd(OAc)2-Catalyzed C–H Acetoxylation with PhI(OAc)28,15
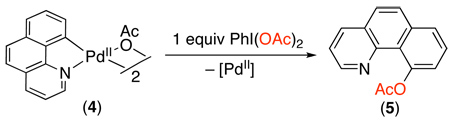 |
(3) |
The mechanism of these catalytic C–H acetoxylation reactions has been studied in detail.19,20 With both 2-benzylpyridine and 2-ortho-tolypyridine as substrates, these transformations showed a zero order dependence on [PhI(OAc)2] in several different solvents (benzene, AcOH/Ac2O, and MeCN). In addition, large 1° intermolecular kinetic isotope effects (between 3.58 and 4.3) were observed (eq. 4 and eq. 5). These studies indicate that cyclopalladation is the rate-limiting step of the catalytic cycle.
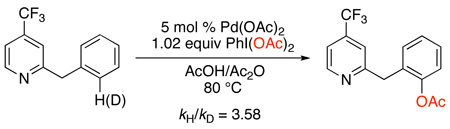 |
(4) |
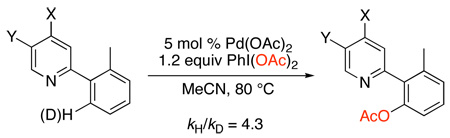 |
(5) |
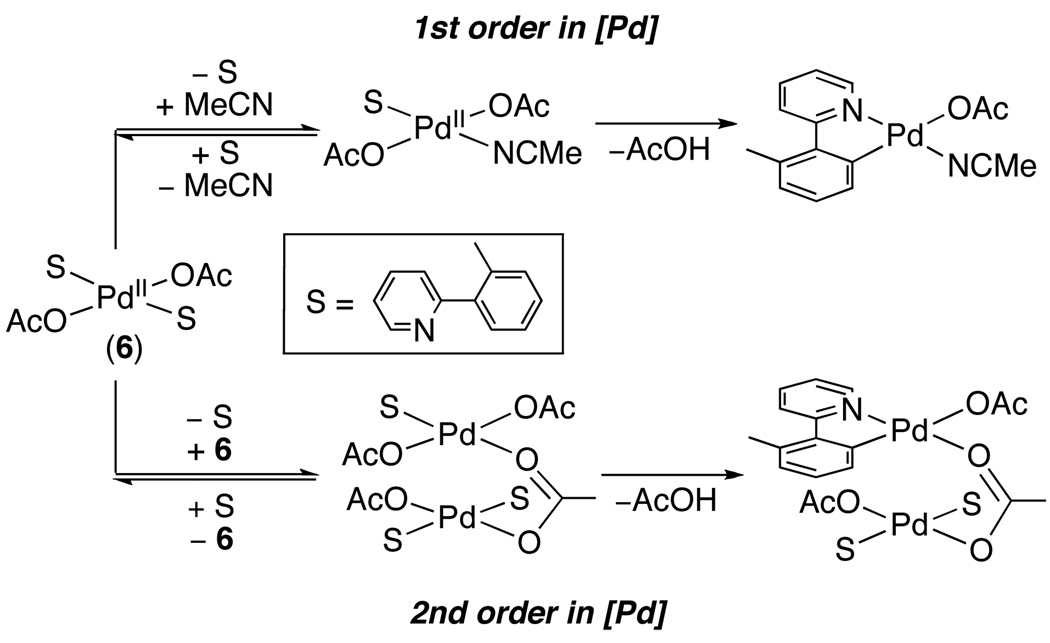
More detailed studies of the turnover-limiting step were recently conducted on the Pd(OAc)2-catalyzed acetoxylation of 2-ortho-tolylpyridine (tolpy) in MeCN.20 This transformation showed a Hammett ρ value of +0.89 upon substitution of X and Y (eq. 5) as well as an inverse 1st order dependence on [tolpy] and a 1.5 order dependence on [Pd]. On the basis of these studies, the catalyst resting state was proposed to be trans-(tolpy)2PdII(OAc)2 (6), and cyclopalladation was proposed to proceed by two competing mechanisms that both involve pre-equilibrium dissociation of the tolpy ligand (Scheme 8).20
Scheme 8.
Proposed Competing Mechanisms of Rate-Limiting Cyclopalladation during Pd(OAc)2-Catalyzed C–H Acetoxylation20
Because cyclopalladation is turnover-limiting, it has proven challenging to directly investigate the proposed oxidation and C–O bond-forming reductive elimination steps of the catalytic cycle in Scheme 7. Thus, studies in this area have focused on the generation of more stable model complexes to assay the viability of both: (1) the two-electron oxidation of PdII to PdIV with PhI(OAc)2 as the well as (2) the feasibility of C–O bond-forming reactions from high oxidation state Pd centers.18,21,22,23 These studies have shown that PhI(OAc)2 reacts with biscyclometalated model complex 7 to afford stable PdIV species 8 (Scheme 9). Further, complex 8 undergoes clean and quantitative C–O bond-forming reductive elimination to form 9. Detailed mechanistic studies of this process (including Hammett plots, Eyring analysis, cross-over studies, and investigations of the influence of solvent and additives) indicate that reductive elimination proceeds by an ionic mechanism involving initial carboxylate dissociation followed by C–O coupling from a 5-coordinate cationic intermediate (Scheme 9).23,24
Scheme 9.
Formation and Reactivity of Model PdIV Complex 823
2.2. Iodine(I) oxidants
The iodine(I) reagent IOAc has been successfully utilized for the Pd(OAc)2-catalyzed acetoxylation of methyl groups in Boc-protected N-methylamine derivatives (eq. 6).25 The IOAc is generated in situ by reaction of I2 with either PhI(OAc)2 or AgOAc. Interestingly, no reaction was observed when either PhI(OAc)2 or I2 was used independently. Various alkyl N-methylamines and N-methylanilines underwent C–H acetoxylation in good to excellent yields, and remarkably high selectivity was observed for functionalization of N-CH3 over N-aryl substituents. The mechanism was proposed to involve the following sequence of steps: (i) amide-directed C–H activation, (ii) oxidation to PdIV, (iii) C–I bond-forming reductive elimination, and finally (iv) nucleophilic displacement of I− by OAc− under the reaction conditions. However, generation of the products via direct C–OAc bond-forming reductive elimination (as proposed in Scheme 7) could not be ruled out.
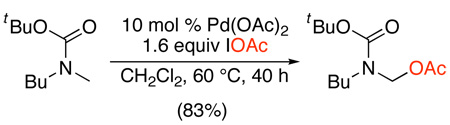 |
(6) |
2.3. Peroxide oxidants
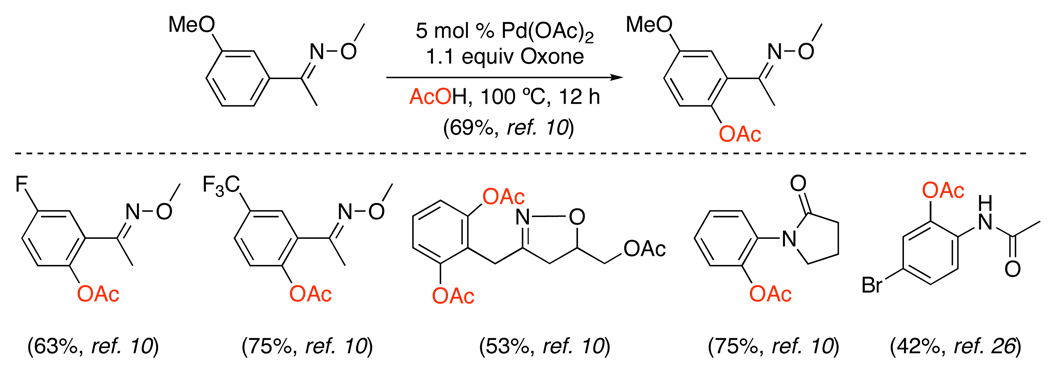
Peroxide oxidants are attractive alternatives to costly iodine-based reagents for Pd-catalyzed ligand-directed C–H oxygenation. The Pd-catalyzed oxygenation of sp2 C–H bonds has been achieved using inorganic peroxides such as Oxone or K2S2O8 in AcOH or MeOH (Scheme 10).10 In this system, the inorganic peroxide serves as the terminal oxidant (proposed to promote oxidation of PdII to PdIV), while the solvent acts as the source of the oxygen functionality. Substrates containing a variety of directing groups including oxime ethers, amides, and isoxazolines react to afford both aryl esters10,26 and aryl ethers.10 Additionally, 2-arylpyridines can be converted directly to phenols using Oxone in conjunction with t-BuOH and PEG-3400.27
Scheme 10.
Pd(OAc)2-Catalyzed sp2 C–H Bond Oxygenation with Inorganic Peroxides10,26
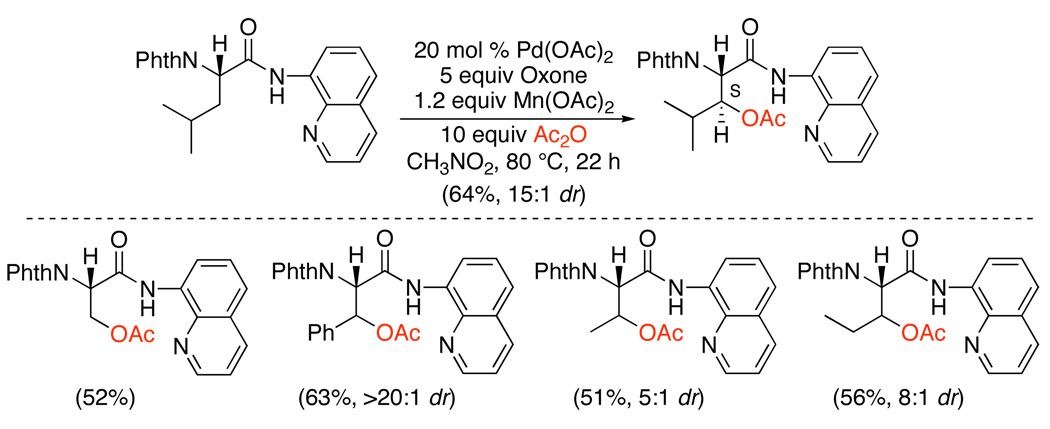
While Oxone and K2S2O8 were highly effective reagents for oxygenation of sp2 C–H bonds, early studies reported only moderate yields with sp3 C–H substrates (eq. 7).10 However, subsequent work showed that the combination of Oxone and Mn(OAc)2 promotes efficient acetoxylation of unactivated 2° sp3 C–H bonds in amidoquinolines (Scheme 11).28 The X-ray structure of a cyclopalladated amidoquinoline showed that both the amide and quinoline moieties were bound to the metal, which may facilitate activation of 2° sp3 C–H bonds. The reaction was applied to a number of amino acid-derived substrates, and generally proceeded in good yield (51–63%) and with high levels of diastereoselectivity (5:1 to >20:1). The authors propose that Mn(OAc)2 reacts with Oxone to afford Mn3O(OAc)7, which then functions as a Lewis acid to increase the reactivity of the PdII catalyst.
Scheme 11.
Acetoxylation of sp3 C–H Bonds with Oxone28
 |
(7) |
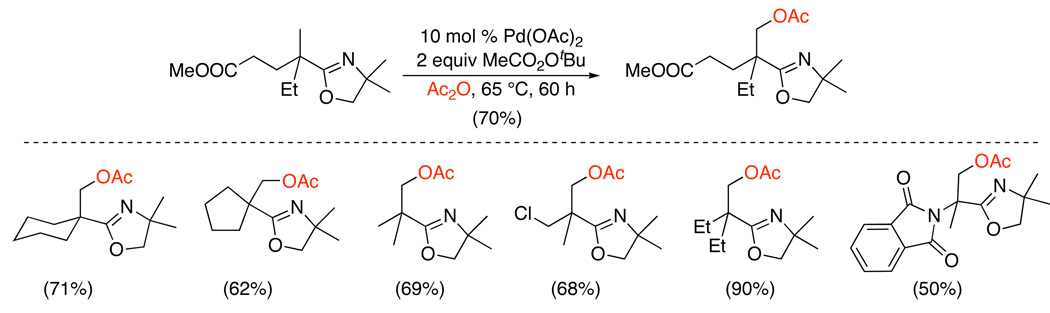
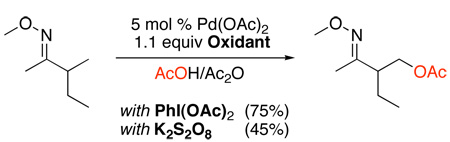
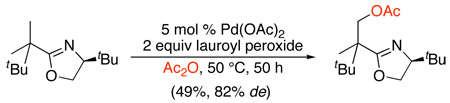
Efficient Pd(OAc)2-catalyzed oxygenation of sp3 C–H bonds has also been achieved using MeCO2Ot-Bu or lauroyl peroxide (Scheme 12).29 The presence of acetic anhydride was essential in this system and was proposed to promote catalytic turnover. Oxazolines were employed as directing groups, and the reaction conditions were compatible with ketals, imides, esters, and alkyl chlorides. Substrates containing diastereotopic methyl groups proximal to a chiral oxazoline directing group afforded acetoxylated products with modest to excellent levels of diastereoselectivity (56:44 to 91:9 dr) (eq. 8). Again, a PdII/IV mechanism was proposed for this system.
Scheme 12.
Oxazoline-Directed sp3 C–H Acetoxylation with MeCO2Ot-Bu or Lauroyl Peroxide29
 |
(8) |
2.4. Dioxygen as a terminal oxidant
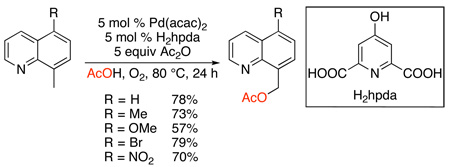
The Pd-catalyzed quinoline-directed acetoxylation of benzylic C–H bonds has recently been achieved using dioxygen as a terminal oxidant (eq. 9).30 This transformation proceeds with Pd(acac)2 as the catalyst in conjunction with a 2,6-pyridinedicarboxylate ligand (H2hpda in eq. 9) in AcOH/Ac2O under an atmosphere of O2. While the reaction is currently restricted to 8-methylquinoline-derived substrates, it is an exciting advance, because O2 is readily available and inexpensive. Furthermore, it raises the intriguing question of whether O2 can be used to generate high oxidation state palladium intermediates.
 |
(9) |
3. Carbon-Sulfur Bond Formation
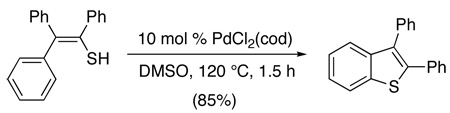
Palladium-catalyzed ligand-directed formation of carbon-sulfur bonds remains relatively rare, and most examples involve intramolecular reactions to generate the C–S linkage. The first report of this type of transformation involved Pd(cod)Cl2-catalyzed conversion of electronically diverse thioenols to substituted benzothiophenes (eq. 10).31 While the detailed mechanism remains to be elucidated, the authors invoked the involvement of a disulfide intermediate (formed in situ by oxidation of the starting material with DMSO).32,33 This disulfide was then proposed to undergo oxidative addition to Pd0, cyclometalation at PdII, and finally C–S bond-forming reductive elimination to release the product. The proposed mechanism is supported by the observation of traces of disulfides in the final reaction mixture, as well as by the demonstration that isolated disulfides undergo smooth Pd-catalyzed annulation to form benzothiophene products.
 |
(10) |
A similar intramolecular C–S bond-forming reaction has been applied to the synthesis of benzothiazoles from thiobenzanilides using catalytic Pd(cod)Cl2 in combination with CuI and Bu4NBr (eq. 11).34 While the role of the additives is not well understood, dramatically lower yields were obtained in their absence (35% without CuI and 3% without NBu4Br). More recently, the related transformation of N-arylthioureas to benzothioxoles was reported using catalytic Pd(PPh3)4 along with O2/MnO2 as oxidants.35 In both cases, PdII/0 mechanisms were proposed, but further studies will be required to delineate the details.
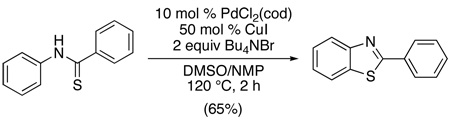 |
(11) |
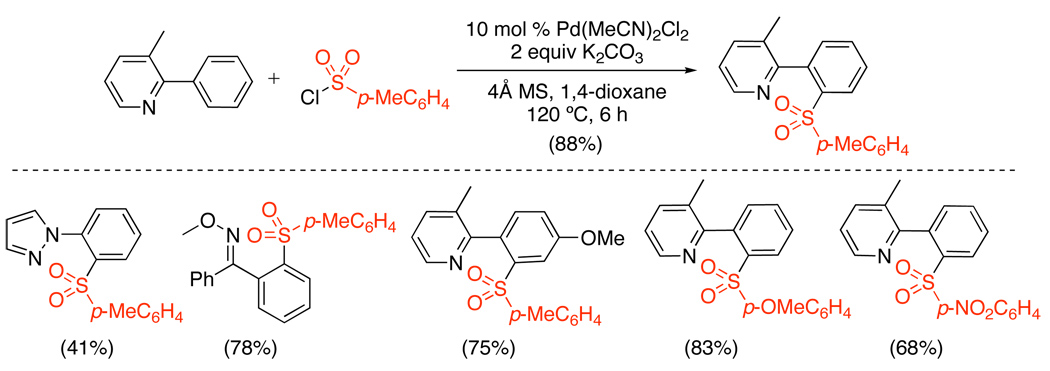
To date, the only example of intermolecular Pd-catalyzed C–H activation/C–S bond formation employed ArSO2Cl as the sulfonating reagent.36 Arylpyridine, arylpyrazole, and aryloxime ether substrates were converted to diarylsulfones using catalytic Pd(MeCN)2Cl2 and stoichiometric ArSO2Cl (Scheme 13). The reaction was tolerant of electron donating (p-MeO) and electron withdrawing (p-CF3) substituents on both the organic substrate and the sulfonyl chloride; however, aliphatic sulfonyl chlorides decomposed under the reaction conditions. Interestingly, tuning of the solvent, co-catalyst, and reaction temperature led to competing C–H chlorination and/or arylation (vide infra). Both PdII/0 and PdII/IV mechanisms are plausible for this transformation.
