Abstract
A common method for collecting behavioral data is through direct observations. However, there is very little information available on how a human observer affects the behavior of the animals being observed. This study assesses the effects of a human observer on the behavior of captive nonhuman primates. The subjects were 19 singly housed baboons (nine male, 10 female) and 20 singly housed rhesus macaques (10 male, 10 female) that were not habituated to the presence of an observer. Four 30-min observations were conducted on each animal. Two observations were conducted with an observer present (“present” condition), while the remaining two observations had no observer present (“absent” condition). All observations were recorded with a video camera and were balanced for time of day, with one of each type of observation taking place in the morning and afternoon. In the presence of an observer, appetitive behavior was significantly reduced in both species [F(1,35) = 8.22, P < 0.01]. When an observer was present, females of both species also rested more and performed fewer manipulative behaviors than males [rest: F(1,35) = 7.10, P < 0.05; manipulative: F(1,35) = 6.66, P < 0.05]. Likewise, macaques rested significantly more [F(1,35) = 11.62, P < 0.005] and exhibited fewer manipulative behaviors in the presence of an observer [F(1,35) = 11.06, P < 0.005], while baboons showed no change. Female macaques showed the greatest decrease in activity while an observer was present [F(1,35) = 4.22, P <0.05]. Based on these results, the presence of a human observer does appear to affect the behavior of unhabituated, singly housed baboons and macaques, but the effect differs by both sex and species.
Keywords: behavior assessment, observer effect, baboon, macaque
1. Introduction
Behavioral data can be recorded using several techniques including direct observations or remote video recording equipment (Line, 1995). Although widely used, direct observations have several drawbacks such as leaving no permanent visual record, observers being prone to fatigue, and the presence of the observer affecting the animal’s behavior without acclimation (Duktig and Meunier, 1990). It is the latter of these drawbacks that this study addresses. Any change in behavior caused by the observer’s visual, olfactory, or auditory presence is referred to as observer effect (Lehner, 1996). To help reduce observer effect, a period of acclimation or habituation should precede any formal observations. Data should not be collected until this period has ended, which is usually signaled by the ability of the observer to approach the subjects without any detectable response (Rasmussen, 1991).
If a subject notices an observer or anything related to the observation process, no matter how unobtrusive, the subject could alter its behavior (Jordan and Burghardt, 1986). Although video equipment eliminates the need for a human observer and creates a permanent visual record, the equipment requires additional costs and may not be appropriate in all research and housing situations (Line, 1995). In addition to these drawbacks, recorded observations can be difficult to score due to the quality of video (e.g., low resolution). In particular situations, the subject might move to an area outside of the camera’s view or may simply situate itself in a way that obscures its behavior.
Regardless of how unobtrusive observers might attempt to be during an observation, they may still have an effect on the animal’s overall behavior by generating a sense of curiosity, anxiety, or stress. This effect may vary by species due in part to differences in temperament. For example, rhesus macaques have been referred to as “belligerent” and “difficult to handle” (Kling and Orbach, 1963; Orbach and Kling, 1964). This species was also referred to as the most “frenetic” when compared to two other species of macaques (MacDonald, 1971). In contrast, baboons have been characterized as both relaxed and highly adaptable to variations in both their physical and social environment (Brent, 2009).
When collecting behavioral data, effects caused by the human observer should be kept to a minimum. Though human observers can affect the behavior of the subjects they are observing, very little research has been conducted to see what these effects are in nonhuman primates (Rasmussen, 1991). This study examined the effect of an observer on two species of singly housed nonhuman primates, baboons (Papio spp.) and rhesus macaques (Macaca mulatta).
