Abstract
GSK-3β, a biologically important signalling protein, is regulated by the Wnt canonical and the PI3K/Akt pathways. We recently reported that mantle cell lymphoma (MCL) frequently shows evidence of GSK-3β inactivation, since GSK-3β is phoshorylated at its functionally critical serine 9 residue in all MCL cell lines and the majority of MCL tumors examined. To further assess the clinical and biological significance of GSK-3β inactivation in MCL, we employed immunohistochemistry to assess the expression of the phosphorylated/inactive form of GSK-3β (pGSK-3β) in 83 paraffin-embedded tumors, and correlated its expression with various biological and clinical parameters. Dichotomizing pGSK-3β into 2 groups produced twenty-seven (32.5%) tumors assessed as negative and fifty-six (67.5%) as positive. Positive pGSK-3β expression correlated significantly with positive nuclear expression of β-catenin and high expression of cyclin D1 (p = 0.0025, 0.0032 Fisher's exact, respectively), both of which have been previously shown to be regulated by GSK-3β regarding their expression levels and/or sub cellular localization in-vitro. However, no significant correlation was found between pGSK-3β and Ki67. Of the clinical parameters, continuous pGSK-3β status had a significant correlation with absolute lymphocyte count in blood (p = 0.0011, Spearman) and negative pGSK-3β expression was significantly correlated with a longer overall survival (p= 0.045, HR = 1.89), but not with age at diagnosis, clinical stage or the international prognostic index. To conclude, our results support the concept that GSK-3β inactivation, found in approximately two-thirds of MCL tumors, is biologically and clinically important in MCL.
Keywords: Mantle cell lymphoma, GSK-3β, immunohistochemistry
Introduction
GSK-3β is an important signalling protein that has been shown to regulate a wide range of cellular functions including apoptosis and cell proliferation. Dysregulation of GSK-3β has been implicated in a wide range of human diseases including diabetes, Alzheimer's, schizophrenia, and cancer [1-4]. One of the major biological functions of GSK-3β is to inhibit β-catenin by sequestration and promotion of its proteosome degradation. GSK-3β itself is known to be regulated by the PI3K/Akt and the Wnt canonical pathways [5-7]. GSK-3β inactivation is mediated by the phosphorylation of its serine 9 residue. Upon inactivation, its inhibitory effect on β-catenin is released, and β-catenin is allowed to accumulate and translocate to the nucleus, where it upregulates the transcription of multiple genes including cyclin D1. GSK-3β also has been shown to directly regulate cyclin D1 via modifying its rate of proteolysis and nuclear export [8, 9].
Mantle cell lymphoma (MCL) is a specific sub-type of aggressive B-cell lymphoma recognized by the World Health Organization Classification Scheme (WHO) [10]. The genetic hallmark of this disease is the recurrent chromosomal abnormality, t(11;14)(q13;q32), which brings the cyclin D1(CCND1) gene under the influence of the enhancer of the immunoglobulin heavy chain (IGH) gene, leading to cyclin D1 overex-pression. Although it has been shown that cyclin D1 overexpression is not sufficient for the induction of lymphoma in animal models, this abnormality is considered to be the primary on-cogenic event in MCL [11, 12]. In view of the roles of GSK-3β in regulating the expression and subcellular localization of cyclin D1, as discussed above, we hypothesized that the activation status of GSK-3β is likely to be relevant to the pathogenesis of MCL. In support of this hypothesis, we have recently shown that GSK-3β is inactivated and phosphorylated in all MCL cell lines and a subset of MCL tumors examined [13]. In another study, using a constitutive active GSK-3β construct, Dal Col et. al. [14] recently provided the first direct evidence that GSK-3β phosphorylates cyclin D1 at Thr286, thereby promoting its nuclear export in MCL. Nevertheless, the biological and clinical significance of GSK-3β in MCL has not been comprehensively examined. Specifically, whether the activation status of GSK-3β correlates with the nuclear expression of β-catenin, the cyclin D1 expression level and/or other clinicopathologic parameters is not known.
