Abstract
Chromatin remodeling is an essential part of transcription initiation. We show that at heat shock gene promoters functional interactions between individual ATP-dependent chromatin remodeling complexes play critical role in both nucleosome displacement and Pol II recruitment. Using HSP12, HSP82 and SSA4 gene promoters as reporters, we demonstrated that while inactivation of SNF2, a critical ATPase of the SWI/SNF complex, primarily affects the HSP12 promoter, depletion of STH1- a SNF2 homolog from the RSC complex reduces histone displacement and abolishes the Pol II recruitment at all three promoters. From these results, we conclude that redundancy between SWI/SNF and RSC complexes is only partial and likely is affecting different chromatin remodeling steps. While inactivation of other individual ATP-dependent chromatin remodeling complexes negligibly affects reporter promoters, combinatorial inactivation of SNF2 and ISW1 has a synergistic effect by diminishing histone loss during heat induction and eliminating Pol II recruitment. Importantly, it also eliminates preloading of HSF on HSP82 and SSA4 promoters before heat shock and diminishes HSF binding during heat shock. These observations suggest that prior action of chromatin remodeling complexes is necessary for the activator binding.
INTRODUCTION
Chromatin remodeling at gene promoters plays a critical role in activation of transcription. It has been demonstrated that these chromatin changes may range from post-translational modifications of individual histones to the complete disassembly and removal of nucleosomes. The importance of chromatin remodeling is underscored by the demonstration that at least some transcriptional activators are dispensable for maintenance of transcription, when nucleosomes are unable to reassemble at a gene promoter (1,2). It has been proposed recently that the elimination of promoter nucleosomes is a critical rate-limiting step in the activation of transcription (3).
One of the central roles in the displacement of nucleosomes during initiation of transcription belongs to a large class of ATP-dependent chromatin remodeling complexes. These protein complexes are divided into families by homology of their protein subunits: SWI/SNF family (SWI/SNF and RSC), ISWI family (ISWI1 and ISWI2), CHD family (Chd1), INO80 family (INO80 and SWR1) (4,5). Since chromatin rearrangements play a crucial role during initiation of transcription, some of these complexes were suggested to play redundant roles (6) and/or functionally interact with each other (7,8). An involvement of individual complexes in chromatin remodeling events had been demonstrated for a number of specific genes, although functional interactions between these complexes were observed only in few instances, such as functional interactions between ISW1 and CHD1 at the ADH1 promoter (7) between ISW1 and SWI/SNF at Gal1-10 locus (9) and genetic interactions between ISW1, NuA4 and SWR1 (8). The mechanistic nature of these interactions remains largely unknown.
Heat shock genes represent an excellent model to investigate chromatin remodeling events, as upon induction these genes undergo the most extensive and rapid nucleosome rearrangements among known gene systems. For instance, at the HSP82 promoter significant nucleosome displacement is observed already during the first seconds after heat induction and reaches maximum nucleosome loss after 8 min (10–12). By contrast, it takes hours to reach maximum nucleosome displacement for other well-studied model systems such as PHO5 and Gal promoters (13–15). The extent of the nucleosome loss is also significantly higher for the HSP promoters in comparison to other gene systems (10,15).
It has been demonstrated that chromatin changes at gene promoters associated with transcriptional activation are generally resilient to inactivation of individual chromatin remodeling activities. For instance, inactivation of ISW1, ISW2 or Chd1 individually (7), did not change significantly expression of ADH2 gene. Even combinatorial inactivation of these activities had minor effects on kinetics of expression and relative nucleosome positioning. Similarly inactivation of SWI/SNF or Ino80 complexes individually or in combination with GCN5 (snf2 gcn5 and ino80 gcn5 double mutants) had either no or minor kinetic effects on PHO5 expression and promoter chromatin remodeling (6).
Chromatin remodeling at heat shock genes is not an exception in resilience to inactivation of individual chromatin remodeling activities. Elimination of SNF2—a critical ATPase of SWI/SNF complex, only slightly delays histone loss without significantly effecting histone elimination at HSP promoters (10,12). Elimination of Gcn5—histone acetylase of SAGA complex, affected basal level of HSP82 expression without an effect on induced levels (D.S. Gross personal communication). It has been demonstrated also that activation of HSP genes bypasses a need for such critical coactivators and general transcription factors as TFIIA, TAF9 (a subunit of TFIID and SAGA), Kin28 (a vital subunit of TFIIH), Med 17 and Med22 (subunits of Mediator complex) (16–19) and even the C-terminal domain of Pol II (20). The resilience of chromatin remodeling and transcriptional activation at HSP and other gene systems might indicate redundancy in function of individual components of machinery, as suggested previously (6), and requires additional investigation.
Recently we reported the involvement of the SWI/SNF complex in the robust chromatin remodeling at the highly inducible HSP12, HSP82 and SSA4 gene promoters (12). Here we present new findings indicating more prominent involvement of the RSC complex possibly affecting promoter Pol II loading and functional interactions between the SWI/SNF and ISW1 complexes. Double inactivation of the SWI/SNF and ISW1 complexes leads to synergistic diminishment of histone displacement, elimination of Pol II recruitment, and abolishment of promoter preloading of HSF—a master regulator of most HSP genes.
MATERIALS AND METHODS
Strains and cultivation conditions
Strains utilized in this study are indicated in Table 1. Strain YIS1 was constructed by substituting the −170 to +5094 SNF2 region (relative to translation start codone) with KanMX cassette PCR amplified from the pUG6 plasmid (21) according to the procedure in the above reference. Correct chromosomal integration was confirmed by a diagnostic PCR using SNF2 (−260 to− 228) and pUG6 primers.
