Abstract
A role for BCL-xL in regulating neuronal activity is suggested by its dramatic effects on synaptic function and mitochondrial channel activity. When recombinant BCL-xL is injected into the giant presynaptic terminal of squid stellate ganglion or applied directly to mitochondrial outer membranes within the living terminal, it potentiates synaptic transmission acutely, and it produces mitochondrial channel activity. The squid, however, is a genetically intractable model, making it difficult to apply genetic tools in squid to explore the role of endogenous BCL-xL in synaptic function. Therefore the small molecule inhibitor ABT-737, a mimetic of the BH3-only protein BAD, binding to the BH3-binding domain pocket, was tested in squid, revealing a dual role for BCL-xL. ABT-737 slowed recovery of synaptic responses after repetitive synaptic activity, indicating that endogenous BCL-xL is necessary for timely recovery of rapidly firing synapses. Unexpectedly, however, ABT-737 also protected neurons from hypoxia-induced synaptic rundown and from increased permeability of the mitochondrial outer membrane during hypoxia. This implies that endogenous BCL-xL or a modified form of BCL-xL, such as the N-truncated, proteolytic, pro-apoptotic cleavage product, ΔN BCL-xL, contributes to injurious responses of the hypoxic synapse. To determine if ABT-737 is also an inhibitor of ΔN BCL-xL, recombinant ΔN BCL-xL protein was injected into the synapse. ABT-737 potently inhibited synaptic rundown induced by recombinant ΔN BCL-xL. These observations support the possibility that endogenous proteolysis or a functionally equivalent modification of BCL-xL is responsible for the deleterious effects of hypoxia on synaptic activity.
INTRODUCTION
Proteins of the BCL-2 family such as BCL-xL and BAX play an important role in the regulation of cell death, but the mechanisms by which they accomplish this are as yet incompletely understood (Adams and Cory 2007). After a death stimulus to a cell, BCL-2 family proteins contribute to, or inhibit the release of, pro-apoptotic factors such as cytochrome c from mitochondria perhaps by influencing ion channel activity (Antonsson et al. 1997; Bonanni et al. 2006; Dejean et al. 2005; Minn et al. 1997; Schendel et al. 1998). Recent evidence indicates that BCL-2 family proteins also modulate the function of neurons that are not undergoing cell death (Fannjiang et al. 2003; Jonas et al. 2003). In particular, within the presynaptic terminal of a neuronal synapse, mitochondria regulate synaptic activity during high-frequency firing by buffering rapid changes in calcium levels and by modulating levels of ATP during and after intense neurotransmission (Hollenbeck 2005; Jonas et al. 2003). In the synapse, acute translocation of recombinant BCL-xL protein into mitochondria produces ion channel activity on mitochondrial outer membranes, potentiates neurotransmitter release, and enhances recovery of depleted vesicle pools after intense synaptic activity, possibly by increasing the availability of ATP within the synaptic compartment (Jonas et al. 2003). BCL-xL may act locally to modulate synaptic transmission, without regulating apoptosis of the cell soma, because the synapse is isolated from the nucleus of the cell (Arnold et al. 1974; Mason et al. 2007).
In addition to their possible constitutive activities, a number of the BCL-2 family proteins such as Bax, BID, BCL-2, and BCL-xL (Cheng et al. 1997; Clem et al. 1998; Condorelli et al. 2001; Fujita et al. 1998; Li et al. 1998; Nakagawa and Yuan 2000; Wood and Newcomb 2000) undergo proteolytic cleavage after a cell death stimulus, enhancing the pro-death activities of these molecules. In the case of BCL-xL, endogenous cleavage by caspases or calpains produces a C-terminal fragment that has Bax-like pro-apoptotic activity. The resulting protein (ΔN BCL-xL) lacks the BH4 domain that is thought to confer protection against apoptosis but still contains the death-inducing BH3 domain (Clem et al. 1998; Jonas et al. 2004). It is likely that conformational rearrangements coincident with removal of the N terminus contribute to the pro-apoptotic activity of the cleaved BCL-xL protein, perhaps especially in the brain, where BCL-xL is highly expressed (Krajewska et al. 2002). ΔN BCL-xL produces large conductance ion channel activity in the outer mitochondrial membrane and may induce cell death in part by contributing to the increase in conductance of this membrane and the release of cytochrome c from mitochondria after a cell death stimulus (Clem et al. 1998; Jonas et al. 2004). In addition to these death-promoting activities, the pro-apoptotic form of BCL-xL, when injected into a presynaptic terminal, causes a decline in synaptic responses that mimics that recorded during acute hypoxic insults to the synapse (Jonas et al. 2003, 2005b).
Cleavage of BCL-xL alters its function from anti- to pro-apoptotic. Both pro- and anti-apoptotic molecules may contribute not only to regulation of cell death, but to other nervous system activities such as synaptic plasticity (Fannjiang et al. 2003; Jonas 2006). The prediction therefore is that acute or long-term inhibition of endogenous BCL-xL would not only alter the response of neurons to cell death stimuli but could impair synaptic function in the absence of a death stimulus. In this study, we disrupt the activities of BCL-xL with ABT-737, a mimetic of the BH3-only protein BAD that binds to BCL-xL with high affinity within a pocket of the three-dimensional structure that usually binds pro-apoptotic proteins containing a BH3 domain (Oltersdorf et al. 2005). The structure of ABT-737, a thioethylamino-2–4-dimethylphenyl analog, was designed to bind to the three-dimensional structure of BCL-xL/ BCL-2. By confocal time-lapsed microscopy experiments, the binding of ABT-737 was found to displace a green fluorescent protein (GFP)-tagged BH3-only protein from BCL-xL at mitochondrial surfaces in intact tumor cells. In cancer cell lines, ABT-737 alone effectively induces cell death possibly via its ability, as a BAD mimetic, to displace from BCL-xL the prebound pro-apoptotic proteins Bax and Bak (Oltersdorf et al. 2005). ABT-737, because of its BAD-like activity, is selective for binding to anti-apoptotic molecules BCL-xL, BCL-2 and BCL-w (van Delft et al. 2006), but it binds BCL-xL with highest affinity in vitro (Oltersdorf et al. 2005). BCL-xL is the most highly expressed of these proteins in the adult brain (Krajewska et al. 2002).
We tested the acute effects of ABT-737 at a neuronal presynaptic terminal. When injected into the presynaptic terminal just before synaptic transmission, ABT-737 inhibits the channel activity of mitochondrial membranes that is normally activated during short-term synaptic plasticity and slows the recovery of neurotransmission following repetitive synaptic activity. When injected before a hypoxic insult of the synapse, however, ABT-737 also prevents the appearance of the large conductance activity of mitochondrial membranes associated with declining synaptic function. Furthermore, ABT-737 attenuates synaptic dysfunction produced by hypoxia or by exogenous injection of ΔN BCL-xL into the synaptic terminal. Taken together, the findings suggest that BCL-xL is necessary for the constitutive functioning of neuronal synapses, and further, raises the possibility that ΔN BCL-xL contributes endogenously to the attenuation of synaptic function after ischemic injury.
