Abstract
The mesodermally derived normal ovarian surface epithelium (OSE) displays both epithelial and mesenchymal characteristics and exhibits remarkable phenotypic plasticity during post-ovulatory repair. The majority of epithelial ovarian carcinomas (EOC) are derived from the OSE and represent the most lethal of all gynecological malignancies, as most patients (~70%) present at diagnosis with disseminated intra-abdominal metastasis. The predominant pattern of EOC metastasis involves pelvic dissemination rather than lymphatic or hematologic spread, distinguishing EOC from other solid tumors. Acquisition of the metastatic phenotype involves a complex series of interrelated cellular events leading to dissociation (shedding) and dispersal of malignant cells. A key event in this process is disruption of cell-cell contacts via modulation of intercellular junctional components. In contrast to most carcinomas that downregulate E-cadherin expression during tumor progression, an unique feature of primary well-differentiated ovarian cancers is a gain of epithelial features, characterized by an increase in expression of E-cadherin. Subsequent reacquisition of mesenchymal features is observed in more advanced tumors with concomitant loss of E-cadherin expression and/or function during progression to metastasis. The functional consequences of this remarkable phenotypic plasticity are not fully understood, but may play a role in modulation of cell survival in suspension (ascites), chemoresistance, and intraperitoneal anchoring of metastatic lesions.
Keywords: ovarian cancer, epithelium, cadherin, keratin, vimentin, epithelial-mesenchymal transition, plasticity
1. Ovarian Cancer—Overview
Tumors arising from the ovarian epithelium account for ~90% of ovarian malignancies and epithelial ovarian carcinoma (EOC) is the leading cause of death from gynecologic malignancy, resulting in approximately 15,280 deaths in the United States alone in 2006 [1]. These distressing statistics are largely due to the low detection rate of disease confined to the ovary (stage 1) when 5-year survival is >90%. Approximately 75% of women are initially diagnosed with disseminated intra-abdominal disease (stage III-IV) and have a 5-year survival of <20%.
Ovarian tumors are pathologically heterogeneous with four major histotypes: serous, endometroid, clear cell and mucinous. These classifications are histogenetically based on distinct morphologic similarities between each histotype and the variety of columnar epithelia of mullerian derivation normally found in areas of the female genital tract [2,3]. While the initiating events in ovarian tumor development are poorly understood [4], recent data support a dualistic model for ovarian tumorigenesis with distinct modes of pathogenesis characterized by specific genetic alterations and unique molecular signatures [5-7]. Clinically, tumors often involve the ovary and omentum, with diffuse, multi-focal intraperitoneal metastases and malignant ascites [8]. Distribution of exfoliated malignant cells is facilitated by the peritoneal fluid, and formation of malignant ascites may further foster metastatic implantation.
The early steps of EOC metastasis involve shedding of the cells from the primary tumor to form free floating cells or multi-cellular aggregates (MCAs) in ascites [Fig. 1]. Cell-cell and cell-matrix adhesion molecules mediate interaction of metastasizing tumor cells with mesothelial cells lining the inner surface of the peritoneal cavity and the sub-mesothelial extracellular matrix (ECM). Localized proteolytic degradation of sub-mesothelial ECM components facilitates migration of tumor cells and anchoring of secondary lesions on adjacent pelvic organs, and at later stages, metastasis to distant organs [9,10]. However, the majority of women with advanced intraperitoneal disease have no clinically apparent hematogenous metastases, implying that a novel mechanism for metastasis is operative in ovarian cancer [11,12]. As the dissemination of ovarian cancer is largely contained within the peritoneal cavity, processes such as cell adhesion, migration and localized intraperitoneal invasion play a predominant role in ovarian cancer pathobiology [Fig. 1].
Figure 1. Schematic of ovarian cancer metastasis.
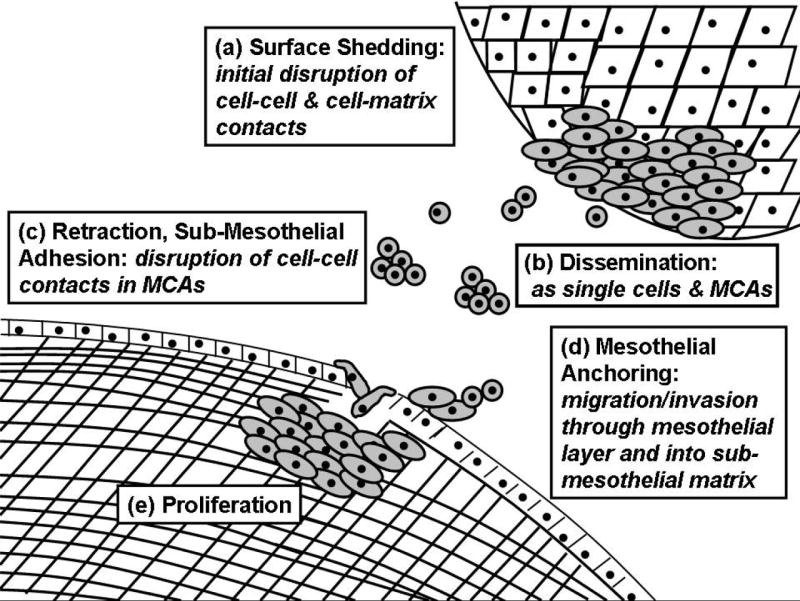
(a) Early events in ovarian cancer metastasis include alteration of cell adhesive properties leading to shedding of tumor cells from the primary tumor. (b) Exfoliated tumor cells can exist as single cells or multi-cellular aggregates (MCAs) or spheroids in the peritoneal cavity. (c) Tumor cell adhesion to the mesothelium induces rapid retraction, exposing the underlying sub-mesothelial matrix. (d) Tumor cells migrate and invade into the submesothelial matrix to anchor secondary lesions followed by (e) proliferation to establish metastatic lesions within the pelvic/abdominal cavity and organs.
In most carcinomas, metastasis is associated with a dysregulated adhesion phenotype, most typically characterized by loss of epithelial (E)-cadherin. In contrast, EOC is unusual because early stage disease reflects an initial gain of epithelial characteristics including E-cadherin expression. This review will cover the unique classical cadherin profiles apparent in EOC and discuss the potential implications of this phenotypic plasticity in ovarian tumor biology.
2. Normal and Neoplastic Ovarian Epithelium
To better understand the changes that occur in EOC, it is important to appreciate distinctions between normal ovarian epithelium and other epithelia. In adult humans, the normal ovarian epithelium is a single cell layer separated by a subepithelial basement membrane from an underlying stroma, the tunica albuginea, which is comprised of dense collagenous connective tissue [13]. The ovarian surface epithelium (OSE) displays epithelial and mesenchymal characteristics, expressing both keratin and vimentin filaments (rare in epithelial cells in situ). In tissue culture, OSE cells express epithelial markers including keratin, desmosomes, tight junctions, laminin, basement membrane type IV collagen and apical microvilli, as well as mesenchymal markers such as vimentin and interstitial collagen types I and III [13,14]. The mesodermally derived OSE expresses unusually low levels of the epithelial cell-cell adhesion molecule E-cadherin [13,15], with expression limited to inclusion cysts or deep clefts [16] where early malignant changes are believed to occur [16,17]. In contrast to other epithelia, OSE epithelial integrity is maintained primarily by neural-(N)-cadherin, consistent with the epithelial-mesenchymal phenotype of this tissue [13, 18-20].
OSE cells exhibit remarkable phenotypic plasticity including alterations in cell shape and cell-cell contact as well as loss of epithelial differentiation markers [13, 21-23]. Such unique phenotypic plasticity suggests that OSE adapts to changes in the cellular microenvironment by transition between epithelial and fibroblastic phenotypes, a characteristic usually limited to immature, regenerating, or neoplastic epithelia. This reversible modulation of ovarian epithelium to a more fibroblastic form occurs during post-ovulatory repair and has been postulated to enhance cell motility and contractility to facilitate wound healing, followed by matrix deposition and associated proliferative responses [13]. It has also been speculated that OSE cells that are trapped within the ovary at ovulation may undergo epithelio-mesenchymal conversion to ovarian stromal fibroblasts to maintain tissue homeostasis [13].
Aberrant differentiation is an unique aspect of ovarian carcinogenesis, as tumors acquire increasingly complex differentiation reminiscent of the highly specialized epithelia of Mullerian duct origin [24,25]. Differentiated primary tumor morphologies acquire characteristics of the fallopian tube (serous carcinoma), endometrium (endometroid carcinoma), endocervix (mucinous carcinoma) and vagina (clear cell carcinoma). Thus, unlike most carcinomas that dedifferentiate during neoplastic progression, ovarian carcinomas undergo transition to a more epithelial phenotype early in tumor progression, with subsequent reacquisition of mesenchymal features in advanced tumors. This pattern of epithelial differentiation followed by de-differentiation distinguishes ovarian cancer from other common carcinomas.