Table 1.
Yeast strains used
| Strain | Genotype | Reference |
|---|---|---|
| FY1350 | MATα leu2Δ0 lys2Δ0 ura3Δ0 | (50) |
| FY1360 (ΔSNF2) | MATα leu2Δ1 snf2::LEU2 his3D200 ura3-52 lys2-173R2 | (50) |
| YTT186 (ΔISW1) | Mata ade2-1 can1-100 his3-11,15leu2-3,112 trp1-1 ura3-1RAD5 + isw1::ADE2 | (8) |
| YIS1 (ΔISW1ΔSNF2) | Same as YTT186 snf2::KanMX | This study |
| Tet-STH1 | pSTH1::kanR-tet07-TATA URA3::CMV-tTA MATa his3-1 leu2-0 met15-0 | Open Biosystems |
Saccharomyces cerevisiae strains were cultivated at 30°C to early log-phase in rich YPD broth supplemented with 0.04 mg/ml adenine. For kinetics experiments instantaneous up-shift was achieved by rapidly mixing equal volumes of 30°C culture with prewarmed 52°C medium and then incubating with shaking at 39°C for the times indicated. If necessary doxycycline was added to the cultivation media in the concentration of 10 µg/ml and cell cultures were grown overnight before heat shock experiments.
ChIP analysis
Chromatin immuno-precipitation (ChIP) was performed essentially as previously described (15) with the exception that protein A magnetic beads were used instead of protein A Sepharose beads to precipitate antigen-antibody complexes. Special attention was paid to the consistency of sonication levels of cell lysates. Before immunoprecipitation all samples were tested for the level of DNA fragmentation and the mean size of DNA fragments was always 500 bp. Antibodies specific for the following epitopes were used: histone H3 total (from AbCam; ab1791); Pol II - YSPT[pS]PS repeat of Pol II C-terminal domain (CTD) (4H8 monoclonal antibody from Upstate Biotechnology, this antibody recognizes both phospho- and non-phospho Pol II according to the manufacturer’s data); HSF [rabbit antibody raised and characterized previously (22)]. Immunoprecipitated DNA samples were used for real-time PCR with SYBR Green dye. Since PHO5 promoter is known to contain positioned nucleosomes (23) and its chromatin context does not change during heat shock (15), signals for individual gene promoters were normalized against the corresponding signal derived from the PHO5 promoter (for histone ChIPs) or the chromosome V intergenic region (in the case of Pol II and HSF ChIPs) and to the input DNA sample. We compared previously (12) normalization of the signal from the HSP12 promoter to either one of these regions and found no differences. For each DNA sample at least three consecutive dilutions of DNA were analyzed making certain that the amplification rate was always optimal and the change in amplification signal was proportional to the change in the amount of DNA. In addition, controls without DNA were always included to verify that primer-dimer formation was not detectable or comparable to the amplification from experimental samples. Experiments were typically repeated three times or more; error bars in the figures indicate standard deviations.
Primers for real-time PCR reactions were selected among a significant number of primers based on the PCR efficiency. Only those primer pairs were used that gave an amplification rate of at least 1.9 per PCR cycle during the linear amplification and did not produce primer-dimers. The sequences of PCR primers used in this study were as follows (coordinates are relative to ATG): PHO5 (from − 214 to − 192; − 20 to − 48), HSP12 (from − 304 to − 279 or − 337 to − 304; − 82 to − 107), HSP82 (from − 193 to − 167; − 37 to − 69), SSA4 (from − 307 to − 279; − 70 to − 98), chromosome V intergenic region (GCAATCAACATCTGAAGAAAAGAAAGTAGT, CATAATCTGCTGAAAAATGGCGTAAAT).
Western blotting
Yeast cells (50 ml) were grown in defined medium to an optical density at 600 nm of 0.7 and western blot analyses were performed using standard techniques. Protein isolations and sample preparation for SDS electrophoresis were done as described in ref. (24). Normalization of protein amount in samples was done by using Bio-Rad Protein Assay kit.
RESULTS
Using promoters of three highly inducible genes—HSP12, HSP82 and SSA4—as reporters of chromatin remodeling during the induction of heat stress, we monitored changes of different parameters during inactivation or depletion of known chromatin remodeling activities. The main method that we utilized was ChIP coupled with quantitative real-time PCR analysis, which allows a side-by-side comparison of events taking place simultaneously at different reporter promoters.
To monitor chromatin remodeling events at the indicated promoters, we used anti-H3 antibody raised against the C-terminal region of H3 (amino acids 125–135). This region is not known to be post-translationally modified, allowing a measure of total histone H3 abundance. Utilization of this antibody for quantification of a change in total H3 has been demonstrated previously by others (13,25). The results of such experiments are traditionally reported as a drop (1–0% or 100–0%) in histone content during gene activation. This form of presentation restricts analysis of the data, since the closer the values are to zero, the more difficult it is to see fine differences. Therefore we have chosen to present the results as an inverse value, which represents the degree of histone displacement and changes from 1 to ∞. This presentation format better conveys fine differential chromatin remodeling and has been utilized by us previously (11). While having the above-mentioned advantages, this form of presentation has a tendency to deemphasize significant histone losses (50–75% of initial level) seen in most of the mutants bearing inactivations of individual chromatin remodeling activities [(6,11,12) and discussed below.
