Abstract
Neurotrophins were christened in consideration of their actions on the nervous system and, for a long time, they were the exclusive interest of neuroscientists. However, more recently, this family of proteins has been shown to possess essential cardiovascular functions. During cardiovascular development, neurotrophins and their receptors are essential factors in the formation of the heart and critical regulator of vascular development. Postnatally, neurotrophins control the survival of endothelial cells, vascular smooth muscle cells, and cardiomyocytes and regulate angiogenesis and vasculogenesis, by autocrine and paracrine mechanisms. Recent studies suggest the capacity of neurotrophins, via their tropomyosin-kinase receptors, to promote therapeutic neovascularization in animal models of hindlimb ischemia. Conversely, the neurotrophin low-affinity p75NTR receptor induces apoptosis of endothelial cells and vascular smooth muscle cells and impairs angiogenesis. Finally, nerve growth factor looks particularly promising in treating microvascular complications of diabetes or reducing cardiomyocyte apoptosis in the infarcted heart. These seminal discoveries have fuelled basic and translational research and thus opened a new field of investigation in cardiovascular medicine and therapeutics. Here, we review recent progress on the molecular signaling and roles played by neurotrophins in cardiovascular development, function, and pathology, and we discuss therapeutic potential of strategies based on neurotrophin manipulation.
I. INTRODUCTION
Neurotrophins (NTs) are a family of proteins that have been implicated in several functions of the nervous system, including axonal growth, synaptic plasticity, survival, differentiation, and myelination (43, 115, 161). Nerve growth factor (NGF), probably the most extensively studied member of NTs, was discovered by Rita Levi-Montalcini and characterized for its ability to stimulate growth, differentiation, and survival of neurons during development and after injury (52, 151). In the early 1980s, Hofer and Barde (111) identified and purified a brain protein that they named brain-derived neurotrophic factor (BDNF). Like NGF, BDNF promotes survival and neurite outgrowth of sensory neurons. Based on the homologies between the primary structures of NGF and BDNF, two years later Jones and Reichardt (124) reported the cloning of a third member of the NTs family, neurotrophin-3 (NT-3). In addition to NGF, BDNF, and NT-3, three other NTs have been identified: neurotrophin-4/5 (NT-4/5) (also known as NT-4) (120) and, limited to fishes, NT-6 and NT-7, which probably do not have mammalian orthologs (94, 189). The components of the NTs family and their signaling receptors are described in sections II and III. Since NTs were discovered by neuroscientists, initial attention focused on their actions on the developing and mature nervous system. However, strong evidence has emerged that NTs exert important cardiovascular functions, which largely exceed their implication in the neural regulation of the heart function (106, 117, 118). Developmental studies (reviewed in sect. IV), which were performed in the late 1990s on global knockout murine models, pointed to the role of NT-3 and BDNF in the formation of the heart and the myocardial vasculature (69, 70, 257, 258). These results represented the kick-off for investigating direct actions of NTs on the adult cardiovascular system and its cellular components. At present, we know that NTs promote angiogenesis and control the survival of adult endothelial cells (EC), vascular smooth muscle cells (VSMC), and cardiomyocytes (sect. V), yet these investigations need to be significantly expanded. Therefore, we auspicate that this review will propel new interest in researching the functions of NTs in cardiovascular physiology and pathology and in characterizing the underpinned signaling pathways. The importance of the discovery that NTs act on cardiovascular cells by autocrine mechanisms has recently been cross-fertilized by the understanding that the prototypic angiogenic factor vascular endothelial growth factor A (VEGF-A) is produced by neurons (195). VEGF-A exerts neuroprotection not only by maintaining the trophism of vasa nervosum (235), but also by directly targeting VEGF receptors expressed by neurons (197). Therefore, although named in agreement with their first identified action, several growth factors look to be acting at multiple cell targets as well as being produced by cells belonging to different systems. In line with this viewpoint, far away from negating a fundamental neural role of NTs, we want to outline the importance of this class of molecules for determining and maintaining the cardiovascular phenotype. Once the role of NTs in cardiovascular physiology is established, this review will progress to address the possible involvement of NTs in pathological angiogenesis and atherosclerosis (sect. VI) and to propose potential clinical targets of NTs (sect. VII). Preclinical studies suggest the capacity of NTs to promote therapeutic neovascularization of ischemic limb muscles (78, 95, 131, 229) and diabetic skin ulcers (95). NTs look particularly promising in treating microvascular complications of diabetes, as they have been shown to reduce diabetes-induced apoptosis of EC and promote vascular regeneration (95, 229). Moreover, in diabetes, cardiovascular complications are usually accompanied by neuropathy, which can also be treated by NTs. It is possible therefore to envisage the use of NTs to cure diabetic cutaneous ulcers and diabetes-associated erectile dysfunction. In view of the antiapoptotic action of NGF on cardiac myocytes, NTs may be a therapeutic target for diabetic cardiomyopathy, which is characterized by exaggerated cardiovascular apoptosis, microvascular rarefaction, and neuropathy. Despite their amazing therapeutic potential, NTs are not considered ideal drug candidates. In fact, they show a poor pharmacokinetic behavior and bind, although with different affinity, two receptor types (trk and p75NTR), which can command opposite actions. Moreover, pain has been described as a side effect of recombinant NGF-based experimental therapy of diabetic neuropathy (8). However, several approaches (described in sect. VII) may allow overcoming these drawbacks.
II. NEUROTROPHINS AND NEUROTROPHIN RECEPTORS
A. Neurotrophins and Pro-Neurotrophins
NGF is a glycoprotein of 118 amino acids, consisting of three subunits (α, β, and γ complex). The α-NGF subunit appears inactive (247). The β-NGF is responsible for the NGF biological activity, while the γ-NGF is a highly specific active protease (26-kDa serine protease of the kallikrein protease group) that is able to process NGF precursor to its mature form. NGF is part of the NT family of molecules, which includes BDNF, NT-3, and NT-4/5. Fishes also possess NT-6 and NT-7. With the exception of NT-4/5, NTs are highly conserved from fishes to mammals (99), thus sharing a similar molecular mass (13.2–15.9 kDa), isoelectric points (range of 9 –10), and 50% identity in primary structure (178). NTs generally function as noncovalently associated homodimers, but at least some NT subunits are able to form heterodimers with other NT subunits. The crystal structures of β-NGF, NT-3, and NT-4/5 homodimers and the BDNF monomer have been determined (30, 172, 222). The structural hallmark of these four proteins is a characteristic arrangement of the disulfide bridges, known as the cystine knot, also identified in other secreted proteins (173). NTs are produced as 30- to 35-kDa proproteins consisting of a preprodomain, a prodomain, and a mature domain that are proteolytically cleaved to produce mature proteins (12–13 kDa). Until recently, little consideration was paid to the NTs prodomain, which was thought to be only involved in protein folding and in regulation of NTs secretion (253). However, following the understanding that pro-NTs are highly secreted in different tissues (29, 81) and that prodomain regions have highly conserved sequences across species, Lee et al. (149) demonstrated that pro-NTs possess biological actions, which are different from those elicited by mature NTs. Furthermore, the presence of a prodomain induces restrictions on the conformation of pro-NTs, thus preventing their interaction with trk receptors (199). Intracellular posttranslational modifications to remove the predomains and glycosylates/glycosulfates specific residues present in the prodomain have been performed (237). These modifications are thought to be responsible for the efficient trafficking of pro-NTs from the endoplasmic reticulum. Subsequently, this trafficking allows the majority of pro-NTs to undergo intracellular cleavage by furin-like proprotein convertases at the consensus site R-X-K/R-R (arginine-unspecified amino acid-lysine/arginine-arginine). The mature proteins form noncovalent symmetrical homodimers and are then secreted into the extracellular environment (44, 178). Lee et al. (149) were the first to demonstrate that also pro-NTs can be secreted from the cell and processed in the extracellular compartment to mature NTs. For example, pro-BDNF can be secreted from neurons (29, 178) and also by a line of EC (149). Mature BDNF can derive from the cleavage of pro-BDNF by extracellular proteases, including the matrix metalloproteinases-3 (MMP-3) and -7 (MMP-7) (149). Another protease that cleaves pro-NTs is the serine protease plasmin (178). This enzyme derives from the proteolytic cleavage of plasminogen by the tissue plasminogen activator (tPA) (207). A recent study demonstrated that NTs upregulate plasminogen gene expression through two Sp1 (Specificity protein 1) binding site on plasminogen promoter, thus creating a positive feedback for the maturation of NTs (97).
B. Neurotrophin Receptors
The search for a receptor for NTs led to the discovery of two distinct ones: the tropomyosin kinase receptors (trks) and the neurotrophin receptor p75 (p75NTR) (123, 127, 138). It is now well established that survival signaling is predominantly associated with trk activation. In contrast, p75NTR, with some exceptions, has a critical role in apoptosis of neuronal and nonneuronal cells.
1. Tropomyosin kinase receptors
The trk receptors A, B, and C are typical tyrosine kinase receptors. TrkA was first cloned by two separate research groups (127, 138), followed later on by trkB and trkC (13, 127). Initially, different NTs were believed to bind preferentially to specific trk receptors: NGF to trkA, BDNF and NT-4/5 to trkB, and NT-3 to trkC. However, NT-3 can also bind to trkA and trkB (51, 252). Ligand specificity can be influenced by inserting variants in the extracellular domain of trkA and trkB. Splice variants have been described for all the three trk receptors, including deletions in the extracellular domain or intracellular truncations of the tyrosine kinase domain (242). Interestingly, the truncated trk splice variants act as dominant negative modulators of trk signaling (75, 191).
In trks, the tyrosine kinase domains are highly conserved (~80% amino acid identity) and the extracellular domains are more different (~30% amino acid identity). The extracellular domain of trk receptors is composed of three leucine-rich motifs flanked by two cysteine clusters, two immunoglobulin-like C2 type domains (Ig-C2), a single transmembrane domain, and a cytoplasmic region with a kinase domain. Binding of NTs to trk receptors occurs mainly through the Ig-C2 domains, helped by the transmembrane domain (206, 263). Like for the other tyrosine kinase receptors, phosphorylation of the cytoplasmatic tyrosines regulates their activity and provides the recruitment of adaptor molecules that mediate initiation of signaling cascades (234).
Trk receptors are present on vascular EC (34, 135, 255), VSMC (132, 184), and cardiomyocytes (36, 128, 154). The finding that vascular cells and myocytes produce and secrete NGF (34) suggests an autocrine control of cardiovascular cell functions by NTs. Unpublished data from our laboratory show that endothelial progenitor cells (EPC) from bone marrow and peripheral blood express trkB and trkA that are responsive to stimulation by BDNF and NGF. In contrast, EPC do not express trkC and are not responsive to stimulation with NT-3 (unpublished observations).
2. p75NTR
The p75NTR, which was the first receptor to be identified for NGF, belongs to the tumor necrosis factor (TNF) receptor superfamily, thus sharing the overall structure of TNF-α p55 receptor, but not the ability to bind TNF-α (123). Similar to the other TNF superfamily members, p75NTR contains four cysteine-rich motifs, a transmembrane domain, and a cytoplasmic death domain. However, unlike other TNF superfamily receptors, p75NTR does not exhibit trimerization. For eliciting its proapoptotic effect, p75NTR must be in its monomeric form, as dimerization or homomultimerization completely suppresses p75NTR-induced apoptosis (212, 271). In addition, the p75NTR death domain conformation, consisting of two perpendicular sets of three helices packed into a globular structure, differs from that of the other TNF receptor family members (152). No catalytic activity has been identified for p75NTR intracellular domain (45), and the majority of the p75NTR signals described to date are mediated by associations with cytoplasmic interactors (see below). Moreover, unlike other death receptors, including Fas/Apo-1/CD95 and the TNF-α p55 receptor, p75NTR does not require the apoptotic adaptor molecules Fas-associated death domain (FADD) and TNF receptor-associated death domain protein (TRADD) to induce apoptosis in neural cells (273). In contrast, the interaction between p75NTR and TRADD is required for activation of NFκB, which controls the antiapoptotic effect of NGF in breast cancer cells (77).
In contrast to the specificity of the trk receptors, each of the mature NTs binds p75NTR with equivalent affinity, but with unique kinetics (224, 225). The crystal structure of NGF in complex with the extracellular domain of p75NTR has been crystallized, revealing a stoichiometry of 2 NGF:1 p75NTR, a sort of trimeric ligand-receptor complex. The interaction between p75NTR and NGF reportedly results in conformational changes in NGF, which prevent the formation of p75NTR dimers (105). Wehrman et al. (275) recently showed that p75NTR and trkA could bind to NGF in a 1:2:1 stoichiometry (1 trkA:2 NGF:1 p75NTR). This ternary complex, in which the receptors are arranged in opposite orientations, requires that the extracellular domains of both receptors are arranged to overlap NGF protein (275). In neural cells, p75NTR and trk receptors are often coexpressed to form a complex that can be immunoprecipitated (20). p75NTR/trkA receptor association leads to the formation of a high-affinity NT binding site (108), which is abolished by mutations in the cytoplasmic or transmembrane domains of any of the two receptors (80). The affinity between NTs and trk receptors is modulated by p75NTR, which in fact increases ligand discrimination by the trk receptors (15, 20, 176).
It has been shown that, in the presence of the coreceptor sortilin (a Vps10-domain receptor), a furin-resistant mutant of pro-NGF binds to p75NTR with five times greater affinity than mature NGF, while the same mutant has no affinity for trkA (149, 193). Similarly, in the presence of sortilin, pro-BDNF binds with high affinity to p75NTR, and it does not activate trkB (256). Both pro-NGF and pro-BDNF, via p75NTR, induce cell apoptosis (149, 256). Secretion of pro-NT-3 or pro-NT-4/5 has not been reported to date. Pro-NGF and pro-BDNF reportedly bind with high affinity to a complex of p75NTR with sortilin. Both sortilin and p75NTR directly participate in binding the prodomain of pro-NGF or pro-BDNF. It has been pre-posed that the presence or absence of sortilin determines whether or not p75NTR acts as a death receptor (193). Recent data demonstrate that sortilin deficiency does not affect developmentally regulated apoptosis of sympathetic neurons, but it prevents their age-dependent degeneration, thus suggesting that sortilin possesses also distinct roles from apoptotic signaling (122). Finally, p75NTR has been proposed to be a dependence receptor, i.e., a receptor which responds to ligand withdrawal by caspase activation and apoptosis, as reviewed by Bresden et al. (27).
p75NTR is scarcely expressed by EC or VSMC cultured under basal culture conditions and in healthy mice (34, 35, 229, 272). Its expression by vascular cells is induced in pathological conditions, such as ischemia, atherosclerosis, and diabetes (34, 35, 229, 272). p75NTR is also present in pericytes and perivascular mesenchymal cells in tumors (82). The expression of p75NTR by healthy or diseased cardiomyocytes has not been investigated, yet. Reports show that pro-BDNF is secreted by cells of a mouse EC line (146) and that exogenous pro-NGF engages p75NTR expressed by a transformed rat brain EC line (RBE4), thus triggering apoptosis. We recently showed that p75NTR transduction induces apoptosis of human umbilical vein EC (HUVEC), microvascular EC (HMVEC), and EPC (35). To date, no evidence exists on whether pro-NT is released from primary cultures of cardiovascular cells or exerts any action on cardiovascular cells.
In summary, mature NTs bind to trk alone or complexed with p75NTR for prosurvival signaling, while pro-NGF and pro-BDNF bind exclusively to p75NTR and trigger apoptotic events. P75NTR can induce apoptosis in the absence of a ligand. In conclusion, the ratio of trk and p75NTR receptors, the proteolytic processing of pro-NTs, and the ability of cross-linking with coreceptor are critical events governing life and death in neural and nonneural cells.
III. SIGNALING OF NEUROTROPHIN RECEPTORS
A. Trk Receptor Signaling
Trks act as typical tyrosine kinase receptors, which, following interaction with mature NT ligands, are activated by dimerization and phosphorylation in trans (115). Trk receptors are not activated by pro-NTs (149). Thus the proteases that control maturation of pro-NTs (see below) also regulate activation of trk receptors.
The cytoplasmic domains of the trk receptors contain five tyrosines (Y490, Y785, Y670, Y674, and Y675) which are essential for receptor signaling. When phosphorylated, the tyrosines constitute the binding sites for adaptor proteins that are intermediates in intracellular signaling cascades (115). In particular, Y490 and Y785 primarily recruit Shc and phospholipase C-γ (PLC-γ), respectively, while Y670, Y674, and Y675 can also engage adaptor proteins, including SH2B, adaptor protein containing PH and SH domains (APS), fibroblast growth factor receptor substrate 2 (Frs2), and growth factor receptor-bound protein 2 (Grb2) (163, 210, 251).
The major pathways activated by the trk receptors are Ras/Rap-mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK), phosphatidylinositol 3-kinase (PI3K)-Akt, and PLC-γ (115). Finally, activation of the small GTPase Ras in response to NTs has been shown and linked to survival and differentiation (98).
1. Ras/Rap-MAPK/ERK
NGF-induced phosphorylation of trkA at Y490 provides a recruitment site for binding of the adaptor protein Shc. Next, Shc recruits Grb2 complexed with SOS which then activates Ras (190). Activation of Ras promotes signaling through several downstream pathways, including PI3K, Raf, and p38 MAPK (266). Raf phosphorylation triggers the sequential activation of MEK1 (meiotic kinase) and/or MEK2 and then the phosphorylation of Erk1 and Erk2 by MEK1 or MEK2 (79). By a negative-feedback loop, MEK activation can stop the signaling of Ras by phosphorylating SOS to disrupt the Grb2-SOS complex (126).
The stimulation of Ras induced by trk receptors promotes only transient MAPK activation (167). In contrast, prolonged Erk activation depends on a distinct signaling pathway and requires the adaptor protein Crk, the guanine nucleotide exchange factor C3G, the small GTPase Rap1, the protein tyrosine phosphatase Shp2, and the serine threonine kinase B-Raf (96, 126). NGF-induced trkA activation leads to Frs2 recruitment at phosphorylated Y490. Frs2 phosphorylation by activated trkA provides binding sites for Grb2 and Crk (175). Association with Crk results in activation of C3G. C3G then activates Rap1, which eventually signals further downstream through B-Raf, thus resulting in a prolonged MAPK signaling (192). Recent studies identified a new ankyrin repeat-rich membrane-spanning protein (ARMS), which represents a link between trkA and Crk (119). Interaction between ARMS and Crk, which increases upon NGF treatment, modulates the activation of Rap1 and subsequently of MAPK pathway (10). The activity of Shp2, in association with Frs2, is also essential for maintaining the MAPK pathway activation by interfering with MAPK phosphatase (278). The MAPK cascade activates different transcription factors that are essential for differentiation and survival, such as cAMP response element-binding protein (CREB), Mads box transcription enhancer factor (MEF2), and Elk1 (160, 203).
The majority of the trk receptor signaling studies were developed in PC12 cells, a rat adrenal pheochromocytoma cell line, which expresses trkA and responds to NGF. Nevertheless, some studies were also performed on cardiovascular cells. In VSMC genetically modified to express high level of trkA, NGF induces a prolonged and profound activation of the MAPK cascade. The activation of this pathway by NGF increases cell migration, but not cell proliferation (141). In addition, in VSMC, NGF induces the expression of MMP-9 trough Erk1/2 activation (132). Increased MMP-9 activity contributes to the migratory response of VSMC. In partial contrast to what is observed in VSMC, NGF treatment of HUVEC causes a rapid phosphorylation of trkA, thus determining a parallel activation of ERK1/2 and subsequently an increase in proliferation (34). In primary cultures of rat neonatal cardiomyocytes, NT-3, via trkC, activated p38MAPK and ERK1/2, thus promoting cell hypertrophy (128).
2. PLC-γ
In response to receptor kinase activation by NTs, PLC-γ is recruited to a docking site surrounding phosphorylated Y785 on trkA (or equivalent sites on trkB and trkC). PLC-γ activation results in the hydrolysis of phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2] and generates inositol 1,4,5-trisphosphate (InsP3) and diacylglycerol (DAG) (194), with subsequent stimulation of different PKC isoforms. The proteins activated as a result of PLC-γ activation include PKC-δ, which is required for NGF-promoted induction of MEK1 and Erk1/2 (54). The PLC-γ pathway has a role in the migration of VSMC, and NGF induces a longer activation of this pathway compared with platelet-derived growth factor (PDGF)-BB (141).
3. PI3K-Akt
The serine-threonine kinase Akt plays a crucial role in the survival activity of NTs. Insulin receptor substrates (IRS-1 and IRS-2) and Grb-associated binder-1 (Gab-1) are the main adaptor proteins that mediate the association and activation of PI3K with trk receptors. Active trk receptors engage Shc, which associates with Grb2. Recruitment of Gab1 by phosphorylated Grb2 permits subsequent binding and activation of PI3K (280).
In addition, trk receptor activation results in direct phosphorylation of IRS, which recruits and activates PI3K (113). Once activated and localized to the membrane, PI3K phosphorylates phosphoinositol lipids on the D3 position of the inositol ring generating phosphatidylinositol 3,4,5-trisphosphates [PtdIns(3,4,5)P3]. These lipids serve to recruit pleckstrin homology (PH) domain-containing enzymes such as Akt and phosphoinositide-dependent kinase 1 (PDK1) to the plasma membrane. After recruitment to the membrane, Akt is phosphorylated and consequently activated by PDK (166). In turn, Akt controls the activities of several proteins through phosphor-ylation. Between the varieties of downstream Akt substrates, Bad, caspase-9, IkB-kinase, and Foxo transcription factors are involved in the antiapoptosis effects of Akt. Cell type-specific Akt substrates also exist, such as endothelial nitric oxide synthase (eNOS), which regulates nitric oxide (NO) biosynthesis in EC, EPC, and cardiomyocytes (236, 241). Numerous reports show that the PI3K/Akt signaling pathway is critically involved in EC survival and migration and in angiogenesis (243). We demonstrated the involvement of Akt in NGF-induced neovascularization and EC survival in vivo (78). Later, Kim et al. (135) reported that BDNF, via stimulation of trkB and activation of the PI3K/Akt pathway, mediates the survival of a brain EC line and induces in vitro angiogenesis (135). A recent study performed in HUVEC demonstrates that NGF stimulates EC invasion and capillary-like EC tube formation by augmenting MMP-2, via the PI3K/Akt signaling pathway and activation of the AP-2 transcription factor (200). Using specific kinase inhibitors, the authors showed that only the PI3K/Akt pathway is critically involved in NGF-induced MMP-2 increased expression, invasion, and EC tube formation. In fact, inhibitors of MEK, p38, or Jun NH2-terminal kinase (JNK) did not effectively suppress these events (200). Moreover, NGF triggers migration of porcine aortic and choroidal EC in vitro through a PI3K/AKT-involving mechanism (213, 250). Furthermore, we established that stimulation of rat newborn cardiomyocytes with NGF induces trkA phosphorylation, followed by Ser-473-phosphorylation and nuclear translocation of Akt. In response to Akt activation, Forkhead transcription factors Foxo-3a and Foxo-1 are phosphorylated and excluded from the nucleus, thus resulting in increased cardiomyocyte survival (36).
