Abstract
Objectives
We explored racial/ethnic differences in perceived cancer risk and determinants of these differences in a nationally representative sample of Whites, Blacks, Hispanics, and Asians.
Methods
Multiple regression techniques, including mediational analyses, were used to identify determinants and quantify racial/ethnic differences in the perception of the risk of developing cancer among 5,581 adult respondents to the 2007 Health Information Trends Survey (HINTS).
Results
Blacks, Hispanics, and Asians reported lower perceived cancer risk than Whites [Bs = − 0.40, −0.34, and −0.69 respectively, (Ps <.001)]. Contributing factors included relatively lower likelihood of reporting a family history of cancer, lower likelihood of having smoked, and a less strong belief that everything causes cancer among non-Whites thanamong Whites. Racial/Ethnic differences in perceived risk were attenuated in older respondents because perceived cancer risk was negatively associated with age for Whites but not for non-Whites.
Conclusions
Non-Whites had lower perceptions of cancer risk than Whites. Some of the racial/ethnic variability in perceived risk may be due to racial and ethnic differences in awareness of one’s family history of cancer, its relevance for cancer risk, experiences with behavioral risk factors, and salience of cancer risk information.
Keywords: Race, Ethnicity, Perceived Risk, Cancer, Psychology
Perceived risk for health problems is a key motivator for taking preventive action (1–2). With respect to cancer, an individual’s perceived cancer risk (PCR) predicts her/his likelihood of engaging in preventive health behaviors, including, cancer screening (3–5). Some evidence indicates that there may be differences in perceived cancer risk as a function of race/ethnicity, with non-Whites reporting lower perceived risk relative to Whites (6–9). Low perceived cancer risk may be a barrier to screening and other prevention behaviors, thus contributing to unnecessary cancer morbidity and mortality among non-Whites.
A small number of studies have investigated racial/ethnic variability in cancer risk perceptions yielding mixed results. Black, Hispanic and other non-White respondents to the 2000 National Health Interview Survey (NHIS) had lower global (all sites) PCR than Whites (9). Among approximately 800 participants in a no-cost oral cancer screening in the New York City area, relative to other racial/ethnic groups, Asians reported lower perceived risk for oral cancer (8). In a diverse sample of women recruited from primary care clinics in San Francisco, Asians reported lower perceived risk for cervical, breast, and colon cancer than Whites, but Hispanics reported higher perceived risk compared to Whites for colon and cervical cancer, and perceptions did not vary between Whites and Blacks (7). Studies have also shown that Blacks are less likely to perceive increased risk due to family history of cancer than Whites, or their Black counterparts without a family history of cancer (6, 10–12).
Overall, Blacks have higher incidence and mortality from cancer (13); therefore, low perception of risk in this population is of particular concern. Although Asians and Hispanics have lower all-site cancer incidence and mortality than Whites (13), lower-than-average risk does not equate to no cancer risk. Even in these groups, inappropriately low perceived cancer risk remains a concern to the extent that it hinders cancer screening or other cancer prevention behaviors. Although not all studies link cancer risk perception and cancer screening, narrative and meta-analytic reviews indicate that, overall, screening is more likely among people with higher perceived risk (3–5). There is ample empirical evidence that non-Whites are less likely than Whites to be screened for breast, cervical, colorectal, and prostate cancer. Specifically, mammography, cervical screening, colorectal cancer screening and prostate cancer screening is lower among Hispanics and Asians than Whites (14–16). Blacks and Whites are equivalently likely to receive prostate and cervical cancer screening (15, 17); however, Blacks may be less likely to receive regular mammograms or colorectal cancer screening (14–15, 17–19). Given lower screening rates among non-Whites and the importance of risk perception to cancer screening utilization, research is needed to better understand the construction of cancer risk perceptions in these groups.
The extant studies on racial and ethnic differences in risk perception have three key limitations. First, most are based on small, non-representative samples; therefore, additional studies of large, nationally representative samples are needed to confirm the generalizability of observed race/ethnic differences in PCR. The present study employs data from the National Cancer Institute’s 2007 nationally representative Health Information National Trends Survey (HINTS). Second, the studies published to date have described differences in risk as a function of race/ethnicity, but have not explored possible factors accounting for those differences. In this study we explored two classes of explanations for why racial/ethnic differences in PCR may exist. First, it is possible that race/ethnicity is associated with differences in predictors of PCR that, in turn, predict racial/ethnic differences in PCR (mediation hypothesis). For example, minorities are less likely to report having a family history of cancer (20–22) which could, in turn, result in lower perceived cancer risk among minorities compared to Whites. To examine this possibility, we identified potential determinants of PCR and tested their role as mediators of racial/ethnic differences in PCR. A second possibility is that the strength or direction of the relationship between factors that typically predict PCR differ as a function of race/ethnicity (moderation hypothesis). For example, if factors that one would expect to increase PCR, such as having a family history of cancer, do not do so among non-Whites, PCR may be lower among non-Whites than Whites. For example, evidence indicates that Blacks with a family history of cancer do not have an increased perception of risk compared to those without a family history of cancer, whereas for Whites, family history is a robust predictor of increased perceived cancer risk (6, 10–12). To test this second explanation, we conducted a series of analyses to test for interactions between race and determinants of PCR. Third, in some previous studies, race/ethnic differences in PCR were not observed (7, 23); however, these previous analyses were performed on data from people who were 45 years or older or 50 years or older. In majority White samples, absolute PCR has been found to be lower among older than younger respondents (24). Racial/Ethnic differences in PCR may be reduced as a function of age if PCR is relatively lower among older Whites but not older non-Whites. In the present work we examined whether this is the case in an attempt to resolve inconsistencies in the literature on racial/ethnic differences in PCR.