Critical role of RSC complex in chromatin remodeling at heat shock gene promoters
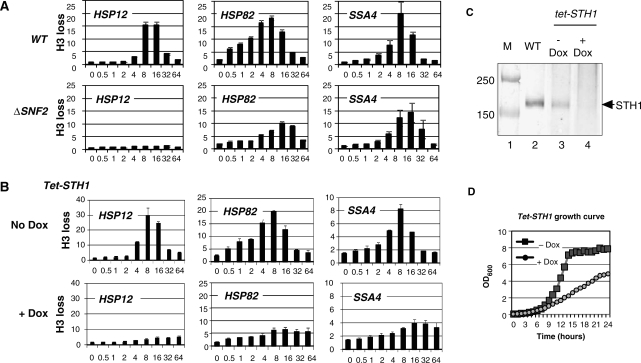
As we showed previously and confirmed with our new data [Figure 1A and ref. (12)], the inactivation of the SNF2—the ATPase subunit of SWI/SNF complex—leads to a mild delay of the histone H3 loss at the HSP82 and SSA4 promoters, while strongly affecting the HSP12 promoter. To test if other ATP-dependent chromatin remodeling complexes are involved and possibly functioning in redundant pathways at the HSP82 and SSA4 promoters, we investigated RSC complex, whose ATPase subunit STH1 is a paralogue of SNF2 (5,26,27). Since inactivation of STH1 is lethal, we employed a conditional knockdown yeast strain bearing a tetracycline regulatable element at the promoter of the STH1 gene. In this strain, the addition of doxycycline (a stable homolog of tetracycline) shuts down the expression of STH1 (Figure 1C). The results depicted in Figure 1B indicate that inactivation of STH1 expression affects all three promoters in two ways. First, the extent of histone H3 loss is diminished, and second, it takes a longer time to reach maximum of the histone displacement level. Since growth of the tet-STH1 strain in presence of doxycycline does not result in lethality as revealed by the complete deletion of the STH1 gene, but rather to a slow growing phenotype (Figure 1D), we conclude that results of Figure 1B reflect a depletion rather than complete elimination of STH1. Thus complete inactivation might have an even stronger effect on chromatin remodeling possibly leading to a complete elimination of histone displacement at all three reporter promoters.
Figure 1.
Inactivation of RSC complex diminishes histone loss at the HSP promoters during heat shock. ChIPs using an antibody raised against the C-terminus of histone H3 were performed in: (A) the ΔSNF2 or isogenic parental (WT) strain; (B) Tet-STH1 strain in presence (+Dox) or absence (no Dox) of doxycycline. The promoter analyzed is indicated in the upper left corner of each panel. y-axis: fold of histone H3 loss. x-axis: time after heat shock (0–64 min.). All real-time PCR values were normalized relative to the input and to the signal from the PHO5 promoter which is known to contain positioned nucleosomes that do not change during heat shock (15). Values represent mean ± SD (n ≥ 3). Note: histone loss value is an inverse value of histone abundance. (C) Western blot stained with anti-STH1 antibody. Lanes: 1, marker; 2, wt strain; 3, tet-STH1 strain grown without doxycycline; 4, tet-SHT1 strain grown in presence of doxycycline. Loading for each lane was normalized to the total protein level. (D) Growth curve of the tet-STH1 strain in presence (+Dox) or Absence (−Dox) of doxycycline. y- axis: optical density of cell culture at λ = 600 nm. x-axis: hours after inoculation.
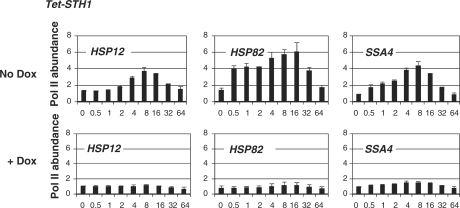
Since nucleosome displacement at the promoter is often a critical step for transcription initiation, we wanted to test if crippling of chromatin remodeling by STH1 knockdown had any effect on the recruitment of the RNA polymerase II (Pol II). To do this experiment we have used an antibody that recognizes both phosphorylated and unphosphorylated forms of Pol II (see ‘Materials and methods’ section). Our ChIPs therefore are able to provide information about transcription initiation events at specific promoters. This approach has an advantage of not having to deal with cross-reactivity issues of probes in canonical northern blot experiments or RT-PCR and not having to deal with the specific mRNA half life. The restriction of this approach is that it does not necessarily give a measure of completed transcription, but rather is a measure of the transcription initiation complex assembly. The data of Figure 2 indicates that all three promoters failed to attract Pol II when the expression of STH1 is shut down by the presence of doxycycline. The effect of STH1 inactivation, even though it is not a complete elimination, is drastically stronger than the effect of SNF2 inactivation on Pol II recruitment at the same reporter promoters described previously (12).
Figure 2.
Inactivation of STH1 eliminates Pol II recruitment at the HSP promoters. ChIPs utilizing antibody against Pol II performed in the tet-STH1 stain in presence or absence of doxycycline. The promoter analyzed is indicated in the upper left corner of each panel. y-axis: abundance of Pol II. x-axis: time after heat shock (0–64 min). All real-time PCR values were normalized relative to the input and to the background values of the intergenic region of chromosome V. Values represent mean ± SD (n ≥ 3).
ISW1 complex affects kinetics of chromatin remodeling
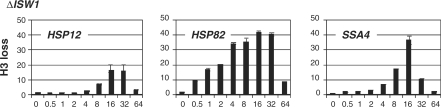
Considering that two ATP-dependent chromatin remodeling complexes—SWI/SNF and RSC—influence chromatin remodeling events at the HSP12, HSP82 and SSA4 promoters, we wanted to test if other ATP-dependent complexes are involved. We systematically tested the effects of inactivation of critical subunits of known chromatin remodeling complexes and found that individual inactivations did not influence significantly the nucleosome displacement events taking place at the reporter promoters. However, one relatively noticeable effect we observed was with the deletion of ISW1, which caused a delay in reaching maximum histone loss at HSP12 and SSA4 promoters or longer maintenance of histone displacement at the HSP82 promoter (Figure 3), similar to what was observed for the HSP82 and SSA4 promoters in the ΔSNF2 strain (Figure 1A).
