Abstract
YKL-40 is a secreted chitinase-like molecule whose expression is associated with glioma grade. Expression is higher in astrocytomas than oligodendrogliomas and has been reported to predict shorter survival and radiation resistance in glioblastomas (GBMs). Whether YKL-40 is directly produced by glioma cells or other admixed nonneo-plastic cells, and whether it correlates with 1p/19q status or other hallmark molecular abnormalities, are unclear. A rank-order list of YKL-40 expression was determined immunohistochemically in 79 untreated high-grade adult glio-mas, including 28 anaplastic oligodendrogliomas (AOs) and 51 GBMs. Relative YKL-40 expression was compared with glioma class, key molecular alterations, and immunohistochemical markers via a series of Spearman rank correlations. YKL-40 mRNA in situ hybridization with colocalization assessment via confocal microscopy was also performed. YKL-40 mRNA was abundant in glioma cells as well as reactive astrocytes, but was low in admixed neurons and macrophages. YKL-40 expression was higher in GBMs than AOs (P < 0.0001) and among GBMs, YKL-40 expression was lower in tumors with either EGFR amplification (P = 0.005) or elevated EGFR expression (P = 0.001). Among AOs, no difference in YKL-40 expression was seen in tumors with 1p19q codeletion (P = 0.3), but loss of heterozygos-ity in 10q23 correlated with increased YKL-40 expression (P = 0.03). These data suggest that YKL-40 is predominantly expressed by neoplastic glial cells and is related to certain key molecular alterations.
Keywords: YKL-40, glioblastoma, oligodendroglioma, EGFR, 1p19q, 10q
Introduction
YKL-40, also known as human chitinase-like protein 1 (HC-gp39), is a secreted inflammatory molecule with no chitinolytic activity. Its gene, CHI3L1, is located on Iq32.1. YKL-40 has no known receptor but is capable of binding to N-acetylglucosamine oligomers and heparin, and appears to be upregulated in a variety of conditions that feature remodeling of the extracellular matrix. For example, elevated serum YKL-40 has been described as a robust biomarker of various inflammatory/fibrotic diseases, including sarcoidosis, rheumatoid arthritis, cirrhosis, and atherosclerotic plaques. It is secreted by microglia and astrocytes in SIV encephalitis and inhibits FGF-2 activity via displacement of ECM-bound FGF-2[1]. In degenerative joint disease YKL-40 is secreted by chondrocytes, inhibits collagen synthesis, and promotes proliferation of synovial cells and chondrocytes through Ras/ MAPK and Akt pathways [2]. Elevated serum YKL-40 expression is also associated with me-tastases, higher stage, and an overall worse outcome in a variety of neoplastic diseases [3-16].
Prior studies have suggested that YKL-40 is an important molecule in gliomas. YKL-40 expression increases with glioma grade and is stronger in astrocytic than oligodendroglial tumors [17-19]. High YKL-40 tumor expression has been shown to correlate with MAPK/Akt activation and 10q deletion, and predicts radiation resistance and shorter survival in GBMs [20-24]. Underscoring its association with behavior, it has recently been identified as part of a 9-gene paraffin tissue-based expression panel that most closely predicts GBM survival[25]. In glioma cell lines YKL-40 is produced in response to hypoxia and radiation[26] and promotes radiation and apoptosis resistance, increased invasiveness, and increased 72 KDa metalloproteinase activity[22]. It also stimulates production of nicotinamide N-methyltransferase in glioma cells[22] which has been shown to facilitate invasiveness[27] and radioresistance [28] in urothelial carcinomas. Blocking VEGF production in glioma cell lines causes a large upregulation of YKL-40, perhaps explaining why anti-VEGF therapy can increase the malignancy of some cancers [23]. Finally, TNF has been shown to suppress YKL-40 via NFkB in GBM but not other cancers[29].
In summary, YKL-40 is expressed in situations wherein extensive tissue remodeling and ECM turnover occurs, including inflammation, joint degeneration, and neoplasias, including gliomas. However, since YKL-40 might be expressed and secreted by reactive astrocytes and microglia which are often admixed within the tumor, it is unclear whether the majority of YKL-40 is directly produced by glioma cells in vivo. Aside from a link with 10q loss [22], it is unknown whether YKL-40 expression correlates with any other molecular abnormalities commonly seen in high grade gliomas. In particular, although anaplastic oligodendrogliomas (AOs) express less YKL-40 than GBMs, it is unknown whether expression in AOs varies according to 1p/19q codeletion status. AOs that carry this codeletion are more sensitive to chemotherapy and radiation, and have longer survival intervals [30].
Herein we demonstrate via immunohistochem-istry and in situ hybridization that YKL-40 mRNA is present within GBM cells and reactive astrocytes. We also show that YKL-40 expression is usually stronger in GBMs than AOs, is independent of 1p19q codeletion in AO, and is inversely correlated with EGFR expression and amplification in GBM.
Material and methods
Cohort organization
This cohort was a retrospective collection of institutional high grade gliomas, including 51 GBMs and 28 AOs. Only cases that were initial biopsies were included; previously treated gliomas were excluded. Formalin-fixed, paraffin-embedded (FFPE) tissues were acquired and de -identified according to an institutional review board-approved protocol, conforming to the provisions of the Declaration of Helsinki. Histology was reviewed and the original diagnosis confirmed for each case. Survival from the time of initial biopsy was determined via the Social Security Death Index, but in only 42 of the 79 cases was definitive survival time available.
Dual mRNA in situ hybridization and immunofluorescence
Antisense YKL-40 DNA templates containing the T7 promoter were generated by PCR from the pUC57 vector (GenScript, Piscataway, NJ) containing the full length human YKL40 cDNA. 35S-labeled RNA probes were generated using MAX-Iscript kit (Ambion, Austin, TX). After deparaffini-zation tissue sections were processed for in situ hybridization (ISH) and then for immunohisto-chemistry as described previously [1].
