Abstract
Background
Hill-Sachs lesions are often present with recurrent shoulder instability and may be a cause of failed Bankart repair.
Hypothesis
Glenohumeral joint stability decreases with increasingly larger humeral head defects.
Study Design
Descriptive laboratory study.
Methods
Humeral head defects, 1/8, 3/8, 5/8, and 7/8 of the humeral head radius, were created in 8 human cadaveric shoulders, simulating Hill-Sachs defects. Testing positions included 45° and 90° of abduction and 40° of internal rotation, neutral, and 40° of external rotation. Testing occurred at each defect size sequentially from smallest to largest for all abduction and rotation combinations. The humeral head was translated at 0.5 mm/s 45° anteroinferiorly to the horizontal glenoid axis until dislocation. Distance to dislocation, defined as humeral head translation until it began to subluxate, was the primary outcome measure.
Results
Significant factors by ANOVA were rotation (P < .001) and defect size (P < .001). There was no difference for the 2 abduction angles. External rotation of 40° significantly reduced distance to dislocation compared with neutral and 40° internal rotation (P < .001). Osteotomies of 5/8 and 7/8 radius significantly decreased distance to dislocation over the intact state (P = .009 and P < .001, respectively). Post hoc analysis determined significant differences for the rotational positions. Decreased distance to dislocation occurred at 5/8 radius osteotomy at 40° external rotation with 90° of abduction (P = .008). For the 7/8 radius osteotomy at 90° abduction, there was a decreased distance to dislocation for neutral and 40° external rotation (P < .001); at 45° abduction, there was a decreased distance to dislocation at 40° external rotation (P < .001). With the humerus internally rotated, there was no significant change in distance to dislocation.
Conclusion
Glenohumeral stability decreases at a 5/8 radius defect in external rotation and abduction. At 7/8 radius, there was a further decrease in stability at neutral and external rotation.
Clinical Relevance
Defects of 5/8 the humeral head radius may require treatment to decrease the failure rate of shoulder instability repair.
Keywords: Hill-Sachs, shoulder instability, Bankart repair, dislocation
INTRODUCTION
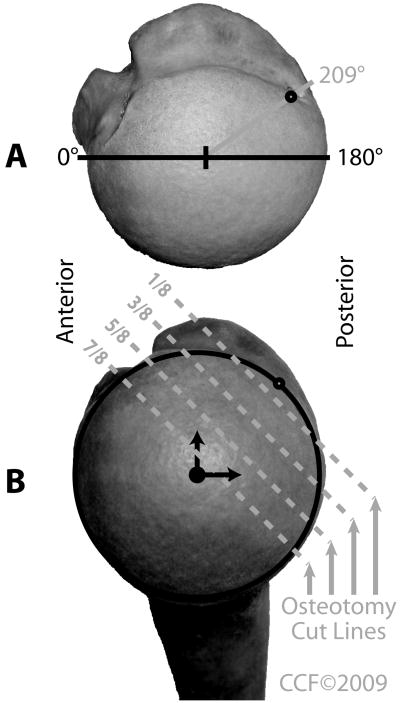
Approximately 95% of patients with an anterior shoulder dislocation sustain either a humeral or glenoid bony lesion.9 Both lesions are known to contribute to recurrent glenohumeral subluxation. Humeral head lesions have been reported in as many as 80% of first-time dislocations, in almost 100% of recurrent shoulder dislocations, and even in many patients with only subluxation.4,26,29 The occurrence of humeral head impression fractures was first documented in 1940 by Hill and Sachs, and such a fracture is thus commonly referred to as a Hill-Sachs lesion.12 These defects typically occur when the posterolateral aspect of the anteriorly dislocated humeral head impacts against the glenoid rim. The center of the defect is located at a mean of 209° along an axial axis of the articular surface, with an average defect arc of 52°.4,25 Hill-Sachs lesions are typically larger with increasing numbers of subsequent dislocations.5
The importance of studying humeral head defects relates to their effect on treatment options for recurrent dislocation. For isolated glenoid defects, the cadaveric studies by Itoi et al13,31 have helped guide the treatment of recurrent glenohumeral dislocation in the setting of glenoid bone deficiency. These findings have been supported by clinical data demonstrating a higher rate of failure of soft tissue Bankart repair alone.1,3,22 As a result, glenohumeral dislocation with large anteroinferior glenoid bone loss resulting in an inverted pear-shaped glenoid is now often treated with restoration of bony support.1,3,6,18 As for humeral defects, clinical data have shown that a significant Hill-Sachs lesion will also lead to higher rates of failure if treated solely with a soft tissue Bankart repair.3-5 Therefore, these lesions must often be treated with either restoration of the humeral head articular arc via bone grafting or hemiarthroplasty.11,14,23,24 In some cases, more complex procedures may be indicated, such as tendon transfers or even a humeral osteotomy (J. B. Willis, 1981, unpublished data).6,7,30 However, no guidelines exist for the treatment of glenohumeral dislocation in the setting of a significant Hill-Sachs lesion. No published cadaveric study has been done to determine the size of the Hill-Sachs lesion that requires treatment beyond what is performed for a patient without such a lesion.
