Abstract
Objectives
The purpose of this study was to evaluate the relationship between calcification in tibial arteries, the degree of limb ischemia, and the near-term risk of amputation.
Background
Determining the amputation risk in patients with peripheral arterial disease (PAD) remains difficult. Developing new measures to identify patients who are at high risk for amputation would allow for targeted interventions and focused trials aimed at limb preservation.
Methods
229 patients underwent evaluation by history, arterial Doppler, and multi-slice (MS) CT of the lower extremities. We then explored the relationship between a tibial artery calcification (TAC), traditional risk factors for PAD, limb status at presentation, and near-term amputation risk.
Results
Increased age and traditional atherosclerosis risk factors were associated with higher tibial artery calcification scores. Patients with critical limb ischemia had the highest TAC scores and increasing TAC scores were associated with worsening levels of limb ischemia in ordinal regression analysis. ROC analysis suggested that the TAC score predicted amputation better than the ABI. Symptomatic patients with a TAC score greater than 400 had a significantly increased risk of amputation. In Cox regression analysis, there was a strong association between the TAC score and the risk of major amputation that remained after adjustment for traditional risk factors and the ABI.
Conclusions
In patients presenting with PAD, the TAC score is associated with the stage of disease and it identifies those who are at high risk for amputation better than traditional risk factors and an abnormal ABI.
Keywords: peripheral arterial disease, calcification, amputation, CT scan
Peripheral arterial disease (PAD) affects approximately 5 million Americans (1) and it is associated with decreased quality of life (2,3), increased risk of death (4,5), and increased risk of limb threatening ischemia (6). The majority of patients with symptoms of early PAD remain stable over time, however, 15–25% develop progressive disease requiring vascular intervention and 1 in 25 will require amputation (7,8). Despite our skill in identifying lower extremity arterial disease using exam and non-invasive testing, our ability to identify the subset of patients with PAD who will ultimately require amputation remains limited.
While extensive investigations on the role of coronary artery calcification and its use as a marker of coronary disease have been made over the last 15 years,(9–11) similar studies using CT-based methods that focus on lower extremity calcification have not been undertaken. When lower extremity arterial calcification is visible on conventional x-rays, it is associated with an increased risk of amputation (12–15) However, arterial calcification is thought to begin insidiously, and it may progress over years or decades before becoming apparent. (16,17) Recent advances in computed tomographic (CT) imaging technology and software have made it possible to rapidly distinguish between minimal, early calcification that is difficult to detect, and the late, systemic calcification that is easily seen on conventional x-rays (18).
We thus established an algorithm for measuring tibial artery calcification (TAC) using multi-detector CT scanning. We evaluated the relationship between the TAC score and the severity of symptoms. We then evaluated the association between the TAC score and the near-term amputation risk in patients presenting with symptomatic PAD. Our results suggest that evaluation of tibial calcification by MSCT scan may be a useful method for identifying those patients who are at high risk for amputation.
Methods
Subjects
Between January 2004 and October 2006, 118 patients with symptomatic lower extremity PAD were recruited from the vascular clinics of Vanderbilt University Hospital and the Nashville VA Hospital. In addition, we recruited a control group of 111 community volunteers without symptomatic PAD. In order to exclude the known effects of renal insufficiency and renal failure on arterial calcification, patients with a creatinine greater than 1.7 were excluded from this study. Also excluded were patients with type I diabetes, patients who did not ambulate, patients who had previously undergone a major amputation, patients with acute limb ischemia, patients who had previously undergone multiple (greater that 2) lower extremity revascularization procedures, and patients presenting with severe ischemia associated with gangrene or tissue loss (Rutherford category 6).
Patient assessment and group assignments
Patients were asked about symptoms of PAD including claudication, ischemic rest pain, and ulcers. A further medical history was obtained that involved assessment of vascular risk factors including a self-reported history of tobacco use, hyperlipidemia, hypertension, and diabetes. Patients then underwent evaluation by pulse exam and non-invasive arterial Doppler testing. Lower extremity physical exam findings and arterial Doppler findings were then used to assign a limb ischemia category according to the criteria set forth by Rutherford et al.(19).
Biochemical measurements
Patients underwent standard laboratory evaluation for measurement of total cholesterol, HDL, LDL, and triglycerides.
Imaging
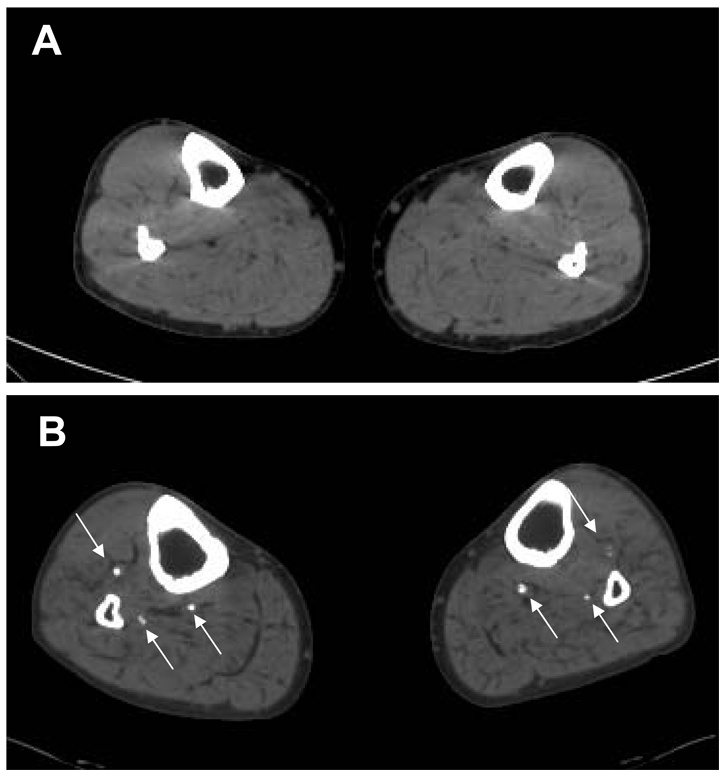
Patients underwent non-contrast CT scanning of the lower extremities on a single 16-slice CT scanner (MX8000 IDT, Philips Medical, Cleveland) that was calibrated daily and twice monthly using standardized protocols. Scans were performed using a 3 mm increment, mAs=200, and kV=120 with a field of view of 350–380 mm yielding typical spatial resolution 0.7 × 0.7 × 3.0 mm3. The scan duration was approximately 15 seconds. From the acquired raw data, the scan was reconstructed in 3 mm slices. The average number of slices was 135 ± 43 slices. Sample images from patients without (A) and with (B) tibial artery calcium are demonstrated in Figure 1.