3. Phenotypic Plasticity in Cancer
Changes in cadherin expression are indicative of the phenotypic plasticity that occurs in epithelial to mesenchymal (EMT) or mesenchymal to epithelial (MET) transitions in the embryo. EMT transforms relatively immobile epithelial cells to motile and invasive cells and this transformation is accompanied by loss of stable cell:cell contacts mediated by E-cadherin and expression of vimentin intermediate filaments (reviewed in [26]). A similar process is common in cancer where metastatic behavior is frequently associated with a loss of epithelial phenotype and decreased or absent intercellular junctions resulting in loss of polarity and reduced cell:cell adhesion (reviewed in 27-31). A hallmark of this transformation is loss of E-cadherin expression and/or function which is clinically associated with poor patient prognosis in many tumors [29]. In certain cases, de novo expression of neural (N)-cadherin is evident in tumors lacking E-cadherin and N-cadherin is frequently used as a marker for the mesenchymal phenotype. Some mechanisms for down-regulation of E-cadherin (ie inactivating mutations or promoter hypermethylation) lead to a persistent mesenchymal phenotype [27,29].
In recent years, it has become clear that partial or incomplete EMT can occur in tumors whereby cells retain some epithelial features and migrate or invade while maintaining cell:cell contacts [32,33]. This intermediate phenotype is observed in normal ovarian epithelium [34], at the invasive front of colon [32] and breast [35] cancer, and in normal epidermal tissue during wound repair [36]. These recent findings suggest that even an “incomplete” EMT may contribute to migratory behavior and tumor invasion. In contrast to the well defined events that characterize EMT in development, tumor-associated EMT is currently viewed as a continuum of phenotypic plasticity and gain of mesenchymal characteristics. Interestingly, a recent gene profiling study of the E-cadherin repressors Snail, Slug, and e47 in MDCK cells found more divergence than similarity in their gene expression profiles. This led the authors to conclude that there are different “EMT programs” corresponding to different states of epithelial plasticity and that specific pathways contribute to distinct aspects of EMT [37]. Thus, tumor phenotype would likely reflect the particular complement of EMT regulatory factors expressed in cells or within the tumor microenvironment [30,31,38] and account for the divergence from a “classical” EMT that is frequently observed in cancer.
There are increasing examples of tumor cells reverting to an epithelial phenotype following EMT and retention of epithelial characteristics in highly invasive tumors [30,33,39]. Among the tumors where a well differentiated phenotype may be detected in metastatic lesions are those of the prostate, breast, squamous cell carcinoma of the head and neck, and colorectal cancer [32,33]. So although an inverse relationship between E-cadherin and invasiveness has been described for many carcinomas, this is not a universal feature of metastatic disease. Microenvironmental factors such as extracellular signals (ie growth factors or cytokines) or ECM environment are believed to contribute to epithelial reversion or MET in metastatic lesions [30,33]. Using colorectal cancer as the paradigm, a “migrating cancer stem cells” (MCS-cells) hypothesis has been proposed [32]. Important aspects of MCS cells are their ability to undergo EMT and capacity to differentiate. In the model proposed by Brabletz et al [32], colorectal tumor progression is dynamic and characterized by a transient EMT followed by an MET leading to re-differentiation at the metastatic site, and potential reinitiation of EMT in the metastases.
The recognition of reversible EMT or phenotypic plasticity in tumor cells is particularly relevant to ovarian cancer and in keeping with known characteristics of the normal tissue described in Section 2 above. Ovarian cancer appears to follow an MET to EMT during progression, in contrast to the more common pattern of EMT to MET in other carcinomas, thereby illustrating the unique nature of EOC progression. In studies of human ovarian tumors, these phenotypic transitions have been largely defined by changes in adhesion molecule (E-cadherin and N-cadherin) and intermediate filament (keratin versus vimentin) expression. The functional consequences of this phenotypic plasticity with respect to ovarian tumor progression are not currently understood.
4. Phenotypic Plasticity and Cadherin Expression in EOC
A. Classical Cadherins
Proteins in the classical cadherin superfamily promote homotypic calcium-dependent cell-cell adhesion in epithelia and are essential for regulation of multiple cellular processes including cell segregation, morphogenesis and maintenance of epithelial integrity. Over 30 classical cadherins (also known as type I cadherins) have been described; however the most prevalent are epithelial (E)-cadherin and neuronal (N)-cadherin. E-cadherin is found in adherens junctions of most polarized epithelial cells while N-cadherin is found in neural tissues, cardiac and skeletal muscle, some mesenchymal tissues and mesothelium [40,41]. Cadherins are comprised of five conserved extracellular tandem repeats, a single pass transmembrane domain and a conserved intracellular catenin binding domain that provides a mechanism for linking cell-cell adhesion to other intracellular structural and signaling complexes [42]. Stable cell-cell adhesions are formed as a consequence of cadherin intracellular domain interactions with beta-or p120-catenin and alpha catenin, which associates with the actin cytoskeleton.
In addition to maintenance of tissue integrity, signal transduction by adherens junction components also occurs via beta-catenin, which plays a dual role as a structural protein at cell-cell junctions and a nuclear transcriptional regulator. E-cadherin is also implicated in regulation of signal transduction through other mediators including Rho family GTPases [43], phosphatidylinositol 3-kinase (PI3K) [44,45], and epidermal growth factor (EGF) receptor [45,46], whereas N-cadherin modulates fibroblast growth factor (FGF) receptor signaling [47] as well as the PI3K/Akt pathway [48,49]. Due to the diverse nature of these dynamic multimolecular interactions, cadherins are properly positioned to functionally integrate cell-cell adhesion with regulation of signal transduction pathways and their associated transcriptional programs to control multiple aspects of cell shape and behavior.
B. Cadherin Expression in EOC
Cell adhesion is essential for tissue cohesion during epithelial remodeling. Dysregulation of adhesion, via alteration in expression and/or function of adhesion molecules, has been implicated in loss of proliferation control and acquisition of a motile, invasive phenotype. Thus, reversible modulation of cellular adhesive events likely plays a critical role in remodeling of the ovarian surface epithelium during tumor progression, resulting in shedding of tumor cells to potentiate intraperitoneal metastasis, invasion and spread [Fig. 2].
Figure 2. Cadherin profiles in ovarian tumor progression.
[1] Genetic changes leading to primary tumor growth are characterized by acquisition of E-cadherin (EC). Early tumors express both epithelial (EC, keratin) and mesenchymal (N-cadherin [NC], vimentin) markers. [2] Tumor cells are shed intra-peritoneally from the primary tumor as single cells and MCAs. It has been speculated that retention of E-cadherin expression enables MCAs to avoid anoikis as cells lose matrix contacts and are suspended in ascites. Unknown mechanisms downregulate EC expression and function in metastatic tumors, although some E-cadherin staining is frequently retained in late stage carcinomas and ascites-derived tumor cells. [3] Intraperitoneal adhesion, invasion and proliferation generate intra-abdominal metastases, but the cadherin composition of the metastatic lesions has not been fully investigated.
N-cadherin
As discussed above, the OSE is mesodermally derived and displays both epithelial and mesenchymal features in the human adult, characteristic of an uncommitted pleuripotent precursor epithelium [24]. In striking contrast to other epithelia, normal OSE epithelial integrity is maintained primarily by N-cadherin [Fig. 2, 16,18,19,24]. Constitutive expression of the mesenchymal intermediate filament vimentin as well as connective tissue collagen types I and III further highlights the epithelio-mesenchymal nature of this tissue.
In other tumors, N-cadherin enhances motility even when co-expressed with E-cadherin [48,50], but the potential role of N-cadherin expression in ovarian cancer is unclear at this time. There are comparatively few studies that evaluate N-cadherin expression in ovarian tumors. N-cadherin has been detected in benign and borderline tumors [51] as well as malignant effusions [52]. Moderate N-cadherin staining of serous and endometroid tumors has been reported, with mucinous and clear cell tumors demonstrating low or undetectable expression [18,53-55]. To date, no relationship between N-cadherin positivity and tumor stage or metastasis has been established [53]. Our data are in agreement with these results, showing positive N-cadherin staining in 33% of tumors analyzed (n=146) with strong immunoreactivity in 16% [Table 1, Fig. 3], predominantly representing serous and endometroid tumors. Similarly, 37% of ovarian tumors also exhibit vimentin immunoreactivity, further highlighting the retention of mesenchymal features in these tissues [Table 1, Fig. 3].
Table 1. Expression of epithelial and mesenchymal markers in ovarian tumors.
