Abstract
The interaction of cells with adhesion proteins in the extracellular matrix (ECM) provides signals which affect the morphology, motility, gene expression and survival of adherent cells. In the present communication we cultured K562 cells in presence of fibronectin to study the fibronectin-integrin mediated signalling and modulation of MMP expression. Our experimental findings demonstrate that exposure of K562 cells in serum free medium in presence of fibronectin up-regulates the expression of pro-MMP-9 within 2 hrs. Phosphorylation of focal adhesion kinase (FAK), ERK, PI-3K and nuclear translocation of EGFR and NF-kB upon FN binding demonstrate possible involvement of FAK/PI-3K/ERK signalling pathways in the fibronectin-integrin mediated up regulation of MMP-9 expression.
Keywords: MMP-9, fibronectin, α5β1 integrin, NF-kB, FAK
Introduction
The interaction of cells with adhesion proteins in the extracellular matrix (ECM) provides signals which affect the morphology, motility, gene expression and survival of adherent cells [1]. The integrin family of glycoprotein are composed of a large family of heterodimeric integral cell surface receptors that mediate cell to ECM and cell-to cell interactions [2]. Lymphocyte in-tegrins have a major role in mediating interactions with ECM proteins during the transmigration process [3]. The integrin cell surface proteins function as receptors for ECM proteins such as fibronectin, vitronectin, collagen IV [4]. The integrins are composed of α and β chain heterocomplex. To date, 8 different β chains and 14 different α-chains have been described, accounting for at least 20 combinations of the heterodimeric receptors [5]. Ligand binding of integrins is thought to be controlled by a mechanism requiring either (a) receptor clustering alone, (b) ligand occupancy plus receptor clustering or (c) clustering, ligand occupancy and tyrosine kinase activation [6]. The process of binding ligands to the integrins involves outside-in signalling initiated by receptor clustering followed by conformational changes in the α/ β chain culminating an affinity modulation for the ligand [7]. Fibronectin is a 440 kD prototype cell adhesion protein widely distributed in the tissues and is a potential ligand for most cell types [8]. It is present as a polymeric network in the ECM and as soluble protomer in body fluids [9]. Two regions in each fibronectin subunit possess cell binding activity: the amino acid sequence -RGD- and -PHSRN-, the site which acts in synergy with the RGD site [10]. The RGD motif in fibronectin and other cell adhesion proteins is the most important recognition site for about half of all known integrins [11]. The RGD loop is critical recognition site for α5β1 integrin receptor; however, the synergy site PHSRN is required for high affinity binding [12]. Upon integrin-ECM protein interaction, focal adhesion kinase (FAK) is recruited to focal adhesion site and autophos-phorylated [13]. FAK binds to a number of signalling and structural proteins and also activates different downstream signalling cascades involving ERK, PI-3K, etc [14]. It has been reported that the ERK and PI-3K pathways regulate MMP-2 expression [15] and FAK transfected cells show enhanced MMP-9 mRNA expression on culture in presence of fibronectin [16]. Thus integrin-mediated signalling initiates cell migration and release of proteolytic enzymes, e.g. matrix metalloproteinases (MMPs) [17, 18]. MMPs are a family of structurally related and highly conserved zinc dependent endopeptidases collectively capable of degrading most of the basement membrane and ECM [19, 20]. MMPs are secreted as zymogens from inside the cell to the cell surface and into the extracellular environment where they are able to degrade both ECM and non-ECM proteins. It is known that invasiveness of many haema-tologic malignancies, including myelo-monocytic leukaemia involves over expression of proteolytic enzymes, such as MMP-2 & MMP-9. MMP-9 is induced and secreted in conditioned media of leukemic cell lines in response to external stimuli, after pre-treatment of cells with chemokines and after cell adhesion to ECM [21, 22]. The importance of tumour cell surface integrin receptors in the regulation of MMP expression and activity has been reported in several studies. In our study, we have reported that binding of α2-mAb to human cervical carcinoma SiHa cell surface modulates MMP-2 activity [23] and culture of SiHa cells in presence of soluble fibronectin activatesMMP-2 & MMP-9 within 2 hrs [24]. The purpose of our study was to determine whether fibronectin are able to modulate MMP expression and activity by human lymphocytes. In this present communication we report that the culture of chronic myelogenic leukemic cell K562, in serum free culture medium in presence of fibronectin up regulate pro-MMP-9 activity.
Materials and methods
Cell culture
K562, human Chronic Myelogenic Leukemic cell line was obtained from National Centre for Cell Sciences (NCCS), Pune, India. K562 cells were grown and maintained in RPMI (Invitrogen Corporation; Cat No. 23400-021) containing 15% FBS in a 5 %C02 incubator at 37°C.
Cell Adhesion Assay
The micro titre plate wells were coated with fibronectin (Roche; Cat No. 11051407001) or laminin (Roche; Cat No. 11243217001) or collagen IV (BD Bio Sciences; Cat No. 35425) (1.56 μg, 3.125 μg, 6.25 μg, 12.5 μg and 25μg Fibronectin or laminin or collagen IV/well). The ligand was allowed to bind for 1½ hrs at 37°C. Wells were blocked with Buffer C (1% BSA, 1mM CaCI2 and 1mM MnCI2 in PBS) for 1hr at 37°C. Cells were collected from culture flasks, washed, suspended in Buffer C and added to micro titre plates (25,000 cells/well) and allowed to bind at 37°C for 1½ hrs. The wells were washed thrice with Buffer C. The bound cells were trypsinised, counted and related to the number of cells seeded (% of adhesion).