Figure 1. Tibial artery calcification.
Non-contrasted MSCT showing patient without (A) and with (B) significant tibial artery calcification. Arrows identify calcified tibial arteries.
Tibial artery calcium scoring
Tibial artery calcium scoring was performed using standardized calcium scoring software by investigators who were blinded to the results of the clinical assessment and Rutherford categories. Automated image analysis was performed using the software on a Dell dual processor computer. On cross sectional images through the lower extremities, areas of calcification along the tibial arteries with a cross sectional area greater than 1 mm2 and with a density of > 130 Hounsfield units (HU) were identified automatically. Regions of interest along the distal popliteal, anterior tibial, posterior tibial, and peroneal arteries were manually selected and labeled. Measurements were started at the bottom of the patella and ended at the widest portion of the tibial and fibular malleoli at the ankle. This usually included the bottom half of the popliteal artery and the entire length of the three tibial arteries down to the ankle. This also allowed us to include all anatomic variants of tibial artery origins. Calciumscores were determined according to the method described by Agatston et al. (9). This method was chosen because it is currently in widespread use and because it provides a simple transition from coronary calcium scoring. Individual calcium values for each artery in each lower extremity were added together to derive a single combined TAC score for each patient. Inter-observer variability was evaluated by two readers in a randomly selected list of 39 scans. The Spearman correlation coefficient was 0.98 with a P <0.001.
Follow-up
Outcomes were determined from patient interviews during routine clinical follow-up. Patients presenting with critical limb ischemia were followed at least monthly until symptoms resolved, healing was complete, or amputation occurred. Additional follow-up data was obtained through an IRB-approved chart review and through a follow-up completion survey performed by phone. Time to follow-up or event was calculated from the date of the CT scan. We were unable to obtain follow-up on 2 patients (1 patient with claudication and 1 patient with CLI) who did not return for management after their CT scan (98% follow-up). The control population was contacted one time for follow-up by phone questionnaire. We were unable to contact 4 patients in this group (96% follow-up). Follow up of at least 3 months was available in 223 patients and the mean follow-up time was 13.8 ± 7.7 months. Minor amputation was defined as any amputation limited to the distal forefoot up to the transmetatarsal level. Major amputation was defined as any amputation above the ankle.
Statistical analysis
Statistical computations were carried out using the SPSS version15.0 and GraphPad Prism 5. Continuous variables were summarized via mean and standard deviation and dichotomous variables were summarized via counts and percents. The normality of continuous variables was checked using the Kolmogorov-Smirnov test. Due to non-normal distributions, comparisons of continuous variables across TAC groups and across limb ischemia categories were performed using the Kruskal-Wallis test and post-hoc comparisons were performed using Dunn’s post test. For comparison of dichotomous variables across TAC groups and limb ischemia categories we used the chi-square test for trend followed by pair-wise comparisons using the chi-square test. Values for TAC scores were significantly skewed and for this reason statistics were performed on log-transformed TAC + 1 scores. Associations of clinical variables with severity of limb ischemia at presentation were assessed using ordinal logistic regression and expressed as hazard ratios with 95% confidence intervals. Receiver operator characteristic (ROC) curves were generated for TAC and ABI versus major and major plus minor amputation. Event free curves for major amputation were generated using Kaplan-Meier analysis and compared using the log-rank test. Predictors of major amputation in patients with PAD were evaluated using Cox proportional hazards regression. Hazard ratios, 95% confidence intervals and p values are listed. In order to maintain the predictive accuracy of different ranges of ankle pressures for limb ischemia, ABI were categorized according to the following scale: 0 for normal ABIs of 0.9–1.4; 1 for diminished ABIs of 0.6–0.9; 2 for ABIs between 0.3–0.6; and 3 for ABIs below 0.3 and for non-compressible vessels.
Results
Patient characteristics at baseline
A total of 229 patients completed the study and were included in the initial analysis of risk factors for tibial artery calcification. (Table 1) Interestingly, total cholesterol levels and LDL were inversely related to the tibial calcification score, however, there was a trend toward increased statin use in patients with higher TAC scores. Patients with higher TAC scores had a higher incidence of PAD symptoms.
Table I.
Patient characteristics according to tibial artery calcification score.
