Abstract
In the fall of 2007 pet food contaminated with melamine and cyanuric acid caused kidney stones in thousands of animals. In the summer of 2008, a more serious outbreak of adulterated dairy food caused the deaths of six infants and sickened about 290,000 children in China. In all cases, melamine was likely added to inflate the apparent protein content of the foods. To determine if we could measure protein without interference from melamine and cyanuric acid we tested these compounds in the Bradford and Ninhydrin assays, two common dye-based assays for protein, as well as by ammonia release, the most common assay used in the food industry. Neither compound was detected in the Ninhydrin and Bradford assays at concentrations of >100 μg/ml. The ammonia assay detected melamine but was inconclusive with respect to cyanuric acid. To develop an accurate test for food that would not detect either chemical as a protein, assays were run on cat food and reconstituted milk powder. The Bradford assay readily measured the protein content of each food, and importantly, the addition of melamine or cyanuric acid to reconstituted milk did not affect the readings. The protein concentrations obtained for reconstituted milk powder were as expected, but those for the cat food were 10 to 30-fold lower, due to its low solubility. We conclude that dye-binding assays can be employed to detect protein in food without interference from melamine and cyanuric acid, thus reducing the incentive to use them as additives.
Keywords: food safety, adulteration, toxin, melamine, protein assay
Introduction
In March 2007, there was a major case of melamine poisoning caused by several pet foods. Hundreds and perhaps thousands of animals, primarily cats, were sickened and killed by wheat gluten from China that was contaminated with melamine and cyanuric acid (Barboza, May 4, 2007). Despite worldwide media attention to the problem, a more serious outbreak occurred in the summer of 2008, when melamine was found in many Chinese dairy products, including infant formula and milk-chocolate. The 2008 outbreak caused the death of at least six infants and sickened about 290,000 Chinese children, about 51,000 of whom were hospitalized with kidney stones. As a result, many countries placed restrictions on Chinese dairy products and several people were convicted (Barboza, January 23, 2009; Barboza, September 30, 2008). Melamine and cyanuric acid were likely added as non-protein nitrogen additives (NPN) to the wheat gluten used in pet foods, and later to the powdered milk, in an illegal effort to increase the apparent level of protein in these products (Barboza & Barrionuevo, April 30, 2007). Both melamine and cyanuric acid are by-products of coal processing and are not approved for use as food additives for humans, although cyanuric acid is used as a NPN additive in some cattle feeds.
Until the recent outbreaks, melamine was considered harmless to humans and is commonly used to make Formica counter tops in kitchens and even plastic eating utensils. Cyanuric acid is often added to swimming pools to stabilize the chlorine from UV light. Melamine rapidly forms hydrogen bonds with cyanuric acid to form melamine cyanurate, a highly ordered insoluble crystal. In fact, melamine precipitation is the basis of tests to measure the cyanuric acid content in swimming pools. When tested on cats, neither chemical is toxic individually; however, when combined they may crystallize in the kidneys to form melamine cyanurate and, thereby, cause renal failure (Puschner, Poppenga, Lowenstine, Filigenzi & Pesavento, 2007; Xin & Stone, 2008).
The food industry uses primarily two methods to measure protein content, the Kjeldahl method and the Dumas method (Thompson, Owen, Wilkinson, Wood & Damant, 2002). Neither of these methods measures protein directly, but instead measures the nitrogen content of samples. The first step in these procedures is to hydrolyze food, with sulfuric acid (Kjeldahl) or through combustion (Dumas), to break down proteins and release nitrogen. The second step is to measure the nitrogen released (Robyt & White, 1987; Rosenberg, 1996). Melamine and cyanuric acid are detected as proteins in these tests because of the large amounts of nitrogen they contain, which includes the nitrogen in the amino groups on melamine.