Immunohistochemical analysis was done retrospectively on tumor tissue microarrays (TMAs) prepared by the Pathology Core Facility of the Robert H. Lurie Comprehensive Cancer Center at Northwestern University assembled from tissue originally taken for routine diagnostic purposes. Samples were cut 3-4 um thick and deparaffinized. The cores were 1 mm in diameter. Immunohistochemical staining was done using standard procedures and analysis was done by light microscopy by an anatomic pathologist (Dr. Brian P. Adley). Scoring was assigned according to the average overall intensity of staining and graded negative (0), weak (1+), moderate (2+) or strong (3+). Staining of <10% of tumor cells was considered negative. TMAs were stained with HECD-1 E-cadherin ectodomain primary antibody; N-cadherin primary antibody (clone 3B9, Zymed), keratin 7 primary antibody (Dako) or vimentin primary antibody (Dako).
| Histotype | E-cadherin | N-cadherin | Keratin | Vimentin | ||||
|---|---|---|---|---|---|---|---|---|
| Pos. | 2-3+ | Pos. | 2-3+ | Pos. | 2-3+ | Pos. | 2-3+ | |
| (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | |
| Serous (n=74) | 64 | 54 | 29 | 23 | 67 | 62 | 20 | 14 |
| (86) | (72) | (39) | (31) | (91) | (84) | (27) | (19) | |
| Endometroid (n=45) | 36 | 29 | 16 | 11 | 42 | 30 | 29 | 29 |
| (80) | (83) | (11) | (0) | (93) | (67) | (22) | (17) | |
| Clear Cell (n=18) | 16 | 15 | 2 | 0 | 17 | 17 | 4 | 3 |
| (89) | (83) | (11) | (0) | (94) | (94) | (22) | (17) | |
| Mucinous (n=9) | 9 | 9 | 1 | 0 | 7 | 7 | 1 | 0 |
| (100) | (100) | (11) | (0) | (78) | (78) | (11) | (0) | |
| Total (n=146) | 125 | 106 | 48 | 23 | 133 | 116 | 54 | 46 |
| (86) | (73) | (33) | (16) | (91) | (79) | (37) | (32) | |
Figure 3. Heterogeneity of epithelial and mesenchymal characteristics in ovarian tumors.
Ovarian tumors exhibit both epithelial and mesenchymal features, as indicated by initial scoring (grouping 1+ to 3+) of serial sections from an ovarian tissue microarray (n=146). Epithelial markers (E-cadherin and keratin) were evident in 86% and 91% of the tumor samples, respectively. Mesenchymal markers (N-cadherin, vimentin) are expressed in 33% and 37%, respectively. Overall, 28% of ovarian tumors in this analysis are positive for both E-cadherin and N-cadherin, although this was less frequent in clear cell or mucinous histotypes. Representative images are shown to illustrate staining patterns. Serous ovarian carcinoma stage IIIb; endometroid carcinoma stage IIIc; clear cell carcinoma stage Ia; mucinous carcinoma stage Ic. Panels were stained with HECD-1 E-cadherin ectodomain primary antibody; N-cadherin primary antibody (clone 3B9, Zymed), keratin 7 primary antibody (Dako) or vimentin primary antibody (Dako) as indicated on the figure. Tumor tissue microarrays were prepared by the Pathology Core Facility of the Robert H. Lurie Comprehensive Cancer Center at Northwestern University assembled from tumor tissue originally taken for routine diagnostic purposes with Institutional Review Board approval. Immunohistochemical stains were scored by anatomic pathologist Dr. Brian P. Adley.
E-Cadherin
An unique feature of EOC is that E-cadherin becomes more abundant in primary differentiated ovarian carcinomas [16,18,20,51,53,56-59], with all histotypes displaying strong immunoreactivity. Our data demonstrate positive E-cadherin staining in 86% of tumors analyzed (n=146) with strong immunoreactivity in 73%, consistent with a gain of epithelial phenotype [Table 1, Fig. 3]. Similarly, 91% of ovarian tumors also exhibit cytokeratin immunoreactivity [Table 1, Fig. 3]. This suggests that E-cadherin may play a role in the early events leading to cellular transformation, a hypothesis supported by data showing that introduction of E-cadherin expression is sufficient for transformation of immortalized OSE [19]. Expression of E-cadherin may also interfere with the response of normal OSE to microenvironmental cues [61]. Acquisition of additional epithelial features including altered cell shape, formation of junctional complexes and expression of secretory products such as mucins and CA125 [19] provide further evidence of the mesenchymal-epithelial transition in early ovarian carcinogenesis.
Interestingly, 28% of ovarian tumors in our analysis exhibit simultaneous positive immunoreactivity for both E-and N-cadherin (serous 33%, endometroid 31%, clear cell 11%, mucinous 11%). Cadherin expression profiles of ovarian carcinoma cell lines reflect the heterogeneity observed in primary patient samples [Fig. 4A]. All cell lines express keratin 8; however immunoblot analysis demonstrates varying E-and N-cadherin expression profiles. Immortalized ovarian surface epithelial (IOSE) cells (generous gift of Dr. Nelly Auersperg, Univ. of British Columbia) do not express detectable E-cadherin or keratin. The cell lines with absent or low E-cadherin expression (DOV13, SKOV3, ES2 [not shown]) display a fibroblastic morphology under phase contrast microscopy [Fig. 4B], while cells expressing E-cadherin exhibit a typical epithelial appearance and tight colony formation [Fig. 4C]. These findings indicate that established ovarian tumor lines display the different phenotypic states observed in vivo and thus represent suitable in vitro models for more detailed mechanistic studies on the functional impact of the diverse cadherin profiles detected in human disease.
Figure 4. Cadherin expression in ovarian cancer cell lines.

(A) Equal protein from whole cell extracts (WCE) was analyzed by western blot and probed with antibodies to E-cadherin (DakoCytomation clone NCH-38), N-cadherin (Zymed 33-3900), keratin-8 (USBiologicals K0199-10) or vimentin (Chemicon CBL202), followed by incubation with a peroxidase-conjugated secondary antibody and ECL detection. IOSE = IOSE 398, ovarian surface epithelium immortalized with SV40 T antigen [109]. Note that the heterogeneity of epithelial (E-cadherin, keratin) and mesenchymal (N-cadherin, vimentin) markers mirrors that observed in human tumors (Fig. 3). β-actin (Sigma A-5441) was used as a loading control. [Note that the lane containing immortalized normal ovarian surface epithelial cell (IOSE) samples was pasted into lane 1 of the figure from another part of the gel.] (B,C) Phenotypes of cells displaying different cadherin profiles. Phase-contrast microscopy highlights the more epithelial phenotype and tight colony morphology of the E-cadherin expressing cells (OVCA 433) relative to the more fibroblastic phenotype of the N-cadherin expressors (DOV13).
Although enhanced E-cadherin expression is a widely recognized feature unique to well-differentiated ovarian tumors; there is less clarity regarding relative E-cadherin levels during ovarian tumor progression and metastasis. In more advanced, poorly differentiated carcinomas, both absent and persistent E-cadherin expression have been reported [16,18,51,59]. While complete loss of E-cadherin expression is uncommon, reduced E-cadherin staining is often detected in late stage carcinomas and in ascites-derived tumor cells [59,62,63]. Correspondingly, ascites cells may be more invasive than paired solid tumor cells from the same patient [60]. Positive E-cadherin staining is reported to be significantly decreased in stage III/IV versus stage I/II tumors [58] and negative E-cadherin is predictive of poor overall survival [21,56]. Collectively, these findings suggest that expression of E-cadherin is generally reduced in advanced ovarian tumors [58]. We have evaluated primary serous ovarian tumors and paired metastatic tissue from the same patient for E-and N-cadherin immunoreactivity (n=17). In this analysis, 47% of the primary tumors show strong E-cadherin staining in the primary tumor (8/17), while 25% (2/8) retain strong E-cadherin staining in the metastatic lesion. In contrast, 71% (12/17) of the primary tumors exhibit strongly positive N-cadherin immunoreactivity that is retained in 67% (8/12) of the paired metastatic lesions [Fig. 5]. These data indicate a trend in which N-cadherin, when high in the primary tumor, is retained in the metastatic lesion while E-cadherin expression is more commonly lost in metastasis. While 41% of tumors exhibited some degree of staining for both N-cadherin and E-cadherin (1+, 2+, or 3+ scoring), only 12% (2/17) showed robust staining for both cadherins (data not shown). Overall the combined data strongly suggest that loss of E-cadherin expression or function is a factor in ovarian cancer progression from well-differentiated lesions to poorly differentiated tumors and metastases, while the role of N-cadherin expression has yet to be resolved. While changes in E-cadherin expression during ovarian tumor progression are well-documented, understanding the interrelationships between altered cadherin expression and/or function and ovarian cancer metastasis will require further mechanistic analyses.