Scheme 13.
Intermolecular C–S Bond Formation with ArSO2Cl36
4. Carbon-Halogen Bond Formation (Halogen = Cl, Br, I)
The earliest report of Pd-catalyzed ligand-directed C–H halogenation involved the ortho-chlorination of azobenzene with Cl2.37 As shown in eq. 12, this reaction afforded a mixture of mono-, di-, tri-, and tetra-chlorinated products. While this work from 1970 elegantly demonstrated the viability of such transformations, the requirement for Cl2 as the oxidant limited its widespread application in organic synthesis. As such, recent work has focused on the use of more practical halogenating reagents (X+).38 These transformations are described below, and are organized based on the X+ reagent used in the reaction.
| (12) |
4.1. N-halosuccinimides
A 2001 patent by Kodama and coworkers demonstrated that the combination of Pd(OAc)2 and N-iodosuccinimide promotes the ortho-iodination of benzoic acids.39 In 2004, similar conditions were applied to the chlorination and bromination of benzo[h]quinoline (eq. 13, i). Interestingly, use of the more reactive “Cl+” source PhICl2 led to chlorination at the 5-position (eq. 13, ii) in lieu of the quinoline-directed transformation.8,40
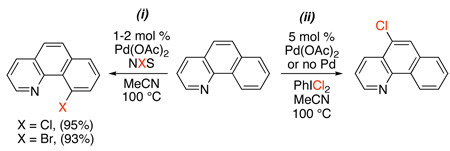 |
(13) |
Pd-catalyzed directed sp2-halogenation with N-halosuccinimides has subsequently been expanded to a wider variety of directing groups, including pyridines, oxime ethers, isoquinolines, amides, and isoxazolines.40,41 In substrates with two available ortho C–H bonds, modest yields were obtained, and competitive di-ortho-halogenation was typically observed. The di-ortho-halogenated products could generally be isolated in high yields utilizing excess oxidant (2.5 equiv), unless a steric bias was introduced into the substrate (for example, the 3-methyl group in 3-methyl-2-phenylpyridine).
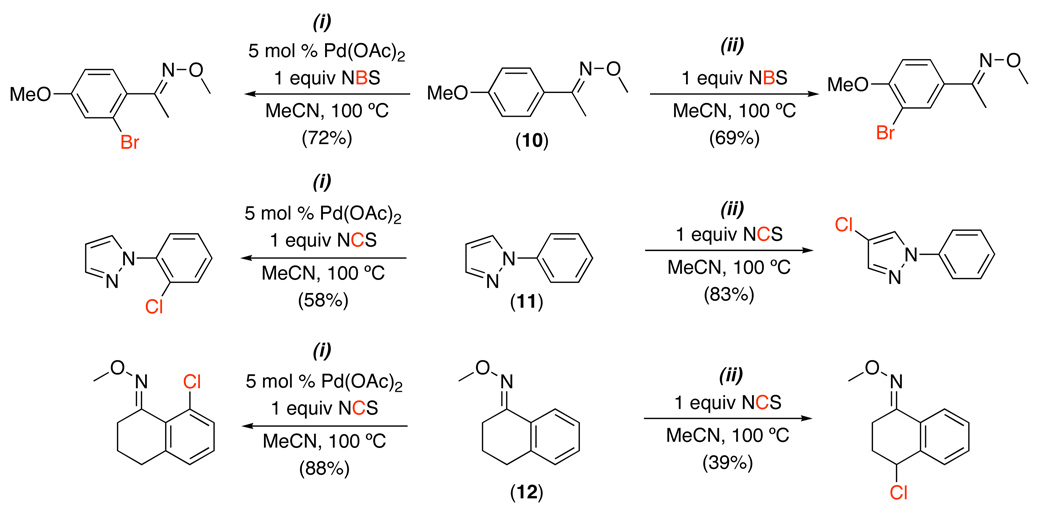
The utility of this methodology is highlighted by substrates that afford different halogenated products in the presence versus the absence of a Pd catalyst.40 For example, with 10–12, ortho-halogenated products were obtained selectively under Pd catalysis (Scheme 15, i). However, in the absence of Pd, products of electrophilic aromatic substitution or benzylic halogenation were generated (Scheme 15, ii). This demonstrates the complementarity of Pd-catalyzed C–H functionalization to more traditional organic halogenation processes.
Scheme 15.
Complementary Halogenation Products in Presence/Absence of Pd40
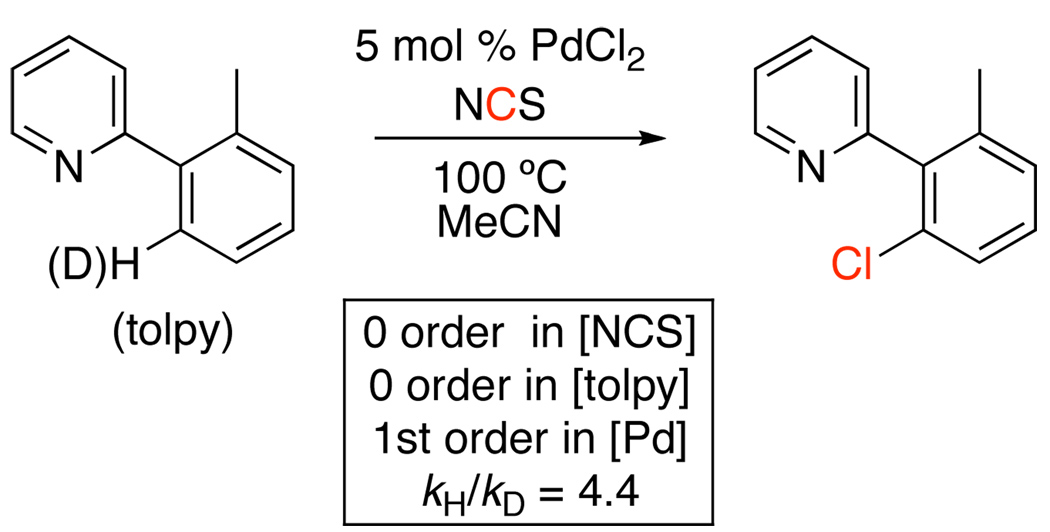
Kinetic studies of the PdCl2-catalyzed chlorination of 2-ortho-tolylpyridine (tolpy) in MeCN showed that the reaction is 1st order in [Pd] and zero order in both [tolpy] and [NCS].20 In addition, a large intermolecular kinetic isotope effect (kH/kD = 4.4) was observed (Figure 1). On the basis of this data, trans-Pd(tolpy)2(Cl)2 was proposed as the catalyst resting state, and cyclopalladation was proposed to be the turnover-limiting step. The zero order dependence on [tolpy] is in interesting contrast to the analogous C–H acetoxylation reactions (Scheme 8).20 This result suggests that cyclopalladation occurs at Pd centers with very different ligand environments in these two reactions.
Figure 1.
Key Mechanistic Data for PdCl2-Catalyzed Chlorination 2-ortho-Tolylpyridine with NCS20
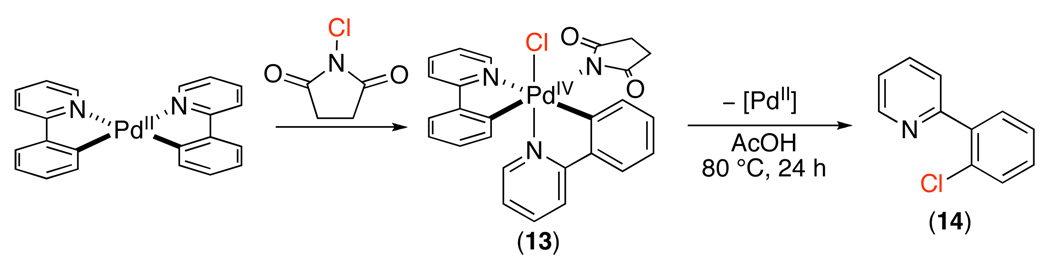
Model studies have been conducted to gain insights into the steps after cyclopalladation in the catalytic cycle. For example, the stoichiometric oxidation of PdII model complex (phpy)2PdII with NCS afforded the PdIV oxidative addition adduct 13. Furthermore, complex 13 underwent C–Cl bond-forming reductive elimination to form 14 at 80 °C (Scheme 16).42 These experiments demonstrate that NCS is a sufficiently strong and kinetically reactive oxidant to promote the oxidation of PdII to PdIV. In addition, these studies illustrate the viability of C–Cl bond-forming reductive elimination from PdIV.
Scheme 16.
Oxidation with N-Chlorosuccinimide and C–Cl Bond-Forming Reductive Elimination from a PdIV Model Complex42
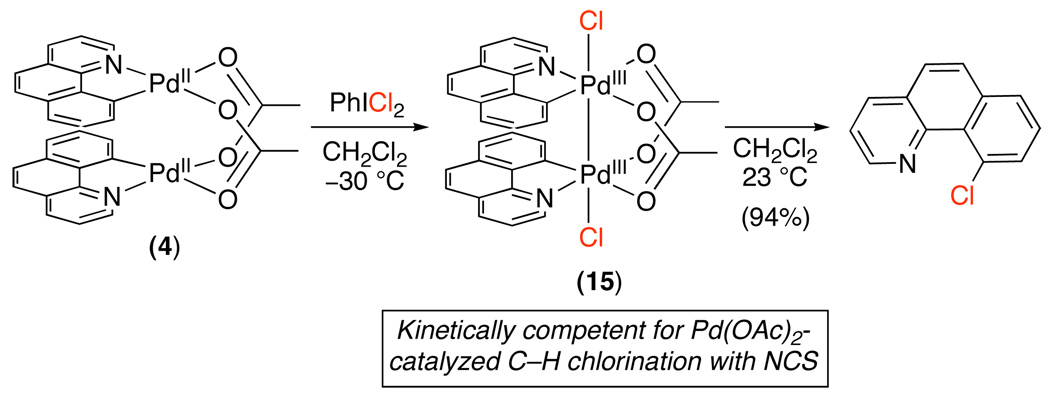
More recent investigations have examined the oxidation of cyclometalated benzo[h]quinoline dimer 4, a proposed intermediate in Pd-catalyzed C–H chlorination.18 Reaction of 4 with PhICl2 resulted in a net two electron oxidation reaction to form symmetrical PdIII~PdIII dimer 15 (Scheme 17). Additionally, this dimer underwent C–Cl bond-forming reductive elimination in high yield at room temperature. While PhICl2 is not a useful oxidant for catalytic C–H chlorination, complex 15 was also shown to be a kinetically competent intermediate in the Pd-catalyzed chlorination of benzo[h]quinoline with NCS. This report, along with a number of other recent studies,16,17,20,43,44 suggests that dimeric or multimeric high oxidation state Pd intermediates are possible in C–H oxidation reactions, particularly when bridging carboxylate ligands could be present.
Scheme 17.
Oxidation of 4 with PhICl218
4.2. CuX2
Pd-catalyzed sp2 C–H halogenation has also been achieved using copper halides as terminal- or co-oxidants. An early study showed that 2-phenyl-3-methylpyridine reacts with CuCl2 in MeCN at 100 °C to afford ortho-chlorinated product 16 in modest (30%) yield (eq. 14).41 More recently, the combination of CuCl2/Cu(OAc)2 has been used for the Pd-catalyzed ortho-chlorination of acetanilides.45 While unselective chlorination of these electron rich substrates was observed with NCS (eq. 15, i), the use of CuCl2/Cu(OAc)2 afforded selective ortho-functionalization for various substituted anilides (eq. 15, ii). A two electron PdII/IV mechanism similar to that in Scheme 7 was suggested, based on the observed catalytic and stoichiometric reactivity of an isolable palladacycle of acetanilide. The formation of closely related PdIII~PdIII dimers should also be considered in this system,16,20,43,44,17 and a one-electron oxidation mechanism involving monomeric PdIII intermediates (Scheme 2) is also possible, particularly with these one-electron Cu-based oxidants.46
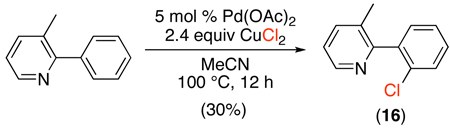 |
(14) |
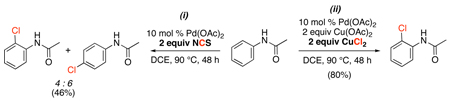 |
(15) |
Pyridine-directed chlorination with CuCl2 has also been achieved using catalytic Pd(MeCN)2Cl2 and stoichiometric ArSO2Cl in DMF (eq. 16).36 Though only four arylpyridine substrates were examined, all underwent ortho-chlorination in high yield (71–85%). Mechanistic studies are not yet available, but the authors speculate that ArSO2Cl reacts with DMF to form an amidinium arenesulfonate salt,47 which may play a role in the chlorination reaction.
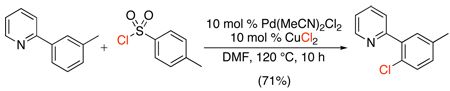 |
(16) |
4.3. Suárez (XOAc) reagents
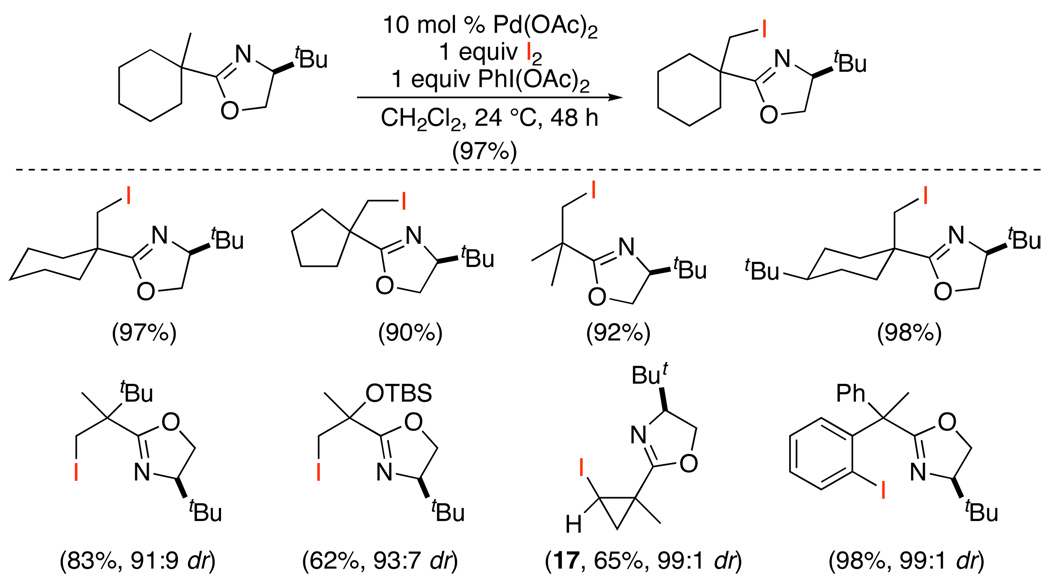
Suárez-type reagents (XOAc) have also been employed for the halogenation of sp2 and sp3 C–H bonds. The use of these reagents was pioneered by Yu, who initially demonstrated that the combination of I2 and PhI(OAc)2 (which generates IOAc in situ) is effective for Pd(OAc)2-catalyzed oxazoline-directed iodination of unactivated alkyl groups (Scheme 18).48 When different types of sp3 C–H bonds were proximal to the directing group, iodination generally occurred selectively at 1° sites, similar to the acetoxylation reactions discussed above. Cyclopropane-containing substrates like 17 were a notable exception to this trend and reacted at a 2° sp3 C–H bond on the ring. The use of chiral directing groups provided iodinated products with good to excellent levels of diastereoselectivity (91:9 to 99:1 dr).
Scheme 18.
sp3 C–H Iodination with IOAc (Formed in situ from PhI(OAc)2/I2)48
Reactions with IOAc were also proposed to proceed via a PdII/IV mechanism, with the key product-forming step involving C–I bond-forming reductive elimination from PdIV. The stoichiometric oxidation of PdII species with IOAc has not been studied in detail. However, mechanistic studies of the catalytic iodination of 18 revealed a modest intramolecular 1° kinetic isotope effect (kH/kD = 1.5) and a Hammett ρ value of −1.6 upon substitution of X (eq. 17).49 On this basis, C–H bond cleavage was proposed to proceed via an electrophilic mechanism.
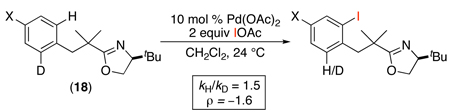 |
(17) |
A similar protocol was developed for the carboxylic acid-directed iodination and bromination of sp2 C-H bonds with XOAc.50 In the presence of catalytic Pd(OAc)2 and 2 equiv of IOAc, substrates with two available ortho-C–H bonds underwent dihalogenation in high yields (eq. 18, ii). The addition of tetraalkylammonium salts could be used to curb this reactivity, and selective mono-halogenation was achieved in the presence of 1 equiv of Bu4NX (eq. 18, i). The tetrabutylammonium carboxylate ion pair is believed to be crucial for reactivity and selectivity in this reaction; however, the exact nature of its interaction with the Pd catalyst has yet to be elucidated.
