Summary
Short-term DC cultures generated with GM-CSF and other cytokines have markedly improved our ability to study the immunobiology of DC. Here we tested 65 cytokines individually for their potentials to promote generation of CD11c+ cells in a murine BM culture system. In addition to several cytokines known to promote DC survival and/or growth, IL-33 was found to augment DC development time- and dose-dependently. Although the resulting CD11c+ cells generated in the presence of IL-33 exhibited a typical dendritic morphology, they expressed MHC class II molecules only at modest levels, showed negligible responses to TLR ligands, produced no detectable IL-12 p70, displayed PD-L1 and PD-L2 on the surface, and failed to activate immunologically naïve T cells efficiently. IL-33-induced expansion of CD11c+ cells was completely blocked by anti-GM-CSF mAb, and GM-CSF mRNA and protein expression in BM culture was markedly elevated by added IL-33, indicating that IL-33 promotes in vitro DC generation indirectly by a GM-CSF-dependent manner. With regard to the cellular source, IL-33-dependent GM-CSF production was observed exclusively within the CD45+/FcεRI+ BM population. Not only do our results reinforce the notion that GM-CSF serves as a primary DC growth factor, they also reveal a previously unrecognized mechanism supporting DC development.
Keywords: dendritic cells, GM-CSF, IL-33
Introduction
DC represent a family of professional APC characterized by their dendritic morphology, unique surface phenotype, and ability to activate immunologically naïve T cells efficiently [1]. Although DC are found in virtually all lymphoid and epithelial tissues, it is technically challenging to isolate DC from respective tissues with a sufficient yield and high purity. Moreover, once isolated from the tissue microenvironment, DC begin to undergo rapid and profound changes that accompany their maturation into fully potent antigen presenting cells. Inaba et al. developed a simple method to generate relatively large numbers of DC from mouse BM cells in the presence of exogenous GM-CSF [2]. Sallusto and Lanzavecchia demonstrated that monocytes isolated from human peripheral blood samples can give rise to DC when cultured in the presence of GM-CSF and IL-4 [3]. Caux et al. reported successful generation of large numbers of DC exhibiting features of epidermal Langerhans cells by culturing CD34+ hematopoietic progenitors in the presence of GM-CSF and TNF-α [4]. These methods for generating short-term DC cultures represent a major technical breakthrough in the field and have been widely used for studying the immunobiology of DC at molecular, biochemical, and cellular levels. In addition to GM-CSF, IL-4 and TNF-α, several cytokines have been shown to promote DC development in the BM culture system – they include Fms-like tyrosine kinase 3 ligand (Flt3L), M-CSF, IL-3, IL-7, and IL-15 [5]. It appears that these DC growth factors were discovered through sporadic research efforts by testing selected cytokines for their potential to promote DC generation in short-term culture. In other words, it remains to be determined whether there exist additional cytokines that are capable of supporting DC development.
In the present study, we sought to test a wide variety of cytokines and growth factors for their impacts on DC generation in a systematic and unbiased manner. By screening 65 cytokines in a standard mouse BM culture system, we identified a previously unrecognized activity of IL-33 to promote DC development. IL-33 is a recently discovered IL-1-like cytokine signaling through ST2 [6]. Unlike other members of the IL-1 family (i.e., IL-1α, IL-1β and IL-18), relatively limited information has been available with regard to biological activities of IL-33. Systemic treatment of mice with IL-33 resulted in Th2-polarized responses characterized eosinophilia, elevated serum IgA and IgE levels, and overproduction of Th2 cytokines [6]. ST2 was identified 20 years ago as a serum- or oncogene-inducible secreted protein characterized by its significant sequence homology to the extracellular domain of mouse IL-1 receptor [7, 8], and a transmembrane form of ST2 was subsequently cloned and termed the ST2L [9]. With regard to function, Th2-mediated, allergic airway inflammatory responses have been blocked in mice by administration of anti-ST2 antibodies or ST2-Fc fusion proteins [10, 11], and ST2-deficient mice showed severely impaired Th2-polarized immune responses in a Schistosoma mansoni-induced granuloma model [12]. Most recently, IL-33 was shown to activate basophils, eosinophils, mast cells, and Th2-polarized T cells [13–16]. Thus, IL-33 signaling via ST2 most likely plays a functional role in driving Th2 immune responses. Here we describe the impact of IL-33 on DC development in murine BM culture. To the best of our knowledge, this is the first report documenting any impact of IL-33 on DC.
Results
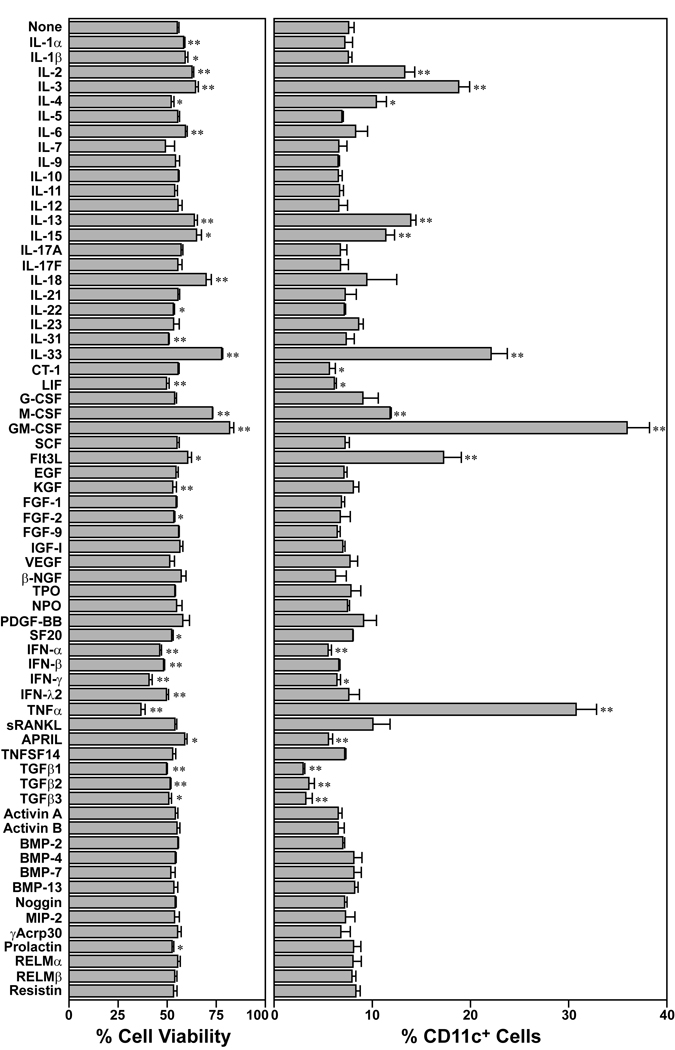
Screening of 65 cytokines for their effects on BM cell survival and CD11c expression
We constructed a test library by purchasing 65 murine recombinant cytokines and growth factors from commercial vendors. Each cytokine was added to a standard short-term BM culture system to test its effect on survival of BM cells and expression of a conventional DC marker CD11c. When cultured in the absence of any added cytokines, BM cells died progressively with their viability declining to 55% after 3 days in culture (left panel in Fig. 1). Addition of a prototypic DC growth factor GM-CSF markedly sustained their survival, with > 80% cell viability observed on day 3. Several other cytokines, including IL-2, IL-3, IL-13, IL-15, IL-18, M-CSF, and Flt3L, also significantly (p < 0.01) improved the cell viability. IL-33 was found to be comparable to these “hematopoietic growth factors” in the capacity to sustain in vitro survival of BM cells. In the absence of added cytokines, only a small fraction (8%) of the viable cells expressed CD11c after 3 days in culture (right panel in Fig. 1). As expected, a larger fraction (> 35%) of the cells expressed CD11c when cultured with GM-CSF. Significant (p < 0.01) increases in the percentage of CD11c+ cells were also observed in BM cell cultures supplemented with IL-2, IL-3, IL-13, IL-15, M-CSF, Flt3L, or TNF-α, which have been reported to promote DC development as well [5]. Strikingly, IL-33 was comparable to these “DC growth factors” in the ability to promote the emergence of CD11c+ cells in BM cultures. We interpreted these screening results to suggest a testable hypothesis that IL-33 supports the development of DC.