Figure 5. Cadherin expression in paired primary tumors and paired peritoneal metastatic tissue.

Tissue was obtained from primary serous tumors localized to the ovary and paired peritoneal metastases from the same patient. Staining for E-cadherin and N-cadherin revealed two predominant patterns illustrated in this figure. One pattern is reduced E-cadherin staining in the metastasis relative to the primary tumor (compare A and B). The other pattern is strong N-cadherin immunoreactivity that is retained in the metastatic lesion (compare C and D). Tumor tissue microarrays were prepared by the Pathology Core Facility of the Robert H. Lurie Comprehensive Cancer Center at Northwestern University assembled from tumor tissue originally taken for routine diagnostic purposes with Institutional Review Board approval. Immunohistochemical stains were scored by anatomic pathologist Dr. Brian P. Adley.
5. Regulation of E-cadherin in ovarian tumors
Numerous mechanisms for down-regulation of E-cadherin protein and/or function including promoter methylation, transcriptional repression, mutation of the E-cadherin gene, and protein internalization have been identified in cancer [see reviews 27-31,64-66]. Although control of E-cadherin expression in EOC has not been studied extensively, examples of mechanisms detected in other tumor types have been identified. Somatic mutations in the E-cadherin gene are rare in ovarian carcinomas [56], but activating mutations of β-catenin are detected in one histologic subtype of ovarian carcinoma, ovarian endometrioid adenocarcinoma [5,55,67,68]. Methylation of the E-cadherin promoter has been detected in epithelial ovarian neoplasms and associated with reduced E-cadherin protein [69-71]. A number of transcription factors that are important regulators of EMT in development act as repressors of E-cadherin expression. Elevated expression of several of these factors including Snail, Slug, SIP1 and Twist have been identified in EOC and associated with less favorable patient outcomes [72-76]. Ectopic expression of Snail, Slug or Twist in ovarian cancer cell lines results in repression of E-cadherin with accompanying EMT, enhanced motility, and invasiveness suggesting that transcriptional regulation of E-cadherin may be operational in EOC [72,75].
On the other hand, it has been reported that reduced E-cadherin protein level in EOC did not correlate with its mRNA levels, suggesting post-transcriptional regulation of E-cadherin [19]. Post-translational mechanisms for modification of E-cadherin function, for example via altered trafficking or proteolytic processing, have received little attention in ovarian cancer. The potential importance of E-cadherin processing is consistent with published reports demonstrating soluble E-cadherin ectodomain (sE-cad) in peripheral blood, ascites and cystic fluids from ovarian cancer patients [51,77]. Further, sE-cad concentrations in ovarian cyst fluid have been shown to differentiate benign from malignant tumors [51,77]. Proteolytic cleavage of the E-cadherin ectodomain, resulting in release of an 80 kDa ectodomain fragment, was first reported in mammary tissue and linked to metalloproteinase activity [78]. Subsequent studies have identified matrix metalloproteinases (MMP)-3 and -7 as well as a disintegrin and metalloproteinase (ADAM)-10 as E-cadherin ectodomain sheddases in mammary cells and keratinocytes [79-81]. Recent data from our laboratories have shown that aggregation of collagen binding integrins, a key event in intraperitoneal metastasis [Fig. 1], induces MMP-9 expression and MMP-9-dependent E-cadherin ectodomain shedding [82] [Fig. 6]. Furthermore, our results show a significant elevation in the levels of sE-cad in ascites from women with ovarian cancer (mean 12.24 +/-5.31 ug/ml) compared with ascites from women with ovarian hyperstimulation syndrome or benign ovarian cysts (mean 2.06 +/-1.87 ug/ml) [82]. Concomitant immunoblot analysis demonstrate the presence of the full length 80 kDa E-cadherin ectodomain, whereas lower molecular weight degradation products were not detected.
Figure 6. Model for modulation of E-cadherin function in ovarian tumor metastasis.
[a] In the well-differentiated primary tumor, cohesion is maintained primarily through E-cadherin. [b] Engagement of the collagen binding integrins (α2β1 or α3β1) leads to upregulation of MMP-9 and E-cadherin cleavage leading to release of the soluble E-cadherin ectodomain into the tumor microenvironment. [c] Destabilization of junctional E-cadherin occurs by multiple mechanisms including ectodomain cleavage and disruption of preformed junctions by soluble E-cadherin in the microenvironment leading to release of junctional β-cateinin for transcriptional co-activation of Tcf/Lef-regulated genes. [d] Transcriptional profiles regulated by Tcf/Lef contribute to EMT.
In contrast to other solid tumors wherein shed E-cadherin ectodomain is released into the circulation, primary ovarian tumors maintain direct contact with sE-cad-rich ascites. This unique microenvironment provides a physiologically relevant model system in which to assess the potential functional contribution of the E-cadherin ectodomain to ovarian cancer pathobiology. To evaluate the effect of sE-cad on endogenous cellular E-cadherin, a recombinant form of the E-cadherin ectodomain (designated hEcad-Fc) was incubated with ovarian cancer cells at concentrations found in human ovarian cancer ascites (12 ug/ml). Our results demonstrate that hEcad-Fc treatment induces changes characteristic of a phenotypic EMT including altered morphology, disruption of cell-cell adhesion with loss of endogenous junctional E-cadherin staining, and increased cell dispersion [82]. These data support a model for increased E-cadherin ectodomain shedding as cells disseminate from primary ovarian tumors and adhere and anchor into the sub-mesothelial matrix [Fig. 6]. As the primary tumor and both suspended and anchored metastatic cells maintain contact with sE-cad-rich ascites, it is interesting to hypothesize that the sE-cad-rich ovarian tumor microenvironment may encourage further disaggregation of cells from the primary tumor or ascitic cancer cell spheroids, thereby inducing a more mesenchymal phenotype, resulting in enhanced intraperitoneal anchoring to facilitate metastatic dissemination.
6. Functional impact of phenotypic plasticity: why gain E-cadherin in EOC?
It is becoming apparent that MET and re-establishment of epithelial characteristics is important to the later stages of metastasis and colonization of distant sites [32,33,39,83]. However the MET of ovarian cancer occurs early in disease progression, suggesting that this event confers a competitive advantage on tumor cells such as enhanced cell survival. In rat and human OSE, disruption of N-cadherin-mediated cell-cell contacts induces apoptosis, suggesting that N-cadherin plays a role in the control of OSE survival during ovulation [84]. Similarly, gain of E-cadherin-mediated cell-cell adhesion is proposed to promote survival of metastatic ovarian tumor cells.
A hallmark of ovarian cancer is dispersal of tumor cells from the ovary into the peritoneal cavity where they contribute to the formation of ascites. Malignant ascites accumulates in advanced ovarian carcinoma and contains a population of non-adherent tumor cells that exist singly and as multicellular aggregates (MCAs) or spheroids [depicted in Fig. 2] ranging in size from 30-200 um in diameter [85]. Although originally considered a non-adhesive subset of ovarian tumor cells, recent data demonstrate that human ovarian cancer ascites-derived spheroids exhibit integrin-dependent adhesion to mesothelial extracellular matrix and can thereby contribute to intra-peritoneal implantation and metastasis[85,86].
Acquisition of E-cadherin expression may facilitate MCA formation and subsequent promotion of anchorage-independent growth. Similarly, expression of a dominant-negative E-cadherin in ovarian carcinoma cells disrupted adherens junctions thereby preventing spheroid formation, suggesting that E-cadherin expression contributes to emergence of multicellular aggregation in EOC [87]. Engagement of E-cadherin in cultured ovarian cancer cells leads to PI3-kinase-dependent Akt activation via ligand-independent activation of the epidermal growth factor receptor [88]. In contrast, downregulation of E-cadherin function causes reduced viability, suggesting that proliferation signals downstream of E-cadherin activation contribute to ovarian cancer cell survival. Similar results have been observed in squamous cell carcinoma [89], hepatoma [90] and Ewing tumor cells [91].
Together these data suggest that acquisition of E-cadherin-mediated adhesion early in tumor progression may represent a compensatory mechanism to suppress ovarian cancer cell anoikis as cells are shed intra-peritoneally from the primary tumor to enable anchorage-independent growth in ascites. Although E-cadherin expression is generally associated with a non-invasive phenotype, there is evidence that spheroids promote ovarian cancer invasion [85,86,92]. Ascites spheroids adhere to mesothelial cells, disaggregate upon contact with ECM components or a mesothelial monolayer, and in some cases invade, suggesting that spheroid formation plays an important function in ovarian cancer dissemination.