2. Materials and Methods
2.1. Subjects
The subjects were 19 singly housed adult baboons (nine male, 10 female) and 20 singly housed adult rhesus macaques (10 male, 10 female). Nine baboons were Papio hamadryas anubis, and the remaining ten were 2-way crosses of P. h. anubis, P. h. cynocephalus, P.h. papio, and P. h. hamadryas (VandeBerg and Cheng, 1986; Williams- Blangero et al. 1990). The baboons ranged in age from 5–13 years and the rhesus macaques (Macaca mulatta) ranged in age from 10–18 years. The subjects were housed at the Southwest National Primate Research Center, an Association for the Assessment and Accreditation of Laboratory Animal Care International accredited institution located in San Antonio, TX, USA. All subjects were born and raised in captivity with no previous experience with the observer in this study. All subjects were housed individually in standard 0.74 m2 cages. Cages were cleaned twice daily, once between 08:00 h and 09:00 h and a second time between 13:00 h and 14:00 h. Animals were fed a biscuit ration twice daily with the first being between 09:00 h and 10:00 h and a second between 14:00 h and 15:00 h. All animals had access to a rubber chew toy or plastic ball in their cage. Daily produce or grain was given as a form of nutritional enrichment. In addition to the daily nutritional enrichment, all subjects were given occupational enrichment (e.g. food puzzles) that were rotated on a semi-weekly schedule, as well as novel food items. The rooms in which the animals were housed were kept on a 12:12 light-dark cycle from 06:00 h to 18:00 h.
2.2. Procedures
Each animal was videotaped in real time with a Panasonic PV-GS320 mini digital recorder (Matushita Electronic Industrial Co. Ltd., Osaka, Japan) for 30 min per day on four separate days with no more than one observation taking place in an animal room in one day. Observations began in September of 2006 and continued until June of 2007. They were balanced for time of day with two observations taking place between 08:00 h and 12:00 h and two observations taking place between 13:00 h and 17:00 h for each subject. For two of the observations (one morning and one afternoon), an observer sat on a small stool behind a digital video recorder that was situated directly in front of the cage approximately 1.0 to 1.5 m away. During this time, the observer averted his gaze from the animal, only occasionally looking up. These observations were referred to as “present”. For the remaining two observations, no observer was in the room, but the video camera was situated in the same location as during the “present” observations. These were referred to as “absent”. All methods and procedures were approved by the Southwest Foundation for Biomedical Research Institutional Animal Care and Use Committee (IACUC).
2.3. Scoring and Data Analysis
Each videotape was scored by one of two scorers that were blind to the presence of the observer. Inter-observer reliability (percentage agreement score) was above 90%. Individual behaviors were scored using a 30-s point sampling method. Due to the low frequency of some individual behaviors, the behaviors recorded were lumped together into six mutually exclusive categories. The categories were rest, active, appetitive, self-directed, manipulative, and abnormal. For a list of the individual behaviors under each category and a definition of those behaviors refer to Table 1.
Table 1.
List of behavior categories, individual behaviors, and definitions
| Category | Behavior | Definition |
|---|---|---|
| Abnormal | Flip | Complete backward revolution of the body, end over end, at least three consecutive times |
| Floating Limb | Apparently random, functionless movement of a limb that appears to be independent of both the animal’s attention and control | |
| Hair Eat | Ingestion of hair obtained from either self or cage surface | |
| Hair Pull | Removing hair from body in tufts or individual strands with hands or teeth | |
| Head Toss | Rapid jerk of the head at the neck in a semi circle motion | |
| Mouth Movements | Repetitive mouth activity unrelated to eating, drinking, or any other apparently functional purpose | |
| Pace | Repetitive walking in a fixed route within the cage for three or more consecutive repetitions | |
| Poke Eye | Pressing finger(s) over eye for no apparent functional purpose. | |
| Regurgitation | Reflux of partially digested food back from the stomach into the mouth and/or cheek pouches | |
| Rock | Front to back or lateral swaying of the torso at least three consecutive times | |
| Self-Bite | Forceful closing of the mouth and contact with the teeth on a body part | |
| Swing | Hanging from any point in the cage using any combination of feet and hands, and swaying back and forth repeatedly for at least three consecutive times | |
| Other Abnormal | Any other behavior not deemed normal but does not fit the description of the above abnormal behaviors | |
| Appetitive | Eating | Physically putting food into the mouth or chewing food |
| Drinking | Placement of the mouth to and activation of the water dispenser | |
| Self-Directed | Self-Directed | Oral or manual manipulation of the hair coat or body parts |
| Masturbate | Any manual manipulation or self stimulation of the genitals excluding grooming or scratching | |
| Manipulative | Cage-Directed | Any interaction with the cage including manipulating cage parts (e.g. locks or latches) or grabbing bars |
| Tactile/Oral Explore | Oral or manual manipulation of objects excluding cage and enrichment items | |
| Enrichment Directed | Oral or manual manipulation of any enrichment device in or on the cage | |
| Active | Active | Any normal locomotion requiring two or more steps to be taken, as well as any change in total body posture including sitting to standing or standing to sitting. |
| Affiliative | Any behavior directed toward the observer or another animal in the bay indicating sociability (e.g. lipsmack) | |
| Aggressive | Any behavior directed toward the observer or another animal in the bay intended to threaten or intimidate (e.g. threat, cage shake) | |
| Submissive | Any behavior directed toward the observer or another animal in the bay indicating deference (e.g. rump present, fear grimace) | |
| Rest | Rest | Inactivity lasting longer than two seconds |
| Yawn | Opening mouth wide in a non-threatening way |
For all behavioral categories, subject means were obtained for each condition (present, absent). Due to non-normality, square root transformations were conducted on the data for abnormal, active, appetitive, manipulative, and self-directed behaviors. Data were analyzed using a mixed design repeated measures analysis of variance with sex and species as between-subjects variables, and condition as a within-subjects variable.