Materials and Methods
Sample
Data for the study were obtained from the 2007 HINTS, a probability-based survey conducted by the National Cancer Institute to study cancer health information seeking, cancer knowledge and health behavior. The survey employed a dual-frame design [Random Digit Dial (RDD) and mail] due to concerns about falling response rates and lack of coverage for cell phone-only households when RDD is used alone. Response rates for the RDD sample were 42.37% for the screener interview and 57.19% for the full interview, yielding an overall response rate of 24.23% (N = 4,081) (25). The overall response rate for the mail sample was 30.99% (N = 3,593). Survey respondents were excluded if they did not self-identify as one of the four races/ethnicities of interest (White, Black, Hispanic or Asian) (n = 718), if they reported having been diagnosed with cancer in the past (n = 942), if they did not respond to the PCR item (n = 222), or if they had missing data on other key variables of interest (gender, marital status, education, family history of cancer, smoking status, perceived health)(n = 211). Analyses were performed on a final sample of 5,581 adult respondents (mail = 2,768, RDD=2,820; White = 4,319, Black = 567, Hispanic = 514, Asian = 181). On average, respondents who were dropped from the analyses due to missing data on PCR or the other variables of interest (433) were older (t = 2.83, P = .006), less educated (t = −3.58, P = .001), less likely to be married (χ2 =10.12, P= .002), had worse perceived health (t = −2.43, P = .02), were more likely to be Black (χ2 =7.08, P = .009), or Hispanic (χ2 = 11.02, P = .001)than White, and perceived their cancer risk to be lower (t = −2.32, P = .02) than respondents whose data were included the study. The groups did not vary with respect to likelihood of reporting a family history of cancer, smoking status or the belief that everything causes cancer (Ps > .15).
Variables
Demographic characteristics
Participants self-classified as Non-Hispanic White, Hispanic, Non-Hispanic Black or African American or Non-Hispanic Asian. The four groups are hereafter referred to as White, Black, Hispanic, and Asian. Gender, age, marital status (married or living with a partner vs. divorced, widowed, separated, or never been married), and education (< high school, high school, some college, college or greater) were assessed via self-report.
Perceived cancer risk
The outcome of interest was perception of one’s overall risk for developing cancer or perceived cancer risk (PCR). Respondents were asked to indicate their level of perceived risk on a scale of absolute risk: “How likely do you think it is that you will develop cancer in the future? Would you say your chance of getting cancer is very low/somewhat low/moderate/somewhat high/very high?” Responses were converted to a 5-point response format with higher scores representing greater PCR.
Potential mediators/moderators
Several factors that have been associated with PCR were examined (reporting a family history of cancer, having been a cigarette smoker in the past or present, perceived health, and beliefs about cancer)(3, 5, 9, 26). Participants were asked, “Have any of your family members ever had cancer?” Respondents were classified as having had one or more family members with cancer or no family members with cancer. Respondents were classified as ever smokers if they had smoked 100 or more cigarettes in their life time or never smokers if they had not. Perceived health was assessed by asking respondents, “in general, would you say your health is excellent/very good/good/fair/poor?” Responses were recoded so that higher responses represented better health. Beliefs about cancer seriousness, early detection, and prevention were assessed with single item measures and 4-point response formats, ranging from strongly agree to strongly disagree, and were reverse coded so that higher responses represented greater agreement. These items were: 1) “When I think of cancer, I automatically think of death,” 2) “Cancer is most often caused by a person’s behavior or lifestyle,” 3) “Getting checked regularly for cancer helps find cancer when it’s easy to treat,” 4) “Cancer is an illness that when detected early can typically be cured,”5) “It seems like everything causes cancer,” 6) “There’s not much you can do to lower your chances of getting cancer,” and 7) “There are so many different recommendations about preventing cancer, it’s hard to know which ones to follow.” Responses to these individual items were typically not correlated (Mean r = .11; Range = 0.00 to 0.33), therefore these items were analyzed individually. An additional cancer belief item asked people, “Overall, how many people who develop cancer do you think survive at least 5 years (less than 25%/about 25%/about 50%/about 75%/nearly all)? Responses were converted to a 5-point response format with higher scores representing greater perceived likelihood of survival.
Data Analysis
All analyses employed data that were weighted to produce nationally representative estimates. Simple logistic and linear regressions were used to assess differences in the categorical and continuous variables as a function of race. Multiple linear regression was used to assess the adjusted effects of hypothesized predictors on PCR. We identified explanatory factors for the hypothesized racial/ethnic differences in PCR employing mediation analysis techniques described by Baron and Kenny (27).1 Mediation is where the predictor variable affects the outcome variable through a third(mediating) variable. According to Baron and Kenny, for there to be statistical mediation, the predictor variable must predict the outcome variable and the hypothesized mediating variable, the mediating variable must predict the outcome variable while controlling for the predictor variable and there should be a significant reduction in the effect of the predictor on the outcome variable when the mediating variable is included in the regression. Linear regression was employed to test most pathways; however, when dichotomous mediators were tested, logistic regression and transformed coefficients were used as appropriate (28). The significance of mediating (indirect) effects were tested employing the Sobel test (29). Tests of interaction hypotheses were conducted employing multiple linear regression. All analyses included sample mode (mail vs. RDD), gender, age, marital status, and education as covariates.2 Income was not included as a covariate due to substantial missing data on this variable (12% of final sample).3 Linear variables included in the interaction terms were mean centered. All analyses were performed using Stata 10(StataCorp, College Station, TX).