Figure 1.
Differential impacts of 65 cytokines on the survival and CD11c expression by BM cells. BM cells freshly procured from adult C57BL/6 mice were cultured for 3 days in triplicate in the presence of each of the indicated cytokines (30 ng/ml). The cells were examined in a semi-automated fashion for their survival by PI uptake (left) and their expression of the CD11c DC marker (right). Data show mean + SD (n = 3) of the % cell viability and the % CD11c+ cells within viable cells. *p<0.05, **p<0.01; compared with control cultures.
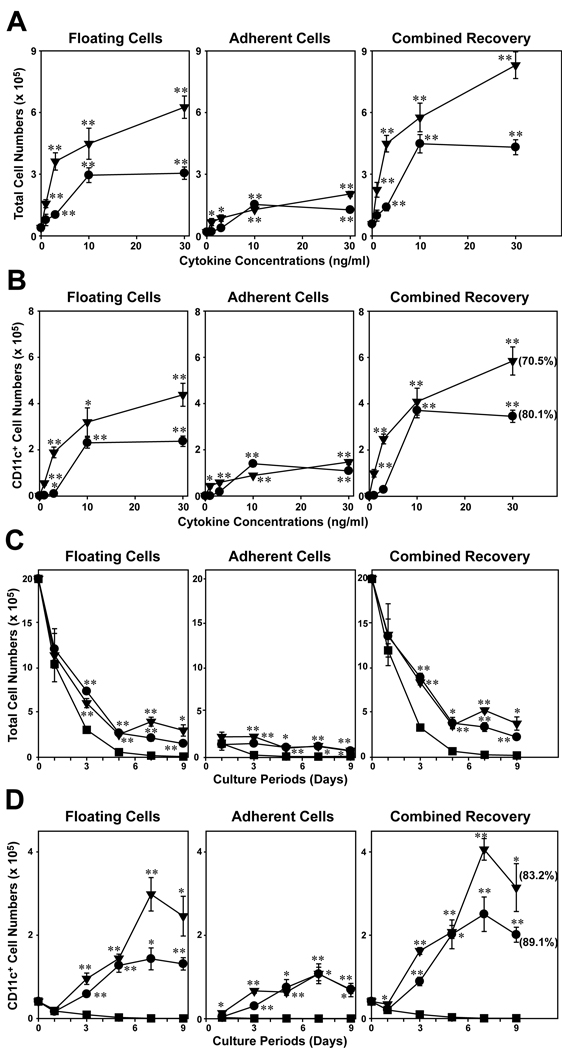
IL-33-induced expansion of CD11c+ cells in BM cell cultures
To test the hypothesis, we cultured BM cells for 7 days in the presence of IL-33 or GM-CSF (serving as a positive control) at different concentrations (1–30 ng/ml), harvested floating cells and adherent cells separately, and then counted total cell numbers (Fig. 2A) and CD11c+ cell numbers in the two fractions independently (Fig. 2B). In the absence of added cytokines, relatively small numbers of viable cells were recovered from the cultures and only few of them expressed CD11c. GM-CSF showed profound and dose-dependent effects on both total cell numbers and CD11c+ cell numbers. Interestingly, a majority of the CD11c+ cells was recovered in the floating cell fraction. IL-33 also produced dose-dependent increases in the recovery of both total cells and CD11c+ cells. Although GM-CSF was much more potent than IL-33 at relatively low doses (1–3 ng/ml), the two cytokines were roughly comparable to each other at 10 ng/ml. GM-CSF showed a more substantial outcome at a higher dose (30 ng/ml), whereas the effect of IL-33 reached to a plateau at 10 ng/ml. These observations implied that added IL-33 increased the number of CD11c+ cells in BM cultures by promoting their generation and/or sustaining their survival.
Figure 2.
IL-33 promotes the survival and CD11c expression by BM cells. (A, B) BM cells were cultured for 7 days with the indicated concentrations of IL-33 (circles) or GM-CSF (triangles). (C, D) BM cells were cultured with 10 ng/ml IL-33 (circles), 10 ng/ml GM-CSF (triangles) or media alone (squares) for the indicated periods. The floating cell fraction (left panels) and adherent cell fraction (middle panels) were examined independently for total cell numbers (A, C) and CD11c+ cell numbers (B, D). The combined recovery data (right panels) were calculated by adding the numbers recovered in the two fractions. The percentages of CD11c+ cells are indicated in the parentheses. Data shown in this figure are representative of at least two independent experiments producing similar results. Error bars indicate mean ± SD from triplicate cultures. *p<0.05, **p<0.01; compared to control cultures without exogenous cytokine.
Time-course experiments revealed that a majority of BM cells died progressively when cultured in the absence of added cytokines (Fig. 2C). Almost no CD11c+ cells were detectable in these control cultures on day 5 and thereafter (Fig. 2D). When BM cells were cultured in the presence of 10 ng/ml GM-CSF, the numbers of CD11c+ cells increased continuously and remarkably during the first 7 days. Similar increases were also observed in the BM cultures supplemented with 10 ng/ml IL-33. Thus, we concluded that GM-CSF and IL-33 both support the generation of CD11c+ cells in a standard BM culture system.
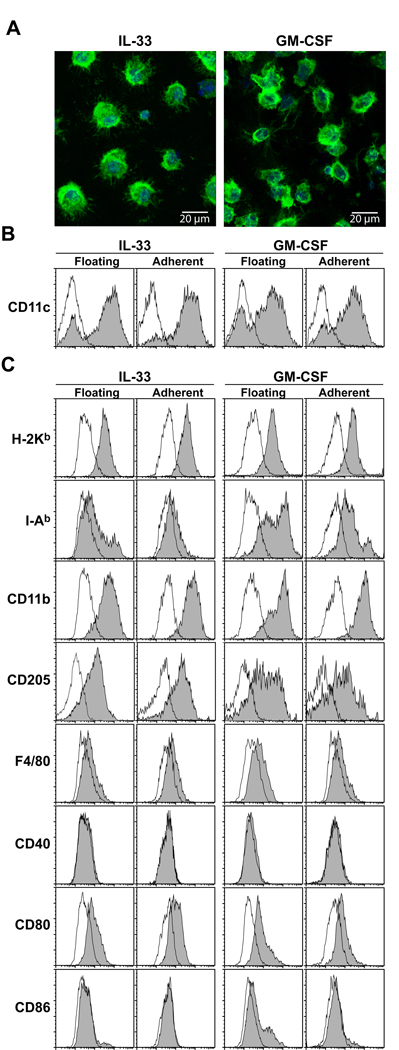
Morphological, phenotypic, and functional characteristics of the DC population generated in the presence of IL-33
A key question concerned the identity of the CD11c+ cells that emerged in IL-33-supplemented BM cultures. The CD11c+ cells purified from these cultures exhibited a characteristic morphology of DC and they were indistinguishable from conventional short-term CD11c+ DC cultures generated from BM cells with GM-CSF (Fig. 3A). A majority (>70%) of the floating cells recovered from IL-33-supplemented BM cultures expressed CD11c and the CD11c+ fraction of this population uniformly expressed MHC class I, CD11b, and CD205 (Fig. 3B, C). This population, however, expressed MHC class II molecules and CD80 only marginally and showed no detectable CD40 or CD86. The adherent cells recovered from IL-33-supplemented cultures showed almost identical surface phenotype profiles. By contrast, surface expression of MHC class II molecules was readily detectable in the CD11c+ populations recovered from GM-CSF-supplemented BM cultures. Thus, we concluded that relatively immature DC emerged from BM cells when cultured in the presence of added IL-33.
