Abstract
Previous studies found that BMPs support osteoclast formation, but it is not clear whether this is a direct effect on osteoclasts or mediated indirectly through osteoblasts. We have shown that a mouse deficient for the BMP antagonist Twisted gastrulation suggested a direct positive role for BMPs on osteoclastogenesis. In this report, we further determine the significance of BMP signaling on osteoclast formation in vitro. We find that BMP2 synergizes with suboptimal levels of RANKL to enhance in vitro differentiation of osteoclast-like cells. The enhancement by BMP2 is not a result of changes in the rate of proliferation or survival of the bone marrow derived cultures, but is accompanied by an increase in expression of genes involved in osteoclast differentiation and fusion. Treatment with BMP2 did not significantly alter expression of RANKL or OPG in our osteoclast cultures, suggesting that the enhancement of osteoclastogenesis is not mediated indirectly through osteoblasts or stromal cells. Consistent with this, we detected phosphorylated SMAD1,5,8 (p-SMAD) in the nuclei of mononuclear and multinucleated cells in osteoclast cultures. Levels of p-SMAD, BMP2 and BMP receptors increased during differentiation. RNAi suppression of Type II BMP receptor inhibited RANKL-stimulated formation of multinuclear TRAP positive cells. The BMP antagonist noggin inhibited RANKL-mediated osteoclast differentiation when added prior to day 3, while addition of noggin on day 3 or later failed to inhibit their differentiation. Taken together, these data indicate that osteoclasts express BMP2 and BMP receptors, and that autocrine BMP signaling directly promotes the differentiation of osteoclasts-like cells.
Keywords: BMP2, osteoclast, BMPR type II, Smad 1/5/8
Bone is a highly dynamic tissue, characterized by a continuous cycle of bone formation by osteoblasts and bone resorption by osteoclasts [Henriksen et al., 2009; Sims and Gooi, 2008]. This cycle permits physiological bone growth, repair of damaged bone, and is important for regulation of systemic calcium and phosphate levels. Dysregulation of the cycle results in numerous pathological conditions such as osteoporosis, Paget's disease, and arthritis [Rodan and Martin, 2000]. It also contributes to the progression and morbidity of osteolytic cancers such as myeloma, osteosarcoma and metastatic breast, lung and prostate tumors [Guise et al., 2006; Roodman, 2009].
Osteoclasts are large, multinucleated cells formed by fusion of mononuclear cells that derive from the monocyte/macrophage lineage [Vaananen and Laitala-Leinonen, 2008]. They produce proteases and other factors to degrade the inorganic mineral and organic protein components of bone, thereby facilitating repair and remodeling [Teitelbaum, 2000]. Two factors that are necessary and sufficient for osteoclast formation are M-CSF [Cecchini et al., 1997] and Receptor Activator of NF-κB Ligand (RANKL) [Wada et al., 2006], both of which are expressed by osteoblasts. M-CSF is required for survival and proliferation of early osteoclast precursors, as the op mutant mouse, which lacks M-CSF, completely lacks osteoclasts [Felix et al., 1990; Wiktor-Jedrzejczak et al., 1990; Yoshida et al., 1990]. Binding of RANKL, produced by osteoblasts, and the RANK receptor on osteoclasts stimulates expression of genes necessary for osteoclast differentiation, cellular fusion and bone resorption. Additionally, osteoblasts express osteoprotegerin- (OPG), a soluble decoy receptor for RANKL, which inhibits activation of RANK by RANKL. The ratio of RANKL to OPG produced by osteoblasts is a major determinant of osteoclast forming activity within the bone microenvironment and allows for close coordination between bone formation and bone resorption under normal physiological conditions. Aberrant expression of RANKL and other osteoclast promoting factors contributes to pathological bone loss associated with cancers, arthritis, and other disease states [Tanaka et al., 2005].
Bone morphogenic proteins (BMPs) are well established as key regulators of osteoblast biology [Canalis et al., 2003; Cao and Chen, 2005]. In addition to multiple important physiological roles in regulating bone formation, they are used clinically to promote localized bone growth and healing in a number of orthopedic and maxillofacial applications [Kirker-Head et al., 2007]. BMPs are members of the TGFβ family of secreted morphogens. They bind to receptor complexes composed of Type I and Type II BMP receptors (BMPR I and BMPR II). Activation of the BMP receptor complex stimulates transcriptional regulation by SMADs 1,5 and 8, and various protein kinase pathways. The signaling activity of BMPs is modulated by secreted molecules such as Chordin, Noggin and Twisted gastrulation (Twsg1), which bind BMPs in the extracellular space, limiting their ability to activate signaling [Canalis et al., 2003]. Although it is well established that BMPs exert multiple effects to promote osteoblast formation, the significance of BMP signaling in the osteoclast lineage is not clear. In vitro application of BMPs during orthopedic procedures has been found to promote a transient increase in osteoclast numbers and osteoclastic bone resorption in some instances [Toth et al., 2009]. A number of studies suggested that BMPs can indirectly promote osteoclasts through enhanced expression of osteoclast-promoting factors by osteoblasts or stromal cells [Abe et al., 2000; Kanatani et al., 1995; Koide et al., 1999; Otsuka et al., 2003; Wutzl et al., 2006]. However, osteoclasts express BMP receptors [Itoh et al., 2001; Kanatani et al., 1995; Kaneko et al., 2000; Onishi et al., 1998], and a growing body of literature suggests that BMPs directly influence the formation and activity of osteoclasts [recently reviewed by Giannoudis et al. Giannoudis et al., 2007; Itoh et al., 2001; Kanatani et al., 1995; Kaneko et al., 2000; Okamoto et al., 2006; Wildemann et al., 2005].