There are other assays for proteins based on dye-binding. The Bradford assay, developed by Marion M. Bradford in 1976, is a common protein assay widely used in molecular biology laboratories, but rarely in the food industry. It determines the amount of protein in a substance by using Coomassie Brilliant Blue Dye, which turns from red to blue when it binds to proteins. The dye is protonated by the amino groups of the basic amino acids lysine and tryptophan and then binds to hydrophobic regions in proteins and turns blue (Bradford, 1976). By measuring absorbance at 595nm in a spectrophotometer and comparing samples to a standard protein such as bovine serum albumin, the amount of protein in a sample can be quantified. The ninhydrin assay is a common laboratory procedure used to detect amino acids. Ninhydrin binds amino groups in amino acids, and fluoresces. When heated, the bound dye turns purple. The assay is used in protein sequencers and in thin layer chromatography applications to identify amino acids (Robyt et al., 1987).
Dye-binding assays differ from the Kjeldahl method and the Dumas method in that they measure protein content directly, instead of indirectly through nitrogen content. We hypothesized that dye-binding assays could be used instead of the ammonia release assays to determine protein content without interference from melamine and cyanuric acid. Thus, we tested melamine and cyanuric acid in Bradford and ninhydrin assays, and found that neither compound was detected. When the Bradford assay was used with cat food pellets, the protein concentrations were 10 to 30-fold lower than reported by the manufacturer, but when the Bradford assay was used with reconstituted milk, the assay yielded concentrations within 2-fold of expected values. Thus the Bradford assay is a rapid and inexpensive substitute for nitrogen release assays. We conclude that there would be less incentive to adulterate food, especially milk, if the food industry employed dye-based assays to measure protein.
Materials and Methods
Melamine was purchased from Fluka and cyanuric acid was purchased from Aldrich; both were prepared as 25mM solutions, 3.15 mg/ml of melamine or 3.23 mg/ml of cyanuric acid dissolved in water. A 5% cat food solution in water was prepared by grinding and sonicating Royal Canon Urinary SO cat food pellets (kibble). Carnation milk powder was purchased from a local supermarket and suspended at a concentration of 11.5 g in 240 ml of water according to the procedure recommended for reconstitution. The sample was diluted 20-fold in water for assays.
Sample hydrolysis/digestion
Some samples were treated with acid to promote the release of nitrogen. Where indicated, melamine, cyanuric acid, and cat food were digested as follows: 100 μl of 97% H2SO4 or 37% HCl, as indicated, was added to 900 μl of each sample solution and then incubated at 100°C for one hour. To neutralize the acid, 100 μl of a 10 M solution of NaOH was added to each sample. To digest samples with proteinase, where indicated (see figures), 1 ml of the 5% cat food solution was combined with 50 μl of a 20mg/ml proteinase K solution and then incubated at 37 °C in a water bath for 1 hr.
Ninhydrin assay
To run a Ninhydrin test, 2 μl of each sample was spotted on a Flexible Thin layer Chromatography (TLC) Plate. After the samples dried, the plate was sprayed with a 0.2% ninhydrin solution prepared by dissolving 0.2 g of ninhydrin in 100 ml of methanol. The plate was air dried and then heated in an oven at 70° for 10 minutes.
Bradford Protein assay
To perform a Bradford assay, test samples were added to microcentrifuge tubes and brought to a volume of 800 μl with water. Next, 200 μl of 5X Bradford reagent (Bio-Rad laboratories catalog number 500-0006) was added to each sample to bring it to a volume of 1 ml. The samples were then analyzed in a Beckman spectrophotometer to determine their absorbance at 595nm. A standard curve was prepared with bovine serum albumin (BSA) as a control for all experiments. To do this, 0, 2, 5, 10 or 20 μl of BSA at a concentration of 1.4 mg/ml or in some assays 2.0 mg/ml, was added to 800 μl of water and then used in the assay.