All data except for the behavioral category “rest” are presented as transformed square root means (SQRmean) ± S.E. with back-transformed SQRmean in parentheses except when otherwise stated. The data for rest were normally distributed and did not require transformation.
3. Results
3.1 Abnormal Behavior
Macaques exhibited more abnormal behavior than baboons [macaque: SQRmean = 2.99 ± 0.52 (14.28), baboon: SQRmean = 0.46 ± 0.33 (2.18); F(1,35) = 18.38, P < 0.001]. There were no condition or sex effects and no interactions.
3.2 Active Behavior
Females were more active overall (P < 0.005). However, there was a condition×species×sex interaction showing that female macaques were significantly more active when the observer was absent [F(1,35) = 4.22, P < 0.05; Fig. 1].
Fig. 1.
Changes in active behavior as demonstrated by a 3-way interaction between condition (with observer present versus video camera only), species (20 macaques and 19 baboons), and sex (20 females and 19 males). The error bars represent standard error.
3.3 Appetitive Behavior
Appetitive behavior was significantly reduced in the presence of an observer [present: SQRmean = 1.44 ± 0.24 (4.18), absent: SQRmean = 2.36 ± 0.32 (9.41); F(1,35) = 8.22, P < 0.01]. There were no sex or species effects and no interactions.
3.4 Manipulative Behavior
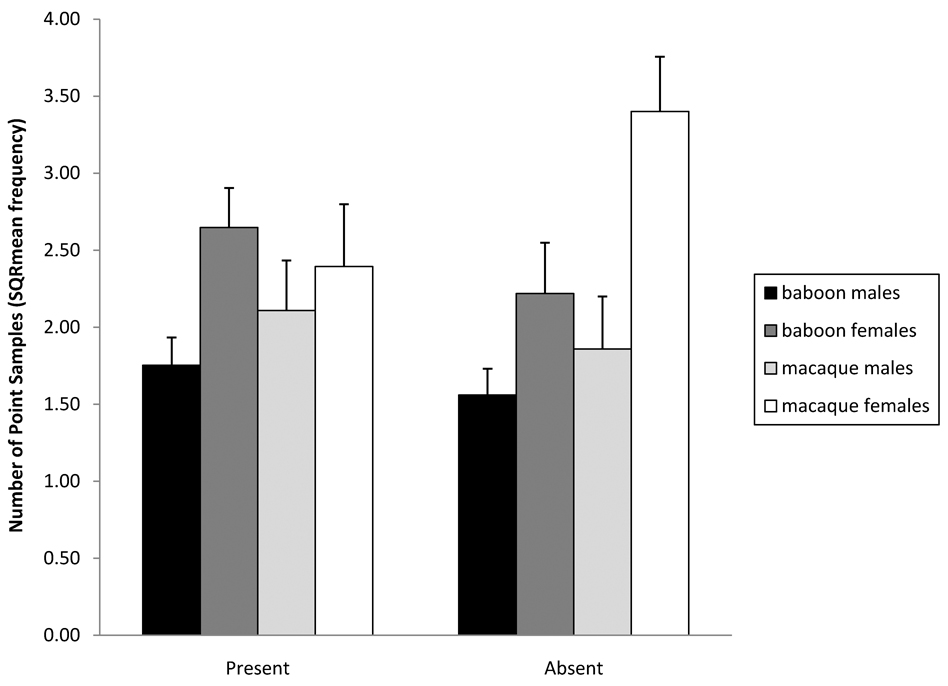
There were condition×species, condition×sex, and species×sex interactions for manipulative behavior. In the presence of an observer, macaques performed significantly fewer manipulative behaviors while baboons showed little change [F(1,35) = 11.06, P < 0.005; Fig. 2]. Likewise, females performed significantly fewer manipulative behaviors while males showed little change [F(1,35) = 6.66, P < 0.05; Fig. 2]. There was also a species×sex interaction in which baboon males were more manipulative than baboon females, while macaque females were more manipulative than macaque males [F(1,35) = 4.84, P < 0.05; Fig. 2].