B. Transactivation of trk Receptors by G Protein-Coupled Receptor
As previously described for the receptors for epidermal growth factor (EGF) and insulin-like growth factor I (IGF-I) (59, 162), trk receptors can be transactivated in response to G protein-coupled receptor (GPCR) signaling. In fact, Lee and co-workers (146, 147) reported that adenosine promotes neuronal survival through phosphorylation of trk receptors. This action is antagonized by the trk inhibitor K252 and requires the adenosine receptor A2A (146, 147). The possibilities that adenosine stimulates NGF production or directly interacts with trk receptors were discounted (146, 147). Four adenosine receptors have been characterized (215), and they are all expressed in myocardial and vascular cells, where they trigger a range of responses, including activation/translocation of PKC, PI3K, and MAPKs (reviewed in Ref. 204). Trk transactivation by adenosine requires a long kinetics (more than 1–2 h from when adenosine binds the GPCR), and the major parts of the transactivated trk receptors are found associated with Golgi membranes (214). Moreover, without concomitant activation of the MAPK cascade, transactivated trkA leads to activation of the PI3K pathway to promote neuronal survival. Inhibitory experimental approaches reveal that members of Src family kinases or intracellular calcium might be the mediators of this transactivation (6, 287). It is also possible that trk receptors are transactivated by additional GPCRs, either in neurons or in cardiovascular cells. The mechanisms behind trk receptors transactivation are not completely understood and have not yet been investigated in cardiovascular cells. Because of the many actions of GPCR on the cardiovascular system and on progenitor cells, this line of investigation may lead to important findings.
C. p75NTR Signaling
The function of p75NTR varies considerably depending on the cellular context in which the receptor is expressed. To date, in vascular cells, p75NTR has only been implicated in apoptosis (35, 135, 139) and in EC cycle arrest (35). The first evidence of p75NTR-mediated apoptosis came from Rabizadeh et al. (211), who showed that p75NTR overexpression induces apoptosis of neural cells in the absence of ligand. We have recently confirmed this by showing the dramatic apoptotic responses of EC and EPC following transduction with p75NTR (35). In our hands, p75NTR-transduced HUVEC (but not HUVEC transduced with control gene) can respond to an exogenously added furin-resistant pro-NGF mutant with a modest increase in apoptosis (unpublished data), but exogenously added ligand is not necessary to initiate a robust apoptotic response (35). In contrast, in VSMC genetically manipulated to express p75NTR and lacking trk receptor expression, treatment with NGF or NT-3 causes a dose-dependent increase of apoptosis (272). It has been proposed that p75NTR overexpression produces cell death in a ligand-dependent fashion as well as following ligand withdrawal. The decision between ligand-dependent or ligand-independent apoptosis might be related to the cellular contest, as well as the presence of trk receptors and of different adaptor proteins (reviewed in Ref. 27). The signaling mechanisms used by p75NTR have remained elusive. The lack of catalytic activity in the cytoplasmic domain of p75NTR suggests that interacting proteins carry out the signaling of this receptor. Two p75NTR regions are crucial in protein-protein interaction: the death domain (273) and the juxtamembrane intracytoplasmatic domain named “chopper” (56). As reviewed by Dempsey et al. (63) and Vilar et al. (267), several different p75NTR interacting molecules, with and without catalytic activity, have been identified. Noncatalytic interactors include scaffolding- and adaptor-like molecules, such as caveolin-1, NADE (p75NTR associated cell death executor) and TNF receptor-associated factor (TRAF) 4 and 6. Transcription factors containing zinc-finger domains, such as NT receptor-interacting factor 1/2 (NRIF1/2), Schwann cell factor 1 (SC-1), and NRAGE (p75NTR-interacting MAGE homolog) can also bind the p75NTR intracellular domain. P75NTR interactors with catalytic activity include serine-threonine kinases involved in interleukin and NFκB signaling, such as interleukin-1 receptor-associated kinase (IRAK) and receptor-interacting protein-2 (RIP2), the protein tyrosine phosphatase Fas-associated phosphatase-1 (FAP-1), and the small GTPase RhoA. Finally, brain-expressed X-linked 1 (Bex-1) has been recently discovered to interact with p75NTR in competition with RIP2 (267). How these p75NTR-interacting proteins connect to downstream signaling pathways and cellular responses has not been extensively elucidated. Moreover, reports linking adaptor proteins to p75NTR actions on mature or progenitor cells of the cardiovascular system are still missing, thus calling for significant investigation. In noncardiovascular cells, ceramide production (67), activation of the transcription factor NFκB (39), and the c-Jun kinases JNK1-3 (40, 86, 102) have been found downstream to p75NTR. One hallmark in p75NTR-mediated apoptosis is reportedly the activation of the JNK signaling cascade (284). Nevertheless, we could never observe increased JNK phosphorylation in p75NTR-transduced HUVEC undergoing apoptosis (unpublished observations). Downstream to JNK, described events involved in p75NTR-induced apoptosis include activation of p53, direct phosphorylation of Bad, release of cytochrome c from mitochondria, and activation of caspases 9, 6, and 3 (19, 196). P75NTR-induced activation of JNK cascade was described to be mediated through Rac GTPase (102). Genetic deletion of TRAF6 prevents p75NTR activation of JNK signaling, thus inhibiting apoptosis in sympathetic neurons (283). NRIF has been proposed to interact with TRAF6 to induce the activation of JNK downstream to p75NTR (41, 92). It has been proposed that NRIF nuclear translocation is necessary for p75NTR-induced cell death. This cytoplasmatic-nuclear shuttling is modulated by TRAF6-mediated polyubiquitination of NRIF at lysine-63 (88, 156). It was reported that NRIF is ubiquitinated following the γ-secretase cleavage of p75NTR in response to proapoptotic ligands and that NRIF ubiquitination is essential for its nuclear entry (129). TRAF-6 also appears to be involved in p75NTR-mediated NFκB activation (134). NRAGE interacts with p75NTR to mediate NT-induced cell death through a mechanism that involves cell cycle arrest, JNK activation, and caspases activation (130). Bronfman et al. (28) reported that binding of NGF induces p75NTR internalization and the subsequent association of p75NTR with NRAGE in recycling endosomes. TRAF-6 also appears to be involved in p75NTR-mediated NFκB activation (134). The possible interactions of p75NTR with NRIF or TRAF-6 and the activity of NRAGE on cardiovascular cells have not been investigated, yet.
In noncardiovascular cells, the interaction of p75NTR with NADE has also been shown to induce apoptosis (179), which is probably modulated by an interaction between NADE and 14 –3-3 protein (136). The effect of p75NTR on survival of noncardiovascular cells is duplex. In fact, several studies have provided evidence that p75NTR can promote survival through activation of NFκB (39, 64, 91, 284). This signaling pathway is activated by NGF, but not BDNF or NT-3 (39), and requires several proteins, including TRAF-6, p62, IRAK, and RIP2. The complex between TRAF6, IRAK, and p62 is necessary for the phosphorylation of IKB and subsequent activation of NFκB (165). Finally, p75NTR mediates sphingomyelin hydrolysis and production of ceramide following NT binding (66). Ceramide has been shown to promote both apoptotic and prosurvival pathways initiated by p75NTR activation (248).
SC-1 and Bex-1 have been involved in the action of p75NTR on cell cycle. The transcription factor SC-1 was the first molecular link between p75NTR and the regulation of cell cycle identified. Following the binding of NGF to p75NTR, SC-1 translocates to the nucleus and induces cell cycle arrest (50). A recent study reports that SC1 forms a complex with histone deacetylases to regulate the levels of cyclins E and B in response to NGF (49). The exact function of Bex-1 is not clear. It was recently described that, in PC12 cells, Bex1 levels oscillated during the cell cycle and may contribute to p75NTR-induced cell cycle arrest (267). Bex-1 looks to mediate p75NTR-induced cell cycle arrest independently to NGF binding (267). We have found that transduction with p75NTR promotes cycle arrest in HUVEC in the absence of exogenously added ligand (35). However, NGF is present in the conditioned culture medium of HUVEC (34). The expression of Bex1 or SC-1 in cardiovascular cells has not been investigated. If Bex1 and SC-1 are present in p75NTR-transduced HUVEC, they may play a role in the antiproliferative effect observed in the absence of added ligands. This hypothesis should be investigated.
Finally, p75NTR also modulates the small GTPase RhoA, a member of the Rho family of proteins that control the organization of the actin cytoskeleton. In neurons, in the absence of NTs, p75NTR interacts with RhoA and activates it to inhibit axonal growth. In contrast, NGF causes dissociation of RhoA from p75NTR, thus blocking RhoA activity and leading to axonal growth (282). In particular, p75NTR facilitates the release of RhoA from inhibition by RhoGDI, thus enabling RhoA to be activated. The activated RhoA then interacts with growth-inhibitory proteins, such as Rho kinase (ROCK) to suppress axonal growth and regeneration (281).
At this stage, it is essential to understand what are the intracellular interactors of p75NTR in mature and progenitor cardiovascular cells either in culture or in vivo. This is a prerequisite toward clarification of the signaling pathways emanating from p75NTR in these cell types.
D. Role of Proteases in p75NTR Activation
Similarly to Notch (62), also p75NTR activation is subject to regulated intramembrane proteolysis (RIP) (125). The first cleavage of RIP is taken over by the metalloproteinase ADAM17/TACE that leaves a membrane-bound COOH-terminal fragment (CTF) and liberates the extracellular domain (ECD). This first cleavage is permissive for the subsequent one. The second cleavage is mediated by the γ-secretase and occurs in the p75NTR-CTF intramembrane region to releases a soluble intracellular domain (ICD) (125). It has been reported that p75NTR cleavage is stimulated by different activators, such as phorbol esters, NGF, and pro-NGF (129, 208, 264). Until now, p75NTR cleavage has not been investigated in cardiovascular cells. It is now clear that the Notch ICD (NICD), which is also generated by γ-secretase, regulates gene expression by forming transcriptionally active complexes (239). Although soluble p75NTR-ICD has been localized in the nucleus of different cells following ligand activation of p75NTR (84, 208), the trancriptional activity of p75NTR-ICD has not been described.
As reported for other members of TNF-α superfamily, a soluble form of p75NTR has been detected in various body fluids (286). In particular, using a semiquantitative assay for human plasma p75NTR, Humpert et al. (116) reported that type 2 diabetic patients had significantly higher plasma levels of p75NTR-ICD and lower levels of p75NTR-ECD.
Currently, investigations linking p75NTR activation with postreceptor signal transduction are missing. The only available report describes that ligand-dependent cleavage of the p75NTR is necessary for NRIF nuclear translocation and apoptosis in sympathetic neurons (129). Significant work in this field is needed.
E. Cross-Talk Between Neurotrophin Signaling Pathways Activated Through trk and p75NTR
The presence of p75NTR increases the affinity of NGF for trkA, due to creation of high-affinity NGF binding-site generation on trkA (108). In turn, several studies suggest that trkA activation modulates p75NTR signaling. Bamji et al. (12) demonstrated that trkA activation inhibits BDNF-induced p75NTR-dependent death of sympathetic neurons. Van der Zee et al. (265) reported that trkA expression is required to counteract the death-inducing effects of p75NTR. Pathways initiated through trk receptors suppress the stimulation of JNK, the major proapoptotic-signaling pathway initiated by p75NTR (284). Moreover, p75NTR-dependent ceramide pathway is blocked by trkA-induced activation of the PI3K pathway (21).
The functional interactions between p75NTR and trk receptor are supported by physical interaction (20, 228) or facilitated by assembly of multiprotein complexes (9, 10). The transmembrane protein ARMS (described previously) has been shown to interact with both p75NTR and trk receptors and to be phosphorylated following trk activation (9, 10).
Caveolin enhances p75NTR-trk complex formation (23). In addition, caveolin overexpression avoids NGF-induced differentiation of PC12 through a direct inhibition of trk receptor activity. This inhibition reportedly correlates with an increased ability of NGF to induce sphingomyelin hydrolysis through p75NTR (22). Interestingly, caveolin-1 is highly expressed in EC, VSMC, and monocytes (EPC are a subpopulation of monocytes). Depending on the cell type and the pathological context, caveolin-1 may positively or negatively influence the development of vascular disease and angiogenesis (reviewed in Ref. 85). Further studies are necessary to understand the nature of interaction between NT receptors and caveolin in cardiovascular cells.
Finally, also the p62-TRAF-6-IRAK complex involved in the p75NTR-activated NFkB pathway functions as a scaffold for association of trk receptors and p75NTR (277).
IV. ROLE OF NEUROTROPHINS IN CARDIOVASCULAR DEVELOPMENT
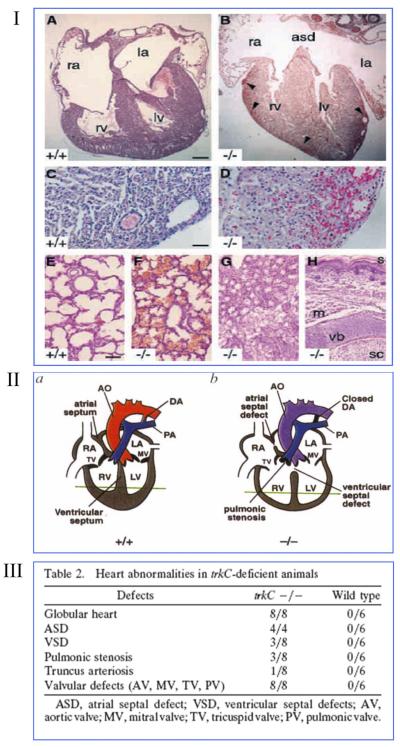
NTs and their receptors are expressed by the developing heart and vessels (18, 233). Seminal studies performed in global knock-out mice implicated NTs in the in utero development of the heart and the coronary vasculature. In particular, BDNF deficiency reduces endothelial cell-cell contact in the mouse embryonic heart, thus leading to intraventricular wall hemorrhage and depressed cardiac contractility (see Fig. 1I) (70). Similarly, trkB−/− mice show a marked reduction of blood vessel density and increased number of TUNEL-positive apoptotic EC, predominantly in the subepicardial region of the developing heart (269). NT-3−/− mice develop abnormalities of the great vessels, including developmental delay in the primitive myofibril organization of the truncus arteriosus (69, 257). Moreover, genetic deficiency of either NT-3 or trkC impaired cardiac morphogenesis in mice (see Fig. 1, II and III) (69, 258). Some of these developmental defects appeared as early as embryonic day 9.5, which is before the onset of cardiac innervation in mice, thus suggesting the existence of a direct control of NTs on cardiovascular development (257). Cardiovascular abnormalities are also present in transgenic mice overexpressing a truncated trkC receptor, which acts as a dominant negative (198). These findings were later expanded in a chicken model of heart development, which demonstrated that NT-3 and trkC are coexpressed by developing cardiomyocytes to mediate their proliferation (154). The series of these developmental studies importantly introduced the concept that NTs have direct effects on cardiovascular cells, which express trk receptors.
FIG. 1.
Genetic deficiency of brain-derived neurotrophic factor (BDNF), neurotropin-3 (NT-3), or tropomyosin-related kinase receptor C (trKC) causes cardiac and vascular defects in the developing mammalian heart. I: BDNF−/− neonate mice exhibit ventricular wall hemorrhage. Histological analyses of BDNF+/+ (A, C, and E) or BDNF−/− (B, D, and F–H) littermates killed at P0. Hematoxylin and eosin-stained sections reveal hemorrhage in the epicardial third of both right and left ventricular walls of BDNF−/− neonates (B and D, arrowheads) and an atrial septal defect (B). Pulmonary hemorrhage is detectable in BDNF−/− (F) but not BDNF+/+ littermates (E). Hemorrhage was not detectable in other organs, such as kidneys (G) as well as skin and spinal cord (H). Ra and la, right and left atria; rv and lv, right and left ventricles; asd, atrial septal defect; s, skin; m, skeletal muscle; vb, vertebral body; sc, thoracic spinal cord. Scale bars, 150 μm (A and B), 50 μm (C and D), 100 μm (E–H). [From Donovan et al. (70), with permission from Development.] II: schematic representation of cardiac abnormalities in NT-3−/− neonate mice. Schematic representations of normal cardiac anatomy(A), the NT-3−/− mutant heart (B), the aorta (AO), ductus arteriousus (DA), right and left atrium (RA, LA), right and left ventricle (RV, LV), tricuspis (TV), and mitral (MV) valve are indicated. [From Donovan et al. (69), reprinted by permission from Macmillan Publishers Ltd.] III: table showing heart abnormalities in trkC−/− mice. [Adapted from Tessarollo et al. (258).]
During prenatal development, p75NTR is present in EC and VSMC of large blood vessels, as demonstrated by immunohistochemistry for p75NTR together with the VSMC marker α-actin and the EC marker PECAM-1 of wild-type murine embryos (at E11.5) (268). Studies aimed at understanding the effect of p75NTR deficiency on vascular development were attempted by von Schack et al. (268), who decided to disrupt exon IV to produce a global null mouse lacking all p75NTR gene products (p75NTRExonIV−/−). The p75NTRExonIV−/− mice suffer from significant death at late gestational stages or around birth. The vascular system of p75NTRExonIV−/− embryos is defective. The dorsal aorta has a thinner wall and increased lumen diameter. Many p75NTRExonIV−/ − embryos show vascular ruptures and blood cell leakage (268). Unfortunately, this genetic model was later proven inappropriate, as it produced a fragment of the p75NTR protein that contains a portion of the extracellular domain and the transmembrane and intracellular domains. This protein derives from aberrant expression of a p75NTR mRNA that is initiated 3′ to the inserted pGK-neo cassette and activates the proapoptotic p75NTR signaling cascades resulting in JNK phosphorylation and cleavage of pro-caspase-3 (202). There is another global p75NTR knock-out model available, which was created by deleting p75NTRExonIII (148). This mouse has been used by many laboratories, but, unfortunately, also p75NTRExonIII−/− mice are not optimal to understand the involvement of the receptor in cardiovascular development. In fact, p75NTRExonIII−/− mice maintain expression of an alternatively spliced form of p75NTR (s-p75NTR) (202, 268).
Mice with global knockout for either trkA or NGF gene can develop to birth, but are smaller and die early, possibly because of their defective nervous phenotype (58, 246). Their cardiovascular phenotype has not been studied. By an interesting “reverse conditional” gene targeting strategy, trkA function was restored specifically in the nervous system to produce mice lacking trkA in nonneuronal tissues, only. These mice are viable and appear grossly normal (53); however, their cardiovascular system was not specifically investigated either during prenatal development or in adulthood. Transgenic mice expressing a neutralizing antibody against NGF (alphaD11) were also developed. These mice show no gross phenotype at birth, even if they later develop skeletal muscle dystrophy, apoptosis in the spleen, and Alzheimer-like neurodegeneration (37, 226). Also for this model, a targeted analysis of the cardiovascular system is still missing.
Additional investigations demonstrated the more obvious concept that NTs can influence cardiovascular development through nerve-mediated actions, as in the case of NGF transgenic mice, which presented cardiac hyper-trophy due to hyperinnervation (104). The generation of new animal models lacking or overexpressing the genes for NT receptors in selected cardiovascular cells should allow elucidating the direct cardiovascular actions of NTs during development and adulthood.
An alternative interesting approach that can help obtain information on the developmental actions of candidate molecules is to test their differentiative activity on embryonic and fetal stem cells. Accordingly, Shmelkov et al. (244) prompted the effect of BDNF on CD133+ stem cells extracted from the human fetal liver. They found that BDNF given alone or together with VEGF-A is able to address CD133+ stem cells to differentiate toward the endothelial lineage as well as giving rise to beating cardiomyocytes, which, once transplanted into the mouse ear, are able to generated electrical action potentials detectable by electrocardiogram tracing (244). The finding that BDNF promotes differentiation into the angiomyogenic lineage prompts new investigations aimed to understand the potential of BDNF and other NTs to improve preparation of cardiomyocytes and EC from embryonic stem cells and from adult stem cells of different sources, such as the bone marrow or adipose tissues. It is worth noting that BDNF, NT-3, and NT-4/5 are produced by human embryonic stem cells (hESC) to mediate their survival by an autocrine mechanism, which engages trk receptors and initiates the PI3K/Akt pathway without affecting MAPK (209). Moreover, it is relevant that addition of NTs to hESC cultures induces a 36-fold improvement in their clonal survival leaving the cells with full conserved developmental potency (209). Furthermore, when hESC are cultured in the presence of NTs on three-dimensional scaffolds, they can form vascular structures throughout the engineered tissues (150).
V. DIRECT ACTIONS OF NEUROTROPHINS ON THE ADULT CARDIOVASCULAR SYSTEM
A. Neurotrophins and Blood Vessel Growth
The development of a complex mature vascular system is a process that requires EC proliferation and migration as well as the fundamental support of periendothelial cells. These accessory cells such as pericytes and VSMC interact with EC to form the complex network of capillaries, arterioles, arteries, and veins. Blood vessels in the embryo form through vasculogenesis, which is in situ differentiation and maturation of vascular cell precursor cells that assemble into a vascular network, and angiogenesis (221). The term angiogenesis was first used to describe the growth of endothelial sprouts from preexisting postcapillary venules. More recently, this term has been used to generally indicate the growth and remodeling process of the primitive vascular network into a complex network. This can involve the enlargement of preexisting vessels which sprout and divide or the formation of capillaries through transendothelial cell bridges (2, 107) as well as intussusception, also known as splitting angiogenesis, where the capillary wall extends into the vascular lumen to split a single vessel in two (65). Angiogenesis and vasculogenesis can also occur in the adult organism. As reviewed by Carmeliet (38), postnatal angiogenesis occurs physiologically in the cycling ovary and the placenta. Moreover, angiogenesis is reactivated during wound healing and repairs and under pathological conditions, such as cancer, ocular, and inflammatory disorders (38). In 1997, Asahara et al. (11) reported that adult bone marrow-derived CD34+ hematopoietic progenitor cells, which can be purified from the mononuclear fraction of peripheral blood cells, could differentiate ex vivo to an endothelial phenotype. These cells, which Ashara named “EPC” (endothelial progenitor cells), showed expression of various endothelial markers and incorporated into neovessels at sites of ischemia (11). Further studies demonstrated that bone marrow and peripheral blood CD133+ hematopoietic stem cells also differentiate to EC in vitro (89, 205).