Ammonia assay
Ammonia assays were performed using a kit from Sigma (catalog number AA0100), in which ammonia reacts with α-ketoglutaric acid and NADPH in the presence of L-glutamate dehydrogenase to form L-gultamate and NAD+. The oxidation of NAD+ is read as a decrease in absorbance at 340nm. Where indicated, the samples were treated with H2SO4, HCl or proteinase K. For the assay, samples were brought to 100 μl with water and then 1 ml of the ammonia assay reagent was added. The samples were incubated for 5 min at 18-35°C and their absorbance was then measured at 340nm. Next, 10μl of L-Glutamate Dehydrogenase solution was added to each sample and after 5min, the absorbance was measured again at 340nm. A standard curve was generated using an ammonia standard and 1 ml of the ammonia assay reagent. The blank was 100 μl of water and 1 ml of the reagent. The data are presented as the change in the absorbance compared to the standard curve with ammonia. Experiments in this study were repeated 2-3 times each.
Results
Melamine and cyanuric acid do not interfere with Bradford assays
Melamine and cyanuric acid are added to food as a means to boost the apparent protein content. To test how these compounds react in protein assays, three assays were employed: the Bradford assay, the Ninhydrin assay, and an ammonia release assay similar to the Kjeldahl method. We first tested samples of melamine and cyanuric acid in the Bradford assay. To calibrate the assay, we used bovine serum albumin (BSA) as a typical protein to create a standard curve (Figure 1A). Figure 1B shows the results of the Bradford assay run on samples of melamine and cyanuric acid. Unlike BSA, the Bradford assay did not detect either compound although almost 10 times as much sample was tested as BSA.
Figure 1. Melamine and cyanuric acid are not detected in a Bradford assay.
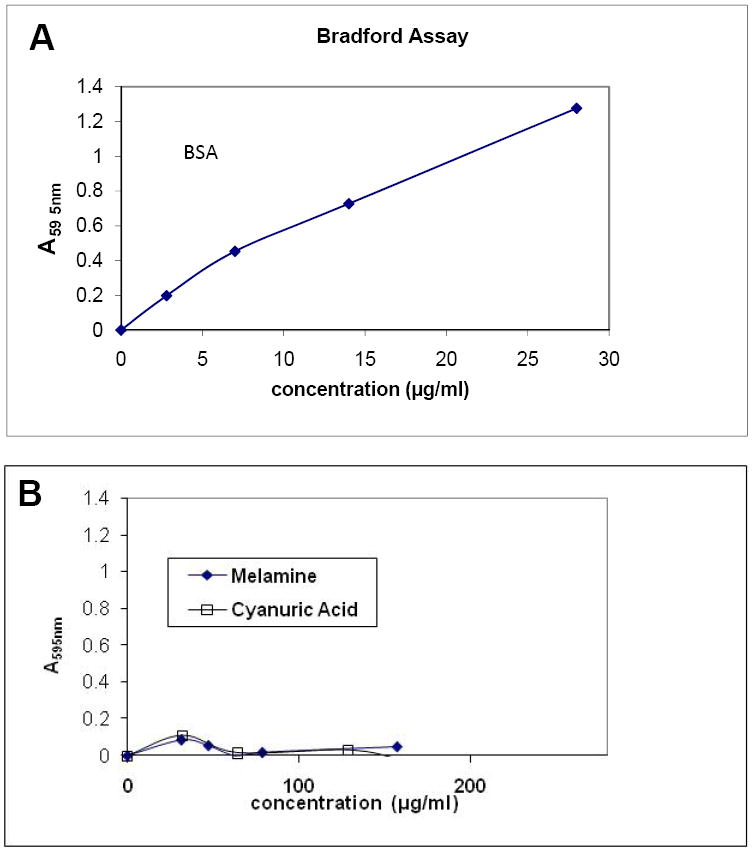
The indicated samples were tested in Bradford protein assays as described in Materials and Methods. (A) BSA protein standard in a Bradford Assay. The indicated amounts of bovine serum albumin (BSA) were used to produce a standard curve. (B) A Bradford Assay with melamine and cyanuric Acid.