Figure 3.
Deletion of ISW1 delays histone H3 displacement at the HSP promoters. ChIPs using antibody against the C-terminus of histone H3 were performed in the ΔISW1 strain. The analyzed promoter is indicated in the upper left corner of each panel. Axis designation is the same as in Figure 1.
SWI/SNF and ISW1 complexes cooperate functionally
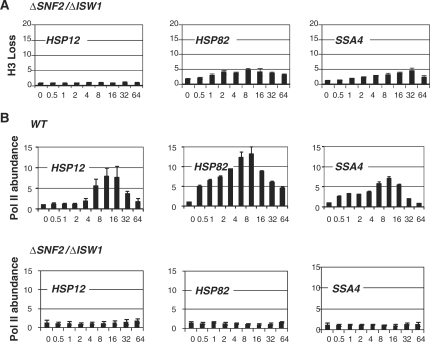
Since the effects we observed were similarly subtle (Figure 1A and 3) and were reflected in a delay of chromatin remodeling, we wanted to test if SWI/SNF and ISW1 complexes were functionally redundant. By inactivation of both SNF2 and ISW1, and analyzing kinetics and the extent of histone loss (Figure 4A) we found a synergistic effect—HSP82 and SSA4 reporter promoters showed drastically lower levels of chromatin remodeling than the histone loss observed after inactivation of SNF2 and ISW1 individually (Figure 1A and 3). For the HSP12 promoter, as we reported pereviously (12), loss of SNF2 alone was enough to abolished chromatin remodeling.
Figure 4.
Combination of SNF2 and ISW1 deletions cripples histone displacement and eliminates Pol II recruitments at the HSP promoters. (A) ChIPs using antibody against the C-terminus of histone H3 were performed in the ΔSNF2 ΔISW1 stain as in Figure 3. (B) ChIPs utilizing antibody against Pol II of the same strain were performed as in Figure 2. WT data refers to the strain FY1350 (Table1).
Since we observed that significant diminishment of chromatin remodeling in case of STH1 knockdown eliminates recruitment of Pol II (Figure 2) we wanted to test if combinatorial inactivation of SNF2 and ISW1 has the same effect on Pol II. Figure 4B shows that Pol II was absent at all three reporter promoters during the time course of heat induction. These results underscore the importance of proper nucleosome displacement for transcription initiation.
Binding of HSF at HSP promoters depends on function of ISW1 or RSC complexes
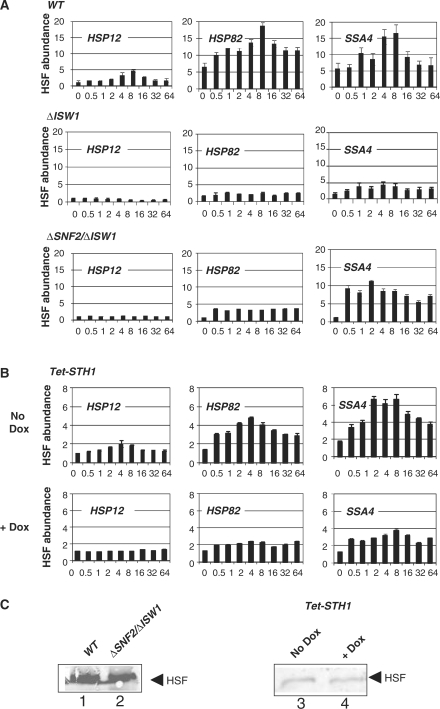
Another important factor determining both chromatin remodeling events and transcription initiation complex assembly is activator binding to the promoter. For the majority of heat shock genes the principal activator is HSF, although some stress genes are regulated with partially redundant Msn2 and Msn4 activators (28). There is a partial overlap between the sets of genes regulated by HSF and Msn2/4, with HSP12 being an example of a gene under the influence of both activator systems with Msn2/4 playing a dominant role (11,28). To test if activator binding is affected by crippling chromatin remodeling machinery, we used HSF ChIPs in the ΔISW1 and ΔSNF2/ΔISW1 mutant strains and compared it to the WT (Figure 5). The results of these experiments show that binding of HSF to the HSP12 promoter was eliminated, while binding to the HSP82 and SSA4 promoters was reduced in both ΔISW1 and ΔSNF2/ΔISW1 strains. Moreover, while HSP82 and SSA4 promoters are characterized by significant preloading of HSF before heat shock (11), the ΔISW1 and ΔSNF2/ΔISW1 strains showed no binding of HSF before heat shock at these promoters. These data suggest that the ISW1 inactivation is likely affecting nucleosome dynamics by diminishing activator binding to the promoters.
Figure 5.
Preloading of HSF at the HSP82 and SSA4 promoters depends on the function of SWI/SNF and ISW1 complexes. (A) ChIPs utilizing antibody against yeast HSF were performed in the ΔISW, ΔSNF2 ΔISW1 or isogenic parental (WT) strain. The promoter analyzed is indicated in the upper left corner of each panel. y-axis: abundance of HSF. x-axis: time after heat shock (0–64 min.). All real-time PCR values were normalized relative to the input and to the background values of the PHO5. Values represent mean ± SD (n ≥ 3). (B) Similar experiments as in panel A were performed in the strain expressing STH1 under tet-dependent promoter in the absence (No Dox) or in the presence ( + Dox) of doxycycline. (C) Western blotting performed with proteins isolated from the indicated strains. For each strain loading was normalized to the total protein level.