Fluorescence in situ hybridization (FISH)
Both FISH and PCR-based microsatellite analyses (see below) were done as part of the routine clinical workup of institutional gliomas. FISH method has been described previously[31]. Briefly, FISH was performed using probes for 1p36, 19q13, 9p21, and EGFR (7p12) (Abbott Molecular, Des Plaines, IL). For ploidy control, centromeric enumeration probes were used for chromosomes 7 (CEP7) and 9 (CEP9), while 1q25 and 19p13 were used as intrachromo-somal controls for 1p and 19q. Approximately 60 cells were analyzed in the targeted region per case. Each tumor was assessed by the average and the maximum numbers of copies of gene per cell and the average ratio of gene to chromosome copy numbers. Amplification was defined as a ratio of gene signals to chromosome centromere signals of >2.0. Deletion was defined if one or both 1p36, 19q13, and 9p21 signals were lost in at least 20% of nuclei. These cutoff points were derived using nonneo-plastic autopsy brain tissue as controls.
PCR-based microsatellite analysis
Manual microdissection of the tissue sample was performed to include tumor tissue. Matched nonneoplastic tissue was available in some cases. Specimens with the minimum of 50% of tumor cells in a microdissection target were accepted for the analysis. DNA was isolated using standard laboratory procedures. Optical density readings were obtained. The assay utilized 7 microsatellite markers on chromosome 1p22-36 (D1S171, DIS 162, D1S199, D1S1172, D1S1161, D1S407 and D1S226), 3 on 19q13(D19S559, D19S112, and D19S206), 3 on 9p21-22 (CDKN2A gene, D9S1748, D9S1679, and D9S251), 2 on 10q23 ﹛PTEN gene, D10S520 and D10S1173), and 3 on 17p13 ﹛TP53 gene, D17S516, D17S768, and D17S1844). PCR was performed and the PCR products were analyzed using capillary gel elec-trophoresis on GeneMapper ABI 3730 (Foster City, CA). Relative fluorescence was determined for individual alleles and the ratio of peaks was calculated. Neoplastic tissue was then analyzed to detect loss of heterozygosity. When normal tissue was not available, peak height ratios falling outside of 2 standard deviations beyond the mean of previously validated normal values for each polymorphic allele paring were scored as showing loss of heterozygosity.
Immunohistochemistry
Immunohistochemical studies were performed on 4-μm-thick sections obtained from paraffin-embedded material. The primary antibodies, including manufacturer, clone, and dilution, were as follows: P53 (Dako, DO-7, 1:100); Ki67 (Dako M7240/ MIB-1/ 1:100); EGFR (Ventana 790-2988/ 3C6/ prediluted). The antibody labeling was performed using the avidin-biotin complex method and visualized using a horseradish peroxidase enzyme label and 2'-diaminobenzamide (DAB, Dako, Carpinteria, CA) as the substrate chromogen (brown).
YKL-40 staining was performed using goat anti-human chitinase 3-like antibody (1:50, R&D Systems, Minneapolis, MN) immunohistochemistry was performed as described previously [1]. A rank-order list of all cases was generated by sorting the relative YKL-40 staining intensity from strongest to weakest while blinded to diagnosis and molecular results. Only tumor cell stainingwas counted duringthe ranking.
P53 expression was scored based on a modified protocol to that reported previously [32]. Briefly, only cases wherein at least 50% of tumor nuclei had dense staining were considered positive because those are the cases most likely to harbor p53 mutations [33].
EGFR expression was semiquantified by assigning tumor cell staining intensity into 1 of 4 numerical groups: 1—negative, 2—weak, 3— moderate, and 4—strong. Distribution was scored as 1—focal or 2—diffuse. The intensity score was then multiplied by the distribution score to produce an EGFR score, from 0 (negative) to 6 (strong and diffuse).
Statistical analysis
The strength of association between YKL-40 and other variables, including demographic, histologic, and molecular characteristics, was determined via a series of nonparametric Spearman rank correlations using GraphPad software (La Jolla, CA). Associations between 2 variables were considered significant when P < 0.05. Linear regression was employed to determine strength of association between genetic variables a part from YKL-40 rank.
Results
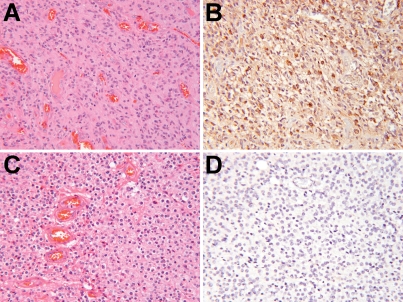
YKL-40 expression has previously been shown to be stronger in astrocytomas than oligoden-drogliomas [18, 19]. To verify this, a rank-order list of YKL-40 immunostaining intensity was compiled using 79 high grade gliomas (see Materials and Methods). Via Spearman rank correlation, GBMs were significantly stronger for YKL-40 expression than AOs (P < 0.0001, Figure 1, Table 1).
Figure 1.
YKL-40 expression is stronger in GBM than AO. 58 GBMs and 21 AOs were immunostained for YKL-40 and ranked according to staining intensity (see Materials and Methods). On average, GBMs (A) showed much stronger YKL-40 expression (B) than did AOs (C & D). P < 0.0001 via Spearman rank correlation. All images are 200× magnification.
Table 1.
Rank-order list of high grade giiomas accordingto relative YKL-40 immunohistochemicai intensity.