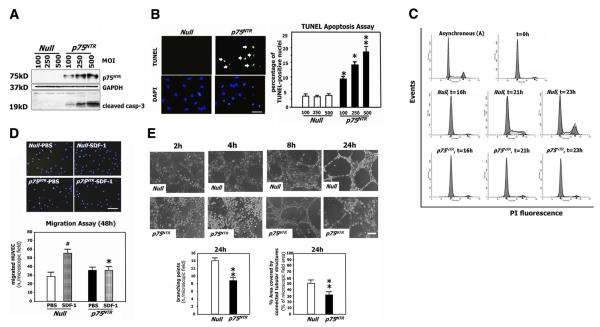
NGF was the first NT to be implicated in postnatal angiogenesis (231). Proliferation, survival, and migration/invasion of EC are all essential to the angiogenesis process. HUVEC and pig aortic EC have the mRNA for NGF and its receptors trkA and p75NTR (34, 213), although the protein expression level of p75NTR is scarce in normally cultured HUVEC (35) and in capillary EC belonging to healthy murine limb muscle (229). In 2001, Raychaudhuri et al. (216) first described the proliferative action of NGF on human dermal microvascular EC, a finding which was later confirmed in HUVEC (34), human choroidal EC (250), and rat brain EC (177). The proliferative action of NGF on EC is reportedly mediated by Erk activation downstream of trkA (34). NGF, via trkA, supports EC survival in vitro and in vivo, an action which is, at least in part, mediated by increased VEGF-A (78, 95, 229). NGF is a chemoattractant for EC, able to induce migration of human and pig aortic EC (68, 213). The chemotactic action of NGF on pig aortic EC is reportedly mediated by the simultaneous activation of the PI3K/Akt and Erk signaling pathways (213). NGF also induces HUVEC invasion through a collagen I-coated filter, by activating MMP-2 (200). The chorioallantoic membrane (CAM) of the chicken embryo and the avascular corneal micropocket of rodent eye are among the primordial assays developed to screen the capacity of molecules to promote blood vessel growth in vivo (103). NGF induces a dose-dependent angiogenic response in the rat cornea (240), and it additionally stimulates blood vessel growth in the chicken and quail CAM (34). NGF-induced CAM neovascularization is partially affected by anti-VEGF-A antibodies, thus suggesting the involvement of VEGF-A in NGF-induced angiogenesis (34, 145). In contrast to trk actions, ligand (NGF and pro-NGF)-dependent activation of p75NTR reportedly induces EC death (135). Moreover, a recent study from our laboratory described impaired proangiogenic capacity of p75NTR-transduced HUVEC (35). Figure 2 illustrates enhanced EC apoptosis and defective EC cycle, migration, and capillary-like structure formation following gene transfer-induced p75NTR expression. Figure 3 summarized the actions and underpinned molecular pathways which activation of trks and p75NTR stimulates in EC.
FIG. 2.
The angiogenic potential of endothelial cells is impaired by p75NTR. A: human umbilical vein endothelial cells (HUVEC) were infected with different concentrations of an adenoviral vector carrying human the p75NTR gene (Ad.p75NTR) or with Ad.Null (control). After 48 h, cell lysates were collected and subjected to Western blotting with antibodies to p75NTR, the apoptosis marker cleaved caspase-3, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (loading control). These analyses provide evidence that p75NTR expression induces EC apoptosis. B: apoptotic nuclei of transduced HUVEC were detected by the TUNEL assay. Fluorescent images are representative of apoptosis rate in Null-HUVEC and p75NTR-HUVEC. Original magnification: ×400; scale bar: 40 μm. Green fluorescence: TUNEL-positive nuclei; blue fluorescence: all the nuclei. Arrows point to TUNEL-positive nuclei. Bar graphs quantify apoptosis, which is expressed as percentage of TUNEL-positive nuclei to total nuclei. Data are presented as means ± SE. *P < 0.05 and **P < 0.001 vs. Ad.Null. C: HUVEC were transduced with p75NTR or Null and syncronized by serum starvation. Following the release from cycle arrest, the cell cycle progression was assessed by flow cytometric analysis, at the indicated time points. The percentage of cells in G1, S, and G2 phases is indicated in figures. These analyses showed defective cell cycle progression in p75NTR-HUVEC. D: migration toward the chemoattractant stroma derived factor-1 (SDF-1; 100 ng/ml) is reduced for p75NTR-HUVEC compared with Null-HUVEC. In bar graph, values are means ± SE. #P < 0.05 vs. Ad.Null combined with PBS, *P < 0. 05 vs. Ad.Null combined with SDF-1. Top panels show representative microscopic fields (original magnification: ×100; scale bar: 100 μm). E: the potential of HUVEC to form vessel-like structures on Matrigel is impaired by p75NTR transduction. Images (original magnification: ×100; scale bar: 100 μm) show the time course (up to 24 h from cell seeding) of cell organization on Matrigel. In bar graphs, the quantification of EC tube network formation at 24 h from seeding is expressed as the number of intersecting points of tubular structures for microscopic field (left) as well as the percent of microscopic field area covered by connected tubular structures (right). Values are means ± SE. **P < 0.01 vs. Ad.Null. [From Caporali et al. (35).]
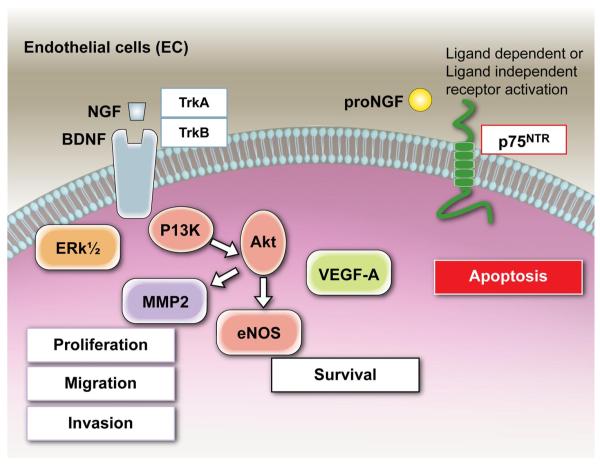
FIG. 3.
Neurotrophin actions on the endothelial cell. Under basal conditions, the neurotrophin receptor trkA and trkB (and, in some tissues, also trkC) are expressed by EC. Trk receptor engagement by mature NTs initiates two major signaling pathways, Erk MAPK and IP3K/Akt. Nerve growth factor (NGF) treatment of EC causes a rapid phosphorylation of trkA, determining a parallel activation of ERK1/2 and a subsequent increase in EC proliferation and migration. Activation of the PI3K/Akt signaling pathway promotes EC survival. Moreover, via the PI3K/Akt, NGF stimulates EC invasion and cord formation by augmenting MMP-2. NGF, via trkA, supports EC survival in vitro and in vivo, an action which is, at least in part, mediated by increased VEGF-A. BDNF, via trkB, also supports EC survival. Under basal conditions, the p75NTR is scarcely expressed by EC. Following p75NTR induction or transduction, the receptor triggers EC apoptosis and inhibits EC cell cycling through mechanisms that may be dependent or not from ligand (mature NT and pro-NGF) activation.
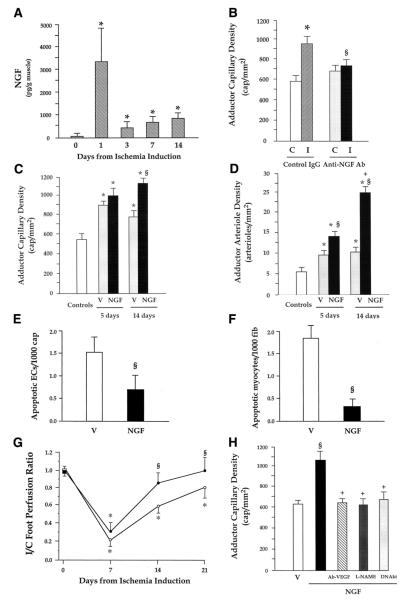
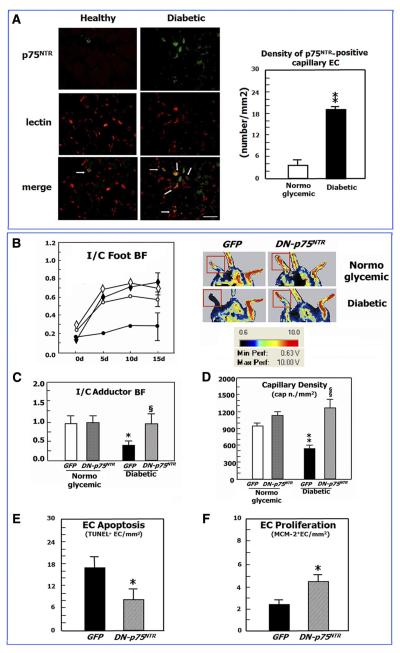
Our group was the first to identify the strong angiogenic action exerted by NTs in a mouse model of limb ischemia, which is instrumental to study the effect of endogenous and exogenous factors on postischemic reparative neovascularization (78). In this model, as illustrated by Figure 4, we showed that NGF is an autocrine proangiogenesis factor, which becomes upregulated together with its trkA receptor following insurgence of ischemia (Fig. 4A) to promote the growth of new capillaries in ischemic muscles. In fact, blockade of endogenous NGF by a neutralizing antibody disrupts the angiogenesis response to muscular ischemia (Fig. 4B), while exogenous NGF supplementation to ischemic muscles enhances the spontaneous formation of capillaries and arterioles in ischemic muscles (Fig. 4, C and D) and accelerates blood flow recovery (Fig. 4G) (78, 229). NGF actions in ischemic muscles extend to include antiapoptosis of capillary EC and skeletal myocytes (Fig. 4, E and F) (78, 229). NGF appears to induce angiogenesis through trkA by increasing the expression level of VEGF-A (33, 78, 101, 230) and possibly VEGF receptors (101) and activating the Akt intracellular pathways leading to NO production and upregulation of MMP-2 expression (78, 200, 213). In fact, in the limb ischemia angiogenesis model, NGF-induced angiogenesis was prevented by a neutralizing antibody for VEGF-A, inhibition of NO synthase (by l-NAME), or gene transfer with a dominant negative mutant form of Akt (Fig. 4H) (78). As reported above, also Erk was implicated in NGF-induced EC proliferation and migration, which are processes potentially utilitarian to angiogenesis. We have more recently found that NGF gene delivery to the infarcted heart of mice and rats can be used to increase capillary density and inhibit EC apoptosis in the peri-infarct area (unpublished data), which may have important therapeutic implications. Moreover, NGF promotes angiogenesis in healing cutaneous wounds, thus favoring cicatrisation (95).
FIG. 4.
NGF promotes angiogenesis and arteriogenesis in ischemic hindlimbs. A: time course of NGF content in adductor muscles following induction of hindlimb ischemia. Values are means ± SE (n = 4 for each time point). *P < 0.05 vs time 0. B: endogenous NGF promotes angiogenesis in ischemic limb muscles. Twenty-one days from ischemia induction, control IgG-treated mice (n = 6) showed higher capillary density in ischemic adductors (I) than in contralateral normoperfused muscles (C). The capillary response to ischemia was prevented in mice given a NGF-neutralizing antibody (anti-NGF Ab, n = 8). Values are means ± SE. *P < 0.05 vs. contralaterals. §P < 0.05 vs IgG. C and D: supplementation of NGF to ischemic limb muscles promotes angiogenesis and increases arteriole numbers. Local daily injections of NGF (full columns) or vehicle (V, dotted columns) in ischemic muscles were repeated over 5 or 14 days, starting from the day of ischemia induction. Capillary (C) and arteriole (D) density was evaluated 14 days thereafter. Microvascular density of untouched adductors (open column) is shown as a reference. Neovascularization was potentiated at arteriole and capillary level by 14-day NGF treatment. Five days of treatment exerted an effect on arteriole growth only. Values are means ± SE. *P < 0.05 vs. controls. §P < 0.05 vs. V; +P < 0.05 vs. 5-day treatment. E and F: supplementation of NGF reduces apoptosis of endothelial cells and skeletal myocytes in ischemic adductor muscles. NGF or vehicle was intramuscularly injected every day starting on the day of femoral artery excision. Muscles were harvested 5 days later. Apoptosis found at the level of capillary endothelial cells (ECs) and skeletal myocytes was expressed as the number of TUNEL-positive cells per 1,000 capillaries (A) or myofiber (B), respectively. Values are means ± SE (n = 4 mice per group). §P < 0.05 vs. V. G: supplementation of NGF to ischemic limb muscles improves postischemic blood flow recovery. Line graph shows the effect of NGF supplementation for 14 days on foot postischemic BF recovery, expressed as ischemic to contralateral BF ratio. NGF (full symbols, n = 8) improved perfusion recovery compared with vehicle (open symbols, n = 10). Values are means ± SE. *P < 0.05 vs. time 0. §P < 0.05 vs. vehicle. H: NGF induces angiogenesis via VEGF-A, Akt, and nitric oxide (NO). Daily injection of 20 μg NGF for 7 days stimulates capillary growth in normoperfused muscles (closed column) compared with vehicle-treated muscles (V, open column). The angiogenic effect of NGF was blocked by a VEGF-neutralizing antibody (Ab-VEGF), the NO synthase inhibitor l-nitroarginine methyl ester (l-NAME), or an adenovirus carrying dominant-negative Akt (DNAkt). Values are means ± SE (at least n = 6 per group). §P < 0.05 vs. vehicle; +P < 0.05 vs NGF alone. [From Emanueli et al. (78).]
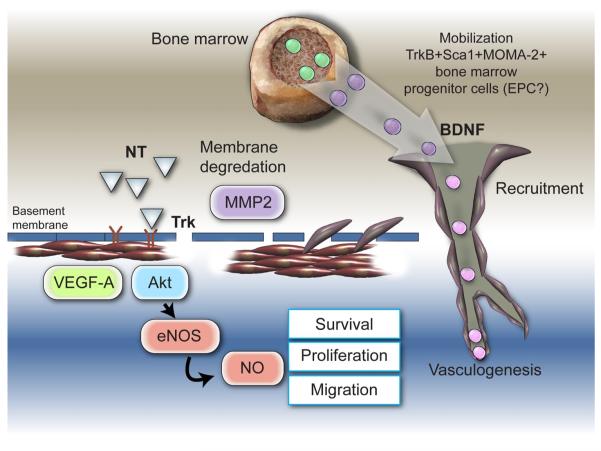
Some of the angiogenesis-related actions of NGF have been later proved to be shared by BDNF. In 2000, Nakahashi et al. (182) found BDNF mRNA and protein in HUVEC. Kim et al. (135) confirmed BDNF expression by EC and additionally showed that EC express trkB and that the level of BDNF transcript is upregulated by hypoxia in EC, a feature which is compatible with a proangiogenesis action of the BDNF. The hypoxia-mediated regulation of BDNF in EC was confirmed by Wang et al. (270) using a mouse brain microvascular EC line (bEnd.3 cells). BEnd.3 cells respond to either sustained or intermittent hypoxia with increased production and release of BDNF in the culture medium. Endogenous BDNF promotes EC survival, while exogenously added recombinant protein stimulates in vitro angiogenesis, via PI3K/Akt (135). Similarly, to NGF, also BDNF, via trkB, reportedly upregulates VEGF-A expression level. According to Nakamura et al. (183), this action is induced by PI3K and hypoxia-inducible factor 1 alpha (HIF-1α). In turn, VEGF-A can upregulate BDNF mRNA level, as observed in myometrial microvascular EC (276). Inhibition of VEGF receptor 2 significantly decreases BDNF expression in brain EC (47). Given the knowledge that VEGF-A, via Akt phosphorylation of eNOS, stimulates NO production from EC (1, 87) and that NO reportedly upregulates BDNF (46), it is possible that eNOS-derived NO is involved in the VEGF-A-induced increase in BDNF. Kermani et al. (131) tested the potential of angiogenesis gene therapy with BDNF in the ischemic limb mouse model. Similar to what was already observed for NGF, BDNF expression is upregulated in ischemic limb muscles to induce phosphorylation of vascular trkB. Gene transfer-mediated BDNF overexpression increases capillary density of ischemic muscle, thus accelerating blood flow recovery (131). Interestingly, intravenous injection of an adenovirus carrying the BDNF gene induces the mobilization of Sca-1+CD11b+ hematopoietic progenitor cells in the peripheral blood of mice. The finding, which is in line with the presence of trkB and other NT family components in the bone marrow (142), was confirmed in vitro by studying the migration toward BDNF of total bone marrow cells. In this system, BDNF proved to predominantly attract MOMA-2+ and Sca-1+ cells. In line, ischemic muscle transduced with BDNF showed increased infiltration of MOMA-2+CD45+ cells (131). The report by Kermani et al. (131) was the first to suggest a supportive effect of NTs on postnatal vasculogenesis. However, a more exhaustive definition of adult progenitor cell populations responding to BDNF and NTs is necessary. In addition, Kermani and colleagues (78, 131) did not demonstrate that increased circulating MOMA-2+ and Sca-1+ cells and enhanced infiltration of MOMA-2+CD45+ cells effectively contribute to BDNF-induced neovascularization of ischemic limb muscles. Unlike NGF, the proangiogenic actions of BDNF are not hampered by neutralizing VEGF. In addition, BDNF increases capillarization, but it has no effect on arterioles or small arteries, although this latter finding is apparently difficult to reconcile with BDNF-induced improved blood flow recovery (78, 131). Another difference from NGF is that BDNF does not induce angiogenesis in the corneal pocket model, which may be explained with the absence of trkB in EC belonging to this district (131, 240). Rather, BDNF induces angiogenesis in both the Matrigel plug and the mouse ear models, with its responses being comparable to those elicited by VEGF-A (131). NT-4/5, which is an additional ligand of trkB, is also able to promote angiogenesis in the Matrigel model (131). Recent findings from our laboratory suggest that BDNF gene transfer promotes neovascularization of the peri-infarct myocardium (unpublished data). Our data are in line with the report that BDNF potentiates basic fibroblast growth factor (bFGF)-induced angiogenesis and improves cardiac function following myocardial infarct (157). Moreover, BDNF induces blood vessel growth in transected adult rat thoracic spinal cord, as demonstrated when using BDNF to impregnate freeze-dried poly(d,l-lactic acid) macroporous guidance scaffolds (201). It is also possible, even if not yet demonstrated, that BDNF plays a role in brain angiogenesis induced by physical exercise. In fact, the levels of brain BDNF and VEGF-A increase and angiogenesis develops in the brain of exercising mice (55). It has been demonstrated that blocking VEGF-A inhibits exercise-induced brain angiogenesis (55). As, out of the brain, BDNF is able to upregulate VEGF-A and vice versa (183, 276), the involvement of BDNF in brain angiogenesis is plausible.
The main receptor for NT-3, trkC, is not expressed by HUVEC, which are the most popular model of EC used by angiogenesis laboratories. This fact possibly explains why NT-3 has not been investigated as a potential angiogenesis mediator. Nevertheless, trkC is highly expressed by human veins and mouse capillary EC (unpublished data). Unpublished data from our laboratory show that adenovirus-mediated NT-3 gene transfer to murine ischemic hindlimbs stimulates the proliferation of capillary EC, thus increasing capillary density (unpublished data). These findings apparently contradict a previous investigation which proved NT-3 to inhibit the mitogenic activity of rat brain EC (255). Gene therapy with NT-3 can promote blood flow recovery to the ischemic foot (unpublished data). Western blot analyses of NT-3-transduced muscles revealed increased levels of phosphorylated trkC, Ser-473-phosphorylated Akt, and Ser-1177-phosphorylated eNOS. Increased eNOS protein level and NO production following stimulation with NT-3 were also reported in rat brain EC (255). In contrast to what was found for NGF, the content of VEGF-A mRNA or protein is unchanged by overexpressing NT-3 (unpublished data). The scheme in Figure 5 summarizes the potential of NTs/trks to induce blood vessel growth by angiogenesis and vasculogenesis.
FIG. 5.
Schematic representation of mechanisms by which NTs stimulate blood vessel growth. NGF promotes angiogenesis through trkA by increasing the expression level of VEGF-A, and through activation of the Akt intracellular pathways leading to NO production and upregulation of matrix metalloproteinase (MMP)-2 expression. Some of the angiogenesis-related actions of NGF have been later proven to be shared by BDNF. Moreover, BDNF induces the mobilization of trkB+Sca1+MOMA-2+ bone marrow progenitor cells, suggesting a promotional role of BDNF on postnatal vasculogenesis, although this hypothesis needs to be validated.
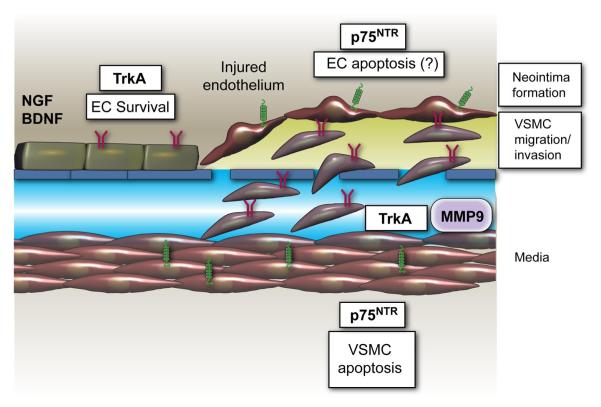
B. Neurotrophins and Vascular Smooth Muscle Cell Control
NGF, BDNF, and NT-3 are expressed in the wall of the aorta and pulmonary artery of embryonic and postnatal rat. The level of NT-3 in blood vessels is relatively constant from the embryonic to adult stage, while levels of BDNF increase and those of NGF decrease (233). Other studies show discrepancies of results about BDNF expression, reporting only faint BDNF mRNA expression in the heart (109, 164, 259).