Although not examined exclusively in the literature, the rate of recurrent dislocation also likely depends on the size of the humeral defect. Some authors have speculated that defects involving less than 20% of the humeral head’s articular surface are of little clinical significance, lesions between 20% and 40% may contribute somewhat to recurrent glenohumeral dislocation, and larger lesions probably result in greater likelihood of dislocation.3,6,15,20,29 Sekiya et al28 did evaluate humeral head defects and concluded that defects of 25% of the humeral head diameter had decreased glenohumeral stability.
The purpose of this study was to investigate the relationship between the size of a humeral head defect and the resulting glenohumeral stability. We used a human cadaveric shoulder model closely adapted from the methods successfully used by Itoi et al13 in their study of glenoid bone loss effects on glenohumeral stability. We hypothesized that larger humeral defects would result in decreased glenohumeral stability.
MATERIALS AND METHODS
Specimen Preparation
This study was approved by the Cleveland Clinic Foundation Institutional Review Board and carried out in accordance with institutional guidelines. Eight fresh-frozen cadaveric shoulder specimens were obtained from the National Disease Research Interchange (Philadelphia, Pennsylvania) for testing. Each specimen was visually inspected to eliminate those with physical abnormalities, glenohumeral osteoarthritis, or prior shoulder surgery. The specimens were stored at −20°C and thawed overnight at room temperature before testing.10 Our specimen preparation and testing methods were adapted from those used by Itoi et al,13 who evaluated the effects of defect size on the glenoid. All soft tissues superficial to the rotator cuff muscles were removed, and the muscles themselves were elevated from the scapula. The tendinous portions of the rotator cuff were bluntly separated from the capsule in a medial-to-lateral direction ending at a level 1 cm lateral to the glenohumeral joint. Further elevation of the rotator cuff tendons was avoided to minimize damage to the capsuloligamentous structures. The muscles of the arm and the periosteum were removed from the humeral shaft, which was cut 25 cm from the humeral head.
The glenohumeral coordinate systems were placed in a clinically relevant location previously shown to be effective in robotic simulations of the shoulder by Debski et al.8 The coordinate system for the glenoid was placed on the center of the glenoid face. The X-axis was defined as perpendicular to the scapular plane and directed anteriorly, the Y-axis was parallel to the scapular plane and directed superiorly, and the Z-axis was obtained from the cross-product of the Xand Y-axes and directed medially. Rotation about the Xaxis defined abduction. The coordinate systemof the humerus was placed at the center of humeral rotation. The X-axis was defined as being parallel to the long axis of the humerus and directed proximally. Rotation about the X-axis defined internal/external rotation.
A custom positioning fixture was used to align the glenoid coordinate system to the coordinate system of the load cell during potting of the specimen. A 2-mm Kirchner wire was drilled perpendicular to the scapular plane to define the X-axis of the glenoid. A second wire was placed perpendicular to this pin and parallel to the scapular plane to define the Y-axis. The scapula was then potted with Woods metal in a steel box with the lateral 3 cm protruding, and the custom positioning fixture was removed. The scapular pot was mounted to a rigidly fixed 6-axis load cell (Theta-series, ATI Industrial Automation, Apex, North Carolina). The humeral shaft was potted with Woods metal (42.5% Bismuth Alloy, McMasterCarr, Cleveland, Ohio) in an aluminum tube and transfixed bicortically. The potted end of the humeral shaft was mounted to a 6 degrees of freedom robot (R2000 Rotopod, Parallel Robotic Systems Corporation, Hampton, New Hampshire) (Figure 1).