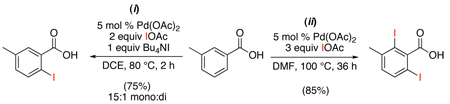 |
(18) |
4.3 Electrochemical oxidation
Electroorganic synthesis techniques have also been utilized to achieve selective halogenation of aryl C–H bonds.51 This unique method of oxidation utilizes aqueous HX as the halogen source in a divided cell with two platinum electrodes. A variety of arylpyridine and arylpyrimidine substrates underwent PdX2-catalyzed chlorination and bromination (X = Cl or Br, respectively) to afford ortho-halogenated products in excellent yields, and the organic products could be isolated in pure form by a simple extraction process. With highly electron rich substrates like 2-(2-methoxyphenyl)pyridine, the site selectivity of halogenation could be controlled by tuning the electric current (and thereby the rate of X+ generation).
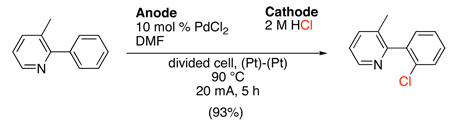 |
(19) |
5. Carbon-Fluorine Bond Formation
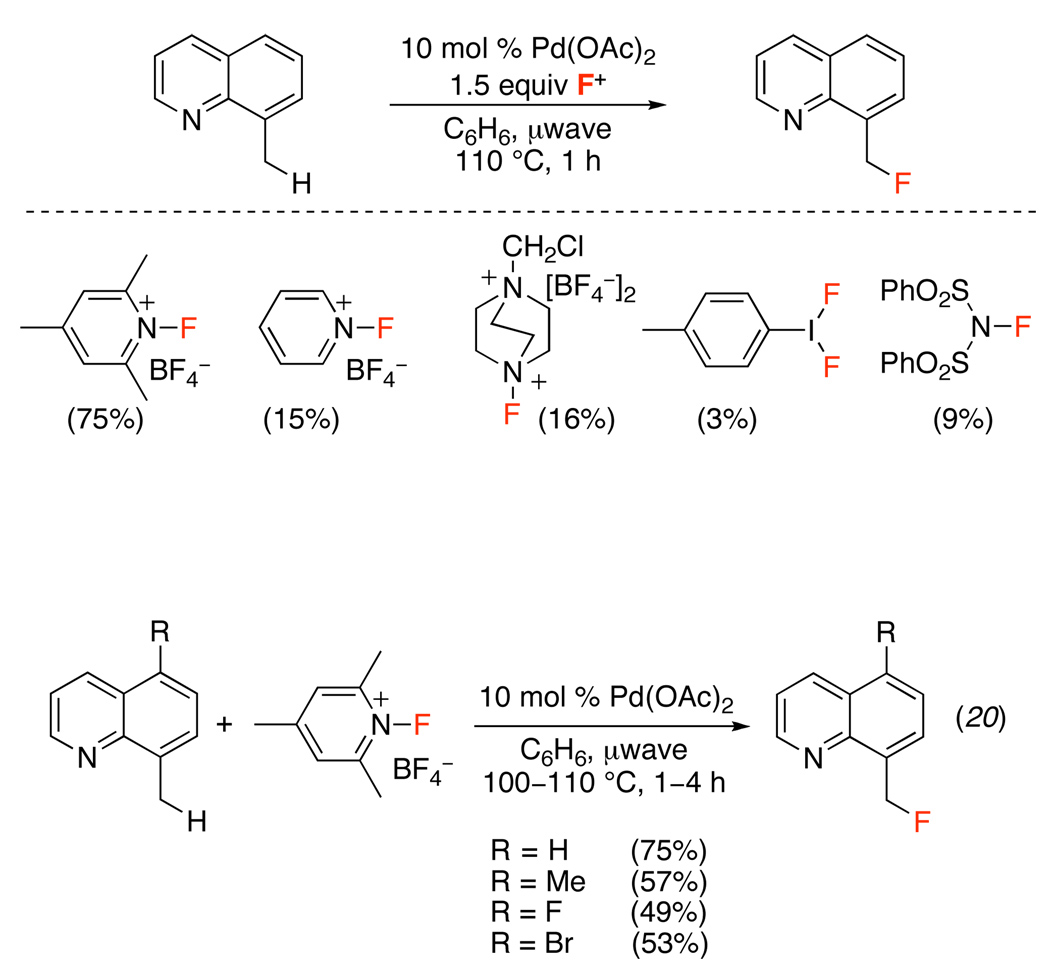
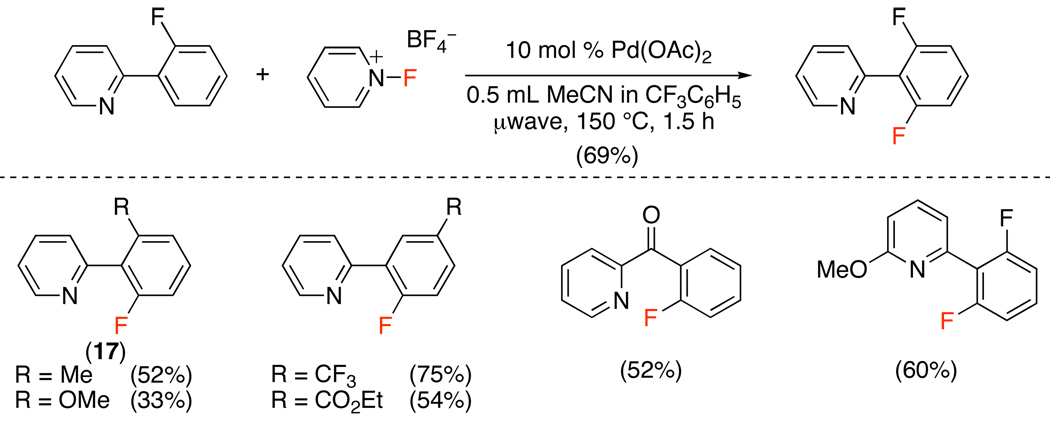
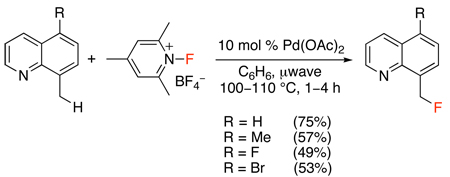
Only two examples of Pd-catalyzed ligand-directed C–H fluorination have been reported to date.52,53 The first study demonstrated that Selectfluor®, N-fluoro-2,4,6-trimethylpyridinium tetrafluoroborate, N-fluoropyridinium tetrafluoroborate, 4-iodotoluenedifluoride, and N-fluorodisulfonamide could all be used for the Pd(OAc)2-catalyzed benzylic fluorination of 8-methylquinoline (Scheme 19). Extensive optimization revealed that N-fluoro-2,4,6-trimethylpyridinium tetrafluoroborate was the best electrophilic fluorinating reagent for this transformation and that microwave heating decreased the reaction time and minimized formation of undesired side products (eq. 20). An analogous transformation (using N-fluoropyridinium tetrafluoroborate) was developed for the ortho-fluorination of 2-arylpyridines (Scheme 20).
Scheme 19.
Electrophilic Fluorinating Reagents for the Benzylic Fluorination of 8-Methylquinoline52
Scheme 20.
Pd(OAc)2-Catalyzed ortho-Fluorination of 2-Arylpyridines with N-Fluoropyridinium Tetrafluoroborate52
 |
(20) |
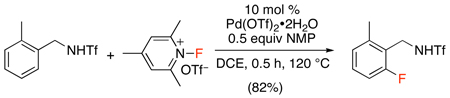
More recent work expanded the scope of catalytic C–H fluorination to benzylamine-based substrates.53 N-Fluoro-2,4,6-trimethylpyridinium triflate was used as the “F+” source along with N-methylpyrrolidinone (NMP) as a key promoter for the Pd(OTf)2-catalyzed fluorination of triflamide-protected benzylamines (eq. 21). This is a valuable expansion of prior work because triflamides can be converted to a broad range of synthetically useful functional groups including benzaldehydes, benzylamines, nitriles, and benzylmalonates, thereby providing access to a variety of functionalized aryl fluoride-containing products.
 |
(21) |
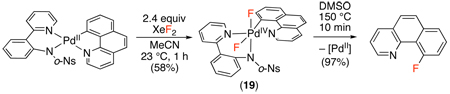
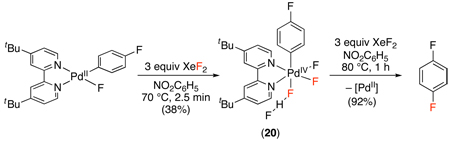
Both of these reports suggested that C–F bond formation might proceed via a PdII/IV mechanism similar to that in Scheme 7, and subsequent work has provided evidence in support of this proposal.54,55 These studies demonstrated the stoichiometric oxidation of PdII aryl complexes with electrophilic fluorinating reagents. In both cases, stable PdIV products (19 in eq. 2254 and 20 in eq. 2355) were isolated and characterized by X-ray crystallography. Furthermore, both species underwent C–F bond formation under conditions milder than those in the catalytic reaction. While 19 and 20 are clearly model complexes (rather than actual catalytic intermediates in the C–H fluorination reactions above), these studies offer evidence for the viability of both the oxidation and C–F bond forming steps of a PdII/IV catalytic cycle for C–H fluorination.
 |
(22) |
 |
(23) |
6. Carbon-Nitrogen Bond Formation
Palladium-catalyzed ligand-directed C–H functionalization has also been utilized for the construction of C–N bonds, which are important features of many biologically active molecules.56,57 Reactions in this class of bond formation fall into two general categories: those where the C–N bond is formed intramolecularly to generate a heterocycle and those where the nitrogen group is delivered from an external reagent.
6.1. Intramolecular C–N bond formation
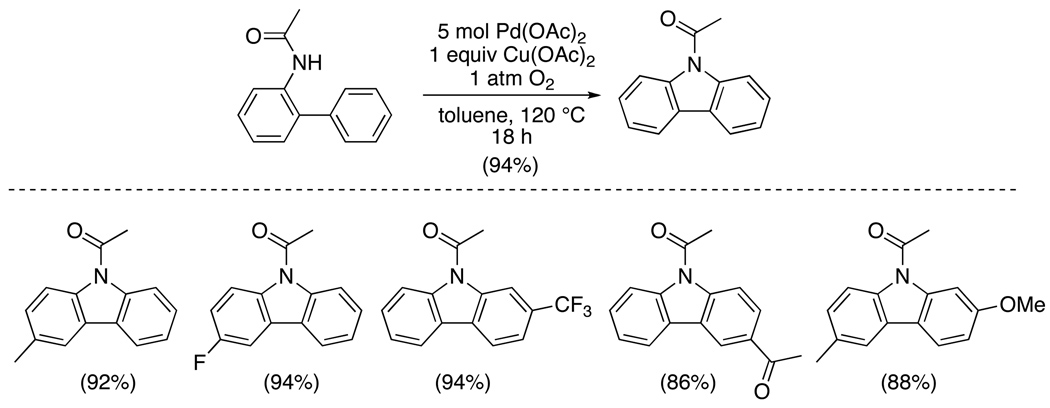
An early example of Pd-catalyzed directed C–H amination involved the construction of carbazoles via intramolecular C–N coupling.58 2-Phenylacetanilides underwent Pd(OAc)2-catalyzed cyclization with Cu(OAc)2/O2 (in toluene)58 or O2 (in DMSO)59 as the oxidant (Scheme 21). An acetyl protecting group on the amine was generally necessary for efficient reaction, although modest yield (12%) was recently reported for a similar reaction with a simple aniline using Pd/C as the catalyst.60
Scheme 21.
Intramolecular C–H Amination to Form Carbazoles58
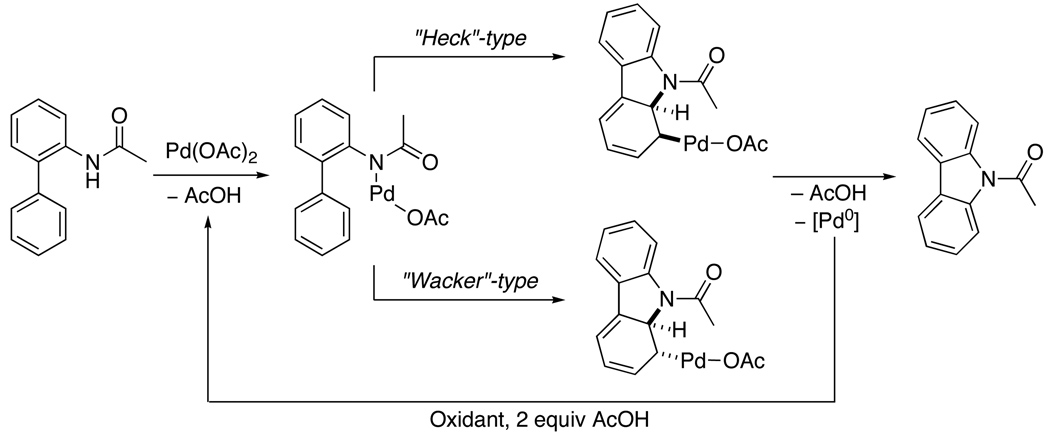
Two possible PdII/0 mechanistic pathways were proposed for this carbazole synthesis (Scheme 22). The first involves coordination of Pd to the amide nitrogen followed by a Heck-type cyclization/β-hydride elimination sequence. A second proposed pathway proceeds via amide-directed Wacker-type cyclization/β-hydride elimination to generate the carbazole.
Scheme 22.
An alternative approach to carbazoles involves the Pd(OAc)2-catalyzed cyclization of N-alkylanilines in the presence of PhI(OAc)2 (eq. 24).61 In contrast to the reactions in Scheme 21, this transformation proceeds efficiently at room temperature, and its substrate scope is highly complementary, allowing synthesis of both N-alkyl and N-allylcarbazoles containing diverse aromatic substituents including aryl iodides (eq. 24). These distinguishing features are believed to result from a difference in mechanism, and a PdII/IV pathway involving intermediate 21 (eq. 24) was proposed.
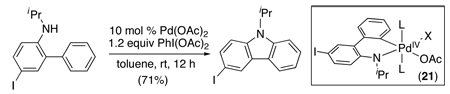 |
(24) |
Tosylhydrazones have been used to direct intramolecular ortho-amination to form indazoles.62 In these systems, Pd(OAc)2 served as the catalyst in conjunction with 1 equiv of Cu(OAc)2 and 2 equiv of AgO2CCF3 (eq. 25). In substrates with two electronically distinct aryl rings, modest to excellent selectivity was observed for cyclization on the more electron rich arene. Interestingly, meta-Br substitution was well-tolerated; however, substrates containing ortho-Br groups underwent facile Ar-Br oxidative addition. A similar enamine-directed intramolecular C–H amination was utilized to construct diarylindoles (eq. 26).63 In both of these reactions, PdII/0 pathways are likely.
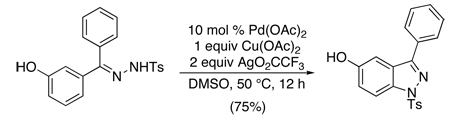 |
(25) |
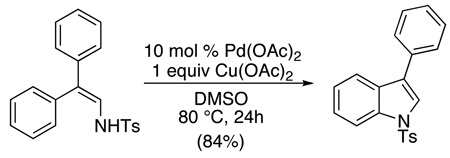 |
(26) |
Very recently, phenethyltriflamides have been used as precursors to indolines (eq. 27).64 N-Fluoro-2,4,6-trimethylpyridinium triflate was employed as the terminal oxidant for these cyclizations, leading to high yields of aminated products and minimal competing fluorination. The reaction showed good functional group compatibility, tolerating halogen, acetyl, cyano, and nitro substituents on the aromatic ring. However, attempts to prepare the corresponding 6-membered tetrahydroquinolines via analogous cyclizations resulted in low yields (28–30%).
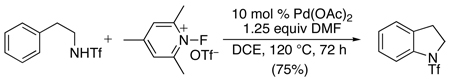 |
(27) |
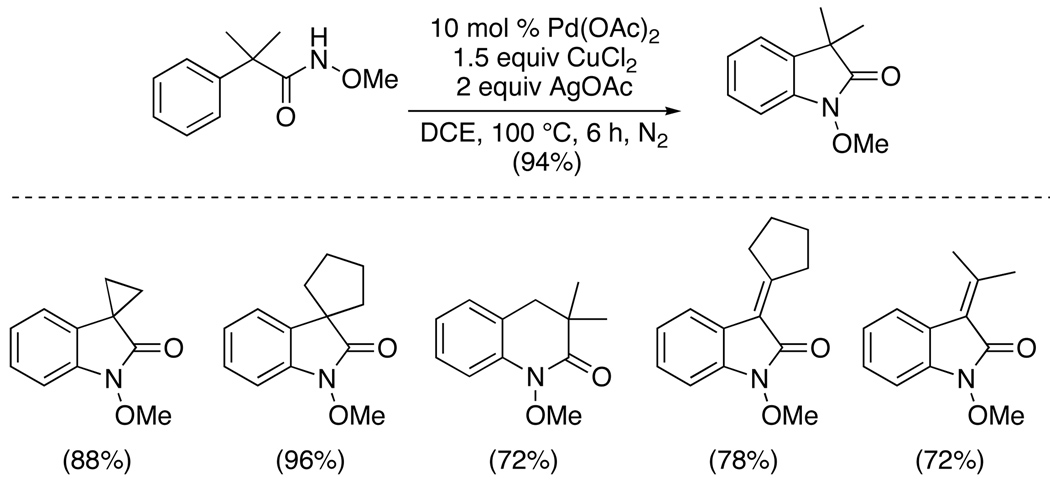
Finally, lactams have been prepared via the intramolecular amination of amides.65 This reaction required the use of catalytic Pd(OAc)2 in combination with 1.5 equiv of CuCl2 and 2 equiv of AgOAc. Diverse β, γ and δ-lactams were formed in high yields; however, the reaction was limited to substrates with a high degree of substitution α to the amide directing group, suggesting that the Thorpe-Ingold effect may play a key role in cyclization. Preliminary investigations implicated a PdII/IV catalytic cycle; however, further studies will be required to obtain a complete mechanistic picture.