METHODS
Intracellular membrane patch-clamp recordings
Experiments were performed on small Loligo pealii at the Marine Biological Laboratory, Woods Hole, MA, as described previously (Jonas et al. 1997, 1999). In brief, isolated squid stellate ganglia were pinned to Sylgard in a Lucite chamber. The bathing solution (in mM: 466 NaCl, 54 MgCl2, 11 CaCl2, 10 KCl, 3 NaHCO3, 10 HEPES, pH 7.2) was cooled, oxygenated with 99.5% O2−0.5% CO2, and perfused over the ganglia. Intracellular membrane pipettes (20–80 MΩ) were filled with intracellular solution containing (in mM) 570 KCl, 1.2 MgCl2, 10 HEPES, 0.07 EGTA, 0.046 CaCl2, and 2 ATP, pH 7.2. The mitochondrial patch electrode was contained in an outer, ensheathing electrode that was used to enter the terminal, after which the outer electrode was retracted, exposing the patch pipette tip (Jonas et al. 1997). Gigaohm seals formed either spontaneously or in response to slight negative pressure. The polarities of potentials reported here refer to those of the patch pipette relative to that of the ground electrode, which was placed in the external medium. As indicated, the mitochondrial patch electrode contained control intracellular solution or ABT-737 (5 µM for patch recordings dissolved in 0.05% DMSO in control intracellular solution). Solutions within the patch pipette were not changed during the recording. Data represent populations of patch recordings exposed to the two different solutions.
Injection of presynaptic terminal and measurement of postsynaptic responses
Intracellular microinjection pipettes were filled with intracellular squid solution alone or with the protein or drug of interest and inserted into the presynaptic terminal. ABT-737 was used at a concentration of 500 µM in the pipette for injection into the presynaptic terminal during postsynaptic recordings. After injection, the final concentration of ABT-737 within the synapse was estimated to be ~5 µM. All injection pipettes also contained FITC-dextran of 3,000 molecular weight (MW; Molecular Probes, 100 µM), which was coinjected with proteins or drugs into the terminal to detect successful injection of peptide and or drug (Morgan et al. 2001). Pulses of positive pressure (20–40 psi, 100 ms), were given with a Picospritzer (General Valve, Parker Hannifin Corp.) to achieve injection of the pipette solution.
Synaptic transmission was evoked by stimulating an external suction electrode attached to the presynaptic nerve. The nerve was stimulated at 0.033 Hz, 20 V, and 0.01 mS to elicit single action potentials. The postsynaptic responses were recorded by an electrode containing 3 M KCl inserted into the postsynaptic nerve. Transmitter release was measured by recording the initial rate of rise of the postsynaptic response (Jonas et al. 2003; Swandulla et al. 1991). The initial rates of rise of the postsynaptic responses were calculated using pClamp 8.0 or 9.0 Clampfit software (Axon Instuments) by placing a cursor at the first onset of the synaptic response, determined by eye, and a second cursor at a time point 100–300 µs later, before any detectable regenerative response occurred (Morgan et al. 2001; Swandulla et al. 1991). To mimic high-frequency events, tetanic stimulation (50 Hz, 2–5 s) was applied to the presynaptic nerve.
To produce hypoxic conditions, the perfusion of the stellate ganglion with oxygenated seawater was stopped. Previous studies have determined, using a Clark type oxygen electrode (Hansatech, Norfolk, UK), that levels of oxygen in the medium surrounding the ganglia fall by 48.1 ± 1.1% in 20 min (Jonas et al. 2005b) after cessation of flow.
RESULTS
We propose that BCL-xL may be one of a number of mitochondrial proteins that regulate acute and long-term synaptic changes (Fannjiang et al. 2003; Jonas et al. 2003, 2004, 2005a) and therefore that an inhibitor of BCL-xL may affect synaptic function. We have shown previously that BCL-xL is present on mitochondria in the stellate ganglion of the squid and that channel activity of mitochondria can be increased either by application of recombinant BCL-xL protein directly to mitochondria or by electrical stimulation of the squid presynaptic nerve (Jonas et al. 1999, 2003).
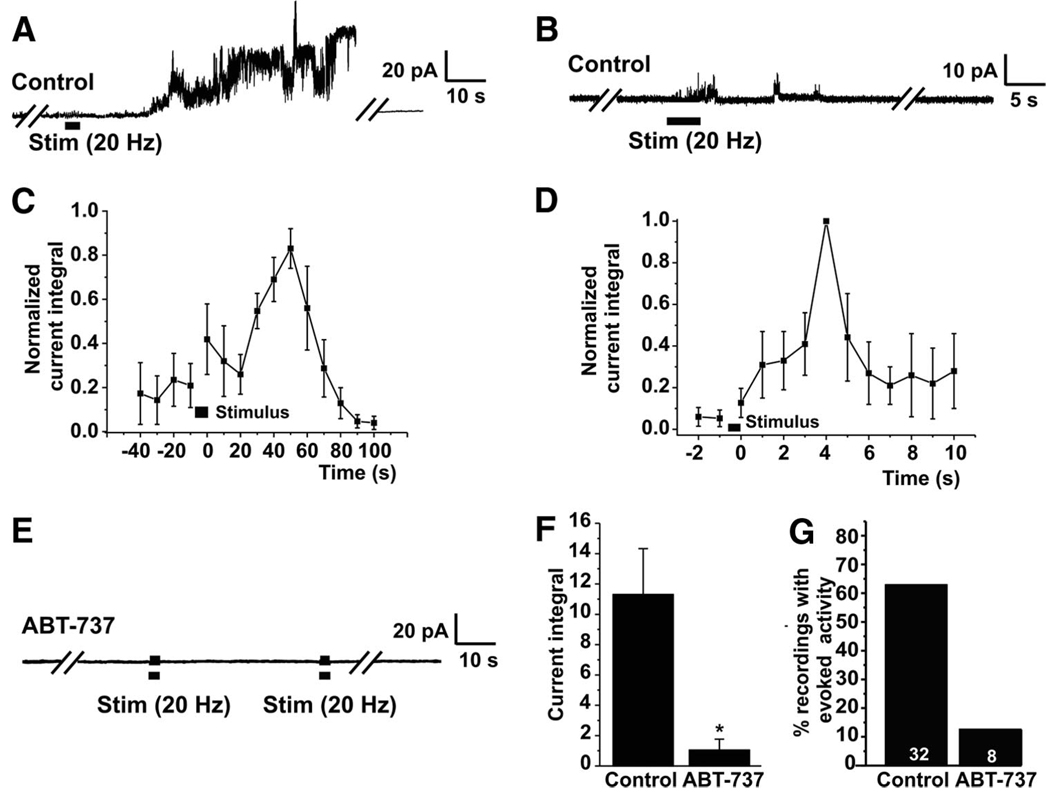
We had previously observed that mitochondrial outer membrane channel activity increased during high-frequency firing of the presynaptic nerve. To test the possibility that channel activity of BCL-xL could be contributing to mitochondrial channel activity during synaptic events, we recorded channel activity of mitochondrial membranes during synaptic stimulation (tetanus), in the presence or absence of the BCL-xL inhibitor, ABT-737 (Oltersdorf et al. 2005). Consistent with previous observations, the new study indicated that, without treatment, mitochondrial channel activity increased during and after the tetanus (Jonas et al. 1999) (Fig. 1 A). The peak amplitude of the stimulus-evoked mitochondrial activity ranged from 2 to 60 times that of control activity recorded just before stimulation (Fig. 1, A and C). The onset of the change in activity was never observed to occur concomitantly with the first action potential but occurred with a delay, suggesting that a diffusible messenger such as calcium present within the presynaptic terminal could be responsible for causing the onset of the change in mitochondrial activity. In addition, mitochondrial activity outlasted the end of the stimulus. There was variability in the time of onset of the response and the duration of the response, perhaps reflecting the position of the mitochondria with respect to the active zone. To illustrate typical time courses, we selected recordings from mitochondria that had long-lasting responses (Fig. 1, A and C; n = 7) or recordings from mitochondria that responded more briefly (Fig. 1, B and D; n = 4). The time course of the activity of long-lasting responders peaked as late as 60 s after the onset of the stimulus, (20 Hz, 5 s; Fig. 1, A and C). As previously reported, synaptic mitochondrial responses were usually reversible, and in the long-lasting group, activity returned to control levels within 100 s of the onset of the stimulus. In the more briefly responding group, responses were smaller, and activity, although it outlasted the stimulus, returned to baseline earlier (Fig. 1, B and D).
