Abstract
Fibrolamellar hepatocellular carcinoma (FLHCC) is an aggressive neoplasm due to high frequency of recurrence after surgical resection and resistance to chemotherapy and radiation therapy. Activation of transcription factor NF-kB signaling pathway has been recognized for involvement in progression of various malignant neoplasms. The role of NF-kB pathway in FLHCC has not been studied to date. Formalin-fixed, paraffin-embedded tissue sections of 8 FLHCC, 10 normal liver tissues (NLT) were evaluated immunohistochemically for the expression of p-NF-kBp65 using phosphospecific antibody directed against phosphorylated (p)-NF-kBp65 (Ser 536). The level of p-NF-kBp65 (Ser 536) expression was categorized into four grades: 0 (background), 1+ (weak), 2+ (moderate), or 3+ (strong) based on intensity of intranuclear staining, and was further assessed using two scales: high expression (2+ or 3+) and low expression (0 or 1+). Only high expression of p-NF-kBp65 (Ser 536) in cells with nuclear translocation was considered as constitutive NF-kB activation. High expression of p-NF-kBp65 (Ser 536) was found in 88 % (7/8) of FLHCC tissue. In contrast, only 10 % (1/10) of NLT showed high expression for p-NF-kBp65 (Ser 536). The differences in p-NF-kBp65 nuclear expression between FLHCC tissue and NLT were significant (P < 0.001). There was no significant correlation between the expression of intranuclear p-NF-kBp65 and the stage of FLHCC. Constitutive NF-kB activation was observed in FLHCC. The findings suggest that NF-kB activation is involved in the tumorigenesis of FLHCC and may represent novel targets for therapeutic intervention to FLHCC.
Keywords: Fibrolamellar hepatocellular carcinoma, nuclear factor-kappa B, immunohistochemistry
Introduction
In 1956, Edmondson reported the first case of fibrolamellar hepatocellular carcinoma (FLHCC) as an uncommon histological variant of hepatocellular carcinoma (HCC) [1]. In 1980, FLHCC was established as a distinct clinicopa-thological entity by Craig et al [2] and Berman et al. [3]. In contrast to the conventional type of HCC, FLHCC is predominantly a disease of young adults and rarely associated with chronic liver disease. Often times, no aetiological factors can be identified [4, 5]. FLHCC is histological ly characterized by large, eosinophilic polygonal tumor cells separated by a marked fibrous stroma arranged in lamellar bands around the tumour cells [6]. Currently, FLHCC is considered as an aggressive neoplasm because of high frequency of recurrence after surgical resection and resistance to chemotherapy and radiation therapy [7, 8]. The major molecular pathogenic processes involved in FLHCC development are still poorly understood.
Nuclear factor-kB (NF-kB) is a transcription factor and functions as regulator of kB light chain expression in mature B lymphocytes and plasma cells [9]. NF-kB is widely used by eukaryotic cells as a regulator of genes that control cell proliferation and cell survival. The activation of transcription factor NF-kB signaling pathway plays a pivotal role in immune and inflammatory responses, developmental processes, cellular growth, and apoptosis [10]. Its constitutive activation is related to progression of various malignant neoplasms [10-13]. Studies also have demonstrated that NF-kB activation is one of key mechanism for chemotherapy resistance, and that inhibition of the NF-kB pathway may enhance the efficacy of cancer therapy. Extensive involvement of NF-kB transcription factors in tumors has established them as targets for therapeutics [14]. Up to now, activation status of NF-kB and its signaling pathway in FLHCC has not been investigated. It has not been known whether or not this pathway is associated with pathogenesis in FLHCC. In this study, we evaluated the immunohisto-chemical expression of phosphorylated (p)-NF-kBp65 (Ser 536) with nuclear translocation in FLHCC and non-neoplastic liver tissue to determine if activation of the NF-kB signaling pathway is present and may play a role in FLHCC.
Materials and methods
Patients and specimens
The study protocol was approved by the institute review board (IRB). The study group was comprised of patients submitted to tumor resection at Memorial Hermann Hospitals, an affiliated hospital of the University of Texas Health Science Center at Houston and University of Texas M. D. Anderson Cancer Center Hospital. Surgical pathology cases were evaluated, including 8 FLHCC and 10 normal liver tissues (NLT). The demographic and clinical profiles for each patient with FLHCC are listed in Table 1. Tumor node metastasis (TNM) stage of the American Joint Commission on Cancer (AJCC) [15] was used for tumor stage of this study. The age of patients with FLHCC ranged from 23 to 80 years, with a mean age of 37. Of the 8 patients, 4 were men and 4 women. None of the patients had any evidence of chronic liver disease. All patients had negative serology for hepatitis A, B and C infection and normal serum alpha-fetoprotein (AFP). 6 patients were T2NOMO stage; 1 was T3N0M0, and 1 was T3N1M1.