We recently characterized the bone phenotype of the Twsg1-deficient mouse (Twsg1−/−), which exhibits significant osteopenia due to increased BMP signaling [Rodriguez et al., 2009]. Surprisingly, osteoblast function was not affected in these mice, nor was RANKL or OPG expression. Rather, the reduced bone mass was attributed to increased osteoclast formation and function. The Twsg1 gene was highly expressed in wild-type osteoclasts. Twsg1−/− osteoclasts were significantly larger that wild-type controls, and had increased levels of phosphorylated SMAD1,5,8 (p-SMAD1,5,8) indicating excessive BMP signaling. Treatment of wild-type osteoclast cultures with recombinant BMP2 phenocopied the enlarged osteoclast phenotype. These observations indicate that the increased osteoclast formation in Twsg1−/− mice stems from enhanced BMP signaling. In these previous studies, the presence of pSMADs in osteoclastic cultures that were not treated with exogenous BMP2 suggested that autocrine BMP signaling may be involved in osteoclast formation. Moreover, prior to day 3 we detected only a low level of pSMAD in osteoclast cultures, and we were unable to detect increased pSMAD in response to exogenous BMP prior to day 3, suggesting that BMP responsiveness may vary during osteoclast formation. The goals of the current study were to determine the temporal requirements of exogenous BMPs on enhanced osteoclastic differentiation in vitro, differentiation, define the profile of BMP receptor and ligand expression during osteoclast formation, and to assess the effect of impaired BMP signaling upon osteoclastogenesis.
Materials and Methods
Cell Culture
Primary osteoclast cultures were performed according to the method described by Kobayashi et al.[Kobayashi et al., 2000]. Bone marrow or spleen-derived cells were obtained from mice at one month old (bone marrow) or one week old (spleen) and cultured for three days on non-tissue culture coated dishes in osteoclast media: phenol red-free α-MEM supplemented with 10% heat-inactivated fetal bovine serum (FBS), 25 units/mL penicillin, 25 μg/mL streptomycin, 400 mM L-glutamine, and 10 ng/mL M-CSF (R&D Systems). The adherent population of cells was then replated at 2×104 cells per cm2 in osteoclast medium further supplemented with RANK Ligand (RANKL) (R&D Systems), BMP2 (R&D Systems), or noggin (R&D Systems) as indicated. Murine primary calvarial osteoblasts were grown in α-MEM supplemented with 10% FBS, penicillin/streptomycin, 200 mM L-glutamine and 50 μg/mL ascorbic acid. RAW264.7 cells were grown in DMEM supplemented with 10% FBS, 25-units/mL penicillin, 25 μg/mL streptomycin.
Cell proliferation and survival
Cell number was determined on each day from triplicate samples using the Cell Titer 96 Aqueous One Solution Assay (Promega) according to the manufacturer's directions. The resulting data were fit to an exponential curve using the least squares method to obtain the growth rate and doubling time.
TRAP Staining
Cells were rinsed in PBS, fixed in 4% paraformaldehyde for 20 minutes and stained using the TRAcP5b kit (Sigma-Aldrich). Cells were photographed and analyzed using Adobe Photoshop to measure the number and size of TRAP-positive osteoclasts.