| Age (yrs) | Gender | Location | Class | Survival (days) | 1p/19q codeletion | 9p21 LOH | CDKN2A deletion | 10q23 LOH | 17p13 LOH | MIB-1 PI (%) | p53 IHC | EGFRIHC score | EGFR amplification | YKL-40 rank |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 72 | M | left frontal lobe | GBM | 176 | no | yes | no | yes | no | 19 | neg | 6 | no | 1 |
| 58 | M | left temporal | GBM | na | no | no | yes | yes | yes | 25 | pos | 1 | no | 2 |
| 50 | M | right frontal lobe | GBM | na | no | yes | yes | yes | no | 25 | neg | 3 | no | 3 |
| 79 | F | left internal capsule | GBM | na | no | no | yes | yes | no | 15 | neg | 0 | no | 4 |
| 60 | M | na | GBM | na | no | no | yes | yes | yes | 25 | neg | 2 | no | 5 |
| 83 | F | right temporal | GBM | 12 | no | no | yes | yes | no | 32 | neg | 2 | no | 6 |
| 60 | M | right parietal | GBM | 517 | no | no | no | yes | yes | 25 | pos | 2 | no | 7 |
| 81 | M | left parietal | GBM | 77 | no | no | yes | no | no | 30 | neg | 2 | no | 8 |
| 72 | M | right frontal lobe | GBM | 154 | no | no | no | yes | no | 25 | neg | 4 | no | 9 |
| 83 | M | left parietal | GBM | 32 | no | no | no | no | yes | 20 | neg | 3 | no | 10 |
| 58 | F | na | AO | na | yes | no | yes | yes | no | 20 | neg | 1 | no | 11 |
| 33 | M | midbrain | GBM | 361 | no | no | no | yes | no | 10 | neg | 2 | no | 12 |
| 37 | F | left hemispehre | AO | na | no | na | na | na | na | 5 | na | 4 | no | 13 |
| 59 | M | na | GBM | na | no | yes | yes | yes | yes | 30 | neg | 6 | yes | 14 |
| 80 | M | left temporal | GBM | 348 | no | no | no | yes | yes | 15 | neg | 4 | no | 15 |
| 80 | M | right parietal | GBM | 285 | no | yes | yes | yes | yes | 50 | pos | 2 | no | 16 |
| 53 | M | frontal | GBM | 519 | no | no | yes | yes | no | 25 | neg | 6 | yes | 17 |
| 68 | M | right frontal lobe | GBM | 64 | no | no | no | no | no | 8 | neg | 1 | no | 18 |
| 76 | F | left temporal | GBM | 60 | no | no | no | yes | yes | 25 | pos | 4 | no | 19 |
| 62 | M | right frontal lobe | GBM | 128 | no | yes | yes | yes | yes | 15 | neg | 3 | no | 20 |
| 60 | F | corpus callosum | GBM | 371 | no | yes | yes | yes | yes | 10 | neg | 6 | yes | 21 |
| 74 | M | left temporal | GBM | na | no | no | yes | yes | no | 20 | neg | 6 | yes | 22 |
| 70 | M | thalamus | AO | na | no | yes | yes | no | yes | 20 | neg | 1 | no | 23 |
| 85 | M | left temporal | GBM | 112 | no | yes | no | yes | no | 55 | neg | 2 | no | 24 |
| 57 | F | occipital | GBM | 230 | no | yes | yes | yes | no | 60 | neg | 4 | no | 25 |
| 64 | M | left frontal lobe | GBM | 255 | no | yes | yes | yes | no | 40 | neg | 6 | yes | 26 |
| 77 | M | left frontal lobe | GBM | 112 | no | yes | yes | yes | no | 15 | neg | 6 | yes | 27 |
| 82 | F | left temporal | GBM | 30 | no | no | no | no | yes | 35 | pos | 4 | no | 28 |
| 16 | F | left tha lam us | GBM | na | no | yes | yes | no | no | 10 | neg | 0 | no | 29 |
| 59 | F | right parietal | AO | na | yes | no | no | yes | no | 5 | neg | 6 | no | 30 |
| 57 | M | rght hemisphere | GBM | 482 | no | no | yes | yes | no | 30 | neg | 6 | yes | 31 |
| 61 | M | na | GBM | 407 | no | no | no | yes | no | 12 | neg | 4 | No | 32 |
| 55 | M | na | GBM | 143 | no | yes | yes | yes | no | 20 | neg | 6 | yes | 33 |
| 70 | M | right temporal | GBM | 190 | no | no | yes | yes | no | 12 | neg | 6 | yes | 34 |
| 80 | M | left hemisphere | GBM | na | no | no | yes | yes | no | 10 | neg | 6 | yes | 35 |
| 61 | M | right temporo-parietal | GBM | na | no | no | no | yes | no | 30 | neg | 6 | yes | 36 |
| 55 | M | left temporal | GBM | na | no | yes | no | yes | no | 20 | neg | 2 | no | 37 |
| 58 | M | right hemisphere | GBM | na | no | no | yes | no | yes | 25 | pos | 1 | no | 38 |
| 55 | M | right temporal | GBM | na | no | no | no | yes | no | 20 | pos | 2 | no | 39 |
| 63 | M | right thalamus | GBM | 221 | no | yes | yes | yes | no | 30 | neg | 6 | yes | 40 |
| 27 | F | na | GBM | na | no | no | no | no | yes | 20 | neg | 2 | no | 41 |
| 85 | M | na | GBM | 62 | no | yes | yes | yes | no | 25 | neg | 6 | yes | 42 |
| 49 | M | right occipital | AO | na | yes | yes | no | no | no | 30 | neg | 4 | no | 43 |
| 50 | M | right frontal | AO | na | yes | no | na | yes | no | 10 | na | 4 | na | 44 |
| 51 | F | left frontal | AO | na | yes | yes | na | no | no | 30 | neg | 6 | na | 45 |
| 49 | M | left temporal | AO | na | yes | na | na | na | na | 15 | neg | 2 | no | 46 |
| 86 | M | left temporal | GBM | 25 | no | yes | yes | yes | no | 10 | neg | 6 | yes | 47 |
| 75 | M | left parietal | AO | 168 | no | yes | no | yes | no | 20 | pos | 4 | no | 48 |
| 43 | F | right thalamus | GBM | 395 | no | no | no | yes | no | 40 | pos | 1 | no | 49 |
| 68 | F | na | GBM | na | no | yes | yes | yes | no | 20 | neg | 6 | yes | 50 |
| 55 | M | right basal ganglia | GBM | 125 | no | yes | no | yes | no | 25 | neg | 6 | yes | 51 |
| 48 | M | left frontal | AO | 960 | yes | yes | na | no | no | 50 | na | 3 | na | 52 |
| 61 | M | right temporal | GBM | 166 | no | yes | yes | yes | no | 20 | neg | 6 | yes | 53 |
| 41 | M | left frontal | AO | na | yes | na | na | na | na | na | na | 6 | no | 54 |