In this study, using immunohistochemistry, we assessed the expression of phosphorylated/inactive form of GSK-3β (pGSK-3β) in 83 formalin-fixed, paraffin-embedded MCL tumors. We then correlated the expression of pGSK-3β with the nuclear expression of β-catenin, the expression level of cyclin D1 and Ki67 labelling in these tumors. We also evaluated whether pGSK-3β correlates with various clinical parameters includingthe overall survival.
Materials and methods
MCL tumors
All cases of MCL were diagnosed at the Cross Cancer Institute (Edmonton, Alberta, Canada) between 1994 and 2007, and the diagnostic criteria were based on those described in the World Health Organization Classification Scheme [10]. All cases were confirmed to express cyclin D1 by immunohistochemistry. Six of these 83 MCL tumors used in this study were blastoid variant. The use of these tissues has been approved by our Institutional Ethics Committee.
Immunohistochemistry
To detect pGSK-3β, β-catenin and Ki67, antigen retrieval was performed using microwave-treated citrate buffer (pH 6.0) for 20 minutes. To detect cyclin D1, antigen retrieval was performed using EDTA (pH=8.0). After antigen retrieval, tissue sections were incubated with 3% hydrogen peroxide (H2O2) for 10 minutes to block endogenous peroxidase activity. Subsequently, sections were incubated overnight at 4°C with a rabbit polyclonal antibody reactive with pGSK-3β (Ser 9)(1:50, Cell Signaling Technology, Danvers, MA), a rabbit polyclonal anti-β-catenin (1:1200 dilution, Sigma, clone C2206, Oakville, Ontario, Canada), anti-cyclin D1 (1:75 dilution, clone SP4, Medicorp, Montreal, Quebec, Canada) or anti-Ki67 (1:60 dilution, clone M7240, Dako Canada Inc., Mississauga, Ontario, Canada). Immunostaining was visualized with a labelled streptavidin-biotin (LSAB) method using DAB as a chromogen (Dako Canada).
Scoring of the markers and statistical analysis
The cytoplasmic expression of pGSK-3β was assessed as 3+ (strong staining), 2+ (moderate staining), 1+ (weak staining), 0 (no staining). For β-catenin, cases showing definitive nuclear immunostaining in >20% of the tumor cells were considered positive, and the β-catenin nuclear staining was scored as 3+ (strong staining), 2+ (moderate staining) and 1+ (weak staining). Score for pGSK-3β was calculated as follows: 3 points were given for 3+ staining, 2 points for 2+ staining, 1 point for 1+ staining and 0 for no staining. Cut point analysis for overall survival was used to dichotomize continuous pGSK-3β with the minimum p-value method applied to estimate the optimum pGSK-3β division. The analysis found a pGSK-3β score of 0.6 is the optimal cut point. For nuclear β-catenin, <20% of the tumor cells showing staining or a score of 1 was considered negative, and a score of 2+ or 3+ was considered positive. The scoring used to assess the expression of cyclin D1 and Ki67 have been previously detailed [15]. All of the staining was reviewed by two observers (RC and RL) independently and discrepant cases were reviewed under a double-headed microscope to reach a consensus.
The correlations between pGSK-3β and the other biological markers were assessed using Fisher's exact test for tables and Spearman rank correlation for continuous variables. Univariate and multivariate Cox regression analysis was used to determine the influence of pGSK-3β and various biological and clinical parameters on overall survival. Overall survival plots of the factor groups were calculated using the Kaplan-Meier method. Statistical tests are two-tailed with a P value <0.05 considered to be statistically significant. The SAS computer program SAS (r) 9.2 (TS1M0) was used to perform the analysis
Results
Clinical characteristics of MCL patients
The clinicopathologic characteristics of the 83 MCL patients included in this study are summarized in Table 1. There were 69 men and 14 women, and the median age at diagnosis was 64 years (range, 41-88 years). Fifty patients died during the follow-up and the median time from the initial diagnosis to either the last follow-up or death was 29 months. Treatment for each MCL patient was determined during our weekly lymphoma conference based on our provincial lymphoma treatment protocol. For first-line treatment, the majority of these patients received CHOP-based (cyclophosphamide, doxorubicin, vincristine, prednisone) chemotherapy, and small subsets of patients received chlorambucil-based chemotherapy or more novel treatments such as proteasome inhibitor PS341 and flavopirodol.
Table 1.