Figure 3.
Morphology and surface phenotype of CD11c+ cells emerging in IL-33-supplemented BM cell cultures. BM cells were cultured for 7 days with IL-33 (10 ng/ml) or GM-CSF (10 ng/ml). (A) FACS-purified CD11c+ cells were stained for F-actin (green) and nuclei (blue) and then examined under confocal microscopy. (B) CD11c expression profiles within the PI-negative populations are shown with filled histograms, whereas background staining profiles with isotype-matched control IgG are shown with open histograms. (C) Data shown are the expression profiles of the indicated markers (filled histograms) or isotype-matched control IgG (open histograms) within the CD11c+ populations. Data are representative of two independent experiments producing similar results.
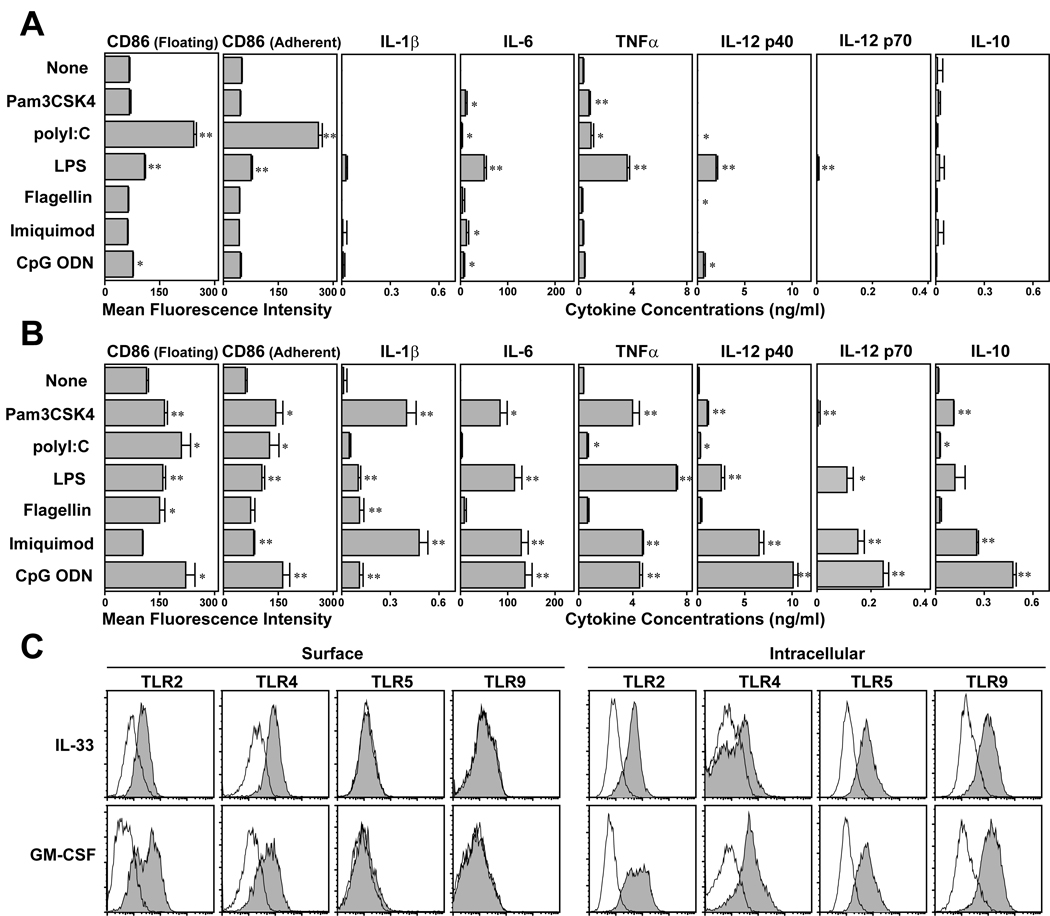
We next examined their responsiveness to TLR ligands. Both floating and adherent cell fractions recovered from IL-33-supplemented BM cell cultures showed significantly elevated CD86 expression when stimulated with polyI:C (Fig. 4A). LPS treatment also upregulated CD86 albeit rather modestly. Those cells emerging in IL-33-supplemented cultures elaborated relatively small amounts of IL-6 and TNF-α after stimulation with polyI:C or LPS and exhibited only marginal, if any, responses to other tested TLR ligands. Importantly, they produced no detectable IL-12 p70 after exposure to any tested TLR ligand. By marked contrast, BM-derived DC cultures generated in the presence of GM-CSF responded to each of the tested TLR ligands by upregulating CD86 expression and secreting readily detectable amounts of IL-1β, IL-6, TNF-α, IL-12 p40, IL-12 p70, and/or IL-10 (Fig. 4B). It should be stated here that some of these features might have reflected the effects of IL-33 on non-DC “contaminants” present in our BM cultures. Nevertheless, these results implied limited responsiveness of IL-33-generated DC to TLR ligands. Interestingly, the observed differences between the two DC cultures could not explained solely by the levels of TLR expression. TLR2 and TLR4 proteins were observed on the surface of CD11c+ cells generated with either IL-33 or GM-CSF, and intracellular TLR5 and TLR9 proteins were also detected in both populations (Fig. 4C). Real-time PCR analyses further revealed that FACS-purified CD11c+ cells from IL-33-supplemented BM cultures did not markedly differ from those purified from GM-CSF-supplemented cultures in mRNA expression of TLR1, TLR3, TLR6, or TLR7 (data not shown).
Figure 4.
Responsiveness of DC generated with IL-33 to TLR ligands. BM cells were cultured for 7 days in the presence of 10 ng/ml IL-33 (A) or 10 ng/ml GM-CSF (B) and stimulated for additional 24 h in the presence of the indicated reagents. The floating cell fraction and adherent cell fraction harvested from these cultures then examined separately for CD86 expression within the CD11c+ populations (left panels). The supernatants collected from the same cultures were examined for the indicated cytokines (right panels). Data show mean + SD from triplicate cultures. *p<0.05, **p<0.01; Asterisks indicate statistically significant increases compared with the control cultures without added reagents (C) Data shown are cell surface or intracellular staining profiles (within the CD11c+ populations) of BM-DC generated with IL-33 or GM-CSF with the indicated anti-TLR mAbs. Open histograms indicate the profiles with isotype-matched control IgG.