| TAC = 0 (N =83) |
TAC 1–503 (N =72) |
TAC > 503 (N =74) |
p value | |
|---|---|---|---|---|
| Age, years | 57 ± 7 | 60 ± 8 | 67 ± 10 | < 0.0001 |
| Male % | 41 | 69 | 76 | < 0.0001 |
| Caucasian % | 89 | 85 | 74 | 0.1128 |
| type 2 diabetes % | 46 | 68 | 74 | 0.0004 |
| Hypertension % | 55 | 72 | 78 | < 0.0001 |
| Hyperlipidemia % | 57 | 63 | 72 | 0.0010 |
| Smoking history | 45 | 74 | 81 | 0.0004 |
| Total cholesterol | 187 ± 35 | 178 ± 43 | 162 ± 42 | 0.0002 |
| HDL-cholesterol | 54 ± 17 | 46 ± 14 | 44 ± 14 | 0.0003 |
| LDL-cholesterol | 99 ± 39 | 88 ± 29 | 79 ± 29 | 0.0026 |
| Triglycerides | 194 ± 115 | 213 ± 150 | 204 ± 137 | 0.6054 |
| BMI | 30.6 ± 7.3 | 29.1 ± 5.4 | 28.7 ± 7.5 | 0.1658 |
| Statin use % | 51 | 61 | 65 | 0.0667 |
| PAD symptoms % | 22 | 53 | 85 | < 0.0001 |
TAC score is associated with severity of limb ischemia at presentation
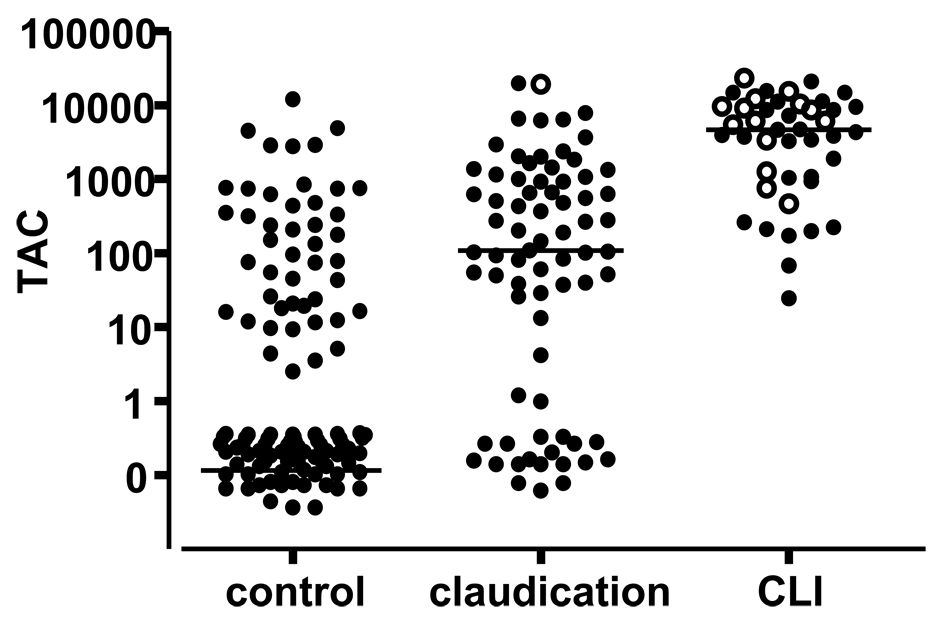
The control population was generally well matched with the claudication group but patients presenting with CLI were older, more likely to be male, and more likely to have type 2 diabetes, hypertension, and history of tobacco use. The TAC score increased significantly between the control and claudication group and between the claudication and CLI group (Figure 2 and Table II).
Figure 2. TAC scores by limb status.
TAC scores in patients without (control) and with PAD (claudication and CLI). Each dot represents a single patient. Open circles represent patients who subsequently underwent major amputation. Bars represents median for each group.
Table II.
Patient characteristics according to severity of limb ischemia at presentation.
| control N=111 |
claudication N=74 |
CLI N=44 |
p value | |
|---|---|---|---|---|
| Age (yrs) | 59 ± 7 | 60 ± 10 | 67 ± 11*† | 0.0001 |
| Male % | 54 | 65 | 73* | 0.0223 |
| Caucasian % | 89 | 77* | 77 | 0.0314 |
| Type 2 dm % | 59 | 55 | 82ठ| 0.0263 |
| Hypertension % | 59 | 72 | 86‡ | 0.0006 |
| Hyperlipidemia % | 53 | 77‡ | 66 | 0.0267 |
| Tobacco use % | 47 | 81‡ | 86‡ | <0.0001 |
| BMI (kg/cm2) | 30.3 ± 6.7 | 29.4 ± 6.5 | 27.7 ± 7.7 | 0.3914 |
| TAC¶ | 0 (0, 12093) | 107(0, 19848)‡ | 4475(24, 23392)‡§ | <0.0001 |
P < 0.05 versus control group
P < 0.05 versus claudication
P < 0.01 versus control group
P < 0.01 versus claudication group
TAC scores are expressed as median (min, max)
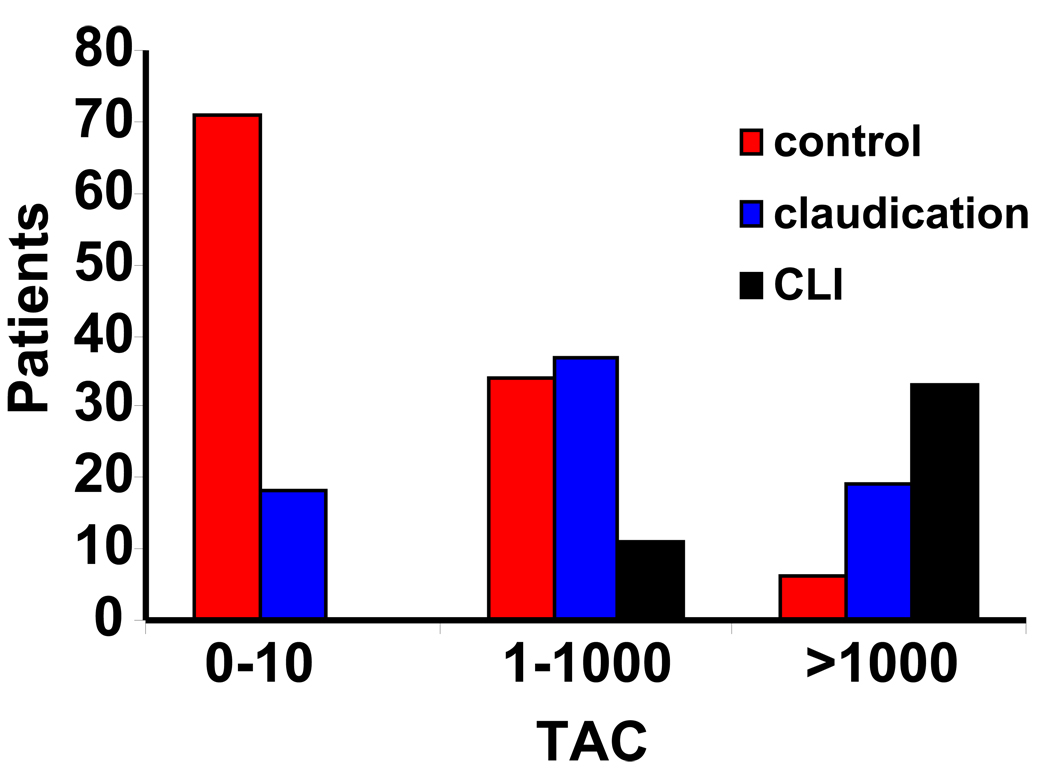
We next assessed the association between the TAC score and the level of ischemia at presentation. When we divided patients according to TAC ranges, the proportion of patients presenting with CLI increased from 0% in the lowest group, to 57% for patients with a TAC score of greater than 1000. (Figure 3). In order to adjust for the possible confounding effects of demographic and risk factors, we used ordinal logistic regression to evaluate the association between the TAC score and limb status at presentation (control, claudication, or CLI). After correcting for age, male gender, type II diabetes, hypertension, hyperlipidemia and tobacco use, the TAC score continued to have a strong association with worsening ischemia.(Table III)
Figure 3.