| 75 | F | right parietal | GBM | na | no | yes | yes | yes | no | 20 | neg | 6 | yes | 55 |
| 58 | M | left frontal lobe | GBM | 285 | no | no | yes | yes | no | 5 | neg | 6 | yes | 56 |
| 59 | M | left frontal lobe | GBM | na | no | no | yes | no | no | 5 | neg | 4 | no | 57 |
| 35 | M | right parietal | AO | na | yes | no | yes | no | na | 75 | neg | 1 | no | 58 |
| 49 | F | right frontal lobe | GBM | 323 | no | yes | yes | yes | no | 30 | neg | 6 | yes | 59 |
| 43 | M | left temporal | GBM | 317 | no | no | no | yes | no | 20 | pos | 4 | no | 60 |
| 55 | F | bifrontal | AO | na | yes | yes | na | na | na | 20 | na | 4 | na | 61 |
| 62 | F | right frontal lobe | GBM | 17 | no | yes | yes | yes | yes | 20 | neg | 6 | no | 62 |
| 30 | F | na | AO | 254 | no | no | na | no | yes | 30 | neg | 6 | na | 63 |
| 42 | F | right frontal | AO | na | yes | yes | na | no | yes | 10 | na | 3 | na | 64 |
| 29 | F | left temporal | AO | na | no | no | na | no | yes | 41 | na | 6 | na | 65 |
| 41 | M | right frontal | AO | 1683 | no | yes | na | yes | no | 30 | na | 6 | na | 66 |
| 74 | M | left occipital | AO | 999 | yes | yes | na | no | yes | 25 | neg | 3 | no | 67 |
| 52 | M | na | AO | 1757 | yes | no | na | no | yes | na | na | 6 | na | 68 |
| 46 | M | left frontal | AO | 124 | yes | no | na | no | yes | 40 | neg | 6 | no | 69 |
| 55 | F | na | GBM | 436 | no | yes | yes | yes | no | 10 | neg | 6 | no | 70 |
| 50 | F | right thalamus | GBM | 109 | no | yes | no | yes | no | 50 | neg | 6 | yes | 71 |
| 38 | M | left frontal | AO | na | no | yes | na | no | no | 10 | pos | 3 | na | 72 |
| 52 | F | right frontal | AO | na | yes | no | na | no | no | 20 | neg | 4 | na | 73 |
| 63 | F | left temporal | AO | na | yes | na | na | na | na | 30 | na | 0 | no | 74 |
| 53 | M | na | AO | na | yes | yes | no | no | no | 25 | neg | 3 | no | 75 |
| 52 | M | right temporal | AO | na | yes | na | na | na | na | 25 | neg | 6 | no | 76 |
| 37 | M | right frontal | AO | na | yes | na | na | na | na | 25 | neg | 6 | no | 77 |
| 47 | F | left temporal | AO | na | yes | yes | no | no | yes | 50 | neg | 4 | no | 78 |
| 42 | F | right frontal | AO | na | yes | no | no | no | no | 20 | neg | 6 | no | 79 |
79 high grade gliomas were ranked according to YKL-40 expression via immunohistochemistry while blinded to all other clinical and pathologic variables (see Materials and Methods). Spearman rank correlations with key molecular and immunohistochemical features were then performed. AO = anaplastic oligodendroglioma; GBM = glioblastoma; IHC = immunohistochemistry; LOH = loss of heterozygosity; PI = proliferation index; na = not available.
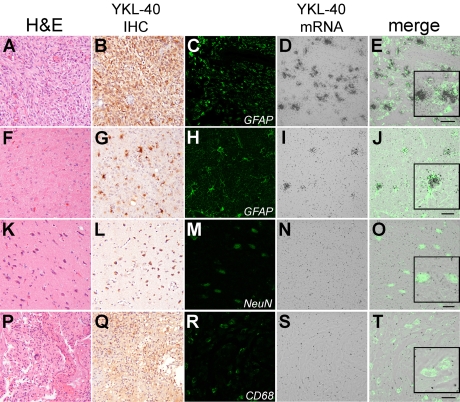
The precise source of YKL-40 mRNA in gliomas was shown to be predominantly in glioma cells (Figure 2A-E), although scattered reactive non-neoplastic astrocytes also produced appreciable amounts of YKL-40 mRNA (Figure 2F-J). Neurons and macrophages/microglia, on the other hand, did not show significant YKL-40 mRNA (Figure 2K-0 and P-T, respectively), although neurons (2L) and macrophages (2Q) did show varying degrees of immunopositivity. Thus, YKL-40 is directly produced by glioma tumor cells and reactive astrocytes, with less contribution from other nonneoplastic elements.
Figure 2.
YKL-40 is directly produced by giioma cells and reactive astrocytes. Confocal microscopy showed thatYKL-40 mRNA colocalizes with GFAP in both giiobiastoma (A-E) and tumor-induced non-neoplastic reactive astrocytosis (F-J). In contrast, although neurons adjacent to giioma are immunopositive for YKL-40, little mRNA is present (K-O). Admixed CD68-positive macrophages and microgiia likewise do not appear to produce YKL-40 mRNA in the neoplastic setting (P-T). Scale bars: A-E=50um; F-J, K-0 and P-T=20um. Insets are higher-magnification images of selected cells within each merged field. The first and second column images (A & B, F & G), K & L, P & Q) are 200× magnification.
EGFR signaling, including gene amplification, is well-known to be a key component of many GBMs [34] and YKL-40 has been shown to correlate with MAPK activation [20, 21]. While the strength of EGFR immunoreactivity positively correlated with EGFR amplification (P < 0.0001), both EGFR immunostaining (P = 0.0012) and gene amplification (P = 0.0054) negatively correlated with YKL-40 rank (Figure 3, Table 2). 17p LOH (P = 0.0298), and increased patient age (P = 0.0356) positively correlated with higher YKL-40 IHC rank. Trends toward positive associations with YKL-40 were identified for 9p21 LOH (P = 0.0558) and male gender (P = 0.0684). No links were identified between GBM YKL-40 and 10q LOH, CDKN2A/ pl6 deletion, p53 accumulation, Ki67 proliferation index (PI), or survival (Table 2), although shorter survival was significantly correlated with increased age (P < 0.0001 via linear regression). Additional significant associations in GBMs were identified between 17p13 LOH and p53 accumulation (P = 0.0031); 9p21 LOH and CDKN2A homozygous deletion (P = 0.022); 10q23 LOH and EGFR amplification (P = 0.0159); and 10q23 LOH and increased EGFR immunoreactivity (P = 0.002).