Table 1.
Clinical profile of 8 patients with FLHCC
| Case | Age(yr) | Race | Sex | Tumor Stage | Chronic Liver disease | α-fetoprotein (ng/mL) |
|---|---|---|---|---|---|---|
| 1 | 80 | White | Female | T2NOMO | No | Normal |
| 2 | 23 | Asia | Male | T2NOMO | No | Normal |
| 3 | 35 | White | Female | T3NOMO | No | Normal |
| 4 | 42 | White | Male | T3N1M1 | No | Normal |
| 5 | 20 | White | Female | T2NOMO | No | Normal |
| 6 | 38 | White | Male | T2NOMO | No | Normal |
| 7 | 27 | Hispanic | Female | T2NOMO | No | Normal |
| 8 | 31 | White | Male | T2NOMO | No | Normal |
Tissue sections from the specimens were fixed in 10% buffered formalin, processed and stained with hematoxylin and eosin. To confirm the diagnosis the cases were reviewed independently by two pathologists.
Immunohistochemical staining
Immunohistochemical stain was performed on formalin-fixed and paraffin-embedded 4-μm sections. The tissue sections were deparaffined, and antigen retrieval conditions included 0.1M citrate buffer (pH 6.0) in an 800-W microwave oven for 15 minutes. The sections were incubated in 3% hydrogen peroxide for 5 minutes to quench endogenous tissue peroxidase. The tissue sections were then incubated with a monoclonal antibody (a phosphospecific probe directed against Serine536, a putative site of activation) against phosphorylated (p)-NF-kBp65 (Ser 536), at 1:600 dilution for 30 minutes at room temperature (Cell Signaling Technology Inc, Danvers, MA). The slides were stained in an automated immunostainer using a standard avidin-biotin complex staining procedure. Immu-nohistochemical reactions were developed with diaminobenzidine as the chromogenic peroxidase substrate, and slides were counters-tained with hematoxylin. Prostatic carcinoma served as the positive control. Negative controls were performed for all cases and consisted of identically prepared slides that were treated with antibody diluent (Dako Corp, Carpinteria, CA) in place of primary antibody, but otherwise subjected to the same immunohistochemical staining protocol.
Assessment of immunohistochemical staining
Positive p-NF-kBp65 expression was defined as nuclear staining, which could be easily identified at low-power magnification (<=100×). Scant faint finely granular background staining, which could not be seen at low-power magnification (<=100×), was interpreted as negative staining (background staining). The level of p-NF -KBp65 intranuclear expression in tumor cells and non-neoplastic cells was categorized into four grades: 0 (background), 1+ (weak), 2+ (moderate), or 3+ (strong) based on intensity of intranuclear staining. The expression of p-NF-kBp65 was further assessed using two scales: high expression (2+ or 3+) and low expression (0 or 1+). Only high expression of p-NF-kBp65 (Ser 536) with nuclear translocation in cells was considered as constitutive NF-kB activation.
Statistics
Mean values and standard deviations were calculated to describe the data population. Statistical analyses were performed using the Fisher exact test or two-tailed t test. A P value of <0.05 was considered to be significant.
Results
The distribution of p-NF-kBp65 (Ser 536) protein expression in liver tissuewas examined by means of immunohistochemical analysis of tissue samples. The intensity of NF-kBp65 staining was scored using two scales: “high expression” represented moderate (2+) or strong (3+) staining while “low expression” indicated background (0) or weak (1+) staining. Only high expression of p-NF-kBp65 (Ser 536) with nuclear translocation in tumor cells is considered as constitutive NF-kB activation. Staining of this protein was detected in the nuclei of both HCC and non-HCC tissue. p-NF-kBp65 (Ser 536) staining intensity was generally low (grade 0 or 1+) in NLT samples (Figure A-B) while the vast majority of HCC samples showed high expres-sion(grade 2+ or 3+) (Figure C-D). The results of p-NF-kBp65 (Ser 536) immunohistochemical staining for FLHCC and NLT are summarized in Table 2. The high expression of p- NF-kBp65 (Ser 536) was present in 88 % (7/8) of FLHCC. In contrast, only 10 % (1 /10) of NLT showed high expression. The difference in high expression between HCC group and NLT group was highly statistically significant (<0.001). There was no significant correlation between the expression of p-NF-kB Ser 536) and the stage of FLHCC. NF-kBp65 phosphorylated on serine 536 showed various degree of intranuclear staining in other types of cells of the liver, including bile duct epithelium, endothelial cells, and stromal cells.