FIG. 1.
ABT-737 prevents the appearance of channels on mitochondrial outer membrane during synaptic stimulation. A: recording on an outer mitochondrial membrane within a squid presynaptic terminal before, during, and after a tetanus. Channel activity increases after the tetanus and remains elevated for many tens of seconds and then returns to baseline (Jonas et al. 1999). B: an example of a recording in which small conductance activity appears briefly during and after stimulation. C: time course of change in normalized mean current integral for large responses. Mean current integral (amplitude times frequency of channel opening) was calculated for each 10-s period before and after a tetanus. Data were normalized to maximum value for each experiment. D: time course of change in normalized mean current integral of mitochondrial membranes during small responses. Normalized mean current integral during each 1-s period was plotted. E: application of 5 µM ABT-737 to the mitochondrial patch before synaptic stimulation prevents the increase in channel activity during and after the tetanus. Recording is representative of 7 of the 8 recordings made with ABT-737 in the patch pipette. F: histogram comparing mean current integral in the10-s period with the maximum evoked channel activity after stimulation in control synapses vs. those exposed to ABT-737 in the patch pipette (*P< 0.03). G: percentage of recordings in which channel activity occurred during synaptic stimulation in controls and ABT-737 exposed mitochondrial patches (n = 32 controls and n = 8 ABT-737-treated synapses; P< 0.02, Fisher’s exact test).
In contrast to control synapses, 62.5% of which responded to stimulation, only one of eight synapses (12.5%) treated with 5 µM ABT-737 in the bath and in the patch pipette responded to stimulation with increased mitochondrial membrane activity during or following the tetanus (P < 0.02, Fisher’s exact test; Fig. 1, E and G). The mean peak current of the response in ABT-737–treated synapses was also less than that of controls (P < 0.02, Mann-Whitney; Fig. 1 F), suggesting that BCL-xL protein, or another target of ABT-737 (BCL-2 or BCL-w), contributes significantly to the modulation of such activity.
Mitochondria are abundant in the synaptic terminals of neurons that are capable of firing at high frequencies (Rowland et al. 2000; Tolbert and Morest 1982) and are known to be involved in presynaptic responses to high-frequency stimulation including posttetanic potentiation (Friel and Tsien 1994; Jonas et al. 1999; Tang and Zucker 1997) and in recovery of neurotransmission after synaptic depression (Billups and Forsythe 2002; Jonas et a. 2003). Injection of BCL-xL into the presynaptic terminal increases the rate of recovery of transmitter release after a period of rapid firing (Jonas et al. 2003), suggesting that it enhances the performance of mitochondria in recovery. We therefore tested the effects of ABT-737 on the rate of recovery from tetanic stimulation.
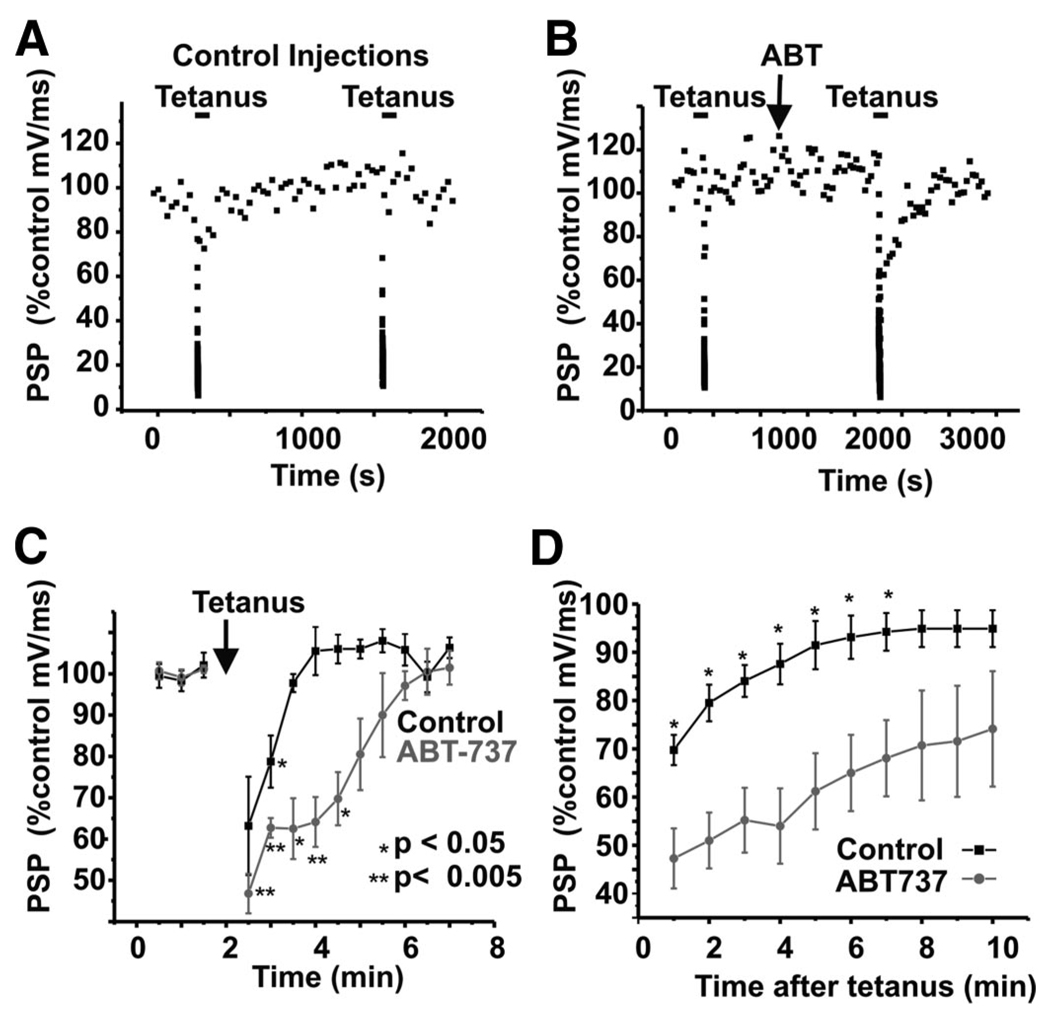
In control untreated synapses, recovery of neurotransmitter release generally occurred in <2 min, such that, within 2 min of the end of a 5-s tetanus, the recorded postsynaptic potentials were indistinguishable from those before the tetanus (Fig. 2, A and C). Moreover, when a second tetanic stimulus was applied at a time 8–30 min after the first, the rate of recovery was unchanged from that observed in the first tetanus (Fig. 2 A; recovery at 1 min was 104 ± 3% of that in first tetanus; n = 6), and in those in which a third tetanus was recorded, recovery was 102.5% of that in the second tetanus (n = 2). In contrast, when ABT-737 was injected into or over the presynaptic terminal just before tetanic stimulation of the synapse, it caused a marked decrease in the rate at which the synapse recovered from high-frequency firing (Fig. 2 B). In the same synapse before and after ABT-737 application (n = 4), recovery remained incomplete for as long as 6 min after the end of the second tetanus (Fig. 2, B and C). In three experiments, a third tetanic stimulus was applied 10–17 min after the second stimulus. The effect of ABT in this third tetanus persisted (time to 50% recovery = 3.5 ± 1.7 min for 1st ABT-737 tetanus and 3.3 ± 1.1 m for 2nd ABT-737 stimulus).