Patient demographics and clinical parameters
| pGSH3-β | |||
|---|---|---|---|
| (negative = <0.6) Total cases = 27 n (%) | (postive = ≥ 0.6) total cases = 56 n (%) | Total cases = 83 | |
| Age [n (mean)] | 27 (63.3) | 56(65.5) | 83 |
| Gender | |||
| Female | 5(18.5) | 9(16.1) | 14 |
| Male | 22(81.5) | 47(83.9) | 69 |
| Stage | |||
| I-II | 4(14.8) | 4(7.1) | 8 |
| III | 6(22.2) | 14(25.0) | 20 |
| IV | 15 (55.6) | 37(56.1) | 52 |
| Missing | 2(7.4) | 1(1.8) | 3 |
| B Symptoms | |||
| Yes | 8(29.6) | 15(26.8) | 23 |
| No | 17(63.0) | 39(69.6) | 56 |
| Missing | 2(7.4) | 2(3.6) | 4 |
| GI Involvement | |||
| Yes | 6(22.2) | 6(10.7) | 12 |
| No | 19(70.4) | 48(85.7) | 67 |
| Missing | 2(7.4) | 2(3.6) | 4 |
| IPI | |||
| 0-2 | 15(55.6) | 36(64.3) | 51 |
| ≥3 | 4(14.8) | 12(21.4) | 16 |
| missing | 8 (29.6) | 8(14.3) | 16 |
| β-catenin | |||
| 0-1 | 23(85.2) | 27(48.2) | 50 |
| 2-3 | 3(70.4) | 23(41.1) | 26 |
| Missing | 1(3.7) | 6(10.7) | 7 |
| Cyclin D1 | |||
| 1-2 | 18(66.7) | 23(41.1) | 41 |
| 3 | 3(11.1) | 26(46.4) | 29 |
| Missing | 6(22.2) | 7(12.5) | 13 |
| KI67 | |||
| 0-3 | 21(77.8) | 37(66.1) | 58 |
| 4 | 4(14.8) | 18(32.1) | 22 |
| Missing | 2(7.4) | 1(1.8) | 3 |
pGSK-3β significantly correlates with β-catenin positivity
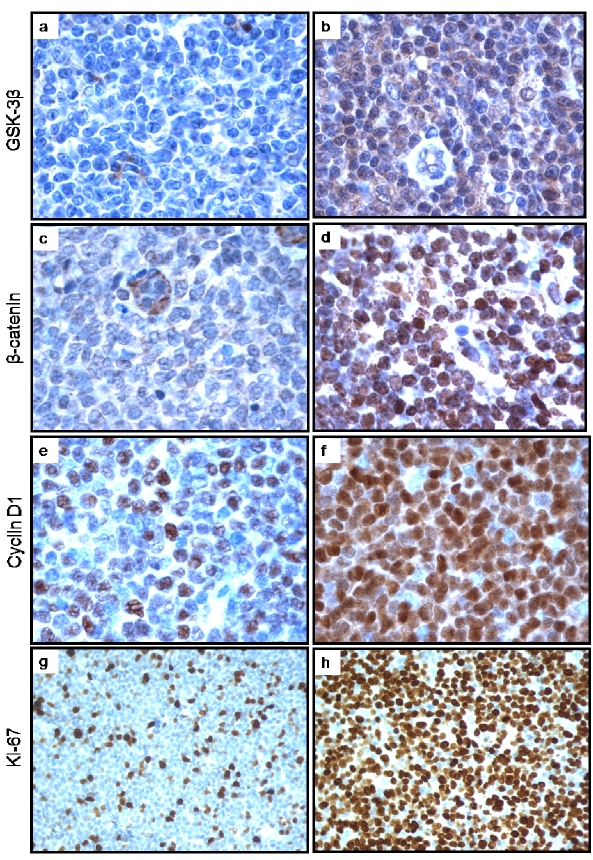
Of the 83 MCL tumors, 56 (67.5%) were considered pGSK-3β positive, based on a minimum p-value cut-off of ≥ 0.6. The expression of pGSK-3β was found to be largely localized to the cytoplasm of the MCL cells (Figure 1 a, b). Of the 76 MCL tumors that were also evaluated for the expression of β-catenin, 26 (32.5%) were assessed positive. In contrast with pGSK-3β, β-catenin was largely expressed in the nuclei of MCL cells (Figure 1 c, d). pGSK-3β positivity significantly correlated with the expression of β-catenin (p = 0.0025, Fisher's exact) (Table 2).