Results
Sample Characteristics and Predictors of Perceived Cancer Risk
Characteristics of the total sample and of each race/ethnicity group are presented in Table 1. There were several statistically significant group differences (P < .05). Hispanic respondents were more likely to be male than White respondents; Black respondents were less likely to be married than White respondents; Blacks and Hispanics had less education than Whites and Asians had more education than Whites; and respondents from the three non-White groups were younger, on average, than White respondents. Blacks, Hispanics and Asians were significantly less likely than Whites to report a family history of cancer; Hispanics and Asians were less likely to have smoked cigarettes than Whites; Blacks and Hispanics perceived that they were less healthy than Whites.
Table 1.
Characteristics and 95% Confidence Intervals of the Weighted Sample (Unweighted N = 5,581)
| Total Sample % | White % | Black % | Hispanic % | Asian % | |
|---|---|---|---|---|---|
| Female | 50.69 (49.94 to 51.44) | 51.23 (50.32 to 52.15) | 57.25 (52.14 to 62.37) | 45.17† (40.65 to 49.69) | 42.16 (32.00 to 52.32) |
| Married | 57.20 (56.35 to 58.06) | 60.31 (59.46 to 61.52) | 36.32* (32.19 to 40.45) | 57.58 (52.49 to 62.67) | 61.08 (49.58 to 72.57) |
| College or greater | 24.91 (24.39 to 25.44) | 27.64 (26.96 to 28.32) | 16.40* (13.16 to 19.64) | 10.23* (7.61 to 12.84) | 45.96* (35.73 to 56.19) |
| Family history of cancer | 70.10 (68.14 to 72.07) | 76.67 (74.55 to 78.80) | 64.86* (59.13 to 70.59) | 51.91* (44.79 to 59.03) | 36.71* (24.43 to 49.00) |
| Ever smoker | 45.65 (43.60 to 47.71) | 48.68 (46.51 to 50.86) | 44.50 (38.55 to 50.45) | 39.99‡ (33.59 to 46.39) | 19.66* (10.97 to 28.35) |
| Total Sample mean | White mean | Black mean | Hispanic mean | Asian mean | |
|---|---|---|---|---|---|
| Age | 44.33 (44.33 to 44.60) | 46.27 (45.95 to 46.59) | 43.85‡ (42.07 to 45.63) | 36.50* (35.01 to 37.92) | 38.73* (35.35 to 42.12) |
| Perceived health (out of 5) | 3.42 (3.38 to 3.56) | 3.48 (3.43 to 3.53) | 3.32‡ (3.21 to 3.43) | 3.22* (3.09 to 3.35) | 3.30 (3.07 to 3.54) |
| Perceived cancer risk (out of 5) | 2.62 (2.58 to 2.67) | 2.73 (2.69 to 2.78) | 2.34* (2.22 to 2.46) | 2.46* (2.32 to 2.59) | 2.09* (1.90 to 2.29) |
subgroup significantly different from Whites P < 0.001
subgroup significantly different from Whites P < 0.01
subgroup significantly different from Whites P < 0.05
Racial Differences in Perceived Cancer Risk
On average, respondents in each of the non-White groups had lower PCR than Whites (Table 1). After adjusting for demographic characteristics (age, gender, education, marital status) and sample mode (mail vs. RDD)(3), being Black (B= −0.40, 95% CI = −0.53 to −0.27), Hispanic (B= −0.34, 95% CI = −0.48 to −0.19), or Asian (B= −0.69, 95% CI = −0.90 to −0.48) compared to White still predicted lower PCR.
Mediators of Racial Differences in Perceived Cancer Risk
Results of the mediational analyses to explore whether racial/ethnic differences in determinants of PCR explained racial/ethnic differences in PCR are presented in Table 2. As can be seen in the table, the race-PCR relation was mediated by family history, smoking status, and the strength of one’s belief that everything causes of cancer. After adjustment for other demographic characteristics, 14.6% of the Black-White difference in PCR was explained by less frequent reporting of family history of cancer among Blacks than Whites. 29.8% of the Hispanic-White difference in PCR was explained by relatively less frequent reporting of a family history of cancer among Hispanics than Whites, and 10.4% of the difference by lower likelihood of having been or being a smoker among Hispanics than among Whites. Twenty-eight percent of the Asian-White difference in PCR was explained by less frequent reporting of a family history of cancer by Asians than Whites and 13.4% by lower likelihood of having been a smoker among Asians than among Whites. All of the above mediating (indirect) effects were statistically significant (see results of Sobel tests presented in Table 2). Table 2 also provides the relevant coefficients from the regression models used to test for mediation.