FIG. 2.
ABT-737 slows recovery from synaptic depression. A: time course of changes in slope of the postsynaptic response in control synapses before and after tetanic stimualtion. The synapse was stimulated at a low rate (0.033 Hz) to monitor control responses. Tetani (50 Hz, 5 s) were applied to induce synaptic depression before and after repeated injections of control intracellular solution into the presynaptic terminal. B: time course of changes in the slope of the postsynaptic response in a synapse before and after treatment with ABT-737. Tetani were applied to induce synaptic depression before and after application of ABT-737 C: group data showing time course of recovery of the slope of the postsynaptic responses after tetani. Shown are paired experiments before and after exposure to ABT-737 in the same terminal (n = 4). D: time course of recovery of the slope of the postsynaptic responses after tetani, in unpaired experiments (n = 10; *P< 0.05).
In addition to experiments in which we compared rates of recovery in the same synapses before and after application of ABT-737, we also compared the mean time courses of recovery in unpaired untreated synapses and ABT-737-treated synapses (Fig. 2 D). Again, the rate of recovery was significantly slower in ABT-737-treated preparations, such that recovery was lower compared with the controls at all time points ≤7 min following stimulation. Although the mean time courses of recovery appeared slightly slower in the unpaired group data, there was no statistically significant difference between control and ABT-737-treated synapses 8 min following tetanic stimulation (Fig. 2 D). The results suggest that BCL-xL or another target of ABT-737 is an endogenous modulator of neurotransmission in the squid presynaptic terminal.
In apoptosis or during insults to the nervous system, there occurs an increase in permeability of the outer mitochondrial membrane (Gross et al. 1999; Halestrap 2005; Jonas et al. 2004; Kroemer and Reed 2000; Nakagawa et al. 2005). Even in the absence of cell death, it is possible that such increases in permeability influence synaptic transmission (Jonas et al. 2005a). In the squid synapse, the effects of hypoxia have served as a model for the effects of a cell death stimulus on synaptic activity (Jonas et al. 2005b). In particular, the presynaptic terminal is sensitive to hypoxia, which attenuates synaptic transmission over 10–30 min (Jonas et al. 2005b). Patchclamp recordings of mitochondrial membranes made during the early phases of decline in synaptic activity showed large conductance channels not found frequently in controls. This activity is correlated with the appearance of ΔN-BCL-xL, which is known to induce cell death and cytochrome c release (Bonanni et al. 2006; Clem et al. 1998; Fujita et al. 1998; Jonas et al. 2004).
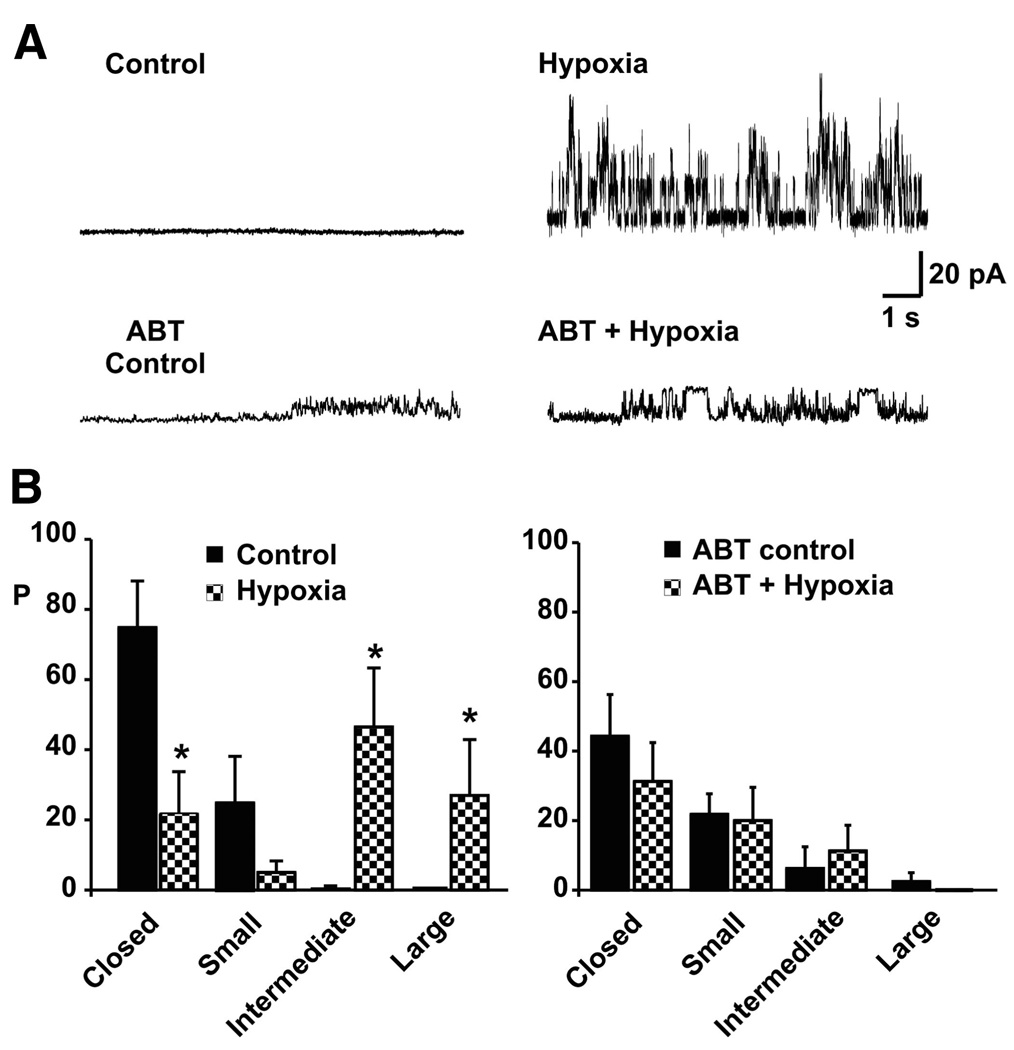
To carry out a more specific test of the hypothesis that ΔN BCL-xL could contribute to synaptic rundown, recordings were performed in the presence of ABT-737. Previous work has shown that hypoxia produces large conductance activity with unitary conductances of >750 pS on mitochondrial membranes (Jonas et al. 2005b). In this study, these findings were again verified (Fig. 3, A and B). Hypoxia produced intermediate (>180 and <760 pS) and large (≥760 pS) conductance channel activity (n = 8) that was not present in controls. In contrast, in the presence of ABT-737, no large conductance activity occurred after hypoxia (Fig. 3; n = 8), although a small, but not statistically significant increase in intermediate conductances (>180 and <760 pS) occurred compared with ABT-treated controls (Fig. 3 B, right). The results suggest that BCL-xL or a proteolytic cleavage product of BCL-xL may contribute to the large conductance activity present after an hypoxic insult.