The lack of positive results from the Bradford assay of melamine and cyanuric acid prompted additional tests, which involved treatments to release nitrogen as ammonia for analysis. We treated samples with HCl and H2S04, the most commonly used means of hydrolyzing proteins in the food industry. Even after these treatments there was no change in the apparent protein as measured by the Bradford assay (Figure 2A, data not shown for cyanuric acid). We conclude that neither melamine nor cyanuric acid is detected in the Bradford assay, even after hydrolysis.
Figure 2. Effects of acid treatment of melamine and cyanuric acid.
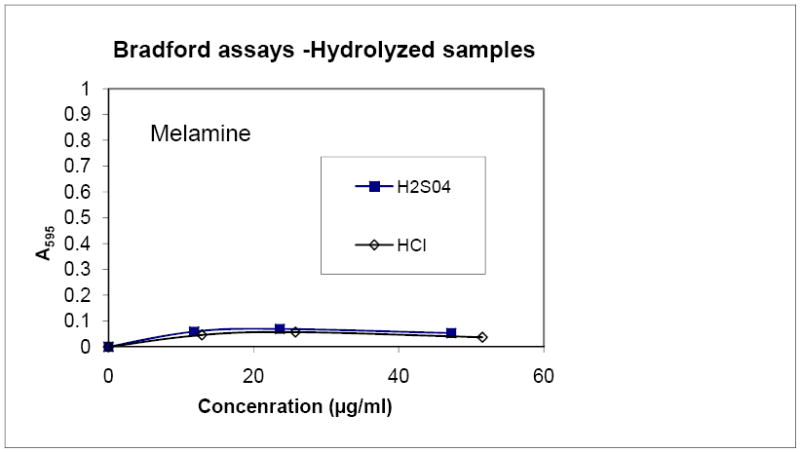
The results of a Bradford assay run with melamine treated with either HCl or H2SO4
A Ninhydrin test to measure protein in test substances
The second protein test we employed on melamine and cyanuric acid was a ninhydrin test. Ninhydrin reacts with amino acids, and when heated becomes purple. This test can also be used to detect fingerprints. We performed the assay by spotting samples onto a thin layer chromatography plate (TLC) and then staining with ninhydrin. We found that neither melamine nor cyanuric reacted with Ninhydrin under conditions that detected several amino acids as well as a fingerprint (Figure 3).
Figure 3. Ninhydrin test of melamine and cyanuric acid.
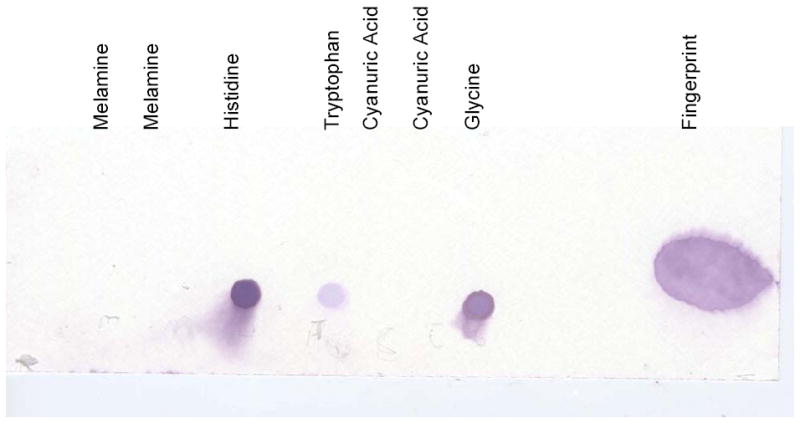
Samples were spotted onto a TLC plate and then treated with Ninhydrin as described in Materials and Methods. The samples were spotted directly below the labels. The ninhydrin turns purple in the presence of amino acids. Histidine, Tryptophan and Glycine were used as control amino acids.
An ammonia assay to measure protein concentration in test substances
Since two common dye-based laboratory reagents did not react with melamine or cyanuric acid, we next employed an ammonia release test similar to the industry protein tests, which are based on nitrogen content (Figure 4). Some samples were treated with HCl and H2SO4 to hydrolyze them prior to nitrogen release. We measured ammonia using an enzyme linked assay in which ammonia reacts with α-ketoglutaric acid and NADPH in the presence of L-glutamate dehydrogenase to form L-gultamate NAD+. The oxidation of NAD+ causes a decrease in absorbance at 340nm. Samples are compared with a standard curve of ammonia.