6.2. Intermolecular C–N bond formation
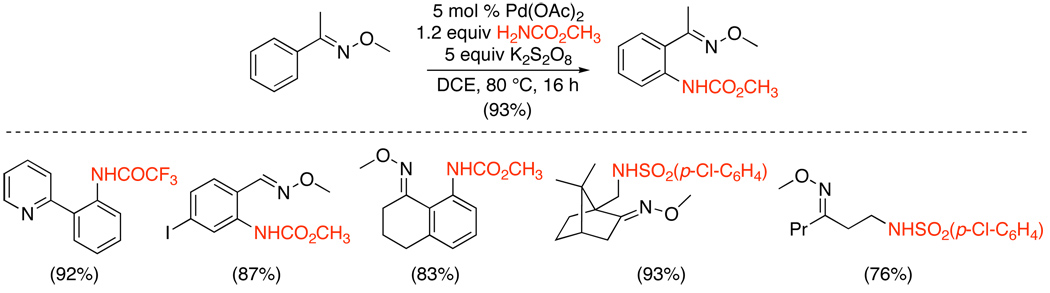
The only example of Pd-catalyzed intermolecular directed C–H amination reported to date involves the functionalization of sp2 and sp3 C–H bonds in pyridine and oxime ether substrates (Scheme 24).66 In this system, Pd(OAc)2 served as the catalyst, and a combination of K2S2O8 and NH2R was used to introduce the nitrogen functionality. Electron deficient primary amides including carbamates, acetamides, and sulfonamides all proved effective as “NHR” sources.
Scheme 24.
Intermolecular Amination of Pyridines and Oxime Ethers66
Mechanisms involving cyclopalladation followed by either direct nitrene insertion or oxidation to a PdIV imido intermediate were proposed. To probe the possibility of a transient nitrene intermediate, benzamide was submitted to the reaction conditions in the presence of MeOH (eq. 28). The major organic product was methyl-N-(2-methoxyphenyl)carbamate (23), which is consistent with a sequence involving: (i) initial generation of a nitrene, (ii) Curtius rearrangement to form the corresponding isocyanate, (iii) trapping with MeOH to generate methyl carbamate 22, and finally (iv) Pd-catalyzed directed C–H oxygenation10 to form 23 (eq. 28). This experiment suggested the viability of a nitrene mechanism; however, the possible intermediacy of a PdIV imido complex (which has been suggested in related stoichiometric reactions of palladacycles)67 could not be excluded.
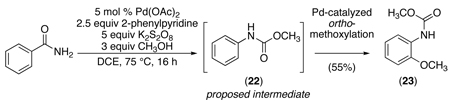 |
(28) |
7. Carbon-Carbon Bond Formation
The most widely studied area in this field is Pd-catalyzed ligand-directed C–H activation followed by C–C coupling to afford ortho-arylated, alkenylated, alkylated, or carbonylated products. Pioneering studies between 1997 and 2004 demonstrated that alcohols, imines, amides, and aldehydes could serve as directing groups for PdII/0 catalyzed ortho-arylation, alkenylation, and alkylation. This and related early work has been extensively reviewed;68,69,70 therefore, the current discussion focuses on advances in the field starting in 2005.
7.1. C–H arylation with prefunctionalized arylating agents
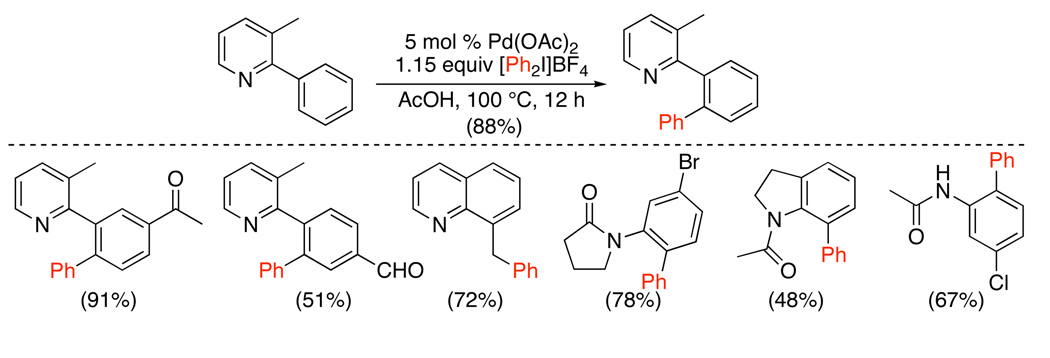
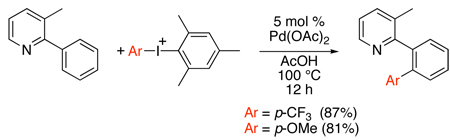
A first key development in 2005 was the demonstration that Pd(OAc)2 catalyzes the ortho-phenylation of diverse arylpyridines, quinolines, pyrrolidinones, oxazolidinones, and benzodiazepines with diphenyliodonium salts (Scheme 25).71,72,73 The unsymmetrical mesityl/aryl-substituted iodonium reagents [Mes–I–Ar]BF4 could be used to install different aryl groups (eq. 29). The large mesityl substituents served as “dummy ligands” on the iodine(III), and the smaller aryl group was transferred with high selectivity (>99 : 1 in most cases). Generally, faster reaction rates and higher yields were observed with electron deficient aryl groups.
Scheme 25.
Pd(OAc)2 Catalyzed sp2 C–H Phenylation with [Ph2I]BF471
 |
(29) |
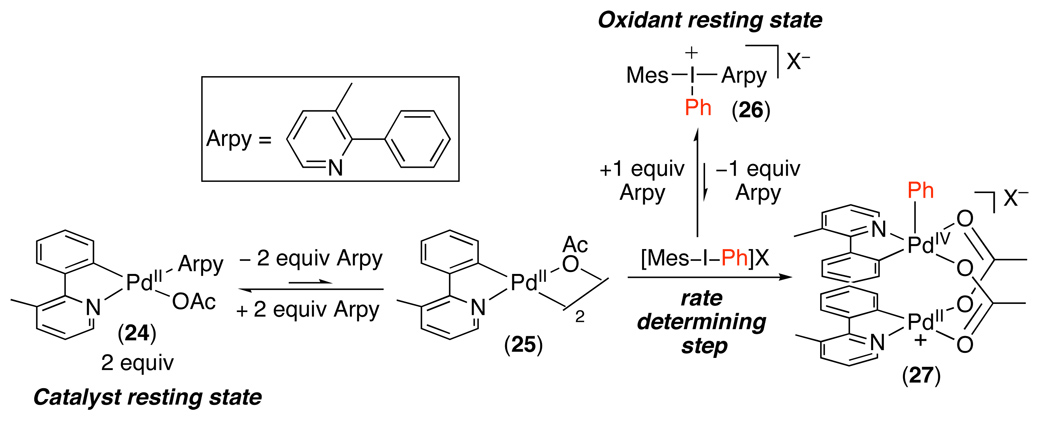
These reactions were initially proposed to proceed by a PdII/IV mechanism analogous to that in Scheme 7.71 Subsequent studies provided more detailed insights into the catalyst resting state, the turnover-limiting step, and the ligand environment of high oxidation state Pd intermediates in this transformation (Scheme 26).17 These investigations showed that, with 2-phenyl-3-methylpyridine as the substrate, the catalyst resting state is monomeric PdII species 24 and the oxidant resting state is arylpyridine-coordinated iodine(III) reagent 26. Pre-equilibrium dissociation of the arylpyridine from both 24 and 26 would produce cyclometalated dimer 25 and free [Mes–I–Ph]BF4. The turnover limiting step was then proposed to proceed via oxidation of 25 by [Mes–I–Ph]BF4 to afford dimeric PdIV~PdII adduct 27 (alternatively formulated as a PdIII~PdIII species, depending on the nature of the Pd–Pd interaction).74 Carbon-carbon bond-forming reductive elimination, ligand exchange, and cyclometalation would complete the catalytic cycle.
Scheme 26.
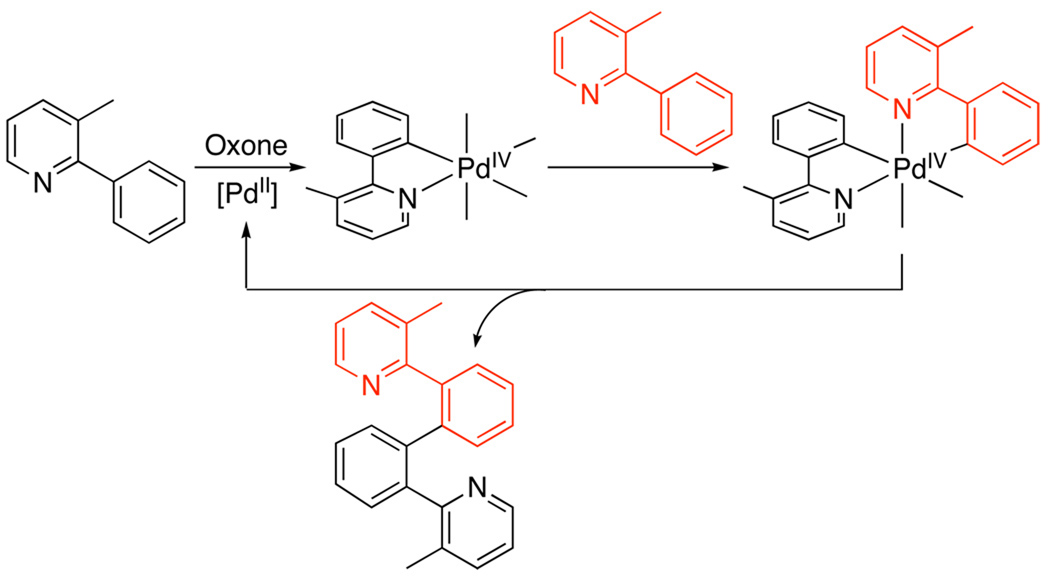
Key Steps in the Proposed Mechanism of sp2 C–H Arylation with [Mes–I–Ph]BF417
Several key experiments provided support for the mechanism in Scheme 26.17 First, the catalytic reaction showed a 1st order dependence on [IIII], a 2nd order dependence on [Pd], and an inverse 3rd order dependence on [2-phenyl-3-methylpyridine] (all consistent with the rate law derived for the sequence in Scheme 26). Second, Hammett studies with para-substituted IIII reagents showed a ρ value of +1.7, consistent with turnover-limiting oxidative addition. Finally, substitution of the ortho-C–H bond with a C–D bond resulted in an intra- but not an intermolecular kinetic isotope effect, indicating that C–H activation occurs after the turnover-limiting step.
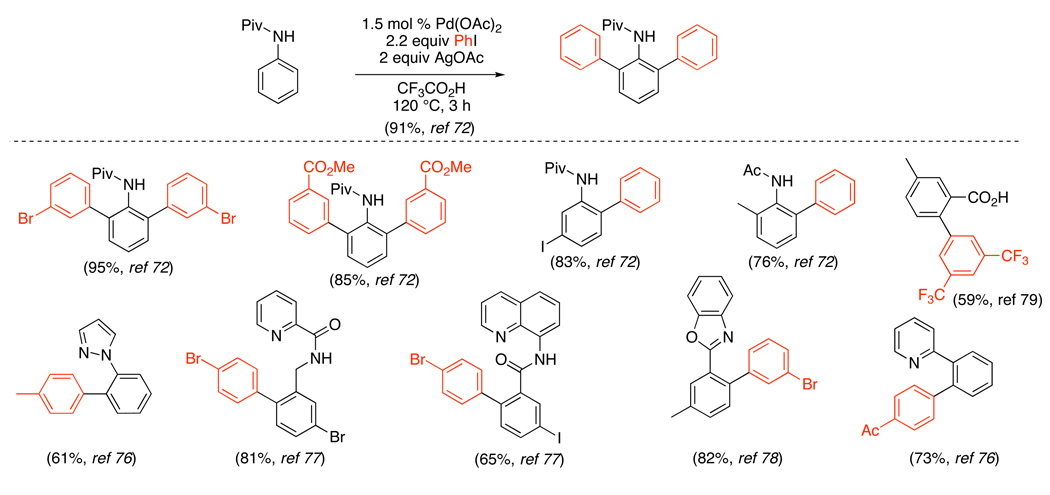
A related Pd(OAc)2-catalyzed sp2 C–H arylation was developed using stoichiometric AgOAc in conjunction with 2–9 equiv of Ar-I.72,75 This method has been applied to the arylation of substituted anilides,72 2-arylpyridines,75,76,77 benzoxazoles,75,78 and benzoic acid derivatives (Scheme 27 and eq. 30).79 Amide and oxime ether-directed versions have also been used as the first step in tandem sequences to generate fluorenones (eq. 31 and eq. 32).80,81 All of these transformations show broad scope and functional group compatibility; most notably, aryl iodides are well-tolerated in the cyclometalating substrate.
Scheme 27.
Pd(OAc)2-Catalyzed sp2 C–H Arylation with AgOAc/Ar-I72,76–79
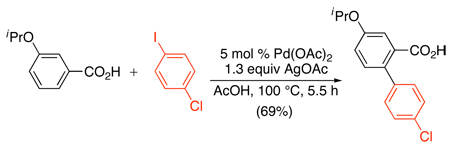 |
(30) |
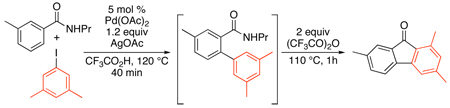 |
(31) |
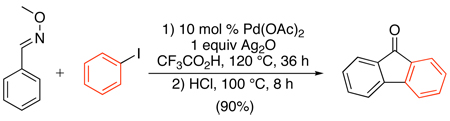 |
(32) |
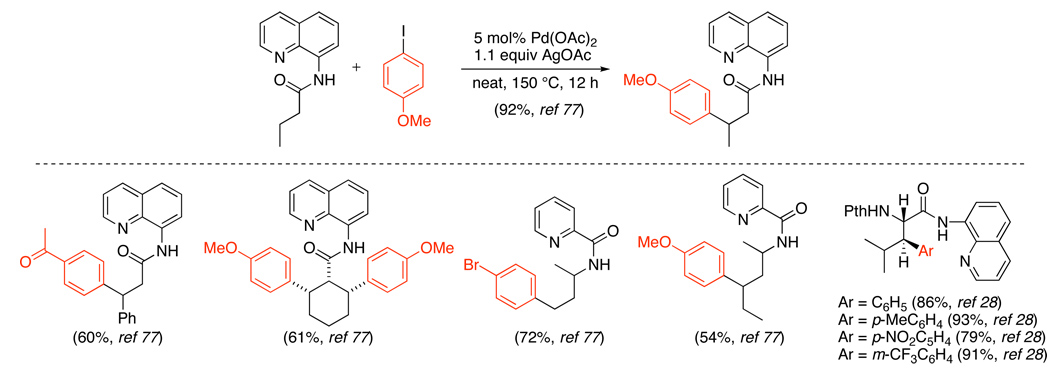
The combination of AgOAc and Ar-I has also been utilized for the Pd-catalyzed arylation of unactivated sp3 C–H bonds (Scheme 28).76,77,28 Directing groups including pyridines, aminoquinolines, and picolinamides were used, and arylation occurred at both 1° and 2° sp3 C–H sites. In substrates containing diastereotopic 2° C–H positions, only one stereoisomer of the product was reported.28
Scheme 28.
The coupling of aryl iodides with sp3 C–H bonds in carboxylic acid derivatives has also been achieved using a slight modification of the above conditions (eq. 33).82 In this system, Ag2CO3 was used as the AgI source in combination with 2 equiv of NaOAc and 1 equiv of K2HPO4. While the reaction yields were generally high, mixtures of mono- and diarylated products (ranging between 3:1 and 5:1) were observed in all cases.
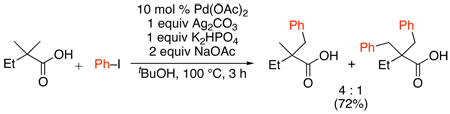 |
(33) |
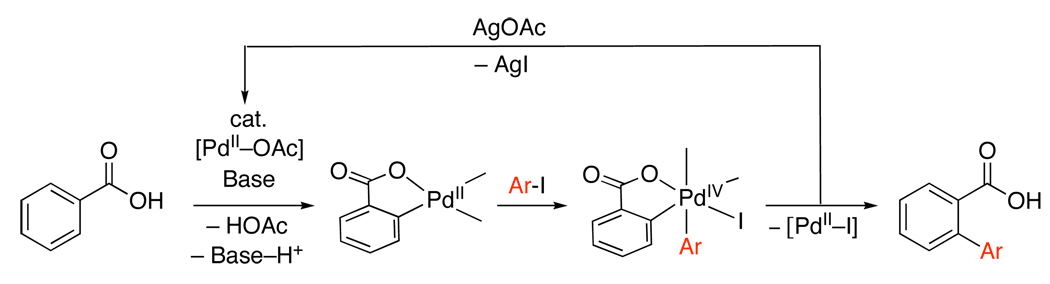
All of these Pd(OAc)2-catalyzed reactions with AgI/ArI have been proposed to proceed by the PdII/IV mechanism outlined in Scheme 29. The only mechanistic studies to date have been conducted with benzoic acid substrates. In these systems, neither of the Pd0 sources Pd2(dba)3 or Pd(tBu3P)2 promoted arylation (eq. 34).79 Furthermore, in the absence of AgOAc, a single turnover was observed (eq. 34), suggesting that the silver salt serves to regenerate the Pd catalyst, rather than activate the Ar-I towards oxidative addition. Intriguingly, qualitative studies also showed that electron rich aryl iodides react faster, which is in contrast to the [Ar2I]BF4 chemistry described above.72,77,83 Though a PdII/IV mechanism was suggested, PdIII radical intermediates have been proposed under similar conditions and cannot currently be ruled out.83,84
Scheme 29.