Figure 1.
a, b: Immunohistochemical staining of pGSK-3β in MCL tumors revealed a subset of negative cases and a subset of positive case (a and b respectively). The staining was predominantly cytoplasmic. c, d: Immunohistochemical staining of β-catenin in MCL tumors revealed a subset of negative cases and a subset of positive case (illustrated in c and d respectively). Of note, the staining in the tumor cells was predominantly nuclear; in contrast, the staining was mostly found in the cytoplasm of the endotheiiai cells. e, f: Immunohistochemical staining of cyclin D1 in MCL tumors was heterogeneous with regard to the proportion of intensely positive cells. The case in 1e was assessed cyclin D1-low, as strongly positive cells were <50% of the neoplastic cell population. The case in figure 1f was assessed cyclin D1-high, as strongly positive cells were ≥ 50% of the neoplastic cell population, g, h: Immunohistochemical staining of Ki67 in MCL tumors also revealed a high degree of heterogeneity in the proportion of positive cells, showing a Ki67-negative case and a Ki67 positive case (gand h respectively).
Table 2.
Correlation between pGSK-3β and β-catenin
| β-catenin | ||||
|---|---|---|---|---|
| Negative (0 or 1) | Positive (2 or 3) | Total | P | |
| pGSK3β | ||||
| negative (< 0.6) | 23 | 3 | 26 | 0.0025 |
| positive (≥ 0.6) | 27 | 23 | 50 | |
| Total | 50 | 26 | 76 | |
pGSK-3β significantly correlates with cyclin D1 expression
As illustrated in Figure 1 e and f, the cyclin D1 immunostaining was largely restricted to the nuclei of MCL cells in all cases. Although all cases were positive for cyclin D1 (as per definition of MCL), the proportion of strongly positive cells was highly variable among tumors. To ensure that the assessment of the cyclin D1 expression status was uniform, we excluded 13 cases for which a different anti-cyclin D1 anti-body was used and insufficient tissues were available to repeat the staining. Of the remaining 70 cases, 29 (41.4%) were considered high cyclin D1-expressing. Statistical analysis revealed that the high-expressing cyclin D1 status correlates significantly with positive pGSK-3β (p = 0.0032) (Table 3).
Table 3.
Correlation between pGSK-3β and cyclin D1
| Cyclin D1 | ||||
|---|---|---|---|---|
| Low (1 or 2) | High (3) | Total | P | |
| pGSK-3β | ||||
| negative (< 0.6) | 18 | 3 | 21 | 0.0032 |
| positive (≥ 0.6) | 23 | 26 | 49 | |
| Total | 41 | 29 | 70 | |
Since the gene expression of cyclin D1 has been shown to be up-regulated by β-catenin in various in-vitro models, we addressed whether there is a significant correlation between the expression of cyclin D1 and β-catenin positivity. Data for both markers was available in 63 cases. In the β-catenin positive group (n=23), 13 (56.5%) were high cyclin D1-expressing. In the β-catenin negative group (n=40), only 12 (30%) were high cyclin D1-expressing; the two markers had a correlation that was not quite significant (p = 0.06) (Table 4).
Table 4.
Correlation between cyclin D1 and β-catenin
| β-catenin | ||||
|---|---|---|---|---|
| Negative (0 or 1) | Positive (2 or 3) | Total | P | |
| Cyclin D1 | ||||
| Low (1 or 2) | 28 | 10 | 38 | 0.0606 |
| High (3) | 12 | 13 | 25 | |
| Total | 40 | 23 | 63 | |
pGSK-3β does not correlate with the Ki67
The percentage of tumor cells with Ki67 expression was assessed in all MCL tumors and categorized into 2 groups: <50% or ≥ 50% (Figure 1 g, h). Three of 83 cases did not have sufficient Ki67 data. Of the remaining 80 cases, 22 had a ≥50% Ki67 labelling. Only 4 (16%) of the 25 pGSK-3β negative cases had a ≥50% Ki67 labelling, as compared to 18 of 55 (49%) pGSK-3β positive cases.
