Abstract
Size of breast cancer is essential in staging cancer to determine type and extent of patient management. This study was conducted to assess accuracy in estimating tumor size by MRI and gross using microscopy as gold standard. A retrospective study was done on 33 patients, 30-75 years, who underwent MRI of breasts with subsequent lumpectomy, 2002-2006, for invasive breast cancer. Size of lesion(s) on MRI and gross were compared with histological size. Of 37 lesions, 27 (73%) were invasive ductal (IDC) and 10 (27%) invasive lobular carcinoma (ILC). Tumor size by MRI matched histological size in 3%, underestimated 27%, and overestimated 70% of cases. Tumor size by gross matched histological size in 22%, underestimated 57%, and overestimated 22% of cases. MRI as an imaging modality and gross pathology both have significant limitations in measuring tumor size particularly in cases of invasive breast carcinoma. Random sectioning of lumpectomy specimen in invasive breast carcinoma may result in inaccurate staging of tumor by leading to false impression of tumor size and multi-focality and/or multi-centricity of tumor particularly in cases of ILC. Microscopic measurements of tumor size are necessary for accurate T-staging and recommended for appropriate patient management.
Keywords: Breast cancer, MRI, microscopic pathology, gross pathology, tumor size
Introduction
Breast cancer accounts for approximately one quarter of all female cancers and is the most common type of non-cutaneous malignancy in women in the United States with one in eight women on average at risk for developing the disease according to American Cancer Society. Considering the physical, emotional, and financial impacts associated with this disease, it is prudent to diagnose breast cancer at an early stage and with greatest accuracy in determining elements involved in assessing the stage.
Tumor size is one of the most important factors in determining disease-free and cause-specific survival in invasive breast cancer particularly, in cases of node-negative breast cancers where tumor size becomes of utmost importance in determining type and extent of subsequent surgical and oncological management. The guidelines of American Joint Committee on Cancer (AJCC) for breast tumors classifies them pathologically into 4 groups for staging purposes where TO refers to tumors that are not grossly visible, T1 for those measuring >2 cm, T2 for those <2-5 cm, and T3 for tumors >5cm in greatest dimension [1]. Therefore, accurate measurement of an invasive breast cancer is crucial for allowing the best outcome in patient management.
There are various methods to determine tumor size including palpation on physical examination and breast-imaging studies such as mammography, ultrasound, and more recently MRI. Pathology however is considered to be the gold standard method for measurement of tumor size. Neither AJCC nor International Union Against Cancer (IUCC) staging manuals specify how breast tumors are to be measured by gross or microscopic methods [1, 2]. College of American Pathologists (CAP), however, recommends that microscopic measurement to be used as the actual tumor size for staging [3]. Microscopic measurement is also known to be the gold standard for determination of breast lesions as described by previous authors [4, 5].
MRI as one of the most sensitive imaging modalities is being used with increasing frequency for detection and measurement of breast lesions. Although its specificity can be limited ranging from 37-97% [6], MRI serves as an additive tool to not only assess the size, multifocality, or multicentricity of lesions but also to help clinicians in making a preoperative treatment plan and assess size of residual tumor after neoadjuvant chemotherapy or to assess recurrence after reconstructive surgery [7]. Positive predictive value of MRI is increased with increasing lesion size [8]. Despite marked progress made in radiologic fields, true size of lesion(s) can be overestimated or underestimated in some patients and may change subsequent course of action. Equally, tumor size by gross examination of specimen can be underestimated or overestimated depending on manner of sectioning and/or by not including contiguous areas of grossly non-invasive lesions.
The objective of this study was first to determine accuracy of MRI and gross pathology in estimating tumor size by comparing measurements given by radiologist and pathologist of the gross tumor size using microscopic pathology as gold standard. The second objective was to assess percentage of changes in T stage when size of tumor by MRI and gross pathology were different from microscopic pathology.
Materials and methods
Breast specimen
A retrospective study was performed on 37 distinct invasive carcinoma from 33 female patients, at UCLA Medical Center. Patients were between 30 and 75 years and all carried a diagnosis of invasive breast cancer with or without in-situ component(s). All underwent bilateral breast MRIs with subsequent lumpectomy between 2002 and 2006. The reason for performing MRI in these patients were multiple and included increased fibroglandular density of breasts, assessment of multifocality or multicentricity of invasive tumor, and high suspicion for invasive carcinoma that was visible on mammogram or ultrasound. All mastectomy cases were excluded from the study.