Proposed Mechanism for Pd-Catalyzed Directed Arylation with AgI/Ar-I79
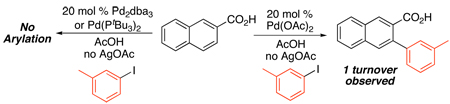 |
(34) |
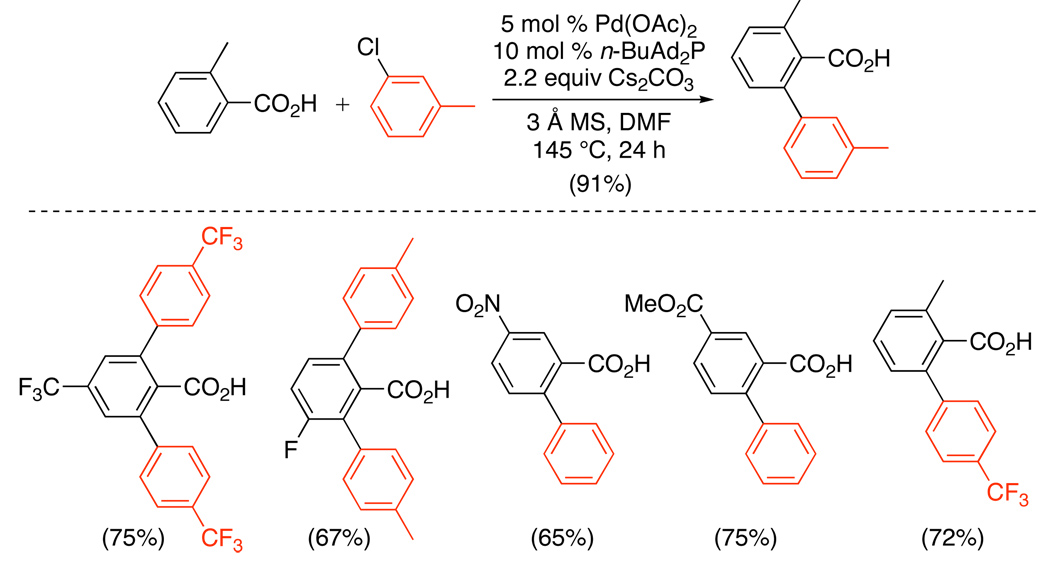
Several PdII/0-catalyzed ortho-arylation methods have also been reported since 2005. For example, the ortho-arylation of benzoic acids with aryl chlorides has been achieved using 5 mol % of Pd(OAc)2, 10 mol % of n-BuAd2P, and 2.2 equiv of Cs2CO3 (Scheme 30). High yields were obtained with electron deficient Ar-Cl and ArCO2H; however, competitive decarboxylation and/or hydrodechlorination was observed with more electron rich substrates. A PdII/0 mechanism was proposed, involving the following steps: (i) oxidative addition of the Ar-Cl at Pd0, (ii) ligand exchange of chloride for benzoate, (iii) directed C–H activation, and (iv) C–C bond-forming reductive elimination to generate the product. A similar procedure has also recently been applied to the ortho-arylation of benzaldehydes with aryl chlorides, using catalytic Pd(OAc)2, an N-heterocyclic carbene ligand, and stoichiometric Cs2CO3 as a base.85
Scheme 30.
PdII/0-Catalyzed ortho-Arylation of Benzoic Acids with Ar-Cl79
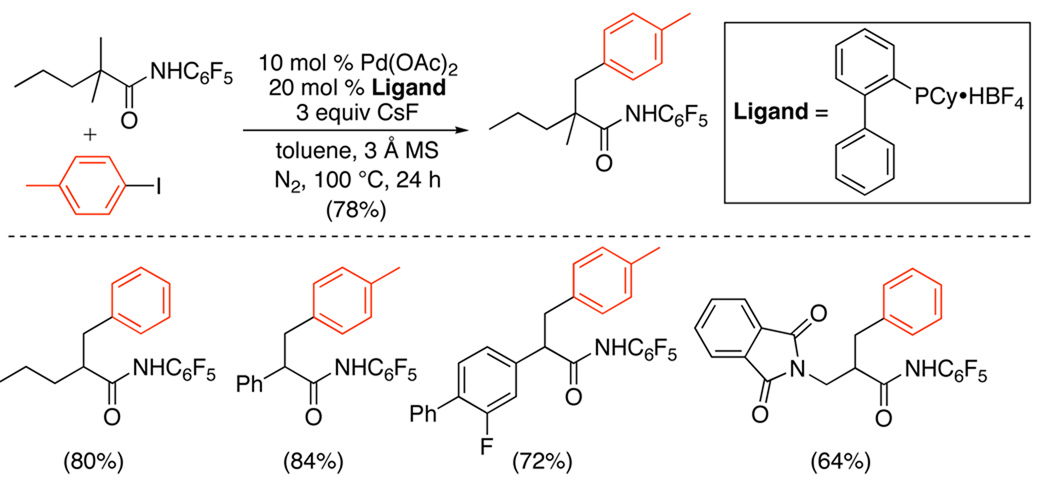
The arylation of sp3 C–H bonds with aryl iodides has been reported using PdII/0 catalysis and phosphine ligands (Scheme 31).86 Pd(OAc)2 was employed as a precatalyst in conjunction with Cy-JohnPhos and CsF to arylate an α-methyl group in N-(perfluorophenyl)acetamides. At least one additional α-substituent (either an alkyl or an aryl group) was generally required to achieve efficient catalysis in these transformations (Scheme 31).
Scheme 31.
PdII/0-Catalyzed sp3 Arylation of Alkylamides with Ar-I86
PdII/0-catalyzed sp3 C–H arylation of carboxylic acids has been achieved with arylboronic esters.82 Pd(OAc)2 was used as the catalyst with benzoquinone as an additive, along with 1.5 equiv of K2HPO4 and 1 equiv of Ag2CO3 (eq. 35). Interestingly, extensive screening revealed that alternative silver oxidants (e.g., Ag2O or AgOAc) and bases (e.g., Na2CO3) afforded <5% yield of the arylated products. While excellent (>98%) selectivity for mono-arylation was observed, the yields were generally modest (20–38%).
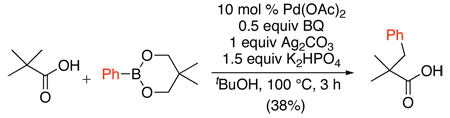 |
(35) |
Boronic acids have also been studied as coupling partners in the Pd-catalyzed ortho-arylation of N-alkyl acetanilides, using Cu(OTf)2 as the oxidant and Ag2O as a key additive (eq. 36).87 A number of commercially available boronic acids proved effective as arylating reagents, particularly those containing electron-donating groups. In contrast, the presence of electron withdrawing substituents or ortho-substitution on the boronic acid resulted in lower yields. A similar reaction with boronic acids was later applied to 2-arylpyridines using 2,2,6,6- tetramethylpiperidine-N-oxyl (TEMPO) as the oxidant.88
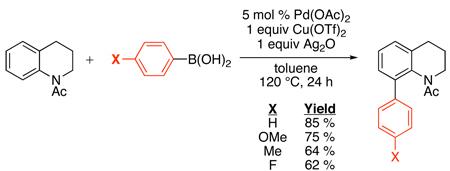 |
(36) |
Arylsilylethers are another class of transmetalating reagents that can be utilized for directed C–H arylation reactions.89,90 For example, the combination of catalytic Pd(OAc)2, 2 equiv of AgF, and 2 equiv of Cu(OTf)2 resulted in clean ortho-arylation of acetanilide substrates with ArSi(OMe)3 (eq. 37). These reactions were proposed to proceed through a PdII/0 manifold. While only trimethoxyphenylsilanes were examined with acetanilides, a variety of different aryl silicon reagents proved effective for the related Pd-catalyzed ortho-arylation of enamides.90
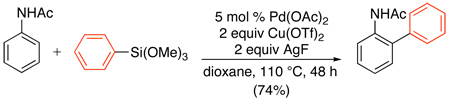 |
(37) |
Finally, aryl acylperoxides have been used to effect pyridine, quinoline, oxime ether, and oxazoline-directed ortho-arylation (eq. 38).91 A mechanism involving decarboxylation to generate aryl radicals (Ar•) followed by reaction of Ar• with cyclopalladated intermediates was proposed. Consistent with this pathway, conducting the catalytic reactions in the presence of ascorbic acid (a potential radical scavenger) led to dramatically decreased conversions to product.
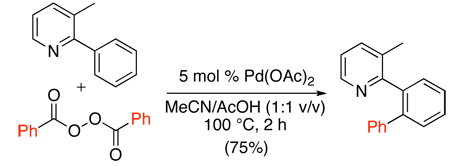 |
(38) |
7.2. C-H arylation without prefunctionalized arylating reagents
A second strategy for Pd-catalyzed ligand-directed C–H arylation involves the direct oxidative coupling of L~C–H with an Ar–H substrate without the requirement for pre-functionalization of either reagent. An early example of this approach involved the Pd(OAc)2-catalyzed dimerization of 2-arylpyridine derivatives using Oxone as the stoichiometric oxidant (eq. 39).92 This homocoupling proceeded in modest to good yield with substituted arylpyridines. With meta-substituents on the aryl ring, low site selectivity was observed, which is in sharp contrast to typical directed C–H activation reactions at PdII.
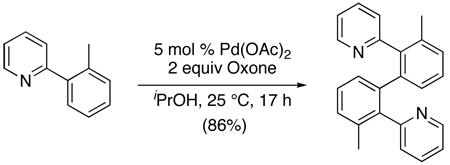 |
(39) |
This unusual selectivity suggested the possibility of an unconventional reaction mechanism. Indeed, detailed investigations provided evidence for a pathway involving the following sequence of steps: (i) initial cyclometalation at PdII, (ii) oxidation of this PdII intermediate to PdIV, (iii) a second cyclometalation at PdIV, and finally (iv) C–C bond-forming reductive elimination to produce the dimeric product (Scheme 32). This appears to be the first example where strong evidence is available in support of C–H activation occurring at a high oxidation state Pd center.
Scheme 32.
Proposed Mechanism for PdII/IV Homocoupling of Arylpyridines92
Pd-catalyzed ligand-directed oxidative cross-coupling reactions have also been described. For example, ferrocenyl oxazolines were selectively ortho-arylated using Pd(OAc)2 as the catalyst, Cu(OAc)2 as the terminal oxidant, K2CO3 as a base, and an arene substrate as the solvent (eq. 40).93
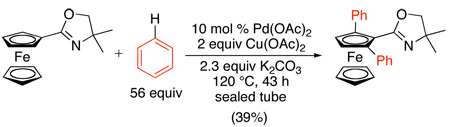 |
(40) |
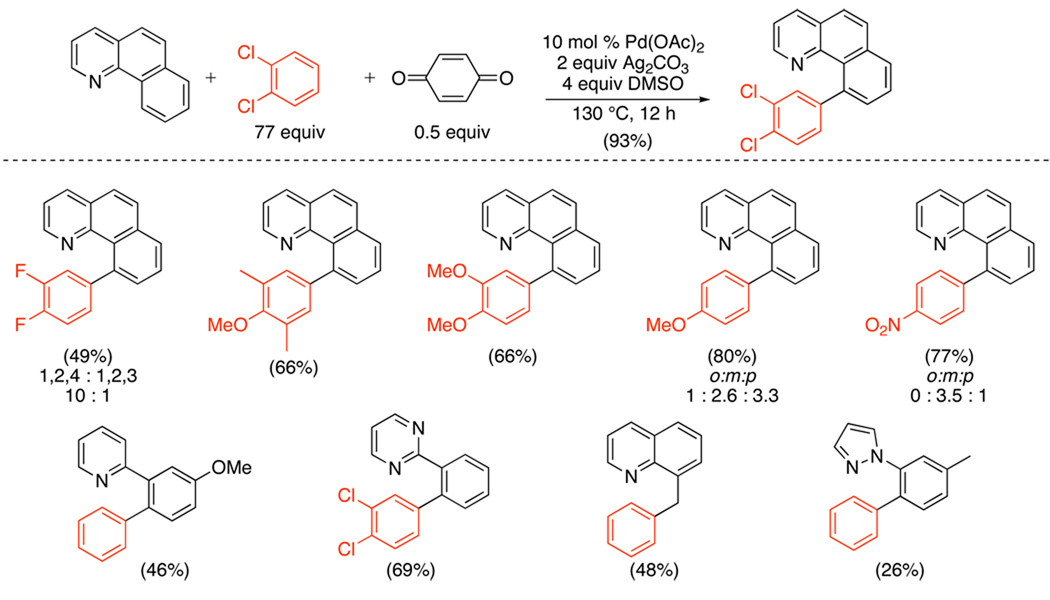
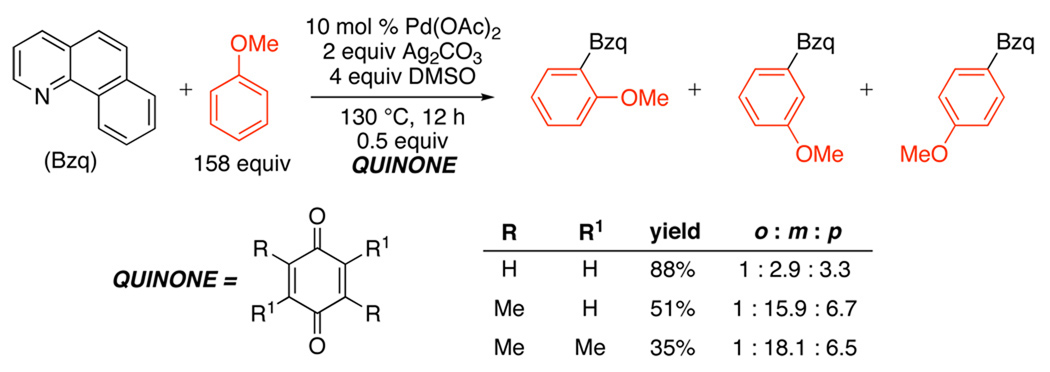
A more general Pd(OAc)2-catalyzed oxidative cross-coupling between L~C–H (L = pyridine, quinoline, pyrimidine, and pyrazole) and Ar–H was subsequently reported (Scheme 33).94 In these transformations, benzoquinone was required as a promoter, and Ag2CO3 was employed as a stoichiometric oxidant. For many 1,2-, 1,3-, and 1,2,3-substituted aromatic substrates, Ar–H activation proceeded with high selectivity at the least sterically hindered site. Site selectivity could be tuned further by changing the structure of the quinone promoter (Scheme 34).
Scheme 33.
Pd-Catalyzed Benzoquinone-Promoted Oxidative L~C–H/Ar–H Cross-Coupling94
Scheme 34.
Tuning Site-Selectivity of L~C–H/Ar–H Cross-Coupling by Variation of Quinone Promoter94
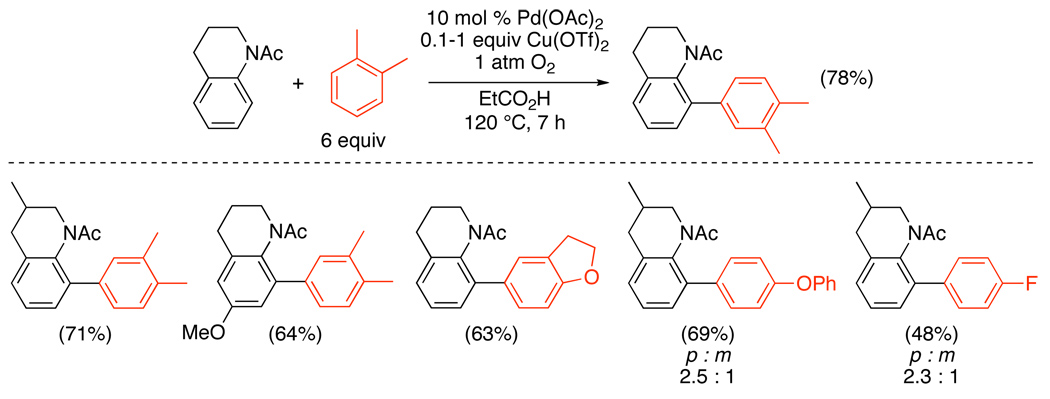
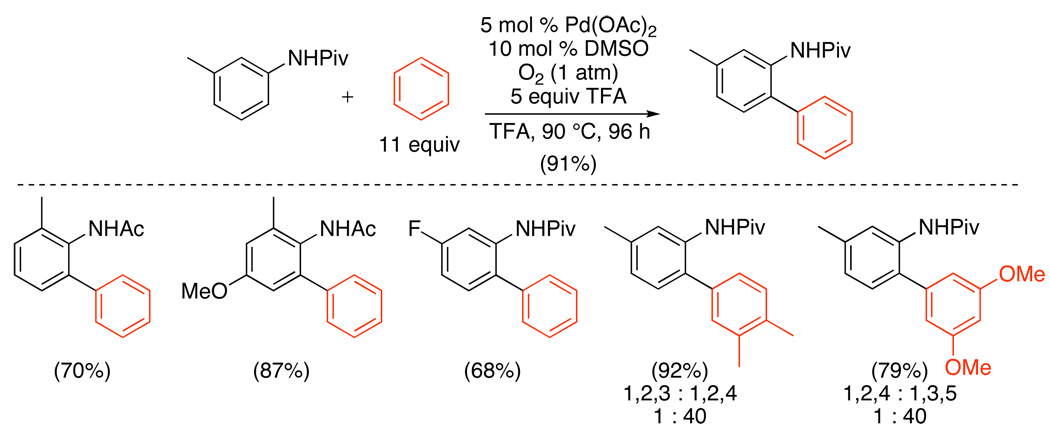
Similar transformations have also been applied to N-acetanilides and N-pivalanilides.95,96 These substrates underwent Pd-catalyzed ortho-arylation with an electronically diverse set of Ar–H using either Cu(OTf)2/O2 (in EtCO2H)95 (Scheme 35) or O2 (in TFA)96 as the oxidant (Scheme 36). No additional promoter was required, and the site selectivity of Ar–H activation appeared to be dominated by steric factors. This methodology was applied to the total synthesis of 4-deoxycarbomycin B (eq. 41).