Table 2.
| Race → PCR† | Race → mediator | Mediator → PCR controlling for race | Race → PCR controlling for mediator | Sobel statistic (P-value) | % Effect | |
|---|---|---|---|---|---|---|
| Hypothesized mediator = family history of cancer | ||||||
| Black | −0.41 (−0.54 to −0.28) | −0.62 (−0.90 to −0.34) | 0.48 (0.39 to 0.57) | −0.35 (−0.48 to −0.22) | −3.99 (<0.001) | 14.6 |
| Hispanic | −0.35 (−0.49 to −0.21) | −1.06 (−1.39 to −0.73) | 0.44 (0.35 to 0.53) | −0.24 (−0.39 to −0.10) | −5.29 (<0.001) | 29.8 |
| Asian | −0.71 (−0.92 to −0.50) | −1.79 (−2.36 to −1.22) | 0.48 (0.38 to 0.57) | −0.52 (−0.73 to −0.30) | −5.34 (<0.00) | 27.2 |
| Hypothesized mediator = smoking status | ||||||
| Black | −0.41 (−0.54 to −0.27) | Ns | ||||
| Hispanic | −0.35 (−0.49 to −0.21) | −0.43 (−0.72 to −0.14) | 0.35 (0.26 to 0.44) | −0.31 (−0.45 to −0.17) | −2.77 (0.006) | 10.4 |
| Asian | −0.71 (−0.92 to −0.50) | −1.23 (−1.79 to −0.68) | 0.38 (0.30 to 0.46) | −0.61 (−0.82 to −0.41) | −4.02 (<0.001) | 13.4 |
| Hypothesized mediator = belief that everything causes cancer | ||||||
| Black | −0.41 (−0.54 to −0.27) | −0.28 (−0.45 to −0.11) | 0.15 (0.11 to 0.19) | −0.37 (−0.50 to −0.24) | −3.03 (0.002) | 8.2 |
| Hispanic | −0.35 (−0.49 to −0.21) | −0.38 (−0.55 to −0.21) | 0.15 (0.11 to 0.20) | −0.28 (−0.43 to −0.14) | −3.66 (0.002) | 17.9 |
| Asian | −0.71 (−0.92 to −0.50) | −0.39 (−0.64 to −0.15) | 0.18 (0.14 to 0.23) | −0.62 (−0.81 to −0.43) | −3.15 (0.002) | 12.3 |
Untransformed coefficients and 95% confidence intervals for pathways that must be statistically significant to support mediation and results of Sobel Test of statistical significance for the mediating effects
PCR = perceived cancer risk
All models included age, marital status, gender, and education as covariates
The associations between beliefs about cancer seriousness, early detection and prevention, and PCR were very modest (|B|s = 0 .04 to 0.17); only one of the cancer beliefs, “it seems like everything causes cancer,” partially mediated race/ethnicity differences in PCR (see Table 2). After adjusting for demographic characteristics, less strong belief that everything causes cancer among Hispanics compared to Whites accounted for 17.9% of the Hispanic-White difference in PCR. Similarly, less strong belief that everything causes cancer among Asians accounted for 12.3% of the difference in the Asian-White difference in PCR. Strength of belief that everything causes cancer did not differ between Blacks and Whites; therefore, we did not test for mediation. Perceived health was associated with race but it did not mediate the effects of race/ethnicity on PCR.
Interactions between Race/Ethnicity and Predictors of Perceived Cancer Risk
Racial/Ethnic differences in PCR could also arise if typical determinants of PCR influence PCR among Whites but not among non-Whites. For this to be the case, one would expect that an interaction between race/ethnicity and a given determinant predicts PCR, and for the determinant to influence PCR (e.g., family history status) among Whites but not among non-Whites (or to a lesser degree among non-Whites). Interactions between race and smoking status and race and perceived health were not significant (Ps >.33). There was a marginally significant interaction between race/ethnicity (Hispanic vs. White) and family history (B = -0.26, 95% CI = -0.56 to 0.04). It was due to the fact that, among Whites, reporting a family history of cancer was positively associated with higher PCR (B = 0.49, 95% CI = 0.40 to 0.59), but among Hispanics reporting a family history of cancer and PCR were not significantly associated with one another(B= 0.19, 95% CI = −0.10 to 0.49). Of note, among Blacks, PCR was positively associated with reporting a family history of cancer (B = 0.43, 95% CI = 0.14 to 0.71). The relationship was weaker and did not reach significance for Asians (B = 0.29, 95% CI = −0.11 to −0.69).
We explored whether race interacted with any of the cancer beliefs to predict PCR. The association between the belief that “everything causes cancer” and PCR varied by race/ethnicity [Black vs. White (B = −0.14, 95% CI = −0.27 to −0.01); Hispanic vs. White (B = −0.12, 95% CI= −0.23 to −0.02)]. These interaction effects were due the fact that among Whites, belief that everything causes cancer was positively associated with PCR (B = 0.17, 95% CI = 0.13 to 0.22), but no such association was found for Blacks (B = 0.03, 95% CI = -0.08 to 0.15) or Hispanics (B = 0.05, 95% CI = −0.06 to 0.16). Also, the effect of believing that cancer is caused by behavior or lifestyle on PCR varied by race/ethnicity (Asian vs. White) (B = 0.30, 95% CI =0.06 to 0.58). This interaction was attributable to the fact that in the Asian group, respondents perceived themselves at greater risk to the extent that they believed that cancer was caused by behavior or lifestyle (B = 0.27, 95% CI = 0.04 to 0.49), but Whites did not (B = 0.03, −0.02 to 0.09). None of the other beliefs significantly interacted with race to predict PCR (Ps > .13).
Cancer Risk Perceptions across the Lifespan
On average, being older was associated with lower PCR; however, this main effect masked racial/ethnic differences in the slopes for PCR as a function of age. Figure 1 displays mean trajectories for PCR as a function of race. Slopes differed significantly between Hispanics and Whites (B = .009, 95% CI = .001 to .018) and between Asians and Whites (B = .02,95% CI = .009 to .039). The Black versus White difference did not reach significance (B = .006, 95% CI = −.002 to .014). Follow-up analysis revealed that for Whites, being older was associated with comparatively lower PCR (B = −.008, 95% CI = −.011 to −.006), but that differences as a function of age did not reach statistical significance for any of the non-White groups (Ps > .52).