FIG. 3.
ABT-737 prevents the appearance of large conductance channel activity in mitochondrial outer membranes after a hypoxic insult to the synapse. A: examples of mitochondrial channel activity before and within 30 min of the onset of hypoxia in the absence of ABT-737 (top) and in the presence of ABT-737 (bottom). Recordings were made at +60 mV. B: group data from all mitochondrial recordings before and 30 min after the onset of hypoxia, in the absence of ABT-737 (left) and in the presence of 5 µM ABT-737 (right). [P represents probability of occurrence of channel openings with small conductance (<180 pS), intermediate conductance (180–750 pS), or large conductance (>750 pS); n = 8 recordings in each group]. There is no statistically significant difference between the probability of observing closed states in controls and in ABT-737–treated mitochondria.
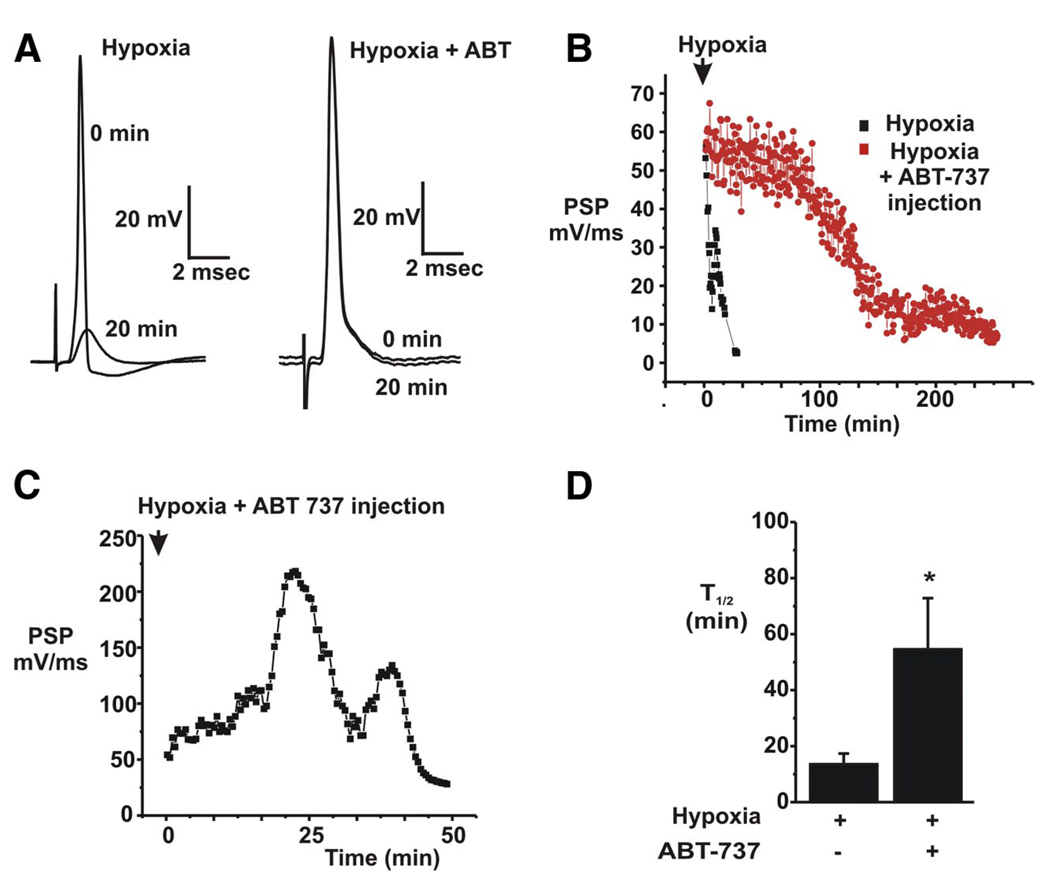
Hypoxia normally causes significant impairment of synaptic transmission in squid (Jonas et al. 2005b). During this synaptic depression, the depolarization of the postsynaptic cell may become insufficient to elicit a postsynaptic action potential, resulting in failure of transsynaptic signaling. The onset of synaptic depression is correlated with a decrease in slope of the initial 100–300 µs of the postsynaptic response (Morgan et al. 2001; Swandulla et al. 1991). When the synapse was exposed to ABT-737 both in the bath and by injection into the presynaptic terminal, rundown of synaptic responses was significantly delayed (Fig. 4), such that 20 min after arresting the flow of oxygenated sea water to the ganglion, a full response to release of transmitter followed by an action potential in the postsynaptic cell could still be observed only in the ABT-737-treated synapses (Fig. 4, A, B, and D). Exposure to ABT-737 (shown in Fig. 4 C) occasionally even transiently enhanced synaptic transmission immediately after the onset of hypoxia, but was unable to enhance transmission when injected into the terminal after full synaptic rundown had already occurred (n = 2).
FIG. 4.
ABT-737 delays decline in synaptic responses during a hypoxic insult to the synapse. A: postsynaptic responses to a suprathreshold stimulus to the presynaptic nerve before and 20 min after the onset of hypoxia in an untreated synapse (left) and in one injected with ABT-737 (right). B: examples of the time course of the decline in slope of the postsynaptic potential in response to hypoxia. Responses of an ABT-737–injected synapse are compared with those of a control, untreated synapse. C: example of an increase in slope over time in a hypoxic synapse previously injected with ABT-737. D: combined data from all experiments comparing the half time of rundown of synaptic function in control vs. ABT-737-injected synapses (*P< 0.03, n = 11 hypoxic controls and n = 9 ABT-737–treated hypoxic synapses).