Scheme 35.
Oxidative Cross-Coupling of Amide Derivatives with Aryl–H using Cu(OTf)2/O2 as Oxidant95
Scheme 36.
Oxidative Cross-Coupling of Amide Derivatives with Ar–H using O2 as Oxidant96
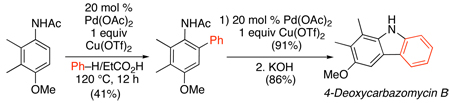 |
(41) |
The mechanism proposed for all of these L~C–H/Ar–H cross-coupling reactions involves the following steps: (i) cyclopalladation of L~C–H, (ii) Ar–H activation at the resulting cyclopalladated intermediate, (iii) C–C bond-forming reductive elimination, and (iv) oxidation of the resulting Pd0 center to PdII (Scheme 37). In the systems where benzoquinone is required, this promoter is believed to facilitate the C–C bond-forming reductive elimination step of the catalytic cycle.94,97 The effect of BQ on site selectivity appears to be due to selective promotion of reductive elimination at less sterically hindered PdII(Ar)(Ar1) complexes.
Scheme 37.
7.3. C–H alkenylation
An early example of Pd-catalyzed directed C–H alkenylation involved the coupling of acetanilides with acrylates (eq. 42).98 Benzoquinone was used as the terminal oxidant, and a solvent mixture of TsOH, N-methylpyrrolidinone, and acetic acid was required for efficient turnover. A variety of acetanilides were effective substrates for ortho-alkenylation with n-butylacrylate, although those bearing electron withdrawing substituents or ortho-substitution afforded lower yields. Additionally, no reaction was observed with N-methylacetanilide. More recently, an analogous transformation has been reported using O2/Cu(OAc)2 as the oxidant.99 N-(2-Pyridyl)sulfonyl protected indoles and pyrroles also show similar reactivity with alkenes using Cu(OAc)2·H2O as a theoxidant and Pd(MeCN)2Cl2 as the catalyst.100
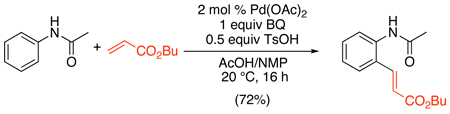 |
(42) |
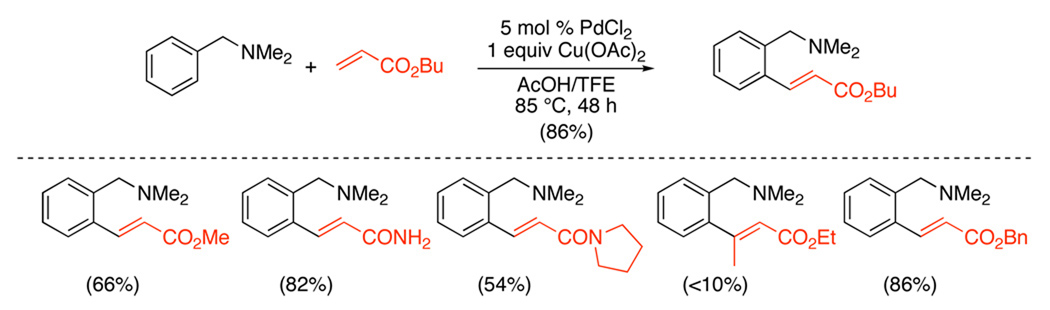
The PdII/0-catalyzed ortho-alkenylation of N,N-dimethylbenzylamines with electron deficient alkenes has also been achieved.101 In this system, catalyst optimization required careful tuning of the electrophilicity of the Pd center as well as the extent of protonation of the directing group, and the optimal conditions used catalytic PdCl2 in conjunction with 1 equiv of Cu(OAc)2 in acetic acid/trifluoroethanol. Terminal α,β-unsaturated esters and amides served as good substrates (Scheme 38); however, substitution at the β-position shut down the reaction. The products could be reduced in one pot to afford ortho-alkylated toluenes, enhancing the synthetic utility of this transformation (eq. 43).
Scheme 38.
ortho-Alkenylation of Benzylamines with α,β-Unsaturated Esters and Amides101
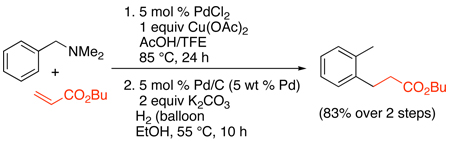 |
(43) |
An alternative approach to Pd-catalyzed ortho-alkenylation of acetanilides precludes the requirement for a stoichiometric oxidant (eq. 44).102 In this system, 3-haloacrylates were used as alkene substrates, and the PdII catalyst was regenerated directly via β-halogen elimination. Though trans-bromo esters provided the highest yields, other halo-olefins were also successfully coupled, with an observed order of reactivity as follows: cis-chloro < trans-chloro ~ cis-iodo < cis-bromo < trans-bromo.
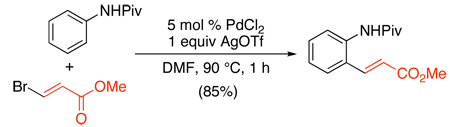 |
(44) |
Finally, a PdII/0-catalyzed alkyne-directed 5-exo-dig intramolecular C–H alkenylation reaction has been developed for the synthesis of fluorenes (eq. 45).103 This transformation proceeded efficiently with both electron rich and electron deficient arenes using 5 mol % of Pd(OAc)2 in conjunction with 7 mol % of 1,1′-bis(diisopropylphosphino)ferrocene (iPrpf). The authors propose a PdII/0 mechanism involving alkyne-directed C–H activation, migratory insertion, and protonation of the resulting Pd–C bond as key steps. Electrophilic attack by the aryl ring on a Pd-alkyne complex was ruled out on the basis of the alkene geometry in the product.
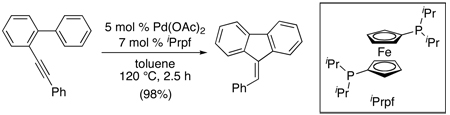 |
(45) |
7.4. C–H alkylation
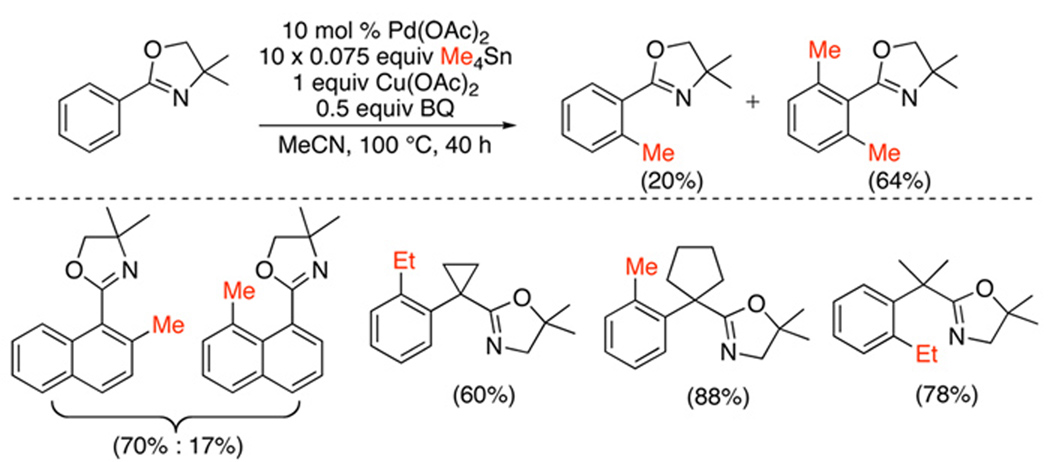
The Pd(OAc)2-catalyzed ortho-alkylation of aryl oxazolines has been accomplished using SnR4 (R = Me or Et) (Scheme 39).104 In these systems, Cu(OAc)2 was used as the terminal oxidant, and benzoquinone (BQ) was a key promoter, believed to accelerate both cyclopalladation and C–C bond formation. Batch-wise addition of the stannane was required to avoid competing alkyl-alkyl coupling. Standard heating protocols resulted in slow reaction (40 h); however, microwave irradiation could be used to reduce the reaction time to 10 h.
Scheme 39.
ortho-Alkylation of Oxazolines with SnR4104
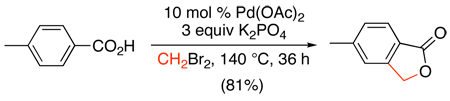
More recently, Pd-catalyzed pyridine-directed sp2 and sp3 C–H alkylation has been reported with methylboroxines and alkylboronic acids.105 Under similar conditions to the SnR4 couplings in Scheme 39, both sp2 and sp3 C-H bonds could be methylated with methylboroxine using Cu(OAc)2 as an oxidant and benzoquinone (BQ) as a promoter (eq. 46). Ethyl- and butylboroxines failed to give the desired alkylated products; however, reoptimization of the conditions showed that the use of a combination of Ag2O and benzoquinone (BQ) resulted in high yields for these substrates (eq. 47). Analogous conditions have also been developed for the Pd-catalyzed ortho-methylation of benzoic acids with methylboronic acid derivatives (eq. 48).82
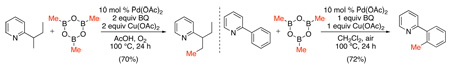 |
(46) |
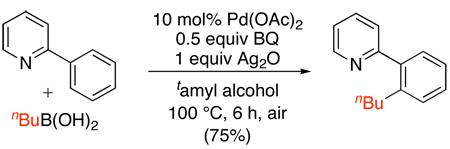 |
(47) |
 |
(48) |
An asymmetric sp2 C–H alkylation reaction has recently been disclosed that uses mono-protected amino acids as chiral ligands and boronic acids as alkylating reagents (eq. 49).106 In this system, (diarylmethyl)pyridine derivatives underwent selective alkylation of one prochiral aryl group in 54–95% ee. A variety of chiral carboxylic acids were screened as ligands, and the best results were obtained with menthoxycarbonyl-protected l-leucine (L in eq. 49). The substrate scope remains somewhat limited; however, these results provide exciting insights for the future development of catalytic asymmetric C–H functionalization reactions.
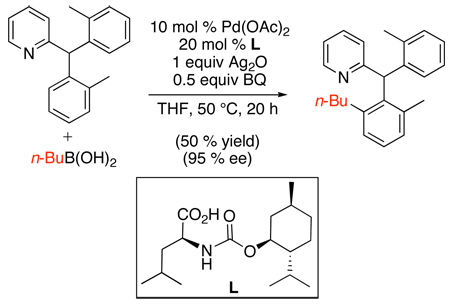 |
(49) |
Dicumylperoxide has also been utilized as a reagent for the Pd-catalyzed ortho-methylation of 2-arylpyridines.107 Mixtures of mono- and di-methylated products were obtained with 2 equiv of the peroxide using Pd(OAc)2 as a catalyst (eq. 50). Though mechanistic studies were not conducted, a PdII/0 pathway was proposed, involving the following sequence: (i) oxidative addition of the peroxide reagent to Pd0, (ii) β-methyl elimination, (iii) cyclopalladation, and (iv) C–C bond forming reductive elimination to yield the product.
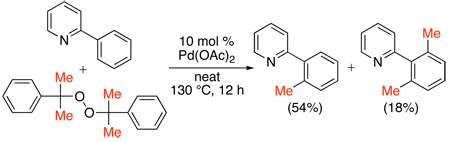 |
(50) |
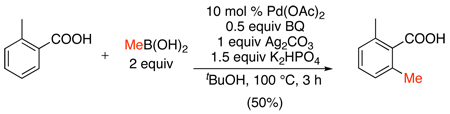
Alkyl halides have also been shown to effect the ortho-alkylation of benzoic acids in the presence of catalytic Pd(OAc)2 and a stoichiometric base.108 In particular, dichloroethane and dibromomethane were effective electrophiles and reacted to form lactone products via an ortho-alkylation/ SN2 cyclization sequence (eq. 51). Competing O-alkylation of the carboxylate salts was observed as a side reaction, particularly with electron rich benzoic acids. However, this undesired transformation could be circumvented by adjusting the temperature along with judicious choice of base (typically K2HPO4, KHCO3, or Na2CO3).
 |
(51) |
7.5 C-H alkynylation
The first and only example of Pd-catalyzed directed C–H alkynylation involves the reaction of anilides with bromoalkynes.109 Using catalytic Pd(OAc)2 along with a silyl protected bromoalkyne, AgOTf, and K2CO3, a number of substituted anilides could be ortho-alkynylated in high yield. The mechanism of this transformation was proposed to proceed via the following series of steps: (i) cyclopalladation, (ii) alkyne insertion into the resulting aryl palladium intermediate, (iii) β-bromide elimination, and (iv) ligand exchange with AgOTf to regenerate the Pd catalyst.
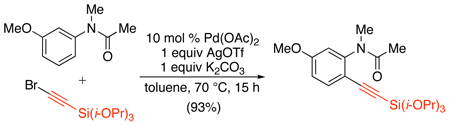 |
(52) |
7.6. C–H carbonylation
An early example of Pd-catalyzed directed C–H carbonylation involved the formation of benzolactam derivatives from benzylamines under an atmosphere of CO.110 Pd(OAc)2 was used as the catalyst and Cu(OAc)2 and O2 served as co-oxidants (eq. 53). Benzylamines underwent significantly faster cyclization than phenethylamines (for example, see substrate 28 in eq. 54), presumably because the formation of 5-membered palladacycles is favored over larger rings.
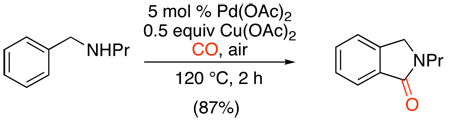 |
(53) |
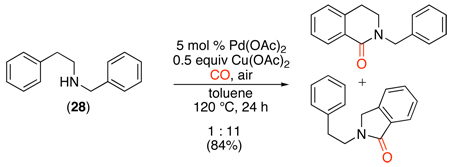 |
(54) |
More recently, an analogous urea-directed reaction was utilized to construct cyclic imidates and methyl anthranilates.111 Benzoquinone (BQ) was used as the stoichiometric oxidant and Pd(MeCN)2(OTs)2 as the catalyst. Notably, TsOH (0.5 equiv) was also required to achieve catalyst turnover in these systems. In CH2Cl2, cyclic imidates were formed in high yields (eq. 55, ii); however, when the solvent was changed to THF/MeOH, anthranilate products of methoxycarbonylation were obtained (eq. 55, i). The latter were proposed to be generated via solvolytic ring-opening of the imidates with MeOH.
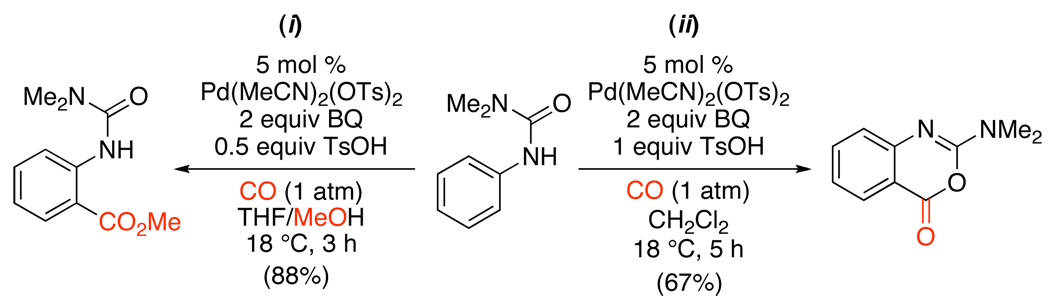 |
(55) |
Carboxylate-directed sp2 C–H activation has also been coupled with carbonylation to afford 1,2- and 1,3-dicarboxylic acids.112 Sodium carboxylates were used to direct cyclopalladation of both benzoic and phenylacetic acids in the presence of catalytic Pd(OAc)2, 2 equiv of NaOAc, and 2 equiv of Ag2CO3 (eq. 56). Anhydrides were proposed to be generated as initial intermediates (29 in eq. 56) via intramolecular trapping of a Pd-acyl intermediate. Hydrolysis under the reaction conditions would then afford the observed dicarboxylic acid products.