Clinical presentation by TAC range. Patients were divided according to TAC categories (0–10, 10–1000, and >1000). Bars represent number of patients with no disease (controls), claudication (gray bars), or CLI (black bars).
Table III.
Adjusted hazard ratios for increasing severity of limb ischemia at presentation.(control, claudication, CLI).
| HR (95% CI) | p value | |
|---|---|---|
| Age | 1.003 (0.970–1.037) | 0.8611 |
| Male | 0.814 (0.426–1.555) | 0.5327 |
| Caucasian | 1.065 (0.486–2.336) | 0.8746 |
| Type 2 diabetes | 0.809 (0.430–1.519) | 0.5091 |
| Hypertension | 1.833 (0.930–3.614) | 0.0800 |
| Hyperlipidemia | 1.229 (0.662–2.282) | 0.5131 |
| Smoking history | 3.683 (1.886–7.194) | 0.0001 |
| TAC Score* | 2.548 (1.945–3.340) | <0.0001 |
log-transformed TAC values were used for statistics.
TAC score predicts amputation
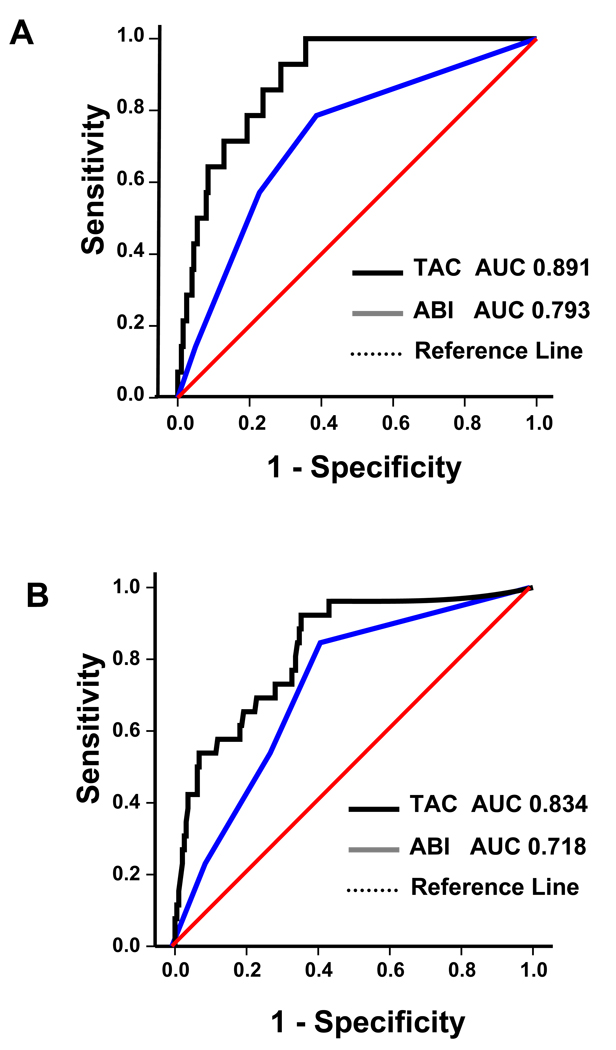
During follow-up, a total of 28 amputations occurred in our vascular patient population while no amputations occurred in the control patient population. In the group of 74 patients initially presenting with claudication, there were 3 amputations (2 minor and 1 major). In the group of 45 patients presenting with CLI, there were 25 amputations. Of these, 15 were major and 10 were minor amputations. ROC curves were generated to evaluate the predictive value of TAC for amputation and to compare it with the ABI. When we evaluated these measures versus major amputation, the area under the curve (AUC) for TAC was greater than that for the ABI (Figure 4A). When we evaluated these measures versus the combined endpoint of major and minor amputation, the AUC for TAC remained greater than that for ABI. (Figure 3B) Thus, ROC curves, while not corrected for possible confounders, indicate that the TAC score is a better marker of amputation risk than the ABI. We identified a TAC cutoff value of 400 which, when applied to our symptomatic vascular patient population, yielded a sensitivity of 94%, specificity of 46%, a positive predictive value of 23% (15 out of 65), and a negative predictive value of 98% (1 out of 59) for major amputation.
Figure 4. Receiver operator characteristic analysis.