Gross measurements
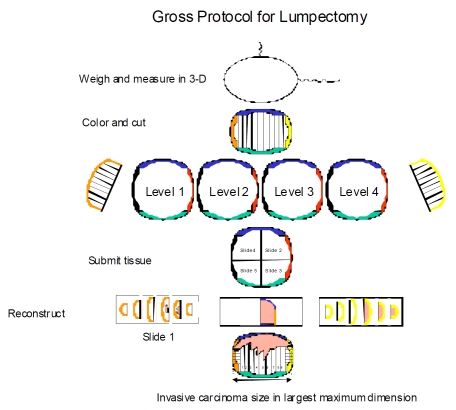
All specimens were received with orientation and grossed in fresh state (Figure 1). All margins were marked with six different colored inks. Specimens were cut into 0.3-0.5 cm thick levels along the longest axis. Grossly identifiable tumors were measured in three dimensions. The gross size was recorded as zero when the dissector was unable to identify a definitive lesion. The most lateral and medial margins cut along the longest axis were each further cut perpendicularly and placed in separate cassettes. The remainders of levels were entirely submitted sequentially for microscopic examination, except for one case of invasive lobular carcinoma (#9) where the lumpectomy size was 11.5 cm which was submitted at alternate levels for histological processing. Formalin fixation happened only after the cut sections of each specimen were placed in cassettes. In this way, tissue shrinkage and the potential change in size of tumor did not occur as a result of specimen fixation prior to grossing.
Figure 1.
Serial sectioning of lumpectomy specimen and submission in their entirety.
Microscopic measurements
Exact tumor size was determined by first measuring in centimeters the microscopic extensions of the invasive carcinoma in six directions, i.e. medial, lateral, superior, inferior, anterior, and posterior. The measurements were then compiled by multiplying number of levels showing invasive cancer by thickness of each level. In cases where maximum dimension of tumor was only on one slide, linear dimension of invasive cancer on that slide was measured and recorded as the maximum tumor size. For purposes of this study, the in-situ components were not included in analyses.
Calculation methods
To compare sizes, only the largest dimension from MRI and gross were used. Size of lesion(s) on MRI and gross pathology were compared with gold standard, i.e., microscopy, by tabulating data and calculating differences among measured sizes in each case. Percentage of cases where MRI and gross had overestimated or underestimated tumor size were calculated. In each case, based on maximum dimension of invasive tumor as assessed by microscopy, a determination was made whether T stage was changed after comparison with MRI and gross sizes, and percentage of such changes were calculated.
Statistical analyses
Mean and standard deviation of each group (MRI, gross, and microscopic sizes) were determined using Microsoft Excel program. Student t-test was performed comparing two of the groups. The p-values were obtained by pairing the two groups using two-tailed distribution. A significant statistical difference was considered when p-value was equal or less than 0.05.
Results
Among 37 lesions, 27 (73%) were invasive ductal carcinoma (IDC) two cases of which were multifocal, and 10 (27%) were invasive lobular carcinoma (ILC) with one case being multifocal. One patient (#2), with IDC originally, was noted to have 3 separate lesions on MRI, but in final pathologic assessment was found to have two lesions. For a patient with IDC (# 20) one lesion was seen on MRI, 3.6 cm in greatest dimension; however two separate lesions were identified on pathologic exam. For purpose of calculations, the same MRI size was used to assess the difference with each of the two lesions. Same situation occurred in a patient with ILC (# 7). (Tables 1, 2)
Table 1.