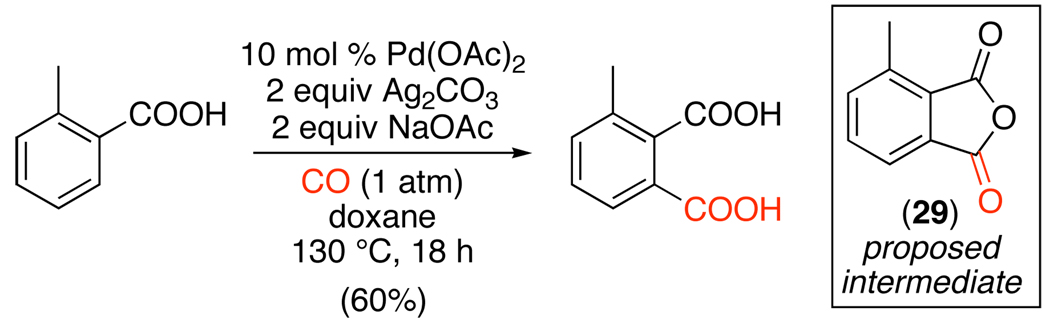 |
(56) |
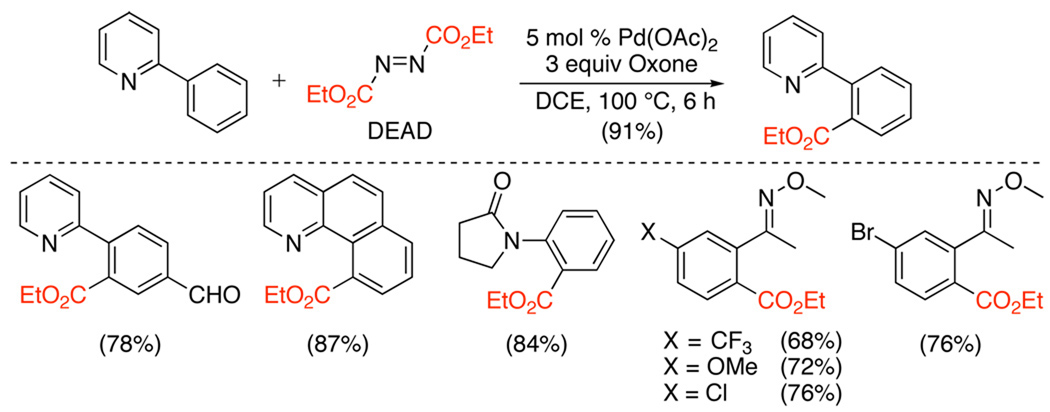
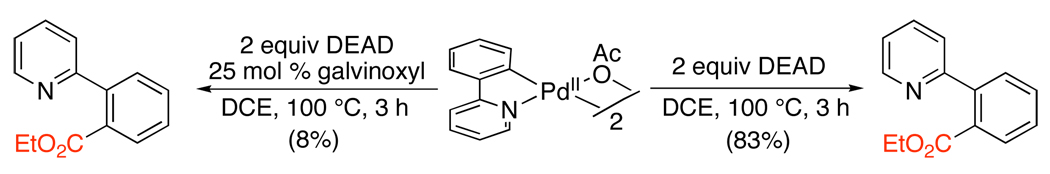
Ortho-esters can also be prepared without the requirement for toxic CO. Instead, diethyl azodicarboxylate (DEAD) has been used as a source of an ethoxycarbonyl group.113 Pyridine, amide, and oxime ether-directed ethoxycarbonylation have been achieved with this reagent using Pd(OAc)2 as the catalyst, Oxone as a terminal oxidant, and batchwise addition of DEAD (Scheme 40). The addition of galvinoxyl (a free radical scavenger) resulted in a dramatically lower yield for the stoichiometric reaction between DEAD and [(phpy)Pd(OAc)]2 (phpy = 2-phenylpyridine) (Scheme 41). Based on this result, a mechanism involving reaction of ethoxylacyl radicals with cyclopalladated intermediates was proposed.
Scheme 40.
Pd-Catalyzed Ethoxycarbonylation with DEAD113
Scheme 41.
Effect of Galvinoxyl on Stoichiometric Ethoxycarbonylation113
Finally, simple aldehydes have recently been shown to be effective reagents for the Pd(OAc)2-catalyzed ortho-acylation of 2-arylpyridines (eq. 57).114 Both electron-withdrawing and donating groups were tolerated on both the arylpyridine and the aldehyde-coupling partner in this transformation. However, alkyl-substituted aldehydes were unreactive under these conditions. A PdII/0 pathway was proposed for this transformation, with aldehyde insertion into a palladacyclic intermediate serving as the key C–C bond forming step.
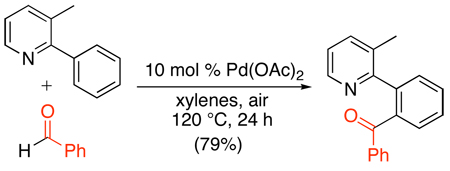 |
(57) |
8. Conclusions and Future Outlook
The field of Pd-catalyzed ligand-directed C–H functionalization has clearly seen a flurry of activity over the past 5 years, and huge advances have been made in both the development of novel reactions and in the mechanistic study of these transformations. The future seems similarly bright, as there are many challenges (both short and long term) that remain to be addressed. For example, it will be important to ascertain whether the reactions described in this review are general with respect to many different directing groups (as each has typically been optimized within a limited class of substrates). Further, it will be synthetically useful to expand the scope of directing groups that can be used in this chemistry to include less basic functionalities like aldehydes, ketones, alcohols, and oxygen heterocycles.68 More general methods for functionalization at remote positions (via 7-, 8-, 9-membered and larger palladacycles)49 as well as at 2° sp3 sites would also greatly expand the utility of these transformations. The application of Pd-catalyzed ligand-directed C–H functionalization to complex, multi-functional substrates also remains a key future objective. The demonstration of efficiency and selectivity in such settings would pave the way for the widespread application of this chemistry for late-stage derivatization of natural products and medical compounds.
Asymmetric catalysis is another clear frontier in this field. Pioneering work by Yu has shown that such reactions are possible.106 However, the design of asymmetric C–H functionalization reactions that are general with respect to directing group, substrate, and functionalizing reagent remains a critical goal. Such transformations will clearly require significant effort in the area of ligand design.
Finally, the future development of novel reactions in this field will be closely tied to mechanistic investigations. As discussed above, our current mechanistic understanding of these transformations and of the complex interplay between catalyst, ligands, solvent, additives, and substrates remains in its infancy. Mechanistic studies are expected to continue to inform the design and discovery of new highly active and selective catalysts for Pd-catalyzed ligand-directed C–H functionalization processes.
Scheme 14.
Halogenation of sp2 C–H Substrates with N-Halosuccinimides40
Scheme 23.
Intramolecular C–H Amination to Form Lactams63
Acknowledgments
We thank the NIH (GM-073836), NSF (CHE-0754639), and DOE (DE-FG02-08ER 15997) for funding various aspects of the work discussed in this review. We also thank the University of Michigan for a graduate fellowship for TWL.
Abbreviations
- Ac
acetyl
- acac
acetylacetonate
- Ac2O
acetic anhydride
- AcOH
acetic acid
- Ad
adamantyl
- Ar
aryl
- atm
atmosphere
- Boc
tert-butoxycarbonyl
- BQ
benzoquinone
- Bn
benzyl
- Bu
butyl
- cod
cyclooctadiene
- Cy-John Phos
Cyclohexyl John Phos ligand
- DCE
dichloroethane
- DCM
dichloromethane
- DEAD
diethyl azodicarboxylate
- d-i-Prpf
1,1’-bis(diisopropylphosphino)ferrocene
- DG
directing group
- DMF
dimethylformamide
- DMSO
dimethyl sulfoxide
- DoL
directed ortho lithiation
- FG
functional group
- H2hpda
4-hydroxypyridine-2,6-dicarboxylic acid
- L
ligand
- MOM
methoxymethyl
- MS
molecular sieves
- NBS
N-bromosuccinimide
- NCS
N-chlorosuccinimide
- NIS
N-iodosuccinimide
- NMP
N-methyl-2-pyrrolidone
- OAc
acetate
- OPiv
pivalate
- Ph
phenyl
- phpy
phenylpyridine
- Phth
phthalyl
- Piv
pivyl
- R
alkyl
- rt
room temperature
- TFA
trifluoroacetic acid
- TFE
trifluoroethanol
- THF
tetrahydrafuran
- Ts
tosyl
- TsOH
p-toluenesulfonic acid
- X
halide
- µwave
microwave irradiation
Biographies

Professor Melanie Sanford received her B.S. and M.S. degrees at Yale University where she carried out undergraduate research in the laboratory of Professor Robert Crabtree. She pursued graduate studies at the California Institute of Technology working with Professor Robert Grubbs. Following postdoctoral work at Princeton University with Professor John Groves, she joined the faculty at the University of Michigan in the summer of 2003 as an Assistant Professor of chemistry. In spring 2007 she was promoted to her current position of Associate Professor of chemistry. Professor Sanford has been recognized with a number of awards, including a Presidential Early Career Award in Sciences and Engineering, an Arthur Cope Scholar award from the American Chemical Society, and the BASF catalysis award. Research in the Sanford group focuses broadly on the development and mechanistic study of new transition metal catalyzed reactions for applications in organic synthesis. More specifically, the group is working to develop a diverse set of transformations for the direct conversion of unactivated carbon-hydrogen bonds into new functional groups with high levels of chemo-, regio-, and stereoselectivity.

Thomas Lyons was born in Ottawa, Illinois in 1983. He received his B.S. with honors in chemistry from DePaul University in 2005 under the mentorship of Prof. Matthew Dintzner. He is currently a Ph.D. candidate in Prof. Melanie Sanford’s research laboratory where he is studying regioselective oxidative coupling reactions.
References
- 1.For pertinent reviews on C–H activation, see: Crabtree RH. Chem. Rev. 1985;85:245. Arndtsen BA, Bergman RG, Mobley TA, Peterson TH. Acc. Chem. Res. 1995;28:154. Shilov AE, Shul’pin GB. Chem. Rev. 1997;97:2879. doi: 10.1021/cr9411886. Dyker G. Angew. Chem., Int. Ed. 1999;38:1698. doi: 10.1002/(SICI)1521-3773(19990614)38:12<1698::AID-ANIE1698>3.0.CO;2-6. Crabtree RH. J. Chem. Soc., Dalton Trans. 2001;2437 Ritleng V, Sirlin C, Pfeffer M. Chem. Rev. 2002;102:1731. doi: 10.1021/cr0104330. Labinger JA, Bercaw JE. Nature. 2002;417:507. doi: 10.1038/417507a. Sezen B, Sames D. In: Handbook of C–H Transformations. Dyker G, editor. Weinheim, Germany: Wiley-VCH Velag GmbH & Co. KGaA; 2005. pp. 3–10. Yu JQ, Giri R, Chen X. Org. Biomol. Chem. 2006;4:4041. doi: 10.1039/b611094k. Godula K, Sames D. Science. 2006;312:67. doi: 10.1126/science.1114731. Kalyani D, Sanford MS. In: Topics in Organometallic Chemistry: Directed Metalation. Chatani N, editor. Vol. 24. New York: Springer Berlin Heidelberg; 2007. pp. 85–116. Bergman RG. Nature. 2007;466:391. doi: 10.1038/446391a. Hartwig JF. Nature. 2008;455:314. doi: 10.1038/nature07369.
- 2.(a) Dunina VV, Zalevskaya OA, Potapov VM. Russ. Chem. Rev. 1988;57:250. [Google Scholar]; (b) Ryabov AD. Chem. Rev. 1990;90:403. [Google Scholar]; (c) Dyker G. Angew. Chem., Int. Ed. 1999;38:1698. doi: 10.1002/(SICI)1521-3773(19990614)38:12<1698::AID-ANIE1698>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]; (d) Dupont J, Consorti CS, Spencer J. Chem. Rev. 2005;105:2527. doi: 10.1021/cr030681r. [DOI] [PubMed] [Google Scholar]
- 3.Cope AC, Siekman RW. J. Am. Chem. Soc. 1965;87:3272. [Google Scholar]
- 4.For examples, see: Davies HML, Beckwith REJ. Chem. Rev. 2003;103:2861. doi: 10.1021/cr0200217. Daugulis O, Zaitsev VG, Shabashov D, Pham QN, Lazareva A. Synlett. 2006:3382. Alberico D, Scott ME, Lautens M. Chem. Rev. 2007;107:174. doi: 10.1021/cr0509760.
- 5.Dick AR, Sanford MS. Tetrahedron. 2006;62:2439. [Google Scholar]
- 6.For an example of Cu-catalyzed ligand-directed C–H functionalization to install diverse groups, see: Chen X, Hao XS, Goodhue CE, Yu JQ. J. Am. Chem. Soc. 2006;128:6790. doi: 10.1021/ja061715q.
- 7.For examples of Pt-catalyzed ligand-directed intramolecular C–H oxygenation, see: Sen A. Acc. Chem. Res. 1998;31:550. Dangel BD, Johnson JA, Sames D. J. Am. Chem. Soc. 2001;123:8149. doi: 10.1021/ja016280f. Lin M, Shen C, Garcia-Zayas, Sen A. J. Am. Chem. Soc. 2001;123:1000. doi: 10.1021/ja001926+.
- 8.Dick AR, Hull KL, Sanford MS. J. Am. Chem. Soc. 2004;126:2300. doi: 10.1021/ja031543m. [DOI] [PubMed] [Google Scholar]
- 9.Kalyani D, Sanford MS. Org. Lett. 2005;7:4149. doi: 10.1021/ol051486x. [DOI] [PubMed] [Google Scholar]
- 10.Desai LV, Malik HA, Sanford MS. Org. Lett. 2006;8:1141. doi: 10.1021/ol0530272. [DOI] [PubMed] [Google Scholar]
- 11.There are rare examples of aldehydes serving a directing groups in Pd-catalyzed C–H functionalization reactions. For examples, see: Terao Y, Kametani Y, Wakui H, Satoh T, Miura M, Nomura M. Tetrahedron. 2001;57:5967. Gürbüz N, Özdemir I, Çetinkaya B. Tetrahedron Lett. 2005;46:2273.
- 12.Kalberer EW, Whitfield SL, Sanford MS. J. Mol. Catal., A. 2006;251:108. [Google Scholar]
- 13.Schardt BC, Hill CL. Inorg. Chem. 1983;22:1563. [Google Scholar]
- 14.Desai LV, Hull KL, Sanford MS. J. Am. Chem. Soc. 2004;126:9542. doi: 10.1021/ja046831c. [DOI] [PubMed] [Google Scholar]
- 15.Yoneyama T, Crabtree RH. J. Mol. Catal., A. 1996;108:35. [Google Scholar]
- 16.Dick AR, Kampf JW, Sanford MS. Organometallics. 2005;24:482. [Google Scholar]
- 17.Deprez NR, Sanford MS. J. Am. Chem. Soc. 2009;131:11234. doi: 10.1021/ja904116k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powers DC, Ritter T. Nat. Chem. 2009;1:302. doi: 10.1038/nchem.246. [DOI] [PubMed] [Google Scholar]
- 19.Desai LV, Stowers KJ, Sanford MS. J. Am. Chem. Soc. 2008;130:13285. doi: 10.1021/ja8045519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stowers KJ, Sanford MS. Org. Lett. 2009 doi: 10.1021/ol901820w. ASAP article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dick AR, Kampf JW, Sanford MS. J. Am. Chem. Soc. 2005;127:12790. doi: 10.1021/ja0541940. [DOI] [PubMed] [Google Scholar]
- 22.Fu Y, Li Z, Liang S, Guo QX, Lui L. Organometallics. 2008;27:3736. [Google Scholar]
- 23.Racowski JA, Dick AR, Sanford MS. J. Am. Chem. Soc. 2009;131:10974. doi: 10.1021/ja9014474. [DOI] [PubMed] [Google Scholar]
- 24.Byers PK, Canty AJ, Crespo M, Puddephatt RJ, Scott JD. Organometallics. 1988;7:1363. [Google Scholar]
- 25.Wang DH, Hao XS, Wu DF, Yu JQ. Org. Lett. 2006;8:3387. doi: 10.1021/ol061384m. [DOI] [PubMed] [Google Scholar]
- 26.Wang GW, Yuan TT, Wu XL. J. Org. Chem. 2008;73:4717. doi: 10.1021/jo8003088. [DOI] [PubMed] [Google Scholar]
- 27.Kim SH, Lee HS, Kim SH, Kim JN. Tetrahedron Lett. 2008;49:5863. [Google Scholar]
- 28.Reddy BVS, Reddy LR, Corey EJ. Org. Lett. 2006;8:3391. doi: 10.1021/ol061389j. [DOI] [PubMed] [Google Scholar]
- 29.Giri R, Liang J, Lei JQ, Li JJ, Wang DH, Chen X, Naggar IC, Guo C, Foxman BM, Yu JQ. Angew. Chem., Int. Ed. 2005;44:7420. doi: 10.1002/anie.200502767. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Khaskin E, Anderson NP, Zavalij PY, Vedernikov AN. Chem. Commun. 2008:3625. doi: 10.1039/b803156h. [DOI] [PubMed] [Google Scholar]
- 31.Inamoto K, Arai Y, Hiroya K, Doi T. Chem. Commun. 2008;5529 doi: 10.1039/b811362a. [DOI] [PubMed] [Google Scholar]
- 32.For examples of DMSO-mediated oxidation of thiols, see: Fristad WE, Peterson JR. Synth. Commun. 1985;15:1. Wallace TJ. J. Am. Chem. Soc. 1964;86:2018. Yiannios CN, Karabinos JV. J. Org. Chem. 1963;28:3246.
- 33.For examples of metal-mediated oxidation of thiols, see: Wallace TJ. J. Org. Chem. 1966;31:1217. Iranpoor N, Zeynizadeh B. Synthesis. 1999:49. Kirihara M, Okubo K, Uchiyama T, Kato Y, Chiai Y, Matsushita S, Hatano A, Kanamori K. Chem. Pharm. Bull. 2004;52:625. doi: 10.1248/cpb.52.625.