Figure 1.
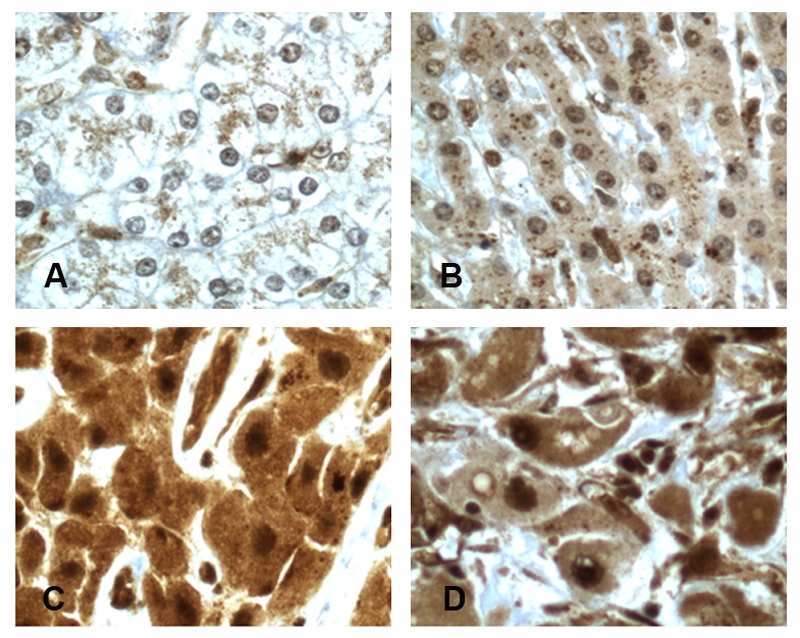
Expression of p-NF-KBp65 (Ser 536) with nuclear translocation in FLHCC and normal liver tissue. A, normal liver tissue (background staining, grade 0). B, normal liver tissue (weakly positive, grade 1+). C, FLHCC (moderately positive, grade 2+). D, FLHCC (strongly positive, grade 3+).
Table 2.
Expression of p-NF-kBp65(Ser536) in FLHCC and normal liver tissue
| p- NF-kBp65 nuclear staining intensity* n (%) |
|||||
|---|---|---|---|---|---|
| Low expression |
High expression |
||||
| 0 | 1+ | 2+ | 3+ | Total No. of Cases | |
| FLHCC | 0(0) | 1(12) | 3(38) | 4(50) | 8 |
| NLT | 4(40) | 5(50) | 1(10) | 0(0) | 10 |
FLHCC. fibrolamellar hepatocellular carcinoma; NLT: normal liver tissue
Nuclear staining intensity is graded as 0 (scant faint finely granular background staining), 1+ (weak), 2+ (moderate), or 3+ (strong).
Discussion
The constitutive activation of NF-kB has been implicated in the pathogenesis and progression of many human malignant neoplasms[16-19]. Although constitutive activation of NF-kB was reported in conventional HCC [20], no prior studies have examined the activation status of NF-kBp65in human FLHCC. In this study, we used immunohistochemical methods to examine expression of p-NF-kBp65 (Ser 536) in FLHCC and normal liver tissue. Our data clearly demonstrated that expression of this protein analyte is significantly enhanced in FLHCC compared with normal liver tissue as regards the intensity of immunostaining. In our study, constitutive NF-kB activation (defined by high expression of NF-kBp65 phosphorylated on a putative site of activation, namely serine 536 and showing nuclear translocation) was observed in 88% of FLHCC and only 10 % of normal liver tissue, suggesting NF-kB activity might play a role in FLHCC tumorigenesis. We have not analyzed the mechanism(s) nor the signal transduction pathways that could lead to constitutive activation of NF-kB in FLHCC. In general, the activation of the NF-kB signal transduction pathway, either by mutation of specific family members or by induction of the pathway through convergent signaling of upstream molecular pathways, is known to contribute to tumorigenesis. It is possible that the consequence of alteration of this pathway is the misguided activation of genes that control tumor cell proliferation, block apoptosis, promote migration and activate angiogenesis [16, 17, 19].