Study groups with their lesion size on MRI, gross, and microscopy
| Tumor size in invasive ductal carcinoma (IDC) | ||||
|---|---|---|---|---|
| Patient # | Lesion # | MRI size (cm) | Gross size (cm) | Microscopic size (cm) |
| 1 | 1 | 1.8 | 1.3 | 1.3 |
| 2 | 2 | 2.7 | 2.6 | 2.6 |
| 2 | 3 | 1 | 1.1 | 0.7 |
| 2 | 4 | 0.8 | 0 | 0 |
| 3 | 5 | 1 | 1.5 | 0.7 |
| 4 | 6 | 1.8 | 1 | 1.2 |
| 5 | 7 | 0.8 | 0.8 | 0.8 |
| 6 | 8 | 1.5 | 1.8 | 1.3 |
| 7 | 9 | 3.1 | 2.3 | 4.2 |
| 8 | 10 | 2.4 | 1.5 | 3.5 |
| 9 | 11 | 1.9 | 1.1 | 1.5 |
| 10 | 12 | 3.8 | 1.5 | 2.5 |
| 11 | 13 | 2.3 | 0.8 | 2.2 |
| 12 | 14 | 3 | 1.5 | 2.3 |
| 13 | 15 | 3.9 | 0 | 0.2 |
| 14 | 16 | 3.7 | 1.7 | 0.5 |
| 15 | 17 | 2.2 | 1.3 | 1.3 |
| 16 | 18 | 1.7 | 1.6 | 1.6 |
| 17 | 19 | 0.9 | 0 | 1.6 |
| 18 | 20 | 1.8 | 0 | 1.6 |
| 19 | 21 | 1 | 0 | 0.6 |
| 20 | 22 | 3.6 | 1.2 | 1.4 |
| 20 | 23 | 3.6 | 2.3 | 1.9 |
| 21 | 24 | 2.1 | 0 | 1.5 |
| 22 | 25 | 3.1 | 2.8 | 2.5 |
| 23 | 26 | 2.3 | 1.9 | 2.5 |
| 24 | 27 | 1.9 | 1.5 | 1.2 |
Table 2.
Study groups with their lesion size on MRI, gross, and microscopy
| Tumor size in invasive lobular carcinoma (ILC) | ||||
|---|---|---|---|---|
| Patient # | Lesion # | MRI size (cm) | Gross size (cm) | Microscopic size (cm) |
| 1 | 1 | 2 | 1.5 | 2.4 |
| 2 | 2 | 2.8 | 1.2 | 3.8 |
| 3 | 3 | 1.7 | 1.2 | 1.2 |
| 4 | 4 | 1.6 | 1.7 | 1.7 |
| 5 | 5 | 3.6 | 0 | 2.5 |
| 6 | 6 | 5 | 1.1 | 5.8 |
| 7 | 7 | 4.5 | 1.6 | 1.9 |
| 7 | 8 | 4.5 | 0.5 | 0.8 |
| 8 | 9 | 3.2 | 5 | 5.5 |
| 9 | 10 | 10.9 | 11 | 11 |
By MRI (Table 3), IDC was underestimated 15% of the time, overestimated 81% of time, and matched exact microscopic size 4% of time. However, MRI underestimated tumor size in cases of ILC in 60% of cases, overestimated it in 40% of cases, and matched exact histologic size in none of the cases. T-stage for IDC changed in 20% of cases after microscopic size was compared to MRI with 100% of these changes going into lower T-stage since IDC was significantly overestimated by MRI. None of the changes in T-stage was into higher level. In case of ILC, T-stage changed 33% of time after microscopic tumor size was compared to MRI size where 67% of cases had to be changed to higher T-stage as MRI had underestimated size of ILC. For ILC, 33% of cases showed a lower T-stage by microscopy.
Table 3.
Percentage of changes in size and T-stage of IDC and ILC after comparison of size assessed by microscopy read against size by MRI and gross pathology
| MRI | IDC | ILC | T-stage | IDC | ILC | |
|---|---|---|---|---|---|---|
| Underestimated | 15% | 60% | Changed | 20% | 33% | |
| Overestimated | 81% | 40% | into higher | 0 | 67% | |
| Same size | 4% | 0% | into lower | 100% | 33% | |
| Gross | Underestimated | 52% | 70% | Changed | 40% | 44% |
| overestimated | 30% | 0 | into higher | 90% | 100% | |
| Same size | 18% | 30% | into lower | 10% | 0 |
By gross pathology, the tumor size of IDC was underestimated in 52% of cases, overestimated in 30% of cases, and matched exact histologic size in 18% of cases. For ILC, gross pathology underestimated 70% of cases, overestimated none of the cases, and matched exact microscopic size in 30% of cases. T-stage for IDC by gross had to be changed 40% of time when compared to microscopic size, 90% into higher stage and 10% into lower. For ILC, T-stage change by gross pathology was overall in 44% of cases, all of which had to be changed into higher T-stage as ILC was underestimated most of the time by gross.