Spheroid cultures exhibit a property termed “multicellular resistance” [93,94], displaying decreased susceptibility to radiation and chemo-therapy similar to that observed in solid tumors in vivo (reviewed in [95-97]). This phenomenon has been attributed to limited drug penetration, reduced fractions of proliferating cells, and enhanced resistance to detachment-induced apoptosis (anoikis) [98]. Disruption of E-cadherin-mediated cell adhesion in spheroids sensitizes tumor cells to chemotherapeutic agents [91,94]. This property of chemoresistance is also observed in ovarian tumor spheroids. Thus the acquisition of E-cadherin early in ovarian cancer confers survival following dispersal from the primary tumor and likely contributes to resistance to therapeutic interventions.
7. Summary and Conclusions
A hallmark of ovarian cancer metastasis is dissemination by exfoliation of cells from the primary tumor into the abdomino-pelvic cavity. This imposes challenges to tumor cell survival because these metastatic cells must escape anoikis. There is evidence that the unique cadherin profiles of EOC in tumor progression, resulting in an initial mesenchymal to epithelial transition with concomitant gain of E-cadherin expression, may be advantageous in the unique ovarian tumor microenvironment. This early gain of E-cadherin may promote ovarian tumor cell survival in ascites as multi-cellular aggregates and foster adhesion to and invasion of the mesothelium. There is also emerging evidence that MCAs are chemoresistant, providing further complications to patient treatment. Collectively, published data suggest a loss of E-cadherin with tumor progression and in metastatic lesions, but our findings demonstrate that a significant fraction (approximately one third) of serous and endometroid tumors co-express E-cadherin and N-cadherin. The role of N-cadherin in in these histotypes is unclear at this time.
Additionally, it is unknown to what extent the loss of E-cadherin is due to epigenetic, transcriptional and/or post-transcriptional mechanisms. Interestingly, factors in the ovarian tumor microenvironment such as lysophosphatidic acid and ligands for the epidermal growth factor receptor down-regulate E-cadherin and modulate cell adhesion [99-102]. This is due, in part, to production of matrix metalloproteinases (MMPs) that cleave E-cadherin [82, 103-106], and we and others have reported that MMPs are abundant in the ascities of ovarian cancer patients [9,107-108]. Another novel mechanism for down-regulation of E-cadherin occurs upon integrin engagement leading to MMP-9 production and E-cadherin ectodomain generation [82]. The ectodomain itself becomes part of the ovarian tumor microenvironment and has been detected in patient ascites. We find that this E-cadherin fragment can disrupt adherens junctions [82], raising the intriguing possibility that the ovarian tumor microenvironment is pivotal in the MET to EMT that occurs later in EOC progression. Thus, while evaluation of E-cadherin expression by immunohistochemistry may detect overall changes in total protein, loss of adhesive function through post-translational mechanisms such as cleavage or trafficking may play a key role in regulation of ovarian tumor cell behavior in vivo and will require further investigation.
Acknowledgments
This work was supported by National Cancer Institute Research Grants RO1CA090492 (LGH), CA109545 (MSS), and CA86984 (MSS). The authors wish to gratefully acknowledge the Robert H. Lurie Comprehensive Cancer Center Pathology Core Facility for ovarian tumor tissues and Dr. Brian P. Adley (Lutheran General Hospital, IL) for scoring of the immunohistochemical analyses.
Literature Cited
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. Clin. 2007 Jan-Feb;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Wheeler JE. Pathology of malignant ovarian epithelial tumors and miscellaneous and rare ovarian and paraovarian neoplasms. In: Rubin SC, Sutton GP, editors. Ovarian Cancer. McGraw Hill; 1993. pp. 87–130. [Google Scholar]
- 3.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–29. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 4.DuBeau L. The cell or origin of ovarian epithelial tumors and the ovarian surface epithelium dogma: does the emperor have no clothes? Gyn Oncol. 1999;72:437–442. doi: 10.1006/gyno.1998.5275. [DOI] [PubMed] [Google Scholar]
- 5.Shedden KA, Kshisagar MP, Schwartz DR, Wu R, Yu H, Misek DE, Hanash S, Katabuchi H, Ellenson LH, Fearon ER, Cho KC. Histologic type, organ or origin and wnt pathway status: effect on gene expression in ovarian and uterine carcinomas. Clin Can Res. 2005;11:2123–31. doi: 10.1158/1078-0432.CCR-04-2061. [DOI] [PubMed] [Google Scholar]
- 6.Shih IM, Kurman RJ. Ovarian Tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Path. 2004;164:1511–18. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz DR, Kardia SLR, Shedden KA, Kuick R, Michailidis G, Taylor JMG, Misek DE, Wu R, Zhai Y, Darrah DM, Reed H, Ellenson LH, Giordano TJ, Rearon ER, Hanash SM, Cho KR. Gene expression in ovarian cancer reflects both morphology and biological behavior, distinguishing clear cell from other poor-prognosis ovarian carcinomas. Can Res. 2002;62:4722–29. [PubMed] [Google Scholar]
- 8.Scully RE, Young RH, Clement PB. Tumors of the ovary, maldeveloped gonads, fallopian tube and broad ligament. Atlas of tumor pathology ,third series, Fascicle 23. 1998 [Google Scholar]
- 9.Ghosh S, Wu Y, Stack MS. Ovarian Cancer Proteinases. Cancer Treat Res. 2002;107:331–51. doi: 10.1007/978-1-4757-3587-1_16. [DOI] [PubMed] [Google Scholar]
- 10.Skubitz APN. Adhesion Molecules. Cancer Treat Res. 2002;107:305–329. doi: 10.1007/978-1-4757-3587-1_15. [DOI] [PubMed] [Google Scholar]
- 11.Hoskins WJ. Prospective on ovarian cancer: Why prevent? J Cell Biochem suppl. 1995;23:189–199. doi: 10.1002/jcb.240590926. [DOI] [PubMed] [Google Scholar]
- 12.Naora H, Montell DJ. Ovarian cancer metastasis: integrating insights from disparate model organisms. Nature Rev Cancer. 2005;5:355–366. doi: 10.1038/nrc1611. [DOI] [PubMed] [Google Scholar]
- 13.Wong AST, Auersperg N. Normal Ovarian Surface Epithelium. Cancer Treat Res. 2002;107:161–183. doi: 10.1007/978-1-4757-3587-1_7. [DOI] [PubMed] [Google Scholar]
- 14.Czernobilsky B, Moll R, Levy M, Franke WW. Co-expression of cytokeratin and vimentin filaments in mesothelial, granulosa and rete ovarii cells of the human ovary. Eur J of Cell Biology. 1985;37:175–190. [PubMed] [Google Scholar]
- 15.Auersperg N, Ota T, Mitchell GW. Early events in ovarian epithelial carcinogenesis: progress and problems in experimental approaches. Int J Gynecol Cancer. 2002;12(6):691–703. doi: 10.1046/j.1525-1438.2002.01152.x. [DOI] [PubMed] [Google Scholar]
- 16.Sundfeldt K, Piontkewitz Y, Ivarsson K, Nilsson O, Hellberg P, Brannstrom M, Janson PO, Enerback S, Hedin L. E cadherin expression in human epithelial ovarian cancer and normal ovary. Int J Can. 1997;74:275–80. doi: 10.1002/(sici)1097-0215(19970620)74:3<275::aid-ijc7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 17.Okamura H, Katabuchi H, Nitta M, Ohtake H. Structural changes and cell properties of human ovarian surface epithelium in ovarian pathophysiology. Microsc Res Tech. 2006;69:469–81. doi: 10.1002/jemt.20306. [DOI] [PubMed] [Google Scholar]
- 18.Peralta-Soler A, Knudsen KA, Tecson-Miguel A, McBrearty FX, Han AC, Salazar H. Expression of E-cadherin and N-cadherin in surface epithelial stromal tumors of the ovary distinguishes mucinous from serous and endometrioid tumors. Human Path. 1997;28:734–9. doi: 10.1016/s0046-8177(97)90184-2. [DOI] [PubMed] [Google Scholar]