This correlation is not statistically significant (p = 0.177, Fisher's exact). Ki67 also did not significantly correlate with β-catenin or cyclin D1.
pGSK-3β significantly correlates with overall survival and absolute lymphocytosis
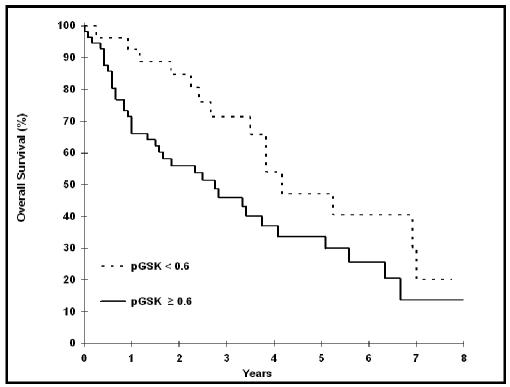
Using a pGSK-3β cut point of ≥ 0.6 univariate Cox survival analysis we found a significant correlation between pGSK-3β and overall survival (p = 0.045, HR = 1.89), with a pGSK-3β negative status associated with a better outcome (Table 5). The Kaplan-Meier survival curves are shown in Figure 2. In keeping with our previously published data cases with ≥50% cells positive for Ki67 significantly correlate with a shorter survival (p<0.0001) (15). Multivariate analysis found the prognostic value of pGSK-3β (p = 0.030, HR = 2.10) was independent of Ki67 (p<0.0001, HR = 4.40). There was no significant interaction effect between pGSK-3β and Ki67 (p = 0.71). Neither cyclin D1 nor β-catenin shows a significant correlation with the overall survival (Table 5).
Table 5.
Cox regression analysis of overall survival
| Unitivariate | Multivariate | ||||
|---|---|---|---|---|---|
| Total cases assessed | HR (95% CI) | P | HR (95% CI) | P | |
| pGSK-3β | |||||
| < 0.6 | 83 | 1.00 | 100 | ||
| ≥ 0.6 | 1.89 (1.01-3.53) | 0.045 | 2.10 (1.075-4.09) | 0.030 | |
| Ki67 | |||||
| 0-3 | 100 | 100 | |||
| 4 | 80 | 4.45 (2.29 - 8.67) | <0.0001 | 4.40 (2.24-8.64) | <0.0001 |
| Cyclin D1 | |||||
| 1 or 2 | 70 | 1.00 | |||
| 3 | 1.17 (0.79-1.73) | 0.442 | |||
| β-catenin | |||||
| 0 or 1 | 76 | 1.00 | |||
| 2 or 3 | 1.06 (0.78-1.44) | 0.732 | |||
| IPI | |||||
| 0-2 | 81 | 1.00 | |||
| 3-5 | 2.08 (1.08-4.03) | 0.030 | |||
Figure 2.
pGSK-3β expression status significantly correlates with overall survival (p=0.045, Log rank).
We also assessed if there is a correlation between the expression of pGSK-3β and various clinical parameters, including clinical stage, the international prognostic index, absolute lymphocytosis, elevation of lactate dehydrogenase, patient age at diagnosis and extra-nodal involvement; only peripheral blood lymphocytosis (using a cut-off of >6 × 109/L) significantly correlates with pGSK-3β status (P = 0.0011, Spearman).
Discussion
GSK-3β is a biologically important signalling protein. In the Wnt canonical pathway, it plays a central role in sequestrating β-catenin and promoting the proteosome destruction of β-catenin [16]. In this study, using pGSK-3β as a surrogate marker detectable by an immunohisto-chemical method, we comprehensively examined the GSK-3β inactivation status in 83 paraffin-embedded MCL tumors. We found evidence that GSK-3β is inactivated in two-thirds in this cohort. We have also provided evidence that the pGSK-3β positive status significantly correlates with the nuclear expression of β-catenin, a relatively high expression level of cyclin D1, absolute lymphocytosis in the peripheral blood, and shorter overall survival in MCL patients. In summary, our data has provided evidence to support that GSK-3β is both biologically and clinically important in MCL.