Figure 1.
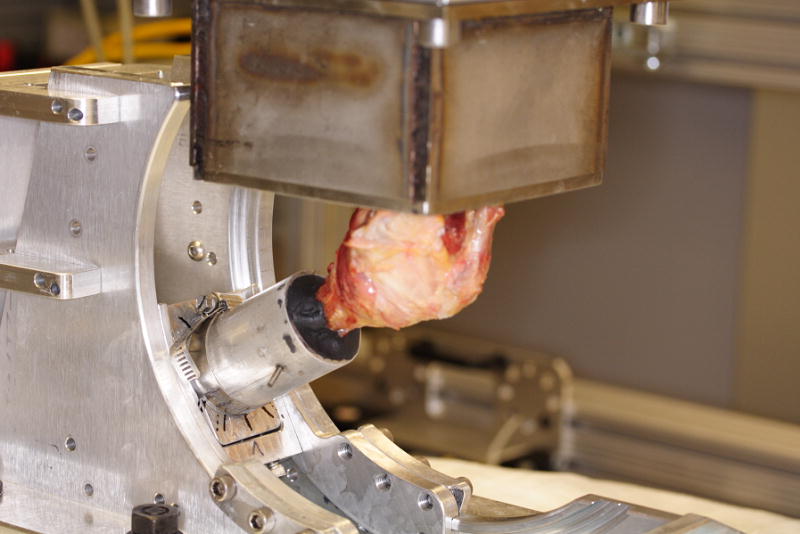
Specimen with scapula mounted to the 6 degrees of freedom load cell and the humeral shaft mounted to a 6 degrees of freedom robot. Reprinted with the permission of the Cleveland Clinic Center for Medical Art & Photography © 2009. All Rights Reserved.
Biomechanical Testing
Each specimen was preconditioned for 5 trials before testing. By the final preconditioning trial, forces were similar for each trial. Each specimen was tested intact, then tested again after the superior half of the subscapularis and the rotator interval were detached from their insertions and repaired with 4 nonabsorbable sutures (size 0 Ethibond, Johnson & Johnson, Somerville, New Jersey). Progressively larger humeral head defects were then created in the posterior superolateral humeral head to simulate Hill-Sachs defects (Figure 2). The progressive defects were created with a customized cutting jig and an oscillating saw. The position of the defects was centered in the area in which Hill-Sachs lesions occur. This is 209° from the anterior border of the humeral head articular cartilage with the humeral head modeled as a circle viewed superiorly, as documented by Richards et al.25 Once the center point of the defect was marked, the cutting guide was aligned perpendicular to the articular surface of the humeral head, parallel to the humeral neck. Then, the defect was created with the oscillating saw. The defect’s depth from the posterior superolateral articular surface, at the previously marked point, corresponded to a predetermined fraction of the radius of the humeral head.
Figure 2.
A, Superior view of a humeral head with the 209° point from the anterior margin of the articular surface marked for the center of the osteotomy. B, View looking directly at the articular surface demonstrating the progressive series of osteotomy cuts used to mimic Hill-Sachs defects. Osteotomy cuts were made at 1/8, 3/8, 5/8, and 7/8 of the projected radius of the humeral head, respectively. Reprinted with the permission of the Cleveland Clinic Center for Medical Art & Photography © 2009. All Rights Reserved.