- 19.Wong AST, Maines-Bandiera SL, Rosen B, Wheelock MJ, Johnson KR, Leung PCK, Roskelley CD, Auersperg N. Constitutive and conditional cadherin expression in cultured human ovarian surface epithelium: influence of family history of ovarian cancer. Int J Can. 1999;81:180–88. doi: 10.1002/(sici)1097-0215(19990412)81:2<180::aid-ijc3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 20.Patel IS, Madan P, Getsios S, Bertrand MA, MacCalman CD. Cadherin switching in ovarian cancer progression. Int J Can. 2003;106:172–177. doi: 10.1002/ijc.11086. [DOI] [PubMed] [Google Scholar]
- 21.Darai E, Scoazec JY, Walker-Combrouze F, Mlika-Cabanne N, Feldmann G, Madelenat P, Potet F. Expression of cadherins in benign, borderline and malignant ovarian epithelial tumors: a clinicopathologic study of 60 cases. Hum Path. 1997;28:922–8. doi: 10.1016/s0046-8177(97)90007-1. [DOI] [PubMed] [Google Scholar]
- 22.Alper O, De Santis ML, Stromberg K, Hacker NF, Cho-Chung YS, Salomon DS. Anti-sense suppression of epidermal growth factor receptor expression alters cellular proliferation, cell-adhesion and tumorigenicity in ovarian cancer cells. Int J Cancer. 2000;88(4):566–74. doi: 10.1002/1097-0215(20001115)88:4<566::aid-ijc8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 23.Alper O, Bergmann-Leitner ES, Bennett TA, Hacker NF, Stromberg K, Stetler-Stevenson WG. Epidermal growth factor receptor signaling and the invasive phenotype of ovarian carcinoma cells. Journal of the National Cancer Institute. 2001;93:1375–84. doi: 10.1093/jnci/93.18.1375. [DOI] [PubMed] [Google Scholar]
- 24.Auersperg N, Maines-Bandiera Sl, Dyck HG, Kruk PA. Characterization of cultured human ovarian surface epithelial cells: phenotypic plasticity and premalignant changes. Lab Invest. 1994;71:510–18. [PubMed] [Google Scholar]
- 25.Naora H. The heterogeneity of epithelial ovarian cancers: reconciling old and new paradigms. Expert Reviews in Molecular Medicine. 2007;9:1–12. doi: 10.1017/S1462399407000324. [DOI] [PubMed] [Google Scholar]
- 26.Hay ED. EMT Concept and Examples from the Vertebrate Embryo. In: Savagner P, editor. Rise and Fall of Epithelial Phenotype: Concepts of Epithelial-Mesenchymal Transition. Springer; Berlin: 2005. pp. 111–134. [Google Scholar]
- 27.Peinado H, Portillo F, Cano A. Transcriptional regulation of cadherins during development and carcinogenesis. Int J Dev Biol. 2004;48:365–75. doi: 10.1387/ijdb.041794hp. [DOI] [PubMed] [Google Scholar]
- 28.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–58. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Van Marck VL, Bracke ME. Epithelial-Mesenchymal Transitions in Human Cancer. In: Savagner P, editor. Rise and Fall of Epithelial Phenotype: Concepts of Epithelial-Mesenchymal Transition. Springer; Berlin: 2005. pp. 111–134. [Google Scholar]
- 30.Guarino M, Rubino B, Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology. 2007;39:305–18. doi: 10.1080/00313020701329914. [DOI] [PubMed] [Google Scholar]
- 31.Tse JC, Kalluri R. Mechanisms of metastasis: Epithelial-to-mesenchymal transition and contribution of tumor microenvironment. J Cell Biochem. 2007;101(4):816–29. doi: 10.1002/jcb.21215. [DOI] [PubMed] [Google Scholar]
- 32.Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells - an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5:744–9. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 33.Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–26. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed N, Maines-Bandiera S, Quinn MA, Unger WG, Dedhar S, Auersperg N. Molecular pathways regulating EGF-induced epithelio-mesenchymal transition in human ovarian surface epithelium. Am J Physiol Cell Physiol. 2006;290(6):C1532–42. doi: 10.1152/ajpcell.00478.2005. [DOI] [PubMed] [Google Scholar]
- 35.Come C, Magnino F, Bibeau F, De Santa Barbara P, Becker KF, Theillet C, Savagner P. Snail and slug play distinct roles during breast carcinoma. Clin Cancer Res. 2006;12:5395–402. doi: 10.1158/1078-0432.CCR-06-0478. [DOI] [PubMed] [Google Scholar]
- 36.Arnoux V, Come C, Kusewitt D, Hudson L, Savagner P. Cutaneous Wound Reepithelializaton: A partial and reversible EMT. In: Savagner P, editor. Rise and Fall of Epithelial Phenotype: Concepts of Epithelial-Mesenchymal Transition. Springer; Berlin: 2005. pp. 111–134. [Google Scholar]
- 37.Moreno-Bueno G, Cubillo E, Sarrio D, Peinado H, Rodriguez-Pinilla SM, Villa S, Bolos V, Jorda M, Fabra A, Portillo F, Palacios J, Cano A. Genetic profiling of epithelial cells expressing e-cadherin repressors reveals a distinct role for snail, slug, and e47 factors in epithelial-mesenchymal transition. Cancer Res. 2006;66:9543–56. doi: 10.1158/0008-5472.CAN-06-0479. [DOI] [PubMed] [Google Scholar]
- 38.Fodde R, Brabletz T. Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol. 2007;19:150–8. doi: 10.1016/j.ceb.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 39.Chaffer CL, Thompson EW, Williams ED. Mesenchymal to epithelial transition in development and disease. Cells Tissues Organs. 2007;185:7–19. doi: 10.1159/000101298. progression. Clin Cancer Res. 12:5395-402. [DOI] [PubMed] [Google Scholar]
- 40.McLachlan RW, Yap AS. Not so simple: the complexity of phosphotyrosine signaling at cadherin adhesive contacts. J Mol Med. 2007;85:545–554. doi: 10.1007/s00109-007-0198-x. [DOI] [PubMed] [Google Scholar]
- 41.Derycke LDM, Bracke ME. N-cadherin in the spotlight of cell-cell adhesion, differentiation, embryogenesis, invasion and signaling. Int J Dev Biol. 2004;48:463–476. doi: 10.1387/ijdb.041793ld. [DOI] [PubMed] [Google Scholar]
- 42.Conacci-Sorrell M, Zhurnisnsky J, Ben-Ze’ev A. The cadherin-catenin adhesion system in signaling and cancer. J Clin Invest. 2002;109:9870991. doi: 10.1172/JCI15429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arthur WT, Noren NK, Burridge K. Regulation of Rho family GTPases by cell-cell and cell-matrix adhesion. Biol Res. 2002;35:239–246. doi: 10.4067/s0716-97602002000200016. [DOI] [PubMed] [Google Scholar]
- 44.Pece S, Chiariello M, Murga C, Gutkind JS. Activation of the protein kinase Akt/PKB by the formation of E-cadherin-mediated cell-cell junctions. J Biol Chem. 1999;274:19347–51. doi: 10.1074/jbc.274.27.19347. [DOI] [PubMed] [Google Scholar]
- 45.Munshi HG, Ghosh S, Mukhopadhyay S, Wu YI, Sen R, Green KJ, Stack MS. Proteinase suppression by E-cadherin-mediated cell-cell attachment in premalignant oral keratinocytes. J Biol Chem. 2002;277:38159–67. doi: 10.1074/jbc.M202384200. [DOI] [PubMed] [Google Scholar]
- 46.Pece S, Gutkind JS. Signaling from E-cadherins to the MAPK pathway by the recruitment and activation of epidermal growth factor receptors upon cell-cell contact formation. J Biol Chem. 2000;275:41227–33. doi: 10.1074/jbc.M006578200. [DOI] [PubMed] [Google Scholar]
- 47.Wheelock MJ, Johnson KR. Cadherin-mediated cellular signaling. Curr Opin Cell Biol. 2003;15:509–14. doi: 10.1016/s0955-0674(03)00101-7. [DOI] [PubMed] [Google Scholar]
- 48.Li G, Satyamoorthy K, Herlyn M. N-cadherin-mediated inter-cellular interactions promote survival and migration of melanoma cells. Cancer Res. 2001;61:3819–25. [PubMed] [Google Scholar]
- 49.Tran NL, Adams DG, Vaillancourt RR, Heimark RL. Signal transduction from N-cadherin increases Bcl-2. Regulation of the phosphatidylinositol 3-kinase/Akt pathway by homophilic adhesion and actin cytoskeletal organization. J Biol Chem. 2002;277:32905–14. doi: 10.1074/jbc.M200300200. [DOI] [PubMed] [Google Scholar]
- 50.Wheelock MJ, Johnson KR. Cadherins as modulators of cellular phenotype. Annu Rev Cell Dev Biol. 2003;19:207–35. doi: 10.1146/annurev.cellbio.19.011102.111135. [DOI] [PubMed] [Google Scholar]