Quantitative real-time PCR
Gene expression in osteoclast cultures was assessed by real-time RT-PCR. Total RNA was isolated from cells using TRIzol reagent (Invitrogen Life Technologies) and quantified by UV spectroscopy. Reverse transcription was performed using 1 μg of RNA and the iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA). Quantitative real-time PCR was performed in triplicate using the MyiQ Single-Color Real-Time PCR Detection System (Bio-Rad) 2x iQ SYBR Green supermix (Bio-Rad) and normalized to L4 or GAPDH. Primer sequences used were: TRAP (Forward) 5'-CGT CTC TGC ACA GAT TGC A; (Reverse) 5'-GAG TTG CCA CAC AGC ATC AC; NFATc1 (Forward) 5'-TCA TCC TGT CCA ACA CCA AA; (Reverse) 5'-TCA CCC TGG TGT TCT TCC TC; Cathepsin K (Forward) 5'-AGG GAA GCA AGC ACT GGA TA; (Reverse) 5'-GCT GGC TGG AAT CAC ATC TT; DC-STAMP (Forward) 5'-GGG CAC CAG TAT TTT CCT GA; (Reverse) 5'-TGG CAG GAT CCA GTA AAA GG; ATV6v0d2 (Forward) 5'-TCA GAT CTC TTC AAG GCT GTG CTG; (Reverse) 5'-GTG CCA AAT GAG TTC AGA GTG ATG; BMP2 (Forward) 5'TGG AAG TGG CCC ATT TAG AG ; (Reverse) 5'-TGA CGC TTT TCT CGT TTG TG ; BMP4 (Forward) 5'-CCT GGT AAC CGA ATG CTG AT ; (Reverse) 5'-AGC CGG TAA AGA TCC CTC AT ; L4 (Forward) 5'-CCT TCT CTG GAA CAA CCT TCT CG; (Reverse) 5'-AAG ATG ATG AAC ACC GAC CTT AGC; RANKL (Forward) 5'-CAG AAG GAA CTG CAA CAC ATT; (Reverse) TGG TAC CAA GAG GAC AGA GTG; OPG (Forward) 5'-CTG CCT GGG AAG AAG ATC AG; (Reverse) 5'-TTG TGA AGC TGT GCA GGA AC; GAPDH (Forward) 5'-TGC ACC ACC AAC TGC TTA G; (Reverse) 5'-GAT GCA GGG ATG ATG TTC
Immunoblotting
Cell protein lysates were prepared in modified RIPA lysate supplemented with protease inhibitor (Roche) and phosphatase inhibitor cocktails (Sigma-Aldrich). Proteins were resolved by SDS-PAGE, transferred to PVDF membrane (Millipore) and incubated overnight at 4°C with primary antibodies against phosphorylated SMAD1,5,8 (Cell Signaling) antibody or anti-total SMAD1,5,8 antibody (Santa Cruz), BMPR 1A (Santa Cruz), BMPR 1B (Santa Cruz), BMPR II (R&D Systems), α-tubulin (Santa Cruz) and horseradish peroxidase conjugated secondary antibodies (Santa Cruz). Immunoreactive bands were visualized using ECL Plus substrate (GE Health Systems).
Immunofluorescence
Cells were grown on glass coverslips and fixed in 4% paraformaldehyde for 20 minutes. They were then permeabilized in PBS/0.3% TritonX-100 for 5 minutes, blocked in immunofluorescence buffer (3% BSA, 20 mM MgCl2, 0.3% Tween20 in PBS) for 20 minutes, and incubated with primary antibodies for 90 minutes in immunofluorescence buffer. Phosphorylated SMAD1,5,8 antibody (Santa Cruz) was used at 1:20, anti-α-tubulin (Sigma-Aldrich) was used at 1:2000. Cells were washed three times in PBS/0.1% Triton X-100, and then incubated for 30 minutes with Alexaconjugated secondary antibodies at 1:800 (Invitrogen). After three washes, cells were stained with DAPI, washed, and mounted in 90% glycerol/ 0.4% N-propyl-gallate. Images were obtained using an Olympus Fluoview 500 confocal microscopy and processed using Adobe Photoshop.
Lentiviral gene suppression
Two lentiviral vectors encoding shRNAs against the Type II BMP receptor, or a control shRNA, together with a GFP marker, were purchased from Open Biosystems and used to produce replication defective lentivirus according to the manufacturer's protocols. Viral stocks were titrated by infection in HeLa cells and assessed by GFP fluorescence. These stocks were used to infect RAW264.7 cells and murine primary osteoclasts according to the manufacturer's recommendations. Following infection, RAW264.7 cells were subjected to puromycin selection at 4 μg/mL for 3 days, expanded, and then treated with RANKL to stimulate osteoclast formation. Following infection, primary osteoclast cultures were stimulated with M-CSF and RANKL without prior puromycin selection, as we were unable to obtain successful differentiation of multinucleated osteoclasts following selection.