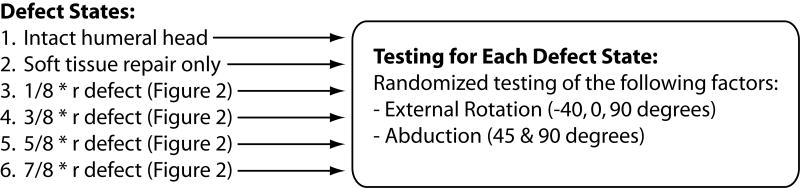
The defects represented 1/8, 3/8, 5/8, and 7/8 of the radius of the humeral head. Secondary experimental factors included humeral abduction angles of 45° and 90°, as well as humeral rotations of 40° internal rotation, neutral, and 40° external rotation. These 2 secondary factors, humeral abduction and rotation, were randomly applied at each testing condition by the robot using a sequence determined with a random number generator within each defect size. Each specimen was tested at each defect size for all possible abduction and rotation combinations (6 for each defect size). After all testing was complete within each defect condition, the next larger size humeral defect was created. This procedure was repeated for each of the 4 humeral head defect sizes sequentially from smallest to largest (Figure 3).
Figure 3.
Protocol outline for testing. Testing was performed sequentially starting with the intact specimen and repeating for each progressively larger humeral defect. The testing for each defect size included all spatial conditions in a randomly applied order.
A reference position was defined for each testing configuration by translating the humeral head 6 mm along both the superior-inferior and anterior-posterior axes. The reference position was defined as the position at which the humeral head was most medial. A constant axial load, relative to the glenoid, of 50 N was applied throughout each testing condition to center the humeral head in the glenoid fossa. This applied load has been successfully used in previous studies and is considered a reasonable estimate of glenohumeral contact force at the time of dislocation.13 Also, it has been shown that 50 N does not cause humeral head damage with dislocation.17
Each experimental trial was performed by translating the humeral head until dislocation in the anteroinferior direction at 45° to the horizontal glenoid fossa axis. The translation was performed at 0.5 mm/s to minimize any viscoelastic effects of the soft tissue stabilizing structures.19 The forces and displacements were recorded throughout each trial by the load cell and robot, respectively. Dislocation was the point at which the humeral head began to translate medially to the glenoid.
Data Analysis
For each trial, the outcome of interest was defined as the normalized distance to dislocation. The distance to dislocation was defined as the distance between the reference position and the point of dislocation along the anteroinferior axis. This was normalized to this distance from the intact test for each configuration. This was chosen as the main outcome measure because it represents when the defect in our model engages the glenoid rim. This distance outcome was analogous to glenoidogram data reported in previous studies that represents the medial-lateral path of the center of the humeral head as it is translated along the glenoid.16 This normally produces a symmetric gull-wing shape but, in the case of glenoid or humeral head defect, would become shortened in the direction of instability and therefore asymmetric. Each trial produced a glenoidogram that was used to determine the point at which the humeral head dislocated. The force to dislocation could not be used because this is mainly dependent on soft tissue tension, which was normalized during the preconditioning trials. A balanced repeatedmeasures analysis of variance(ANOVA) was used to identify the significance of each factor (defect state, abduction angle, rotation angle) on the normalized distance to dislocation. Tukey post hoc analyses were used to determine significance of differences between factor levels. Statistical significance was set at α = .05.
RESULTS
Results of the ANOVA demonstrated significant factors, including the amount of humeral rotation (P < .001) and the size of the defect (P < .001). A Tukey post hoc analysis was used to determine significance of differences between factor levels. At 40° of external rotation, there was a significant reduction in distance to dislocation compared with both neutral and 40° of internal rotation (P < .001). The 5/8 radius osteotomy had a decreased distance to dislocation compared with the intact state (P = .008), as did the 7/8 radius osteotomy (P < .001). No significant difference was found between the 2 abduction angles.