Figure 3.
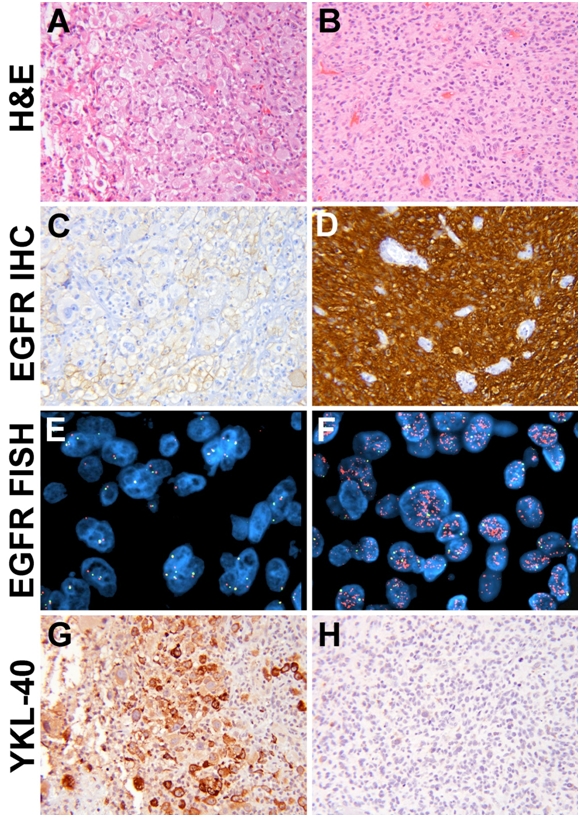
YKL-40 expression is reduced in GBMs with EGFR amplification. Tumors with weak EGFR expression (A, C) also tended to lack EGFR amplification (E) but produced YKL-40 (G). In contrast, tumors strong for EGFR (B, D) were likely to show EGFR amplification (F) but not YKL-40 (H). (P = 0.003 via FISH and = 0.001 via EGFR IHC). Orange signal = 7q34, green = CEP7. All H & E and immunohistochemical images are 200× magnification; both FISH images are 1000× magnification.
Table 2.
Strength of associations with YKL-40 immunohistochemical intensity.
| Association with YKL-40 IHC rank (P) |
|||
|---|---|---|---|
| Parameter | All gliomas | GBM only | AO only |
| Patient age | < 0.0001 | 0.0356 | 0.3953 |
| Gender | 0.0882 | 0.0684 | 0.9281 |
| Glioma type | < 0.0001 | NA | NA |
| Survival | 0.1612 | 0.8630 | 0.7131 |
| 1p19q codeletion | < 0.0001 | NA | 0.3097 |
| 9pL0H | 0.0602 | 0.0558 | 0.9493 |
| Homozygous CDKN2A deletion | 0.4536 | 0.7296 | 0.1328 |
| 10q23 LOH | 0.0006 | 0.8783 | 0.0276 |
| 17p13 LOH | 0.5380 | 0.0298 | 0.5023 |
| Ki67 (MIB-1) PI | 0.3723 | 0.3795 | 0.1141 |
| P53 accumulation | 0.6380 | 0.7702 | > 0.9999 |
| EGFR positive | 0.0034 | 0.0012 | 0.2361 |
| EGFR amplification | 0.3671 | 0.0054 | NA |
79 high grade gliomas were ranked according to YKL-40 expression via immunohistochemistry while blinded to all other clinical and pathologic variables (see Materials and Methods). Spearman rank correlations with key clinical and molecular features were then performed. AO = anaplastic oligodendroglioma; GBM = glioblas-toma; LOH = loss of heterozygosity; PI = proliferation index; NA = not applicable (none of the GBMs showed 1p19q codeletion and none of the AOs showed EGFR amplification).
Twenty-one of 28 AOs (75%) in this cohort had 1p/19q codeletion but showed no correlation with YKL-40 expression (P = 0.3097, Table 2). On the other hand, 10q23 LOH correlated with stronger YKL-40 staining in AOs (P = 0.0276). No associations were found between YKL-40 and age, 9p21 LOH, 17p13 LOH, CDKN2A deletion, p53 accumulation, EGFR expression, Ki67 PI, or survival in AOs (Table 2).
Discussion
YKL-40 has recently attracted attention as a biomarker of metastatic cancers and chronic inflammatory conditions, as well as a possible effector molecule contributing to specific features that are characteristic of neoplastic glial cells (e.g. invasiveness, radioresistance). Our data suggest that YKL-40 expression is stronger in GBMs compared to AOs and is chiefly produced by neoplastic glial cells and reactive as-trocytes. YKL-40 expression is inversely associated with EGFR in GBMs. In contrast, YKL-40 tends to be higher in AOs with 10q23 LOH.
There appears to be a wide variety of cells that secretes YKL-40, including stromal vascular fraction cells in adipose tissue[35], chondro-cytes[2], carcinoma cells, tumor associated macrophages, neutrophils, and mast cells[36]. Herein we demonstrate that the YKL-40 in gliomas is actively being transcribed and translated within the tumor cells, with reactive astrocytes also producing YKL-40. Macrophages, microglia, and neurons, while sometimes showing YKL-40 immunopositivity, do not appear to actively produce the molecule. However, given that macrophages and microglia have already been shown to produce and secrete YKL-40 in viral encephalitis [1], it is possible that these cells contribute to the YKL-40 pool in gliomas in a more temporally limited manner.