Results
BMP2 synergizes with RANKL on days 3–4 to enhance in vitro differentiation of osteoclast precursors
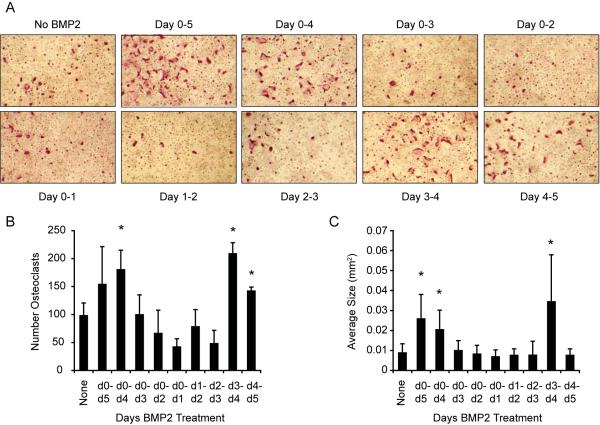
We previously reported that treatment with BMP2 synergistically enhanced the in vitro differentiation of bone marrow cells cultured in the presence of suboptimal levels of RANKL (30 ng/mL, which is half our optimal dose) into TRAP-positive multinucleated osteoclast-like cells [Rodriguez et al., 2009]. To determine when during differentiation BMP2 acts to promote osteoclast formation, we treated bone marrow derived cultures with suboptimal levels of RANKL and added or removed BMP2 at defined intervals. After five days of culture, we performed TRAP staining to visualize differentiated osteoclast-like cells (OCLs). As shown in Fig. 1A, cells treated with suboptimal RANKL alone gave a modest number of relatively small multinuclear cells, while the continuous addition of BMP2 during the differentiation resulted in many more TRAP-positive OCLs. Treatment with BMP2 from day 0 to day 4 showed a similar positive effect on OCL formation. In contrast, when BMP2 was removed on or before day 3, we did not detect enhanced OCL formation. Similarly, although BMP2 treatment on just day 0 to day 1, day 1 to day 2, or day 2 to day 3 failed to enhance multinuclear cell formation, the induction of TRAP-positive cells was enhanced by BMP2 treatment from day 3 to day 4 and to a lesser extent from day 4 to day 5. No TRAP-positive cells were detected in the absence of RANKL or in cultures treated with BMP2 only (data not shown). These results are quantified in Fig. 1B–C, which show that BMP2 treatments that include days 3 to 5, the average number of OCLs per field is increased by approximately 60%, and the average size of the 20 largest OCLs in each field was increased 2–3 fold. These data indicate that treatment with BMP2 between day 3 and 5, synergizes with suboptimal levels of RANKL to induce the differentiation of multinucleated OCLs.
Figure 1. BMP2 enhances suboptimal RANKL-stimulated differentiation of osteoclast precursors.
A. TRAP staining of bone marrow-derived osteoclasts differentiated with suboptimal (30 ng/mL) RANKL and BMP2 at 30 ng/mL for five days. BMP2 was added or removed on the indicated days of the differentiation, with day 0 defined as the day RANKL was added to cells. After five days, cells were fixed and stained for TRAP. Three fields were photographed and used for quantitative analysis in B–C. Representative images are shown in A. B. The number of TRAP-stained cells per field; C. the average size of the 20 largest osteoclasts in each field. * p≤0.05 vs. no BMP2 treatment.
BMP2 does not affect OCL cell number
To determine if the observed enhancement of OCL formation by BMP2 was mediated by increased proliferation or survival; we incubated bone marrow cultures in the presence or absence of BMP2. The cell number was measured using an MTS-based assay, and plotted to obtain the growth rate and doubling time of the cultures (Fig. 2). We detected very little difference between BMP2-treated and control cultures as control cultures treated with M-CSF and RANKL exhibited a doubling time of 68.5 hours, and cultures treated with BMP2 exhibited a doubling time of 65.8 hours. From this, we conclude that BMP2 does not enhance OCL formation by stimulating proliferation or promoting the survival of OCL precursors.
Figure 2. BMP2 does not affect OCL proliferation and survival.
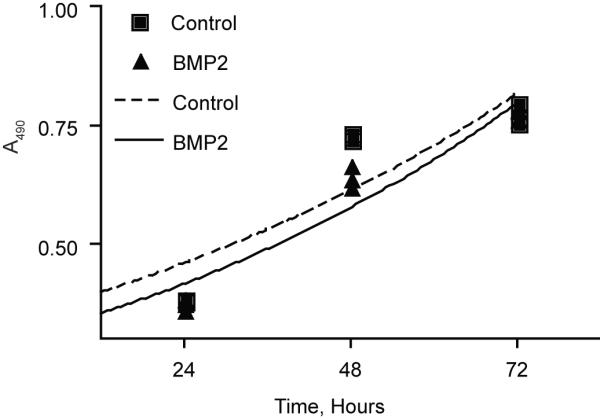
Bone marrow cultures were incubated in the presence (BMP2) or absence (control) of BMP2 for 3 days. Cell number was number was determined on each day from triplicate samples using the Cell Titer 96 Aqueous One Solution Assay.