Fig. 2.
Changes in manipulative behavior as demonstrated by three 2-way interactions: (1) condition (with observer present versus video camera only) by species (20 macaques and 19 baboons), (2) condition (with observer present versus video camera only) by sex (20 females and 19 males), and (3) species (20 macaques and 19 baboons) by sex (20 females and 19 males). The error bars represent standard error.
3.5 Rest
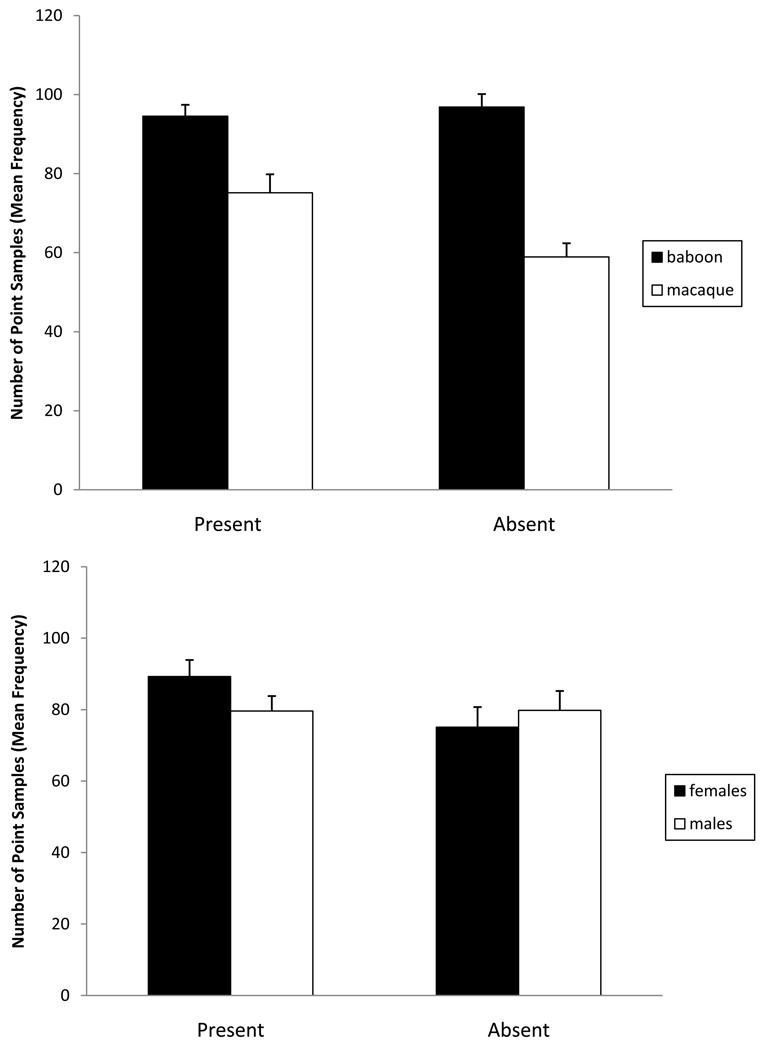
Rest was significantly higher when an observer was present (P < 0.05) and was significantly higher in baboons when compared to macaques (P < 0.001). However, there was a condition×species and a condition×sex interaction. In the presence of an observer, macaques rested significantly more while baboons showed little change [F(1,35) = 11.62, P < 0.005; Fig. 3]. Likewise, females rested significantly more while males showed little change [F(1,35) = 7.10, P <0.05; Fig. 3].