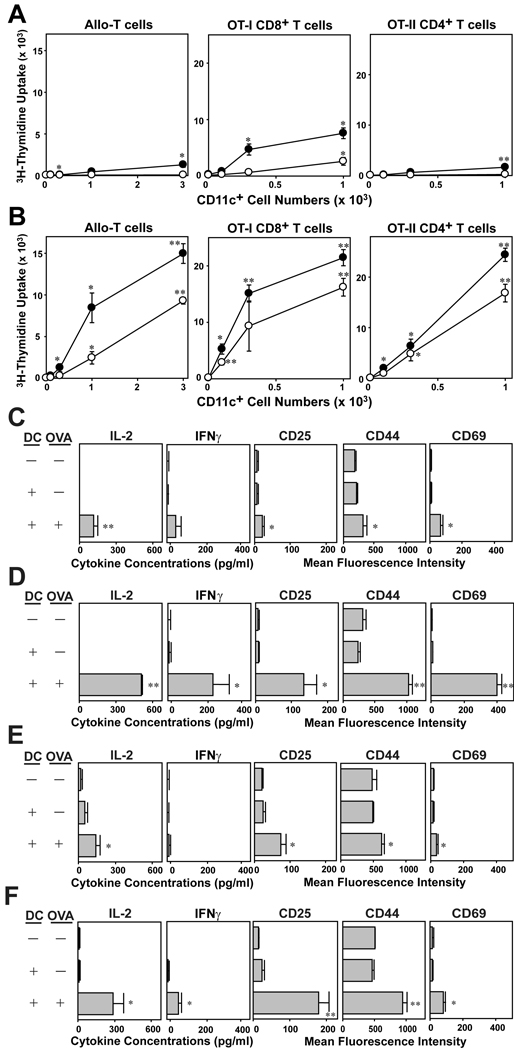
FACS-purified CD11c+ DC from IL-33-supplemented versus GM-CSF-supplemented BM cultures were then compared for their abilities to activate allogeneic T cells and present relevant OVA peptides to CD8 and CD4 T cells freshly procured from OT-I and OT-II TCR Tg mice. IL-33-generated DC exhibited only a negligible T cell stimulatory capacity in any of the three T cell activation assays even when tested after LPS pre-treatment (Fig. 5A). By contrast, CD11c+ DC generated in the presence of GM-CSF efficiently activated all three T cell populations (Fig. 5B). To confirm these observations, we next measured cytokine production and surface phenotype of the responding T cells. Both OT-I T cells (Fig. 5C, D) and OT-II T cells (Fig. 5E, F) produced significance amounts of IL-2 and IFNγ and markedly elevated CD25, CD44, and CD69 expression when stimulated with CD11c+ DC generated in the presence of GM-CSF (Fig. 5D, F). By contrast, only marginal changes were observed when stimulated with DC generated in the presence of IL-33 (Fig. 5C, E). Thus, we concluded that DC generated with IL-33 exhibit a relatively limited T cell-stimulatory capacity compared to conventional DC cultures established with GM-CSF.
Figure 5.
T cell-stimulatory potentials of DC generated with IL-33. BM cells procured from C57BL/6 mice were cultured for 7 days in the presence of 10 ng/ml IL-33 (A, C, E) or 10 ng/ml GM-CSF (B, D, F). (A, B) After 24 h of additional culture in the presence (closed circles) or absence (open circles) of 10 ng/ml LPS, CD11c+ cells were FACS-purified and cocultured with allogeneic CD3+ T cells purified from BALB/c mice, CD8+ T cells purified from OT-I Tg mice, or CD4+ T cells purified from OT-II Tg mice. CD11c+ cells were added at the indicated cell numbers to micro-cultures of responder T cells (5 × 104 cells/well). To test the ability to activate OT-I and OT-II T cells, CD11c+ cells were pulsed for 1 h with OVA257–264 peptide (10 µg/ml) or OVA323–339 peptide (10 µg/ml) and washed extensively before addition to T cell micro-cultures. Data shown mean ± SD (n = 3) of 3H-thymidine uptake on day 3. *p<0.05, **p<0.01; compared to the levels of proliferation of allogeneic T cells in the absence of CD11c+ cells or the base line levels of OT-I and OT-II T cells stimulated by CD11c+ cells in the absence of OVA peptide pulsing. (C–F) FACS-purified CD11c+ cells from the above BM cultures were pulsed with relevant OVA peptide or PBS alone and then co-cultured with OT-I T cells for 24 h (C, D) or with OT-II T cells for 60 h (E, F). Culture supernatants were then tested for the indicated cytokines, and CD3+ T cells were examined for surface expression of the indicated activation markers. Data show mean ± SD (n = 3). *p< 0.05, **p<0.01; compared to the control panels in which T cells were co-cultured with DC in the absence of OVA peptide pulsing.
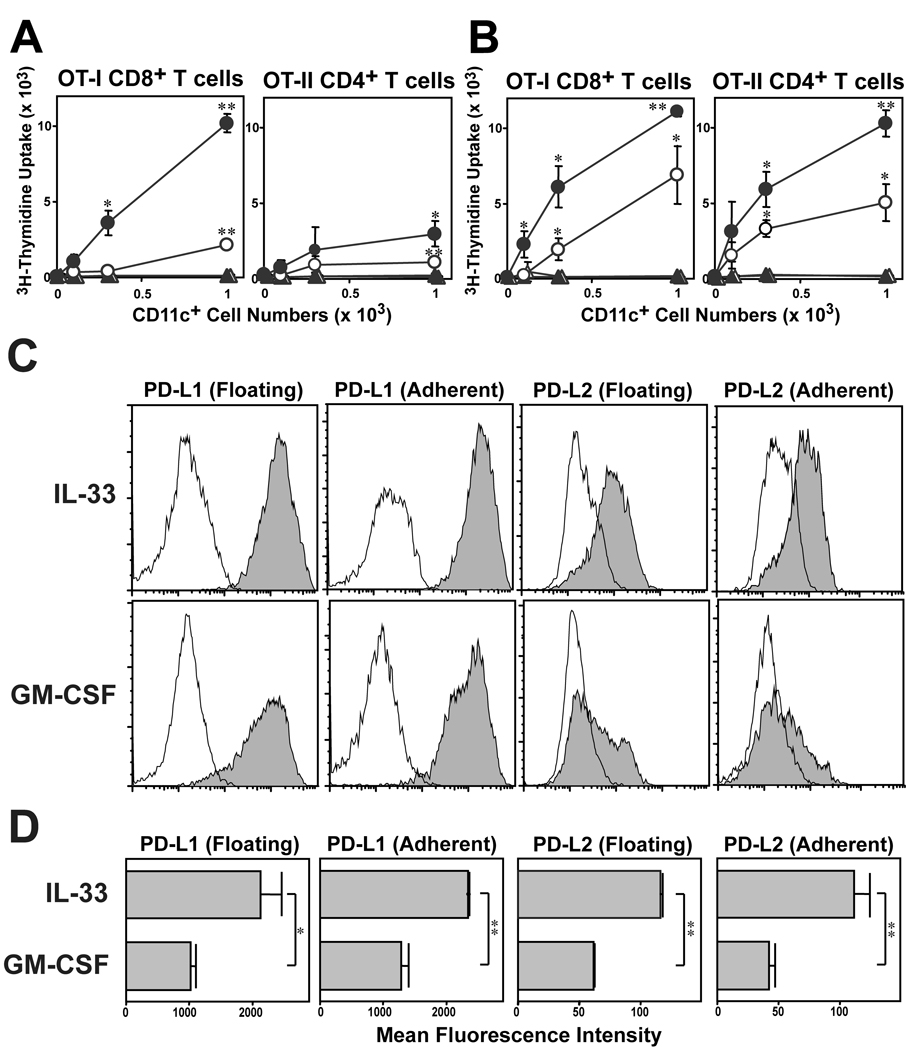
Because DC generated with IL-33 failed to produce IL-12 p70 at detectable levels (Fig 4A), we sought to determine whether one could restore full T cell-stimulatory function by adding IL-12 p70 exogenously. In fact, CD11c+ DC purified from IL-33-supplemented BM cultures were roughly comparable to the counterpart from GM-CSF-supplemented cultures in their efficiency to drive OT-I T cell proliferation in the presence of added IL-12 (Fig. 6A. B). On the other hand, IL-12 failed to restore the ability of IL-33-generated DC to activate OT-II T cells, suggesting additional mechanism(s). The magnitude of DC-dependent T cell activation is also regulated negatively via ligation of the programmed death 1 (PD-1) receptor on T cells with it ligands, PD-L1 and PD-L2 [17]. In complete agreement with the recent report by Benedict et al. [18], conventional BM-DC preparations generated with GM-CSF expressed PD-L1 at high levels, whereas PD-L2 was barely detectable (Fig. 6C). By contrast, DC generated with IL-33 exhibited both PD-L1 and PD-L2 at significantly higher levels (Fig. 6C, D), suggesting that constitutive and elevated expression of PD-1 ligands may partially account for the limited T cell-stimulatory capacity of IL-33-generated DC.