ROC curves for the predictive value of TAC and ABI on major amputation (A) and on major and minor amputation (B). The bold line represents the ROC curve for TAC score. The continuous line represents ROC curve for ABI. The dotted line represents no effect. AUC = area under the curve. TAC = tibial artery calcium score. ABI = ankle brachial index.
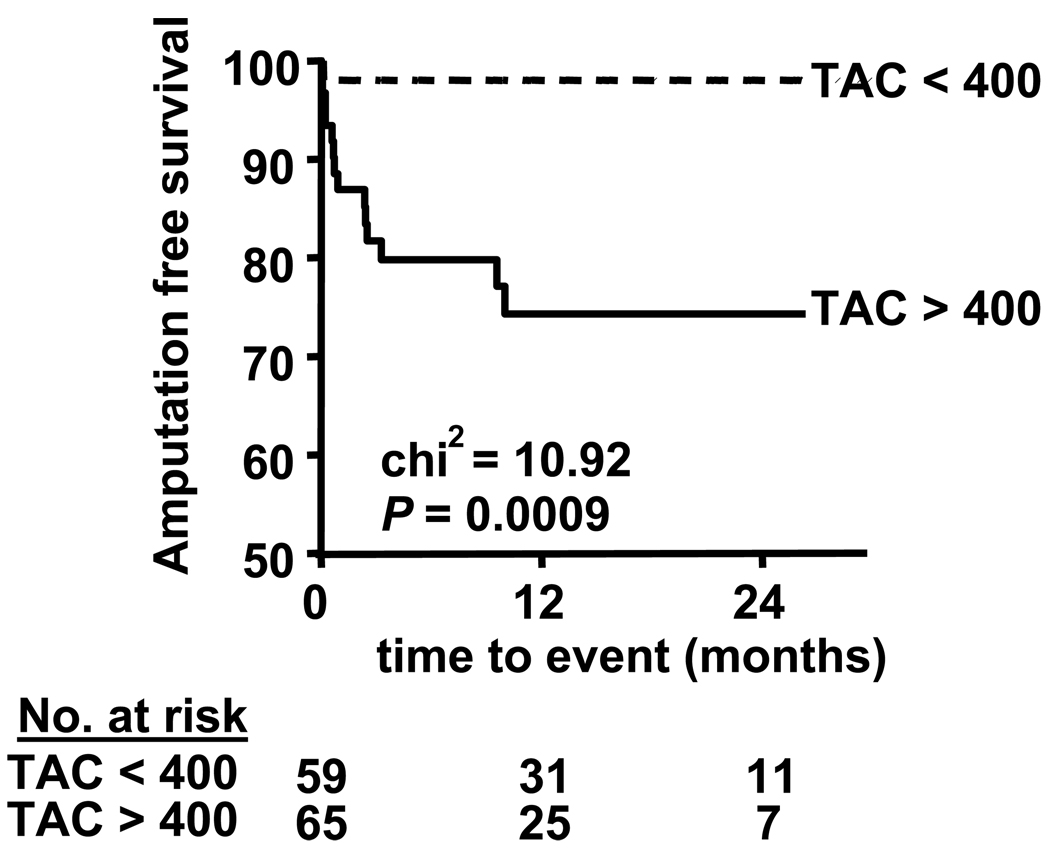
Kaplan-Meier curve analysis was performed using a cutoff TAC value of 400 for the prediction of major amputation in symptomatic vascular patients. The log-rank test showed that patients presenting with vascular symptoms and a TAC score > 400 had a significantly higher major amputation rate compared to patients with a TAC score < 400 (Figure 4).
In our final analysis, we used Cox proportional hazards analysis to evaluate predictors of major amputation including TAC > 400 in our symptomatic vascular patient population (Rutherford categories 1–5). Interestingly, in univariate analysis, traditional risk factors and the ABI lost their predictive value while a TAC > 400 remained predictive. In multivariate analysis, after adjusting for age and ABI, the hazard ratio for a TAC > 400 was 11.27 (p value = 0.025). (Table IV). These findings suggest that in patient presenting to the vascular service with symptomatic PAD, a TAC score > 400 predicts amputation better than demographics, risk factors, and the ABI.
Table IV.
Hazard ratios for major amputation in patients presenting with PAD.
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age | 1.04 (0.996–1.086) | 0.079 | 1.001 (0.955–1.049) | 0.980 |
| Male | 2.27 (0.645–7.965) | 0.202 | ||
| Caucasian | 0.87 (0.281–2.705) | 0.813 | ||
| Type 2 diabetes | 1.04 (0.390–2.771) | 0.938 | ||
| Hypertension | 1.42 (0.406–5.002) | 0.581 | ||
| Hyperlipidemia | 1.63 (0.466–5.737) | 0.443 | ||
| Smoking history | 0.82 (0.232–2.865) | 0.751 | ||
| ABI | 1.73 (0.971–3.084) | 0.063 | 1.33 (0.732–2.433) | 0.346 |
| TAC > 400 | 14.40 (1.90–109.112) | 0.010 | 11.27 (1.353–93.842) | 0.025 |
Discussion
Our study showed for the first time that MSCT can be used to quantify calcification in the tibial arteries and that TAC is strongly associated with the stage of lower extremity vascular disease and the near-term risk of major amputation. This association was preserved after correction for traditional risk factors and the ankle brachial index, an indirect measure of pedal perfusion. Patients without tibial calcification did not require amputation, while 1 in 5 patients presenting with ischemic symptoms and a TAC score > 400 underwent major amputation. Our study suggests that tibial artery calcium may be a useful measure to stratify patients into risk categories and to guide therapy aimed at limb preservation.
The initial diagnosis of PAD in patients is uncomplicated. Once the diagnosis of PAD is made, however, determining which patients have a poor limb prognosis with ultimate need for major amputation remains problematic. While an abnormal ABI can predict all-cause and cardiovascular specific mortality (20), it correlates poorly with symptoms(21), it can give misleading results in patients with calcified vessels (22), and it cannot predict healing after forefoot amputation (23). Our results suggest that the TAC score may be most useful in this at-risk patient cohort with known vascular disease. Patients with low or normal TAC scores could be safely managed with conventional medical management, whereas patients with a TAC score above a certain threshold might benefit from more intensive medical therapy, custom shoes, more frequent foot examination, and they may also be more suitable for participation in clinical trials aimed at preventing amputation.