Fig. 3.
Changes in resting behavior as demonstrated by two 2-way interactions: (1) condition (with observer present versus video camera only) by species (20 macaques and 19 baboons) and (2) condition (with observer present versus video camera only) by sex (20 females and 19 males). The error bars represent standard error.
3.6 Self-directed
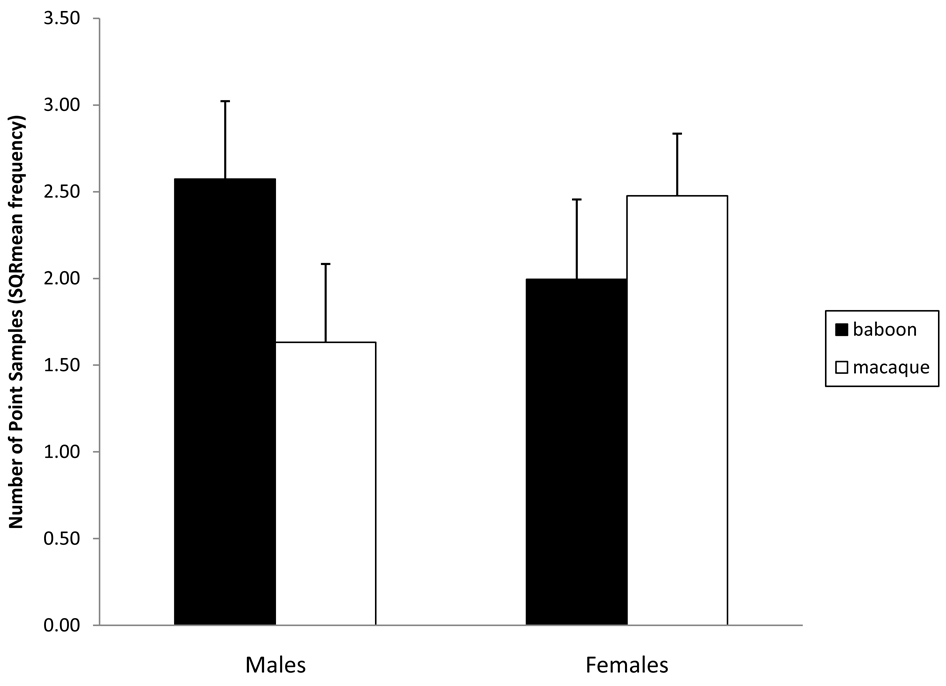
Macaques showed more self-directed behavior than baboons (P < 0.005) and males exhibited more self-directed behavior than females (P < 0.05). However, there was a significant species×sex interaction. Macaque males exhibited more self-directed behavior than macaque females, while there was no sex difference in baboons [F(1,35) = 4.83, P < 0.05; Fig. 4].
Fig. 4.
Species (20 macaques and 19 baboons) and sex (20 females and 19 males) differences in self-directed behavior as demonstrated by a 2-way interaction. The error bars represent standard error.
4. Discussion
The presence of a human observer does appear to affect the behavior of unhabituated baboons and rhesus macaques. In the present study, an overall decrease in appetitive behavior was seen when an observer was present. This is similar to results of a previous study on squirrel monkeys in which the presence of a human observer inhibited eating (Candland et al., 1972). Although cortisol levels were not monitored in this study, stress could have been a factor since appetite suppression has been associated with stress (Crockett et al., 2000).
The present study also found that rest, defined as at least two seconds of inactivity, increased significantly during the “present” condition in macaques and in females. These results are similar to those found by Line (1995) in rhesus macaques in which movement decreased significantly during sessions when an observer was present compared to those in which the animals were videotaped only. As with Line (1995), the animals in the present study may have been focusing on or inhibited by the observer and therefore less likely to move around the cage. However, in comparison to macaques, baboons showed little behavioral change, which may be due to their more relaxed demeanor (Brent, 2009).