Figure 6.
Mechanisms for poor T cell-stimulatory capacity of IL-33-generated DC. T cell-stimulatory potentials of DC generated with IL-33. BM cells procured from C57BL/6 mice were cultured for 7 days in the presence of 10 ng/ml IL-33 (A) or 10 ng/ml GM-CSF (B). FACS-purified CD11c+ cells were pulsed with relevant OVA peptide (circles) or PBS (triangles) and then co-cultured with OT-I T cells in the presence (closed symbols) or absence (open symbols)of 30 ng/ml IL-12 p70. Data show mean ± SD (n = 3) of 3H-thymidine uptake on day 3. *p<0.05, **p<0.01; compared to the control panels in which T cells were co-cultured with DC in the absence of OVA peptide pulsing. (C, D) Floating and adherent ells harvested from day 7 BM cultures with 10 ng/ml IL-33 or 10 ng/ml GM-CSF were examined for surface expression of the indicated molecules. (C) FACS profiles for PD-L1 or PD-L2 (within the CD11c+ populations). Open histograms indicate the profiles with isotype-matched control IgG. (D) Data show mean ± SD (n = 3) of MFI of PD-L1 and PD-L2 expression. *p<0.05, **p<0.01, between the two DC preparations.
IL-33 supports the generation of CD11c+ DC by a GM-CSF-dependent mechanism
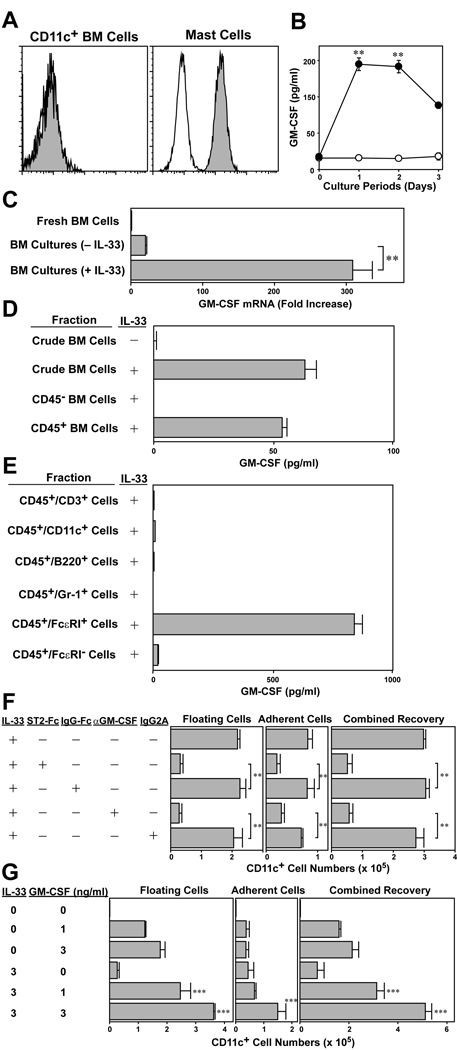
To determine whether IL-33 might act on DC directly, we tested surface expression of ST2, a relevant receptor for IL-33 (Fig. 7A). Surface expression of ST2 was barely detectable on CD11c+ DC freshly isolated from BM, whereas it was clearly expressed by mast cells, which are known to express ST2 at high levels [19]. Interestingly, significant amounts of GM-CSF were found in the supernatants of IL-33-supplemented BM cell cultures. By contrast, no GM-CSF was detected in control cultures without IL-33 (Fig. 7B). Likewise, GM-CSF mRNA expression became detectable in BM cells after 15 h incubation with IL-33 (Fig. 7C). In an attempt to identify the cellular source for GM-CSF, we first fractionated BM cells into CD45− and CD45+ populations and cultured them in the presence of IL-33. Significant amounts of GM-CSF were observed only in the culture of CD45+ BM cells (Fig. 7D). CD45+ BM cells were then fractioned into five populations based on surface expression of CD3, CD11c, B220, Gr-1, and FcεRI. Only the CD45+/ FcεRI+ population produced large amounts of GM-CSF in the presence of IL-33, and conversely, CD45+/FcεRI− fractions failed to produce significant GM-CSF (Fig. 7E). Since c-kit expression was barely detectable on the CD45+/ FcεRI+ BM population, we interpreted our results to indicate that IL-33 induces GM-CSF production primarily by basophils and/or their precursors in BM cultures.
Figure 7.
GM-CSF-dependency of IL-33-induced DC development. (A) Freshly procured BM cells (left panel) and 5 wk-old mast cell cultures (right panel) were stained with mAb against ST2 (filled histograms) or isotype-matched control IgG (open histograms). Data shown are the staining profiles within the CD11c+ populations (left) and the CD117+/FcεRI+ populations (right). (B) BM cells were cultured (2×106 cells/ml) for the indicated periods in the presence (closed circles) or absence (open circles) of 10 ng/ml IL-33. Culture supernatants were then tested for GM-CSF by ELISA. Data show mean ± SD of triplicate cultures. **p<0.01, compared with the control cultures without added reagents. (C) BM cells were cultured for 15 h in the presence or absence of 10 ng/ml IL-33 and then examined for GM-CSF mRNA expression. **p<0.01; compared with the control cultures without IL-33. (D, E) Crude BM cells or the indicated BM fractions purified by FACS were cultured (106 cells/ml) for 24 h in the presence or absence of 10 ng/ml IL-33. Culture supernatants were then examined for GM-CSF. Data show mean + SD of triplicate cultures. (F) BM cells were cultured with for 5 days with 10 ng/ml IL-33 in the presence of ST2-Fc (5 µg/ml) or anti-GM-CSF mAb (5 µg/ml). IgG-Fc and isotype-matched control IgG were added to serve as controls. The floating cell fraction (left panels) and adherent cell fraction (middle panels) were examined independently for CD11c+ cell numbers. The combined recovery data (right panels) were calculated by adding the numbers recovered in the two fractions. Data show mean + SD from triplicate cultures. **p<0.01. (G) BM cells were cultured for 7 days in the presence of IL-33 and/or GM-CSF added at the indicated concentrations. Data show mean + SD (n = 3) of CD11c+ cell numbers. ***p<0.001, compared between IL-33 and GM-CSF based on ANOVA and multiple comparisons by Ryan’s method.
Finally, we sought to study functional contribution(s) of newly produced GM-CSF to DC development in IL-33-supplemented BM cultures. IL-33-induced development of CD11c+ cells was blocked almost completely not only by a ST2 antagonist (ST2-Fc fusion protein), but also by neutralizing mAb against GM-CSF (Fig. 7F). Furthermore, when added together at relatively low concentrations, IL-33 and GM-CSF promoted the generation of CD11c+ DC in synergistic manners. For example, the numbers of CD11c+ DC emerging in BM cell cultures supplemented with 1 ng/ml or 3 ng/ml of GM-CSF were almost doubled by addition of 3 ng/ml of IL-33, which alone showed an only modest activity at this concentration (Fig. 7G). Thus, we concluded that IL-33 and GM-CSF synergistically support the generation of DC.