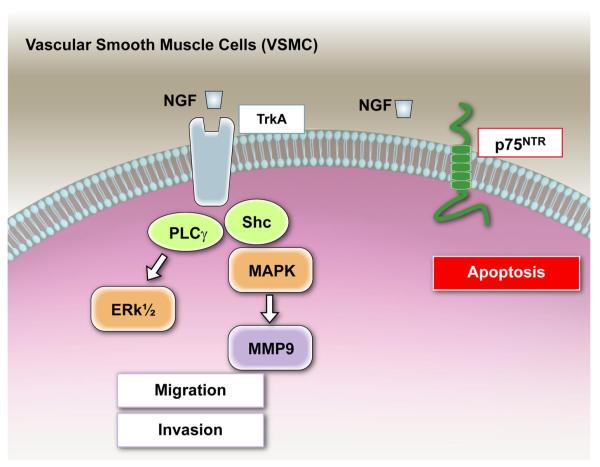
Cultured VSMC express NTs and their receptors and secrete NTs in a regulated manner (71, 184, 185, 261). VSMC possess proprotein convertases (PC), such as furin, PC5, and PC7, which are deputed to the maturation of pro-NTs in their active form (249). NGF does not stimulate VSMC proliferation, but it is a potent chemotactic agent for human aortic VSMC (71). NGF-induced VSMC migration is mediated by PI3K and Akt, as it can be blocked by either LY294002 or wortmannin (141). The NGF/trkA receptor signal, via Shc/MAPK pathway, induces MMP-9 expression in primary rat aortic VSMC and in a VSMC line genetically manipulated to express trkA. MMP-9 may favor VSMC migration and invasion (132). Stimulation with NGF, NT-3 and, to a less extent, BDNF of p75NTR-expressing VSMC promotes apoptotic death (272). The role of trkA in VSMC apoptosis is still controversial (26, 272).
Figure 6 illustrates the actions and underpinned molecular pathways that could be stimulate by NTs in VSMC.
FIG. 6.
Neurotrophin actions on vascular smooth muscle cells. Human and rodent VSMC express NTs, p75NTR, and trk receptors in vivo and in culture. Activaction of trkA by NGF, via PI3K and Akt, promotes chemotaxis of human aortic VSMC, without influencing VSMC proliferation. Moreover, the NGF/trkA receptor signal, via Shc/MAPK pathway, induces MMP-9 expression in primary rat aortic VSMC, which, in vivo, may favor the invasion of VSMC through the extracellular matrix and endothelial basal membrane. Stimulation with NGF of p75NTR-expressing VSMC promotes apoptotic death.
C. Actions of Neurotrophins on Cardiac Myocytes
The first evidence of secretion of neurotrophic factors by heart cells was obtained in 1979, when Eberdal et al. (73) showed that heart explants support neurite extension from sensory neurons in vitro. All NTs are expressed by the mammalian heart, as demonstrated by different techniques (112, 164, 238, 259). Truncated form of trkB and trkC receptors have also been reported in human or rat hearts with Northern blotting or RNase protection assay (242, 260).
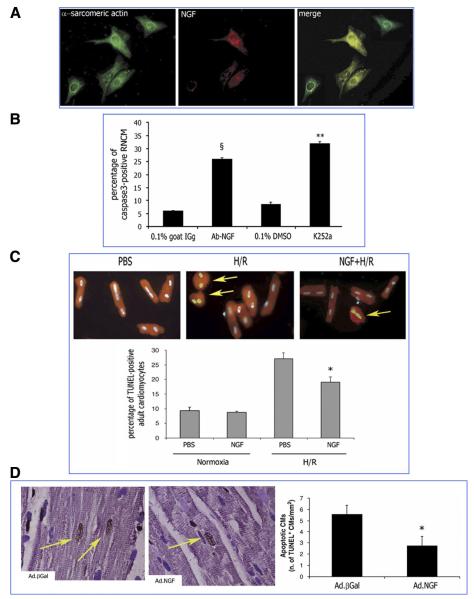
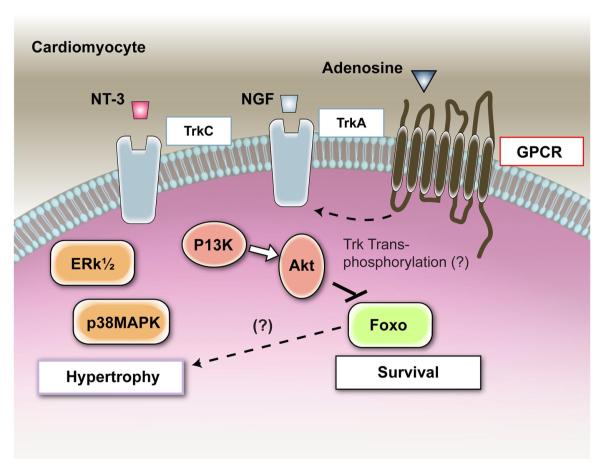
We have recently identified that NGF is an autocrine prosurvival factor for cardiomyocytes (36). Rat neonatal cardiomyocytes express and release NGF (36, 117) and possess trkA (36) (Fig. 7). Moreover, cardiomyocytes undergo apoptosis when treated with a neutralizing antibody for NGF or the trkA inhibitor K252a (Fig. 7B). Furthermore, NGF expression in cultured cardiomyocytes increases following either hypoxia/reoxygenation (H/R) or incubation with high ANG II levels, two conditions inducing cardiomyocyte apoptosis. When, under each of those conditions, the NGF/trk receptor signaling is inhibited, apoptosis is dramatically augmented, thus reinforcing the idea of an antiapoptotic role for this NT (36). Increased NGF level obtained by either gene transfer or supplementation with a NGF recombinant protein protects neonatal and adult cardiomyocytes from apoptosis induced by either H/R (Fig. 7C) or ANG II (36). The prosurvival signal of NGF in cardiac myocytes encompasses Akt phosphorylation and the nuclear/cytosolic shuttling of Akt and Forkhead transcription factors Foxo-3a and Foxo-1 (36). Finally, in a rat model of myocardial infarction, NGF gene transfer supports cardiomyocytes survival (Fig. 7D) (36). Akt promotes both survival and (physiological and pathological) hypertrophy of cardiomyocytes (reviewed in Ref. 42). Anversa and Sussmann (42) proposed the theory that extranuclear Akt plays the double role of promoting survival and growth by acting on several redundant downstream targets, including Bad and glycogen synthase kinase-3, whereas nuclear Akt combats apoptosis without interfering with the control of cell size. Indeed, the knowledge that Foxo is a nuclear target of Akt (274) and that Foxo-3a and Foxo-1 are negative regulators of myocyte hypertrophy (187, 245) requires a new look at the role of nuclear Akt in growth. Although the impact of NGF on cardiomyocyte size was not the objective of our recent study (36), we consider this issue of high relevance. Interestingly, NT-3, via trkC, reportedly promotes cardiomyocyte hypertrophy, possibly by acting on MAPK (128). We found that also trkB is expressed by rat neonatal cardiomyocytes (unpublished results), thus suggesting that BDNF may trigger responses in these cells. Further investigations are needed to improve our understanding of the full range of NT actions on the developing and adult cardiomyocytes.
FIG. 7.
Prosurvival activity of NGF on cardiac myocytes. A: rat neonatal cardiomyocytes (RNMCs) express NGF and trkA. Immunofluorescence analysis is shown of the cardiac marker α-sarcomeric actin (green fluorescence) and NGF (red fluorescence) and merged images (costaining results in yellow fluorescence). B: NGF is an endogenous prosurvival factor for RNCMs. RNCMs were incubated for 48 h in serum-free medium with a goat-raised anti-NGF neutralizing antibody (Ab-NGF) or the trkA inhibitor K252a. Controls consisted of nonimmune goat serum (0.1% in PBS) or 0.1% DMSO, respectively. Apoptosis was detected by cleaved caspase-3 immunostaining (green fluorescence). Nuclei were counterstained by DAPI (blue fluorescence). The fluorescent images (×400) are representative of the experiment. Bar graph shows the percentage of cleaved caspase 3-positive RNCMs. Data are presented as means ± SE (n = 3). §P < 0.05 vs. 0.1% goat IgG; **P < 0.01 vs. 0.1% DMSO. C: NGF inhibits apoptosis in rat adult cardiomyocytes. A, isolated rat adult cardiomyocytes were maintained under normoxia or submitted to 6 h of hypoxia followed by 18 h reoxygenation and cotreated with NGF (50 ng/ml) or its vehicle PBS. Apoptotic nuclei were identified by TUNEL staining (green fluorescence). α-Sarcomeric actin stains cardiomyocytes (red fluorescence). The pictures were captured at ×400 magnification. Arrows point to TUNEL-positive cardiomyocytes. Bar graphs quantify apoptosis, which is expressed as percentage of TUNEL-positive cardiomyocyte. Data are presented as means ± SE (n = 3). *P < 0.05 vs. PBS plus H/R (B) D: local NGF gene transfer prevents apoptosis of cardiomyocytes in the rat infarcted heart. Myocardial infarction was induced in adult Wistar rat. Ad.NGF or Ad.βGal (each at 108 p.f.u.) was injected in the peri-infarct myocardium. After 7 days, the heart was arrested in diastole and perfusion/fixed. Apoptosis of cardiomyocytes (CMs) was revealed by double staining for TUNEL (TUNEL-positive nuclei are stained in dark brown) and the cardiac marker α-sarcomeric actin (in purple). Nuclei were counterstained with hematoxylin. In the pictures captured (optical microscopy, ×1,000) from Ad.NGF and Ad.βGal specimens, TUNEL-positive apoptotic cardiomyocytes are pointed by arrows. Graph quantifies apoptosis of cardiomyocytes per mm2 of peri-infarct myocardium section. Values are means ± SE (n = 7) *P < 0.05 vs. Ad.βGal. [From Caporali et al. (36).]
Figure 8 illustrates the actions and signaling pathways emanating from trk receptors in the cardiomyocyte.
FIG. 8.
Neurotrophin actions and signalling in cardiomyocytes. NGF is an autocrine prosurvival factor for cardiomyocytes, and an increased level of this neurotrophin protects cardiomyocytes from apoptosis. The prosurvival signal of NGF in cardiac myocytes is mediated by trkA and downsream Akt phosphorylation. In response to Akt activation by NGF, Forkhead transcription factors Foxo are phosphorylated and excluded from the nucleus, thus resulting in increased cardiomyocyte survival. In primary-cultured rat neonatal cardiomyocytes, NT-3, via trkC, activates p38 MAPK and Erk1/2, thus resulting in increased cell size. Interestingly, in neural cells, the possibility that trk receptors can be transactivated in response to G protein-coupled receptor (GPCR) signaling has been proven. The GPCR adenosine receptors are expressed by cardiomyocytes, where they trigger a range of responses, including activation PI3K. We speculate that transactivation of trk receptors by adenosine receptors may happen in cardiomyocytes.
VI. NEUROTROPHINS IN CARDIOVASCULAR PATHOLOGY
A. Neurotrophins and Cancer Angiogenesis
Angiogenesis is essential for tumor development and metastasis. To initiate angiogenesis, tumor cells make an angiogenic switch by perturbing the local balance of pro- and antiangiogenesis factors and proteases (83). Being strong promoters of angiogenesis and able to activate VEGF-A and metalloproteases, NTs may contribute to the growth and diffusion of cancers (17, 72, 121, 188), even if this hypothesis has not been exhaustively validated, yet. It may be interesting to remember that the trk gene has originally been cloned as an oncogene fused with the tropomyosin gene in the extracellular domain conferring constitutive activation of its tyrosine kinase activity to induce continuous cell proliferation (14, 180). Surprisingly, this gene was discovered to code for a high-affinity receptor for NGF (180). Trk receptors regulate growth, differentiation, and survival of tumors with neuronal origin, such as neuroblastoma and medulloblastoma (180). High expression of BDNF and trkB is considered a poor prognosis marker in neuroblastoma, one of the most common pediatric neoplasms (7, 169). Furthermore, trkB activation by BDNF, via HIF-1α and PI3K/mTOR, induces VEGF-A in neuroblastoma cells (183). In contrast, high expression of trkA is known to be a marker of good prognosis in the same tumor type (181). This discrepancy was explained in terms of the dependence of trkA-expressing neuroblastoma cells on NGF, which, when present in abundance, pushes them to mature or, when NGF is too low, to die (180). However, it was also shown that NGF-mediated activation of trkA decreases the content of proangiogenesis factors in the conditioned medium of neuroblastoma cells (74). Opposite results to neuroblastoma were found for medullary thyroid carcinoma (MTC). Compared with control cells, trkB-overexpressing MTC cells produce less VEGF-A in vitro and develop less and smaller cancers when implanted in mice, while trkA-overexpressing MTC cells give more VEGF-A and promote tumorigenesis and cancer growth in vivo (174).
Moreover, NGF is produced by hepatocellular carcinoma cells, and trkA was found expressed in the vascular walls of tumor-associated arteries, thus suggesting that NGF/trkA signaling may participate in cancer angiogenesis (137). Further support for this hypothesis comes from the finding that, in ovarian carcinoma, activated trkA is present on the EC membrane, both inside the tumor and in its vicinity (60). NGF is also produced by human melanomas (170), and both NT-3 and trkC are expressed by EC in or around human gliomas (32). In addition, malignant glioma-released NT-3 has the capacity to attract bone marrow-derived stroma cell (24), which may have implications for tumor vasculogenesis.
All these findings suggest the possibility that NTs can control cancer angiogenesis and consequently the growth and diffusion of primary tumors. However, a systemic evaluation of this concept was never developed, and cause-effect studies are especially important at this stage.
B. Neurotrophins and Angiogenesis Associated With Chronic Inflammation
Angiogenesis is intrinsic to psoriasis and arthritis. Psoriasis is a chronic inflammatory skin disorder characterized by accelerated proliferation and altered differentiation of keratinocytes and microvessels undergoing angiogenesis (57). NGF is produced by healthy and psoriatic epidermal keratinocytes and fibroblasts (4, 218), as well as by dermal microvascular EC (216). In a murine model of cutaneous pressure ulcers, NGF promotes angiogenesis (95), thus accelerating wound healing (95). A similar wound closure effect by NGF has been reported in full-thickness skin wounds in mice (168). In a SCID mouse-human skin chimeras xenograft model, NGF was seen to stimulate EC proliferation and induction of the intercellular adhesion molecule (ICAM-1) on EC (217, 219). In the same experimental model, either NGF neutralization or inhibition of trk receptors by K252a improved psoriasis (219). In addition, NGF upregulates the expression levels of substance P (155), which is a strong proangiogenesis inducer (285) involved in psoriasis (219). These findings, which need to be confirmed and expanded in future studies, suggest a role of NGF in psoriasis-associated angiogenesis. To the best of our knowledge, no report linking other NTs or their receptors to psoriasis are currently available.
Angiogenesis in the synovial membrane is considered an important early step in the pathogenesis and progression of inflammatory arthritis, particularly rheumatoid arthritis. Angiogenesis is required for the development of the rheumatoid pannus, which then leads to extensive synovial inflammation and joint destruction (133, 254). Consequently, it is relevant that NTs and their receptors are present at both mRNA and protein levels in inflamed peripheral joints of rheumatoid arthritis and spondyloarthritis patients (220). Both trkA and p75NTR are expressed in the endothelium of synovial tissue biopsy specimens obtained from clinically affected knee joints of patients with rheumatoid arthritis, spondyloarthritis, and osteoarthritis (220). Notably, NGF appears to be elevated in the synovial fluids of rheumatoid arthritis patients in comparisons with normal human synovial fluids (100). Investigations addressing the causative role of NTs in arthritis-linked angiogenesis have not been carried out.
C. Neurotrophins in Cardiovascular Inflammation, Atherosclerosis, and Vascular Wall Remodeling
Atherosclerosis is a complex, chronic inflammatory process of the arterial wall that involves the recruitment and activation of cells in a developing intimal lesion. Among the events triggering this process is the EC activation by inflammatory cytokines and oxidized lipoproteins, followed by the increased adhesion of blood-circulating monocytes to the activated endothelium and the migration of VSMC into a developing neointima. Then, lipid accumulation and modulation of vascular cell phenotypes by extracellular matrix proteins (especially metalloproteases) stimulate the development of an atherosclerotic plaque (140), which progressively obstructs the vascular lumen, thus reducing blood flow. The attempts to reestablish lumen patency by percutaneous transluminal angioplasty can result in the damage of the EC and the vascular internal elastic lamina, which in turn activates the shift of VSMC from a contractile to a synthetic phenotype and the VSMC migration to the lumen. This process culminates in neointima formation and restenosis of the vessel (186). Figure 9 illustrates the possible role played by NTs in neointima formation. Human and rat VSMC express NTs, p75NTR, and trk receptors in vivo and in culture (71, 141, 272). The expression levels of NGF, BDNF, trkA, and trkB are dramatically upregulated by arterial balloon injury in rats, and increased levels persist during neointima formation (71). BDNF, NT-3, NT-4/5, trkB, trkC, and p75NTR were also found expressed in VSMC of human atherosclerotic lesions (71, 139), thus suggesting an implication of NTs in regulating VSMC response to vascular injury (71). This hypothesis is further supported by the knowledge that NGF promotes VSMC migration, via trkA (71, 141), while p75NTR stimulates VSMC apoptosis (139, 272). Furthermore, in VSMC, NGF induces the expression of the gelatinase MMP-9 (132), which has prominent activity against basement membrane components and becomes rapidly activated following mechanical injury (186). MMPs are major players in intimal thickening and atherosclerotic plaque development and rupture (186). Kraemer et al. (140) crossed trkB+/− with the atherosclerosis apolipoprotein E gene knock out mouse model (ApoE−/−) to find that reduction in trkB signaling slows the appearance of lipid deposition and neointima formation in mice fed with a proatherogenic diet (140). Unexpectedly, MMP-9 levels were identical in the vascular lesions of trkB+/− ApoE−/− and trkB+/+ ApoE−/− mice (140). However, other matrix proteases might be regulated in their expression and activity by trkB. Bone marrow-derived inflammatory cells are less in vascular lesion developed by trkB+/− ApoE−/− mice compared with trkB+/+ ApoE−/− mice. This difference could not be explained with the reduced trkB presence in bone marrow cells. In fact, results of experiments with bone marrow cross-transplantation between trkB+/− ApoE−/− and trkB+/+ ApoE−/− mice disclaimed the importance of trkB presence in bone marrow for infiltration of inflammatory cells in atherosclerotic lesions (140). The latter finding is in apparent contradiction with another report of the same group, showing a prominent role of BDNF in stimulating mobilization of trkB+Sca-1+MOMA2+ from the bone marrow to engraft in tissue expressing high level of BDNF (131). We should not forget that BDNF/trkB activates an important prosurvival signaling for EC (135), and the integrity of luminar EC is fundamental to avoid adhesion of monocytes and to maintain VSMC in a contractile rather than proliferative status. Therefore, it is possible that the role of trkB in vascular wall remodeling and atherosclerosis is more complex. New models separately targeting trkB expression in EC, VSMC, and monocytes may provide more substantial information.
FIG. 9.
Neurotrophin actions could be involved in neointima formation. Neurotrophins, which, via trk receptors and p75NTR, regulate survival of both EC and VSMC may have a dual role in neointima formation. In fact, the trk-mediated survival of EC may contribute to avoid intravascular adhesion of monocytes and to maintain VSMC in a contractile rather than proliferative status. In contrast, p75NTR triggers apoptosis of both EC (negative event) and VSMC (possibily a positive event, as it may inhibit VSMC growth). Moreover, NGF is a potent chemotactic agent for human aortic VSMC and, by inducing MMP-9 expression, it may also favor VSMC invasion through the basal membrane and internal elastic lamina. Arterial balloon injury upregulates the expression levels of NGF, BDNF, trkA, and trkB. Increased expression levels of NTs and trk receptors persist during neointima formation.
In support of a proinflammatory effect of trkB is a phage-display study, which identified increased trkB expression in the aging rodent heart (31). This was associated with functional studies revealing that age-associated alterations in the cardiac BDNF/trkB signaling enhance inflammation and increase myocardial injury after myocardial infarction in the aging heart (31). A role for BDNF in the pathogenesis of coronary artery disease has been also proposed (76). According to Ejiri et al. (76), BDNF derives mainly from diseased coronary arteries and contributes to plaque instability through enhancing local NADPH activity and superoxide production. These assumptions derived from finding higher BDNF concentrations in the EDTA-containing plasma of the coronary sinus than in the aortic root of patients with acute coronary syndrome (76). However, high levels of BDNF are stored in human platelets. Increased blood flow velocity and shear stress in the presence of significant narrowing of epicardial coronary arteries lead to platelet activation, and this may justify the BDNF released in the circulation. Therefore, it is possible that BDNF is not released by diseased vessels, but by activated platelets (158). It is also possible that, since BDNF is a prosurvival factor for EC, diseased vessels increase its production and release for their self-protection.
D. p75NTR-Induced Impairment in Postischemic Reparative Neovascularization in Diabetes
Type 1 diabetes downregulates the content of NGF and trkA in ischemic skeletal muscles and concomitantly induces p75NTR expression in capillary EC (35, 36, 229) (see Fig. 10A). Recent data from our group provide evidence that p75NTR is responsible for diabetes-induced impairment in neovascularization of ischemic limb muscles. In fact, when diabetic mice were treated with an adenovirus carrying a dominant negative p75NTR mutant form in their ischemic limbs, the postischemic angiogenesis process and blood perfusion recovery were normalized to levels similar to what was observed in normoglycemic mice (see Fig. 10B) (35). Interestingly, NGF supplementation, rather than initiating apoptosis of diabetic EC via p75NTR, downregulates p75NTR expression, by a mechanism which has not yet not been clarified, and promotes EC survival and vascular regeneration (95, 229).
FIG. 10.
p75NTR is implicated in diabetes-induced impairment in reparative neovascularization of ischemic limb muscles. A: diabetes upregulates the content of endogenous p75NTR protein in capillary EC of ischemic limb muscles. Staining of ischemic muscular sections is shown from diabetic and normoglycemic mice with an antibody for mouse p75NTR (green fluorescence) and with the endothelial marker isolectin-B4 (red fluorescence). Capillary EC which are positive for p75NTR express yellow fluorescence (from merging of the red and green fluorescence) and are pointed by arrows. Representative sections show higher density of p75NTR-expressing capillary EC in the ischemic muscles of diabetics in comparison with normoglycemic mice. (Original magnification: ×400; scale bar: 500 μm.). B–F: inhibition of p75NTR signaling in diabetic limb muscles restores proper neovascularization and BF recovery following limb ischemia. Unilateral limb ischemia was induced in diabetic and normoglycemic mice before an adenovirus carrying a dominant negative mutant of p75NTR (Ad.DN-p75NTR) or Ad.GFP (control) was delivered to the ischemic adductor. B, left: time course of postischemic BF recovery (calculated by laser color Doppler flowmetry) in diabetic and normoglycemic mice treated with Ad.DN-p75NTR (◆, diabetic; ◇, normoglycemic) or Ad.GFP (●, diabetic; ○, normoglycemic). Right: representative laser Doppler images taken at 14 days after induction of ischemia are shown. Squares include the ischemic feet. C: recovery of BF (measured by Oxford Optronic) is expressed as the ratio between the BF to ischemic muscle and the BF to the contralateral muscle at 14 days after ischemia induction. Values are means ± SE. *P < 0.05 vs. normoglycemic mice with Ad.GFP. §P < 0.05 vs. diabetic with Ad.GFP. D: capillary density in the ischemic adductor at 14 days postischemia. Values are means ± SE. **P < 0.01 vs. normoglycemic mice with Ad.GFP. §§P < 0.05 vs. diabetic with Ad.GFP. Apoptosis (revealed by TUNEL assay) (E) and proliferation (revealed by immunhistochemistry for the proliferation antigen MCM-2) (F) of capillary EC in diabetic limb muscles at 14 days postischemia. Values are means ± SE. *P < 0.05 vs. Ad.GFP. [From Caporali et al. (35).]