BMP2 enhances RANKL-mediated expression of osteoclast genes
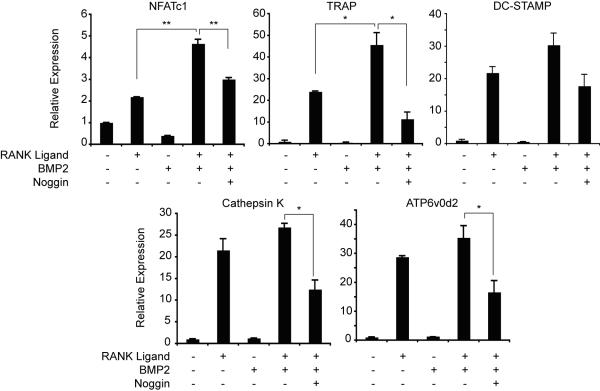
To further determine the effect of BMP signaling on OCLs, we treated bone marrow cultures with combinations of RANKL and BMP2 and used real-time PCR to measure expression of genes involved in osteoclast differentiation. RANKL induced expression of osteoclast marker genes NFAT-c1, TRAP, DC-STAMP, cathepsin K and ATP6v0d2, (Fig. 3). Although BMP2 was unable to induce expression of these genes in the absence of RANKL, it enhanced expression of all these genes in the presence of suboptimal RANKL. Treatment with the BMP-antagonist, noggin, impaired the BMP2-mediated increase in gene expression. These results further demonstrate that BMP2 enhances OCL formation in the presence of RANKL.
Figure 3. Expression of osteoclast marker genes.
Bone marrow-derived osteoclast precursors were cultured in media containing 30 ng/mL RANKL, 30 ng/mL BMP2, and 1 μg/mL noggin as indicated for three days. Gene expression was determined by real-time reverse transcription-PCR, normalized to L4 expression, and is graphed relative to the expression level in osteoclast media without RANKL or BMP2. * p≤0.05; ** p≤0.005.
BMP2-enhanced OCL formation is not associated with altered RANKL and OPG expression
Previous studies suggested that BMPs could enhance OCL formation by an indirect mechanism that involves BMP2-altered RANKL and OPG expression in osteoblasts or bone marrow stromal cells [Kanatani et al., 1995; Kaneko et al., 2000; Onishi et al., 1998]. To establish whether our observed enhancement of OCL differentiation by BMP2 is mediated indirectly through contaminating bone marrow stromal cells or osteoblasts, we compared expression of RANKL and OPG in bone marrow-derived cultures treated with suboptimal RANKL or suboptimal RANKL plus BMP2 for three days (Fig. 4). RANKL was detected in cultures of primary osteoblasts and at approximately six-fold lower levels in the OCL cultures. OPG expression in bone marrow derived cultures was 70 to 90 fold lower than primary osteoblast cultures. Treatment with BMP2 had no significant effect on expression of either RANKL or OPG in OCL cultures. These data indicate that altered RANKL or OPG expression does not account for the BMP2-mediated increase in OCL formation that we observe in our in vitro cultures, but are consistent with direct stimulation of OCL by BMP2.
Figure 4. BMP2 does not affect RANKL or OPG expression in osteoclast cultures in vitro.
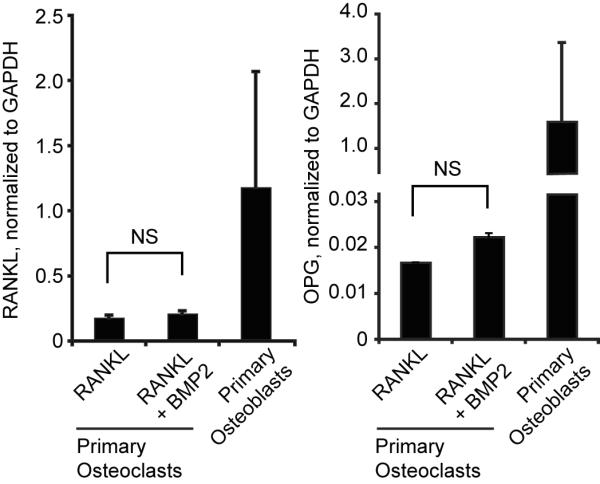
Real-time RT-PCR was used to quantify RANKL and OPG expression in bone marrow-derived osteoclast precursor cells cultured for three days in the presence of 30 ng/mL RANKL and 30 ng/mL BMP2 as indicated, or in murine primary calvarial osteoblasts. NS, not significant.
BMP signaling becomes activated during in vitro OCL differentiation
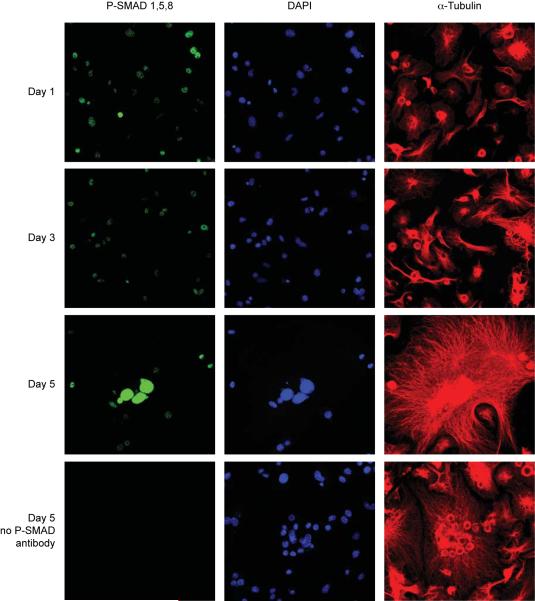
To further test whether BMP signaling acts directly on OCLs, we performed immunofluorescence against phosphorylated SMAD (p-SMAD1,5,8) on bone marrow derived OCL precursors during RANKL-stimulated differentiation. We counterstained the cells with DAPI to visualize nuclei and with α-tubulin to reveal the overall cellular morphology. As shown in Fig. 5, weak nuclear P-SMAD staining was detected in mononucleated cells after one and three days in RANKL. Nuclear staining for P-SMAD was greatly increased in multinucleated cells after five days differentiation. No staining was observed in control slides stained without p-SMAD antibody. This increase in p-SMAD1,5,8 levels throughout OCL differentiation was confirmed by immunoblotting (Fig. 7A). The detection of p-SMAD1,5,8 in the nuclei of mononuclear and multinucleated OCL indicates that BMP signaling is activated in OCL during their differentiation.