- 34.Inamoto K, Hasegawa C, Hiroya K, Doi T. Org. Lett. 2008;10:5147. doi: 10.1021/ol802033p. [DOI] [PubMed] [Google Scholar]
- 35.Joyce LL, Batey RA. Org. Lett. 2009;11:2792. doi: 10.1021/ol900958z. [DOI] [PubMed] [Google Scholar]
- 36.Zhao X, Dimitrijevic E, Dong VM. J. Am. Chem. Soc. 2009;131:3466. doi: 10.1021/ja900200g. [DOI] [PubMed] [Google Scholar]
- 37.(a) Fahey DR. J. Chem. Soc., Chem. Commun. 1970:417. [Google Scholar]; (b) Fahey DR. J. Organomet. Chem. 1971;27:283. [Google Scholar]
- 38.(a) Wong PK, Stille JK. J. Organomet. Chem. 1974;70:121. [Google Scholar]; (b) Bäckvall J. Tetrahedron Lett. 1977;18:467. [Google Scholar]; (c) Bäckvall J. Acc. Chem. Res. 1983;16:335. [Google Scholar]; (d) Kubota M, Boegeman SC, Keil RN, Webb CG. Organometallics. 1989;8:1616. [Google Scholar]; (e) Alsters PL, Engel PF, Hogerheide MP, Marinus PH, Copijn M, Spek AL, van Koten G. Organometallics. 1993;12:1831. [Google Scholar]; (f) Alsters PL, Boersma J, van Koten G. Organometallics. 1993;12:1629. [Google Scholar]; (g) Lagunas MC, Gossage RA, Spek AL, van Koten G. Organometallics. 1998;17:731. [Google Scholar]; (h) van Belzen R, Elsevier CJ, Dedieu A, Velman N, Spek AL. Organometallics. 2003;22:722. [Google Scholar]
- 39.Kodama H, Katsuhira T, Nishida T, Hino T, Tsubata K. Chem. Abstr. 2001;135:344284. [Google Scholar]
- 40.Kalyani D, Dick AR, Anani WQ, Sanford MS. Tetrahedron. 2006;62:11483. doi: 10.1021/ol060747f. [DOI] [PubMed] [Google Scholar]
- 41.Kalyani D, Dick AR, Anani WQ, Sanford MS. Org. Lett. 2006;8:2523. doi: 10.1021/ol060747f. [DOI] [PubMed] [Google Scholar]
- 42.Whitfield SR, Sanford MS. J. Am. Chem. Soc. 2007;129:15142. doi: 10.1021/ja077866q. [DOI] [PubMed] [Google Scholar]
- 43.Canty AJ, Gardiner MG, Jones RC, Rodemann T, Sharma M. J. Am. Chem. Soc. 2009;131:7236. doi: 10.1021/ja902799u. [DOI] [PubMed] [Google Scholar]
- 44.Canty AJ. Dalton Trans. 2009 Advance online publication (DOI: 10.1039/b914080h) [Google Scholar]
- 45.Wan X, Ma Z, Li B, Zhang K, Cao S, Zhang S, Shi Z. J. Am. Chem. Soc. 2006;128:7416. doi: 10.1021/ja060232j. [DOI] [PubMed] [Google Scholar]
- 46.For one electron oxidation of NiII complexes promoted by CuX2, see: Grove DM, Van Koten G, Zoet R, Murrall NW, Welch AJ. J. Am. Chem. Soc. 1983;105:1379. Grove DM, Van Koten G, Mul P, Zoet R, van der Linden JGM, Legters J, Schmitz JEJ, Murrall NW, Welch AJ. Inorg. Chem. 1988;27:2466.
- 47.Ulery JE. J. Org. Chem. 1965;30:2464. [Google Scholar]
- 48.(a) Giri R, Chen X, Yu JQ. Angew. Chem., Int. Ed. 2005;44:2112. doi: 10.1002/anie.200462884. [DOI] [PubMed] [Google Scholar]; (b) Giri R, Chen X, Hao XS, Li JJ, Liang J, Fan ZP, Yu JQ. Tetrahedron Asymm. 2005;16:3502. [Google Scholar]
- 49.Li JJ, Giri R, Yu JQ. Tetrahedron. 2008;64:6979. [Google Scholar]
- 50.Mei TS, Giri R, Maugel N, Yu JQ. Angew. Chem., Int. Ed. 2008;47:5215. doi: 10.1002/anie.200705613. [DOI] [PubMed] [Google Scholar]
- 51.Kakiuchi F, Kochi T, Mutsutani H, Kobayashi N, Urano S, Sato M, Nishiyama S, Tanabe T. J. Am. Chem. Soc. 2009;131:11310. doi: 10.1021/ja9049228. [DOI] [PubMed] [Google Scholar]
- 52.Hull KL, Anani WQ, Sanford MS. J. Am. Chem. Soc. 2006;128:7134. doi: 10.1021/ja061943k. [DOI] [PubMed] [Google Scholar]
- 53.Wang X, Mei TS, Yu JQ. J. Am. Chem. Soc. 2009;131:7520. doi: 10.1021/ja901352k. [DOI] [PubMed] [Google Scholar]
- 54.Furuya T, Ritter T. J. Am. Chem. Soc. 2008;130:10060. doi: 10.1021/ja803187x. [DOI] [PubMed] [Google Scholar]
- 55.Ball ND, Sanford MS. J. Am. Chem. Soc. 2009;131:3796. doi: 10.1021/ja8054595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.(a) Hartwig JF. In: Handbook of Organopalladium Chemistry for Organic Synthesis. Negishi EI, de Meijere A, editors. Vol. 1. New York: Wiley-Interscience; 2002. p. 1051. [Google Scholar]; (b) Ley SV, Thomas AW. Angew. Chem., Int. Ed. 2003;42:5400. doi: 10.1002/anie.200300594. [DOI] [PubMed] [Google Scholar]; (c) Beletskaya IP, Cheprakov AV. Coord. Chem. Rev. 2004;248:2337. [Google Scholar]; (d) Jiang L, Buchwald SL. In: Metal-Catalyzed Cross-Coupling Reactions. 2nd. Diederich F, Stang PJ, editors. Weinheim, Germany: Wiley-VCH; 2004. p. 699. [Google Scholar]; (e) Thomas JS, Wolfe JP. Curr. Org. Chem. 2005;9:625. [Google Scholar]; (f) Corbet JP, Mignani G. Chem. Rev. 2006;106:2651. doi: 10.1021/cr0505268. [DOI] [PubMed] [Google Scholar]
- 57.Thansandote P, Lautens M. Chem. Eur. J. 2009;15:5874. doi: 10.1002/chem.200900281. [DOI] [PubMed] [Google Scholar]
- 58.Tsang WCP, Zheng N, Buchwald SL. J. Am. Chem. Soc. 2005;127:14560. doi: 10.1021/ja055353i. [DOI] [PubMed] [Google Scholar]
- 59.Tsang WCP, Munday RH, Brasche G, Zheng N, Buchwald SL. J. Org. Chem. 2008;73:7603. doi: 10.1021/jo801273q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamamoto M, Matsubara S. Chem. Lett. 2007;36:172. [Google Scholar]
- 61.Jordon-Hore JA, Johansson CCC, Gulia M, Beck EM, Gaunt MJ. J. Am. Chem. Soc. 2008;130:16184. doi: 10.1021/ja806543s. [DOI] [PubMed] [Google Scholar]
- 62.Inamoto K, Saito T, Katsuno M, Sakamoto T, Hiroya K. Org. Lett. 2007;9:2931. doi: 10.1021/ol0711117. [DOI] [PubMed] [Google Scholar]
- 63.Inamoto K, Saito T, Hiroyo K, Doi T. Synlett. 2008:3157. [Google Scholar]
- 64.Mei TS, Wang X, Yu JQ. J. Am. Chem. Soc. 2009;131:10806. doi: 10.1021/ja904709b. [DOI] [PubMed] [Google Scholar]
- 65.Li JJ, Mei TS, Yu JQ. Angew. Chem., Int. Ed. 2008;47:6452. doi: 10.1002/anie.200802187. [DOI] [PubMed] [Google Scholar]
- 66.Thu HY, Yu WY, Che CM. J. Am. Chem. Soc. 2006;128:9048. doi: 10.1021/ja062856v. [DOI] [PubMed] [Google Scholar]
- 67.Dick AR, Remy MS, Kampf JW, Sanford MS. Organometallics. 2007;26:1365. [Google Scholar]
- 68.(a) Alberico D, Scott ME, Lautens M. Chem. Rev. 2007;107:174. doi: 10.1021/cr0509760. [DOI] [PubMed] [Google Scholar]; (b) Chen X, Engle KM, Wang DH, Yu JQ. Angew. Chem., Int. Ed. 2009;48:5094. doi: 10.1002/anie.200806273. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) McGlacken GP, Bateman LM. Chem. Soc. Rev. 2009;38:2447. doi: 10.1039/b805701j. [DOI] [PubMed] [Google Scholar]
- 69.Beccalli EM, Broggini G, Martinelli M, Sottocornola S. Chem. Rev. 2007;107 doi: 10.1021/cr068006f. 5318 and the references therein. [DOI] [PubMed] [Google Scholar]
- 70.Daugulis O, Do HQ, Shabashov D. Acc. Chem. Res. 2009;42:1074. doi: 10.1021/ar9000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.(a) Kalyani D, Deprez NR, Desai LV, Sanford MS. J. Am. Chem. Soc. 2005;127:7330. doi: 10.1021/ja051402f. [DOI] [PubMed] [Google Scholar]; (b) Deprez NR, Sanford MS. Inorg. Chem. 2007;46:1924. doi: 10.1021/ic0620337. [DOI] [PubMed] [Google Scholar]
- 72.Daugulis O, Zaitsev VG. Angew. Chem., Int. Ed. 2005;44:4046. doi: 10.1002/anie.200500589. [DOI] [PubMed] [Google Scholar]
- 73.Spencer J, Chowdhry BZ, Mallet AI, Rathnam RP, Adatia T, Bashall A, Rominger F. Tetrahedron. 2008;64:6082. [Google Scholar]
- 74.Gary JB, Deprez NR, Sanford MS. Preliminary DFT calculations suggest that the Pd–Pd interaction is minimal in this structure. unpublished results. [Google Scholar]
- 75.Yang F, Wu Y, Li Y, Wang B, Zhang J. Tetrahedron. 2009;65:914. [Google Scholar]
- 76.Shabashov D, Daugulis O. Org. Lett. 2005;7:3657. doi: 10.1021/ol051255q. [DOI] [PubMed] [Google Scholar]
- 77.Zaitsev VG, Shabashov D, Daugulis O. J. Am. Chem. Soc. 2005;127:13154. doi: 10.1021/ja054549f. [DOI] [PubMed] [Google Scholar]
- 78.Yang F, Wu Y, Zhu Z, Zhang J, Li Y. Tetrahedron. 2008;64:6782. [Google Scholar]
- 79.Chiong HA, Pham QN, Daugulis O. J. Am. Chem. Soc. 2007;129:9879. doi: 10.1021/ja071845e. [DOI] [PubMed] [Google Scholar]
- 80.Shabashov D, Malina Moldonado JR, Daugulis O. J. Org. Chem. 2008;73:7818. doi: 10.1021/jo801300y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thirunavukkarasu VS, Parthasarathy K, Cheng CH. Angew. Chem., Int. Ed. 2008;47:9462. doi: 10.1002/anie.200804153. [DOI] [PubMed] [Google Scholar]
- 82.Giri R, Maugel N, Li JJ, Wang DH, Breazzano SP, Saunders LB, Yu JQ. J. Am. Chem. Soc. 2007;129:3510. doi: 10.1021/ja0701614. [DOI] [PubMed] [Google Scholar]
- 83.Stille JK, Lau KSY. Acc. Chem. Res. 1977;10:434. [Google Scholar]
- 84.Kraatz HB, van der Boom ME, Ben-David Y, Milstein D. Isr. J. Chem. 2001;41:163. [Google Scholar]
- 85.Dogan Ö, Gürbüz N, Özdemir I, Çetinkaya B. Heteroatom Chem. 2008;19:569. [Google Scholar]
- 86.Wasa M, Engle KM, Yu JQ. J. Am. Chem. Soc. 2009;131:9886. doi: 10.1021/ja903573p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shi Z, Li B, Wan X, Cheng J, Fang Z, Cao B, Qin C, Wang Y. Angew. Chem. Int. Ed. 2007;46:5554. doi: 10.1002/anie.200700590. [DOI] [PubMed] [Google Scholar]
- 88.Kirchberg S, Vogler T, Studer A. Synlett. 2008:2841. [Google Scholar]
- 89.Yang S, Li B, Wan X, Shi Z. J. Am. Chem. Soc. 2007;129:6066. doi: 10.1021/ja070767s. [DOI] [PubMed] [Google Scholar]
- 90.Zhou H, Xu YH, Chung WJ, Loh TP. Angew. Chem. Int. Ed. 2009;48:5355. doi: 10.1002/anie.200901884. [DOI] [PubMed] [Google Scholar]
- 91.Yu WY, Sit WN, Zhou Z, Chan ASC. Org. Lett. 2009;11:3174. doi: 10.1021/ol900756g. [DOI] [PubMed] [Google Scholar]
- 92.Hull KL, Lanni EL, Sanford MS. J. Am. Chem. Soc. 2006;128:14047. doi: 10.1021/ja065718e. [DOI] [PubMed] [Google Scholar]
- 93.Xia JB, You SL. Organometallics. 2007;26:4869. [Google Scholar]
- 94.Hull KL, Sanford MS. J. Am. Chem. Soc. 2007;129:11904. doi: 10.1021/ja074395z. [DOI] [PubMed] [Google Scholar]
- 95.(a) Li BJ, Tian SL, Fang Z, Shi ZJ. Angew. Chem., Int. Ed. 2008;47:1115. doi: 10.1002/anie.200704092. [DOI] [PubMed] [Google Scholar]; (b) Li BJ, Yang SD, Shi ZJ. Synlett. 2008:949. [Google Scholar]
- 96.Brasche G, García-Fortanet J, Buchwald SL. Org. Lett. 2008;10:2207. doi: 10.1021/ol800619c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hull KL, Sanford MS. J. Am. Chem. Soc. 2009;131:9651. doi: 10.1021/ja901952h. [DOI] [PubMed] [Google Scholar]
- 98.Boele MDK, van Strijdonck GPF, de Vries AHM, Kamer PCJ, de Vries JG, van Leeuwen PWNM. J. Am. Chem. Soc. 2002;124:1586. doi: 10.1021/ja0176907. [DOI] [PubMed] [Google Scholar]
- 99.Wang JR, Yang CT, Liu L, Guo QX. Tetrahedron Lett. 2007;48:5449. [Google Scholar]
- 100.García-Rubia A, Arrayás RG, Carretero JC. Angew. Chem. Int. Ed. 2009;48:6511. doi: 10.1002/anie.200902802. [DOI] [PubMed] [Google Scholar]
- 101.Cai G, Fu Y, Li Y, Wan X, Shi Z. J. Am. Chem. Soc. 2007;129:7666. doi: 10.1021/ja070588a. [DOI] [PubMed] [Google Scholar]
- 102.Zaitsev VG, Daugulis O. J. Am. Chem. Soc. 2005;127:4156. doi: 10.1021/ja050366h. [DOI] [PubMed] [Google Scholar]
- 103.Chernyak N, Gevorgyan V. J. Am. Chem. Soc. 2008;130:5636. doi: 10.1021/ja8006534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen X, Li JJ, Hao XS, Goodhue CE, Yu JQ. J. Am. Chem. Soc. 2006;128:78. doi: 10.1021/ja0570943. [DOI] [PubMed] [Google Scholar]
- 105.Chen X, Goodhue CE, Yu JQ. J. Am. Chem. Soc. 2006;128:12634. doi: 10.1021/ja0646747. [DOI] [PubMed] [Google Scholar]
- 106.Shi BF, Maugel N, Zhang YH, Yu JQ. Angew. Chem., Int. Ed. 2008;47:4882. doi: 10.1002/anie.200801030. [DOI] [PubMed] [Google Scholar]
- 107.Zhang Y, Feng J, Li CJ. J. Am. Chem. Soc. 2008;130:2900. doi: 10.1021/ja0775063. [DOI] [PubMed] [Google Scholar]
- 108.Zhang YH, Shi BF, Yu JQ. Angew. Chem. Int. Ed. 2009;48:6097. doi: 10.1002/anie.200902262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tobisu M, Ano Y, Chatani N. Org. Lett. 2009;11:3250. doi: 10.1021/ol901049r. [DOI] [PubMed] [Google Scholar]
- 110.Orito K, Horibata A, Nakamura T, Ushito H, Nagasaki H, Yuguchi M, Yamashita S, Tokuda M. J. Am. Chem. Soc. 2004;126:14342. doi: 10.1021/ja045342+. [DOI] [PubMed] [Google Scholar]
- 111.Houlden CE, Hutchby M, Bailey CD, Ford JG, Tyler SNG, Gagné MR, Lloyd-Jones GC, Booker-Milburn KI. Angew. Chem., Int. Ed. 2009;48:1830. doi: 10.1002/anie.200805842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Giri R, Yu JQ. J. Am. Chem. Soc. 2008;130:14082. doi: 10.1021/ja8063827. [DOI] [PubMed] [Google Scholar]
- 113.Yu WY, Sit WN, Lai KM, Zhou Z, Chan ASC. J. Am. Chem. Soc. 2008;130:3304. doi: 10.1021/ja710555g. [DOI] [PubMed] [Google Scholar]
- 114.Jia X, Zhang S, Wang W, Luo F, Cheng J. Org. Lett. 2009;11:3120. doi: 10.1021/ol900934g. [DOI] [PubMed] [Google Scholar]