Zymography
K562 cells were initially grown in RPMI supplemented with 15 % FBS for 2 weeks. K562 cells (300,000 cells/1ml) were then maintained in petridishes (35 mm), washed thrice with serum free culture medium (SFCM) and treated with either a) increasing concentrations of fibronectin (10 μg & 20μg/ml SFCM), b) GRGDSP (Life technologies; Cat No. 12135-018) (20μg/ml SFCM) and c) collagen IV (20μg/ml SFCM) or laminin (20μg/ml SFCM) for 2 hrs, and the respective SFCMs were collected by centrifugation at 389 g for 5 min. K562 cells (300,000 cells/ml) were also grown in SFCM in presence of fibronectin (20 μg/ml SFCM) and the SFCM was collected after 1 or 2 hrs. All the experiments were done in triplicate and separately in separate petridishes. Control cells (300,000 cells/ml) were grown without fibronectin for 2 hrs and SFCM was collected. In another set of experiment petridishes were first coated with fibronectin (20 μg/l ml SFCM) and then K562 cells were grown in the fibronectin coated petridishes in SFCM for 2 hrs. The culture supernatant was collected. To test the specificity of fibronectin, the effects of other ECM proteins, collagen IV (20 μg/ml SFCM) and laminin (20 μg/ml SFCM) were investigated. K562 cells (300,000cells/ml) were maintained in presence of laminin (20 μg/ml SFCM) and collagen IV (20 μg/ml SFCM) for 2 hrs in RPMI SFCM. The SFCMs were collected. The matrix metalloproteinases were concentrated from conditioned SFCM (750μl) by binding to gelatin Sepharose 4B beads (Amersham Biosciences; Cat No. 17095601) for 2hrs at 4C. The beads were washed thrice with Tris-buffered saline (10 mM, pH 7.4) with Tween-20 (20μl/100ml) (TBST) and suspended in 50μl of 1× sample buffer (0.075 gm Tris, 0.2 gm SDS in 10 ml water, pH 6.8). Total 50μl suspension was incubated for 30 min at 37°C and then centrifuged at 876 g to precipitate the sepharose bead for 5 min and the 50μl suspension was collected in a fresh tube and the total 50μl suspension was then subjected to zymography on 10% SDS-PAGE copolymerized with 0.1% gelatin. Gel was washed in 2.5% Triton-X-100 for 30 min to remove SDS and was then incubated overnight in reaction buffer (50 mM Tris-HCI pH 7, 4.5 mM CaCI2, 0.2 M NaCI). After incubation, the gel was stained with 0.5% Coomassie Blue in 30% methanol and 10% glacial acetic acid. The bands were visualized by destaining the gel with water. To determine the α5 and α4 integrin mediated MMP expression and activity: K562 cells (300,000 cells/1ml) were initially grown in RPMI supplemented with 15 % FBS in pet-ridishes, washed thrice with serum free culture medium (SFCM) and incubated with anti-α5 (Santa crutz; Cat No. sc 10729) and anti-α4 (Invitrogen; Cat No. 10391-019) antibody (5 μg/1ml SFCM) for 1 hr. Subsequently the SFCM was removed and the cells were washed and incubated with fresh SFCM (1 ml) supplemented with 20μg fibronectin, for 2 hrs. The metalloproteinases were concentrated with gelatin Sepharose B beads and subjected to zymography as described above. anti-α5 and anti-α4 antibody treated K562 cells were also collected for cell adhesion assay as described above. K562 cells were grown in the presence of ERK inhibitor PD98059 (Promega; Cat No. V1191) (50μM), PI-3K inhibitor LY294002 (Promega; Cat No. V1201) (50μM), U0126 MEK inhibitor (Promega; Cat No. V1121) (50μM), SB203580 p38 inhibitor (Promega; Cat No. V1161) (50μM) in SFCM for hrs for NF-kB inhibitor (Alexies Biochemical; Cat No. ALX-0270-220) (5μM & 10μM) 1 hr or in SFCM for 24hrs and then for 2 hrs in presence of fibronectin (20μg /1 ml SFCM). The gelatinases were again concentrated and subjected to zymography as described above.
Enzyme linked immunosorbent assay (ELISA)
K562 cells (300,000/1 ml) were maintained in absence and in presence of 20μg fibronectin for 2 hours in RPMI SFCM. The culture supernatants were collected by centrifugation at 389 g for 5 min. Cells were collected and extracted with 250μl cell extraction buffer (37.5 mM Tris, 75 mM NaCI, 0.5% tritonX-100 & complete, mini, EDTA-free one tablet (Roche) per 7 ml extraction buffer). The wells of micro titre plate (96 well flat bottom sterile tissue culture plate with lid from greiner) were coated in triplicate with 50μl culture SFCM (for MMP-9 and TIMP-1) and 50μg of protein (for α5 & β1) from both control and experimental set and kept at 37°C for one and half hour (plate was wrapped in wrap to prevent evaporation). Blank wells (with SFCM for MMP-9 & TIMP-1 and with cell extraction buffer for α5 & β1) were also prepared. Wells were washed with blocking buffer (1% BSA in PBS) to block non-specific binding sites and incubated for 1 hr at 37°C. Then the wells were washed thrice with Washing Buffer (0.5% NP-40 & 0.5% BSA dissolved in PBS). Anti-MMP-9 (Cat No. sc 6840), anti-TIMP-1 (Cat No. sc 5538), anti-α5 , anti-β1 (BD Biosciences; 610468) primary antibody solution (1:1000 dilution) was added to respective wells and incubated at 37°C for 1 hr. Wells were washed thrice with Washing Buffer. Respective second antibody (Cat No.sc 2005/ sc 2768/ sc 2004) solution (1:1000 dilution buffer) was added to wells and incubated at 37 °C for 1 hr. Wells were washed six times with washing buffer (3 -5 min per wash). 50 μl substrate (TMB) was added to the wells (in dark) and kept as long as required (i.e. until colour developed begins to become too intense).Then 25μl 1M H2SO4 stop solution was added and reading was taken in ELISA reader at 450 nm. Only control sets (K562 cells maintained for 2 hrs in RPMI SFCM in absence of fibronectin) were developed for α5 & β1.
Immunocytochemistry
K562 cells were allowed to maintain on pet-ridishes in presence and in absence of 20μg fibronectin for 2 hrs in SFCM. The cells were collected in IX PBS and then smeared on cover slips and air dried. Cells were fixed with 3.5% formaldehyde, permeabilised with 0.5% Triton-X100 and the nonspecific sites were blocked with 1% BSA. The coverslips were then treated with anti- α5, anti-ERK (Cat No. sc 93), anti-p-ERK (Cat No. 7383), anti PI-3K (Cat No. sc 1637), anti-p-PI-3K (Cat No. sc 12929), anti FAK (Cat No. sc 557), anti-NF-kB (Cat No. sc 109), anti-ILK (Cat no. sc 13075), anti-EGFR (BD Biosciences; Cat No. 610017) and anti-p-EGFR (sc 12351) antibodies followed by respective FITC-coupled second antibody (Cat No. sc 1020/ sc 2012/ sc 2777) at 37° C in a humidified chamber. After washing 6 times in PBS, the cover slips were mounted on glass slides and observed under fluorescence microscope.
Western blot of ERK, p-ERK, PI-3K, p-PI-3K, FAK, p-FAK, ILK from direct cell lysate
Total protein was extracted from control K562 cells and K562 cells exposed to fibronectin for 2 hrs. 75μg of protein from each extract were subjected to run on 7.5 % SDS-PAGE and were transferred onto a nitrocellulose membrane. Membranes were then blocked, and reacted with anti-ERK, anti-p-ERK, anti-PI-3K, anti-p-PI-3K, anti-FAK, anti-p-FAK (Cat No. sc 11765-R), anti-ILK antibodies. After washing membranes were incubated with respective secondary antibodies (sc 2008/ sc 2771/ sc 2007). Bands were visualized by using NBT/BCIP substrate. Actin (Cat No. sc 8432) was used as internal control and was detected on the same membrane by reprobing.
Preparation of nuclear extract
K562 cells (300,000/ 1 ml) were grown with or without (control) fibronectin (20μg/ml) for 2 hrs at 370C. Cells were collected in 1 ml ice cold 1 X PBS and was pelleted by centrifugation at 9727 g for 5 min at 40C. Supernatant was removed and the cells pellet was resuspended in 1 ml of hypotonic buffer (10 mM Hepes pH 7.9, 1.5 mM MgCI2, 10 mM KCI, 0.5 mM PMSF, 0.5 mM DTT). Cells were pelleted by centrifugation at 19064 g for 10 min at 40°C and lysed for 10 min on ice in 40 μl of hypotonic buffer containing 0.5 % NP-40. Lysate was centrifuged as before and the cytoplasmic extract was collected. The nuclear pellet was lysed in 30 μl of lysis buffer (20 mM Hepes (pH 7.9), 420 mM NaCI, 1.5 mM MgCI2, 0.2 mM EDTA, 0.5 mM PMSF, 25 % v/v glycerol) for 15 min on ice. After centrifugation at 19064 g for 10 min at 4°C, nuclear extract was collected into 70μl of storage buffer (10 mM Hepes pH 7.9, 50 mM KCI, 0.2 mM EDTA, 20% v/v glycerol, 0.5 mM PMSF, 0.5 mM DTT) [25]. Western blot was performed as discussed above using 100μg of protein from both the cytoplasmic and nuclear extract of control and fibronectin treated cells. The bolt was developed with anti-NF-kB antibody, followed by incubation with respective secondary antibody and substrate to visualise the bands. Actin was used as internal control for the cytoplasmic fraction and B23 was used as internal control for nuclear fraction.