VII. NEUROTROPHIN-BASED THERAPEUTIC PERSPECTIVES
As illustrated above, NTs can promote reparative neovascularization. Therefore, NTs could be of therapeutic value for ischemic diseases, such as peripheral ischemia and myocardial infarct, which would benefit from strategies able to improve blood flow. Moreover, because of their capacity to promote neuroprotection and neurogenesis together with cardiovascular protection and angiogenesis, we consider NTs particularly appealing for treatment of conditions in which both microangiopathy and neuropathy are pathogenetically involved. This is the case, for example, of the diabetic foot. Loss of nociception, axon-reflex vasodilation, and microangiopathy leading to ischemia contribute to cutaneous ulceration in diabetic subjects. Interestingly, NGF, which is trophic to sensory and sympathetic fibers, is reduced in diabetic nerves, and endogenous NGF deprivation results in hypoalgesia (3, 5). NGF, BDNF, and NT-3 have all been found to be reduced in early diabetic neuropathy skin (3). Recombinant NGF has been tested in a clinical trial of diabetic polyneuropathy, showing promise at the phase II level (8). Unfortunately, the only phase III trial attempted to date showed negative results, which stopped the investigation, even when the trial design had been questioned (8). Nevertheless, cases of diabetic patients with limb ulcers unresponsive to conventional therapies responding positively to topical application of NGF have been described (90). Moreover, NGF-based therapy proved beneficial for stimulating angiogenesis and healing of skin wounds in diabetic mice (95). NGF additionally showed the potential to cure several forms of skin ulcers in humans, such as foot pressure ulcers (144), ischemic skin lesion of the foot in a child with Crush syndrome (48), and chronic vasculitic ulcers associated with rheumatoid arthritis (262). In all these cases, even if it was not specifically assessed, it is possible that NGF-induced neoangiogenesis may have contributed to the healing action of the NT. NGF additionally proved its ability to treat neurotrophic (25, 143) and herpetic corneal ulcer (171).
The diabetic heart is also expected to benefit from NGF supplementation. In fact, the diabetic heart suffers from exaggerated apoptosis of cardiomyocytes and vascular cells (223), which NGF is able to combat (78, 229). Furthermore, a main cause of sudden death in diabetes is silent myocardial ischemia, caused by cardiac sensory nerve impairment. Ieda et al. (118) interestingly demonstrated that NGF is critical for cardiac sensory innervation and that NGF gene therapy to the heart rescues neuropathy in diabetic rats.
Another clinical condition in which we foresee a particular potential for NT-based therapies is erectile dysfunction, where neurogenic and vasculogenic causes are both involved. Diabetes and hypercholesterolemia are possible causes of erectile dysfunctions. NTs and receptors are expressed by penis-projecting neurons of the major pelvic ganglion as well as by penile sensory neurons, which are all involved in erections (110). Angiogenesis gene therapy of the corpus cavernous with VEGF-A and angiopoietin-1 has been recently shown to improve erectile function in hypercholesterolemic rats (227), thus anticipating the possibility that proangiogenic NTs may be ideal drug candidates for treating this disabling condition. In agreement, NT-3 delivery to the cavernous nerve of type 1 diabetic rats significantly enhances intracavernous pressure (16), which may have been partially due to neoangiogenesis under the stimulation of secreted NT-3. Moreover, combined VEGF-A and BDNF improved the erectile response in a nerve-crush rat model (114) and in erectile dysfunction induced by hyperlipidemia (93). Also in these cases, the proangiogenesis action of NTs may have played a role.
NTs are not considered good drug candidates, because of their poor pharmacokinetic behavior and, in case the target is the brain, lack passage across the blood-brain barrier. Moreover, NTs, even if with lower affinity than pro-NTs, can still activate the proapoptotic p75NTR (153, 232). Some of these considerations may in part justify the partial disappointment with the trial using recombinant NGF in diabetic neuropathy (8). Apart from gene therapy or cell-based gene delivery, alternative strategies to the use of NT proteins may consist of using small molecule activators of trks. Recently, cell-permeable small compounds able to activate trkA (but not p75NTR) in the absence of NGF have been developed starting from the screening of libraries of asterriquinones and mono-indolyl-quinones using a 96-well enzyme-linked immunosorbant assay that detects phosphorylated trkA (153). By similar or alternative approaches, small molecule agonists for trkB and trkC could be developed for antiapoptosis and proangiogenesis therapies. The same experimental platform could be also exploited to develop antagonist compounds able to block NT-induced activation of trks. In this case, the obvious therapeutic target would be represented by pathological angiogenesis associated with naturopathic pain and inflammation, such as arthritis and psoriasis, or cancer. In addition, the team led by Longo and Massa (159) has prepared NGF small molecule mimetics, able to act selectively at p75NTR or trkA receptors and activate partially distinct patterns of intracellular signaling cascades, compared with those activated by NGF. Of these molecules, the ones selectively activating trkA may deserve to be tested for the ability to promote angiogenesis in ischemic tissues. Moreover, trk function can also be improved by inhibition of protein-tyrosine phosphatase (279). Conversely, small molecules able to sequestrate NTs, thus impeding interaction with their receptors, could have a scope in pathological angiogenesis. This may be the case for the recombinant domain 5 of trkA (trkAd5), which binds to NGF, thus inhibiting NGF action (61). TrkAd5 showed a therapeutic potential in preclinical models of inflammatory pain and asthma (61). In general, drugs able to block trk signaling at receptorial or postreceptorial level might have a role in inhibiting angiogenesis.
VIII. SUMMARY AND PERSPECTIVES
In this review, we have described the components of the NTs family, showing that mature NTs and proforms of NTs can bind, with different affinities, to two receptor types: the tropomyosin kinase receptors (trks) and the p75NTR. We have reported expression of both types of receptors by cardiovascular cells, which is an essential prerequisite for direct cardiovascular actions of NTs. We have also described the signaling mechanisms downstream of NT receptors. The first hints of the existence of direct cardiovascular actions of NTs stemmed from pioneering work on development of mice with global gene knockout for NT-3, trkC, BDNF, and trkB. These studies, together with more recent data on the role of NTs in cardiovascular development and stem cell differentiation into the angiomyogenic lineage, have been extensively described by this review. Next, we have illustrated the roles of NTs in cardiovascular physiology during adulthood. In developing and adult organisms, endogenous NTs control the growth and maintenance of blood vessels as well as the survival of cardiac myocytes. On the other side, NTs can also participate in pathological events, such as cancer angiogenesis and angiogenesis associated with other situations of chronic inflammation (psoriasis, arthritis, etc.). NTs also contribute to athersclerosis and vascular wall remodeling following intraluminar vascular injury or changes in blood flow. Recent data from our laboratory provide novel evidence that p75NTR induces EC apoptosis and cycle arrest and contributes to diabetes-induced impairment in reparative angiogenesis following induction of peripheral ischemia (35). We have finally discussed potential disease targets that can benefit from therapies based on NTs, NT-mimetic, and enhancers of trk receptor actions to promote cardiovascular protection or, vice versa, trk inhibition or antagonism to arrest pathological angiogenesis.
We hope that this review will serve to encourage additional research in the cardiovascular actions of NTs.
ACKNOWLEDGMENTS
This review contains unpublished data from Dr. Marco Meloni, Dr. Elisabetta Pani, Brunella Cristofaro, and Paola Campagnolo, who are present or past PhD students or post docs at the Experimental Cardiovascular Medicine Division of the Bristol Heart Institute. We thank Prof. Paolo Madeddu and Prof. Saadeh Suleiman (both from our same institute) for revision of the manuscript.
GRANTS C. Emanueli holds a British Heart Foundation (BHF) Basic Science Lectureship (RFLS. RJ4430) and is Reader in Experimental Cardiovascular Medicine at the University of Bristol. A. Caporali is a BHF PhD student (RFLS. SM6266). Their laboratory is a member of the European Vascular Genomic Network of Excellence.
REFERENCES
- 1.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, Walsh K, Sessa WC. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115:2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 3.Anand P. Neurotrophic factors and their receptors in human sensory neuropathies. Prog Brain Res. 2004;146:477–492. doi: 10.1016/S0079-6123(03)46030-5. [DOI] [PubMed] [Google Scholar]
- 4.Anand P, Foley P, Navsaria HA, Sinicropi D, Williams-Chestnut RE, Leigh IM. Nerve growth factor levels in cultured human skin cells: effect of gestation and viral transformation. Neurosci Lett. 1995;184:157–160. doi: 10.1016/0304-3940(94)11195-o. [DOI] [PubMed] [Google Scholar]
- 5.Anand P, Terenghi G, Warner G, Kopelman P, Williams-Chestnut RE, Sinicropi DV. The role of endogenous nerve growth factor in human diabetic neuropathy. Nat Med. 1996;2:703–707. doi: 10.1038/nm0696-703. [DOI] [PubMed] [Google Scholar]
- 6.Andreev J, Galisteo ML, Kranenburg O, Logan SK, Chiu ES, Okigaki M, Cary LA, Moolenaar WH, Schlessinger J. Src and Pyk2 mediate G-protein-coupled receptor activation of epidermal growth factor receptor (EGFR) but are not required for coupling to the mitogen-activated protein (MAP) kinase signaling cascade. J Biol Chem. 2001;276:20130–20135. doi: 10.1074/jbc.M102307200. [DOI] [PubMed] [Google Scholar]
- 7.Aoyama M, Asai K, Shishikura T, Kawamoto T, Miyachi T, Yokoi T, Togari H, Wada Y, Kato T, Nakagawara A. Human neuroblastomas with unfavorable biologies express high levels of brain-derived neurotrophic factor mRNA and a variety of its variants. Cancer Lett. 2001;164:51–60. doi: 10.1016/s0304-3835(00)00715-1. [DOI] [PubMed] [Google Scholar]
- 8.Apfel SC. Nerve growth factor for the treatment of diabetic neuropathy: what went wrong, what went right, and what does the future hold? Int Rev Neurobiol. 2002;50:393–413. doi: 10.1016/s0074-7742(02)50083-0. [DOI] [PubMed] [Google Scholar]
- 9.Arevalo JC, Waite J, Rajagopal R, Beyna M, Chen ZY, Lee FS, Chao MV. Cell survival through Trk neurotrophin receptors is differentially regulated by ubiquitination. Neuron. 2006;50:549–559. doi: 10.1016/j.neuron.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 10.Arevalo JC, Yano H, Teng KK, Chao MV. A unique pathway for sustained neurotrophin signaling through an ankyrin-rich membrane-spanning protein. Embo J. 2004;23:2358–2368. doi: 10.1038/sj.emboj.7600253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 12.Bamji SX, Majdan M, Pozniak CD, Belliveau DJ, Aloyz R, Kohn J, Causing CG, Miller FD. The p75 neurotrophin receptor mediates neuronal apoptosis and is essential for naturally occurring sympathetic neuron death. J Cell Biol. 1998;140:911–923. doi: 10.1083/jcb.140.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbacid M. The Trk family of neurotrophin receptors. J Neurobiol. 1994;25:1386–1403. doi: 10.1002/neu.480251107. [DOI] [PubMed] [Google Scholar]
- 14.Barbacid M, Lamballe F, Pulido D, Klein R. The trk family of tyrosine protein kinase receptors. Biochim Biophys Acta. 1991;1072:115–127. doi: 10.1016/0304-419x(91)90010-i. [DOI] [PubMed] [Google Scholar]
- 15.Benedetti M, Levi A, Chao MV. Differential expression of nerve growth factor receptors leads to altered binding affinity and neurotrophin responsiveness. Proc Natl Acad Sci USA. 1993;90:7859–7863. doi: 10.1073/pnas.90.16.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett NE, Kim JH, Wolfe DP, Sasaki K, Yoshimura N, Goins WF, Huang S, Nelson JB, de Groat WC, Glorioso JC, Chancellor MB. Improvement in erectile dysfunction after neurotrophic factor gene therapy in diabetic rats. J Urol. 2005;173:1820–1824. doi: 10.1097/01.ju.0000158056.66236.1f. [DOI] [PubMed] [Google Scholar]
- 17.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernd P, Miles K, Rozenberg I, Borghjid S, Kirby ML. Neurotrophin-3 and TrkC are expressed in the outflow tract of the developing chicken heart. Dev Dyn. 2004;230:767–772. doi: 10.1002/dvdy.20084. [DOI] [PubMed] [Google Scholar]
- 19.Bhakar AL, Howell JL, Paul CE, Salehi AH, Becker EB, Said F, Bonni A, Barker PA. Apoptosis induced by p75NTR overexpression requires Jun kinase-dependent phosphorylation of Bad. J Neurosci. 2003;23:11373–11381. doi: 10.1523/JNEUROSCI.23-36-11373.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bibel M, Hoppe E, Barde YA. Biochemical and functional interactions between the neurotrophin receptors trk and p75NTR. Embo J. 1999;18:616–622. doi: 10.1093/emboj/18.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bilderback TR, Gazula VR, Dobrowsky RT. Phosphoinositide 3-kinase regulates crosstalk between Trk A tyrosine kinase and p75(NTR)-dependent sphingolipid signaling pathways. J Neurochem. 2001;76:1540–1551. doi: 10.1046/j.1471-4159.2001.00171.x. [DOI] [PubMed] [Google Scholar]
- 22.Bilderback TR, Gazula VR, Lisanti MP, Dobrowsky RT. Caveolin interacts with Trk A and p75(NTR) and regulates neurotrophin signaling pathways. J Biol Chem. 1999;274:257–263. doi: 10.1074/jbc.274.1.257. [DOI] [PubMed] [Google Scholar]
- 23.Bilderback TR, Grigsby RJ, Dobrowsky RT. Association of p75(NTR) with caveolin and localization of neurotrophin-induced sphingomyelin hydrolysis to caveolae. J Biol Chem. 1997;272:10922–10927. doi: 10.1074/jbc.272.16.10922. [DOI] [PubMed] [Google Scholar]
- 24.Birnbaum T, Roider J, Schankin CJ, Padovan CS, Schichor C, Goldbrunner R, Straube A. Malignant gliomas actively recruit bone marrow stromal cells by secreting angiogenic cytokines. J Neurooncol. 2007;83:241–247. doi: 10.1007/s11060-007-9332-4. [DOI] [PubMed] [Google Scholar]
- 25.Bonini S, Lambiase A, Rama P, Caprioglio G, Aloe L. Topical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology. 2000;107:1347–1351. doi: 10.1016/s0161-6420(00)00163-9. [DOI] [PubMed] [Google Scholar]
- 26.Bono F, Lamarche I, Herbert JM. NGF exhibits a pro-apoptotic activity for human vascular smooth muscle cells that is inhibited by TGFbeta1. FEBS Lett. 1997;416:243–246. doi: 10.1016/s0014-5793(97)01215-5. [DOI] [PubMed] [Google Scholar]
- 27.Bredesen DE, Mehlen P, Rabizadeh S. Apoptosis and dependence receptors: a molecular basis for cellular addiction. Physiol Rev. 2004;84:411–430. doi: 10.1152/physrev.00027.2003. [DOI] [PubMed] [Google Scholar]
- 28.Bronfman FC, Tcherpakov M, Jovin TM, Fainzilber M. Ligand-induced internalization of the p75 neurotrophin receptor: a slow route to the signaling endosome. J Neurosci. 2003;23:3209–3220. doi: 10.1523/JNEUROSCI.23-08-03209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruno MA, Cuello AC. Activity-dependent release of precursor nerve growth factor, conversion to mature nerve growth factor, and its degradation by a protease cascade. Proc Natl Acad Sci USA. 2006;103:6735–6740. doi: 10.1073/pnas.0510645103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butte MJ, Hwang PK, Mobley WC, Fletterick RJ. Crystal structure of neurotrophin-3 homodimer shows distinct regions are used to bind its receptors. Biochemistry. 1998;37:16846–16852. doi: 10.1021/bi981254o. [DOI] [PubMed] [Google Scholar]
- 31.Cai D, Holm JM, Duignan IJ, Zheng J, Xaymardan M, Chin A, Ballard VL, Bella JN, Edelberg JM. BDNF-mediated enhancement of inflammation and injury in the aging heart. Physiol Gen. 2006;24:191–197. doi: 10.1152/physiolgenomics.00165.2005. [DOI] [PubMed] [Google Scholar]
- 32.Calatozzolo C, Salmaggi A, Pollo B, Sciacca FL, Lorenzetti M, Franzini A, Boiardi A, Broggi G, Marras C. Expression of cannabinoid receptors and neurotrophins in human gliomas. Neurol Sci. 2007;28:304–310. doi: 10.1007/s10072-007-0843-8. [DOI] [PubMed] [Google Scholar]
- 33.Calza L, Giardino L, Giuliani A, Aloe L, Levi-Montalcini R. Nerve growth factor control of neuronal expression of angiogenetic and vasoactive factors. Proc Natl Acad Sci USA. 2001;98:4160–4165. doi: 10.1073/pnas.051626998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cantarella G, Lempereur L, Presta M, Ribatti D, Lombardo G, Lazarovici P, Zappala G, Pafumi C, Bernardini R. Nerve growth factor-endothelial cell interaction leads to angiogenesis in vitro and in vivo. FASEB J. 2002;16:1307–1309. doi: 10.1096/fj.01-1000fje. [DOI] [PubMed] [Google Scholar]
- 35.Caporali A, Pani E, Horrevoets AJ, Kraenkel N, Oikawa A, Sala-Newby GB, Meloni M, Cristofaro B, Graiani G, Leroyer AS, Boulanger CM, Spinetti G, Yoon SO, Madeddu P, Emanueli C. Neurotrophin p75 receptor (p75NTR) promotes endothelial cell apoptosis and inhibits angiogenesis. Implications for diabetes-induced impaired neovascularization in ischemic limb muscles. Circ Res. doi: 10.1161/CIRCRESAHA.108.177386. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caporali A, Sala-Newby GB, Meloni M, Graiani G, Pani E, Cristofaro B, Newby AC, Madeddu P, Emanueli C. Identification of the prosurvival activity of nerve growth factor on cardiac myocytes. Cell Death Differ. 2008;15:299–311. doi: 10.1038/sj.cdd.4402263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Capsoni S, Ruberti F, Di Daniel E, Cattaneo A. Muscular dystrophy in adult and aged anti-NGF transgenic mice resembles an inclusion body myopathy. J Neurosci Res. 2000;59:553–560. doi: 10.1002/(SICI)1097-4547(20000215)59:4<553::AID-JNR11>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 38.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 39.Carter BD, Kaltschmidt C, Kaltschmidt B, Offenhauser N, Bohm-Matthaei R, Baeuerle PA, Barde YA. Selective activation of NF-kappa B by nerve growth factor through the neurotrophin receptor p75. Science. 1996;272:542–545. doi: 10.1126/science.272.5261.542. [DOI] [PubMed] [Google Scholar]
- 40.Casaccia-Bonnefil P, Carter BD, Dobrowsky RT, Chao MV. Death of oligodendrocytes mediated by the interaction of nerve growth factor with its receptor p75. Nature. 1996;383:716–719. doi: 10.1038/383716a0. [DOI] [PubMed] [Google Scholar]
- 41.Casademunt E, Carter BD, Benzel I, Frade JM, Dechant G, Barde YA. The zinc finger protein NRIF interacts with the neurotrophin receptor p75(NTR) and participates in programmed cell death. Embo J. 1999;18:6050–6061. doi: 10.1093/emboj/18.21.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Catalucci D, Condorelli G. Effects of Akt on cardiac myocytes: location counts. Circ Res. 2006;99:339–341. doi: 10.1161/01.RES.0000239409.90634.a9. [DOI] [PubMed] [Google Scholar]
- 43.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 44.Chao MV, Bothwell M. Neurotrophins: to cleave or not to cleave. Neuron. 2002;33:9–12. doi: 10.1016/s0896-6273(01)00573-6. [DOI] [PubMed] [Google Scholar]
- 45.Chao MV, Hempstead BL. p75 and Trk: a two-receptor system. Trends Neurosci. 1995;18:321–326. [PubMed] [Google Scholar]