Figure 1.
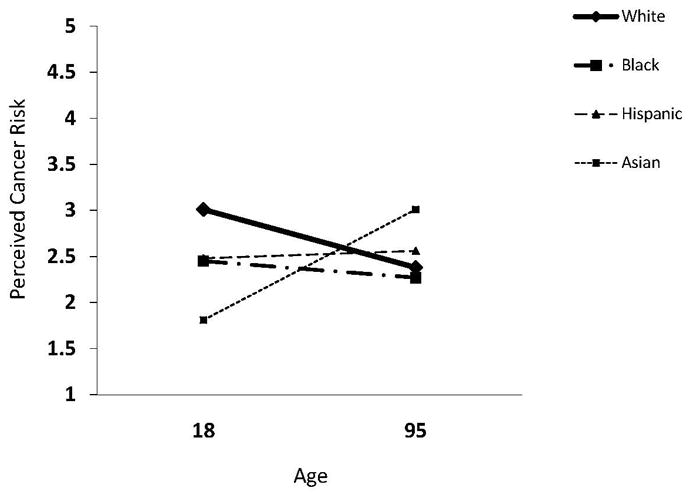
Perceived Cancer Risk as a Function of Age, by Race
Discussion
Black, Hispanic, and Asian respondents to HINTS perceived themselves at lower risk for getting cancer (lower PCR) than White respondents. These differences remained after controlling for age, education, marital status, gender and the mode in which the survey was administered (mail vs. RDD). Blacks perceived their cancer risk to be lower than Whites, in part, because they were less likely to have or know that they have a family history of cancer, and because they were less likely to believe that everything causes cancer than Whites. Similar patterns were found for Hispanics and Asians, except that being less likely to have smoked also accounted for lower PCR among Hispanics and Asians with respect to Whites. Although adding these mediators to the adjusted model of PCR significantly reduced the association between race and PCR, even after accounting for these effects, being Black, Hispanic, or Asian remained significantly associated with lower PCR. Additional research is needed to identify other factors that contribute to racial/ethnic differences in PCR.
The relation between race and reported family history of cancer is consistent with past research (20), including a previous study in which we found that racial/ethnic minorities, and especially immigrants within these groups, were less likely to report a family history of cancer than Whites and non-immigrants (22). Given disparities in cancer incidence and prevalence, Blacks should have a greater likelihood of reporting that they have or had a family member who was diagnosed with cancer than Whites. Although other Asians and Hispanics have lower cancer incidence, in our previous work we suggested that the differences in family history of cancer reporting were in excess of what would be expected due to racial/ethnic differences in cancer incidence (22), and that these differences are likely due, in part, to minorities and immigrants being relatively less likely to know their family history of cancer. We predicted that less knowledge of family history of cancer among immigrants and racial/ethnic minorities could result in health care providers and non-Whites themselves underestimating their cancer risk. Results from the present study support this hypothesis. Lower reporting of family history of cancer among non-Whites, compared to Whites, did predict relatively lower PCR in non-Whites.
Hispanics and Asians were less likely than Whites to have smoked during their lifetimes, which, in turn, was associated with lower PCR compared to Whites. It is realistic for people who have never smoked to appraise their cancer risk as lower than those who have; however, future research should investigate whether people distinguish between smoking as a risk factor for cancers that are strongly associated with smoking (e.g., lung cancer, bladder cancer) and others that are less strongly associated with smoking (e.g., breast cancer, prostate cancer). It is possible that generalized beliefs that, as a nonsmoker, one is at relatively lower risk for all cancers could impede appropriate screening and other prevention behaviors. Physical inactivity and low fruit and vegetable consumption are associated with increased risk for several kinds of cancer (30). According to the 2005 Behavioral Risk Factor Surveillance System survey, there are racial/ethnic differences in physical activity and fruit and vegetable consumption (31). However, self-reported physical activity is higher among Whites than among Blacks, Hispanics or Asian/Pacific Islanders, a pattern that would not account for lower perceptions of risk among non-Whites compared to Whites. Asians/Pacific Islanders consume more fruits and vegetables than Whites (no differences across the other three races). Fruit and vegetable consumption was not assessed in the full HINTS 2007 sample; therefore, we were not able to explore whether fruit and vegetable consumption accounted for lower PCR among Asians compared to Whites.
We tested a second model of racial/ethnic differences in PCR: whether factors that typically predict PCR only do so for Whites, contributing to the lower PCR among non-Whites with respect to Whites. For example, if non-White groups have had less access to personally relevant cancer risk information, do not believe that cancer risk information is personally applicable, or do not trust cancer risk information (32–33), their PCR may not be calibrated to known risk factors (e.g., family history of cancer, smoking). This explanation received some support. Among Hispanics, PCR varied less strongly as a function of family history of cancer than among Whites and Blacks. Hispanics may be less aware of family history risk due to language and other barriers that can make the dissemination of health information difficult. The finding is important for qualifying previous reports that people with a family history of cancer perceive themselves at higher risk for cancer (3, 9, 23): this may not be true among Hispanics. In combination, lower reporting (and knowledge) of family history of cancer and lack of influence of family history on PCR among Hispanics, suggest that tailored public health and health care provider-delivered messages about the importance of family history for cancer risk and encouraging family communication about cancer may be a means of increasing awareness of cancer risk among non-Whites.