Electro mobility shift assay (EMSA)
Nuclear extract was prepared as discussed above.
Labeling of probes: The probes of the double stranded oligonucleotides (Operon) for NF-kB binding sequence on human MMP-9 promoter sequence as follows: 5’ - TGG AAT TCC CAG -3'. The complementary oligonucleotides were annealed using annealing buffer (10 mM Tris pH 8, 50 mM NaCI, lmM EDTA) by heating at 90°C for 2 min followed by slow cooling to room temperature for 60 min. NF-kB oligonucleotides was end labelled with [ y-32P] ATP using T4 polynucleotide kinase incubating for 1 hr at 37°C. 10 μg of nuclear protein from control and treated cells were incubated with 32P labeled oligonucleotide probes using 2X binding buffer (25mM Hepes (pH7.6), 1mM EDTA, 0.5mM DTT, 9 5mM MgCI2, and 75 mM KCI, 10% glycerol) for 30 min at room temperature in a final volume of 20 μl. After binding the protein- DNA complexes were electrophoresed on a native 5% polyacrylamide gel using 0.5 X TBE buffer. Each gel was then dried and subjected to auto radiography at - 80°C [26].
RT-PCR
RNA was extracted from 1× 106 K562 cells (experiment was done in 3 sets to get 1×106 cells) grown with fibronectin (20g/μ1 ml SFCM) or without fibronectin for 2 hrs. (Control). The sequence of the primers used for PCR were: hMMP-9: 5’ - CGC TAC CAC CTC GAA CTT TG -3’ (forward) and 5’ - GCC ATT CAC GTC GTC CTT AT - 3’ (reverse); hTIMP-1: 5'-CAC CCA CAG ACG GCC TTC TGC - 3’ (forward) and 5'- AGT GTA GGT CTT GGT GAA GCC - 3’ (reverse); hFAK: 5'-GCG CTG GCT GGA AM AGA A -3’ (forward) and 5'- TCG GTG GGT GCT GGC TGG TAG G -3’ (reverse), hα5: 5'- CAT TTC CGA GTC TGG GCC AA -3’ (forward) and 5'- CAA AAC AGC CAG TAG CAA CAA -3’ (reverse), hβ1: 5'- TGT TCA GTG CAG AGC CTT CA -3’ (forward) and 5'- CCT CAT ACT TCG GAT TGA CC -3’ (reverse). GAPDH primers 5’ - CGG AGT CAA CGG ATT TGG TCG TAT -3’ (forward) and 5’ - AGC CTT CTC CAT GGT GGT GAA GAC - 3’ (reverse) were used as control to normalize for mRNA integrity and equal loading. RT-PCR was carried out using two-step RT-PCR kit (Ambion, USA Cat No. AM1710). RNA and RT components were incubated at 42°C for 15 mins and then 52°C for 45 min. and at 92°C for 10 min (to inactivate the reverse transcriptase). Conditions used for PCR consisted of 40 cycles for MMP-9 & TIMP-1, 35 cycles for α5 & β1 at 94°C for 30 sec, 58°C for 30 sec and 72° C for 1 min 30 sec with a final incubation at 72° C for 7 min in DNA thermal cycler (Perkin Elmer). The predicted size of the PCR products was 198 base pairs (bp) for MMP-9, 345 bp for TIMP-1, 324 bp for 5 & 452 bp for β1. For FAK (475 bp), PCR consisted of 25 cycles of 30 sec at 94° C, 30 sec at 60 °C and 1 min 30 sec at 72 °C.
Transwell migration assay
24 well transwell plate (Corning) with 12 inserts were taken and the lower chamber of each well was poured with 600μl RPMI SFCM. Control and fibronectin treated K562 cells (100,000 cells/insert) were seeded in triplicate on membrane in the upper chamber of the insert. Cells were then allowed to grow for 24 & 48 hrs. After 24 & 48 hrs of incubation, media was pipette out from membrane. SFCMs from lower chambers were collected and centrifuged at 3000 rpm for 3 min. The membranes of the inserts were washed thrice with PBS. Cells were then fixed with 4% formaldehyde solution, followed by washing with PBS. Cells were then stained with Gill's hematoxylin for 10 min. Membranes were then washed thoroughly in running water. The upper side of the membranes were scraped with buds; membranes were then cut and mounted with glycerol. The cells migrated through the membrane pore were observed under microscope.
Results
K562 cell adhesion to fibronectin, laminin, vollagen IV
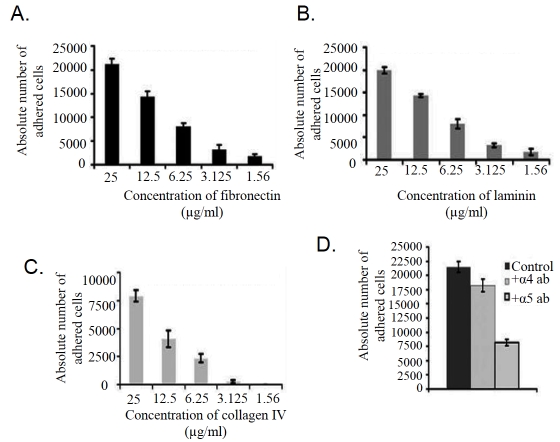
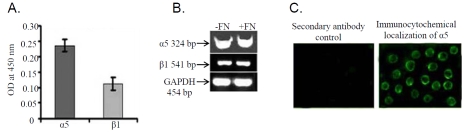
Cell adhesion assay showed that K562 cell bind to fibronectin (Figure 1A) and laminin (Figure 1B) efficiently. Binding of cells to collagen IV (Figure 1C) was observed to be much less than that of fibronectin and laminin. However the incubation of K562 cells with anti-α5 monoclonal antibody (+Alpha 5 antibody) for 1hr at 37°C (Figure 1D) inhibits the adhesion of cell to fibronectin appreciably. Binding of cells were not found to be altered significantly when cell were treated with anti-α4 monoclonal antibody (+Alpha 4 antibody; Figure 1D), confirming the binding of K562 cells through α5β1 integrin receptor. ELISA (Figure 2A) and RT -PCR (Figure 2B) analysis showed that K562 cells express both α5 and β1 subunit of α5β1 integrin receptor. Immunocytochemical study (Figure 2C) with α5 monoclonal antibody confirms its membrane localization in K562 cells.
Figure 1.
Cell adhesion assay: K562 cells (25,000/well) (Control) were added to wells coated with different amounts of Fibronectin (A), laminin (B) and collagen IV (C) (25μg/ml, 12.5μg/ml, 6.25 μg/ml, 3.125 μg/ml, and 1.56 μg/ml) in triplicate. Wells were incubated at 37°C for 1.5 hrs. The wells were then washed thrice and cells were trypsinized. The cells were counted in a haemocytometer and cell adhesion was expressed in absolute number of cells recovered. (D) K562 cells grown in absence (Control) and in presence of 1μg alpha5 (+alpha 5 antibody) and alpha4 (+alpha 4 antibody) antibody for 1 hr were allowed to bind to the fibronectin (25μg/ml) and number of adhered cells in both control and treated cells were calculated as described above.
Figure 2.