Although the female macaques in the present study reduced their activity more than the other subjects when an observer was present, the manner in which they were inactive showed signs that other factors might have been affecting their activity levels. Some of the female macaques would suspend themselves from the upper back corner of the cage for most of the observation. This behavior is similar to behaviors described by Bernstein et al. (1963) and Singh and Manocha (1966) where rhesus monkeys were found to become immobilized and inattentive in the presence of novel objects. Similarly, Kalin (1993) noted that infant macaques appear to “freeze,” remaining completely still for long periods of time in a frightening situation. The inactivity of the females in the present study may indicate that this behavior was a result of fear or stress with regard to an unfamiliar observer. After further habituation to the observer, the amount of activity and appetitive behaviors may increase.
5. Conclusion
The results of this study indicate that the technique used to obtain behavioral data does have an impact on the behaviors exhibited, and reactivity to an observer could have an adverse effect on the data collected. These effects can be seen not only overall, but also to varying degrees by species and sex. Therefore, when collecting behavioral data, one must not only consider the data collection method utilized, but also the sex and species of the subjects in order to minimize the effects of the observation.
Acknowledgments
This research was supported by NCRR grant #RR013986. We would like to thank two anonymous reviewers for their helpful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bernstein IS, Schusterman RJ, Sharpe LG. A comparison of rhesus monkey and gibbon responses to unfamiliar situations. J. of Comp. and Physiol. Psychol. 1963;56(5):914–916. doi: 10.1037/h0048631. [DOI] [PubMed] [Google Scholar]
- Brent L. The study of captive baboon behavior. In: VandeBerg JL, Williams-Blangero S, Tardiff SD, editors. The Baboon in Biomedical Research. New York: Springer; 2009. pp. 21–34. [Google Scholar]
- Candland DK, Dresdale L, Leiphart J, Johnson C. Videotape as a replacement for the human observer in studies of nonhuman primate behavior. Behav. Res. Meth. and Instru. 1972;4(1):24–26. [Google Scholar]
- Crockett CM, Shimoji M, Bowden DM. Behavior, appetite, and urinary cortisol responses by adult female pigtailed macaques to cage size, cage level, room change, and ketamine sedation. Am. J. of Primatol. 2000;52:63–80. doi: 10.1002/1098-2345(200010)52:2<63::AID-AJP1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Duktig JT, Meunier LD. Using videotape cameras to quantify data in primate environmental enrichment studies. Lab Anim. 1990;19(7):30–31. [Google Scholar]
- Jordan RH, Burghardt GM. Employing an ethogram to detect reactivity of black bears (Ursus americanus) to the presence of humans. Ethology. 1986;73:89–115. [Google Scholar]
- Kalin NH. The neurobiology of fear. Sci. Am. 1993;268(5):94–101. doi: 10.1038/scientificamerican0593-94. [DOI] [PubMed] [Google Scholar]
- Kling A, Orbach J. Plasma 17-hydroxycorticosteroid levels in the stump-tailed monkey and two other macaques. Psychol. Rep. 1963;13:863–865. [Google Scholar]
- Lehner PN. Handbook of Ethological Methods. 2nd ed. NewYork: Cambridge University Press; 1996. pp. 210–212. [Google Scholar]
- Line SW. Effects of observation techniques on the behavior of adult rhesus macaques. Contemp. Top. Lab. Anim. Sci. 1995;34(6):61–65. [PubMed] [Google Scholar]
- MacDonald GJ. Reproductive patterns of three species of macaques. Fertil. Steril. 1971;22(6):373–377. [PubMed] [Google Scholar]
- Orbach J, Kling A. The stump-tailed macaque: a docile Asiatic monkey. Anim. Behav. 1964;12:343–347. [Google Scholar]
- Rasmussen DR. Observer influence on range use of Macaca arctoides after 14 years of observation. Lab. Primate Newslett. 1991;30(3):6–11. [Google Scholar]
- Singh SD, Manocha SN. Reactions of the rhesus monkey and the langur in novel situations. Primates. 1966;7(2):259–262. [Google Scholar]
- VandeBerg JL, Cheng ML. The Genetics of Baboons in Biomedical Research. In: Else JG, Lee PC, editors. Primate Evolution. New York: Cambridge University Press; 1986. pp. 317–327. [Google Scholar]
- Williams- Blangero S, VandeBerg JL, Blangero J, Konigsberg L, Dyke B. Genetic differentiation between baboon subspecies: Relevance for biomedical research. Am. J. of Primatol. 1990;20:67–81. doi: 10.1002/ajp.1350200202. [DOI] [PubMed] [Google Scholar]