With regard to the mechanism by which GSK-3β is inactivated, our group has recently published evidence of constitutive activation of the Wnt canonical pathway in MCL cell lines and patient tumours, which is likely to be attributed to the autocrine and/or paracrine stimulation of various Wnt ligands including Wnt3 [13]. These findings echo those of two recently published studies. In the first study, the authors showed that RNA-interference to abrogate the expression of FZD2, a receptor for Wnt ligands, decreases cell proliferation in a MCL cell line [17]. In the second study, the authors employed gene expression profiling experiments and identified a significant upregulation of several genes in the Wnt canonical pathway in MCL compared to naive B cells [18]. In addition to the Wnt canonical pathway, the PI3K/Akt signalling pathway may also contribute to GSK-3β inactivation. To this end, it has been previously reported that the PI3K/Akt signalling pathway is constitutively activated in a subset of MCL [19].
In various in-vitro experimental models, β-catenin has been shown to accumulate and translocate to the nucleus upon inactivation of GSK-3β. This appears to be the case for MCL, since we observed that the pGSK-3β positive status significantly correlates with the nuclear expression of β-catenin (i.e. p = 0.0025, Fisher's exact). In this regard, β-catenin nuclear accumulation coupled with GSK-3β inactivation is also observed in a number of other types of cancer, including chronic myeloid leukemia in blast crisis, precursor B-cell acute lymphoblastic leukemia and specific types of epithelial cancers [20-22]. Nevertheless, in our study, a substantial proportion of pGSK-3β positive cases, namely 27 of 50 (54%) cases, did not have detectable β-catenin. One likely explanation is that additional mechanisms may be required for the nuclear transport of β-catenin, and these mechanisms are not operational in some MCL tumors.
The finding of heterogeneous cyclin D1 immunostaining in paraffin-embedded MCL tissues is commonly observed by diagnostic hematopathologists. It is highly unlikely that this staining heterogeneity is due to variations in fixation or other technical reasons, since strongly cyclin D1-positive cells were seen in almost all cases. Thus, we believe that there is a genuine biological difference in the protein expression level of cyclin D1. Our findings of a significant correlation between a high level of cyclin D1 expression and the pGSK-3β positive status (p = 0.0032, Fisher's exact) support the concept that GSK-3β plays an active role in regulating the protein level of cyclin D1. Variation in the cyclin D1 protein level in MCL is also likely to be related to the variations in the cyclin D1 mRNA levels, as previously reported by multiple groups [23].
Of various clinical parameters analyzed in this study, only absolute lymphocytosis (arbitrarily defined as >6.0 × 109/L in this study) and overall survival significantly correlate with pGSK-3β. It is unlikely that the correlation between lymphocytosis and pGSK-3β is related to the tumor burden, since the pGSK-3β status does not significantly correlate with the clinical stage. In view of the fact that GSK-3β has been shown to regulate cell motility and cell-to-cell adhesions [3, 24-26], we have considered the possibility that GSK-3β may directly modulate the cell migratory and adhesion properties of MCL cells, such that inactivation of GSK-3β predisposes to a leukemic phase.
One of the key findings in this study is the observation that GSK-3β inactivation is significantly associated with a worse clinical outcome in MCL patients. Since pGSK-3β did not significantly correlate with Ki67, it is unlikely that its association with a worse clinical outcome is directly linked to increased cell proliferation. In view of the biologic importance of GSK-3β in various normal cellular functions and the pathogenesis of various cancers, we believe that this finding is rather not too surprising. Since the expression of β-catenin does not show a significant correlation with survival, the prognostic value of GSK-3β is likely to be mediated via downstream effectors other than β-catenin.
In contrast with two other previous studies [27, 28], we did not identify a significant correlation between the high cyclin D1 expression status and overall survival. A plausible explanation for this discrepancy is related to the difference in the experimental approach employed. While we measured the protein level expression of cyclin D1 using immunohistochemistry, the other two studies assessed the levels of cyclin D1 mRNA in MCL tumors. Of note, while MCL cells express two cyclin D1 mRNA species, namely cyclin D1a and cyclin D1b, only cyclin D1a is translated into protein in MCL cells [24]. Moreover, the anti-cyclin D1 antibody used in this immunohis-tochemical study recognizes only the cyclin D1a isoform. These two factors may have contributed to this discrepancy of our conclusions.