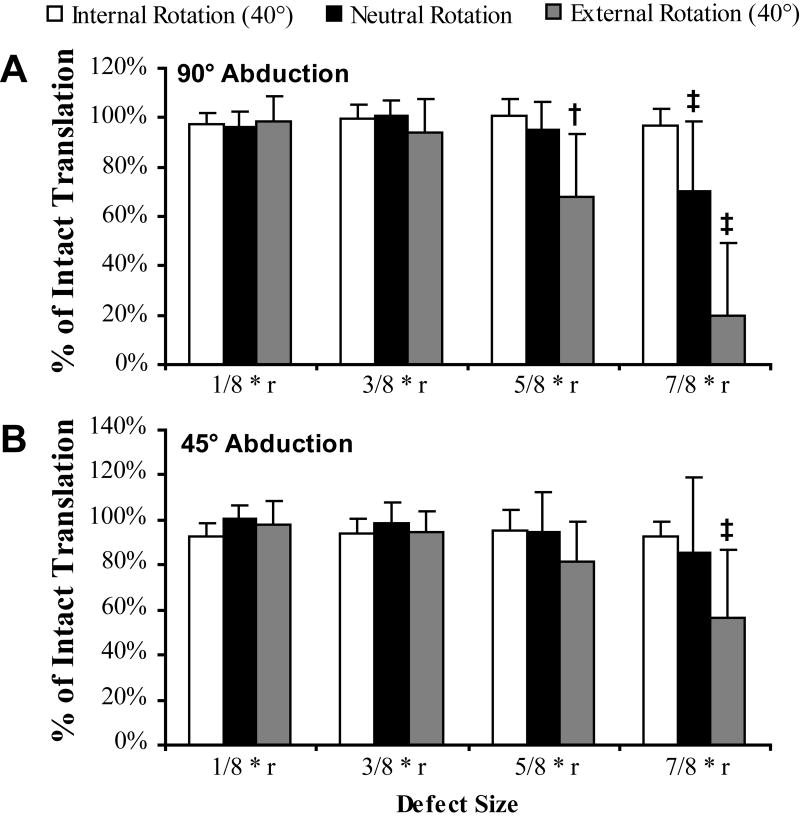
Figure 4 illustrates the effect of defect size, abduction angle, and rotation angle on the normalized distance to dislocation. A Tukey post hoc analysis was used to determine significant differences between the intact state and the defect state for each arm position. There was a decreased distance to dislocation at the 5/8 radius osteotomy at 40° of external rotation with 90° of abduction only (P = .008). For the 7/8 radius osteotomy at 90° of abduction, there was a significant decrease in the distance to dislocation for both neutral rotation and 40° of external rotation (P < .001). For the same osteotomy at 45° of abduction, there was a significantly decreased distance to dislocation at 40° of external rotation (P < .001). In all cases, with the humerus internally rotated, there was no significant change in the distance to dislocation.
Figure 4.
The effect of humeral head defect size on anteroinferior translation at 90° (A) and 45° (B) of abduction (mean ± 95% confidence interval). A defect size of “1/8 * r” represents a defect of 1/8 of the radius of the humeral head. Significance calculation is based on a post hoc analysis of the variation from the intact humeral head for each rotation (‡P < .001, †P = .008).
DISCUSSION
In our cadaveric study of simulated Hill-Sachs defects, we found that with increasing size of defects, glenohumeral stability decreased. This effect was pronounced with humeral external rotation and abduction. A large defect of 5/8 the humeral head radius was found to decrease glenohumeral stability in the abducted and externally rotated position. The larger 7/8 radius defect led to a decrease in stability at lower abduction levels and neutral rotation.
To date, there has been no published basic science research that answers the question of when and how to treat humeral head defects. One study not yet published found that defects 25% the radius of the humeral head decreased stability. This study differed from ours in that it evaluated shoulders only in abduction and only at neutral and 60° external rotation. Also, they studied the shoulders completely devoid of soft tissue and any effects of the capsulolabral complex. Lastly, their study was performed under load control, so not all the shoulders dislocated during testing.28
Some clinical series have reported a higher postsurgical failure rate associated with large Hill-Sachs defects. In the series of arthroscopic capsulorrhaphy reported by Burkhart and De Beer,3 each patient with what they termed an ‘‘engaging’’ Hill-Sachs lesion had failed repair. Burkhart and Danaceau2 reported on a snowboarder who had recurrent shoulder instability despite a healed Bankart repair in the setting of an engaging Hill-Sachs lesion. Boileau et al1 found that a large Hill-Sachs lesion as seen arthroscopically was a significant (P < .05) risk factor associated with recurrence of instability. Rowe et al27 also found that a large Hill-Sachs lesion was a risk factor for recurrent instability after open repair. None of these studies defined what was considered a large humeral head defect.