To test if activity of RSC complex similarly affects activator loading at the reporter HSP promoters we performed HSF ChIPs in the tet-STH1 strain. We found that the depletion of STH1 by shutting down the STH1 promoter with doxycycline affects HSF binding to the promoters. Considering, as we argued above comparing survivability of the tet-STH1 strain and lethality of the STH1 deletion, that STH1 is only severely depleted (Figure 1C) but not completely eliminated, the effect of complete inactivation of RSC might be even stronger. The lower overall abundance of HSF at the reporter promoters (Figure 5B—no Dox) in comparison with the WT strain (Figure 5A) can be explained by lower STH1 expression even without doxycycline (Figure 1C). These results again suggest that chromatin remodeling might be an important factor for activator binding.
Since chromatin remodeling might be gene specific, it can change the balance between proteins thus affecting relative HSF level in the cell. To test this possibility we performed western blotting analysis (Figure 5C), which showed that there is no significant change in HSF level during inactivation of either chromatin remodeling activity. That might indicate that chromatin remodeling is not so critical for the constitutive expression of HSF1 gene.
DISCUSSION
Related SWI/SNF and RSC complexes play partially overlapping but not redundant function at heat shock gene promoters
In this study we tested the involvement of known ATP-dependent chromatin remodeling complexes in rapid and extensive nucleosome displacement at the highly inducible yeast heat shock gene promoters. We observed a significant difference in the dependence of reporter promoters on the activity of particular complexes. Inactivation of specific complexes generally at least for the PHO5 promoter had either no or mild gene specific effects (6), which might suggests a redundancy in functional involvement. By far the strongest effect we have detected was with the depletion of STH1. Only in this case the depletion of the RSC complex affects all three reporter promoters which is reflected in diminishment of chromatin remodeling and more importantly in the elimination of Pol II recruitment (Figures 2 and 3). These data are in agreement with the reported association between the Rcs4 component and Rpb5, a conserved subunit shared by all three nuclear RNA polymerases (29). Although the involvement of RSC complex in chromatin remodeling at gene promoters was reported previously (30), we have demonstrated for the first time that RSC complex is critical for chromatin remodeling and Pol II recruitment at the HSP promoters.
The idea of redundancy in the action of chromatin remodeling complexes contradicts to the different outcomes of SWI/SNF and RSC inactivation. The RSC and SWI/SNF complexes are very related complexes with five shared subunits and critical ATPase subunits SNF2 and STH1 sharing some structural motifs (26,31–33). Yet we observed significant differences in effects of inactivation of SNF2 and STH1. The deletion of SNF2 has an adverse effect on both chromatin remodeling and Pol II recruitment at the HSP12 promoter, while the effect on the HSP82 and SSA4 is very mild, comprising a slight diminishment and a delay of both processes (12). This promoter specificity likely stems from the dominance of promoters with different activators—HSF for the HSP82 and SSA4 promoters, and Msn2/4 for the HSP12 promoter (12,28). Additionally, while HSP82 and SSA4 promoters are partially occupied with HSF before heat shock, the HSP12 promoter likely is free of an activator and requires assistance from the SWI/SNF complex (11). The situation is different for the RSC complex, since the inactivation of this complex similarly affects all three reporter promoters. These effects might be a result of association of RSC with Pol II and not due to recruitment by a gene-specific activator. This general action of the RSC complex is consistent with it being the only essential ATP-dependent chromatin remodeler.
Cooperation between SWI/SNF and ISW1 complexes
A synergistic effect of SNF2 and ISW1 inactivation observed in our study is another indication of functional interactions between ATP-dependent chromatin remodeling complexes. Based on DNA microarray results, the ISW1 deletion has only subtle effects resulting in 1.5–2-fold change in gene expression level (34). This subtlety suggests that ISW1 might be involved in some form of functionally interactions with other complexes. For example, functional interaction between ISW1 and CHD1 is required for ADH2 activation (7), and functional interactions between ISW1 and SWI/SNF is necessary for transcriptional memory at the yeast Gal1-10 gene cluster (9). In addition, tripartite genetic interactions between ISW1, NuA4 and SWR1 complexes are important to repress Msn2/4 regulated genes (8).
An involvement of SWI1 in chromatin remodeling has been demonstrated for the HIS3 and SER3 promoters (35,36). Importantly, in both cases mild effects of SWI1 inactivation correlated with independent requirement of the SWI/SNF complex for chromatin remodeling. Here we report for the first time that inactivation of both SNF2 and ISW1 has a strong synergistic effect, resulted in diminishment of histone loss and elimination of Pol II recruitment at all three reporter HSP promoters. Since it has been demonstrated that SWI/SNF complex is recruited to gene promoters by gene specific activators (37), and ISW1 is required for phosphorylation of Pol II CTD, and influences elongation-phase of transcription (38), it is reasonable to hypothesize that cooperation between SWI/SNF and ISW1 complexes stems from chromatin remodeling necessary for both promoter opening and Pol II firing.
One possible mechanistic explanation for cooperation between SWI/SNF and ISW1 complexes can be based on the reported differences in modes of nucleosome remodeling for these two complexes. While SWI/SNF complex causes disordered nucleosome positioning, thereby promoting transcription factor binding and gene activation (39), ISWI type remodelers space nucleosomes with regular distance from one another (40,41). Considering these mechanistic differences, we hypothesize that the SWI/SNF complex creates and maintains an original nucleosome free window distorting uniform nucleosome spacing, while ISW1 by equilibrating the distances between nucleosomes propagates nucleosome movement along the entire gene.