Acknowledgments
This study is supported by a research operating grant from the Canadian cancer Society and the National Cancer institute of Canada awarded to RL. MA is a research fellow of the Terry Fox Foundation through an award from the National Cancer Institute of Canada. PG is a recipient of the fellowship award from the Lymphoma Research Foundation of Canada. We also would like to thank Mr. John Hanson for his assistance in the statistical analysis of this study.
References
- 1.Karim R, Tse G, Putti T, Scolyer R, Lee S. The significance of the Wnt pathway in the pathology of human cancers. Pathology. 2004;36:120–128. doi: 10.1080/00313020410001671957. [DOI] [PubMed] [Google Scholar]
- 2.Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Bioohem Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Jope RS, Yuskaitis CJ, Beurel E. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem Res. 2007;32:577–595. doi: 10.1007/s11064-006-9128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim L, Kimmel AR. GSK3, a master switch regulating cell-fate specification and tumorigenesis. Curr Opin Genet Dev. 2000;10:508–514. doi: 10.1016/s0959-437x(00)00120-9. [DOI] [PubMed] [Google Scholar]
- 5.Joshi MB, Ivanov D, Philippova M, Erne P, Resink TJ. Integrin-linked kinase is an essential mediator for T-cadherin-dependent signaling via Akt and GSK3beta in endothelial cells. Faseb J. 2007;21:3083–3095. doi: 10.1096/fj.06-7723com. [DOI] [PubMed] [Google Scholar]
- 6.Failor KL, Desyatnikov Y, Finger LA, Firestone GL. Glucocorticoid-induced degradation of glycogen synthase kinase-3 protein is triggered by serum- and glucocorticoid-induced protein kinase and Akt signaling and controls beta-catenin dynamics and tight junction formation in mammary epithelial tumor cells. Mol Endocrinol. 2007;21:2403–2415. doi: 10.1210/me.2007-0143. [DOI] [PubMed] [Google Scholar]
- 7.Manoukian AS, Woodgett JR. Role of glycogen synthase kinase-3 in cancer: regulation by Wnts and other signaling pathways. Adv Cancer Res. 2002;84:203–229. doi: 10.1016/s0065-230x(02)84007-6. [DOI] [PubMed] [Google Scholar]
- 8.Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi-Yanaga F, Sasaguri T. GSK-3beta regulates cyclin D1 expression: a new target for chemotherapy. Cell Signal. 2008;20:581–589. doi: 10.1016/j.cellsig.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. Classification of Tumours of Haematopoietic and Lymphoid Tissues, Fourth Edition. WHO Classification of Tumours, Volume 2 IARC. 2008;2:229–232. [Google Scholar]
- 11.Diehl JA. Cycling to cancer with cyclin D1. Cancer Biol Ther. 2002;1:226–231. doi: 10.4161/cbt.72. [DOI] [PubMed] [Google Scholar]
- 12.Jares P, Colomer D, Campo E. Genetic and molecular pathogenesis of mantle cell lymphoma: perspectives for new targeted therapeutics. Nat Rev Cancer. 2007;7:750–762. doi: 10.1038/nrc2230. [DOI] [PubMed] [Google Scholar]
- 13.Gelebart P, Anand M, Armanious H, Peters AC, Dien Bard J, Amin HM, Lai R. Constitutive activation of the Wnt canonical pathway in mantle cell lymphoma. Blood. 2008;112:5171–5179. doi: 10.1182/blood-2008-02-139212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dal Col J, Dolcetti R. GSK-3beta inhibition: at the crossroad between Akt and mTOR constitutive activation to enhance cyclin D1 protein stability in mantle cell lymphoma. Cell Cycle. 2008;7:2813–2816. doi: 10.4161/cc.7.18.6733. [DOI] [PubMed] [Google Scholar]
- 15.Hui D, Reiman T, Hanson J, Linford R, Wong W, Belch A, Lai R. Immunohisto-chemical detection of cdc2 is useful in predicting survival in patients with mantle cell lymphoma. Mod Pathol. 2005;18:1223–1231. doi: 10.1038/modpathol.3800409. [DOI] [PubMed] [Google Scholar]