No guidelines exist at this time for the surgical treatment of humeral head defects. There is anecdotal evidence without supporting scientific data that humeral head defects equivalent to 20% to 40%of the humeral head may affect glenohumeral stability and the lesions larger than 40% do affect stability.3,6,15,20,29 This convention is not specifically defined in the literature. However, assuming that it involves modeling the humeral head as a circle (as would be seen on a computed tomography or magnetic resonance imaging scan) and the humeral head articular surface is approximately 180°, defects of 20% and 40% of the humeral head would correspond to defects of 1/5 and 2/3 the humeral head radius, respectively, in our model. The 5/8 radius defect that we found to affect glenohumeral stability would fall close to the larger end of the 20% to 40% range (approximately 38%).
There is no consensus regarding which defects need to be surgically fixed. Burkhart and De Beer3 introduced the concept of the engaging Hill-Sachs lesion. An engaging Hill-Sachs lesion is a humeral head defect that the glenoid rim falls into with the shoulder in a functional position and leads to symptoms of recurrent instability. Whether any Hill-Sachs lesion engages, however, must be determined by both the orientation and the size of the defect. Larger defects likely engage the glenoid rim with less humeral external rotation and abduction. This interrelationship is evident from our study, in which larger defects decreased the distance to glenohumeral dislocation. Our results suggest that humeral head defects of 5/8 the humeral head radius lead to decreased glenohumeral stability. This finding provides a biomechanical basis for developing a surgical treatment algorithm for shoulder instability with Hill-Sachs lesions in much the same way that Itoi et al contributed to the treatment of glenoid bone defects.
Another issue is that significant humeral head defects and glenoid bone defects rarely exist in isolation. It is unclear how the presence of such combinations of bony defects affects glenohumeral stability. It is possible that defects of the glenoid or humeral head that individually are small enough in isolation to allow for purely ligamentous repair may, when present together, lead to a higher failure rate of soft tissue repair. The interaction between the effects of glenoid and humeral head defects is unknown at this time.
Miniaci and Gish21 have recommended performing humeral external rotation in 45° of abduction as a means to detect Hill-Sachs lesions that are of greater clinical significance. They found that patients with larger, more significant Hill-Sachs lesions will have apprehension in this position as well as with 90° of abduction in the position the shoulder is routinely examined. Our study supports this notion in that the largest defect group had a decreased distance to dislocation at both 45° and 90°.
Limitations of our study include all those that are inherent to cadaveric studies. The defects created were flat osteotomy defects based on the desired depth to be easily reproducible. However, in some cases, a Hill-Sachs lesion may be cavitary shaped. The most important limitation, however, is that this was a study of the bony effects of humeral head defects only. There was undoubtedly capsuloligamentous stretch of the anterior inferior band of the glenohumeral ligament with each dislocation. We therefore performed preconditioning on each specimen before formal testing, which normalized the soft tissue forces resisting dislocation. We also tried to minimize this effect by using a slow rate of translation, in a manner similar to that of prior studies. We also stopped each test immediately at the point of dislocation of the humeral head and did not allow the entire head to sit completely dislocated anterior and inferior to the glenoid, as occurs in most cases of actual shoulder dislocations. Lastly, there was no conceivable way to retighten the soft tissues once they underwent plastic deformation. On the other hand, our study therefore represents the case of chronic recurrent anterior glenohumeral instability with a deficient inferior glenohumeral ligament and a Hill-Sachs lesion.
In summary, we performed a cadaveric study to determine the effects of humeral head bony defects on glenohumeral stability. We demonstrated that large defects with a depth of 5/8 the radius of the humeral head from the posterior superolateral articular surface were associated with a decreased distance to anteroinferior dislocation. The effects of the humeral head bone defects were more pronounced with external rotation and at higher angles of abduction. Our study is the first to provide basic science data that can be used for the treatment of Hill-Sachs lesions.
Acknowledgments
This study was supported by a grant from the Research Program Committee of the Cleveland Clinic (PI: Fening). The Cleveland Clinic Musculoskeletal Core Center, in which 3 of the authors participate (S.D.F., M.H.J., and A.M.), is funded in part by Core Center Grant 1P30 AR- 050953 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases/NIH. The authors thank Robert (Sam) Butler of Cleveland Clinic’s Department of Quantitative Health Sciences for statistical analysis.