Figure 5. Phosphorylated SMAD1,5,8 Immunofluorescence.
Osteoclasts were differentiated by RANKL for 1, 3 or 5 days and subjected to immunofluorescence staining against phosphorylated SMAD1,5,8 (green). Cells were costained against α-tubulin (red) and with DAPI (dark blue) to show cell outline and nuclei, respectively. Parallel slides from each day were stained without P-SMAD antibody as a negative control (bottom row and not shown). All images are at equal magnification and were acquired and processed identically.
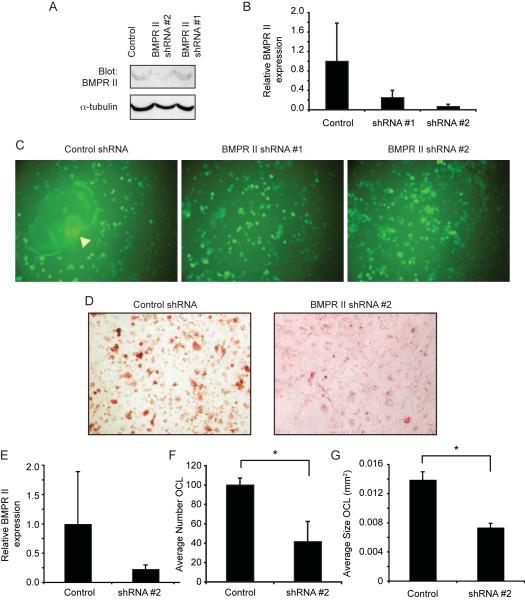
Figure 7. Suppression of BMPR II expression inhibits osteoclast formation.
A–B. Western blot (A) and real-time RT-PCR (B) showing expression of Type II BMP Receptor in RAW264.7 cells following infection 20ith control shRNA or BMPR II shRNA lentiviral vectors and puromycin selection. C. Fluorescence micrographs showing BMPR II and control-infected RAW264.7 cells that have been stimulated with RANKL for 6 days. Note the large multinucleated osteoclast indicated by the arrowhead in the “Control” panel. D. TRAP staining of Control and BMPR II shRNA -infected primary osteoclasts treated with RANKL for five days. E. Real time RT-PCR analysis of BMPR II expression in shRNA primary osteoclasts, plotted relative to control shRNA cells. F–G. Quantitative analysis showing the number (F) and size (G) of differentiated BMPR II suppressed primary osteoclasts following RANKL differentiation. *, p≤0.002.
Dynamic expression of BMP Receptors and BMP2 ligand in osteoclasts
The functional BMP receptor is a heteromeric complex composed of Type I and Type II receptors (referred to as BMPR IA, BMPR IB and BMPR II). Binding of BMP ligands to the Type II receptors activates the Type I receptors, which phosphorylate downstream intracellular effectors including SMADs. Expression of BMP ligands and BMP receptors in osteoclasts was previously reported [Anderson et al., 2000; Itoh et al., 2001; Kanatani et al., 1995; Kaneko et al., 2000; Onishi et al., 1998; Spector et al., 2001; Zoricic et al., 2003], but whether their expression in OCLs changes during differentiation has not been described. To determine whether changes in expression of BMP receptors or BMP ligands by OCLs could account for the stage-specific activation of BMP signaling during their differentiation, we performed immunoblots against each of the BMP receptors between day 0 and day 5 using optimal amounts of M-CSF and RANKL. Expression of BMPR 1A was first weakly detected on day 2 and increased on subsequent days (Fig. 7A). BMPR 1B was readily detected at all time points examined and showed relatively little change. BMPR II was barely detected on days 1 to 3, but increased on days 4 and 5. We measured expression of BMP2 and BMP4 ligands by real time RT-PCR. BMP2 was weakly expressed on day 1 and increased by 6 to 8-fold on subsequent days (Fig. 6B). Although BMP4 was robustly detected in several positive controls, we were unable to detect expression of BMP4 in OCL-like cell cultures at any time point examined (data not shown).
Figure 6. The expression of BMP receptors and BMP2 ligand increase during osteoclast differentiation.