Figure 4. Ammonia release assays for protein.
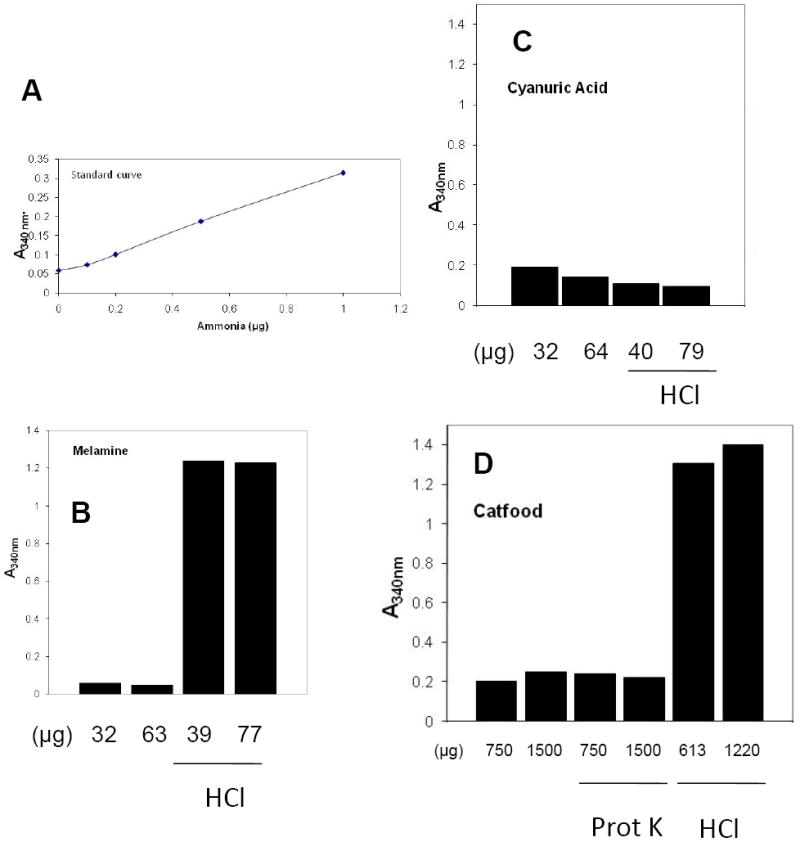
Samples were tested for ammonia content as described in Materials and Methods. (A) Standard curve. (B) Ammonia detected in melamine and melamine treated with HCl. Note that the ammonia concentration in melamine was beyond the linear range of the assay. (C) Ammonia detected in cyanuric acid and cyanuric treated with HCl. (D) Ammonia assay of cat food and cat food treated with proteinase K or HCl.
The sample of melamine was not detected when tested by itself in the ammonia assay, but yielded readings off the scale when it was treated with HCl. Cyanuric acid yielded much lower readings which did not change after hydrolysis. Experiments with H2SO4 hydrolysis were inconsistent, probably because of incomplete neutralization of the acid after the treatment (data not shown). We also tested cat food pellets in the ammonia release experiment (Figure 4D). As with the melamine, the untreated samples did not yield any ammonia but pretreatment with HCl released ammonia; Proteinase K did not release ammonia. We conclude that melamine is detected by nitrogen release assays but not by two dye-based assays.
Bradford assays of food
The results suggested that neither melamine nor cyanuric acid is detected in Bradford assays. To determine if the Bradford assay could determine the protein content of cat food, we tested a sample of cat food (Figure 5). We found that the sample was difficult to dissolve and did not yield a reliable signal, even after extensive mechanical disruption. Therefore, we employed several methods to dissolve the protein--proteinase K digestion, HCl hydrolysis and H2SO4 hydrolysis. Each of these increased the detection of protein in the sample with a linear dose response in the assay, with hydrochloric acid resulting in the strongest signal. We noted that, although all treatments caused protein to be detected in the assay, the calculated protein concentration was less than the 30% reported by the manufacturer. For proteinase K and HCl treated samples, the calculated value was 1% of the sample by weight, and for HCl treated samples, the value was 3.8%. Thus, although the Bradford assay detects the protein in cat food, it is not very efficient.