FLHCC is rare histological subtype comprising approximately 4% of all HCC. Currently surgical resection is the treatment of first choice for FLHCC. Multimodality treatments including chemotherapy are considered for patients with recurrent disease in addition to repeated resection [21]. However, there is still no proven effective adjuvant systemic therapy for FLHCC because tumor cells are either inherently resistant to chemotherapy, or they develop resistance during the course of therapy [8, 22]. Several studies have suggested the NF-kB activation may play a key role in chemotherapy resistance, and that inhibition of NF-kB significantly enhances tumor cell response to chemothera-peutic agents [23]. Baldwin et al. reported that treatments to HT 1080 fibrosarcoma cells with chemotherapeutic agent, daunorubicin induce strong nuclear accumulation of accumulation of the NF-[kappa] B p50-p65 heterodimer. Inhibition of NF-kB by expression of the super-repressor form of I[kappa] B [alpha] dramatically improves the apoptotic response to ionizing radiation or daunorubicin [24]. It is likely that constitutive activation of NF-kB in FLHCC share similar pathogenic mechanisms of tumor cell resistance to chemotherapeutic agents. Further studies are needed to clarify this possibility.
The significance of our findings is that it may have a potential target of therapy in FLHCC. High expression of p-NF-kBp65 (Ser 536) which is present in a high percentage of FLHCC, but in only small percentage or rarely in non-HCC tissue, suggests that NF-kB signaling pathway may be a target for cancer treatment. NF-kB functions as a key molecule that control apop-tosis. The anti-apoptotic response of NF-kB may depend on the expression of its downstream target genes, which can be influenced greatly by other signaling pathways. Inhibition of constitutive NF-kB activity is able to amplify apoptotic response in tumor cells treated with other therapeutic agents [25]. Studies by Wiestner et al. showed that constitutive activation of the NF-kB signaling pathway is observed in diffuse large B-cell lymphoma, and interference with this pathway selectively kills these lymphoma cells [26].
Finally, since there is significant differential expression of NF-kB between FLHCC and non-neoplastic liver, NF-kB, as a tumor biomarker, may offer diagnostic aid in differential diagnosis of FLHCC and non-neoplastic liver, which sometimes is difficult in small biopsy specimens.
In summary, NF-kB pathway was activated con-stitutively in FLHCC tissues, and may be involved in FLHCC development and progression. Molecular targeted therapy against NF-kB activation may be effective in FLHCC, especially those with constitutive NF-kB activation. In addition, NF-kB may have potential diagnostic value in differential diagnosis of FLHCC of small liver biopsy specimens. The molecular mechanism(s) of NF-kB signaling pathway in FLHCC merits further investigation.
Acknowledgments
Authors thank Ms. Bheravi Patel for secretary assistance, and Ms. Pamela K Johnston for immunohistochemistry
References
- 1.Edmondson HA. Differential diagnosis of tumors and tumor-like lesions of liver in infancy and childhood. AMA J Dis Child. 1956;91:168–186. doi: 10.1001/archpedi.1956.02060020170015. [DOI] [PubMed] [Google Scholar]
- 2.Craig JR, Peters RL, Edmondson HA, Omata M. Fibrolamellar carcinoma of the liver: A tumour of adolescents and young adults with distinctive clinico-pathologic features. Cancer. 1980;46:372–9. doi: 10.1002/1097-0142(19800715)46:2<372::aid-cncr2820460227>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 3.Berman MM, Libbey NP, Foster JH. Hepatocellular carcinoma: Polygonal cell type with fibrous stroma: An atypical variant with a favorable prognosis. Cancer. 1980;46:1448–55. doi: 10.1002/1097-0142(19800915)46:6<1448::aid-cncr2820460626>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 4.Soyer P, Roche A, Rougier P, Levesque M. CT of fibrolamellar hepatocellular carcinoma. J. Comput. Assist. Tomogr. 1991;15:533–8. doi: 10.1097/00004728-199107000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Imai T, Yokoi H, Noguchi H, Kawarada Y, Mizumoto R. Fibrolamellar carcinoma of the liver: A case report. Gastroenterol Jpn. 1991;26:382–9. doi: 10.1007/BF02781929. [DOI] [PubMed] [Google Scholar]