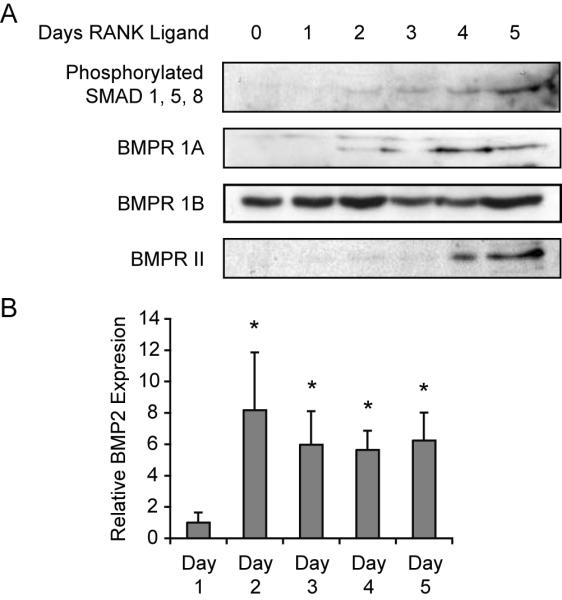
A. Immunoblot analysis of osteoclast precursors showing protein expression of BMP receptors and levels of phosphorylated SMAD 1, 5, 8 on the indicated days of differentiation. B. Real-time PCR quantitation of BMP2 expression in osteoclast precursors on each day of differentiation. Gene expression was determined in triplicate, normalized with GAPDH and is graphed relative to expression on Day 1. * p≤0.001 vs Day 1.
Inhibition of BMP signaling inhibits OCL formation
Based on the observation that exogenous BMP2 enhances osteoclast-like cell formation, we hypothesized that decreasing expression of BMP receptors should inhibit OCL differentiation. We made use of shRNA against the BMPR II, as this should block signaling through either type IA or type IB receptor. To ensure that any effect was due to knockdown of BMPR II rather than to any unintended target, we obtained lentiviral vectors from Open Biosystems that encode two different shRNAs against BMPR II and a control lentivirus. We began by infecting RAW264.7 cells, which are a common model for osteoclast/ macrophage/ monocyte differentiation, with control shRNA or BMPR II shRNA lentivirus, subjecting them to puromycin selection, and measuring expression of BMPR II. Both western blotting (Fig. 7A) and real-time RT-PCR (Fig. 7B) indicated that expression of BMPR II is reduced by both BMPR II shRNAs, although shRNA #2 gave a stronger reduction. Further indicating successful infection with the lentivirus and puromycin selection, we readily detected the GFP marker in almost all cells. When the control-infected RAW264.7 cells were stimulated with RANKL, they formed large, multinucleated cells as expected (Fig. 7C, yellow arrow). In contrast, treatment of the BMPR II shRNA cells with RANKL failed to stimulate formation of multinucleated cells.
We next infected bone marrow-derived OCLs with control lentivirus or the BMPR II shRNA #2 virus. We only used BMPRII shRNA #2 to infect primary bone marrow derived cells because both shRNAs gave a similar phenotype in RAW 264.7 cells but shRNA#2 showed higher ability to knockdown expression of BMPRII in RAW 264.7 cells. Stimulation of the control-infected cells with M-CSF and RANKL induced differentiation of multinucleated TRAP-positive OCLs (Fig. 7D). Expression of the BMPR II mRNA in these cultures was reduced by over 76% even though these cells were not subjected to puromycin selection. Quantitative analysis of these cells revealed that the differentiation of BMPR II shRNA-infected bone marrow cultures was greatly impaired relative to control-infected cells (Fig. 7E). The number of TRAP-positive OCLs was reduced from 100 to 42 cells per field (Fig. 7F) and the average size of TRAP-positive OCLs was reduced from 0.014 mm2 to 0.007 mm2 (Fig. 7G). These data indicate that a reduced level of BMP receptors in bone marrow derived OCL cultures impairs their differentiation into mature, multinucleated cells.