Abdominal, pelvic, and lower extremity calcification have previously been shown to reflect advanced occlusive disease and increased risk of cardiovascular events (12–15,24–27). Recent studies have focused on patients with chronic kidney disease (28), and there is a well-known association of increased systemic vascular calcification in patients on dialysis (29–33). Relationships between the macroscopic arterial calcification that is visible on conventional x-rays and glucose tolerance (34), neuropathy (35), mortality rates, and complications of diabetes including amputation have also been demonstrated (12). However, the utility of quantitative assessment of vascular calcification by MSCT has not been evaluated.
We propose a scoring protocol for lower extremities based on the extensive investigations previously performed on coronary artery calcium (9). Our rationale for assessing arterial calcification in the tibial vessels was related to previous work suggesting an association between distal calcific disease and amputation (12). It was also based on the known relationships between diabetes, tibial atherosclerosis, and limb loss (36). While there are significant risk factor associations for calcified atherosclerosis in different vascular beds (37), we suspected that the amount of calcific disease in the tibial vessels would most strongly reflect the degree of blood-flow impairment to the foot. We excluded calcification in the aortoiliac and femoral regions based on the fact that chronic, single-level disease above the knee is infrequently associated with critical limb ischemia (38), while tibial artery disease, even in an isolated form, can lead to amputation (39). Additionally, the risk factors for aortoiliac and femoropopliteal disease are known to be different from those for tibial occlusive disease (40), and we did not want to confound the associations between the TAC score, risk factors, and endstage events.
In order to remain consistent with the methods used for scoring coronary calcium, we did not attempt to distinguish between intimal and medial calcification. Previous studies have suggested that medial, but not intimal, calcification predicts cardiovascular events (12–14). We are unable to determine if our results would have been different had we focused on medial calcification, however, the predictive ability of the TAC score remains strong. A comparison of the total calcification score with a scoring system based solely on medial calcification may yield insight into to pathophysiologic importance of the two types of calcification. Finally, the progression rate of tibial artery calcification is unknown. It was previously thought that PAD patients progressed consecutively through the stages of ischemia from mild to severe claudication then to rest pain or ulceration. However, we now know that many patients presenting with critical limb ischemia progressed rapidly, and previous studies suggest that more than half of CLI patients are asymptomatic 6 months prior (41). Our data shows that many patients with high TAC scores had minimal symptoms of lower extremity vascular disease. Further studies will be needed to determine if this subgroup of patients with high TAC and minimal or no symptoms are indeed the same patients who will go on to develop critical limb ischemia.
Study limitations
Our study has several limitations. First, we chose to modify the Agatston scoring system for use in the tibial arteries because of its widespread integration in coronary artery scoring; however, it is possible that other scoring methods such as the volumetric method of Callister (42) or the mass scoring method initially suggested by Detrano and associates (43) would be better for comparing scores at different centers and for tracking calcium changes over time. Future work will be needed to address this issue. We have not fully addressed the variability in the performance or interpretation of scans. However, our inter-observer variability was excellent with a Spearman correlation coefficient of 0.98 and in coronary arteries, the technique is remarkably robust (44). We chose to use parameters similar to those used for coronary artery calcium scoring including a 3 mm slice thickness and non-overlapping segments; however, optimization of parameters for efficient comparison and tracking of TAC scores will be needed. The scoring method will also need to be validated against other calcium assessment systems and against the total amount of occlusive disease. We did not demonstrate utility of the TAC score in patients within specific Rutherford groups due to the limited number of events; however, when we assessed the predictive value of TAC for major and minor amputations in patients presenting with Rutherford categories 1 – 4, TAC was a significant factor (p = 0.010); however, there were only 3 amputations in this group (2 minor and 1 major). When we included the presence or absence of CLI as a variable in logistic regression analysis, the p-value for TAC approached significance (p = 0.060). Larger and longer-term studies will be needed to determine the associative and predictive aspects of TAC scoring in individual patient subpopulations. There was selection bias in our population in that over half of our subjects came from a referral vascular surgery practice. While we tried to compensate for this by recruiting asymptomatic volunteers from the community, our results cannot be extrapolated to the overall population. We did not include toe pressures or a toe-brachial index in our analysis of predictors for major amputation and this may be a better measure of limb ischemia in patients with calcified vessels.(22) Additionally, our study does not take into account the various efforts at limb salvage and, in particular, we have not categorized the attempts at revascularization, wound care, or other treatments that were offered to the patients in our study. TAC scores, however, were unknown to physicians recommending amputation and they were not used for clinical decision-making.
In conclusion, we have demonstrated that 1) tibial artery calcium scores are higher in patients with PAD than in asymptomatic controls; 2) increasing calcium scores are associated with increasing severity of PAD; and 3) the TAC score predicts the short-term risk of amputation, independent of other risk factors and the ankle brachial index. Further efforts to evaluate the pathophysiologic mechanisms relating tibial artery calcium accumulation to critical limb ischemia are warranted, as are clinical investigations aimed at preventing lower extremity amputation in this high-risk population.
Figure 5. Major amputation events according to TAC score for patients with PAD.
Symptomatic vascular patients were stratified by TAC scores greater than or less than 400. Kaplan-Meier curves were derived for major amputation-free survival. The p value is derived using the log-rank test. Number of patients at risk at each time point is listed on bottom.
Acknowledgments
This work was funded by grants from the NIH, HL069926 and DK06736, and a research award from the Lifeline Foundation and the William J. von Liebig Foundation. We also would like to acknowledge contributions from members of the Division of Vascular Surgery and the Department of Radiology at Vanderbilt University Medical Center.