Figure 5. Bradford Assays of cat food.
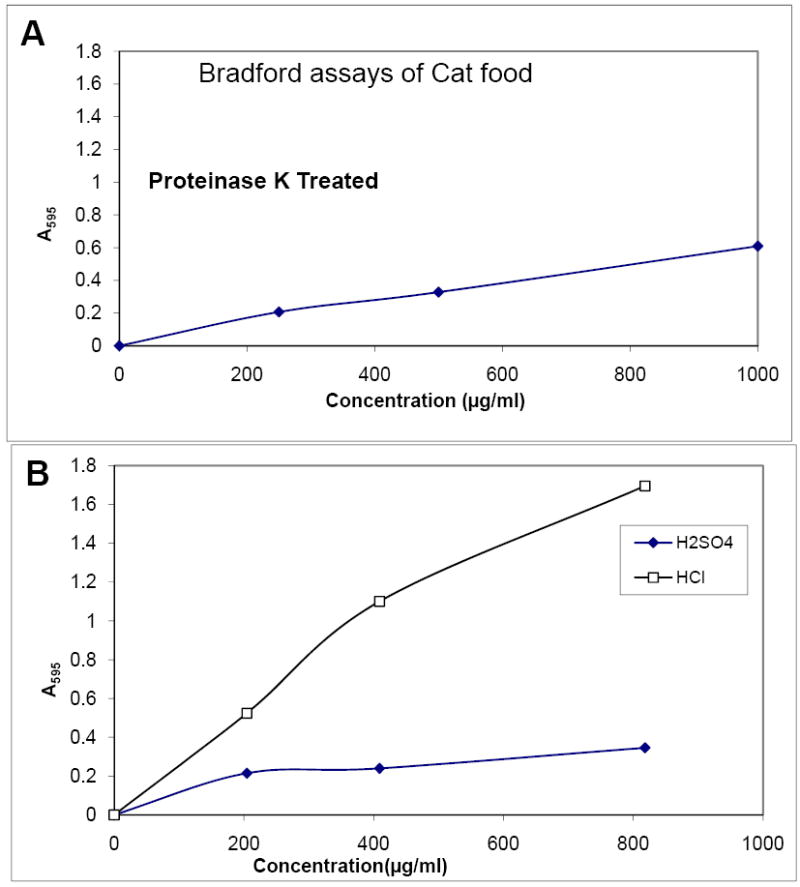
Samples of cat food were hydrolyzed with proteinase K or acid, prior to testing, since attempts using untreated samples were unsuccessful due to the difficulty in dissolving the cat food. (A) Food treated with proteinase K. The calculated percentage of protein in the cat food was about 1%, considerably less than the manufacturer’s claim of 30%. (B) Bradford assay of acid treated cat food. The cat food was not fully dissolved for this test, but its calculated concentration was 3-6% for the HCl treated and 1% for the H2SO4 treated food.
We next tested reconstituted milk powder. When we tested the reconstituted milk directly we found the concentration was too high and beyond the linear range of the standard, so we diluted the samples 20-fold for subsequent tests. The milk gave a highly linear dose response in the Bradford assay (R2=.9987) with calculated protein concentrations that were about half those reported by the manufacturer (Figure 6A). We note that all protein assays compare a standard with unknown samples and that, because of their unique sequences, there are typically 2-fold variations between different standard proteins. Next, to test if the Bradford assay could be used in the presence of melamine, we treated samples of milk with melamine and cyanuric acid prior to testing the milk in the Bradford assay (Figure 6B). Despite adding about twice as much melamine as milk protein, there was no interference in the assay. Therefore, the Bradford assay is a quantitative method to measure the protein in milk powder, and will yield accurate measures of protein even in samples contaminated with melamine or cyanuric acid.