Grant Support: This study was supported by a grant from the Research Program Committee of the Cleveland Clinic (PI: Fening). The Cleveland Clinic Musculoskeletal Core Center, in which 3 of the authors participate (S.D.F., M.H.J., and A.M.), is funded in part by Core Center Grant 1P30 AR- 050953 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases/NIH.
References
- 1.Boileau P, Villalba M, Hery JY, Balg F, Ahrens P, Neyton L. Risk factors for recurrence of shoulder instability after arthroscopic Bankart repair. J Bone Joint Surg Am. 2006;88(8):1755–1763. doi: 10.2106/JBJS.E.00817. [DOI] [PubMed] [Google Scholar]
- 2.Burkhart SS, Danaceau SM. Articular arc length mismatch as a cause of failed Bankart repair. Arthroscopy. 2000;16(7):740–744. doi: 10.1053/jars.2000.7794. [DOI] [PubMed] [Google Scholar]
- 3.Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill- Sachs lesion. Arthroscopy. 2000;16(7):677–694. doi: 10.1053/jars.2000.17715. [DOI] [PubMed] [Google Scholar]
- 4.Calandra JJ, Baker CL, Uribe J. The incidence of Hill-Sachs lesions in initial anterior shoulder dislocations. Arthroscopy. 1989;5(4):254–257. doi: 10.1016/0749-8063(89)90138-2. [DOI] [PubMed] [Google Scholar]
- 5.Cetik O, Uslu M, Ozsar BK. The relationship between Hill-Sachs lesion and recurrent anterior shoulder dislocation. Acta Orthop Belg. 2007;73(2):175–178. [PubMed] [Google Scholar]
- 6.Chen AL, Hunt SA, Hawkins RJ, Zuckerman JD. Management of bone loss associated with recurrent anterior glenohumeral instability. Am J Sports Med. 2005;33(6):912–925. doi: 10.1177/0363546505277074. [DOI] [PubMed] [Google Scholar]
- 7.Connolly JF. Humeral head defects associated with shoulder dislocations: their diagnostic and surgical significance. Instr Course Lect. 1972;21:42–54. [Google Scholar]
- 8.Debski RE, Wong EK, Woo SL, Sakane M, Fu FH, Warner JJ. In situ force distribution in the glenohumeral joint capsule during anteriorposterior loading. J Orthop Res. 1999;17(5):769–776. doi: 10.1002/jor.1100170523. [DOI] [PubMed] [Google Scholar]
- 9.Edwards TB, Boulahia A, Walch G. Radiographic analysis of bone defects in chronic anterior shoulder instability. Arthroscopy. 2003;19(7):732–739. doi: 10.1016/s0749-8063(03)00684-4. [DOI] [PubMed] [Google Scholar]
- 10.Fening SD, Kovacic J, Kambic H, McLean S, Scott J, Miniaci A. The effects of modified posterior slope on anterior cruciate ligament strain and knee kinematics. J Knee Surg. 2008;21(3):205–211. doi: 10.1055/s-0030-1247820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flatow EL, Miller SR, Neer CS. Chronic anterior dislocation of the shoulder. J Shoulder Elbow Surg. 1993;2:2–10. doi: 10.1016/S1058-2746(09)80131-6. [DOI] [PubMed] [Google Scholar]
- 12.Hill HA, Sachs MD. The grooved defect of the humeral head. Radiology. 1940;35:690–700. [Google Scholar]
- 13.Itoi E, Lee SB, Berglund LJ, Berge LL, An KN. The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: a cadaveric study. J Bone Joint Surg Am. 2000;82(1):35–46. doi: 10.2106/00004623-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Kropf EJ, Sekiya JK. Osteoarticular allograft transplantation for large humeral head defects in glenohumeral instability. Arthroscopy. 2007;23(3):e321–325. doi: 10.1016/j.arthro.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 15.Kropf EJ, Tjoumakaris FP, Sekiya JK. Arthroscopic shoulder stabilization: is there ever a need to open? Arthroscopy. 2007;23(7):779–784. doi: 10.1016/j.arthro.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Lazarus MD, Sidles JA, Harryman DT, 2nd, Matsen FA., 3rd Effect of a chondral-labral defect on glenoid concavity and glenohumeral stability: a cadaveric model. J Bone Joint Surg Am. 1996;78(1):94–102. doi: 10.2106/00004623-199601000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Lippitt SB, Vanderhooft E, Harris SL, Sidles JA, Harryman DT, II, Matsen FA., III Glenohumeral stability from concavity-compression: a quantitative analysis. J Shoulder Elbow Surg. 1993;2:27–35. doi: 10.1016/S1058-2746(09)80134-1. [DOI] [PubMed] [Google Scholar]