Race Differences in Perceived Cancer Risk Across the Lifespan
The findings are consistent with and expand on previous research that demonstrated that older age is associated with lower PCR (9) by showing that the effect varies as a function of race. Perceived cancer risk was lower in older Whites than younger Whites, but similar downward slopes were not found among non-Whites. This, in effect, narrowed the gap between PCR in older Whites and Non-Whites. Racial/Ethnic differences in age-related trajectories would explain why one might not find racial/ethnic differences in PCR in samples populated primarily by older adults; this would not be due to high PCR among older non-Whites, but rather to low PCR among older Whites. The age-related differences in PCR among Whites could be a cohort effect if large-scale cancer prevention awareness efforts initiated in the early 1980s (34) only reached certain sectors of the public. Efforts to communicate about cancer and cancer risk and prevention may have been more successful at reaching Whites coming into adulthood during this period, but less successful at reaching non-Whites and Whites who were older at the time.
Do Individual-Level Cancer Risk Perceptions Reflect Community-Level Cancer Salience?
The existence of racial/ethnic differences in PCR suggests that community-level dynamics influence PCR. Individual-level perceptions of cancer risk may vary as a function of the salience of the disease in one’s larger racial/ethnic community and, or peer groups. In the United States, social networks tend to be racially homogenous (35)and race/ethnicity is a central component of identity and social categorization (36); therefore, people may be most likely to base their cancer risk perceptions on the experiences and beliefs of same-race/ethnic peers and models, and images of members of their race/ethnic group. Cancer risk salience may reflect cancer incidence and mortality in one’s racial/ethnic group, prevalence of cancer in one’s social network, and the degree to which cancer is discussed openly within one’s network and larger community. Cultural variability in openness about cancer (37–40) may also contribute to the relative salience of cancer risk in a given community. Risk perceptions, and even whether risk perception is a meaningful construct for a given group, are shaped by culturally-informed systems of beliefs about health and illness (41). These systems of beliefs may be inconsistent with those promoted by the mainstream U.S. medical establishment, which could reduce the relevance and effectiveness of traditional risk and prevention communication in some communities (41).
Strengths, Limitations and Future Directions
There has been limited research on variability in cancer risk perceptions across racial/ethnic groups even though cancer screening is known to be lower in non-White groups, and low PCR can be a barrier to screening and other cancer prevention behaviors. In the present study we examined whether, in a nationally representative sample, non-Whites had lower PCR than Whites and tested several possible explanations for these differences. Results indicated that racial/ethnic differences in awareness of family history, experience with risk behaviors (i.e., smoking) and salience of cancer risk information could cause racial/ethnic differences in cancer risk perceptions; however, these results should be considered suggestive because we used correlational, cross-sectional data to test these pathways. Laboratory-based experimental and, or community-based quasi-experimental research is needed to confirm the causal pathways proposed on the basis of our findings. Fortunately, logical plausibility also lends support to the proposed pathways. It is reasonable that people who have never smoked might perceive their cancer risk to be lower; the reverse, that low PCR causes lower likelihood of being a smoker, is far less plausible. Similarly, although lower PCR could, to an extent, contribute to people being less attentive to information about, and less able to report their family history of cancer, this is probably less likely than knowing (or not knowing) that one has a family history of cancer affecting perceptions of risk. Finally, we proposed that racial/ethnic differences in the belief that everything causes cancer predict PCR. Although beliefs about the causes of cancer would seem to be antecedent to beliefs about risk perception, it is possible that the two constructs could be reciprocally related if global perceptions of risk shape how people process health information about cancer risk-factors.
The study has a number of other limitations. Although the White, Black, and Hispanic sub-samples were relatively large, the Asian sample was relatively small (N = 181), resulting in relatively less stable estimates for comparisons between Asians and Whites. Also, approximately 8%of the eligible cases were dropped from the analysis due to missing values on one or more of the key variables of interest. There were significant differences between participants who were dropped versus those retained with respect to demographic and other characteristics. We do not believe, however, that dropping these cases substantially affected the results of the study. If anything, they may have weakened the findings. Participants who were dropped were, on average, more likely to be non-White and perceive their risk to be lower than those who were retained.
Despite the constraints of using a dataset not originally designed for the purpose of the present work, it was possible to identify several determinants of lower PCR among non-Whites compared to Whites, perhaps most importantly, lower likelihood of reporting family history of cancer among non-Whites relative to Whites. However, many additional hypotheses merit examination. Qualitative research has generated several possible explanations for why cancer risk perceptions are lower in Black communities, including mistrust of a predominantly White medical establishment, lack of personally relevant cancer information, and lack of perceived personal control over health outcomes (32). This qualitative work ought to be extended to other non-White populations, and survey work is needed to test whether these explanations are born out in larger representative samples. Finally, we studied perceptions of global cancer risk. Perhaps most the pressing need is for research that examines racial/ethnic differences in PCR for specific cancers, including more fine-grained analyses of different aspects of risk (e.g., comparative versus absolute risk; risk of developing cancer versus risk of dying of cancer; or affective versus cognitive components of risk perception), and whether these risk perceptions are associated with cancer screening rates and prevention behavior.
Acknowledgments
Author Note
We thank John Gaeddert for his assistance with the preparation of this manuscript.