Overall, tumor size by MRI matched exactly same histological size in only 3%, underestimated 27%, and overestimated 70% of cases. By MRI analysis, T stage was altered 24% of time, 6% into higher and 18% into lower T-stage. Overall, tumor size by gross examination matched exactly the same histological size in 22%, underestimated 57%, and overestimated 22% of cases. By gross pathologic size, T stage was altered 42% of time, 39% into higher and 3% into lower T-stage.
Table 4 shows that differences seen in tumor size for IDC among MRI, gross pathology and microscopic pathology are all statistically significant with a p-value <0.05. In contrast, for ILC difference obtained in tumor sizes between MRI and microscopy is not statistically significant with p-value of 0.58.
Table 4.
p-values calculated using student t-test for comparison of MRI and gross, MRI and microscopic, and gross and microscopic tumor sizes
| IDC | ILC | |
|---|---|---|
| MRI - gross | p= 0.002 | p= 0.04270 |
| MRI - Microscopic | p= 0.00701 | p= 0.58019 |
| Gross - Microscopic | p= 0.02998 | p= 0.04229 |
Discussion
Based on our study both MRI and gross pathology are less accurate in predicting tumor size when compared with microscopic tumor size. In this study our objective was to demonstrate to what extent breast MRI and gross exam of lumpectomy specimen deviate from the closest estimate of tumor size that we can achieve, that being microscopic pathology. We have accomplished this task by submitting lumpectomy specimens in their entirety in a sequential manner (except for one case of ILC) in an attempt to demonstrate that random sectioning of lumpectomy specimen should be avoided.
This study shows that MRI tends to overestimate tumor size in IDC (81%) affecting T-stage in 20% of the cases. In contrast, MRI tends to underestimate tumor size in ILC (60%) affecting T-stage in 33% of the cases. Regarding gross measurements, tumor size in IDC is more commonly underestimated (52%) than overestimated (30%), thus affecting T-stage in 40% of the cases. Similar to MRI, gross measurement tends to underestimate ILC (70%) leading to changes of T-stage in 44% of cases. These alterations in T-stage are quite high and essentially translate into changes in patient management in 33% of IDC cases and 44% of ILC cases had microscopic pathology not been used for accurate size measurements. Analyzing this issue from a different angle more in favor of MRI, T-stage of invasive tumor was more accurately predicted by MRI (76%) than gross pathology (42%).
The fact that differences observed in tumor size between MRI and microcopy did not reach a statistical significance for ILC can be attributed partially to small sample size. It could also be argued that statistical significance of other paired groups may not be valid as sample size was small, and larger cohort is required for such conclusions.
Wiberg et al in their study of invasive breast cancers were able to demonstrate that although MRI can be of value in detecting multifocality and multicentricity, when considering mixed lesions such as IDC along with ductal carcinoma in situ (DCIS), MRI tends to underestimate the size of the lesion [9]. Although we disregarded in-situ lesions in this study, Wiberg's analysis is in favor of the proposal that MRI cannot be relied on for accurate tumor staging without correlation with microscopic pathology.
We did not take into account degree to which measurements on MRI and gross were due to carcinoma in-situ. Since a few of the cases had extensive in-situ components, it is conceivable that part of overestimation or underestimation of tumor size by MRI and gross were due to presence of carcinoma in-situ. Analyses of how much the presence of in-situ component contributes to errors in estimation of invasive tumor size would be in order for future studies.
In general, size of IDC is measured more accurately than size of ILC on MRI and gross exams. In other words, both MRI and gross pathology are better in predicting actual tumor size for IDC than ILC. This is explained best by acknowledging pattern of growth of most ILCs where tumor cells infiltrate singly or in files into adjacent benign appearing fibrous tissue frequently without inciting desmoplasia.
Moatamed et al in their study of patients with ILC clearly demonstrated importance of microscopic pathology by systematic and sequential submission of lumpectomy and mastectomy specimen as a way to accurately measure tumor size particularly in tumors ≤. 5 cm. Their conclusion that 50% of the specimen with ILC show different dimensions on microscopy compared to the initial gross measurement is quite alarming [10].