The actions of YKL-40, while still mostly unknown, are becoming clearer. As a secreted molecule it can displace FGF-2 from the extracellular matrix and inhibit its actions [1], link membrane-bound syndecan-1 with integrins [37], and inhibit collagen degradation via inhibition of matrix metalloproteinases[35, 38], though other work has shown upregulation of metalloproteinase activity[22]. It may also promote collagen synthesis[35], but another model found opposing results[39]. Recent work has identified YKL-40 as a promoter of angiogenesis in neoplasms, including activating the MAPK/ ERK pathway in endothelial cells[37]. Interestingly, blocking VEGF sharply upregulates YKL-40 expression [23]. These findings are intriguing in light of the fact that microvascular proliferation is used as a diagnostic criterion for GBM and, to a lesser extent, AO.
Prior work has indicated a correlation between YKL-40 expression and activation of MAPK and Akt pathways [2, 20, 37, 38]. Because EGFR can signal through both pathways, initially it was postulated that tumors with strong EGFR expression and EGFR amplification might also exhibit higher YKL-40 expression. Finding the opposite result (Figure 3, Table 2) suggests that, while YKL-40 may activate MAPK and/or Akt, it may itself be negatively regulated by EGFR. Further mechanistic studies to address YKL-40 regulation will be of interest.
The association between YKL-40 expression and 10q23 LOH in high grade gliomas, specifically AOs, is similar to what has been reported in GBMs [22], strengthening the association between 10q and YKL-40. Similar association in this GBM subgroup was not seen, perhaps because the vast majority (84%) had 10q23 LOH, making correlation with YKL-40 rank difficult. In AOs, given that 10q deletion is more common in GBMs than AOs, and AOs with 10q deletion often show more aggressive behavior with shorter survival [40-43], it is possible that AOs with 10q deletion are more like GBMs in terms of genetics and biology, including YKL-40 expression.
In summary, YKL-40 is more abundant in GBMs than AOs, is directly produced by neoplastic cells, and accounts for the majority of YKL-40 in high-grade gliomas. An inverse relationship exists between EGFR and YKL-40 in GBMs, while a direct correlation exists with 10q23 LOH and YKL-40 in AOs. This molecule appears to be an important factor in many gliomas and, as the mechanisms of YKL40 action and regulation become more precisely defined, the importance of this molecule in understanding of glioma biology will be better understood.
Acknowledgments
Additional thanks to Colleen Lovellfor histologic support; Mark Stauffer for YKL-40 immunohisto-chemical assistance; Kathy Cieply, Carol Sherer, Kathy Cumbie, and John Salvatore for FISH data; and Kim Fuhrerfor EGFR immunostaining. CH was supported by a Callie Rohr American Brain Tumor Association Fellowship during this project. CAW was supported by K24 (MH01717).
References
- 1.Bonneh-Barkay D, Bissel SJ, Wang G, Fish KN, Nicholl GC, Darko SW, Medina-Flores R, Murphey -Corb M, Rajakumar PA, Nyaundi J, Mellors JW, Bowser R, Wiley CA. YKL-40, a marker of simian immunodeficiency virus encephalitis, modulates the biological activity of basic fibro-blast growth factor. Am J Pathol. 2008;173:130–143. doi: 10.2353/ajpath.2008.080045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Recklies AD, White C, Ling H. The chitinase 3 -like protein human cartilage glycoprotein 39 (HC-gp39) stimulates proliferation of human connective-tissue cells and activates both extracellular signal-regulated kinase- and protein kinase B-mediated signalling pathways. Biochem J. 2002;365:119–126. doi: 10.1042/BJ20020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johansen JS, Bojesen SE, Mylin AK, Frikke-Schmidt R, Price PA, Nordestgaard BG. Elevated Plasma YKL-40 Predicts Increased Risk of Gastrointestinal Cancer and Decreased Survival After Any Cancer Diagnosis in the General Population. J Clin Oncol. 2009;27:572–578. doi: 10.1200/JCO.2008.18.8367. [DOI] [PubMed] [Google Scholar]
- 4.Mitsuhashi A, Matsui H, Usui H, Nagai Y, Tate S, Unno Y, Hirashiki K, Seki K, Shozu M. Serum YKL-40 as a marker for cervical adenocarci-noma. Ann Oncol. 2009;20:71–77. doi: 10.1093/annonc/mdn552. [DOI] [PubMed] [Google Scholar]
- 5.Yamac D, Ozturk B, Coskun U, Tekin E, Sancak B, Yildiz R, Atalay C. Serum YKL-40 levels as a prognostic factor in patients with locally advanced breast cancer. Adv Ther. 2008;25:801–809. doi: 10.1007/s12325-008-0082-2. [DOI] [PubMed] [Google Scholar]
- 6.Kucur M, Isman FK, Balci C, Onal B, Hacibekiro-glu M, Ozkan F, Ozkan A. Serum YKL-40 levels and chitotriosidase activity as potential bio-markers in primary prostate cancer and benign prostatic hyperplasia. Urol Oncol. 2008;26:47–52. doi: 10.1016/j.urolonc.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Mylin AK, Abildgaard N, Johansen JS, Andersen NF, Heickendorff L, Standal T, Gimsing P, Knudsen LM. High serum YKL-40 concentration is associated with severe bone disease in newly diagnosed multiple myeloma patients. Eur J Haematol. 2008;80:310–317. doi: 10.1111/j.1600-0609.2007.01027.x. [DOI] [PubMed] [Google Scholar]