- 46.Chen J, Zacharek A, Zhang C, Jiang H, Li Y, Roberts C, Lu M, Kapke A, Chopp M. Endothelial nitric oxide synthase regulates brain-derived neurotrophic factor expression and neurogenesis after stroke in mice. J Neurosci. 2005;25:2366–2375. doi: 10.1523/JNEUROSCI.5071-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, Katakowski M, Lu M, Chopp M. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiaretti A, Piastra M, Caresta E, Nanni L, Aloe L. Improving ischaemic skin revascularisation by nerve growth factor in a child with crush syndrome. Arch Dis Child. 2002;87:446–448. doi: 10.1136/adc.87.5.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chittka A, Arevalo JC, Rodriguez-Guzman M, Perez P, Chao MV, Sendtner M. The p75NTR-interacting protein SC1 inhibits cell cycle progression by transcriptional repression of cyclin E. J Cell Biol. 2004;164:985–996. doi: 10.1083/jcb.200301106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chittka A, Chao MV. Identification of a zinc finger protein whose subcellular distribution is regulated by serum and nerve growth factor. Proc Natl Acad Sci USA. 1999;96:10705–10710. doi: 10.1073/pnas.96.19.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clary DO, Reichardt LF. An alternatively spliced form of the nerve growth factor receptor TrkA confers an enhanced response to neurotrophin 3. Proc Natl Acad Sci USA. 1994;91:11133–11137. doi: 10.1073/pnas.91.23.11133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohen S, Levi-Montalcini R, Hamburger V. A nerve growth-stimulating factor isolated from sarcomas 37 and 180. Proc Natl Acad Sci USA. 1954;40:1014–1018. doi: 10.1073/pnas.40.10.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coppola V, Barrick CA, Southon EA, Celeste A, Wang K, Chen B, Haddad el B, Yin J, Nussenzweig A, Subramaniam A, Tessarollo L. Ablation of TrkA function in the immune system causes B cell abnormalities. Development. 2004;131:5185–5195. doi: 10.1242/dev.01383. [DOI] [PubMed] [Google Scholar]
- 54.Corbit KC, Foster DA, Rosner MR. Protein kinase Cdelta mediates neurogenic but not mitogenic activation of mitogen-activated protein kinase in neuronal cells. Mol Cell Biol. 1999;19:4209–4218. doi: 10.1128/mcb.19.6.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 56.Coulson EJ, Reid K, Baca M, Shipham KA, Hulett SM, Kilpatrick TJ, Bartlett PF. Chopper, a new death domain of the p75 neurotrophin receptor that mediates rapid neuronal cell death. J Biol Chem. 2000;275:30537–30545. doi: 10.1074/jbc.M005214200. [DOI] [PubMed] [Google Scholar]
- 57.Creamer D, Sullivan D, Bicknell R, Barker J. Angiogenesis in psoriasis. Angiogenesis. 2002;5:231–236. doi: 10.1023/a:1024515517623. [DOI] [PubMed] [Google Scholar]
- 58.Crowley C, Spencer SD, Nishimura MC, Chen KS, Pitts-Meek S, Armanini MP, Ling LH, McMahon SB, Shelton DL, Levinson AD. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 1994;76:1001–1011. doi: 10.1016/0092-8674(94)90378-6. [DOI] [PubMed] [Google Scholar]
- 59.Daub H, Weiss FU, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 60.Davidson B, Reich R, Lazarovici P, Nesland JM, Skrede M, Risberg B, Trope CG, Florenes VA. Expression and activation of the nerve growth factor receptor TrkA in serous ovarian carcinoma. Clin Cancer Res. 2003;9:2248–2259. [PubMed] [Google Scholar]
- 61.Dawbarn D, Fahey M, Watson J, Tyler S, Shoemark D, Sessions R, Zhang R, Brady L, Willis C, Allen SJ. NGF receptor TrkAd5: therapeutic agent and drug design target. Biochem Soc Trans. 2006;34:587–590. doi: 10.1042/BST0340587. [DOI] [PubMed] [Google Scholar]
- 62.De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 63.Dempsey PW, Doyle SE, He JQ, Cheng G. The signaling adaptors and pathways activated by TNF superfamily. Cytokine Growth Factor Rev. 2003;14:193–209. doi: 10.1016/s1359-6101(03)00021-2. [DOI] [PubMed] [Google Scholar]
- 64.Descamps S, Toillon RA, Adriaenssens E, Pawlowski V, Cool SM, Nurcombe V, Le Bourhis X, Boilly B, Peyrat JP, Hondermarck H. Nerve growth factor stimulates proliferation and survival of human breast cancer cells through two distinct signaling pathways. J Biol Chem. 2001;276:17864–17870. doi: 10.1074/jbc.M010499200. [DOI] [PubMed] [Google Scholar]
- 65.Djonov V, Schmid M, Tschanz SA, Burri PH. Intussusceptive angiogenesis: its role in embryonic vascular network formation. Circ Res. 2000;86:286–292. doi: 10.1161/01.res.86.3.286. [DOI] [PubMed] [Google Scholar]
- 66.Dobrowsky RT, Jenkins GM, Hannun YA. Neurotrophins induce sphingomyelin hydrolysis. Modulation by co-expression of p75NTR with Trk receptors. J Biol Chem. 1995;270:22135–22142. doi: 10.1074/jbc.270.38.22135. [DOI] [PubMed] [Google Scholar]
- 67.Dobrowsky RT, Werner MH, Castellino AM, Chao MV, Hannun YA. Activation of the sphingomyelin cycle through the low-affinity neurotrophin receptor. Science. 1994;265:1596–1599. doi: 10.1126/science.8079174. [DOI] [PubMed] [Google Scholar]
- 68.Dolle JP, Rezvan A, Allen FD, Lazarovici P, Lelkes PI. Nerve growth factor-induced migration of endothelial cells. J Pharmacol Exp Ther. 2005;315:1220–1227. doi: 10.1124/jpet.105.093252. [DOI] [PubMed] [Google Scholar]
- 69.Donovan MJ, Hahn R, Tessarollo L, Hempstead BL. Identification of an essential nonneuronal function of neurotrophin 3 in mammalian cardiac development. Nat Genet. 1996;14:210–213. doi: 10.1038/ng1096-210. [DOI] [PubMed] [Google Scholar]
- 70.Donovan MJ, Lin MI, Wiegn P, Ringstedt T, Kraemer R, Hahn R, Wang S, Ibanez CF, Rafii S, Hempstead BL. Brain derived neurotrophic factor is an endothelial cell survival factor required for intramyocardial vessel stabilization. Development. 2000;127:4531–4540. doi: 10.1242/dev.127.21.4531. [DOI] [PubMed] [Google Scholar]
- 71.Donovan MJ, Miranda RC, Kraemer R, McCaffrey TA, Tessarollo L, Mahadeo D, Sharif S, Kaplan DR, Tsoulfas P, Parada L. Neurotrophin and neurotrophin receptors in vascular smooth muscle cells Regulation of expression in response to injury. Am J Pathol. 1995;147:309–324. [PMC free article] [PubMed] [Google Scholar]
- 72.Duffy M, McGowan P, Gallagher W. Cancer invasion and metastasis: changing views. J Pathol. 2007 doi: 10.1002/path.2282. [DOI] [PubMed] [Google Scholar]
- 73.Ebendal T, Belew M, Jacobson CO, Porath J. Neurite outgrowth elicited by embryonic chick heart: partial purification of the active factor. Neurosci Lett. 1979;14:91–95. doi: 10.1016/0304-3940(79)95350-3. [DOI] [PubMed] [Google Scholar]
- 74.Eggert A, Grotzer MA, Ikegaki N, Liu XG, Evans AE, Brodeur GM. Expression of the neurotrophin receptor TrkA down-regulates expression and function of angiogenic stimulators in SH-SY5Y neuroblastoma cells. Cancer Res. 2002;62:1802–1808. [PubMed] [Google Scholar]
- 75.Eide FF, Vining ER, Eide BL, Zang K, Wang XY, Reichardt LF. Naturally occurring truncated trkB receptors have dominant inhibitory effects on brain-derived neurotrophic factor signaling. J Neurosci. 1996;16:3123–3129. doi: 10.1523/JNEUROSCI.16-10-03123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ejiri J, Inoue N, Kobayashi S, Shiraki R, Otsui K, Honjo T, Takahashi M, Ohashi Y, Ichikawa S, Terashima M, Mori T, Awano K, Shinke T, Shite J, Hirata K, Yokozaki H, Kawashima S, Yokoyama M. Possible role of brain-derived neurotrophic factor in the pathogenesis of coronary artery disease. Circulation. 2005;112:2114–2120. doi: 10.1161/CIRCULATIONAHA.104.476903. [DOI] [PubMed] [Google Scholar]
- 77.El Yazidi-Belkoura I, Adriaenssens E, Dolle L, Descamps S, Hondermarck H. Tumor necrosis factor receptor-associated death domain protein is involved in the neurotrophin receptor-mediated antiapoptotic activity of nerve growth factor in breast cancer cells. J Biol Chem. 2003;278:16952–16956. doi: 10.1074/jbc.M300631200. [DOI] [PubMed] [Google Scholar]
- 78.Emanueli C, Salis MB, Pinna A, Graiani G, Manni L, Madeddu P. Nerve growth factor promotes angiogenesis and arteriogenesis in ischemic hindlimbs. Circulation. 2002;106:2257–2262. doi: 10.1161/01.cir.0000033971.56802.c5. [DOI] [PubMed] [Google Scholar]
- 79.English J, Pearson G, Wilsbacher J, Swantek J, Karandikar M, Xu S, Cobb MH. New insights into the control of MAP kinase pathways. Exp Cell Res. 1999;253:255–270. doi: 10.1006/excr.1999.4687. [DOI] [PubMed] [Google Scholar]
- 80.Esposito D, Patel P, Stephens RM, Perez P, Chao MV, Kaplan DR, Hempstead BL. The cytoplasmic and transmembrane domains of the p75 and Trk A receptors regulate high affinity binding to nerve growth factor. J Biol Chem. 2001;276:32687–32695. doi: 10.1074/jbc.M011674200. [DOI] [PubMed] [Google Scholar]
- 81.Fahnestock M, Abdalati W, Joughin I, Brozena J, Gogineni P. High geothermal heat flow, basal melt, and the origin of rapid ice flow in central Greenland. Science. 2001;294:2338–2342. doi: 10.1126/science.1065370. [DOI] [PubMed] [Google Scholar]
- 82.Fanburg-Smith JC, Miettinen M. Low-affinity nerve growth factor receptor (p75) in dermatofibrosarcoma protuberans and other nonneural tumors: a study of 1,150 tumors and fetal and adult normal tissues. Hum Pathol. 2001;32:976–983. doi: 10.1053/hupa.2001.27602. [DOI] [PubMed] [Google Scholar]
- 83.Folkman J. Fundamental concepts of the angiogenic process. Curr Mol Med. 2003;3:643–651. doi: 10.2174/1566524033479465. [DOI] [PubMed] [Google Scholar]
- 84.Frade JM. Nuclear translocation of the p75 neurotrophin receptor cytoplasmic domain in response to neurotrophin binding. J Neurosci. 2005;25:1407–1411. doi: 10.1523/JNEUROSCI.3798-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Frank PG, Hassan GS, Rodriguez-Feo JA, Lisanti MP. Caveolae and caveolin-1: novel potential targets for the treatment of cardiovascular disease. Curr Pharm Des. 2007;13:1761–1769. doi: 10.2174/138161207780831202. [DOI] [PubMed] [Google Scholar]
- 86.Friedman WJ. Neurotrophins induce death of hippocampal neurons via the p75 receptor. J Neurosci. 2000;20:6340–6346. doi: 10.1523/JNEUROSCI.20-17-06340.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Geetha T, Jiang J, Wooten MW. Lysine 63 polyubiquitination of the nerve growth factor receptor TrkA directs internalization and signaling. Mol Cell. 2005;20:301–312. doi: 10.1016/j.molcel.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 89.Gehling UM, Ergun S, Schumacher U, Wagener C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N, Kluge K, Schafer B, Hossfeld DK, Fiedler W. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95:3106–3112. [PubMed] [Google Scholar]
- 90.Generini S, Tuveri MA, Cerinic M Matucci, Mastinu F, Manni L, Aloe L. Topical application of nerve growth factor in human diabetic foot ulcers. A study of three cases. Exp Clin Endocrinol Diabetes. 2004;112:542–544. doi: 10.1055/s-2004-821313. [DOI] [PubMed] [Google Scholar]
- 91.Gentry JJ, Casaccia-Bonnefil P, Carter BD. Nerve growth factor activation of nuclear factor kappaB through its p75 receptor is an antiapoptotic signal in RN22 schwannoma cells. J Biol Chem. 2000;275:7558–7565. doi: 10.1074/jbc.275.11.7558. [DOI] [PubMed] [Google Scholar]
- 92.Gentry JJ, Rutkoski NJ, Burke TL, Carter BD. A functional interaction between the p75 neurotrophin receptor interacting factors, TRAF6 and NRIF. J Biol Chem. 2004;279:16646–16656. doi: 10.1074/jbc.M309209200. [DOI] [PubMed] [Google Scholar]
- 93.Gholami SS, Rogers R, Chang J, Ho HC, Grazziottin T, Lin CS, Lue TF. The effect of vascular endothelial growth factor and adeno-associated virus mediated brain derived neurotrophic factor on neurogenic and vasculogenic erectile dysfunction induced by hyperlipidemia. J Urol. 2003;169:1577–1581. doi: 10.1097/01.ju.0000055120.73261.76. [DOI] [PubMed] [Google Scholar]
- 94.Gotz R, Koster R, Winkler C, Raulf F, Lottspeich F, Schartl M, Thoenen H. Neurotrophin-6 is a new member of the nerve growth factor family. Nature. 1994;372:266–269. doi: 10.1038/372266a0. [DOI] [PubMed] [Google Scholar]
- 95.Graiani G, Emanueli C, Desortes E, Van Linthout S, Pinna A, Figueroa CD, Manni L, Madeddu P. Nerve growth factor promotes reparative angiogenesis and inhibits endothelial apoptosis in cutaneous wounds of Type 1 diabetic mice. Diabetologia. 2004;47:1047–1054. doi: 10.1007/s00125-004-1414-7. [DOI] [PubMed] [Google Scholar]
- 96.Grewal SS, York RD, Stork PJ. Extracellular-signal-regulated kinase signalling in neurons. Curr Opin Neurobiol. 1999;9:544–553. doi: 10.1016/S0959-4388(99)00010-0. [DOI] [PubMed] [Google Scholar]
- 97.Gutierrez-Fernandez A, Parmer RJ, Miles LA. Plasminogen gene expression is regulated by nerve growth factor. J Thromb Haemost. 2007;5:1715–1725. doi: 10.1111/j.1538-7836.2007.02636.x. [DOI] [PubMed] [Google Scholar]
- 98.Hagag N, Halegoua S, Viola M. Inhibition of growth factor-induced differentiation of PC12 cells by microinjection of antibody to ras p21. Nature. 1986;319:680–682. doi: 10.1038/319680a0. [DOI] [PubMed] [Google Scholar]
- 99.Hallbook F, Ibanez CF, Persson H. Evolutionary studies of the nerve growth factor family reveal a novel member abundantly expressed in Xenopus ovary. Neuron. 1991;6:845–858. doi: 10.1016/0896-6273(91)90180-8. [DOI] [PubMed] [Google Scholar]
- 100.Halliday DA, Zettler C, Rush RA, Scicchitano R, McNeil JD. Elevated nerve growth factor levels in the synovial fluid of patients with inflammatory joint disease. Neurochem Res. 1998;23:919–922. doi: 10.1023/a:1022475432077. [DOI] [PubMed] [Google Scholar]
- 101.Hansen-Algenstaedt N, Algenstaedt P, Schaefer C, Hamann A, Wolfram L, Cingoz G, Kilic N, Schwarzloh B, Schroeder M, Joscheck C, Wiesner L, Ruther W, Ergun S. Neural driven angiogenesis by overexpression of nerve growth factor. Histochem Cell Biol. 2006;125:637–649. doi: 10.1007/s00418-005-0111-z. [DOI] [PubMed] [Google Scholar]
- 102.Harrington AW, Kim JY, Yoon SO. Activation of Rac GTPase by p75 is necessary for c-jun N-terminal kinase-mediated apoptosis. J Neurosci. 2002;22:156–166. doi: 10.1523/JNEUROSCI.22-01-00156.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hasan J, Shnyder SD, Bibby M, Double JA, Bicknel R, Jayson GC. Quantitative angiogenesis assays in vivo–a review. Angiogenesis. 2004;7:1–16. doi: 10.1023/B:AGEN.0000037338.51851.d1. [DOI] [PubMed] [Google Scholar]
- 104.Hassankhani A, Steinhelper ME, Soonpaa MH, Katz EB, Taylor DA, Andrade-Rozental A, Factor SM, Steinberg JJ, Field LJ, Federoff HJ. Overexpression of NGF within the heart of transgenic mice causes hyperinnervation, cardiac enlargement, and hyperplasia of ectopic cells. Dev Biol. 1995;169:309–321. doi: 10.1006/dbio.1995.1146. [DOI] [PubMed] [Google Scholar]
- 105.He XL, Garcia KC. Structure of nerve growth factor complexed with the shared neurotrophin receptor p75. Science. 2004;304:870–875. doi: 10.1126/science.1095190. [DOI] [PubMed] [Google Scholar]
- 106.Heath BM, Xia J, Dong E, An RH, Brooks A, Liang C, Federoff HJ, Kass RS. Overexpression of nerve growth factor in the heart alters ion channel activity and beta-adrenergic signalling in an adult transgenic mouse. J Physiol. 1998;512:779–791. doi: 10.1111/j.1469-7793.1998.779bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Heil M, Schaper W. Insights into pathways of arteriogenesis. Curr Pharm Biotechnol. 2007;8:35–42. doi: 10.2174/138920107779941408. [DOI] [PubMed] [Google Scholar]
- 108.Hempstead BL, Martin-Zanca D, Kaplan DR, Parada LF, Chao MV. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature. 1991;350:678–683. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
- 109.Hiltunen JO, Arumae U, Moshnyakov M, Saarma M. Expression of mRNAs for neurotrophins and their receptors in developing rat heart. Circ Res. 1996;79:930–939. doi: 10.1161/01.res.79.5.930. [DOI] [PubMed] [Google Scholar]
- 110.Hiltunen JO, Laurikainen A, Klinge E, Saarma M. Neurotrophin-3 is a target-derived neurotrophic factor for penile erection-inducing neurons. Neuroscience. 2005;133:51–58. doi: 10.1016/j.neuroscience.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 111.Hofer MM, Barde YA. Brain-derived neurotrophic factor prevents neuronal death in vivo. Nature. 1988;331:261–262. doi: 10.1038/331261a0. [DOI] [PubMed] [Google Scholar]
- 112.Hohn A, Leibrock J, Bailey K, Barde YA. Identification and characterization of a novel member of the nerve growth factor/brain-derived neurotrophic factor family. Nature. 1990;344:339–341. doi: 10.1038/344339a0. [DOI] [PubMed] [Google Scholar]
- 113.Holgado-Madruga M, Moscatello DK, Emlet DR, Dieterich R, Wong AJ. Grb2-associated binder-1 mediates phosphatidylinositol 3-kinase activation and the promotion of cell survival by nerve growth factor. Proc Natl Acad Sci USA. 1997;94:12419–12424. doi: 10.1073/pnas.94.23.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hsieh PS, Bochinski DJ, Lin GT, Nunes L, Lin CS, Lue TF. The effect of vascular endothelial growth factor and brain-derived neurotrophic factor on cavernosal nerve regeneration in a nerve-crush rat model. BJU Int. 2003;92:470–475. doi: 10.1046/j.1464-410x.2003.04373.x. [DOI] [PubMed] [Google Scholar]
- 115.Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 116.Humpert PM, Kopf S, Djuric Z, Laine K, Korosoglou G, Rudofsky G, Jr, Hamann A, Morcos M, von Eynatten M, Nawroth PP, Bierhaus A. Levels of three distinct p75 neurotrophin receptor forms found in human plasma are altered in type 2 diabetic patients. Diabetologia. 2007;50:1517–1522. doi: 10.1007/s00125-007-0683-3. [DOI] [PubMed] [Google Scholar]
- 117.Ieda M, Fukuda K, Hisaka Y, Kimura K, Kawaguchi H, Fujita J, Shimoda K, Takeshita E, Okano H, Kurihara Y, Kurihara H, Ishida J, Fukamizu A, Federoff HJ, Ogawa S. Endothelin-1 regulates cardiac sympathetic innervation in the rodent heart by controlling nerve growth factor expression. J Clin Invest. 2004;113:876–884. doi: 10.1172/JCI19480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ieda M, Kanazawa H, Ieda Y, Kimura K, Matsumura K, Tomita Y, Yagi T, Onizuka T, Shimoji K, Ogawa S, Makino S, Sano M, Fukuda K. Nerve growth factor is critical for cardiac sensory innervation and rescues neuropathy in diabetic hearts. Circulation. 2006;114:2351–2363. doi: 10.1161/CIRCULATIONAHA.106.627588. [DOI] [PubMed] [Google Scholar]
- 119.Iglesias T, Cabrera-Poch N, Mitchell MP, Naven TJ, Rozengurt E, Schiavo G. Identification and cloning of Kidins220, a novel neuronal substrate of protein kinase D. J Biol Chem. 2000;275:40048–40056. doi: 10.1074/jbc.M005261200. [DOI] [PubMed] [Google Scholar]
- 120.Ip NY, Ibanez CF, Nye SH, McClain J, Jones PF, Gies DR, Belluscio L, Le Beau MM, Espinosa R, 3rd, Squinto SP. Mammalian neurotrophin-4: structure, chromosomal localization, tissue distribution, and receptor specificity. Proc Natl Acad Sci USA. 1992;89:3060–3064. doi: 10.1073/pnas.89.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 122.Jansen P, Giehl K, Nyengaard JR, Teng K, Lioubinski O, Sjoegaard SS, Breiderhoff T, Gotthardt M, Lin F, Eilers A, Petersen CM, Lewin GR, Hempstead BL, Willnow TE, Nykjaer A. Roles for the pro-neurotrophin receptor sortilin in neuronal development, aging and brain injury. Nat Neurosci. 2007;10:1449–1457. doi: 10.1038/nn2000. [DOI] [PubMed] [Google Scholar]
- 123.Johnson D, Lanahan A, Buck CR, Sehgal A, Morgan C, Mercer E, Bothwell M, Chao M. Expression and structure of the human NGF receptor. Cell. 1986;47:545–554. doi: 10.1016/0092-8674(86)90619-7. [DOI] [PubMed] [Google Scholar]
- 124.Jones KR, Reichardt LF. Molecular cloning of a human gene that is a member of the nerve growth factor family. Proc Natl Acad Sci USA. 1990;87:8060–8064. doi: 10.1073/pnas.87.20.8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kanning KC, Hudson M, Amieux PS, Wiley JC, Bothwell M, Schecterson LC. Proteolytic processing of the p75 neurotrophin receptor and two homologs generates C-terminal fragments with signaling capability. J Neurosci. 2003;23:5425–5436. doi: 10.1523/JNEUROSCI.23-13-05425.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kao S, Jaiswal RK, Kolch W, Landreth GE. Identification of the mechanisms regulating the differential activation of the mapk cascade by epidermal growth factor and nerve growth factor in PC12 cells. J Biol Chem. 2001;276:18169–18177. doi: 10.1074/jbc.M008870200. [DOI] [PubMed] [Google Scholar]
- 127.Kaplan DR, Hempstead BL, Martin-Zanca D, Chao MV, Parada LF. The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science. 1991;252:554–558. doi: 10.1126/science.1850549. [DOI] [PubMed] [Google Scholar]