Figure 6. Bradford assay of Milk.
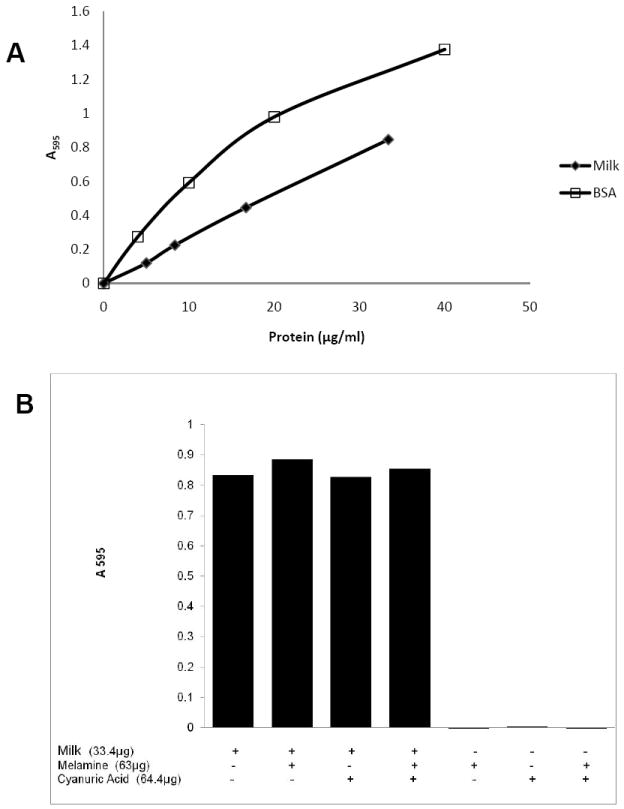
Powdered milk was reconstituted as recommended by the manufacturer and then tested as described in Materials and Methods. (A) Assay of milk in the Bradford assay. The protein concentration of the milk was calculated from the manufacturers report. (B) The effect of melamine and cyanuric acid on a Bradford assay of milk.
Discussion
In 2007 there was a serious outbreak of melamine toxicity affecting thousands of animals. Despite the publicity, a more serious case occurred in the fall of 2008. It is likely that the melamine was deliberately added to boost the apparent protein content and that cyanuric acid was a byproduct of melamine preparation. Since the underlying cause of the adulteration was to substitute melamine as a non-protein source of nitrogen, one strategy to insure the safety of food is to test for melamine. However, melamine tests are expensive to implement since most utilize liquid chromatography mass spectroscopy. Although other melamine tests are based on antibodies, they are still considerably more expensive than the dye-binding based assays for protein (Filigenzi, Tor, Poppenga, Aston & Puschner, 2007; Garber, 2008). The 2008 outbreak illustrated that direct melamine testing is not yet practical on a large scale. A second strategy is to develop rapid, inexpensive protein assays that are not subject to interference from melamine and related substances. We suggest two tests, the Bradford and Ninhydrin assays, as substitutes for nitrogen release assays, but other dye-based assays are also practical.
There are some limitations to using the Bradford assay as noted here. Although it measured the protein in milk accurately, we found that the assay was less reliable with solid food, such as cat food. However, pretreatment of the cat food by proteinase K digestion or acid hydrolysis improved the efficiency. Importantly, similar treatments to melamine did not cause it to react in the Bradford assay, so these digestion steps could be used with food samples. We also note that the overall sensitivity of the Bradford assay for solid foods was about an order of magnitude less than expected. The low value for the cat food probably reflects a need for more extensive digestion or hydrolysis than the treatments that we used. Laboratories routinely calibrate instruments with specific foods to compensate for their heterogeneous nature, so with appropriate standards, dye-based assays should be readily adapted to measuring solid foods (Thompson et al., 2002). In contrast to the low values with solid food, we found that no pretreatment of samples is required in order to use the Bradford assay with liquid samples, such as milk formulas.