- 8.Roslind A, Johansen JS, Christensen IJ, Kiss K, Balslev E, Nielsen DL, Bentzen J, Price PA, Andersen E. High serum levels of YKL-40 in patients with squamous cell carcinoma of the head and neck are associated with short survival. Int J Cancer. 2008;122:857–863. doi: 10.1002/ijc.23152. [DOI] [PubMed] [Google Scholar]
- 9.Johansen JS, Brasso K, Iversen P, Teisner B, Garnero P, Price PA, Christensen IJ. Changes of biochemical markers of bone turnover and YKL-40 following hormonal treatment for metas-tatic prostate cancer are related to survival. Clin Cancer Res. 2007;13:3244–3249. doi: 10.1158/1078-0432.CCR-06-2616. [DOI] [PubMed] [Google Scholar]
- 10.Kim SH, Das K, Noreen S, Coffman F, Hameed M. Prognostic implications of immuno-histochemically detected YKL-40 expression in breast cancer. World J Surg Oncol. 2007;5:17. doi: 10.1186/1477-7819-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diefenbach CS, Shah Z, lasonos A, Barakat RR, Levine DA, Aghajanian C, Sabbatini P, Hensley ML, Konner J, Tew W, Spriggs D, Fleisher M, Thaler H, Dupont J. Preoperative serum YKL-40 is a marker for detection and prognosis of endometrial cancer. Gynecol Oncol. 2007;104:435–442. doi: 10.1016/j.ygyno.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt H, Johansen JS, Gehl J, Geertsen PF, Fode K, von der Maase H. Elevated serum level of YKL-40 is an independent prognostic factor for poor survival in patients with metas-tatic melanoma. Cancer. 2006;106:1130–1139. doi: 10.1002/cncr.21678. [DOI] [PubMed] [Google Scholar]
- 13.Bergmann OJ, Johansen JS, Klausen TW, Mylin AK, Kristensen JS, Kjeldsen E, Johnsen HE. High serum concentration of YKL-40 is associated with short survival in patients with acute myeloid leukemia. Clin Cancer Res. 2005;11:8644–8652. doi: 10.1158/1078-0432.CCR-05-1317. [DOI] [PubMed] [Google Scholar]
- 14.Jensen BV, Johansen JS, Price PA. High levels of serum HER-2/neu and YKL-40 independently reflect aggressiveness of metastatic breast cancer. Clin Cancer Res. 2003;9:4423–4434. [PubMed] [Google Scholar]
- 15.Cintin C, Johansen JS, Christensen IJ, Price PA, Sorensen S, Nielsen HJ. High serum YKL-40 level after surgery for colorectal carcinoma is related to short survival. Cancer. 2002;95:267–274. doi: 10.1002/cncr.10644. [DOI] [PubMed] [Google Scholar]
- 16.Bi J, Lau SH, Lv ZL, Xie D, Li W, Lai YR, Zhong JM, Wu HQ, Su Q, He YL, Zhan WH, Wen JM, Guan XY. Overexpression of YKL-40 is an independent prognostic marker in gastric cancer. Hum Pathol. 2009;40:1790–1797. doi: 10.1016/j.humpath.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Tanwar MK, Gilbert MR, Holland EC. Gene expression microarray analysis reveals YKL-40 to be a potential serum marker for malignant character in human glioma. Cancer Res. 2002;62:4364–4368. [PubMed] [Google Scholar]
- 18.Nutt CL, Betensky RA, Brower MA, Batchelor TT, Louis DN, Stemmer-Rachamimov AO. YKL-40 is a differential diagnostic marker for histologic subtypes of high-grade gliomas. Clin Cancer Res. 2005;11:2258–2264. doi: 10.1158/1078-0432.CCR-04-1601. [DOI] [PubMed] [Google Scholar]
- 19.Rousseau A, Nutt CL, Betensky RA, lafrate AJ, Han M, Ligon KL, Rowitch DH, Louis DN. Expression of oligodendroglial and astrocytic lineage markers in diffuse gliomas: use of YKL-40, ApoE, ASCL1, and NKX2-2. J Neuropathol Exp Neurol. 2006;65:1149–1156. doi: 10.1097/01.jnen.0000248543.90304.2b. [DOI] [PubMed] [Google Scholar]
- 20.Pelloski CE, Lin E, Zhang L, Yung WK, Colman H, Liu JL, Woo SY, Heimberger AB, Suki D, Prados M, Chang S, Barker FG, 3rd, Fuller GN, Al-dape KD. Prognostic associations of activated mitogen-activated protein kinase and Akt pathways in glioblastoma. Clin Cancer Res. 2006;12:3935–3941. doi: 10.1158/1078-0432.CCR-05-2202. [DOI] [PubMed] [Google Scholar]
- 21.Pelloski CE, Mahajan A, Maor M, Chang EL, Woo S, Gilbert M, Colman H, Yang H, Ledoux A, Blair H, Passe S, Jenkins RB, Aldape KD. YKL-40 expression is associated with poorer response to radiation and shorter overall survival in glioblastoma. Clin Cancer Res. 2005;11:3326–3334. doi: 10.1158/1078-0432.CCR-04-1765. [DOI] [PubMed] [Google Scholar]
- 22.Nigro JM, Misra A, Zhang L, Smirnov I, Colman H, Griffin C, Ozburn N, Chen M, Pan E, Koul D, Yung WK, Feuerstein BG, Aldape KD. Integrated array-comparative genomic hybridization and expression array profiles identify clinically relevant molecular subtypes of glioblastoma. Cancer Res. 2005;65:1678–1686. doi: 10.1158/0008-5472.CAN-04-2921. [DOI] [PubMed] [Google Scholar]
- 23.Saidi A, Javerzat S, Bellahcene A, De Vos J, Bello L, Castronovo V, Deprez M, Loiseau H, Bikfalvi A, Hagedorn M. Experimental anti-angiogenesis causes upregulation of genes associated with poor survival in glioblastoma. Int J Cancer. 2008;122:2187–2198. doi: 10.1002/ijc.23313. [DOI] [PubMed] [Google Scholar]
- 24.Pelloski CE, Ballman KV, Furth AF, Zhang L, Lin E, Sulman EP, Bhat K, McDonald JM, Yung WK, Colman H, Woo SY, Heimberger AB, Suki D, Prados MD, Chang SM, Barker FG, 2nd, Buckner JC, James CD, Aldape K. Epidermal growth factor receptor variant III status defines clinically distinct subtypes of glioblastoma. J Clin Oncol. 2007;25:2288–2294. doi: 10.1200/JCO.2006.08.0705. [DOI] [PubMed] [Google Scholar]