Although we cannot formally exclude indirect effects caused by inactivation of subunits of chromatin remodeling complexes, there are data which contradict this idea. First, we and others previously showed that SWI/SNF complex is directly recruited the heat shock gene promoters during heat induction (10,12,42). The RSC complex also was shown to be directly involved in chromatin remodeling at HSP and other promoters (43). Physical interactions between RSC and Pol II (29) also suggest that the RSC complex is directly involved at least in single nucleosome events at gene promoters (30).
Chromatin remodeling by action of either ISW1 or RSC complexes is necessary for preloading of HSF at the HSP promoters
Another important aspect of our study is a demonstration that preliminary HSF binding to the cognate promoters is dependent on the function of chromatin remodeling complexes. While in higher eukaryotes, HSF binds to corresponding promoters in a heat shock dependent manner, in yeast S. cerevisiae it was originally proposed that this binding is constitutive (44–46). Later investigation showed that yeast HSF at least partially binds to gene promoters in the heat inducible manner (11,47–49). But even these studies showed that certain HSP promoters, including HSP82 and SSA4, are partially occupied with HSF before heat shock and the abundance of HSF only slightly increases upon heat shock (11,49). Here we show that occupancy of HSP82 and SSA4 promoters with HSF depends on the function of both ISW1 and RSC complexes, as depletion or elimination of either one causes greatly decreased occupancy. While binding of HSF at the HSP82 and SSA4 promoters was not affected significantly in the ΔSNF2 (12), the binding of HSF before heat shock to all three reporter promoters was absent in the ΔISW1 and ΔSNF2ΔISW1 strains and during depletion of STH1 and increased upon heat shock only slightly. Our data suggest that a pre-emptive action of chromatin remodeling complexes is necessary for activator binding to the promoters at least for HSF in case of some HSP promoters. Perhaps this action is encompassed in directing consistent nucleosome movement creating a ‘window’ of opportunity for activator binding. In sum, our experimental data suggest that chromatin remodeling is a critical part of activation of HSP genes. Crippling of chromatin remodeling impedes two essential steps of gene activation—binding of the activator molecule and assembly of the transcription initiation complex.
FUNDING
Grants awarded to A.M.E. from the National Science Foundation [MCB- 0845297]; and the NIH [P20 RR016479] from INBRE Program of the National Center for Research Resources. Funding for open access charges: National Science Foundation.
Conflict of interest statement. None declared.
ACKNOWLEDGEMENTS
The authors thank David Gross and Evelyn Schlenker for critical reading of the manuscript and helpful suggestions, Fred Winston for yeast strains and Brad Cairns for anti-STH1 antibody.
REFERENCES
- 1.Adkins MW, Tyler JK. Transcriptional activators are dispensable for transcription in the absence of Spt6-mediated chromatin reassembly of promoter regions. Mol. Cell. 2006;21:405–416. doi: 10.1016/j.molcel.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Zhang H, Reese JC. Exposing the core promoter is sufficient to activate transcription and alter coactivator requirement at RNR3. Proc. Natl Acad. Sci. USA. 2007;104:8833–8838. doi: 10.1073/pnas.0701666104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boeger H, Griesenbeck J, Kornberg RD. Nucleosome retention and the stochastic nature of promoter chromatin remodeling for transcription. Cell. 2008;133:716–726. doi: 10.1016/j.cell.2008.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gangaraju VK, Bartholomew B. Mechanisms of ATP dependent chromatin remodeling. Mutat. Res. 2007;618:3–17. doi: 10.1016/j.mrfmmm.2006.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith CL, Peterson CL. ATP-dependent chromatin remodeling. Curr. Top Dev. Biol. 2005;65:115–148. doi: 10.1016/S0070-2153(04)65004-6. [DOI] [PubMed] [Google Scholar]
- 6.Barbaric S, Luckenbach T, Schmid A, Blaschke D, Horz W, Korber P. Redundancy of chromatin remodeling pathways for the induction of the yeast PHO5 promoter in vivo. J. Biol. Chem. 2007;282:27610–27621. doi: 10.1074/jbc.M700623200. [DOI] [PubMed] [Google Scholar]
- 7.Xella B, Goding C, Agricola E, Di Mauro E, Caserta M. The ISWI and CHD1 chromatin remodelling activities influence ADH2 expression and chromatin organization. Mol. Microbiol. 2006;59:1531–1541. doi: 10.1111/j.1365-2958.2005.05031.x. [DOI] [PubMed] [Google Scholar]