It was somewhat surprising that melamine, which contains so many amino groups, did not react with the Coomassie Blue (Bradford reagent) or Ninhydrin, since these dyes are reported to react with amino groups. However their reactions with proteins, which are more complex than simple binding to amino groups, require hydrophobic side chain binding after protonation by the amino group. The aromatic ring of melamine may prevent protonation of the Coomassie Blue. Alternatively, melamine may protonate the Coomassie dye, but the protonated dye may be unable to bind melamine and elicit a response.
Although there are many dye-based assays for protein determination, Biuret, Lowry, Bradford, Ninhydrin, Amido Black and Ponceau S, all have some limitations that reduce their effectiveness under specific conditions. While the Bradford assay is clearly superior to the nitrogen based tests, there are some compounds that interfere with it. It is subject to interference by detergents such as sodium dodecyl sulfate (SDS), Triton X-100 and commercial glassware detergents (Bradford, 1976). Interference can be avoided by using only small amounts of these detergents when dissolving food samples. More of a concern would be detergents deliberately substituted for protein in food adulteration schemes. We note that other protein determination methods such as the Lowry method and Amido black methods are not prone to detergent interference (Robyt et al., 1987; Rosenberg, 1996).
We conclude that the use of dye-binding assays provide a rapid and inexpensive way to reduce the incentives to adulterate food with melamine. However, the best strategy would be to use multiple protein assays because, although each assay may be subject to interference by deliberate tampering, it is not likely that all assays can be circumvented by adulteration.
Acknowledgments
We thank the students and mentors of the TREES (Teen Research and Education in Environmental Science) program of the University of Pennsylvania. Supported, in part, by a grant from the NIH (R25-ES-016146).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barboza D. Death sentences in Chinese milk case. The New York Times; New York: Jan 23, 2009. [Google Scholar]
- Barboza D. China Makes arrest in Pet food Case. The New York Times; New York: May 4, 2007. [Google Scholar]
- Barboza D. China detains 22 in Tainted-Milk Case. The New York Times; New York: Sep 30, 2008. [Google Scholar]
- Barboza D, Barrionuevo A. Filler in Animal Feed is Open Secret in China. The New York Times; New York: Apr 30, 2007. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Filigenzi MS, Tor ER, Poppenga RH, Aston LA, Puschner B. The determination of melamine in muscle tissue by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21(24):4027–4032. doi: 10.1002/rcm.3289. [DOI] [PubMed] [Google Scholar]
- Garber EA. Detection of melamine using commercial enzyme-linked immunosorbent assay technology. J Food Prot. 2008;71(3):590–594. doi: 10.4315/0362-028x-71.3.590. [DOI] [PubMed] [Google Scholar]
- Puschner B, Poppenga RH, Lowenstine LJ, Filigenzi MS, Pesavento PA. Assessment of melamine and cyanuric acid toxicity in cats. J Vet Diagn Invest. 2007;19(6):616–624. doi: 10.1177/104063870701900602. [DOI] [PubMed] [Google Scholar]
- Robyt JF, White BJ. Biochemical Techniques Theory and Practice. Prospect Heights, Illinois: Waveland Press, Inc; 1987. [Google Scholar]
- Rosenberg IM. Protein Analysis and Purification Benchtop Techniques. Boston: Birkhäuser; 1996. [Google Scholar]
- Thompson M, Owen L, Wilkinson K, Wood R, Damant A. A comparison of the Kjeldahl and Dumas methods for the determination of protein in foods, using data from a proficiency testing scheme. Analyst. 2002;127(12):1666–1668. doi: 10.1039/b208973b. [DOI] [PubMed] [Google Scholar]
- Xin H, Stone R. TAINTED MILK SCANDAL: Chinese Probe Unmasks High-Tech Adulteration With Melamine. Science. 2008;322(5906):1310–1311. doi: 10.1126/science.322.5906.1310. [DOI] [PubMed] [Google Scholar]