Footnotes
More sophisticated and powerful bootstrapping techniques for estimating indirect effects have been developed since Baron and Kenny first introduced their approach for testing statistical mediation;however, these techniques have not been developed for STATA for use with complex survey data. Figure 1. Perceived Cancer Risk as a Function of Age, by Race
All analyses included sample mode as a covariate. Participants in the final sample of mail respondents had higher education than those in the final sample of RDD respondents (Ps < .05). In addition, after controlling for other variables included in the multivariate analyses (age, marital status, education, gender, family history of cancer, smoking status) PCR varied as a function of an interaction between race/ethnicity and mode (−0.43, −0.73 to −0.10). This was attributable to the fact that Asians in the RDD sample reported lower PCR (M = 1.85, 95% CI = 1.53 to 2.17) than Asians in the mail sample (M = 2.33, 95% CI = 2.11 to 2.56). It should be noted, however, that the Asian subsamples were small (119 in the mail sample and 62 in the RDD sample). There were no other significant interaction effects between sample mode and the variables included in the analyses on PCR (all Ps > .2).
We doubt that not including income as a covariate significantly impacted the results of the analyses. Income was not associated with PCR. In addition, we included education which covaried with income (r = 0.44, P < .001).
During the preparation of this manuscript Dr. Kiviniemi was supported by NIH grant K07CA106225 and Dr. Underwood was supported by an Astella/AUA Foundation Rising Star in Urology Award
References
- 1.Janz NK, Becker MH. The Health Belief Model: A decade later. Health Educ Q. 1984;11:1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- 2.Weinstein N. The precaution adoption process. Health Psychol. 1988;7:355–86. doi: 10.1037//0278-6133.7.4.355. [DOI] [PubMed] [Google Scholar]
- 3.Katapodi MC, Lee KA, Facione NC, Dodd MJ. Predictors of perceived breast cancer risk and the relation between perceived risk and breast cancer screening: A meta-analytic review. Prev Med. 2004;38:388–402. doi: 10.1016/j.ypmed.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 4.McCaul KD, Branstetter AD, Schroeder DM, Glasgow RE. What is the relationship between breast cancer risk and mammography screening? A meta-analytic review. Health Psychol. 1996;15:423–9. doi: 10.1037//0278-6133.15.6.423. [DOI] [PubMed] [Google Scholar]
- 5.Vernon SW. Risk perception and risk communication for cancer screening behaviors: a review. J Natl Cancer Inst Monog. 1999;1999:101–19. doi: 10.1093/oxfordjournals.jncimonographs.a024184. [DOI] [PubMed] [Google Scholar]
- 6.Hughes C, Lerman C, Lustbader E. Ethnic differences in risk perception among women at increased risk for breast cancer. Breast Cancer Res Treat. 1996;40:25–35. doi: 10.1007/BF01806000. [DOI] [PubMed] [Google Scholar]
- 7.Kim SE, Perez-Stable EJ, Wong S, et al. Association Between Cancer Risk Perception and Screening Behavior Among Diverse Women. Arch Intern Med. 2008;168:728–34. doi: 10.1001/archinte.168.7.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hay JL, Ostroff JS, Cruz GD, LeGeros RZ, Kenigsberg H, Franklin DM. Oral Cancer Risk Perception among Participants in an Oral Cancer Screening Program. Cancer Epidemiol Biomarkers Prev. 2002;11:155–8. [PubMed] [Google Scholar]
- 9.Honda K, Neugut AL. Associations between perceived cancer risk and established risk factors in a national community sample. Cancer Detect Prev. 2004;28:1–7. doi: 10.1016/j.cdp.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Griffith KA, McGuire DB, Royak-Schaler R, Plowden KO, Steinberger EK. Influence of family history and preventive health behaviors on colorectal cancer screening in African Americans. Cancer. 2008;113:276–85. doi: 10.1002/cncr.23550. [DOI] [PubMed] [Google Scholar]
- 11.Haas JS, Kaplan CP, Des Jarlais G, et al. Perceived risk of breast cancer among women at average and increased risk. J Womens Health. 2005;14:845–51. doi: 10.1089/jwh.2005.14.845. [DOI] [PubMed] [Google Scholar]
- 12.Bloom JR, Stewart SL, Oakley-Girvans I, et al. Family history, perceived risk, and prostate cancer screening among African American men. Cancer Epidemiol Biomarkers Prev. 2006;15:2167–73. doi: 10.1158/1055-9965.EPI-05-0738. [DOI] [PubMed] [Google Scholar]
- 13.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC) National Health Interview Survey in Health, United States 2007. Centers for Disease Control and Prevention; 2007. Available from: http://www.cdc.gov/cancer/breast/statistics/screening.htm. [Google Scholar]
- 15.Ponce NA, Babey SH, Etzioni D, Spencer BA, Brown ER, Chawla N. Cancer screening in California: Findings from the 2001 California Health Interview Survey. Los Angeles: UCLA Center for Health Policy Research; 2003. [PubMed] [Google Scholar]
- 16.Wee CC, McCarthy EP, Phillips RS. Factors associated with colon cancer screening: the role of patient factors and physician counseling. Prev Med. 2005;41:23–9. doi: 10.1016/j.ypmed.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Ward E, Jemal A, Cokkinides V, et al. Cancer Disparities by Race/Ethnicity and Socioeconomic Status. CA Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 18.Edwards QT, Li AX, Pike MC, et al. Ethnic differences in the use of regular mammography: the multiethnic cohort. Breast Cancer Res Treat. 2009;115:163–70. doi: 10.1007/s10549-008-0049-7. [DOI] [PubMed] [Google Scholar]