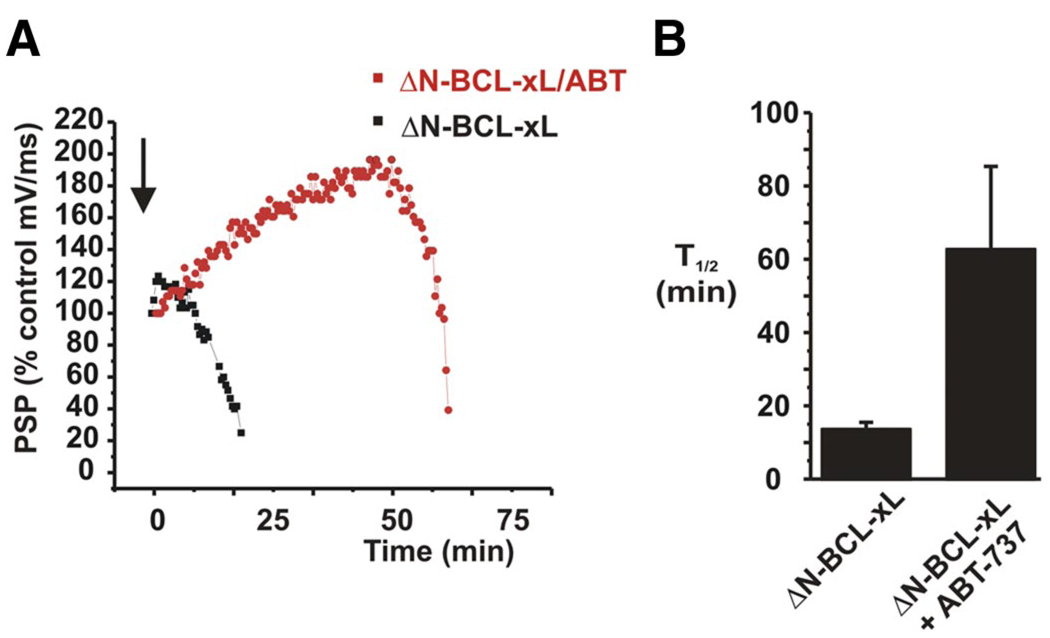
The large conductance mitochondrial activity following hypoxia and the rundown of synaptic transmission have been correlated with specific proteolytic cleavage of BCL-xL (Jonas et al. 2004, 2005b). We therefore recorded synaptic responses after injection of recombinant ΔN-BCL-xL protein into the synapse and compared these responses to those of synapses treated with both ΔN-BCL-xL protein and ABT-737 (Fig. 5). ΔN BCL-xL enhanced the rate of decline of synaptic responses in oxygenated sea water as previously described (Jonas et al. 2004) (Fig. 5, A and B). In contrast, such rundown was significantly delayed by ABT-737 (Fig. 5, A and B). In some cases, as described above for hypoxia, postsynaptic potentials were transiently enhanced in the presence of ATP-737 with ΔN BCL-xL before eventual rundown (Fig. 5 A).
FIG. 5.
ABT-737 delays decline in synaptic potentials after injection of 20 µg/ml recombinant ΔN BCL-xL into the presynaptic terminal. A: examples of change in slope of postsynaptic potentials over time after injection of ΔN BCL-xL or ΔN BCL-xL/ABT-737 solution. B: combined data comparing the half time of rundown of synaptic function in ΔN BCL-xL-injected synapses vs. synapses injected with ΔN BCL-xL + ABT-737 (n = 3 for ΔN BCL-xL alone and n = 5 for ΔN BCL-xL/ABT-737.
DISCUSSION
Here we report that BCL-xL has seemingly opposite effects on synaptic transmission. Modulation of BCL-xL by the BAD mimetic ABT-737 prevents the onset of stimulation-induced mitochondrial channel activity and slows the rate of recovery of synaptic responses after intense synaptic activity. Thus BCL-xL function seems to be required for the normal change in conductance of the outer mitochondrial membrane during synaptic transmission and also for the normal rate of recovery of neurotransmission after synaptic depression. In contrast, during hypoxia, BCL-xL seems to enhance synaptic rundown. In such hypoxic synapses, treatment with ABT-737 slows the decline in synaptic function and attenuates the appearance of large conductance channels in mitochondrial outer membranes within the presynaptic terminal.
The finding that ABT-737 inhibits ion channel activity on mitochondrial membranes during a tetanus suggests that such activity is produced in part by BCL-xL. The importance of the BH3 domain of BCL-xL for its channel-forming activity has been described by us previously (Jonas et al. 2004), and it is intriguing that a mimetic of the BH3-only protein BAD, when bound into the BH3-containing pocket of BCL-xL, inhibits channel activity. One might speculate that binding of another protein “ligand” in the BCL-xL pocket is required for channel activity and that ABT-737 inhibits this interaction. BCL-xL itself may form a channel in the outer membrane (Minn et al. 1997) or form a subunit of a multi-protein complex that conducts ions and metabolites through the outer membrane (Vander Heiden et al. 2001).
Another finding of this study is that ABT-737 slows recovery of synaptic transmission after synaptic depression. Our previous work suggested that injection of recombinant BCL-xL protein into the synapse speeds recovery from synaptic depression by regulating the refilling of specific neurotransmitter-containing pools within the presynaptic terminal (Jonas et al. 2003). In that study, we suggested that BCL-xL may enhance the availability of ATP within the presynaptic terminal possibly to stimulate an ATP dependent step in the refilling of specific pools (Jonas et al. 2003). ATP is known to be required for a myriad of cellular processes, and certain steps in synaptic vesicle mobilization, release, and recycling may be compromised by the lack of locally generated ATP. For example, enzyme-dependent steps in synaptic transmission include refilling of single vesicles with neurotransmitter (Takamori et al. 2000), membrane fission during endocytosis (Heidelberger 2001), coated pit formation (Faundez and Kelly 2000; Smythe et al. 1989), and fast compensatory membrane retrieval (Heidelberger 2001). Previous work using Drosophila melanogaster mutants has suggested that mitochondria are necessary for normal vesicle recycling. A mutation of Drp1, which encodes a mitochondrial fission protein, was associated with the absence of mitochondria at presynaptic sites, abnormal synaptic transmission, and decreased activity of an ATP-sensitive myosin light chain kinase that is necessary for vesicle mobilization (Guo et al. 2005; Verstreken et al. 2005). In the absence of mitochondria, synaptic transmission and vesicle recycling can take place, but more slowly, especially during times of high demand (high-frequency events) when the vesicle pools become rapidly depleted. It is therefore likely that mitochondrial ion channel activity plays a specific role in regulating the restoration of certain pools of vesicles but that other pools may not be so acutely dependent on mitochondrial activity. Undoubtedly, additional as yet unknown ATP-dependent molecular processes of vesicle recycling may be regulated by the presence of BCL-xL at synaptic sites.