A point noteworthy of mention is changes that potentially can occur in tumor size at several stages during specimen processing. Artifactual tissue shrinkage and expansion from formalin fixation to tissue embedding can alter ultimate microscopic measurements. In one study, decrease in size of tumor was noted in 40% of 50 invasive breast cancer cases after final processing and mounting while tissue expansion occurred in 19% [11]. Gross measurement was the main tactic in measuring tumor size, and mean differences in size from original fresh state ranged from 1.7 to 2.4 mm. In our study, we partially eliminated most of such artifactual changes by cutting the specimen in a fresh state.
Although focus of this study was comparison of differences in tumor size among MRI, gross pathology and microscopy, it can be argued that just as much as it is possible to miss maximum tumor size or contiguous areas of tumor by not submitting entire lumpectomy specimen, margins where invasive carcinoma is present can be missed with grave consequences. A study of lumpectomy specimen for assessment of margins comparing two groups, one where lumpectomies are totally submitted and another with representative sections submitted would be insightful.
MRI as an imaging modality and gross pathology both have significant limitations in measuring tumor size particularly in cases of invasive breast carcinoma. Random sectioning of lumpectomy specimen in invasive breast carcinoma may result in inaccurate staging of tumor by leading to false impression of tumor size and multi-focality and/or multi-centricity of tumor particularly in cases of ILC. Microscopic measurements of tumor size are necessary for accurate T-staging and recommended for appropriate patient management.
References
- 1.Greene FL, Page DL, Fleming ID, Firtz AG, Balch CM, Haller DG, Marrow M, et al., editors. 6th ed. New York: Springer; 2002. American Joint Committee on Cancer (AJCC) Cancer Staging Manual; pp. 221–40. [Google Scholar]
- 2.Sobin LH, Wittekind CH. 6th edn. New York: Wiley; 2002. TNM Classification of Malignant Tumors. [Google Scholar]
- 3.Fitzgibbons PL, Page DL, Weaver D, Thor AD, Allred DC, Clark GM, et al. Prognostic factors in breast cancer: College of American Pathologists consensus statement 1999. Arch Pathol Lab Med. 2000;124:966–78. doi: 10.5858/2000-124-0966-PFIBC. [DOI] [PubMed] [Google Scholar]
- 4.Pritt B, Ashikaga T, Oppenheimer RG, Weaver DL. Influence of breast cancer histology on the relationship between ultrasound and pathology tumor size measurements. Mod Pathol. 2004;17(8):905–10. doi: 10.1038/modpathol.3800138. [DOI] [PubMed] [Google Scholar]
- 5.Apple SK, Suthar F. How do we measure a residual tumor size in istopathology (gold standard) after neoadjuvant chemotherapy? Breast. 2005;15(3):370–6. doi: 10.1016/j.breast.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Bartella L, Smith CS, Dershaw DD, Liberman L. Imaging breast cancer. Radiol Clin North Am. 2007;45(1):45–67. doi: 10.1016/j.rcl.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Esserman L, Hylton N, Yassa L, Barclay J, Frankel S, Sickles E. Utility of magnetic resonance imaging in the management of breast cancer: evidence for improved preoperative staging. J Clin Oncol. 1999;17(1):110–19. doi: 10.1200/JCO.1999.17.1.110. [DOI] [PubMed] [Google Scholar]
- 8.Liberman L, Mason G, Morris EA, Dershaw DD. Does size matter? Positive predictive value of MRI detected breast lesion size. AJR. 2006;186:426–30. doi: 10.2214/AJR.04.1707. [DOI] [PubMed] [Google Scholar]
- 9.Kristoffersen Wiberg MK, Aspelin P, Sylvan M, Bone B. Comparison of lesion size estimated by dynamic MR imaging, mammography, and histopathology in breast neoplasms. Eur Radiol. 2003;13:1207–12. doi: 10.1007/s00330-002-1718-2. [DOI] [PubMed] [Google Scholar]
- 10.Moatamed NA, Apple SK. extensive sampling changes T-staging of infiltrating lobular carcinoma of breast: A comparative study of gross versus microscopic tumor sizes. Breast J. 2006;12(6):511–17. doi: 10.1111/j.1524-4741.2006.00338.x. [DOI] [PubMed] [Google Scholar]
- 11.Pritt B, Tessitore JJ, Weaver DL, Blaszyk H. The effect of tissue fixation and processing on breast cancer size. Human Pathol. 2005;36:756–60. doi: 10.1016/j.humpath.2005.04.018. [DOI] [PubMed] [Google Scholar]