- 8.Lindstrom KC, Vary JC, Jr, Parthun MR, Delrow J, Tsukiyama T. Isw1 functions in parallel with the NuA4 and Swr1 complexes in stress-induced gene repression. Mol. Cell Biol. 2006;26:6117–6129. doi: 10.1128/MCB.00642-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kundu S, Horn PJ, Peterson CL. SWI/SNF is required for transcriptional memory at the yeast GAL gene cluster. Genes Dev. 2007;21:997–1004. doi: 10.1101/gad.1506607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao J, Herrera-Diaz J, Gross DS. Domain-Wide Displacement of Histones by Activated Heat Shock Factor Occurs Independently of Swi/Snf and Is Not Correlated with RNA Polymerase II Density. Mol. Cell Biol. 2005;25:8985–8999. doi: 10.1128/MCB.25.20.8985-8999.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erkina TY, Erkine AM. Displacement of histones at promoters of Saccharomyces cerevisiae heat shock genes is differentially associated with histone H3 acetylation. Mol. Cell Biol. 2006;26:7587–7600. doi: 10.1128/MCB.00666-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erkina TY, Tschetter PA, Erkine AM. Different requirements of the SWI/SNF complex for robust nucleosome displacement at promoters of heat shock factor and Msn2- and Msn4-regulated heat shock genes. Mol. Cell Biol. 2008;28:1207–1217. doi: 10.1128/MCB.01069-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kristjuhan A, Svejstrup JQ. Evidence for distinct mechanisms facilitating transcript elongation through chromatin in vivo. Embo J. 2004;23:4243–4252. doi: 10.1038/sj.emboj.7600433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reinke H, Horz W. Histones are first hyperacetylated and then lose contact with the activated PHO5 promoter. Mol. Cell. 2003;11:1599–1607. doi: 10.1016/s1097-2765(03)00186-2. [DOI] [PubMed] [Google Scholar]
- 15.Erkine AM, Gross DS. Dynamic chromatin alterations triggered by natural and synthetic activation domains. J. Biol. Chem. 2003;278:7755–7764. doi: 10.1074/jbc.M211703200. [DOI] [PubMed] [Google Scholar]
- 16.Apone LM, Virbasius CA, Holstege FC, Wang J, Young RA, Green MR. Broad, but not universal, transcriptional requirement for yTAFII17, a histone H3-like TAFII present in TFIID and SAGA. Mol. Cell. 1998;2:653–661. doi: 10.1016/s1097-2765(00)80163-x. [DOI] [PubMed] [Google Scholar]
- 17.Chou S, Chatterjee S, Lee M, Struhl K. Transcriptional activation in yeast cells lacking transcription factor IIA. Genetics. 1999;153:1573–1581. doi: 10.1093/genetics/153.4.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee D, Lis JT. Transcriptional activation independent of TFIIH kinase and the RNA polymerase II mediator in vivo. Nature. 1998;393:389–392. doi: 10.1038/30770. [DOI] [PubMed] [Google Scholar]
- 19.Moqtaderi Z, Keaveney M, Struhl K. The histone H3-like TAF is broadly required for transcription in yeast. Mol. Cell. 1998;2:675–682. doi: 10.1016/s1097-2765(00)80165-3. [DOI] [PubMed] [Google Scholar]
- 20.McNeil JB, Agah H, Bentley D. Activated transcription independent of the RNA polymerase II holoenzyme in budding yeast. Genes Dev. 1998;12:2510–2521. doi: 10.1101/gad.12.16.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guldener U, Heck S, Fielder T, Beinhauer J, Hegemann JH. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 1996;24:2519–2524. doi: 10.1093/nar/24.13.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erkine AM, Adams CC, Diken T, Gross DS. Heat shock factor gains access to the yeast HSC82 promoter independently of other sequence-specific factors and antagonizes nucleosomal repression of basal and induced transcription. Mol. Cell Biol. 1996;16:7004–7017. doi: 10.1128/mcb.16.12.7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korber P, Luckenbach T, Blaschke D, Horz W. Evidence for histone eviction in trans upon induction of the yeast PHO5 promoter. Mol. Cell Biol. 2004;24:10965–10974. doi: 10.1128/MCB.24.24.10965-10974.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kushnirov VV. Rapid and reliable protein extraction from yeast. Yeast. 2000;16:857–860. doi: 10.1002/1097-0061(20000630)16:9<857::AID-YEA561>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 25.Lee CK, Shibata Y, Rao B, Strahl BD, Lieb JD. Evidence for nucleosome depletion at active regulatory regions genome-wide. Nat. Genet. 2004;36:900–905. doi: 10.1038/ng1400. [DOI] [PubMed] [Google Scholar]
- 26.Du J, Nasir I, Benton BK, Kladde MP, Laurent BC. Sth1p, a Saccharomyces cerevisiae Snf2p/Swi2p homolog, is an essential ATPase in RSC and differs from Snf/Swi in its interactions with histones and chromatin-associated proteins. Genetics. 1998;150:987–1005. doi: 10.1093/genetics/150.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Vugt JJ, Ranes M, Campsteijn C, Logie C. The ins and outs of ATP-dependent chromatin remodeling in budding yeast: biophysical and proteomic perspectives. Biochim. Biophys. Acta. 2007;1769:153–171. doi: 10.1016/j.bbaexp.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 28.Boy-Marcotte E, Lagniel G, Perrot M, Bussereau F, Boudsocq A, Jacquet M, Labarre J. The heat shock response in yeast: differential regulations and contributions of the Msn2p/Msn4p and Hsf1p regulons. Mol. Microbiol. 1999;33:274–283. doi: 10.1046/j.1365-2958.1999.01467.x. [DOI] [PubMed] [Google Scholar]