One surprising finding of the study is that, under certain circumstances, the inhibitor of BCL-xL, rather than attenuating synaptic responses, actually enhances transmission. This is apparent after a traditional death stimulus, that of hypoxia, which has been shown previously to produce synaptic decline (Jonas et al. 2005b). Many BCL-2 family proteins undergo proteolytic cleavage after a cell death stimulus, which serves to activate pro-apoptotic features of the molecules (Cheng et al. 1997; Clem et al. 1998; Fujita et al. 1998; Nakagawa and Yuan 2000). From previous work, we know that cell death stimuli promote the N-terminal proteolytic cleavage of BCL-xL to form AN-BCL-xL, which induces cell death, cytochrome c release, and large conductance channel activity in outer mitochondrial membranes (Bonanni et al. 2006; Clem et al. 1998; Fujita et al. 1998; Jonas et al. 2004). Indeed, ABT-737 inhibits both the large conductance channel activity of mitochondrial outer membranes and synaptic dysfunction induced by hypoxia, raising the possibility that ABT-737 binds to AN-BCL-xL or prevents specific proteolytic cleavage of BCL-xL. Therefore the synaptic dysfunction of hypoxic synaptic terminals could be a result of the activity of proteolytically altered BCL-xL that has formed a new kind of channel in outer mitochondrial membranes (Bonanni et al. 2006; Jonas et al. 2004). ABT-737, by inhibiting large conductance activity of mitochondrial membranes during hypoxia, could possibly prevent the release of factors from mitochondria that could in turn cause synaptic failure.
A traditional view of anti-apoptotic molecules such as BCL-xL is that they regulate and prevent cell death after a death stimulus (Antonsson et al. 1997; Cheng et al. 2001; Kluck et al. 1997; Oltersdorf et al. 2005; Plas and Thompson 2002; Plas et al. 2001; Vander Heiden et al. 2001). Therefore inhibition of BCL-xL might produce injurious effects on synaptic function. Inhibition of full-length BCL-xL in the setting of hypoxia could, in and of itself, be expected to enhance the synaptic decline produced by hypoxia. These data do not support this notion, but are rather in keeping with the interpretation that the proteolytic cleavage product of BCL-xL, ΔN BCL-xL, is the predominant form of BCL-xL present after the hypoxic stimulus. Indeed, in the presence of ABT-737 during either hypoxia or exposure to recombinant ΔN BCL-xL, synaptic responses actually improved transiently, suggesting that the intermediate mitochondrial conductances resulting from the combination of the activity of the pro-apoptotic molecule and ABT were beneficial to the synapse, perhaps by transiently releasing ATP or other metabolites from mitochondria.
Our observations underscore the importance of BCL-xL as a modulator of both physiological and pathological functions of neurons. The effects of ABT-737 suggest that both BCL-xL and ΔN-BCL-xL could help balance synaptic transmission between under-and overactivity, to protect against disuse leading to synaptic degeneration or elimination and overexcitation leading to excitotoxic death. In addition, the outer mitochondrial membrane ion channel activity of BCL-xL seems to participate in changes of mitochondrial conductance that are correlated with short-term synaptic plasticity and recovery of neurotransmission after repetitive firing. The consequences of these positive actions of BCL-xL at the synapse are as yet unclear, but it is likely that they could involve calcium buffering and the regulated release of ATP from mitochondria. Since ABT-737 mimics a natural ligand (BAD) for BCL-xL, it is possible that control of synaptic function by BCL-xL is normally controlled by BCL-xL-binding ligands. A protein such as BCL-xL that is so integrally related to mitochondrial function inside the synapse could also serve as sensor of synaptic activity, to provide for physiologically important changes in the properties of the synapse necessary for the eventual long-term changes in synaptic efficacy that underlie memory and learning.
REFERENCES
- Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod JJ, Mazzei G, Maundrell K, Gambale F, Sadoul R, Martinou JC. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- Arnold JM, Summers WC, Gilbert DL, Manalis RS, Daw NW, Lasek RJ. A Guide to Laboratory Use of the Squid Loligo Pealei. Woods Hole, MA: Marine Biological Laboratory; 1974. pp. 55–65. [Google Scholar]
- Billups B, Forsythe ID. Presynaptic mitochondrial calcium sequestration influences transmission at mammalian central synapses. J Neurosci. 2002;22:5840–5847. doi: 10.1523/JNEUROSCI.22-14-05840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanni L, Chachar M, Jover-Mengual T, Li H, Jones A, Yokota H, Ofengeim D, Flannery RJ, Miyawaki T, Cho CH, Polster BM, Pypaert M, Hardwick JM, Sensi SL, Zukin RS, Jonas EA. Zinc-dependent multi-conductance channel activity in mitochondria isolated from ischemic brain. J Neurosci. 2006;26:6851–6862. doi: 10.1523/JNEUROSCI.5444-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng EH, Kirsch DG, Clem RJ, Ravi R, Kastan MB, Bedi A, Ueno K, Hardwick JM. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278:1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Molec Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- Clem RJ, Cheng EH, Karp CL, Kirsch DG, Ueno K, Takahashi A, Kastan MB, Griffin DE, Earnshaw WC, Veliuona MA, Hardwick JM. Modulation of cell death by Bcl-XL through caspase interaction. Proc Natl Acad Sci USA. 1998;95:554–559. doi: 10.1073/pnas.95.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condorelli F, Salomoni P, Cotteret S, Cesi V, Srinivasula SM, Alnemri ES, Calabretta B. Caspase cleavage enhances the apoptosis-inducing effects of BAD. Molec Cell Biol. 2001;21:3025–3036. doi: 10.1128/MCB.21.9.3025-3036.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejean LM, Martinez-Caballero S, Guo L, Hughes C, Teijido O, Ducret T, Ichas F, Korsmeyer SJ, Antonsson B, Jonas FA, Kinnally KW. Oligomeric BAX is a component of the putative cytochrome c release channel MAC, mitochondrial apoptosis-induced channel. Molec Biol Cell. 2005;16:2424–2432. doi: 10.1091/mbc.E04-12-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fannjiang Y, Kim CH, Huganir RL, Zou S, Lindsten T, Thompson CB, Mito T, Traystman RJ, Larsen T, Griffin DE, Mandir AS, Dawson TM, Dike S, Sappington AL, Kerr DA, Jonas EA, Kaczmarek LK, Hardwick JM. BAK alters neuronal excitability and can switch from anti- to pro-death function during postnatal development. Developmental Cell. 2003;4:575–585. doi: 10.1016/s1534-5807(03)00091-1. [DOI] [PubMed] [Google Scholar]
- Faundez VV, Kelly RB. The AP-3 complex required for endosomal synaptic vesicle biogenesis is associated with a casein kinase Ialpha-like isoform. Molec Biol Cell. 2000;11:2591–2604. doi: 10.1091/mbc.11.8.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel DD, Tsien RW. An FCCP-sensitive Ca2+ store in bullfrog sympathetic neurons and its participation in stimulus-evoked changes in [Ca2+]i. J Neurosci. 1994;14:4007–4024. doi: 10.1523/JNEUROSCI.14-07-04007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita N, Nagahashi A, Nagashima K, Rokudai S, Tsuruo T. Acceleration of apoptotic cell death after the cleavage of Bcl-XL protein by caspase-3-like proteases. Oncogene. 1998;17:1295–1304. doi: 10.1038/sj.onc.1202065. [DOI] [PubMed] [Google Scholar]
- Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Develop. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- Guo X, Macleod GT, Wellington A, Hu F, Panchumarthi S, Schoenfield M, Marin L, Charlton MP, Atwood HL, Zinsmaier KE. The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron. 2005;47:379–393. doi: 10.1016/j.neuron.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Halestrap A. Biochemistry: a pore way to die. Nature. 2005;434:578–579. doi: 10.1038/434578a. [DOI] [PubMed] [Google Scholar]
- Heidelberger R. ATP is required at an early step in compensatory endocytosis in synaptic terminals. J Neurosci. 2001;21:6467–6474. doi: 10.1523/JNEUROSCI.21-17-06467.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbeck PJ. Mitochondria and neurotransmission: evacuating the synapse. Neuron. 2005;47:331–333. doi: 10.1016/j.neuron.2005.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas E. BCL-xL regulates synaptic plasticity. Molec Interventions. 2006;6:208–222. doi: 10.1124/mi.6.4.7. [DOI] [PubMed] [Google Scholar]
- Jonas EA, Buchanan J, Kaczmarek LK. Prolonged activation of mitochondrial conductances during synaptic transmission. Science. 1999;286:1347–1350. doi: 10.1126/science.286.5443.1347. [DOI] [PubMed] [Google Scholar]
- Jonas EA, Hardwick JM, Kaczmarek LK. Actions of BAX on mitochondrial channel activity and on synaptic transmission. Antioxidants Redox Signal. 2005a;7:1092–1100. doi: 10.1089/ars.2005.7.1092. [DOI] [PubMed] [Google Scholar]
- Jonas EA, Hickman JA, Chachar M, Polster BM, Brandt TA, Fannjiang Y, Ivanovska I, Basanez G, Kinnally KW, Zimmerberg J, Hardwick JM, Kaczmarek LK. Proapoptotic N-truncated BCL-xL protein activates endogenous mitochondrial channels in living synaptic terminals. Proc Natl Acad Sci USA. 2004;101:13590–13595. doi: 10.1073/pnas.0401372101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas EA, Hickman JA, Hardwick JM, Kaczmarek LK. Exposure to hypoxia rapidly induces mitochondrial channel activity within a living synapse. J Biol Chem. 2005b;280:4491–4497. doi: 10.1074/jbc.M410661200. [DOI] [PubMed] [Google Scholar]
- Jonas EA, Hoit D, Hickman JA, Brandt TA, Polster BM, Fannjiang Y, McCarthy E, Montanez MK, Hardwick JM, Kaczmarek LK. Modulation of synaptic transmission by the BCL-2 family protein BCL-xL. J Neurosci. 2003;23:8423–8431. doi: 10.1523/JNEUROSCI.23-23-08423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas EA, Knox RJ, Kaczmarek LK. Giga-ohm seals on intracellular membranes: a technique for studying intracellular ion channels in intact cells. Neuron. 1997;19:7–13. doi: 10.1016/s0896-6273(00)80343-8. [DOI] [PubMed] [Google Scholar]
- Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- Krajewska M, Mai JK, Zapata JM, Ashwell KW, Schendel SL, Reed JC, Krajewski S. Dynamics of expression of apoptosis-regulatory proteins Bid, Bcl-2, Bcl-X, Bax and Bak during development of murine nervous system. Cell Death Differentiation. 2002;9:145–157. doi: 10.1038/sj.cdd.4400934. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Reed JC. Mitochondrial control of cell death. Nature Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S, Kelly PN, Ekert PG, Metcalf D, Roberts AW, Huang DC, Kile BT. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173–1186. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- Minn AJ, Velez P, Schendel SL, Liang H, Muchmore SW, Fesik SW, Fill M, Thompson CB. Bcl-x(L) forms an ion channel in synthetic lipid membranes. Nature. 1997;385:353–357. doi: 10.1038/385353a0. [DOI] [PubMed] [Google Scholar]
- Morgan JR, Prasad K, Jin S, Augustine GJ, Lafer EM. Uncoating of clathrin-coated vesicles in presynaptic terminals: roles for Hsc70 and auxilin. Neuron. 2001;32:289–300. doi: 10.1016/s0896-6273(01)00467-6. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000;150:887–894. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, Joseph MK, Kitada S, Korsmeyer SJ, Kunzer AR, Letai A, Li C, Mitten MJ, Nettesheim DG, Ng S, Nimmer PM, O’Connor JM, Oleksijew A, Petros AM, Reed JC, Shen W, Tahir SK, Thompson CB, Tomaselli KJ, Wang B, Wendt MD, Zhang H, Fesik SW, Rosenberg SH. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- Plas DR, Talapatra S, Edinger AL, Rathmell JC, Thompson CB. Akt and Bcl-xL promote growth factor-independent survival through distinct effects on mitochondrial physiology. J Biol Chem. 2001;276:12041–12048. doi: 10.1074/jbc.M010551200. [DOI] [PubMed] [Google Scholar]
- Plas DR, Thompson CB. Cell metabolism in the regulation of programmed cell death. Trends Endocrinol Metab. 2002;13:75–78. doi: 10.1016/s1043-2760(01)00528-8. [DOI] [PubMed] [Google Scholar]
- Rowland KC, Irby NK, Spirou GA. Specialized synapse-associated structures within the calyx of Held. J Neurosci. 2000;20:9135–9144. doi: 10.1523/JNEUROSCI.20-24-09135.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendel SL, Montal M, Reed JC. Bcl-2 family proteins as ion-channels. Cell Death Differentiation. 1998;5:372–380. doi: 10.1038/sj.cdd.4400365. [DOI] [PubMed] [Google Scholar]
- Smythe E, Pypaert M, Lucocq J, Warren G. Formation of coated vesicles from coated pits in broken A431 cells. J Cell Biol. 1989;108:843–853. doi: 10.1083/jcb.108.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swandulla D, Hans M, Zipser K, Augustine GJ. Role of residual calcium in synaptic depression and posttetanic potentiation: fast and slow calcium signaling in nerve terminals. Neuron. 1991;7:915–926. doi: 10.1016/0896-6273(91)90337-y. [DOI] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000;407:189–194. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- Tang Y, Zucker RS. Mitochondrial involvement in post-tetanic potentiation of synaptic transmission. Neuron. 1997;18:483–491. doi: 10.1016/s0896-6273(00)81248-9. [DOI] [PubMed] [Google Scholar]
- Tolbert LP, Morest DK. The neuronal architecture of the anteroventral cochlear nucleus of the cat in the region of the cochlear nerve root: electron microscopy. Neuroscience. 1982;7:3053–3067. doi: 10.1016/0306-4522(82)90229-9. [DOI] [PubMed] [Google Scholar]
- van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, Willis SN, Scott CL, Day CL, Cory S, Adams JM, Roberts AW, Huang DC. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Li XX, Gottleib E, Hill RB, Thompson CB, Colombini M. Bcl-xL promotes the open configuration of the voltage-dependent anion channel and metabolite passage through the outer mitochondrial membrane. J Biol Chem. 2001;276:19414–19419. doi: 10.1074/jbc.M101590200. [DOI] [PubMed] [Google Scholar]
- Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Wood DE, Newcomb EW. Cleavage of Bax enhances its cell death function. Exp Cell Res. 2000;256:375–382. doi: 10.1006/excr.2000.4859. [DOI] [PubMed] [Google Scholar]