- 128.Kawaguchi-Manabe H, Ieda M, Kimura K, Manabe T, Miyatake S, Kanazawa H, Kawakami T, Ogawa S, Suematsu M, Fukuda K. A novel cardiac hypertrophic factor, neurotrophin-3, is paradoxically downregulated in cardiac hypertrophy. Life Sci. 2007;81:385–392. doi: 10.1016/j.lfs.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 129.Kenchappa RS, Zampieri N, Chao MV, Barker PA, Teng HK, Hempstead BL, Carter BD. Ligand-dependent cleavage of the P75 neurotrophin receptor is necessary for NRIF nuclear translocation and apoptosis in sympathetic neurons. Neuron. 2006;50:219–232. doi: 10.1016/j.neuron.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 130.Kendall SE, Goldhawk DE, Kubu C, Barker PA, Verdi JM. Expression analysis of a novel p75(NTR) signaling protein, which regulates cell cycle progression and apoptosis. Mech Dev. 2002;117:187–200. doi: 10.1016/s0925-4773(02)00204-6. [DOI] [PubMed] [Google Scholar]
- 131.Kermani P, Rafii D, Jin DK, Whitlock P, Schaffer W, Chiang A, Vincent L, Friedrich M, Shido K, Hackett NR, Crystal RG, Rafii S, Hempstead BL. Neurotrophins promote revascularization by local recruitment of TrkB+ endothelial cells and systemic mobilization of hematopoietic progenitors. J Clin Invest. 2005;115:653–663. doi: 10.1172/JCI200522655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Khan KM, Falcone DJ, Kraemer R. Nerve growth factor activation of Erk-1 and Erk-2 induces matrix metalloproteinase-9 expression in vascular smooth muscle cells. J Biol Chem. 2002;277:2353–2359. doi: 10.1074/jbc.M108989200. [DOI] [PubMed] [Google Scholar]
- 133.Khong TL, Larsen H, Raatz Y, Paleolog E. Angiogenesis as a therapeutic target in arthritis: learning the lessons of the colorectal cancer experience. Angiogenesis. 2007;10:243–258. doi: 10.1007/s10456-007-9081-1. [DOI] [PubMed] [Google Scholar]
- 134.Khursigara G, Orlinick JR, Chao MV. Association of the p75 neurotrophin receptor with TRAF6. J Biol Chem. 1999;274:2597–2600. doi: 10.1074/jbc.274.5.2597. [DOI] [PubMed] [Google Scholar]
- 135.Kim H, Li Q, Hempstead BL, Madri JA. Paracrine and autocrine functions of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) in brain-derived endothelial cells. J Biol Chem. 2004;279:33538–33546. doi: 10.1074/jbc.M404115200. [DOI] [PubMed] [Google Scholar]
- 136.Kimura MT, Irie S, Shoji-Hoshino S, Mukai J, Nadano D, Oshimura M, Sato TA. 14–3-3 is involved in p75 neurotrophin receptor-mediated signal transduction. J Biol Chem. 2001;276:17291–17300. doi: 10.1074/jbc.M005453200. [DOI] [PubMed] [Google Scholar]
- 137.Kishibe K, Yamada Y, Ogawa K. Production of nerve growth factor by mouse hepatocellular carcinoma cells and expression of TrkA in tumor-associated arteries in mice. Gastroenterology. 2002;122:1978–1986. doi: 10.1053/gast.2002.33581. [DOI] [PubMed] [Google Scholar]
- 138.Klein R, Jing SQ, Nanduri V, O’Rourke E, Barbacid M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell. 1991;65:189–197. doi: 10.1016/0092-8674(91)90419-y. [DOI] [PubMed] [Google Scholar]
- 139.Kraemer R. Reduced apoptosis and increased lesion development in the flow-restricted carotid artery of p75(NTR)-null mutant mice. Circ Res. 2002;91:494–500. doi: 10.1161/01.res.0000035245.83233.2a. [DOI] [PubMed] [Google Scholar]
- 140.Kraemer R, Baker PJ, Kent KC, Ye Y, Han JJ, Tejada R, Silane M, Upmacis R, Deeb R, Chen Y, Levine DM, Hempstead B. Decreased neurotrophin TrkB receptor expression reduces lesion size in the apolipoprotein E-null mutant mouse. Circulation. 2005;112:3644–3653. doi: 10.1161/CIRCULATIONAHA.105.587980. [DOI] [PubMed] [Google Scholar]
- 141.Kraemer R, Nguyen H, March KL, Hempstead B. NGF activates similar intracellular signaling pathways in vascular smooth muscle cells as PDGF-BB but elicits different biological responses. Arterioscler Thromb Vasc Biol. 1999;19:1041–1050. doi: 10.1161/01.atv.19.4.1041. [DOI] [PubMed] [Google Scholar]
- 142.Labouyrie E, Dubus P, Groppi A, Mahon FX, Ferrer J, Parrens M, Reiffers J, de Mascarel A, Merlio JP. Expression of neurotrophins and their receptors in human bone marrow. Am J Pathol. 1999;154:405–415. doi: 10.1016/S0002-9440(10)65287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lambiase A, Rama P, Bonini S, Caprioglio G, Aloe L. Topical treatment with nerve growth factor for corneal neurotrophic ulcers. N Engl J Med. 1998;338:1174–1180. doi: 10.1056/NEJM199804233381702. [DOI] [PubMed] [Google Scholar]
- 144.Landi F, Aloe L, Russo A, Cesari M, Onder G, Bonini S, Carbonin PU, Bernabei R. Topical treatment of pressure ulcers with nerve growth factor: a randomized clinical trial. Ann Intern Med. 2003;139:635–641. doi: 10.7326/0003-4819-139-8-200310210-00006. [DOI] [PubMed] [Google Scholar]
- 145.Lazarovici P, Gazit A, Staniszewska I, Marcinkiewicz C, Lelkes PI. Nerve growth factor (NGF) promotes angiogenesis in the quail chorioallantoic membrane. Endothelium. 2006;13:51–59. doi: 10.1080/10623320600669053. [DOI] [PubMed] [Google Scholar]
- 146.Lee FS, Chao MV. Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proc Natl Acad Sci USA. 2001;98:3555–3560. doi: 10.1073/pnas.061020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lee FS, Rajagopal R, Kim AH, Chang PC, Chao MV. Activation of Trk neurotrophin receptor signaling by pituitary adenylate cyclase-activating polypeptides. J Biol Chem. 2002;277:9096–9102. doi: 10.1074/jbc.M107421200. [DOI] [PubMed] [Google Scholar]
- 148.Lee KF, Li E, Huber LJ, Landis SC, Sharpe AH, Chao MV, Jaenisch R. Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell. 1992;69:737–749. doi: 10.1016/0092-8674(92)90286-l. [DOI] [PubMed] [Google Scholar]
- 149.Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- 150.Levenberg S, Burdick JA, Kraehenbuehl T, Langer R. Neurotrophin-induced differentiation of human embryonic stem cells on three-dimensional polymeric scaffolds. Tissue Eng. 2005;11:506–512. doi: 10.1089/ten.2005.11.506. [DOI] [PubMed] [Google Scholar]
- 151.Levi-Montalcini R, Hamburger V. Selective growth stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J Exp Zool. 1951;116:321–361. doi: 10.1002/jez.1401160206. [DOI] [PubMed] [Google Scholar]
- 152.Liepinsh E, Ilag LL, Otting G, Ibanez CF. NMR structure of the death domain of the p75 neurotrophin receptor. Embo J. 1997;16:4999–5005. doi: 10.1093/emboj/16.16.4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lin B, Pirrung MC, Deng L, Li Z, Liu Y, Webster NJ. Neuroprotection by small molecule activators of the nerve growth factor receptor. J Pharmacol Exp Ther. 2007;322:59–69. doi: 10.1124/jpet.106.118034. [DOI] [PubMed] [Google Scholar]
- 154.Lin MI, Das I, Schwartz GM, Tsoulfas P, Mikawa T, Hempstead BL. Trk C receptor signaling regulates cardiac myocyte proliferation during early heart development in vivo. Dev Biol. 2000;226:180–191. doi: 10.1006/dbio.2000.9850. [DOI] [PubMed] [Google Scholar]
- 155.Lindsay RM, Harmar AJ. Nerve growth factor regulates expression of neuropeptide genes in adult sensory neurons. Nature. 1989;337:362–364. doi: 10.1038/337362a0. [DOI] [PubMed] [Google Scholar]
- 156.Linggi MS, Burke TL, Williams BB, Harrington A, Kraemer R, Hempstead BL, Yoon SO, Carter BD. Neurotrophin receptor interacting factor (NRIF) is an essential mediator of apoptotic signaling by the p75 neurotrophin receptor. J Biol Chem. 2005;280:13801–13808. doi: 10.1074/jbc.M410435200. [DOI] [PubMed] [Google Scholar]
- 157.Liu Y, Sun L, Huan Y, Zhao H, Deng J. Application of bFGF and BDNF to improve angiogenesis and cardiac function. J Surg Res. 2006;136:85–91. doi: 10.1016/j.jss.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 158.Lommatzsch M, Virchow JC. Letter regarding article by Ejiri et al, “possible role of brain-derived neurotrophic factor in the pathogenesis of coronary artery disease. Circulation. 2006;113:e724–725. doi: 10.1161/CIRCULATIONAHA.105.594135. [DOI] [PubMed] [Google Scholar]
- 159.Longo FM, Massa SM. Neuroprotective strategies in Alzheimer’s disease. NeuroRx. 2004;1:117–127. doi: 10.1602/neurorx.1.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- 161.Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- 162.Luttrell LM, Daaka Y, Lefkowitz RJ. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr Opin Cell Biol. 1999;11:177–183. doi: 10.1016/s0955-0674(99)80023-4. [DOI] [PubMed] [Google Scholar]
- 163.MacDonald JI, Gryz EA, Kubu CJ, Verdi JM, Meakin SO. Direct binding of the signaling adapter protein Grb2 to the activation loop tyrosines on the nerve growth factor receptor tyrosine kinase, TrkA. J Biol Chem. 2000;275:18225–18233. doi: 10.1074/jbc.M001862200. [DOI] [PubMed] [Google Scholar]
- 164.Maisonpierre PC, Belluscio L, Friedman B, Alderson RF, Wiegand SJ, Furth ME, Lindsay RM, Yancopoulos GD. NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron. 1990;5:501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- 165.Mamidipudi V, Li X, Wooten MW. Identification of interleukin 1 receptor-associated kinase as a conserved component in the p75-neurotrophin receptor activation of nuclear factor-kappa B. J Biol Chem. 2002;277:28010–28018. doi: 10.1074/jbc.M109730200. [DOI] [PubMed] [Google Scholar]
- 166.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 168.Matsuda H, Koyama H, Sato H, Sawada J, Itakura A, Tanaka A, Matsumoto M, Konno K, Ushio H, Matsuda K. Role of nerve growth factor in cutaneous wound healing: accelerating effects in normal and healing-impaired diabetic mice. J Exp Med. 1998;187:297–306. doi: 10.1084/jem.187.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Matsumoto K, Wada RK, Yamashiro JM, Kaplan DR, Thiele CJ. Expression of brain-derived neurotrophic factor and p145TrkB affects survival, differentiation, and invasiveness of human neuroblastoma cells. Cancer Res. 1995;55:1798–1806. [PubMed] [Google Scholar]
- 170.Mattei S, Colombo MP, Melani C, Silvani A, Parmiani G, Herlyn M. Expression of cytokine/growth factors and their receptors in human melanoma and melanocytes. Int J Cancer. 1994;56:853–857. doi: 10.1002/ijc.2910560617. [DOI] [PubMed] [Google Scholar]
- 171.Mauro C, Pietro L, Emilio CC. The use of nerve growth factor in herpetic keratitis: a case report. J Med Case Reports. 2007;1:124. doi: 10.1186/1752-1947-1-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.McDonald NQ, Blundell TL. Crystallization and characterization of the high molecular weight form of nerve growth factor (7 S NGF) J Mol Biol. 1991;219:595–601. doi: 10.1016/0022-2836(91)90655-p. [DOI] [PubMed] [Google Scholar]
- 173.McDonald NQ, Hendrickson WA. A structural superfamily of growth factors containing a cystine knot motif. Cell. 1993;73:421–424. doi: 10.1016/0092-8674(93)90127-c. [DOI] [PubMed] [Google Scholar]
- 174.McGregor LM, McCune BK, Graff JR, McDowell PR, Romans KE, Yancopoulos GD, Ball DW, Baylin SB, Nelkin BD. Roles of trk family neurotrophin receptors in medullary thyroid carcinoma development and progression. Proc Natl Acad Sci USA. 1999;96:4540–4545. doi: 10.1073/pnas.96.8.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Meakin SO, MacDonald JI, Gryz EA, Kubu CJ, Verdi JM. The signaling adapter FRS-2 competes with Shc for binding to the nerve growth factor receptor TrkA. A model for discriminating proliferation and differentiation. J Biol Chem. 1999;274:9861–9870. doi: 10.1074/jbc.274.14.9861. [DOI] [PubMed] [Google Scholar]
- 176.Mischel PS, Smith SG, Vining ER, Valletta JS, Mobley WC, Reichardt LF. The extracellular domain of p75NTR is necessary to inhibit neurotrophin-3 signaling through TrkA. J Biol Chem. 2001;276:11294–11301. doi: 10.1074/jbc.M005132200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Moser KV, Reindl M, Blasig I, Humpel C. Brain capillary endothelial cells proliferate in response to NGF, express NGF receptors and secrete NGF after inflammation. Brain Res. 2004;1017:53–60. doi: 10.1016/j.brainres.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 178.Mowla SJ, Farhadi HF, Pareek S, Atwal JK, Morris SJ, Seidah NG, Murphy RA. Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J Biol Chem. 2001;276:12660–12666. doi: 10.1074/jbc.M008104200. [DOI] [PubMed] [Google Scholar]
- 179.Mukai J, Shoji S, Kimura MT, Okubo S, Sano H, Suvanto P, Li Y, Irie S, Sato TA. Structure-function analysis of NADE: identification of regions that mediate nerve growth factor-induced apoptosis. J Biol Chem. 2002;277:13973–13982. doi: 10.1074/jbc.M106342200. [DOI] [PubMed] [Google Scholar]
- 180.Nakagawara A. Trk receptor tyrosine kinases: a bridge between cancer and neural development. Cancer Lett. 2001;169:107–114. doi: 10.1016/s0304-3835(01)00530-4. [DOI] [PubMed] [Google Scholar]
- 181.Nakagawara A, Arima-Nakagawara M, Scavarda NJ, Azar CG, Cantor AB, Brodeur GM. Association between high levels of expression of the TRK gene and favorable outcome in human neuroblastoma. N Engl J Med. 1993;328:847–854. doi: 10.1056/NEJM199303253281205. [DOI] [PubMed] [Google Scholar]
- 182.Nakahashi T, Fujimura H, Altar CA, Li J, Kambayashi J, Tandon NN, Sun B. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett. 2000;470:113–117. doi: 10.1016/s0014-5793(00)01302-8. [DOI] [PubMed] [Google Scholar]
- 183.Nakamura K, Martin KC, Jackson JK, Beppu K, Woo CW, Thiele CJ. Brain-derived neurotrophic factor activation of TrkB induces vascular endothelial growth factor expression via hypoxia-inducible factor-1alpha in neuroblastoma cells. Cancer Res. 2006;66:4249–4255. doi: 10.1158/0008-5472.CAN-05-2789. [DOI] [PubMed] [Google Scholar]
- 184.Nemoto K, Fukamachi K, Nemoto F, Miyata S, Hamada M, Nakamura Y, Senba E, Ueyama T. Gene expression of neurotrophins and their receptors in cultured rat vascular smooth muscle cells. Biochem Biophys Res Commun. 1998;245:284–288. doi: 10.1006/bbrc.1998.8418. [DOI] [PubMed] [Google Scholar]
- 185.Nemoto K, Sekimoto M, Fukamachi K, Nemoto F, Miyata S, Nakamura Y, Hamada M, Senba E, Ueyama T, Degawa M. A possible mechanism of TPA-mediated downregulation of neurotrophin-3 gene expression in rat cultured vascular smooth muscle cells. Brain Res. 1999;68:186–189. doi: 10.1016/s0169-328x(99)00088-1. [DOI] [PubMed] [Google Scholar]
- 186.Newby AC. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol Rev. 2005;85:1–31. doi: 10.1152/physrev.00048.2003. [DOI] [PubMed] [Google Scholar]
- 187.Ni YG, Berenji K, Wang N, Oh M, Sachan N, Dey A, Cheng J, Lu G, Morris DJ, Castrillon DH, Gerard RD, Rothermel BA, Hill JA. Foxo transcription factors blunt cardiac hypertrophy by inhibiting calcineurin signaling. Circulation. 2006;114:1159–1168. doi: 10.1161/CIRCULATIONAHA.106.637124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Nico B, Mangieri D, Benagiano V, Crivellato E, Ribatti D. Nerve growth factor as an angiogenic factor. Microvasc Res. 2008;75:135–141. doi: 10.1016/j.mvr.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 189.Nilsson AS, Fainzilber M, Falck P, Ibanez CF. Neurotrophin-7: a novel member of the neurotrophin family from the zebrafish. FEBS Lett. 1998;424:285–290. doi: 10.1016/s0014-5793(98)00192-6. [DOI] [PubMed] [Google Scholar]
- 190.Nimnual AS, Yatsula BA, Bar-Sagi D. Coupling of Ras and Rac guanosine triphosphatases through the Ras exchanger Sos. Science. 1998;279:560–563. doi: 10.1126/science.279.5350.560. [DOI] [PubMed] [Google Scholar]
- 191.Ninkina N, Adu J, Fischer A, Pinon LG, Buchman VL, Davies AM. Expression and function of TrkB variants in developing sensory neurons. Embo J. 1996;15:6385–6393. [PMC free article] [PubMed] [Google Scholar]
- 192.Nosaka Y, Arai A, Miyasaka N, Miura O. CrkL mediates Ras-dependent activation of the Raf/ERK pathway through the guanine nucleotide exchange factor C3G in hematopoietic cells stimulated with erythropoietin or interleukin-3. J Biol Chem. 1999;274:30154–30162. doi: 10.1074/jbc.274.42.30154. [DOI] [PubMed] [Google Scholar]
- 193.Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, Kliemannel M, Schwarz E, Willnow TE, Hempstead BL, Petersen CM. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427:843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- 194.Obermeier A, Halfter H, Wiesmuller KH, Jung G, Schlessinger J, Ullrich A. Tyrosine 785 is a major determinant of Trk-substrate interaction. Embo J. 1993;12:933–941. doi: 10.1002/j.1460-2075.1993.tb05734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ogunshola OO, Antic A, Donoghue MJ, Fan SY, Kim H, Stewart WB, Madri JA, Ment LR. Paracrine and autocrine functions of neuronal vascular endothelial growth factor (VEGF) in the central nervous system. J Biol Chem. 2002;277:11410–11415. doi: 10.1074/jbc.M111085200. [DOI] [PubMed] [Google Scholar]
- 196.Okuno S, Saito A, Hayashi T, Chan PH. The c-Jun N-terminal protein kinase signaling pathway mediates Bax activation and subsequent neuronal apoptosis through interaction with Bim after transient focal cerebral ischemia. J Neurosci. 2004;24:7879–7887. doi: 10.1523/JNEUROSCI.1745-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Oosthuyse B, Moons L, Storkebaum E, Beck H, Nuyens D, Brusselmans K, Van Dorpe J, Hellings P, Gorselink M, Heymans S, Theilmeier G, Dewerchin M, Laudenbach V, Vermylen P, Raat H, Acker T, Vleminckx V, Van Den Bosch L, Cashman N, Fujisawa H, Drost MR, Sciot R, Bruyninckx F, Hicklin DJ, Ince C, Gressens P, Lupu F, Plate KH, Robberecht W, Herbert JM, Collen D, Carmeliet P. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet. 2001;28:131–138. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- 198.Palko ME, Coppola V, Tessarollo L. Evidence for a role of truncated trkC receptor isoforms in mouse development. J Neurosci. 1999;19:775–782. doi: 10.1523/JNEUROSCI.19-02-00775.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Paoletti F, Konarev PV, Covaceuszach S, Schwarz E, Cattaneo A, Lamba D, Svergun DI. Structural and functional properties of mouse proNGF. Biochem Soc Trans. 2006;34:605–606. doi: 10.1042/BST0340605. [DOI] [PubMed] [Google Scholar]
- 200.Park MJ, Kwak HJ, Lee HC, Yoo DH, Park IC, Kim MS, Lee SH, Rhee CH, Hong SI. Nerve growth factor induces endothelial cell invasion and cord formation by promoting matrix metalloproteinase-2 expression through the phosphatidylinositol 3-kinase/Akt signaling pathway and AP-2 transcription factor. J Biol Chem. 2007;282:30485–30496. doi: 10.1074/jbc.M701081200. [DOI] [PubMed] [Google Scholar]
- 201.Patist CM, Mulder MB, Gautier SE, Maquet V, Jerome R, Oudega M. Freeze-dried poly(d,l-lactic acid) macroporous guidance scaffolds impregnated with brain-derived neurotrophic factor in the transected adult rat thoracic spinal cord. Biomaterials. 2004;25:1569–1582. doi: 10.1016/s0142-9612(03)00503-9. [DOI] [PubMed] [Google Scholar]
- 202.Paul CE, Vereker E, Dickson KM, Barker PA. A pro-apoptotic fragment of the p75 neurotrophin receptor is expressed in p75NTRExonIV null mice. J Neurosci. 2004;24:1917–1923. doi: 10.1523/JNEUROSCI.5397-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Pearson G, Robinson F, Gibson T Beers, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 204.Peart JN, Headrick JP. Adenosinergic cardioprotection: multiple receptors, multiple pathways. Pharmacol Ther. 2007;114:208–221. doi: 10.1016/j.pharmthera.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 205.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 206.Perez P, Coll PM, Hempstead BL, Martin-Zanca D, Chao MV. NGF binding to the trk tyrosine kinase receptor requires the extra-cellular immunoglobulin-like domains. Mol Cell Neurosci. 1995;6:97–105. doi: 10.1006/mcne.1995.1010. [DOI] [PubMed] [Google Scholar]
- 207.Plow EF, Herren T, Redlitz A, Miles LA, Hoover-Plow JL. The cell biology of the plasminogen system. FASEB J. 1995;9:939–945. doi: 10.1096/fasebj.9.10.7615163. [DOI] [PubMed] [Google Scholar]