Discussion
By testing a total of 65 cytokines, we have identified a previously unrecognized biological activity of IL-33 to promote DC generation in short-term BM cultures. Only limited information has been available with regard to immunoregulatory function of IL-33, which is a new member of the family of IL-1-like cytokines [6]. Prior to the discovery of IL-33 as a ST2 ligand, several in vivo data suggested functional roles played by ST2. Experimentally induced allergic airway inflammatory responses were inhibited by ST2 antagonists, including neutralizing antibodies against ST2 and ST2-Fc fusion proteins [10, 11]. Likewise, ST2 knockout mice failed to develop Th2-mediated pulmonary granuloma formation following initial administration of Schistosoma mansoni eggs. A subsequent challenge with the same antigen resulted in marked production of IL-4 and IL-5 in draining lymph nodes in wild-type mice, but not in the ST2 deficient animals [12]. Corroborating with these observations, IL-33 has been found in vitro to induce preferential activation of Th2 effector leukocytes, including basophils, eosinophils, mast cells, and Th2 cells [13–16]. Th2-polarized T cells showed elevated production of IL-5 and IL-13 in vitro when stimulated with anti-CD3 and anti-CD28 antibodies in the presence of added IL-33 [6]. Elevated gene expression for Th2 cytokines (e.g., IL-4, IL-5, and IL-13) has been induced in mice by systemic administration of a large amount of IL-33 [6]. These reports all support the current concept that IL-33 signaling through ST2 plays crucial roles in the induction of Th2-biased immune responses [20]. Most recently, IL-33/ST2 pathway has been further implicated in the pathophysiology of rheumatoid arthritis, anaphylaxis, and cardiomyocyte hypertrophy [21–23]. Our unbiased screening has now unveiled a new activity of IL-33 to promote DC development in BM cultures. It is important to point out that IL-33 also functions as a nuclear factor associated with heterochromatin and mitotic chromosomes and possesses a potent transcriptional repressor activity [24, 25]. A similar dual functionality has been reported for IL-1α, another member of the IL-1-like family of cytokines [26]. Thus, it appears that IL-33 is capable of regulating a broader spectrum of biological functions beyond Th2 immunity.
The following observations support our conclusion that IL-33 promotes DC development in vitro, at least in part, by triggering GM-CSF production by other BM leukocyte populations: a) CD11c+ BM cells expressed no detectable ST2, b) BM cells produced GM-CSF when cultured in the presence of IL-33, c) IL-33-induced GM-CSF production occurred exclusively within the CD45+/FcεRI+ BM cell fraction, and d) IL-33-dependent DC generation in BM cultures was blocked almost completely not only by ST2-Fc fusion protein, but also by anti-GM-CSF mAb. Physiological relevance of our in vitro observations remains to be elucidated in living animals. For example, it will be important to determine whether the number, tissue distribution, maturational status, and/or functional property of DC can be altered by administering exogenous IL-33 or blocking endogenous IL-33 with ST2-Fc fusion protein. Markedly elevated IL-33 production has been observed in skin lesions of atopic dermatitis [22], synovial fibroblasts from patients with rheumatoid arthritis patients [21], bronchoalveolar lavage fluid collected from asthmatic mice experimentally induced by intratracheal administration of house dust mite allergen [27], and pressure-overloaded rat cardiac fibroblasts [23]. Thus, it is tempting to speculate that IL-33 produced locally under these pathogenic conditions may promote DC development by triggering GM-CSF production. In fact, human CD34+ hematopoietic progenitor cells have been shown to express abundant GM-CSF mRNA and secrete significant amounts of GM-CSF in vitro in response to IL-33 treatment [28]. Our in vitro findings now provide a conceptual basis for studying physiological and pathogenic roles of IL-33 in regulating DC development.
We observed that DC generated in IL-33-supplemented BM cultures remained relatively immature – they barely expressed MHC II or co-stimulatory molecules, responded poorly to TLR ligands, and failed to activate naïve T cells efficiently. In this regard, Turnquist et al. reported that exposure of murine BM-derived DC to rapamycin converted them into “tolerogenic” DC characterized by immature surface phenotype, unresponsiveness to TLR ligands, and a poor T cell-stimulatory capacity [29]. Interestingly, rapamycin treatment triggered surface expression of ST2L (a transmembrane form of ST2) by DC, and rapamycin-conditioned DC generated from ST2-deficient mice exhibited elevated CD86 expression and augmented TLR responses compared to those from wild-type mice [30]. Likewise, Brint et al. reported that macrophages isolated from ST2-deficient mice exhibited significantly augmented cytokine production after LPS stimulation than did macrophages from wild-type mice [31]. On the other hand, Rank et al. reported that murine BM-derived DC secreted IL-6, but not other tested cytokines (including IL-12), upon 24 h exposure to IL-33 [32]. IL-33 treatment also induced modest upregulation of MHC class II and CD86 expression by the same DC preparation, in which ST2 protein was detected only intracellularly [32]. Espinassous et al. reported that IL-33 treatment enhanced LPS-triggered IL-1β, IL-6, and TNF-α secretion by murine macrophages [33]. Thus, it appears that experimental outcomes of genetic deletion of endogenous ST2 versus short-term exposure to exogenous IL-33 suggested opposing impacts of IL-33/ST2 pathway on DC and macrophages. Obviously, further studies are required to define how IL-33 and ST2 regulate adaptive immunity by altering the function of APC populations.
Of the tested 65 cytokines, 10 cytokines (IL-2, IL-3, IL-4, IL-13, IL-15, IL-33, M-CSF, GM-CSF, Flt3L, and TNF-α) were found to produce statistically significant (p < 0.05) increase in the number of CD11c+ cells above vehicle-treated control cultures. Except for IL-33, all these cytokines have been reported to promote the generation of mouse and/or human DC [2, 3, 34–42]. We also tested the same cytokines in the same short-term BM cell culture system, but in combination with 10 ng/ml GM-CSF. Although several cytokines were found to significantly reduce the number of CD11c+ cells compared to the control panel cultured with GM-CSF alone, no cytokine was found to augment GM-CSF-induced generation of CD11c+ cells (Supporting Information Fig. 1). The relatively high concentration of GM-CSF added to the BM cell cultures most likely accounted for our failure to identify additive and/or synergistic effects. In fact, statistically significant synergy was observed between IL-33 and GM-CSF only when GM-CSF was added at suboptimal concentrations (1–3 ng/ml). With regard to mechanisms, IL-33-induced expansion of CD11c+ cells was blocked almost completely by anti-GM-CSF mAb and significant GM-CSF mRNA and protein expression was observed in IL-33-supplemented BM cultures. With regard to the identity of GM-CSF-producers in BM cultures, our results are in complete agreement with the recent report that human basophils secret significant amounts of GM-CSF in vitro in response to IL-33 stimulation [16]. Taken together, not only do our data reinforce the concept that GM-CSF serves as a primary DC growth factor, they also demonstrate a new mechanism by which IL-33 and GM-CSF synergistically promote DC development.
Materials and Methods
A library of murine cytokines
We prepared a test library by purchasing a total of 65 murine recombinant cytokines from the following commercial venders: a) PeproTech (Rocky Hill, NJ) for IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-11, IL-12, IL-13, IL-15, IL-17A, IL-17F, IL-21, IL-22, IL-31, IL-33, cardiotrophin-1 (CT-1), G-CSF, M-CSF, GM-CSF, SCF, Flt3L, EGF, keratinocyte growth factor (KGF), FGF-1, FGF-2, FGF-9, IGF-I, vascular endothelial growth factor (VEGF), β-nerve growth factor (β-NGF), thrombopoietin (TPO), neuropoietin (NPO), PDGF-BB, bone marrow stroma-derived growth factor (SF) 20, IFN-γ, IFN-λ2, TNF-α, soluble receptor activator of NF-κB ligand (sRANKL), a proliferating-inducing ligand (APRIL), TNF- ligand superfamily 14 (TNFSF14), TGF-β1, TGF-β2, TGF-β3, activin A, activin B, bone morphogenic protein (BMP)-2, BMP-4, BMP-7, BMP-13, noggin, MIP-2, grobular domain of adipocyte complement related protein of 30kDa (γAcrp30), prolactin, resistin-like molecule (RELM)α, RELMβ, resistin from; b) MBL (Woburn, MA) for IL-18; c) R&D systems (Minneapolis, MN) for IL-23; d) STEMCELL technologies (Vancouver, Canada) for leukemia inhibitory factor (LIF); e) AbD Serotech (Oxford, UK) for IFN-α and IFN-β. Each cytokine was tested at 30 ng/ml unless otherwise mentioned.