- 18.Lo IK, Parten PM, Burkhart SS. The inverted pear glenoid: an indicator of significant glenoid bone loss. Arthroscopy. 2004;20(2):169–174. doi: 10.1016/j.arthro.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 19.Malicky DM, Soslowsky LJ, Blasier RB, Shyr Y. Anterior glenohumeral stabilization factors: progressive effects in a biomechanical model. J Orthop Res. 1996;14(2):282–288. doi: 10.1002/jor.1100140217. [DOI] [PubMed] [Google Scholar]
- 20.Millett PJ, Clavert P, Warner JJ. Open operative treatment for anterior shoulder instability: when and why? J Bone Joint Surg Am. 2005;87(2):419–432. doi: 10.2106/JBJS.D.01921. [DOI] [PubMed] [Google Scholar]
- 21.Miniaci A, Gish M. Management of anterior glenohumeral instability associated with large Hill-Sachs defects. Tech Shoulder Elbow Surg. 2004;5(3):170–175. [Google Scholar]
- 22.Mologne TS, Provencher MT, Menzel KA, Vachon TA, Dewing CB. Arthroscopic stabilization in patients with an inverted pear glenoid: results in patients with bone loss of the anterior glenoid. Am J Sports Med. 2007;35(8):1276–1283. doi: 10.1177/0363546507300262. [DOI] [PubMed] [Google Scholar]
- 23.Pritchett JW, Clark JM. Prosthetic replacement for chronic unreduced dislocations of the shoulder. Clin Orthop Relat Res. 1987;216:89–93. [PubMed] [Google Scholar]
- 24.Re P, Gallo RA, Richmond JC. Transhumeral head plasty for large Hill-Sachs lesions. Arthroscopy. 2006;22(7):798, e791–794. doi: 10.1016/j.arthro.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 25.Richards RD, Sartoris DJ, Pathria MN, Resnick D. Hill-Sachs lesion and normal humeral groove: MR imaging features allowing their differentiation. Radiology. 1994;190(3):665–668. doi: 10.1148/radiology.190.3.8115607. [DOI] [PubMed] [Google Scholar]
- 26.Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63(6):863–872. [PubMed] [Google Scholar]
- 27.Rowe CR, Zarins B, Ciullo JV. Recurrent anterior dislocation of the shoulder after surgical repair: apparent causes of failure and treatment. J Bone Joint Surg Am. 1984;66(2):159–168. [PubMed] [Google Scholar]
- 28.Sekiya JK, Wickwire AC, Stehle JH, Debski RE. Hill-Sachs defects and repair using osteoarticular allograft transplantation: biomechanical analysis using a joint compression model. Am J Sports Med. 2009 Sep 2; doi: 10.1177/0363546509341576. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Taylor DC, Arciero RA. Pathologic changes associated with shoulder dislocations: arthroscopic and physical examination findings in firsttime, traumatic anterior dislocations. Am J Sports Med. 1997;25(3):306–311. doi: 10.1177/036354659702500306. [DOI] [PubMed] [Google Scholar]
- 30.Weber BG, Simpson LA, Hardegger F. Rotational humeral osteotomy for recurrent anterior dislocation of the shoulder associated with a large Hill-Sachs lesion. J Bone Joint Surg Am. 1984;66(9):1443–1450. [PubMed] [Google Scholar]
- 31.Yamamoto N, Itoi E, Abe H, et al. Effect of an anterior glenoid defect on anterior shoulder stability: a cadaveric study. Am J Sports Med. 2009;37(5):949–954. doi: 10.1177/0363546508330139. [DOI] [PubMed] [Google Scholar]