- 208.Podlesniy P, Kichev A, Pedraza C, Saurat J, Encinas M, Perez B, Ferrer I, Espinet C. Pro-NGF from Alzheimer’s disease and normal human brain displays distinctive abilities to induce processing and nuclear translocation of intracellular domain of p75NTR and apoptosis. Am J Pathol. 2006;169:119–131. doi: 10.2353/ajpath.2006.050787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Pyle AD, Lock LF, Donovan PJ. Neurotrophins mediate human embryonic stem cell survival. Nat Biotechnol. 2006;24:344–350. doi: 10.1038/nbt1189. [DOI] [PubMed] [Google Scholar]
- 210.Qian X, Ginty DD. SH2-B and APS are multimeric adapters that augment TrkA signaling. Mol Cell Biol. 2001;21:1613–1620. doi: 10.1128/MCB.21.5.1613-1620.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Rabizadeh S, Oh J, Zhong LT, Yang J, Bitler CM, Butcher LL, Bredesen DE. Induction of apoptosis by the low-affinity NGF receptor. Science. 1993;261:345–348. doi: 10.1126/science.8332899. [DOI] [PubMed] [Google Scholar]
- 212.Rabizadeh S, Ye X, Sperandio S, Wang JJ, Ellerby HM, Ellerby LM, Giza C, Andrusiak RL, Frankowski H, Yaron Y, Moayeri NN, Rovelli G, Evans CJ, Butcher LL, Nolan GP, Assa-Munt N, Bredesen DE. Neurotrophin dependence domain: a domain required for the mediation of apoptosis by the p75 neurotrophin receptor. J Mol Neurosci. 2000;15:215–229. doi: 10.1385/JMN:15:3:215. [DOI] [PubMed] [Google Scholar]
- 213.Rahbek UL, Dissing S, Thomassen C, Hansen AJ, Tritsaris K. Nerve growth factor activates aorta endothelial cells causing PI3K/Akt- and ERK-dependent migration. Pflügers Arch. 2005;450:355–361. doi: 10.1007/s00424-005-1436-0. [DOI] [PubMed] [Google Scholar]
- 214.Rajagopal R, Chen ZY, Lee FS, Chao MV. Transactivation of Trk neurotrophin receptors by G-protein-coupled receptor ligands occurs on intracellular membranes. J Neurosci. 2004;24:6650–6658. doi: 10.1523/JNEUROSCI.0010-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 216.Raychaudhuri SK, Raychaudhuri SP, Weltman H, Farber EM. Effect of nerve growth factor on endothelial cell biology: proliferation and adherence molecule expression on human dermal microvascular endothelial cells. Arch Dermatol Res. 2001;293:291–295. doi: 10.1007/s004030100224. [DOI] [PubMed] [Google Scholar]
- 217.Raychaudhuri SP, Dutt S, Raychaudhuri SK, Sanyal M, Farber EM. Severe combined immunodeficiency mouse-human skin chimeras: a unique animal model for the study of psoriasis and cutaneous inflammation. Br J Dermatol. 2001;144:931–939. doi: 10.1046/j.1365-2133.2001.04178.x. [DOI] [PubMed] [Google Scholar]
- 218.Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84–86. doi: 10.1080/000155598433368. [DOI] [PubMed] [Google Scholar]
- 219.Raychaudhuri SP, Sanyal M, Weltman H, Kundu-Raychaudhuri S. K252a, a high-affinity nerve growth factor receptor blocker, improves psoriasis: an in vivo study using the severe combined immunodeficient mouse-human skin model. J Invest Dermatol. 2004;122:812–819. doi: 10.1111/j.0022-202X.2003.12602.x. [DOI] [PubMed] [Google Scholar]
- 220.Rihl M, Kruithof E, Barthel C, De Keyser F, Veys EM, Zeidler H, Yu DT, Kuipers JG, Baeten D. Involvement of neurotrophins and their receptors in spondyloarthritis synovitis: relation to inflammation and response to treatment. Ann Rheum Dis. 2005;64:1542–1549. doi: 10.1136/ard.2004.032599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 222.Robinson RC, Radziejewski C, Stuart DI, Jones EY. Structure of the brain-derived neurotrophic factor/neurotrophin 3 heterodimer. Biochemistry. 1995;34:4139–4146. doi: 10.1021/bi00013a001. [DOI] [PubMed] [Google Scholar]
- 223.Rodrigues B, McNeill JH. The diabetic heart: metabolic causes for the development of a cardiomyopathy. Cardiovasc Res. 1992;26:913–922. doi: 10.1093/cvr/26.10.913. [DOI] [PubMed] [Google Scholar]
- 224.Rodriguez-Tebar A, Dechant G, Barde YA. Binding of brain-derived neurotrophic factor to the nerve growth factor receptor. Neuron. 1990;4:487–492. doi: 10.1016/0896-6273(90)90107-q. [DOI] [PubMed] [Google Scholar]
- 225.Rodriguez-Tebar A, Dechant G, Gotz R, Barde YA. Binding of neurotrophin-3 to its neuronal receptors and interactions with nerve growth factor and brain-derived neurotrophic factor. Embo J. 1992;11:917–922. doi: 10.1002/j.1460-2075.1992.tb05130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ruberti F, Capsoni S, Comparini A, Di Daniel E, Franzot J, Gonfloni S, Rossi G, Berardi N, Cattaneo A. Phenotypic knockout of nerve growth factor in adult transgenic mice reveals severe deficits in basal forebrain cholinergic neurons, cell death in the spleen, and skeletal muscle dystrophy. J Neurosci. 2000;20:2589–2601. doi: 10.1523/JNEUROSCI.20-07-02589.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ryu JK, Cho CH, Shin HY, Song SU, Oh SM, Lee M, Piao S, Han JY, Kim IH, Koh GY, Suh JK. Combined angiopoietin-1 and vascular endothelial growth factor gene transfer restores cavernous angiogenesis and erectile function in a rat model of hypercholesterolemia. Mol Ther. 2006;13:705–715. doi: 10.1016/j.ymthe.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 228.Salehi A, Verhaagen J, Dijkhuizen PA, Swaab DF. Co-localization of high-affinity neurotrophin receptors in nucleus basalis of Meynert neurons and their differential reduction in Alzheimer’s disease. Neuroscience. 1996;75:373–387. doi: 10.1016/0306-4522(96)00273-4. [DOI] [PubMed] [Google Scholar]
- 229.Salis MB, Graiani G, Desortes E, Caldwell RB, Madeddu P, Emanueli C. Nerve growth factor supplementation reverses the impairment, induced by Type 1 diabetes, of hindlimb post-ischaemic recovery in mice. Diabetologia. 2004;47:1055–1063. doi: 10.1007/s00125-004-1424-5. [DOI] [PubMed] [Google Scholar]
- 230.Samii A, Unger J, Lange W. Vascular endothelial growth factor expression in peripheral nerves and dorsal root ganglia in diabetic neuropathy in rats. Neurosci Lett. 1999;262:159–162. doi: 10.1016/s0304-3940(99)00064-6. [DOI] [PubMed] [Google Scholar]
- 231.Santos PM, Winterowd JG, Allen GG, Bothwell MA, Rubel EW. Nerve growth factor: increased angiogenesis without improved nerve regeneration. Otolaryngol Head Neck Surg. 1991;105:12–25. doi: 10.1177/019459989110500103. [DOI] [PubMed] [Google Scholar]
- 232.Saragovi HU, Gehring K. Development of pharmacological agents for targeting neurotrophins and their receptors. Trends Pharmacol Sci. 2000;21:93–98. doi: 10.1016/s0165-6147(99)01444-3. [DOI] [PubMed] [Google Scholar]
- 233.Scarisbrick IA, Jones EG, Isackson PJ. Coexpression of mRNAs for NGF, BDNF, and NT-3 in the cardiovascular system of the pre- and postnatal rat. J Neurosci. 1993;13:875–893. doi: 10.1523/JNEUROSCI.13-03-00875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 235.Schratzberger P, Schratzberger G, Silver M, Curry C, Kearney M, Magner M, Alroy J, Adelman LS, Weinberg DH, Ropper AH, Isner JM. Favorable effect of VEGF gene transfer on ischemic peripheral neuropathy. Nat Med. 2000;6:405–413. doi: 10.1038/74664. [DOI] [PubMed] [Google Scholar]
- 236.Seddon M, Shah AM, Casadei B. Cardiomyocytes as effectors of nitric oxide signalling. Cardiovasc Res. 2007;75:315–326. doi: 10.1016/j.cardiores.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 237.Seidah NG, Benjannet S, Pareek S, Chretien M, Murphy RA. Cellular processing of the neurotrophin precursors of NT3 and BDNF by the mammalian proprotein convertases. FEBS Lett. 1996;379:247–250. doi: 10.1016/0014-5793(95)01520-5. [DOI] [PubMed] [Google Scholar]
- 238.Selby MJ, Edwards R, Sharp F, Rutter WJ. Mouse nerve growth factor gene: structure and expression. Mol Cell Biol. 1987;7:3057–3064. doi: 10.1128/mcb.7.9.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Selkoe D, Kopan R. Notch and Presenilin: regulated intramembrane proteolysis links development and degeneration. Annu Rev Neurosci. 2003;26:565–597. doi: 10.1146/annurev.neuro.26.041002.131334. [DOI] [PubMed] [Google Scholar]
- 240.Seo K, Choi J, Park M, Rhee C. Angiogenesis effects of nerve growth factor (NGF) on rat corneas. J Vet Sci. 2001;2:125–130. [PubMed] [Google Scholar]
- 241.Sessa WC. eNOS at a glance. J Cell Sci. 2004;117:2427–2429. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- 242.Shelton DL, Sutherland J, Gripp J, Camerato T, Armanini MP, Phillips HS, Carroll K, Spencer SD, Levinson AD. Human trks: molecular cloning, tissue distribution, and expression of extracellular domain immunoadhesins. J Neurosci. 1995;15:477–491. doi: 10.1523/JNEUROSCI.15-01-00477.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Shiojima I, Walsh K. Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev. 2006;20:3347–3365. doi: 10.1101/gad.1492806. [DOI] [PubMed] [Google Scholar]
- 244.Shmelkov SV, Meeus S, Moussazadeh N, Kermani P, Rashbaum WK, Rabbany SY, Hanson MA, Lane WJ, St Clair R, Walsh KA, Dias S, Jacobson JT, Hempstead BL, Edelberg JM, Rafii S. Cytokine preconditioning promotes codifferentiation of human fetal liver CD133+ stem cells into angiomyogenic tissue. Circulation. 2005;111:1175–1183. doi: 10.1161/01.CIR.0000157155.44008.0F. [DOI] [PubMed] [Google Scholar]
- 245.Skurk C, Izumiya Y, Maatz H, Razeghi P, Shiojima I, Sandri M, Sato K, Zeng L, Schiekofer S, Pimentel D, Lecker S, Taegtmeyer H, Goldberg AL, Walsh K. The FOXO3a transcription factor regulates cardiac myocyte size downstream of AKT signaling. J Biol Chem. 2005;280:20814–20823. doi: 10.1074/jbc.M500528200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Smeyne RJ, Klein R, Schnapp A, Long LK, Bryant S, Lewin A, Lira SA, Barbacid M. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature. 1994;368:246–249. doi: 10.1038/368246a0. [DOI] [PubMed] [Google Scholar]
- 247.Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001;24:1217–1281. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- 248.Song MS, Posse de Chaves EI. Inhibition of rat sympathetic neuron apoptosis by ceramide. Role of p75NTR in ceramide generation. Neuropharmacology. 2003;45:1130–1150. doi: 10.1016/s0028-3908(03)00284-3. [DOI] [PubMed] [Google Scholar]
- 249.Stawowy P, Marcinkiewicz J, Graf K, Seidah N, Chretien M, Fleck E, Marcinkiewicz M. Selective expression of the proprotein convertases furin, pc5, pc7 in proliferating vascular smooth muscle cells of the rat aorta in vitro. J Histochem Cytochem. 2001;49:323–332. doi: 10.1177/002215540104900306. [DOI] [PubMed] [Google Scholar]
- 250.Steinle JJ, Granger HJ. Nerve growth factor regulates human choroidal, but not retinal, endothelial cell migration and proliferation. Auton Neurosci. 2003;108:57–62. doi: 10.1016/S1566-0702(03)00151-6. [DOI] [PubMed] [Google Scholar]
- 251.Stephens RM, Loeb DM, Copeland TD, Pawson T, Greene LA, Kaplan DR. Trk receptors use redundant signal transduction pathways involving SHC and PLC-gamma 1 to mediate NGF responses. Neuron. 1994;12:691–705. doi: 10.1016/0896-6273(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 252.Strohmaier C, Carter BD, Urfer R, Barde YA, Dechant G. A splice variant of the neurotrophin receptor trkB with increased specificity for brain-derived neurotrophic factor. Embo J. 1996;15:3332–3337. [PMC free article] [PubMed] [Google Scholar]
- 253.Suter U, Heymach JV, Jr, Shooter EM. Two conserved domains in the NGF propeptide are necessary and sufficient for the biosynthesis of correctly processed and biologically active NGF. Embo J. 1991;10:2395–2400. doi: 10.1002/j.1460-2075.1991.tb07778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Szekanecz Z, Koch AE. Mechanisms of disease: angiogenesis in inflammatory diseases. Nat Clin Pract Rheumatol. 2007;3:635–643. doi: 10.1038/ncprheum0647. [DOI] [PubMed] [Google Scholar]
- 255.Takeo C, Nakamura S, Tanaka T, Uchida D, Noguchi Y, Nagao T, Saito Y, Tatsuno I. Rat cerebral endothelial cells express trk C and are regulated by neurotrophin-3. Biochem Biophys Res Commun. 2003;305:400–406. doi: 10.1016/s0006-291x(03)00770-8. [DOI] [PubMed] [Google Scholar]
- 256.Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, Kraemer RT, Nykjaer A, Hempstead BL. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci. 2005;25:5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Tessarollo L. Pleiotropic functions of neurotrophins in development. Cytokine Growth Factor Rev. 1998;9:125–137. doi: 10.1016/s1359-6101(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 258.Tessarollo L, Tsoulfas P, Donovan MJ, Palko ME, Blair-Flynn J, Hempstead BL, Parada LF. Targeted deletion of all isoforms of the trkC gene suggests the use of alternate receptors by its ligand neurotrophin-3 in neuronal development and implicates trkC in normal cardiogenesis. Proc Natl Acad Sci USA. 1997;94:14776–14781. doi: 10.1073/pnas.94.26.14776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Timmusk T, Belluardo N, Metsis M, Persson H. Widespread and developmentally regulated expression of neurotrophin-4 mRNA in rat brain and peripheral tissues. Eur J Neurosci. 1993;5:605–613. doi: 10.1111/j.1460-9568.1993.tb00526.x. [DOI] [PubMed] [Google Scholar]
- 260.Tsoulfas P, Soppet D, Escandon E, Tessarollo L, Mendoza-Ramirez JL, Rosenthal A, Nikolics K, Parada LF. The rat trkC locus encodes multiple neurogenic receptors that exhibit differential response to neurotrophin-3 in PC12 cells. Neuron. 1993;10:975–990. doi: 10.1016/0896-6273(93)90212-a. [DOI] [PubMed] [Google Scholar]
- 261.Tuttle JB, Etheridge R, Creedon DJ. Receptor-mediated stimulation and inhibition of nerve growth factor secretion by vascular smooth muscle. Exp Cell Res. 1993;208:350–361. doi: 10.1006/excr.1993.1256. [DOI] [PubMed] [Google Scholar]
- 262.Tuveri M, Generini S, Matucci-Cerinic M, Aloe L. NGF, a useful tool in the treatment of chronic vasculitic ulcers in rheumatoid arthritis. Lancet. 2000;356:1739–1740. doi: 10.1016/S0140-6736(00)03212-8. [DOI] [PubMed] [Google Scholar]
- 263.Urfer R, Tsoulfas P, O’Connell L, Shelton DL, Parada LF, Presta LG. An immunoglobulin-like domain determines the specificity of neurotrophin receptors. Embo J. 1995;14:2795–2805. doi: 10.1002/j.1460-2075.1995.tb07279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Urra S, Escudero CA, Ramos P, Lisbona F, Allende E, Covarrubias P, Parraguez JI, Zampieri N, Chao MV, Annaert W, Bronfman FC. TrkA receptor activation by nerve growth factor induces shedding of the p75 neurotrophin receptor followed by endosomal gamma-secretase-mediated release of the p75 intracellular domain. J Biol Chem. 2007;282:7606–7615. doi: 10.1074/jbc.M610458200. [DOI] [PubMed] [Google Scholar]
- 265.Van der Zee CE, Ross GM, Riopelle RJ, Hagg T. Survival of cholinergic forebrain neurons in developing p75NGFR-deficient mice. Science. 1996;274:1729–1732. doi: 10.1126/science.274.5293.1729. [DOI] [PubMed] [Google Scholar]
- 266.Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- 267.Vilar M, Murillo-Carretero M, Mira H, Magnusson K, Besset V, Ibanez CF. Bex1, a novel interactor of the p75 neurotrophin receptor, links neurotrophin signaling to the cell cycle. Embo J. 2006;25:1219–1230. doi: 10.1038/sj.emboj.7601017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Von Schack D, Casademunt E, Schweigreiter R, Meyer M, Bibel M, Dechant G. Complete ablation of the neurotrophin receptor p75NTR causes defects both in the nervous and the vascular system. Nat Neurosci. 2001;4:977–978. doi: 10.1038/nn730. [DOI] [PubMed] [Google Scholar]
- 269.Wagner N, Wagner KD, Theres H, Englert C, Schedl A, Scholz H. Coronary vessel development requires activation of the TrkB neurotrophin receptor by the Wilms’ tumor transcription factor Wt1. Genes Dev. 2005;19:2631–2642. doi: 10.1101/gad.346405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Wang H, Ward N, Boswell M, Katz DM. Secretion of brain-derived neurotrophic factor from brain microvascular endothelial cells. Eur J Neurosci. 2006;23:1665–1670. doi: 10.1111/j.1460-9568.2006.04682.x. [DOI] [PubMed] [Google Scholar]
- 271.Wang JJ, Rabizadeh S, Tasinato A, Sperandio S, Ye X, Green M, Assa-Munt N, Spencer D, Bredesen DE. Dimerization-dependent block of the proapoptotic effect of p75(NTR) J Neurosci Res. 2000;60:587–593. doi: 10.1002/(SICI)1097-4547(20000601)60:5<587::AID-JNR3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 272.Wang S, Bray P, McCaffrey T, March K, Hempstead BL, Kraemer R. p75(NTR) mediates neurotrophin-induced apoptosis of vascular smooth muscle cells. Am J Pathol. 2000;157:1247–1258. doi: 10.1016/S0002-9440(10)64640-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Wang X, Bauer JH, Li Y, Shao Z, Zetoune FS, Cattaneo E, Vincenz C. Characterization of a p75(NTR) apoptotic signaling pathway using a novel cellular model. J Biol Chem. 2001;276:33812–33820. doi: 10.1074/jbc.M010548200. [DOI] [PubMed] [Google Scholar]
- 274.Webster KA. Aktion in the nucleus. Circ Res. 2004;94:856–859. doi: 10.1161/01.RES.0000126699.49835.5D. [DOI] [PubMed] [Google Scholar]
- 275.Wehrman T, He X, Raab B, Dukipatti A, Blau H, Garcia KC. Structural and mechanistic insights into nerve growth factor interactions with the TrkA and p75 receptors. Neuron. 2007;53:25–38. doi: 10.1016/j.neuron.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 276.Weston GC, Haviv I, Rogers PA. Microarray analysis of VEGF-responsive genes in myometrial endothelial cells. Mol Hum Reprod. 2002;8:855–863. doi: 10.1093/molehr/8.9.855. [DOI] [PubMed] [Google Scholar]
- 277.Wooten MW, Seibenhener ML, Mamidipudi V, Diaz-Meco MT, Barker PA, Moscat J. The atypical protein kinase C-interacting protein p62 is a scaffold for NF-kappaB activation by nerve growth factor. J Biol Chem. 2001;276:7709–7712. doi: 10.1074/jbc.C000869200. [DOI] [PubMed] [Google Scholar]
- 278.Wright JH, Drueckes P, Bartoe J, Zhao Z, Shen SH, Krebs EG. A role for the SHP-2 tyrosine phosphatase in nerve growth-induced PC12 cell differentiation. Mol Biol Cell. 1997;8:1575–1585. doi: 10.1091/mbc.8.8.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Xie Y, Massa SM, Ensslen-Craig SE, Major DL, Yang T, Tisi MA, Derevyanny VD, Runge WO, Mehta BP, Moore LA, Brady-Kalnay SM, Longo FM. Protein-tyrosine phosphatase (PTP) wedge domain peptides: a novel approach for inhibition of PTP function and augmentation of protein-tyrosine kinase function. J Biol Chem. 2006;281:16482–16492. doi: 10.1074/jbc.M603131200. [DOI] [PubMed] [Google Scholar]
- 280.Yamada M, Ohnishi H, Sano S, Araki T, Nakatani A, Ikeuchi T, Hatanaka H. Brain-derived neurotrophic factor stimulates interactions of Shp2 with phosphatidylinositol 3-kinase and Grb2 in cultured cerebral cortical neurons. J Neurochem. 1999;73:41–49. doi: 10.1046/j.1471-4159.1999.0730041.x. [DOI] [PubMed] [Google Scholar]
- 281.Yamashita T, Tohyama M. The p75 receptor acts as a displacement factor that releases Rho from Rho-GDI. Nat Neurosci. 2003;6:461–467. doi: 10.1038/nn1045. [DOI] [PubMed] [Google Scholar]
- 282.Yamashita T, Tucker KL, Barde YA. Neurotrophin binding to the p75 receptor modulates Rho activity and axonal outgrowth. Neuron. 1999;24:585–593. doi: 10.1016/s0896-6273(00)81114-9. [DOI] [PubMed] [Google Scholar]
- 283.Yeiser EC, Rutkoski NJ, Naito A, Inoue J, Carter BD. Neurotrophin signaling through the p75 receptor is deficient in traf6−/− mice. J Neurosci. 2004;24:10521–10529. doi: 10.1523/JNEUROSCI.1390-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Yoon SO, Casaccia-Bonnefil P, Carter B, Chao MV. Competitive signaling between TrkA and p75 nerve growth factor receptors determines cell survival. J Neurosci. 1998;18:3273–3281. doi: 10.1523/JNEUROSCI.18-09-03273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, Geppetti P, Ledda F. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest. 1994;94:2036–2044. doi: 10.1172/JCI117557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Zupan AA, Osborne PA, Smith CE, Siegel NR, Leimgruber RM, Johnson EM., Jr Identification, purification, and characterization of truncated forms of the human nerve growth factor receptor. J Biol Chem. 1989;264:11714–11720. [PubMed] [Google Scholar]
- 287.Zwick E, Wallasch C, Daub H, Ullrich A. Distinct calcium-dependent pathways of epidermal growth factor receptor transactivation and PYK2 tyrosine phosphorylation in PC12 cells. J Biol Chem. 1999;274:20989–20996. doi: 10.1074/jbc.274.30.20989. [DOI] [PubMed] [Google Scholar]