Funding sources: This work was funded by grants from the NIH, DK06736 and HL069926 and a research award from the Lifeline Foundation and the William J. von Liebig Foundation.
Abbreviations
- TAC
Tibial artery calcification
- ABI
ankle brachial index
- MSCT
multi-slice computed tomography
- CI
confidence interval
- HU
Hounsfield units
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None
References
- 1.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999– 2000. Circulation. 2004;110:738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 2.Izquierdo-Porrera AM, Gardner AW, Bradham DD, et al. Relationship between objective measures of peripheral arterial disease severity to self-reported quality of life in older adults with intermittent claudication. J Vasc Surg. 2005;41:625–630. doi: 10.1016/j.jvs.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Long J, Modrall JG, Parker BJ, Swann A, Welborn MB, 3rd, Anthony T. Correlation between ankle-brachial index, symptoms, and health-related quality of life in patients with peripheral vascular disease. J Vasc Surg. 2004;39:723–727. doi: 10.1016/j.jvs.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Feringa HH, Bax JJ, van Waning VH, et al. The long-term prognostic value of the resting and postexercise ankle-brachial index. Arch Intern Med. 2006;166:529–535. doi: 10.1001/archinte.166.5.529. [DOI] [PubMed] [Google Scholar]
- 5.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 6.Dormandy J, Heeck L, Vig S. Predicting which patients will develop chronic critical leg ischemia. Semin Vasc Surg. 1999;12:138–141. [PubMed] [Google Scholar]
- 7.Harris LM, Peer R, Curl GR, Pillai L, Upson J, Ricotta JJ. Long-term follow-up of patients with early atherosclerosis. J Vasc Surg. 1996;23:576–580. doi: 10.1016/s0741-5214(96)80035-8. discussion 581. [DOI] [PubMed] [Google Scholar]
- 8.Weitz JI, Byrne J, Clagett GP, et al. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: a critical review. Circulation. 1996;94:3026–3049. doi: 10.1161/01.cir.94.11.3026. [DOI] [PubMed] [Google Scholar]
- 9.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 10.Bellasi A, Raggi P. Diagnostic and prognostic value of coronary artery calcium screening. Curr Opin Cardiol. 2005;20:375–380. doi: 10.1097/01.hco.0000172704.83016.26. [DOI] [PubMed] [Google Scholar]
- 11.Fuster V, Fayad ZA, Moreno PR, Poon M, Corti R, Badimon JJ. Atherothrombosis and High-Risk Plaque: Part II: Approaches by Noninvasive Computed Tomographic/Magnetic Resonance Imaging. Journal of the American College of Cardiology. 2005;46:1209–1218. doi: 10.1016/j.jacc.2005.03.075. [DOI] [PubMed] [Google Scholar]
- 12.Everhart JE, Pettitt DJ, Knowler WC, Rose FA, Bennett PH. Medial arterial calcification and its association with mortality and complications of diabetes. Diabetologia. 1988;31:16–23. doi: 10.1007/BF00279127. [DOI] [PubMed] [Google Scholar]
- 13.Niskanen L, Siitonen O, Suhonen M, Uusitupa MI. Medial artery calcification predicts cardiovascular mortality in patients with NIDDM. Diabetes Care. 1994;17:1252–1256. doi: 10.2337/diacare.17.11.1252. [DOI] [PubMed] [Google Scholar]
- 14.Lehto S, Niskanen L, Suhonen M, Ronnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arteriosclerosis, Thrombosis & Vascular Biology. 1996;16:978–983. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- 15.Mayfield JA, Caps MT, Boyko EJ, Ahroni JH, Smith DG. Relationship of medial arterial calcinosis to autonomic neuropathy and adverse outcomes in a diabetic veteran population. J Diabetes Complications. 2002;16:165–171. doi: 10.1016/s1056-8727(01)00178-7. [DOI] [PubMed] [Google Scholar]
- 16.Ardehali R, Nasir K, Kolandaivelu A, Budoff MJ, Blumenthal RS. Screening patients for subclinical atherosclerosis with non-contrast cardiac CT. Atherosclerosis. 2007;192:235–242. doi: 10.1016/j.atherosclerosis.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Kronmal RA, McClelland RL, Detrano R, et al. Risk Factors for the Progression of Coronary Artery Calcification in Asymptomatic Subjects. Results From the Multi-Ethnic Study of Atherosclerosis (MESA) Screening patients for subclinical atherosclerosis with non-contrast cardiac CT. Circulation. 2007;192:235–242. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 18.Nasir K, Budoff MJ, Post WS, et al. Electron beam CT versus helical CT scans for assessing coronary calcification: current utility and future directions. Am Heart J. 2003;146:969–977. doi: 10.1016/S0002-8703(03)00450-2. [DOI] [PubMed] [Google Scholar]
- 19.Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26:517–538. doi: 10.1016/s0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]
- 20.Resnick HE, Lindsay RS, McDermott MM, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation. 2004;109:733–739. doi: 10.1161/01.CIR.0000112642.63927.54. [DOI] [PubMed] [Google Scholar]
- 21.Szuba A, Oka RK, Harada R, Cooke JP. Limb hemodynamics are not predictive of functional capacity in patients with PAD. Vasc Med. 2006;11:155–163. doi: 10.1177/1358863x06074828. [DOI] [PubMed] [Google Scholar]