(A) K562 cells were collected from culture flask by centrifugation and were extracted in cells extraction buffer. 50μg of protein was added into microtiter well and allowed to coat for 1.5 hrs. Wells were then blocked with 1% BSA followed by anti-α5 and anti-β1 antibody treatment respectively. After washing thrice with washing buffer well were reacted with respective HRP-coupled secondary antibody for 1 hr at 37°C. Wells were washed thoroughly and substrate was allowed to react until colour development. Reactions were stop with 1M H2SO4 and the OD was measured at 450nm in ELISA reader (Biorad). (B) K562 cells (300,000 cells/ml) were grown in absence (lane C) and in presence of 20μg/ml fibronectin (lane E) for 2 hrs in SFCM. Cells were washed in PBS and total RNA were extracted, followed by two-step RT-PCR with equal amounts of total RNA using specific primers for PCR (alpha 5, beta 1). PCR products were run on a 2% agarose gel and bands were visualized under UV. GAPDH primers were used to confirm equal loading. (C). K562 cells were smeared on coverslip and fixed with3.5 % formaldehyde, followed by incubation with 0.5% triton-X100. 1% BSA was used for blocking and coverslips were then allowed to react with anti-α5 antibody for 1.5 hrs at 37°C. Respective FITC coupled secondary antibody was added after washing. Coverslips were washed thoroughly with PBS and mounted on glass slides with glycerol.
Fibronectin induces pro-gelatinase B (MMP-9) activity in K562 cells
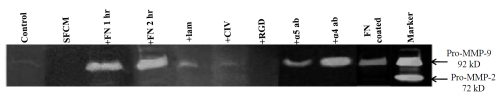
Figure 2 shows a comparative zymogram. K562 cells (300,000) grown in SFCM (1 ml) without fibronectin for 2 hrs (Figure 3; lane Control) do not show virtually any MMP-9 activity. When 1 ml of SFCM (harvested from K562 cells) was incubated at 37°C with 20μg of fibronectin and processed along with experimental samples, no MMP activity was detectable (Figure 3; lane SFCM). However, when K562 cells were maintained in the presence of 20 μg fibronectin for 1 hr (Figure 3; lane +FN 1hr) or 2 hrs (Figure 3; lane +FN 2hrs) gelatinolytic activity of pro-MMP -9 was very prominent at both 1hr and 2 hrs. It is interesting to note that only a weak pro-MMP-9 gelatinolytic band was observed when K562 cells were cultured with another ECM protein laminin (Figure 3; lane +Lam) and collagen IV (Figure 3; lane +C IV) at the same concentration (20μg/ml) and time (2 hrs) as fibronectin.When K562 cells were grown in SFCM with 20μg -GRGDSP - hexapeptide MMP-9 activity was virtually undetectable (Figure 3; lane +RGD). When K562 cells were grown in the presence of anti-α5 antibody for lh and subsequently in the presence of fibronectin (20 μg/1 ml SFCM) for 2 hrs (Figure 3; lane + α5 ab), pro-MMP-9 showed substantial reduction in activity. However, fibronectin induced pro-MMP-9 activity was not found to be altered appreciably in presence of anti-α4 antibody (Figure 3; lane + α4 ab). When K562 cells were allowed to grow on fibronectin coated surface (Figure 3; FN coated), MMP-9 activity was induced, however, the response was not much stronger. Figure 3; lane Marker is MMP-9/MMP-2 marker (culture supernatant of HT-1080 cells grown for 24 h in SFCM).
Figure 3.
K562 cells (300,000 cells/ml) were grown in SFCM in absence (lane Control) and in presence of 20μg/ml fibronectin for 1 hr (lane +FN 1hr), 2 hrs (lane +FN 2hr). 1 ml RPMI SFCM collected from K562 cells was treated with 20μg/ml for 2 hrs (lane SFCM). K562 cells (300,000 cells/ml) were grown in presence of 20μg/ml laminin (lane +Lam), 20μg/ml collagen IV (lane +CIV) and 20μg/ml -GRGDSP- (lane +RGD) for 2 hrs. K562 cells were grown in presence of anti-α5 antibody (lane + α5ab) and anti-α4 antibody (lane + α4ab) prior to 20μg/ml fibronectin for 2 hrs in SFCM. Lane FN coated represents pro-MMP-9 activity in the SFCM, collected from 20μg/ml fibronectin coated culture dish. Lane Marker is MMP-9/MMP-2 marker; obtained from culture supernatant of HT-1080 cells grown for 24 h in SFCM. In all the cases culture supernatant were separated by centrifugation and MMPs secreted were concentrated by gelatin sepharose 4B bead. MMPs were eluted from bead and run in 7.5 % SDS-PAGE copolymerized with 0.1% gelatin. Gels were shaked with 2.5 % tritonX-100 followed by incubation with reaction buffer for 24 hrs at 37°C. Gels were stained with coomassie blue staining solution to visualize the bands.
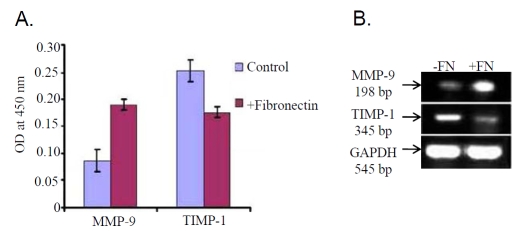
Modulation of MMP-9 and TIMP-1
ELISA report (Figure 4A) showed that MMP-9 expression was increased appreciably in the SFCM upon culturing cells in presence of 20μg fibronectin (+Fibronectin) for 2 hrs, however, TIMP-1 expression was observed to be down regulated appreciably. Furthermore, RT-PCR result (Figure 4B) also showed increased expression of MMP-9, but inhibition of TIMP-1 in K562 cells treated with 20μg fibronectin for 2 hrs (lane +FN) compared to the control cells (lane-FN).
Figure 4.
(A) SFCM collected from K562 cells cultured in presence (+Fibronectin) and in absence (Control) of 20μg/ml fibronectin was coated in microtitre wells. Wells were blocked with 1% BSA followed by incubation with anti-MMP-9 and anti-TIMP-1 primary antibody and respective secondary antibodies. OD was taken at 450nm as described above. (B) Total RNA was extracted from 20μg/ml fibronectin treated (lane +FN) and untreated (lane -FN) K562 (300,000 cells/ml) cells. RT-PCR was done as described above using MMP-9 and TIMP-1 primer and bands were visualized in 2% agarose gel under UV. GAPDH was used to confirm total RNA integrity and equal loading.
Role of ERK, PI-3K, NF-kB in the fibronectin mediated induction of pro-MMP-9 activity
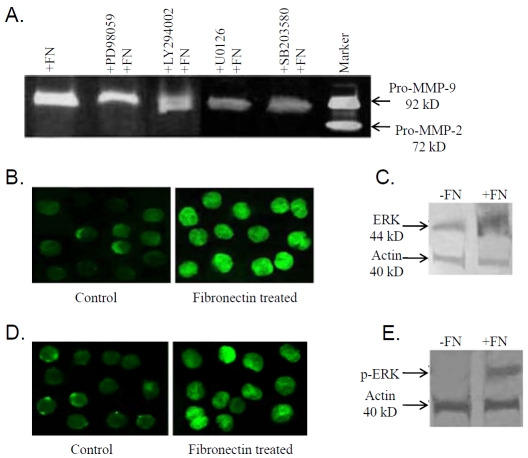
To study the importance of any cell signaling pathway(s) that are activated in fibronectin induced pro-MMP-9 activity, K562 cells were grown in presence of 50μM ERK inhibitor [PD 98059] (Figure 5A; lane +PD98059+FN), 50μM PI-3K inhibitor [LY 294002] (Figure 5A; lane + LY 294002+FN), MEK inhibitor [U0126] (Figure 5A; lane +U0126+FN) and p38 inhibitor [SB203580] (Figure 5A; 12 lane +SB203580+FN) for 1 hr followed by 20μg fibronectin treatment for 2 hrs. Comparative zy-mograms showed partial inhibition of pro-MMP-9 activity in the SFCM collected from ERK as well as PI-3K inhibitor treated K562 cells. Control cells were treated with 20 μg fibronectin for 2 hrs. (Figure 5A; lane +FN). Lane Marker is MMP-9/MMP-2 marker (culture supernatant of HT-1080 cells grown for 24 h in SFCM).