Automated assay system to monitor DC development
BM cells isolated from C57BL/6 mice (6-8 wks old female) were cultured in round-bottom 96 well-plates (4×105 cells/200 µl/well) in complete RPMI1640 [43] in the presence or absence of a test cytokine. On day 3, the cells were stained in the 96-well plates with allophycocyanin (APC)-conjugated anti-CD11c mAb and analyzed for CD11c expression and PI uptake in a semi-automated fashion with FACSCalibur equipped with a HTP sampler device (BD Biosciences, San Jose, CA).
Medium-scale BM culture system
BM cells isolated from C57BL/6 mice were cultured in 24-well plates (2×106 cells/ml/well) in the presence of IL-33 or GM-CSF at various concentrations. On days 3 and 5, one half of culture medium was gently replaced with fresh complete RPMI containing IL-33 or GM-CSF at the same concentrations. On day 7, floating cells were collected by repeated pipetting, whereas adherent cells were harvested by a cell scraper. The two cell fractions recovered in this manner were then examined independently for total cell numbers, cell viability, and CD11c expression. In some experiments, crude BM cells or FACS-fractionated BM subpopulations were cultured in the presence or absence of IL-33 to test its effects on GM-CSF production. The levels of GM-CSF protein were measured by ELISA (R&D Systems). GM-CSF mRNA expression was examined real-time PCR using a primer set purchased from SABiosciences (Frederick, MD). Expression levels were normalized by the house keeping gene control, GAPDH.
Morphological, phenotypic, and functional analyses
BM cell cultures generated with IL-33 or GM-CSF were examined for surface phenotype and cytokine production before and after treatment with ligands of selected TLRs. They included LPS (10 ng/ml), Pam3CSK4 (1 µg/ml), polyI:C (10 µg/ml), flagellin (1 µg/ml), imiquimod (10 µg/ml), and CpG oligonucleotide (CpG ODN, 100 nM), which were all purchased from InvivoGen (San Diego, CA). The cells were double-stained with APC-labeled anti-CD11c mAb and FITC-labeled mAb against various surface molecules and then analyzed with FACSCalibur. TLR expression profiles were examined at protein levels by FACS in the presence or absence of cell permeabilization procedure and at mRNA levels by real-time PCR. In some experiments, CD11c+ cells purified with FACSAria (BD Biosciences) were examined for morphology under a confocal microscope after staining with Alexa Fluor 488-phalloidin and DAPI. To test the APC function, the CD11c+ cells were pulsed for 1 h with one of the two OVA-derived peptides (10 µg/ml each), OVA 257–264 and OVA323–339. As responder cells, CD8 and CD4 T cells were purified from OT-I or OT-II TCR Tg mice (Jackson Laboratories, Bar Harbor, ME), respectively, by negative selection with Dnyabeads (Invitorgen, San Diego, CA), followed by further depletion of MHC class II-positive cells by MACS Microbeads (Miltenyi Biotec, Auburn, CA).
Other materials and reagents
In some experiments, BM cells were cultured with IL-33 in the presence of 5 µg/ml ST2-Fc fusion protein (R&D Systems) or 5 µg/ml anti-GM-CSF mAb (R&D Systems). To generate mast cell cultures, BM cells from C57BL/6 mice were cultured for 5 wks in the presence of IL-3 (10 ng/ml) and SCF (10 ng/ml) [44]. ELISA kits for various cytokines were purchased from R&D Systems.
Statistical analyses
All the experiments were performed in triplicates and repeated more than three times to test reproducibility. The results were analyzed for statistical significance by a two-tailed Student t-test. Synergy between IL-33 and GM-CSF was analyzed by ANOVA and multiple comparison by Ryan’s method [45].
Supplementary Material
Acknowledgments
This work was supported by NIH grants (RO1-AI46755, RO1-AR35068, RO1-AR43777, and RO1-AI43232 to A.T.).
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Steinman RM, Banchereau J. Taking dendritic cells in to medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 2.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J. Exp. Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-α cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360:258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 5.Conti L, Gessani S. GM-CSF in the generation of dendritic cells from human blood monocyte precursors: recent advances. Immunobiol. 2008;213:859–870. doi: 10.1016/j.imbio.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Tominaga S. A putative protein of a growth specific cDNA from BALB/c-3T3 cells is highly similar to the extracellular portion of mouse interleukin 1 receptor. FEBS Lett. 1989;258:301–304. doi: 10.1016/0014-5793(89)81679-5. [DOI] [PubMed] [Google Scholar]
- 8.Klemenz R, Hoffmann S, Werenskiold AK. Serum- and oncoprotein-mediated induction of a gene with sequence similarity to the gene encoding carcinoembryonic antigen. Proc. Natl. Acad. Sci. U S A. 1989;86:5708–5712. doi: 10.1073/pnas.86.15.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yanagisawa K, Takagi T, Tsukamoto T, Tetsuka T, Tominaga S. Presence of a novel primary response gene ST2L, encoding a product highly similar to the interleukin 1 receptor type 1. FEBS Lett. 1993;318:83–87. doi: 10.1016/0014-5793(93)81333-u. [DOI] [PubMed] [Google Scholar]
- 10.Lohning M, Stroehmann A, Coyle AJ, Grogan JL, Lin S, Gutierrez-Ramos J-C, Levinson D, et al. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc. Natl. Acad. Sci. U S A. 1998;95:6930–6935. doi: 10.1073/pnas.95.12.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayakawa H, Hayakawa M, Kume A, Tominaga S. Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. J. Biol. Chem. 2007;282:26369–26380. doi: 10.1074/jbc.M704916200. [DOI] [PubMed] [Google Scholar]
- 12.Townsend MJ, Fallon PG, Matthews DJ, Jolin HE, McKenzie ANJ. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J. Exp. Med. 2000;191:1069–1075. doi: 10.1084/jem.191.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iikura M, Suto H, Kajiwara N, Oboki K, Ohno T, Okayama Y, Saito H, et al. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab. Invest. 2007;87:971–978. doi: 10.1038/labinvest.3700663. [DOI] [PubMed] [Google Scholar]
- 14.Pecaric-Petkovic T, Didichenko SA, Kaempfer S, Spiegl N, Dahinden CA. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1-family member IL-33. Blood. 2009;113:1526–1534. doi: 10.1182/blood-2008-05-157818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzukawa M, Koketsu R, Iikura M, Nakae S, Matsumoto K, Nagase H, Saito H, et al. Interleukin-33 enhances adhesion, CD11b expression and survival in human eosinophils. Lab. Invest. 2008;88:1245–1253. doi: 10.1038/labinvest.2008.82. [DOI] [PubMed] [Google Scholar]
- 16.Smithgall MD, Comeau MR, Yoon B-RP, Kaufman D, Armitage R, Smith DE. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int. Immunol. 2008;20:1019–1030. doi: 10.1093/intimm/dxn060. [DOI] [PubMed] [Google Scholar]