- 22.Brooks B, Dean R, Patel S, Wu B, Molyneaux L, Yue DK. TBI or not TBI: that is the question. Is it better to measure toe pressure than ankle pressure in diabetic patients? Diabet Med. 2001;18:528–532. doi: 10.1046/j.1464-5491.2001.00493.x. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen TH, Gordon IL, Whalen D, Wilson SE. Transmetatarsal amputation: predictors of healing. Am Surg. 2006;72:973–977. [PubMed] [Google Scholar]
- 24.Witteman JC, Kok FJ, van Saase JL, Valkenburg HA. Aortic calcification as a predictor of cardiovascular mortality. Lancet. 1986;2:1120–1122. doi: 10.1016/s0140-6736(86)90530-1. [DOI] [PubMed] [Google Scholar]
- 25.Niskanen LK, Suhonen M, Siitonen O, Lehtinen JM, Uusitupa MI. Aortic and lower limb artery calcification in type 2 (non-insulin-dependent) diabetic patients and non-diabetic control subjects. A five year follow-up study. Atherosclerosis. 1990;84:61–71. doi: 10.1016/0021-9150(90)90009-8. [DOI] [PubMed] [Google Scholar]
- 26.Wilson PW, Kauppila LI, O'Donnell CJ, et al. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103:1529–1534. doi: 10.1161/01.cir.103.11.1529. [DOI] [PubMed] [Google Scholar]
- 27.Reaven PD, Sacks J. Coronary artery and abdominal aortic calcification are associated with cardiovascular disease in type 2 diabetes. Diabetologia. 2005;48:379–385. doi: 10.1007/s00125-004-1640-z. [DOI] [PubMed] [Google Scholar]
- 28.Sigrist M, Bungay P, Taal MW, McIntyre CW. Vascular calcification and cardiovascular function in chronic kidney disease. Nephrol. Dial. Transplant. 2006;21:707–714. doi: 10.1093/ndt/gfi236. [DOI] [PubMed] [Google Scholar]
- 29.Moe SM, Chen NX. Pathophysiology of vascular calcification in chronic kidney disease. Circ Res. 2004;95:560–567. doi: 10.1161/01.RES.0000141775.67189.98. [DOI] [PubMed] [Google Scholar]
- 30.Shanahan CM. Vascular calcification. Curr Opin Nephrol Hypertens. 2005;14:361–367. doi: 10.1097/01.mnh.0000172723.52499.38. [DOI] [PubMed] [Google Scholar]
- 31.Ketteler M, Westenfeld R, Schlieper G, Brandenburg V. Pathogenesis of vascular calcification in dialysis patients. Clin Exp Nephrol. 2005;9:265–270. doi: 10.1007/s10157-005-0385-4. [DOI] [PubMed] [Google Scholar]
- 32.London GM, Marchais SJ, Guerin AP, Metivier F. Arteriosclerosis, vascular calcifications and cardiovascular disease in uremia. Curr Opin Nephrol Hypertens. 2005;14:525–531. doi: 10.1097/01.mnh.0000168336.67499.c0. [DOI] [PubMed] [Google Scholar]
- 33.Campean V, Neureiter D, Varga I, et al. Atherosclerosis and vascular calcification in chronic renal failure. Kidney Blood Press Res. 2005;28:280–289. doi: 10.1159/000090182. [DOI] [PubMed] [Google Scholar]
- 34.Neubauer B. A quantitative study of peripheral arterial calcification and glucose tolerance in elderly diabetics and non-diabetics. Diabetologia. 1971;7:409–413. doi: 10.1007/BF01212055. [DOI] [PubMed] [Google Scholar]
- 35.Edmonds ME, Morrison N, Laws JW, Watkins PJ. Medial arterial calcification and diabetic neuropathy. Br Med J (Clin Res Ed) 1982;284:928–930. doi: 10.1136/bmj.284.6320.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faglia E, Favales F, Quarantiello A, et al. Angiographic evaluation of peripheral arterial occlusive disease and its role as a prognostic determinant for major amputation in diabetic subjects with foot ulcers. Diabetes Care. 1998;21:625–630. doi: 10.2337/diacare.21.4.625. [DOI] [PubMed] [Google Scholar]
- 37.Allison MA, Criqui MH, Wright CM. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:331–336. doi: 10.1161/01.ATV.0000110786.02097.0c. [DOI] [PubMed] [Google Scholar]
- 38.Martinez BD, Hertzer NR, Beven EG. Influence of distal arterial occlusive disease on prognosis following aortobifemoral bypass. Surgery. 1980;88:795–805. [PubMed] [Google Scholar]
- 39.Wolfle KD, Bruijnen H, Reeps C, et al. Tibioperoneal arterial lesions and critical foot ischaemia: successful management by the use of short vein grafts and percutaneous transluminal angioplasty. Vasa. 2000;29:207–214. doi: 10.1024/0301-1526.29.3.207. [DOI] [PubMed] [Google Scholar]
- 40.Vogt MT, Wolfson SK, Kuller LH. Segmental arterial disease in the lower extremities: Correlates of disease and relationship to mortality. Journal of Clinical Epidemiology. 1993;46:1267–1276. doi: 10.1016/0895-4356(93)90091-e. [DOI] [PubMed] [Google Scholar]
- 41.Dormandy J, Belcher G, Broos P, et al. Prospective study of 713 below-knee amputations for ischaemia and the effect of a prostacyclin analogue on healing. Hawaii Study Group. Br J Surg. 1994;81:33–37. doi: 10.1002/bjs.1800810110. [DOI] [PubMed] [Google Scholar]
- 42.Callister TQ, Cooil B, Raya SP, Lippolis NJ, Russo DJ, Raggi P. Coronary artery disease: improved reproducibility of calcium scoring with an electron-beam CT volumetric method. Radiology. 1998;208:807–814. doi: 10.1148/radiology.208.3.9722864. [DOI] [PubMed] [Google Scholar]
- 43.Detrano R, Kang X, Mahaisavariya P, et al. Accuracy of quantifying coronary hydroxyapatite with electron beam tomography. Invest Radiol. 1994;29:733–738. doi: 10.1097/00004424-199408000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Lawler LP, Horton KM, Scatarige JC, et al. Coronary artery calcification scoring by prospectively triggered multidetector-row computed tomography: is it reproducible? J Comput Assist Tomogr. 2004;28:40–45. doi: 10.1097/00004728-200401000-00006. [DOI] [PubMed] [Google Scholar]