Figure 5.
(A) K562 cells (300,000 cells/ml) were maintained in absence (lane +FN) and in presence of ERK (lane +PD98059+FN), PI-3K (lane +LY294002+FN), MEK (lane +U0126+FN) and P38 (lane +SB203580+FN) inhibitors for 45 min prior to 20μg/ml fibronectin for 2 hrs in SFCM. Lane Marker is MMP-9/MMP-2 marker; obtained from culture supernatant of HT-1080 cells grown for 24 h in SFCM. MMPs were concentrated using gelatin sepharose 4B bead and gelatin zymography was performed. (B) Control and 20μg/ml fibronectin treated K562 cells were smeared on coverslips and allowed to air dry prior to formaldehyde fixation. Coverslips were then reacted with 0.5 % triton X-100 for 10 min followed by incubation with anti-ERK primary antibody. FITC coupled secondary antibody was added to coverslips after washing thrice with PBS. Coverslips were then washed thoroughly and mounted on glass slides with glycerol. (C) Total protein was extracted from K562 cells (300,000 cells/ml) grown in presence (lane +FN) and in absence (lane -FN) of 20μg fibronectin for 2 hrs in SFCM. 75μg of protein from each extracts were subjected to analyze by western transfer. The gels were transferred onto nitrocellulose membranes. Membranes were blocked with 4% BSA, reacted with anti-ERK primary antibody, followed by alkaline phosphatase coupled secondary antibody and NBT/BCIP substrate. Actin was used to confirm equal loading. (D) Immunocytochemical study of control and fibronectin treated K562 cells were done using anti-p-ERK primary antibody and respective secondary antibody. (E) Western blot was performed with K562 cells grown in absence (lane -FN) and in presence (lane +FN) of 20μg fibronectin for 2 hrs. Membrane was reacted with anti-p-ERK primary antibody, followed by alkaline phosphatase coupled respective secondary antibody and bands were visualized with NBT/BCIP substrate. Actin was used to confirm equal loading.
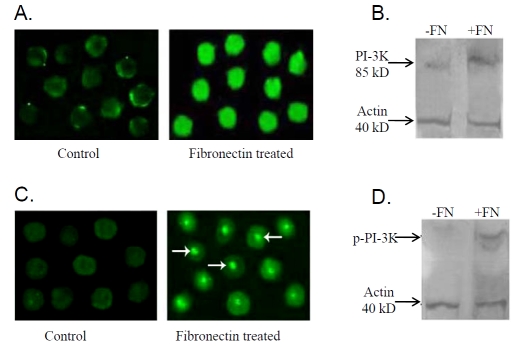
To confirm the role of ERK, PI-3K and NF-kB in fibronectin mediated MMP-9 up regulation ICC and western blot of ERK, PI-3K, & NF-kB were performed. Figure 5B, 5C, 5D, 5E showed increased expression (Figure 5B, 5C) and phos-phorylation of ERK (Figure 5D, 5E) upon fibronectin treatment (Fibronectin treated) compared to the cells grown in absence of fibronectin (Control). Western blot analysis for Pl-3K (Figure 6B) and p-PI-3K (Figure 6D) showed increased expression and phosphorylation of Pl-3K in presence of fibronectin. Immunocytochemical study of PI-3K (Figure 6A) and p-PI-3K (Figure 6C) showed nuclear translocation in presence of fibronectin. Pro-MMP-9 activity was appreciably inhibited upon 10μM (Figure 7A; lane + BAY11-7085 (10 μM) +FN) NF-kB inhibitor [BAY11-7085] treatment rather than 5μM (Figure 7A; lane BAY11-7085 (5 μM)+FN) NF-kB treatment for 24 hrs followed by 20μg fibronectin treatment for 2 hrs, compared to the control cells (Figure 7A; lane +FN), maintained in absence of NF-kB inhibitor for 24 hrs and then exposed to 20μg fibronectin treatment for 2 hrs. Figure 7B demonstrates nuclear translocation of NF-kB. In control K562 cells NF-kB is mainly located in the cytosol (Control). When K562 cells were grown in presence of 20μg fibronectin (Fibronectin treated), NF-kB is shown to be translocated into the nuclei within 2 hrs. Western blot analysis of both the cytoplasmic (Figure 7C) and nuclear fraction (Figure 7D) of control (lane-FN) and fibronectin treated (lane +FN) cells showed expression of NF-kB in both cytoplasmic and nuclear fraction of control K562 cells. However, when cells were grown in presence of fibronectin (20μg/ml), NF-kB expression was mainly detected in the nuclear fraction and a very less or no expression was found in cytoplasmic fraction. Figure 7E is the representative of electrophoretic mobility shift assay (EMSA) of NF-kB. EMSA showed appreciable increase in binding of NF-kB with MMP-9 promoter in fibronectin treated K562 cells (lane 3) comparing the control cells (lane 2). Lane 1 indicates NF-kB binding sequences without nuclear extract.
Figure 6.
(A) Immunocytochemistry of PI-3K was performed in fibronectin treated and untreated (Control) K562 cells. (B) PI-3K expression was studied with 75μg of whole cell extracts collected from K562 cells (300,000 cells/ml) grown in presence (lane +FN) and in absence (lane -FN) of 20μg fibronectin for 2 hrs in SFCM with actin as internal control by western blot. (C) Immunocytochemical localization of p-PI-3K was done in K562 cells cultured in presence (Fibronectin treated) and in absence (Control) of 20μg fibronectin for 2 hrs. (D) Total protein was extracted from K562 cells (300,000 cells/ml) grown in presence (lane +FN) and in absence (lane -FN) of 20μg fibronectin for 2 hrs in SFCM. 75μg of protein from each extracts was treated with sample buffer and mercaptoethanol and run on 7.5 % SDA-PAGE. The gels were then transferred onto nitrocellulose membranes. Membranes were blocked with 4% BSA, reacted with anti-p-PI-3K antibody. After washing with TBS-T membranes were reacted with respective alkaline phos-phatase coupled secondary antibodies. Bands were visualized using NBT/BCIP substrate. Actin confirms equal amount of protein loading.
Figure 7.