- 25.Aldape K. Multiple markers increase overall predictive power as compared to any single marker (presentation) New Orleans: Society for Neuro-Oncology. 2009 [Google Scholar]
- 26.Junker N, Johansen JS, Hansen LT, Lund EL, Kristjansen PE. Regulation of YKL-40 expression during genotoxic or microenvironmental stress in human glioblastoma cells. Cancer Sci. 2005;96:183–190. doi: 10.1111/j.1349-7006.2005.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Y, Siadaty MS, Berens ME, Hampton GM, Theodorescu D. Overlapping gene expression profiles of cell migration and tumor invasion in human bladder cancer identify metallothionein IE and nicotinamide N-methyltransferase as novel regulators of cell migration. Oncogene. 2008;27:6679–6689. doi: 10.1038/onc.2008.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kassem H, Sangar V, Cowan R, Clarke N, Margison GP. A potential role of heat shock proteins and nicotinamide N-methyl transferase in predicting response to radiation in bladder cancer. Int J Cancer. 2002;101:454–460. doi: 10.1002/ijc.10631. [DOI] [PubMed] [Google Scholar]
- 29.Bhat KP, Pelloski CE, Zhang Y, Kim SH, deLaCruz C, Rehli M, Aldape KD. Selective repression of YKL-40 by NF-kappaB in glioma cell lines in-volves recruitment of histone deacetylase-1 and -2. FEBS Lett. 2008;582:3193–3200. doi: 10.1016/j.febslet.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Nutt CL. Molecular genetics of oligodendroglio-mas: a model for improved clinical management in the field of neurooncology. Neurosurg Focus. 2005;19:E2. doi: 10.3171/foc.2005.19.5.3. [DOI] [PubMed] [Google Scholar]
- 31.Horbinski C, Dacic S, McLendon RE, Cieply K, Datto M, Brat DJ, Chu CT. Chordoid Glioma: A Case Report and Molecular Characterization of Five Cases. Brain Pathol. 2009;19:439–448. doi: 10.1111/j.1750-3639.2008.00196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pollack IF, Finkelstein SD, Woods J, Burnham J, Holmes EJ, Hamilton RL, Yates AJ, Boyett JM, Finlay JL, Sposto R. Expression of p53 and prognosis in children with malignant gliomas. N Engl J Med. 2002;346:420–427. doi: 10.1056/NEJMoa012224. [DOI] [PubMed] [Google Scholar]
- 33.Pollack IF, Hamilton RL, Finkelstein SD, Campbell JW, Martinez AJ, Sherwin RN, Bozik ME, Gollin SM. The relationship between TP53 mutations and overexpression of p53 and prognosis in malignant gliomas of childhood. Cancer Res. 1997;57:304–309. [PubMed] [Google Scholar]
- 34.Kleihues P, Ohgaki H. Primary and secondary glioblastomas: from concept to clinical diagnosis. Neuro Oncol. 1999;1:44–51. doi: 10.1093/neuonc/1.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iwata T, Kuwajima M, Sukeno A, Ishimaru N, Hayashi Y, Wabitsch M, Mizusawa N, Itakura M, Yoshimoto K. YKL-40 secreted from adipose tissue inhibits degradation of type I collagen. Biochem Biophys Res Commun. 2009;388:511–516. doi: 10.1016/j.bbrc.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 36.Hogdall EV, Ringsholt M, Hogdall CK, Christen-sen IJ, Johansen JS, Kjaer SK, Blaakaer J, Os-tenfeld-Moller L, Price PA, Christensen LH. YKL-40 tissue expression and plasma levels in patients with ovarian cancer. BMC Cancer. 2009;9:8. doi: 10.1186/1471-2407-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shao R, Hamel K, Petersen L, Cao QJ, Arenas RB, Bigelow C, Bentley B, Yan W. YKL-40, a secreted glycoprotein, promotes tumor angio-genesis. Oncogene. 2009 doi: 10.1038/onc.2009.292. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ling H, Recklies AD. The chitinase 3-like protein human cartilage glycoprotein 39 inhibits cellular responses to the inflammatory cytokines interleukin-1 and tumour necrosis factor-alpha. Biochem J. 2004;380:651–659. doi: 10.1042/BJ20040099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Badariotti F, Kypriotou M, Lelong C, Dubos MP, Renard E, Galera P, Favrel P. The phyloge-netically conserved molluscan chitinase-like protein 1 (Cg-Clpl), homologue of human HC-gp39, stimulates proliferation and regulates synthesis of extracellular matrix components of mammalian chondrocytes. J Biol Chem. 2006;281:29583–29596. doi: 10.1074/jbc.M605687200. [DOI] [PubMed] [Google Scholar]
- 40.Lavon I, Zrihan D, Zelikovitch B, Fellig Y, Fuchs D, Soffer D, Siegal T. Longitudinal assessment of genetic and epigenetic markers in oligoden-drogliomas. Clin Cancer Res. 2007;13:1429–1437. doi: 10.1158/1078-0432.CCR-06-2050. [DOI] [PubMed] [Google Scholar]
- 41.Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64:479–489. doi: 10.1093/jnen/64.6.479. [DOI] [PubMed] [Google Scholar]
- 42.Sanson M, Leuraud P, Aguirre-Cruz L, He J, Marie Y, Cartalat-Carel S, Mokhtari K, Duffau H, Delat-tre JY, Hoang-Xuan K. Analysis of loss of chromosome 10q, DMBT1 homozygous deletions, and PTEN mutations in oligodendroglio-mas. J Neurosurg. 2002;97:1397–1401. doi: 10.3171/jns.2002.97.6.1397. [DOI] [PubMed] [Google Scholar]
- 43.Ueki K, Nishikawa R, Nakazato Y, Hirose T, Hi-rato J, Funada N, Fujimaki T, Hojo S, Kubo O, Ide T, Usui M, Ochiai C, Ito S, Takahashi H, Mukasa A, Asai A, Kirino T. Correlation of histology and molecular genetic analysis of 1p, 19q, 10q, TP53, EGFR, CDK4, and CDKN2A in 91 astrocytic and oligodendroglial tumors. Clin Cancer Res. 2002;8:196–201. [PubMed] [Google Scholar]