- 51.Darai E, Bringuier AF, Walker-Combrouze F, Feldmann G, Madelenat P, Scoaze JY. Soluble adhesion molecules in serum and cyst fluid from patients with cystic tumors of the ovary. Hu Reprod. 1998;13:2831–5. doi: 10.1093/humrep/13.10.2831. [DOI] [PubMed] [Google Scholar]
- 52.Silvertsen S, Berner A, Michael CW, Bedrossian C, Davidson B. Cadherin expression in ovarian carcinoma and malignant mesothelioma cell effusions. Acta Cytol. 2006;50:603–7. doi: 10.1159/000326027. [DOI] [PubMed] [Google Scholar]
- 53.Roveri Marques F, Fonsechi-Carvasan GA, Andrade LAL, Luiz FB. Immunohistochemical patterns for α and β catenin, E and N cadherin expression in ovarian epithelial tumors. Gyn Onc. 2004;94:16–24. doi: 10.1016/j.ygyno.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 54.Marques RF, Fonsechi-Carvasan GA, De Angelo Andrade LA, Bottcher-Luiz F. Immunohistochemical patterns for alpha-and beta-catenin, E-and N-cadherin expression in ovarian epithelial tumors. Gynecol Oncol. 2004;94:16–24. doi: 10.1016/j.ygyno.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 55.Sarrio D, Moreno-Bueno G, Sanchez-Estevez C, Banon-Rodriguez I, Hernandez-Cortes G, Hardisson D, Palacios J. Expression of cadherins and catenins correlates with distinct histologic types of ovarian carcinomas. Hum Pathol. 2006;37:1042–9. doi: 10.1016/j.humpath.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 56.Faleiro-Rodrigues C, Macedo-Pinto I, Pereira D, Lopes CS. Prognostic value of E cadherin. 2004 doi: 10.1093/annonc/mdh387. [DOI] [PubMed] [Google Scholar]; Fujioka T, Takebayashi Y, Kihana T, Kusanagi Y, Haada K, Ochi H, Uchida T, Fukumoto M, Ito M. Expression of Ecadherin and β-catenin in primary and peritoneal metastatic ovarian carcinoma. Oncol Rep. 2001;8:249–255. [PubMed] [Google Scholar]
- 57.Maines-Bandiera SL, Auersperg N. Increased E-cadherin expression in ovarian surface epithelium: an early step in metaplasia and dysplasia? Int J Gynecol Path. 1997;16:250–255. doi: 10.1097/00004347-199707000-00010. [DOI] [PubMed] [Google Scholar]
- 58.Imai T, Horiuchi A, Shiozawa T, Osada R, Kikuchi N, Ohira S, Oka K, Konishi I. Elevated expression of E-cadherin and alpha-, beta-, and gamma-catenins in metastatic lesions compared with primary epithelial ovarian carcinomas. Hum Pathol. 2004;35(12):1469–7. doi: 10.1016/j.humpath.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 59.Davies BR, Worsley SD, Ponder BA. Expression of E cadherin, α-catenin and β-catenin in normal ovarian surface epithelium and epithelial ovarian cancers. Histopath. 1998;32:69–80. doi: 10.1046/j.1365-2559.1998.00341.x. [DOI] [PubMed] [Google Scholar]
- 60.Veatch AL, Carson LF, Ramakrishnan S. Differential expression of the cell-cell adhesion molecule E cadherin in ascites and solid human ovarian tumor cells. Int J Can. 1994;58:393–9. doi: 10.1002/ijc.2910580315. [DOI] [PubMed] [Google Scholar]
- 61.Qian X, Karpova T, Sheppard AM, McNally J, Lowy DR. E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. EMBO J. 2004;23:1739–48. doi: 10.1038/sj.emboj.7600136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ho EY, Choi Y, Chae SW, Sohn JH, Ahn GH. Immunohistochemical study of the expression of adhesion molecules in ovarian serous neoplasms. Pathol Int. 2006;56:62–70. doi: 10.1111/j.1440-1827.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 63.Voutilainen KA, Anttila MA, Sillanpaa SM, Ropponen KM, Saarikoski SV, Juhola MT, Kosma VM. Prognostic significance of E-cadherin-catenin complex in epithelial ovarian cancer. J Clin Pathol. 2006;59:460–7. doi: 10.1136/jcp.2005.029876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bryant DM, Stow JL. The ins and outs of E-cadherin trafficking. Trends Cell Biol. 2004;14:427–34. doi: 10.1016/j.tcb.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 65.D’Souza-Schorey C. Disassembling adherens junctions: breaking up is hard to do. Trends Cell Biol. 2005;15:19–26. 67. doi: 10.1016/j.tcb.2004.11.002. [DOI] [PubMed] [Google Scholar]; Palacios J, Gamallo C. Mutations in the beta-catenin gene (CTNNB1) in endometrioid ovarian carcinomas. Cancer Res. 1998;58:1344–7. [PubMed] [Google Scholar]
- 66.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–28. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 67.Wu R, Zhai Y, Fearon ER, Cho KR. Diverse mechanisms of beta-catenin deregulation in ovarian endometroid carcinomas. 2001 [PubMed] [Google Scholar]
- 68.Oliva E, Sarrio D, Brachtel EF, Sanchez-Estevez C, Soslow RA, Moreno-Bueno G, Palacios J. High frequency of beta-catenin mutations in borderline endometrioid tumours of the ovary. J Pathol. 2006;208:708–13. doi: 10.1002/path.1923. [DOI] [PubMed] [Google Scholar]
- 69.Rathi A, Virmani AK, Schorge JO, Elias KJ, Maruyama R, Minna JD, Mok SC, Girard L, Fishman DA, Gazdar AF. Methylation profiles of sporadic ovarian tumors and nonmalignant ovaries from high-risk women. Clin Cancer Res. 2002;8:3324–31. [PubMed] [Google Scholar]
- 70.Makarla PB, Saboorian MH, Ashfaq R, Toyooka KO, Toyooka S, Minna JD, Gazdar AF, Schorge JO. Promoter hypermethylation profile of ovarian epithelial neoplasms. Clin Cancer Res. 2005;11:5365–9. doi: 10.1158/1078-0432.CCR-04-2455. [DOI] [PubMed] [Google Scholar]
- 71.Yuecheng Y, Hongmei L, Xiaoyan X. Clinical evaluation of E-cadherin expression and its regulation mechanism in epithelial ovarian cancer. Clin Exp Metastasis. 2006;23:65–74. doi: 10.1007/s10585-006-9020-3. [DOI] [PubMed] [Google Scholar]
- 72.Kurrey NK, A K, Bapat SA. Snail and Slug are major determinants of ovarian cancer invasiveness at the transcription level. Gynecol Oncol. 2005;97:155–65. doi: 10.1016/j.ygyno.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 73.Elloul S, Elstrand MB, Nesland JM, Trope CG, Kvalheim G, Goldberg I, Reich R, Davidson B. Snail, Slug, and Smad-interacting protein 1 as novel parameters of disease aggressiveness in metastatic ovarian and breast carcinoma. Cancer. 2005;103:1631–43. doi: 10.1002/cncr.20946. [DOI] [PubMed] [Google Scholar]
- 74.Elloul S, Silins I, Trope CG, Benshushan A, Davidson B, Reich R. Expression of E-cadherin transcriptional regulators in ovarian carcinoma. Virchows Arch. 2006;449:520–8. doi: 10.1007/s00428-006-0274-6. [DOI] [PubMed] [Google Scholar]
- 75.Terauchi M, Kajiyama H, Yamashita M, Kato M, Tsukamoto H, Umezu T, Hosono S, Yamamoto E, Shibata K, Ino K, Nawa A, Nagasaka T, Kikkawa F. Possible involvement of TWIST in enhanced peritoneal metastasis of epithelial ovarian carcinoma. Clin Exp Metastasis. 2007 May 9; doi: 10.1007/s10585-007-9070-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 76.Hosono S, Kajiyama H, Terauchi M, Shibata K, Ino K, Nawa A, Kikkawa F. Expression of Twist increases the risk for recurrence and for poor survival in epithelial ovarian carcinoma patients. Br J Cancer. 2007;96:314–20. doi: 10.1038/sj.bjc.6603533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sundfeldt K, Ivarsson K, Rask K, et al. higher levels of soluble E-cadherin in cyst fluid from malignant ovarian tumors than in benign cysts. Anticancer Res. 2001;21:65–70. [PubMed] [Google Scholar]
- 78.Wheelock MJ, Buck CA, Bechtol KM, Damsky CH. Soluble 80-kd fragment of cell-CAM120/80 disrupts cell-cell adhesion. J Cell Biochem. 1983;34:187–202. doi: 10.1002/jcb.240340305. [DOI] [PubMed] [Google Scholar]
- 79.Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol. 1997;139:1861–72. doi: 10.1083/jcb.139.7.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Noe V, Fingleton B, Jacobs K, et al. release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci. 2001;114:111–8. doi: 10.1242/jcs.114.1.111. [DOI] [PubMed] [Google Scholar]