(A) K562 cells (300,000 cells/ml) were maintained in absence (lane +FN) and in presence of 5μM (lane +Bayll-7085 (5μM) +FN), 10μM (lane +Bayll-7085 (10μM) +FN) NF-kB inhibitor for 24 hrs prior to 20μg/ml fibronectin for 2 hrs in SFCM. Lane Marker is MMP-9/MMP-2 marker; obtained from culture supernatant of HT-1080 cells grown for 24 h in SFCM. MMPs were concentrated using gelatin sepharose 4B bead and gelatin zymography was performed as described before. (B) Control and 20μg/ml fibronectin treated K562 cells were smeared on cover-slips and allowed to air dry prior to formaldehyde fixation. Coverslips were then reacted with 0.5 % triton-100, followed by incubation with anti-NF-kB primary antibody and respective FITC coupled secondary antibody. Coverslips were then washed thoroughly and mounted on glass slides with glycerol. Nuclear extracts (D) were prepared from K562 cells (300,000 cells/ml) cultured with (lane +FN) or without (lane -FN) 20μg fibronectin for 2 hrs in SFCM. Cytoplasmic (C) extracts were also collected from both fibronectin treated (lane +FN) and untreated (lane -FN) K562 cells as described in the methods. 50μg of protein from each extracts were subjected to run on 7.5% SDS-PAGE as discussed above. Gel was transferred on nitrocellulose membrane. Membrane was blocked, followed by reaction with anti -NF-kB primary antibody and respective secondary antibody and western blot was developed keeping actin as internal control in case of cytoplasmic fraction (C) and in case of nuclear fraction (D) B23 was used as internal control. (E) K562 (300,000 cells/ml) cells were grown with (lane 3) or without (lane 2) 20μg fibronectin for 2 hrs in SFCM and nuclear extract were prepared. Oligonucleotides containing NF-kB binding sequence was end labeled with [ y-32P] ATP using T4 polynucleotide kinase and was incubated with 10 μg of nuclear extract from control (lane 2) and FN treated (lane 3) K562 cells. Then protein-DNA complexes were electrophoresed on 5% polyacrylamide gel, gel was then dried and subjected to autoradiography at − 80°C. Lane 1 represents NF-kB binding sequences without nuclear extract.
Fibronectin modulates FAK, p-FAK, ILK, EGFR and p-EGFR
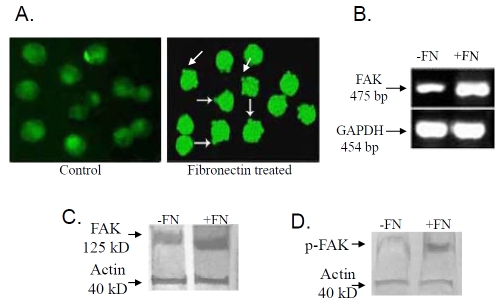
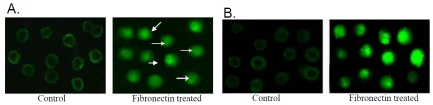
Immunocytochemical localization (Figure 8A) demonstrated localization of FAK protein at the newly formed focal adhesion sites in 20μg fibronectin treated cells (Fibronectin treated). In control cells grown without fibronectin for 2 hrs (Control) FAK was found to be located throughout the cytoplasm. Comparative RT-PCR (Figure 8B) and western blot analysis of FAK (Figure 8C I) demonstrated increased FAK m-RNA and protein expression in 20μg fibronectin treated K562 cells (lane +FN) than that of the control cells (lane -FN). Figure 8C II shows increased level of FAK phosphorylation in 20μg fibronectin treated (lane +FN) K562 cells, compared to the cells grown in absence of fibronectin (lane -FN) for 2 hrs. Immunocytochemical localization (Figure 9A) of ILK showed punctuate localization of ILK at the cell membrane in fibronectin treated K562 cells (Fibronectin treated) compared to the control cells grown in absence of fibronectin (Control). Expression (Figure 9B) of ILK also get induced upon 20μg fibronectin treatment for 2 hrs (lane +FN), comparing to the control cells grown in absence of fibronectin. Immunocytochemical analysis of EGFR and p-EGFR are shown in Figure 10A and Figure 10B respectively. EGFR (Figure 10A) showed translocation into the nucleus upon 20μg fibronectin treatment for 2 hrs (Fibronectin treated). However EGFR is mainly located in the cell membrane in control K562 cells grown without fibronectin (Control). Phosphorylation of EGFR (Figure 10B) also gets induced in K562 cells treating with 20μg fibronectin for 2 hrs (Fibronectin treated) as compared to the control.
Figure 8.
(A) 20μg fibronectin (for 2 hrs) treated (Fibronectin treated) and untreated (Control) K562 cells were smeared on coverslips and immunocytochemistry was performed as discussed before with anti-FAK primary antibody, followed by respective FITC-coupled secondary antibody. (B) Total RNA was extracted from K562 cells (300,000 cells/ml) grown in absence (lane -FN) and in presence (lane +FN) of 20μg fibronectin for 2 hrs. RT-PCR was performed as before using FAK primer and bands were visualized in 2% agarose gel under UV. (C & D) K562 cells (300,000 cells/ml) were cultured in presence (lane +FN) and in absence (lane -FN) of 20μg fibronectin for 2 hrs in SFCM. Cells were collected, extracted and cell extracts (75μg protein) were subjected to run on 7.5% SDS-PAGE as before. Transferring the gels on nitrocellulose membrane, membranes were blocked and reacted with anti-FAK (C) anti-p-FAK (D) primary antibody. All the membranes were incubated with respective secondary antibodies and NBT/ BCIP substrate was added to visualize the band. Actin was used to confirm equal loading.
Figure 9.
(A) 20μg fibronectin (for 2 hrs) treated (Fibronectin treated) and untreated (Control) K562 cells were smeared on coverslips and immunocytochemistry was performed as discussed before with anti-ILK primary antibody and respective secondary antibody. (B) Western analysis was performed in K562 cells cultured with (lane +FN) or without (lane - FN) fibronectin as described before with ILK primary antibody and respective secondary antibodies. Actin was used as internal control.
Figure 10.
(A) 20μg fibronectin (for 2 hrs) treated (Fibronectin treated) and untreated (Control) K562 cells were smeared on coverslips and immunocytochemistry was performed as discussed before with anti-EGFR primary antibody. Respective secondary antibody was added and coverslips were mounted on glass slides with glycerol to visualize the localization of specific proteins under fluorescence microscope. (B) Immunocytochemical localization of p-EGFR was performed in fibronectin treated and untreated (Control) K562 cells using p-EGFR primary antibody and respective FITC coupled secondary antibody.
Effect of fibronectin on K562 cell migration
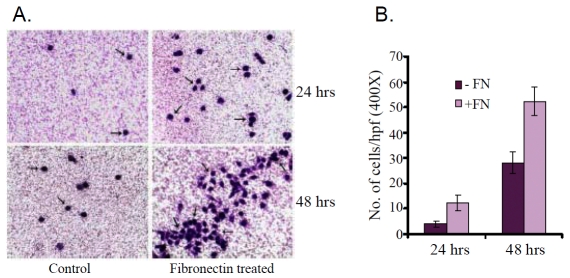
When control (Control) and fibronectin treated (Fibronectin treated) K562 cells were seeded on the membrane of transwell insert (Figure 11A), the fibronectin treated cells were observed to be migrated through the membrane after 24 hrs and 48 hrs. Figure 11B showed that after an incubation of 24 hrs total number of 28±3 fibronectin treated cell (+FN) were migrated, compared to control (-FN) cells (4±1). The migration rate was appreciably higher when cells were allowed to grow for 48 hrs. 52±5 fibronectin treated (+FN) cells were migrated through the membrane, compared to the control (-FN) cells (12±4). The data presented as mean number of net migrated cells counted in 10 microscopic high power field (×400).
Figure 11.