- 19.Ananthakrishnan AN, Schellhase KG, Sparapani RA, Laud PW, Neuner JM. Disparities in Colon Cancer Screening in the Medicare Population. Arch Intern Med. 2007;167:258–64. doi: 10.1001/archinte.167.3.258. [DOI] [PubMed] [Google Scholar]
- 20.Ramsey SD, Yoon P, Moonesinghe R, Khoury MJ. Population-based study of the prevalence of family history of cancer: implications for cancer screening and prevention. Genet Med. 2006;8:571–5. doi: 10.1097/01.gim.0000237867.34011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinsky PF, Kramer BS, Reding D, Buys S. Reported family history of cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. Am J Epidemiol. 2003;157:792–9. doi: 10.1093/aje/kwg043. [DOI] [PubMed] [Google Scholar]
- 22.Orom H, Coté ML, Gonzalez HM, Underwood W, 3rd, Schwartz AG. Family history of cancer: Is it an accurate indicator of cancer risk in the immigrant population? Cancer. 2008;112:399–406. doi: 10.1002/cncr.23173. [DOI] [PubMed] [Google Scholar]
- 23.Hay J, Coups E, Ford J. Predictors of perceived risk for colon cancer in a national probability sample in the United States. J Health Commun. 2006;11:71–92. doi: 10.1080/10810730600637376. [DOI] [PubMed] [Google Scholar]
- 24.Honda K, Neugut AI. Associations between perceived cancer risk and established risk factors in a national community sample. Cancer Detect Prev. 2004;28:1–7. doi: 10.1016/j.cdp.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Cantor D, Coa K, Crystal-Mansour SD, Davis T, Dipko S, Sigman R. Final report. Rockland, MD: Westat; 2009. Health Information National Trends Survey (HINTS) 2007. [Google Scholar]
- 26.McQueen A, Swank PR, Bastian LA, Vernon SW. Predictors of perceived susceptibility of breast cancer and changes over time: a mixed modeling approach. Health Psychol. 2008;27:68–77. doi: 10.1037/0278-6133.27.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic and statistical considerations. J Pers Soc Psychol. 1986:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 28.MacKinnon DP, Dwyer JH. Estimating mediated effects in prevention studies. Eval Rev. 1993;17:144–58. [Google Scholar]
- 29.Sobel ME. Asymptotic intervals for indirect effects in structural equations models. In: Leinhart S, editor. Sociological methodology. San Francisco: Jossey-Bass Bloom; 1982. pp. 290–312. [Google Scholar]
- 30.Danaei G, Ding EL, Mozaffarian D, et al. The Preventable Causes of Death in the United States: Comparative Risk Assessment of Dietary, Lifestyle, and Metabolic Risk Factors. PLoS Med. 2009;6:e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention (CDC) Prevalence of Fruit and Vegetable Consumption and Physical Activity by Race/Ethnicity--United States, 2005. MMWR Morb Mortal Wkly Rep. 2007;56:301–4. [PubMed] [Google Scholar]
- 32.Salant T, Gehlert S. Collective memory, candidacy, and victimisation: community epidemiologies of breast cancer risk. Sociol Health Illn. 2008;30:599–615. doi: 10.1111/j.1467-9566.2007.01079.x. [DOI] [PubMed] [Google Scholar]
- 33.Shavers VL, Underwood W, Moser RP. Race/ethnicity and the perception of the risk of developing prostate cancer. Am J Prev Med. 2009;37:64–7. doi: 10.1016/j.amepre.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Office of Communications and Public Liason OIB. NIH Almanac 2008–2009. Bethesda, MD: 2008. Available from: http://www.nih.gov/about/almanac/ [Google Scholar]
- 35.McPherson M, Smith-Lovin L, Cook JM. Birds of a Feather: Homophily in Social Networks. Annu Rev Sociol. 2001;27:415–44. [Google Scholar]
- 36.Omi M, Winant H. Racial Formation in the United States: From the 1960s to the 1990s. 2. New York: Routledge; 1992. [Google Scholar]
- 37.Wong-Kim E, Sun A, DeMattos MC. Assessing cancer beliefs in a Chinese immigrant community. Cancer Control. 2003;10:22–8. doi: 10.1177/107327480301005s04. [DOI] [PubMed] [Google Scholar]
- 38.Matthews AK, Sellergren SA, Manfredi C, Williams M. Factors influencing medical information seeking among African American cancer patients. J Health Commun. 2002;7:205–19. doi: 10.1080/10810730290088094. [DOI] [PubMed] [Google Scholar]
- 39.Bottorff JL, Johnson JL, Bhagat R, et al. Beliefs related to breast health practices: the perceptions of South Asian women living in Canada. Soc Sci Med. 1998;47:2075–85. doi: 10.1016/s0277-9536(98)00346-3. [DOI] [PubMed] [Google Scholar]
- 40.Erwin DO, Johnson VA, Trevino M, Duke K, Feliciano L, Jandorf L. A comparison of African American and Latina social networks as indicators for culturally tailoring a breast and cervical cancer education intervention. Cancer. 2007;109:368–77. doi: 10.1002/cncr.22356. [DOI] [PubMed] [Google Scholar]
- 41.Joseph G, Burke NJ, Tuason N, Barker JC, Pasick RJ. Perceived susceptibility to illness and perceived benefits of preventive care: An exploration of behavioral theory constructs in a transcultural context. Health EducBehav. 2009;36:71S–91S. doi: 10.1177/1090198109338915. [DOI] [PMC free article] [PubMed] [Google Scholar]