- 16.Staal FJ, Clevers HC. WNT signalling and haematopoiesis: a WNT-WNT situation. Nat Rev Immunol. 2005;5:21–30. doi: 10.1038/nri1529. [DOI] [PubMed] [Google Scholar]
- 17.Ortega-Paino E, Fransson J, Ek S, Borre-baeck CA. Functionally associated targets in mantle cell lymphoma as defined by DNA microarrays and RNA interference. Blood. 2008;111:1617–1624. doi: 10.1182/blood-2007-02-068791. [DOI] [PubMed] [Google Scholar]
- 18.Rizzatti EG, Falcao RP, Panepucci RA, Proto-Siqueira R, Anselmo-Lima WT, Oka-moto OK, Zago MA. Gene expression profiling of mantle cell lymphoma cells reveals aberrant expression of genes from the PI3K-AKT, WNT and TGFbeta signalling pathways. Br J Haematol. 2005;130:516–526. doi: 10.1111/j.1365-2141.2005.05630.x. [DOI] [PubMed] [Google Scholar]
- 19.Rudelius M, Pittaluga S, Nishizuka S, Pham TH, Fend F, Jaffe ES, Quintanilla-Martinez L, Raffeld M. Constitutive activation of Akt contributes to the pathogenesis and survival of mantle cell lymphoma. Blood. 2006;108:1668–1676. doi: 10.1182/blood-2006-04-015586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radich JP, Dai H, Mao M, Oehler V, Schel-ter J, Druker B, Sawyers C, Shah N, Stock W, Willman CL, Friend S, Linsley PS. Gene expression changes associated with progression and response in chronic myeloid leukemia. Proc Natl Acad Sci USA. 2006;103:2794–2799. doi: 10.1073/pnas.0510423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, Gotlib J, Li K, Manz MG, Keating A, Sawyers CL, Weissman IL. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N EnglJ Med. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 22.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 23.Marzec M, Kasprzycka M, Lai R, Gladden AB, Wlodarski P, Tomczak E, Nowell P, De-primo SE, Sadis S, Eck S, Schuster SJ, Diehl JA, Wasik MA. Mantle cell lymphoma cells express predominantly cyclin D1a isoform and are highly sensitive to selective inhibition of CDK4 kinase activity. Blood. 2006;108:1744–1750. doi: 10.1182/blood-2006-04-016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Etienne-Manneville S, Hall A. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature. 2003;421:753–756. doi: 10.1038/nature01423. [DOI] [PubMed] [Google Scholar]
- 25.Bianchi M, De Lucchini S, Marin O, Turner DL, Hanks SK, Villa-Moruzzi E. Regulation of FAK Ser-722 phosphorylation and kinase activity by GSK3 and PP1 during cell spreading and migration. Biochem J. 2005;391:359–370. doi: 10.1042/BJ20050282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 27.Yatabe Y, Suzuki R, Tobinai K, Matsuno Y, Ichinohasama R, Okamoto M, Yamaguchi M, Tamaru J, Uike N, Hashimoto Y, Morishima Y, Suchi T, Seto M, Nakamura S. Significance of cyciin D1 overexpression for the diagnosis of mantle cell lymphoma: a clinicopa-thologic comparison of cyciin D1-positive MCL and cyciin D1-negative MCL-like B-cell lymphoma. Blood. 2000;95:2253–2261. [PubMed] [Google Scholar]
- 28.Rosenwald A, Wright G, Wiestner A, Chan WC, Connors JM, Campo E, Gascoyne RD, Grogan TM, Muller-Hermelink HK, Smeland EB, Chiorazzi M, Giltnane JM, Hurt EM, Zhao H, Averett L, Henrickson S, Yang L, Powell J, Wilson WH, Jaffe ES, Simon R, Klausner RD, Montserrat E, Bosch F, Greiner TC, Weisenburger DD, Sanger WG, Dave BJ, Lynch JC, Vose J, Armitage JO, Fisher RI, Miller TP, LeBlanc M, Ott G, Kvaloy S, Holte H, Delabie J, Staudt LM. The proliferation gene expression signature is a quantitative integrator of oncogenic events that predicts survival in mantle cell lymphoma. Cancer Cell. 2003;3:185–197. doi: 10.1016/s1535-6108(03)00028-x. [DOI] [PubMed] [Google Scholar]