(A) Fibronectin treated and untreated (Control) K562 cells were seeded into transwell insert and were allowed to grow for 24 and 48 hrs. Figure shows K562 cell migration through membrane. (B) Number of control (-FN) and fibronectin treated (+FN) K562 cell migrated through transwell insert were counted per high power field after 24 and 48 hrs of cell seeded.
Discussion
In this present communication we report that exposure of K562 cells to fibronectin resulted in expression of pro-MMP-9 within 1-2 hrs in SFCM. In previous studies it has been reported that exposure of cells to fibronectin in SFCM induce MMP-9 and also MMP-2 expression and activity [23, 24, 27]. Interestingly masking of integrin α5 with α5 mAb suppresses the pro-MMP-9 activity appreciably, indicating important role of α5β1 integrin, a major fibronectin binding integrin in the fibronectin-induced MMP expression and activity. The molecular motif of fibronectin for binding to α5β1 integrin receptor is -RGD- [11] showed no pronounced activity of pro-MMP-9. Werb et al (1989) also reported that binding of α5β1 integrin receptor to fibronectin triggers events different from those triggered by binding the native fibronectin ligand. This result perhaps indicates that a mul-tivalent ligand receptor interaction rather that simple ligand occupancy is required for pro-MMP-9 expression and activity. Moreover, this induced pro-MMP-9 activity of K562 cells was not found in case of other ECM ligands, such as laminin and collagen IV. Migration assay shows fibronectin promotes K562 cell migration which may occur due to increased pro-MMP-9 activity. Reduction in TIMP-1 (inhibitor of MMP-9) expression indicates increase in MMP-9/TIMP-1 ratio [28]. This imbalance of MMP-9/TIMP-1ratio may lead to up regulation of fibronectin induced pro-MMP-9 activity. Binding of ECM proteins with their respective integrin receptors initiates signalling cascades that modulates different gene regulatory pathways. The function of integrins depends on their ability to shift between active and inactive ligand binding states by alteration of the conformation of extracellular domain [29]. In adherent cells integrins are mainly in the active state. However, in circulating cells like K562, Chronic Myelogenic Leukemic cells, the integrins are present on the cell surface in an inactive conformation until the cells are exposed to factors, such as fibronectin, which trigger intracellular reactions leading to activation of the integrins [30, 31]. Integrin-linked kinase (ILK) is a novel ankyrin repeat containing serine/threonine protein kinase. ILK interacts with the β1 cytoplasmic domain via the carboxy terminal region of the kinase catalytic domain [32]. The kinase activity of ILK is important in the stimulation of fibronectin matrix assembly [33]. ILK mediates the fibronectin-α5β1 interactions and promotes the assembly of focal adhesion proteins to the newly formed focal adhesion sites. Increased ILK expression in fibronectin treated K562 cells may indicate fibronectin- α5β1 mediated downstream signalling cascade via ILK to the focal adhesion points. The binding of fibronectin to its corresponding α5β1 integrin receptor also results in phosphorylation of focal adhesion kinase (FAK). FAK is reported to be in a critical position as a receptor proximal component of both integrin and growth factor. Localization of FAK to sites of integrin-receptor clustering is also important for cell motility [34]. Binding of K562 cells to fibronectin results in localization of FAK at newly formed focal adhesion sites and increased expression and phosphorylation of FAK initiates its downstream signalling cascades which may activate ERK/PI-3K pathways. PI-3K binds to the phosphorylated Y397 of FAK [15]. Increased level of ERK, PI-3K expression and phosphorylation indicate that transduction of α5β1 integrinmediated signals upon fibronectin binding for modulation of pro-MMP-9 activity in K562 cells may occur via FAK/ERK/PI-3K pathways. The clustering of integrin α5β1 receptors by fibronectin resulted in the access of activated EGFR to Shc which connects the activated EGFR to Ras-ERK pathway and activates it [35]. Activation of EGFR results in nuclear translocation of EGFR. EGFR activation upon fibronectin-integrin interaction may occur through a ligand dependent process such as intracrine EGFR activation [36]. Integrin mediated anchorage plays an important role in trafficking NF-kB between the cytoplasm and the Nucleus. Fibronectin treatment activates NF-kB and facilitates its entry into the nucleus [37], where it binds directly to the promoter region of MMP-9 gene to initiate MMP-9 mRNA production [38, 39]. As a result of this pro-MMP-9 is up regulated in presence of fibronectin. Interestingly in a different study Munoz et al reported up regulation of MMP-9 by α4β1 integrin in B-CLL, but in K562 masking of α4β1 integrin with a4 mAb did not affect the fibronectin induced MMP-9 expression and activity. However, the ligand [fibronectin fragment H89 (FN-H89)] used by Munoz et al differs from our ligand, which is a native fibronectin molecule of 440 kD (Roche, Germany). It may suggest that integrin mediated MMP-9 regulation may vary depending on the cell types and the difference in ligand. Our experimental findings in human chronic myelogenic leukaemia cells (K562) indicate that exposure of K562 cells to fibronectin generates a α5β1 integrin mediated signalling cascade which leads to the up regulation of MMP-9. These findings demonstrate possible involvement of FAK/ERK/PI-3K/NF-kB signalling pathways in the fibronectin-integrin mediated up regulation of MMP-9. This study may help to understand the role of alpha5 beta1 as a modulator of MMP-9 and may have clinical and therapeutic potentials in the management of the disease.
Acknowledgments
The authors wish to express their thanks to the Director, Chittaranjan National Cancer Institute for his continuous inspiration and financial support.