- 6.Ang PT, Evans H, Pazdur R. Fibrolamellar hepatocellular carcinoma. Am. J. Clin. Oncol. 1991;14:175–8. [PubMed] [Google Scholar]
- 7.Maniaci V, Davidson BR, Rolles K, Dhillon AP, Hackshaw A, Begent RH, Meyer T. Fibrolamellar hepatocellular carcinoma: prolonged survival with multimodality therapy. Eur J Surg Oncol. 2009;35:617–621. doi: 10.1016/j.ejso.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Moreno-Luna LE, Arrieta O, García-Leiva J, Martínez B, Torre A, Uribe M, León-Rodríguez E. Clinical and pathologic factors associated with survival in young adult patients with fibrolamellar hepatocarcinoma. BMC Cancer. 2005;5:142. doi: 10.1186/1471-2407-5-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46:705–716. [PubMed] [Google Scholar]
- 10.Garg A, Aggarwal BB. Nuclear transcription factor -kappaB as a target for cancer drug development. Leukemia. 2002;16:1053–1068. doi: 10.1038/sj.leu.2402482. [DOI] [PubMed] [Google Scholar]
- 11.Yu HG, Yu LL, Yang Y, Luo HS, Yu JP, Meier JJ, Schrader H. Increased expression of RelA/ nuclear factor-kappa B protein correlates with colorectal tumorigenesis. Oncology. 2003;65:37–45. doi: 10.1159/000071203. [DOI] [PubMed] [Google Scholar]
- 12.Liptay S, Weber CK, Ludwig L, Wagner M, Adler G, Schmid RM. Mitogenic and antiapoptotic role of constitutive NF-êB/Rel activity in pancreatic cancer. Int J Cancer. 2003;105:735–746. doi: 10.1002/ijc.11081. [DOI] [PubMed] [Google Scholar]
- 13.Nair A, Venkatraman M, Maliekal TT, Nair B, Karunagaran D. NF-êB is constitutive ly activated in high-grade squamous intraepithelial lesions and squamous cell carcinomas of the human uterine cervix. Oncogene. 2003;22:50–58. doi: 10.1038/sj.onc.1206043. [DOI] [PubMed] [Google Scholar]
- 14.Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J Clin Invest. 2001;107:241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anonymous American Joint Committee on Cancer. Liver. 2002;14:131–8. [Google Scholar]
- 16.Sovak MA, Bellas RE, Kim DW, Zanieski GJ, Rogers AE, Traish AM. Aberrant NF- KB/Rel expression and the pathogenesis of breast cancer. J Clin Invest. 1997;100:2952–60. doi: 10.1172/JCI119848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mori N, Fujii M, Ikeda S, Yamada Y, Tomonaga M, Ballard D. Constitutive activation of NF-kB in primary adult leukemia cells. Blood. 1999;93:2360–8. [PubMed] [Google Scholar]
- 18.Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ, Jr, Sledge GW., Jr Constitutive activation of NF-kappa B during progression of breast cancer to hormone-independent growth. Mol Cell Biol. 1997;17:3629–39. doi: 10.1128/mcb.17.7.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang W, Abbruzzese JL, Evans DB, Larry L, Cleary KR, Chiao PJ. The nuclear factor-kB RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5:119–27. [PubMed] [Google Scholar]
- 20.Wang JH, Huang QK, Chen MX. The role of NF-kB in hepatocellular carcinoma cell. Chin Med J. 2003;116:747–752. [PubMed] [Google Scholar]
- 21.Epstein BE, Pajak TF, Haulk TL, Herpst JM, Order SE, Abrams RA. Metastatic nonresectable fibrolamellar hepatoma. Am J Clin Oncol. 1999;22:22–8. doi: 10.1097/00000421-199902000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Maniaci V, Davidson BR, Rolles K, Dhillon AP, Hackshaw A, Begent RH, Meyer T. Fibrolamellar hepatocellular carcinoma: prolonged survival with multimodality therapy. Eur J Surg Oncol. 2009;35:617–621. doi: 10.1016/j.ejso.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Ma MH, Yang HH, Parker K. The proteasome inhibitor PS-341 markedly enhances sensitivity of multiple myeloma tumor cells to chemotherapeutic agents. Clin Cancer Res. 2003;9:1136–1144. [PubMed] [Google Scholar]
- 24.Wang CY, Mayo M, Baldwin A. TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-[kappa]B. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 25.Foo SY, Nolan GP. NF-kB to the rescue: RELS, apoptosis and cellular transformation. Trends Genet. 1999;15:229–35. doi: 10.1016/s0168-9525(99)01719-9. [DOI] [PubMed] [Google Scholar]
- 26.Wiestner A, Staudt LM. Towards molecular diagnosis and targeted therapy of lymphoid malignancies. Semin Hematol. 2003;40(4):296–307. doi: 10.1016/s0037-1963(03)00194-x. [DOI] [PubMed] [Google Scholar]