- 29.Soutourina J, Bordas-Le Floch V, Gendrel G, Flores A, Ducrot C, Dumay-Odelot H, Soularue P, Navarro F, Cairns BR, Lefebvre O, et al. Rsc4 connects the chromatin remodeler RSC to RNA polymerases. Mol. Cell Biol. 2006;26:4920–4933. doi: 10.1128/MCB.00415-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parnell TJ, Huff JT, Cairns BR. RSC regulates nucleosome positioning at Pol II genes and density at Pol III genes. Embo J. 2008;27:100–110. doi: 10.1038/sj.emboj.7601946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cairns BR, Erdjument-Bromage H, Tempst P, Winston F, Kornberg RD. Two actin-related proteins are shared functional components of the chromatin-remodeling complexes RSC and SWI/SNF. Mol. Cell. 1998;2:639–651. doi: 10.1016/s1097-2765(00)80162-8. [DOI] [PubMed] [Google Scholar]
- 32.Cairns BR, Schlichter A, Erdjument-Bromage H, Tempst P, Kornberg RD, Winston F. Two functionally distinct forms of the RSC nucleosome-remodeling complex, containing essential AT hook, BAH, and bromodomains. Mol. Cell. 1999;4:715–723. doi: 10.1016/s1097-2765(00)80382-2. [DOI] [PubMed] [Google Scholar]
- 33.Laurent BC, Yang X, Carlson M. An essential Saccharomyces cerevisiae gene homologous to SNF2 encodes a helicase-related protein in a new family. Mol. Cell Biol. 1992;12:1893–1902. doi: 10.1128/mcb.12.4.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vary JC, Jr, Gangaraju VK, Qin J, Landel CC, Kooperberg C, Bartholomew B, Tsukiyama T. Yeast Isw1p forms two separable complexes in vivo. Mol. Cell Biol. 2003;23:80–91. doi: 10.1128/MCB.23.1.80-91.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim Y, McLaughlin N, Lindstrom K, Tsukiyama T, Clark DJ. Activation of Saccharomyces cerevisiae HIS3 results in Gcn4p-dependent, SWI/SNF-dependent mobilization of nucleosomes over the entire gene. Mol. Cell Biol. 2006;26:8607–8622. doi: 10.1128/MCB.00678-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martens JA, Winston F. Evidence that Swi/Snf directly represses transcription in S. cerevisiae. Genes Dev. 2002;16:2231–2236. doi: 10.1101/gad.1009902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neely KE, Hassan AH, Brown CE, Howe L, Workman JL. Transcription activator interactions with multiple SWI/SNF subunits. Mol. Cell Biol. 2002;22:1615–1625. doi: 10.1128/MCB.22.6.1615-1625.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morillon A, Karabetsou N, O'S;ullivan J, Kent N, Proudfoot N, Mellor J. Isw1 chromatin remodeling ATPase coordinates transcription elongation and termination by RNA polymerase II. Cell. 2003;115:425–435. doi: 10.1016/s0092-8674(03)00880-8. [DOI] [PubMed] [Google Scholar]
- 39.Schnitzler GR, Cheung CL, Hafner JH, Saurin AJ, Kingston RE, Lieber CM. Direct imaging of human SWI/SNF-remodeled mono- and polynucleosomes by atomic force microscopy employing carbon nanotube tips. Mol. Cell Biol. 2001;21:8504–8511. doi: 10.1128/MCB.21.24.8504-8511.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitehouse I, Tsukiyama T. Antagonistic forces that position nucleosomes in vivo. Nat. Struct. Mol. Biol. 2006;13:633–640. doi: 10.1038/nsmb1111. [DOI] [PubMed] [Google Scholar]
- 41.Gangaraju VK, Bartholomew B. Dependency of ISW1a chromatin remodeling on extranucleosomal DNA. Mol. Cell Biol. 2007;27:3217–3225. doi: 10.1128/MCB.01731-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shivaswamy S, Iyer VR. Stress-dependent dynamics of global chromatin remodeling in yeast: dual role for SWI/SNF in the heat shock stress response. Mol. Cell Biol. 2008;28:2221–2234. doi: 10.1128/MCB.01659-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Damelin M, Simon I, Moy TI, Wilson B, Komili S, Tempst P, Roth FP, Young RA, Cairns BR, Silver PA. The genome-wide localization of Rsc9, a component of the RSC chromatin-remodeling complex, changes in response to stress. Mol. Cell. 2002;9:563–573. doi: 10.1016/s1097-2765(02)00475-6. [DOI] [PubMed] [Google Scholar]
- 44.Gross DS, English KE, Collins KW, Lee SW. Genomic footprinting of the yeast HSP82 promoter reveals marked distortion of the DNA helix and constitutive occupancy of heat shock and TATA elements. J. Mol. Biol. 1990;216:611–631. doi: 10.1016/0022-2836(90)90387-2. [DOI] [PubMed] [Google Scholar]
- 45.Jakobsen BK, Pelham HR. Constitutive binding of yeast heat shock factor to DNA in vivo. Mol. Cell Biol. 1988;8:5040–5042. doi: 10.1128/mcb.8.11.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sorger PK, Lewis MJ, Pelham HR. Heat shock factor is regulated differently in yeast and HeLa cells. Nature. 1987;329:81–84. doi: 10.1038/329081a0. [DOI] [PubMed] [Google Scholar]
- 47.Erkine AM, Magrogan SF, Sekinger EA, Gross DS. Cooperative binding of heat shock factor to the yeast HSP82 promoter in vivo and in vitro. Mol. Cell Biol. 1999;19:1627–1639. doi: 10.1128/mcb.19.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giardina C, Lis JT. Dynamic protein-DNA architecture of a yeast heat shock promoter. Mol. Cell Biol. 1995;15:2737–2744. doi: 10.1128/mcb.15.5.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hahn JS, Hu Z, Thiele DJ, Iyer VR. Genome-wide analysis of the biology of stress responses through heat shock transcription factor. Mol. Cell Biol. 2004;24:5249–5256. doi: 10.1128/MCB.24.12.5249-5256.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martens JA, Wu PY, Winston F. Regulation of an intergenic transcript controls adjacent gene transcription in Saccharomyces cerevisiae. Genes Dev. 2005;19:2695–2704. doi: 10.1101/gad.1367605. [DOI] [PMC free article] [PubMed] [Google Scholar]