References
- 1.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. 1992;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 2.Huhtala P, Humphries MJ, McCarthy JB, Tremble PM, Werb Z, Damsky CH. Cooperative signaling by alpha 5 beta 1 and alpha 4 beta 1 integrins regulates metalloproteinase gene expression in fibroblasts adhering to fibronectin. J Cell Biol. 1995;129(3):867–79. doi: 10.1083/jcb.129.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin H, Varner J. Integrins. roles in cancer development and as treatment targets. Br J Cancer. 2004;90(3):561–5. doi: 10.1038/sj.bjc.6601576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esparza J, Vilardell C, Calvo J, Juan M, Vives J, Urbano-Márquez A, Yagüe J, Cid MC. Fibronectin upregulates gelatinase B (MMP-9) and induces coordinated expression of gelatinase A (MMP-2) and its activator MT1-MMP (MMP-14) by human T lymphocyte cell lines. A process repressed through RAS/MAP kinase signaling pathways. Blood. 1999;94(8):2754–66. [PubMed] [Google Scholar]
- 5.Stefanidakis M, Koivunen E. Cell-surface association between matrix metalloproteinases and integrins: role of the complexes in leukocyte migration and cancer progression. Blood. 2006;108(5):1441–50. doi: 10.1182/blood-2006-02-005363. [DOI] [PubMed] [Google Scholar]
- 6.Bosman FT. Integrins: cell adhesives and modulators of cell function. Histochem J. 1993;25(7):469–77. doi: 10.1007/BF00159282. [DOI] [PubMed] [Google Scholar]
- 7.Akiyama SK. Integrins in cell adhesion and signaling Hum Cell. 1996;9(3):181–6. [PubMed] [Google Scholar]
- 8.Hato T, Pampori N, Shattil SJ. Complementary roles for receptor clustering and conformational change in the adhesive and signaling functions of integrin alphallb beta3. J Cell Biol. 1998;141(7):1685–95. doi: 10.1083/jcb.141.7.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hynes RO. Fibronectin. New York Sringer Verlag. 1990 [Google Scholar]
- 10.Johansson S. Non-collagenous matrix proteins. In: Comper WE, editor. Extracellular matrix, 1 ed, vol 2. Amsterdam: Harwood academic publishers; 1996. pp. 68–94. [Google Scholar]
- 11.Ruoslahti E. Fibronectin and its integrin receptors in cancer. Adv Cancer Res. 1999;76:1–20. doi: 10.1016/s0065-230x(08)60772-1. [DOI] [PubMed] [Google Scholar]
- 12.Smith JW, Ruggeri ZM, Kunicki TJ, Cheresh DA. Interaction of integrins alpha v beta 3 and glycoprotein llb-llla with fibrinogen. Differential peptide recognition accounts for distinct binding sites. J Biol Chem. 1990;265(21):12267–71. [PubMed] [Google Scholar]
- 13.Koivunen E, Gay DA, Ruoslahti E. Selection of peptides binding to the alpha 5 beta 1 integrin from phage display library. J Biol Chem. 1993;268:20205–10. [PubMed] [Google Scholar]
- 14.Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309(5963):30–3. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 15.Kornberg L, Earp HS, Parsons JT, Schaller M, Juliano RL. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J Biol Chem. 1992;267(33):23439–42. [PubMed] [Google Scholar]
- 16.Juliano RL. Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu Rev Pharmacol Toxicol. 2002;42:283–323. doi: 10.1146/annurev.pharmtox.42.090401.151133. [DOI] [PubMed] [Google Scholar]
- 17.Zhang D, Bar-Eli M, Meloche S, Brodt P. Dual regulation of MMP-2 expression by the type 1 insulin-like growth factor receptor: the phosphatidylinositol 3-kinase/Akt and Raf/ERK pathways transmit opposing signals. J Biol Chem. 2004;279(19):19683–90. doi: 10.1074/jbc.M313145200. [DOI] [PubMed] [Google Scholar]
- 18.Segarra M, Vilardell C, Matsumoto K, Esparza J, Lozano E, Serra-Pages C, Urbano-Márquez A, Yamada KM, Cid MC. Dual function of focal adhesion kinase in regulating integrin-induced MMP-2 and MMP-9 release by human T lymphoid cells. FASEB J. 2005;19(13):1875–7. doi: 10.1096/fj.04-3574fje. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann UB, Westphal JR, Van Kraats AA, Ruiter DJ, Van Muijen GN. Expression of integrin alpha (v)beta(3) correlates with activation of membrane-type matrix metalloproteinase-1 (MT1-MMP) and matrix metalloproteinase-2 (MMP-2) in human melanoma cells in vitro and in vivo. Int J Cancer. 2000;87(1):12–9. doi: 10.1002/1097-0215(20000701)87:1<12::aid-ijc3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 20.Brakebusch C, Bouvard D, Stanchi F, Sakai T, Fässler R. Integrins in invasive growth. J Clin Invest. 2002;109(8):999–1006. doi: 10.1172/JCI15468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2(3):161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 22.Klein G, Vellenga E, Fraaije MW, Kamps WA, de Bont ES. The possible role of matrix metalloproteinase (MMP)-2 and MMP-9 in cancer, e.g. acute leukemia. Crit Rev Oncol Hematol. 2004;50(2):87–100. doi: 10.1016/j.critrevonc.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Mitra A, Chakrabarti J, Banerji A, Chatterjee A. Binding of alpha2 monoclonal antibody to human cervical tumor cell (SiHa) surface alpha2beta1 integrin modulates MMP-2 activity. Gynecol Oncol. 2004;94(1):33–9. doi: 10.1016/j.ygyno.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 24.Mitra A, Banerji A, Das S, Chatterjee A. Culture of human cervical cells in presence of fibronectin activated MMP-2. J Can Res Clin Oncol. 2006;132(8):505–13. doi: 10.1007/s00432-006-0096-6. [DOI] [PubMed] [Google Scholar]
- 25.Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acid Res. 1983;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han S, Sidell A, Roser-Page S, Roman J. Fibronectin stimulates human lung carcinoma cell growth by inducing cyclooxygenase-2 (COX-2) expression. Int J cancer. 2004;111:322–331. doi: 10.1002/ijc.20281. [DOI] [PubMed] [Google Scholar]
- 27.Gargi Maity, Shabana Fahreen, Aniruddha Banerji, Paromita Roy Choudhury, Triparna Sen, Anindita Dutta, Amitava Chatterjee. Fibronectin-integrin mediated signaling in human cervical cancer cells (SiHa) Mol Cell Biochem. doi: 10.1007/s11010-009-0256-5. (in press) [DOI] [PubMed] [Google Scholar]
- 28.Frankenberger M, Hanck RW, Frankenberger B, Häu Binger K, Maier KL. All-trans Retinoic acid selectively down regulates matrix metalloproteinase-9 (MMP-9) and up-regulates tissue inhibitor of metalloproteinase-1 (TIMP-1) in human bronchoalveolar lavage cells. Molecular medicine. 2001;7(4):263–270. [PMC free article] [PubMed] [Google Scholar]
- 29.Humphries MJ. Integrin activation: the link between ligand binding and signal transduction. Curr Opin Cell Biol. 1996;8:632–640. doi: 10.1016/s0955-0674(96)80104-9. [DOI] [PubMed] [Google Scholar]
- 30.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 31.Staffan Johansson, Gunbjørg Svineng, Krister Wennerberg, Annika Armulik, Lars Lohikangas. Fibronectin-integrin interactions. Frontiers in Biosci. 1997;2:dl26, 146. doi: 10.2741/a178. [DOI] [PubMed] [Google Scholar]
- 32.Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, Bell JC, Dedhar S. Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin- linked protein kinase. Nature. 1996;379:91–96. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- 33.Wu C, Keighetly SY, Leung-Hagesteijn C, Radeva G, Coppolino M, Goicoechea S, McDonald JA, Dedhar S. Integrin-linked protein kinase regulates fibronectin-matrix assembly, E-cadherin expression and tumorigenicity. J Biol Chem. 1998;273(1):528–36. doi: 10.1074/jbc.273.1.528. [DOI] [PubMed] [Google Scholar]
- 34.Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2(5):249–56. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- 35.Scott K. Integrin alpha5 Beta1 mediates fibronectin dependent epithelial cell proliferation through epithelial growth factor receptor activation. Mol Biol Cell. 2000;11:2485–96. doi: 10.1091/mbc.11.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao H. Functional nuclear epithelial growth factor receptors in human choriocarcinoma JEG-3 cells and normal human placenta. 1995;136:3163–72. doi: 10.1210/endo.136.7.7540549. [DOI] [PubMed] [Google Scholar]
- 37.Das S, Banerji A, Frie E, Chatterjee A. Rapid expression and activation of matrix metalloproteinase-2 and -9 upon exposure of human breast cancer cells (MCF-7) to fibronectin in serum free culture medium. Life Sci. 2008;82:467–76. doi: 10.1016/j.lfs.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 38.Frisch SM, Morisaki JH. Positive and Negative Transcriptional Elements of the Human Type IV Collagenase Gene. Mol Cell Biol. 1990;10(12):6524. doi: 10.1128/mcb.10.12.6524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshizaki T, Sato H, Furukawa M, Pagano JS. The expression of matrix metalloproteinase 9 is enhanced by Epstein-Barr virus latent membrane protein 1. Proc Natl Acad Sci. 1998;95:3621. doi: 10.1073/pnas.95.7.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]