- 81.Maretzky T, Reiss K, Ludwig A, et al. ADAM10 mediated E-cadherin shedding and regulates epithelial cell-cell adhesion, migration and b-catenin translocation. Proc Natl Acad Sci USA. 2005;102:9182–7. doi: 10.1073/pnas.0500918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Symowicz J, Adley BP, Gleason KJ, Johnson JJ, Ghosh S, Fishman DA, Hudson LG, Stack MS. Engagement of collagen-binding integrins promotes matrix metalloproteinase-9-dependent e-cadherin ectodomain shedding in ovarian carcinoma cells. Cancer Res. 2007;67:2030–9. doi: 10.1158/0008-5472.CAN-06-2808. [DOI] [PubMed] [Google Scholar]
- 83.Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW, Williams ED. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res. 2006;66:11271–8. doi: 10.1158/0008-5472.CAN-06-2044. [DOI] [PubMed] [Google Scholar]
- 84.Pon YL, Auersperg N, Wong AS. Gonadotropins regulate N-cadherin-mediated human ovarian surface epithelial cell survival at both post-translational and transcriptional levels through a cyclic AMP/protein kinase A pathway. J Biol Chem. 2005;280:15438–48. doi: 10.1074/jbc.M410766200. [DOI] [PubMed] [Google Scholar]
- 85.Burleson KM, Casey RC, Skubitz KM, Pambuccian SE, Oegema TR, Skubitz AP. Ovarian carcinoma ascites spheroids adhere to extracellular matrix components and mesothelial cell monolayers. Gyn Oncol. 2004;93:170–81. doi: 10.1016/j.ygyno.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 86.Burleson KM, Boente MP, Pambuccian SE, Skubitz AP. Disaggregation and invasion of ovarian carcinoma ascites spheroids. J Transl Med. 2006;4:6. doi: 10.1186/1479-5876-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu C, Cipollone J, Maines-Bandiera S, Tan C, Karsan A, Auersperg N, Roskelley CD. The morphogenic function of E-cadherin-mediated adherens junctions in epithelial ovarian carcinoma formation and progression. Differentiation. 2007 Jul 2; doi: 10.1111/j.1432-0436.2007.00193.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 88.Reddy P, Liu L, Ren C, Lindgren P, Boman K, Shen Y, Lundin E, Ottander U, Rytinki M, Liu K. Formation of E-cadherin mediated cell-cell adhesion activates AKT and MAPK via PI3kinase and ligand-independent activation of epidermal growth factor receptor in ovarian cancer cells. Mol Endocrinol. 2005;19:2564–78. doi: 10.1210/me.2004-0342. [DOI] [PubMed] [Google Scholar]
- 89.Shen X, Kramer RH. Adhesion-mediated squamous cell carcinoma survival through ligand-independent activation of epidermal growth factor receptor. Am J Pathol. 2004;165:1315–29. doi: 10.1016/S0002-9440(10)63390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lin Rz, Chou LF, Chien CC, Chang HY. Dynamic analysis of hepatoma spheroid formation: roles of E-cadherin and beta1-integrin. Cell Tissue Res. 2006;324:411–422. doi: 10.1007/s00441-005-0148-2. [DOI] [PubMed] [Google Scholar]
- 91.Kang HG, Jenabi JM, Zhang J, Keshelava N, Shimada H, May WA, Ng T, Reynolds CP, Triche TJ, Sorensen PH. E-cadherin cell-cell adhesion in weing tumor cells mediates suppression of anoikis through activation of the ErbB4 tyrosine kinase. Cancer Res. 2007;67:3094–105. doi: 10.1158/0008-5472.CAN-06-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shield K, Riley C, Quinn MA, Rice GE, Ackland ML, Ahmed N. alpha2beta1 integrin affects metastatic potential of ovarian carcinoma spheroids by supporting disaggregation and proteolysis. J Carcinog. 2007;6:11. doi: 10.1186/1477-3163-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sutherland RM. Cell and environment interactions in tumor microregions: the multicell spheroid model. Science. 1988;240:177–84. doi: 10.1126/science.2451290. [DOI] [PubMed] [Google Scholar]
- 94.Green SK, Francia G, Isidoro C, Kerbel RS. Anti-adhesive antibodies targeting E-cadherin sensitize multicellular tumor spheroids to chemotherapy in vitro. Mol Cancer Ther. 2004;3:149–59. [PubMed] [Google Scholar]
- 95.Desoize B, Jardillier J. Multicellular resistance: a paradigm for clinical resistance? Crit Rev Oncol Hematol. 2000;36:193–207. doi: 10.1016/s1040-8428(00)00086-x. [DOI] [PubMed] [Google Scholar]
- 96.Bates RC, Edwards NS, Yates JD. Spheroids and cell survival. Crit Rev Oncol Hematol. 2000;36:61–74. doi: 10.1016/s1040-8428(00)00077-9. [DOI] [PubMed] [Google Scholar]
- 97.Minchinton AI, Tannock IF. Drug penetration in solid tumors. Nat Rev Cancer. 2006;6:583–92. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 98.Frankel A, Rosen K, Filmus J, Kerbel RS. Induction of anoikis and suppression of human ovarian tumor growth in vivo by down-regulation of Bcl-X(L) Cancer Res. 2001;61:4837–41. [PubMed] [Google Scholar]
- 99.Ning Y, Buranda T, Hudson LG. Activated epidermal growth factor receptor induces integrin alpha2 internalization via caveolae/raft-dependent endocytic pathway. J Biol Chem. 2006;282:6380–7. doi: 10.1074/jbc.M610915200. [DOI] [PubMed] [Google Scholar]
- 100.Zeineldin R, Rosenberg M, Ortega D, Buhr C, Chavez MG, Stack MS, Kusewitt DF, Hudson LG. Mesenchymal transformation in epithelial ovarian tumor cells expressing epidermal growth factor receptor variant III. Mol Carcinog. 2006;45:851–60. doi: 10.1002/mc.20237. [DOI] [PubMed] [Google Scholar]
- 101.Ning Y, Zeineldin R, Liu Y, Rosenberg M, Stack MS, Ludson LG. down-regulation of integrin alpha2 surface expression by mutant epidermal growth factor receptor (EGFRvIII) induces aberrant cell spreading and focal adhesion formation. Cancer Res. 2005;65:9280–6. doi: 10.1158/0008-5472.CAN-05-0407. [DOI] [PubMed] [Google Scholar]
- 102.Do TV, Symowicz JC, Berman DM, Liotta LA, Petricoin EF, Stack MS, Fishman DA. Lysophosphatidic acid down-regulates stress fibers and up-regulates pro-matrix metalloproteinase-2 activation in ovarian cancer cells. Mol Cancer Res. 2007;5:121–31. doi: 10.1158/1541-7786.MCR-06-0319. [DOI] [PubMed] [Google Scholar]
- 103.Cowden-Dahl KD, Zeineldin R, Hudson LG. PEA3 is necessary for optimal epidermal growth factor receptor-stimulated matrix metalloproteinase expression and invasion of ovarian cancer cells. Mol Cancer Res. 2007;5:413–21. doi: 10.1158/1541-7786.MCR-07-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fishman DA, Liu Y, Ellerbroek SM, Stack MS. Lysophosphatidic acid promotes matrix metalloproteinase (MMP) activation and MMP-dependent invasion in ovarian cancer cells. Cancer Res. 2001;61:3194–9. [PubMed] [Google Scholar]
- 105.Ellerbroek SM, Hudson LG, Stack MS. Proteinase requirements of epidermal growth factor-induced ovarian cancer cell invasion. Int J Cancer. 1998;78:331–7. doi: 10.1002/(SICI)1097-0215(19981029)78:3<331::AID-IJC13>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 106.Ellerbroek SM, Halbleib JM, Benavidez M, Warmka JK, Wattenberg EV, Stack MS, Hudson LG. Phosphatidylinositol 3-kinase activity in epidermal growth factor-stimulated matrix metalloproteinase-9 production and cell surface association. Cancer Res. 2001;61:1855–61. [PubMed] [Google Scholar]
- 107.Fishman DA, Bafetti LM, Banionis S, Kearns AS, Chilukuri K, Stack MS. Production of extracellular matrix-degrading proteinases by primary cultures of human epithelial ovarian carcinoma cells. Cancer. 1997;80:1457–63. doi: 10.1002/(sici)1097-0142(19971015)80:8<1457::aid-cncr13>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 108.Young TN, Rodriguez GC, Rindhart AR, Bast RC, Jr, Pizzo SV, Stack MS. Characterization of gelatinases linked to extracellular matrix invasion in ovarian adenocarcinoma: purification of matrix metalloproteinase 2. Gynecol Oncol. 1996;62:89–99. doi: 10.1006/gyno.1996.0195. [DOI] [PubMed] [Google Scholar]
- 109.Choi JH, Choi KC, Auersperg N, Leung PC. Overexpression of follicle-stimulating hormone receptor activates oncogenic pathways in preneoplastic ovarian surface epithelial cells. J Clin Endocrinol metab. 2004;89:5508–16. doi: 10.1210/jc.2004-0044. [DOI] [PubMed] [Google Scholar]