- 17.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benedict CA, Loewendorf A, Garcia Z, Blazar BR, Janssen EM. Dendritic cell programming by cytomegalovirus stunts naive T cell responses via the PD-L1/PD-1 pathway. J.Immunol. 2008;180:4836–4847. doi: 10.4049/jimmunol.180.7.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moritz DR, Rodewald H-R, Gheyselinck J, Klemenz R. The IL-1 receptor-related T1 antigen is expressed on immature and mature mast cells and on fetal blood mast cell progenitors. J. Immunol. 1998;161:4866–4874. [PubMed] [Google Scholar]
- 20.Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat. Rev. Drug Discov. 2008;7:827–840. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer G, Talabot-Ayer D, Lamacchia C, Toy D, Seemayer CA, Viatte S, Finckh A, Smith DE, Gabay C. Inhibition of interleukin-33 signaling attenuates the severity of experimental arthritis. Arthritis Rheum. 2009;60:738–749. doi: 10.1002/art.24305. [DOI] [PubMed] [Google Scholar]
- 22.Pushparaj PN, Tay HK, H'ng SC, Pitman N, Xu D, McKenzie A, Liew FY, Melendez AJ. The cytokine interleukin-33 mediates anaphylactic shock. Proc. Natl. Acad. Sci. U. S. A. 2009;106:9773–9778. doi: 10.1073/pnas.0901206106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J.Clin.Invest. 2007;117:1538–1549. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carriere V, Roussel L, Ortega N, Lacorre D-A, Americh L, Aguilar L, Bouche G, Girard J-P. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc. Natl. Acad. Sci. U S A. 2007;104:282–287. doi: 10.1073/pnas.0606854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haraldsen G, Balogh J, Pollheimer J, Sponheim J, Kuchler AM. Interleukin-33 - cytokine of dual function or novel alarmin? Trends Immunol. 2009;30:227–233. doi: 10.1016/j.it.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Werman A, Werman-Venkert R, White R, Lee J-K, Werman B, Krelin Y, Voronov E, et al. The precursor form of IL-1α is an intracrine proinflammatory activator of transcription. Proc. Natl. Acad. Sci. U S A. 2004;101:2434–2439. doi: 10.1073/pnas.0308705101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat. Med. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allakhverdi Z, Comeau MR, Smith DE, Toy D, Endam LM, Desrosiers M, Liu YJ, et al. CD34+ hemopoietic progenitor cells are potent effectors of allergic inflammation. J.Allergy Clin.Immunol. 2009;123:472–478. doi: 10.1016/j.jaci.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 29.Turnquist HR, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J. Immunol. 2007;178:7018–7031. doi: 10.4049/jimmunol.178.11.7018. [DOI] [PubMed] [Google Scholar]
- 30.Turnquist HR, Sumpter TL, Tsung A, Zahorchak AF, Nakao A, Nau GJ, Liew FY, et al. IL-1β-driven ST2L expression promotes maturation resistance in rapamycin-conditioned dendritic cells. J. Immunol. 2008;181:62–72. doi: 10.4049/jimmunol.181.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brint EK, Xu D, Liu H, Danne A, McKenzie ANJ, O’Neill LAJ, Liew FY. ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat. Immunol. 2004;5:373–379. doi: 10.1038/ni1050. [DOI] [PubMed] [Google Scholar]
- 32.Rank MA, Kobayashi T, Kozaki H, Bartemes KR, Squillace DL, Kita H. IL-33-activated dendritic cells induce an atypical TH2-type response. J. Allergy Clin. Immunol. 2009;123:1047–1054. doi: 10.1016/j.jaci.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Espinassous Q, Garcia-de-Paco E, Garcia-Verdugo I, Synguelakis M, von AS, Sallenave JM, McKenzie AN, Kanellopoulos J. IL-33 enhances lipopolysaccharide-induced inflammatory cytokine production from mouse macrophages by regulating lipopolysaccharide receptor complex. J.Immunol. 2009;183:1446–1455. doi: 10.4049/jimmunol.0803067. [DOI] [PubMed] [Google Scholar]
- 34.Bykovskaja SN, Buffo MJ, Bunker M, Zhang H, Majors A, Herbert M, Lokshin A, et al. Interleukin-2-induces development of denditric cells from cord blood CD34+ cells. J. Leukoc. Biol. 1998;63:620–630. doi: 10.1002/jlb.63.5.620. [DOI] [PubMed] [Google Scholar]
- 35.Sanarico N, Ciaramella A, Sacchi A, Bernasconi D, Bossu P, Mariani F, Colizzi V, Vendetti S. Human monocyte-derived dendritic cells differentiated in the presence of IL-2 produce proinflammatory cytokines and prime Th1 immune response. J. Leukoc. Biol. 2006;80:555–562. doi: 10.1189/jlb.1105690. [DOI] [PubMed] [Google Scholar]
- 36.Caux C, Vanbervliet B, Massacrier C, Durand I, Banchereau J. Interleukin-3 cooperates with tumor necrosis factor alpha for the development of human dendritic/Langerhans cells from cord blood CD34+ hematopoietic progenitor cells. Blood. 1996;87:2376–2385. [PubMed] [Google Scholar]
- 37.Piemonti L, Bernasconi S, Luini W, Trobonjaca Z, Minty A, Allavena P, Mantovani A. IL-13 supports differentiation of dendritic cells from circulating precursors in concert with GM-CSF. Eur. Cytokine Netw. 1995;6:245–252. [PubMed] [Google Scholar]
- 38.Mohamadzadeh M, Berard F, Essert G, Chalouni C, Pulendran B, Davoust J, Bridges G, et al. Interleukin 15 skews monocyte differentiation into dendritic cells with features of Langerhans cells. J. Exp. Med. 2001;194:1013–1020. doi: 10.1084/jem.194.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saikh KU, Khan AS, Kissner T, Ulrich RG. IL-15-induced conversion of monocytes to mature dendritic cells. Clin. Exp. Immunol. 2001;126:447–455. doi: 10.1046/j.1365-2249.2001.01672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mollah ZU, Aiba S, Nakagawa S, Hara M, Manome H, Mizuhashi M, Ohtani T, et al. Macrophage colony-stimulating factor in cooperation with transforming growth factor-β1 induces the differentiation of CD34+ hematopoietic progenitor cells into Langerhans cells under serum-free conditions without granulocyte-macrophage colony-stimulating factor. J. Invest. Dermatol. 2003;120:256–265. doi: 10.1046/j.1523-1747.2003.12036.x. [DOI] [PubMed] [Google Scholar]
- 41.Strobl H, Bello-Fernandez C, Riedl E, Pickl WF, Majdic O, Lyman SD, Knapp W. flt3 ligand in cooperation with transforming growth factor-beta1 potentiates in vitro development of Langerhans-type dendritic cells and allows single-cell dendritic cell cluster formation under serum-free conditions. Blood. 1997;90:1425–1434. [PubMed] [Google Scholar]
- 42.Reid CD, Stackpoole A, Meager A, Tikerpae J. Interactions of tumor necrosis factor with granulocyte-macrophage colony-stimulating factor and other cytokines in the regulation of dendritic cell growth in vitro from early bipotent CD34+ progenitors in human bone marrow. J. Immunol. 1992;149:2681–2688. [PubMed] [Google Scholar]
- 43.Matsue H, Matsue K, Walters M, Okumura K, Yagita H, Takashima A. Induction of antigen-specific immunosuppression by CD95L cDNA-transfected "killer" dendritic cells. Nat. Med. 1999;5:930–937. doi: 10.1038/11375. [DOI] [PubMed] [Google Scholar]
- 44.Kirshenbaum AS, Goff JP, Kessler SW, Mican JM, Zsebo KM, Metcalfe DD. Effect of IL-3 and stem cell factor on the appearance of human basophils and mast cells from CD34+ pluripotent progenitor cells. J. Immunol. 1992;148:772–777. [PubMed] [Google Scholar]
- 45.Ryan TA. Significance tests for multiple comparison of proportions, variances, and other statistics. Psychol. Bull. 1960;57:318–328. doi: 10.1037/h0044320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.