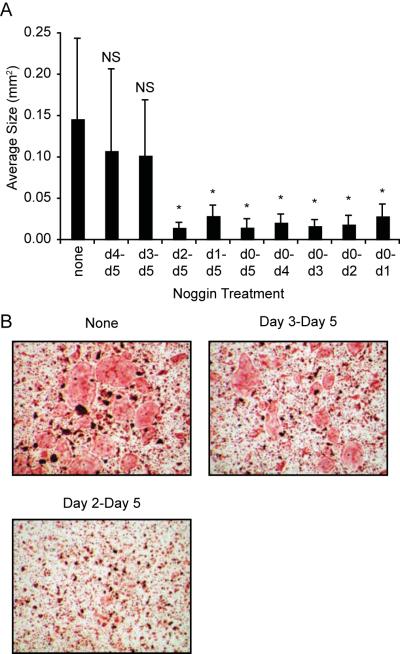
To further confirm whether endogenous BMP signaling is necessary for OCL formation and the timing of this signaling, we differentiated OCL with optimal amount of RANKL in the presence of the BMP antagonist, noggin. Noggin was selected for these studies rather than TWSG1 to simplify interpretation. While BMP-agonist activity has been reported for TWSG1 in some circumstances [Oelgeschlager et al., 2003; Ross et al., 2001], only BMP-antagonist activity has been described for noggin. RANKL-stimulated bone marrow derived cultures were treated with noggin for defined intervals, and stained for TRAP on day 5. The average number of TRAP-positive cells was not significantly altered by any regimen of noggin treatment (not shown). OCLs treated with only RANKL exhibited an average size of 0.15 mm2. In agreement with our previous studies [Rodriguez et al., 2009], treatment with noggin for the entire 5-day period reduced the average size of TRAP-positive multinucleated cells from 0.15 mm2 to approximately 0.02 mm2, as did treatment from day 1 to 5 and day 2 to 5 (significantly different from RANKL-only, p≤0.002) (Fig. 8). In contrast, OCLs treated with noggin beginning on day 3 or day 4 averaged 0.10–0.11 mm2 (not significantly different from RANKL-only). These observations are consistent with an endogenous BMP signal that enhances OCL formation beginning around day 3. Unexpectedly, treatment with noggin from day 0 to day 1, day 0 to day 2 and day 0 to day 3 was equally effective as noggin treatment from day 0 to day 5. These data indicate that inhibition of endogenous BMP signaling by noggin impairs the differentiation of osteoclast-like cells.
Figure 8. Noggin inhibits osteoclast differentiation.
Spleen-derived osteoclast precursors were cultured with 60 ng/mL RANKL supplemented with 250 ng/mL noggin for the indicated days. A. Average size of TRAP-positive multinucleated osteoclasts. B. Photographs showing TRAP stained osteoclast precursors treated with noggin for the indicated days. * p≤0.001 versus no noggin; NS, not significant versus no noggin.
Discussion
Despite their well-established functions promoting osteoblast development and function in bone formation, maintenance, and repair, the significance of BMPs during osteoclast formation remains poorly understood. In this study, we find that BMP2 directly enhances in vitro differentiation of murine osteoclasts in the presence of RANKL, that BMP signaling becomes activated in osteoclasts during their late stages of differentiation by an autocrine mechanism, and that inhibition of BMP signaling impairs osteoclast formation.
Emerging evidence indicates that BMPs act directly on cells of the osteoclast lineage to promote their development and/or activity. Okamoto et al. showed that increased BMP4 signaling in vivo stimulated both osteoclasts and osteoblasts, while the BMP antagonist noggin inhibited osteoclast formation. They also reported that exogenous BMP4 stimulated SMAD phosphorylation in macrophage cultures [Okamoto et al., 2006]. In an important in vitro study, Itoh et al. showed that treatment of bone marrow-derived osteoclast precursors with BMP2 enhanced RANKL-mediated differentiation into mature osteoclasts [Itoh et al., 2001]. Addition of a soluble form of the BMPR-IA receptor, which inhibits signaling through the membrane-associated receptors, attenuated osteoclast formation. Kaneko et al. showed that BMP2 directly enhanced the activity of purified rabbit mature osteoclasts [Kaneko et al., 2000]. In our previous work, we showed that mice mutant for the BMP antagonist TWSG1 exhibit osteopenia that results from increased numbers and size of osteoclasts. Twsg1−/− osteoclasts exhibited higher levels of p-SMAD1,5,8 than wild-type osteoclasts beginning on the third day of differentiation with RANKL, and treatment of wild-type osteoclast precursors with exogenous BMP2 increased p-SMAD levels in osteoclasts only after three days pre-incubation with RANKL [Rodriguez et al., 2009]. These observations led us to hypothesize that the effect of BMPs on osteoclasts may be restricted to specific times during their differentiation.
In this report, we find that exogenous BMP2 enhanced differentiation of OCLs in the presence of suboptimal RANKL beginning on day 3, shortly before mononuclear cells begin to fuse into mature multinucleated OCLs. BMP2 did not induce OCL formation in the absence of RANKL, confirming that BMP signaling is not sufficient to induce OCL differentiation by itself. Expression of various BMPs by OCLs was noted in earlier studies [Anderson et al., 2000; Itoh et al., 2001; Onishi et al., 1998; Paul et al., 2009; Spector et al., 2001; Zoricic et al., 2003], although the expression profile of BMPs in OCLs during their differentiation was not previously reported. Our data are novel in that they define a changing profile of responsiveness to BMPs during OCL differentiation and establish that the change in responsiveness arises from increased expression of type IA and II BMP receptors. We are also the first to demonstrate that we can detect pSMAD in the nucleus of osteoclasts.
In addition to responsiveness to exogenous BMP2, our data indicate the presence of an autocrine BMP signal during osteoclast-like cell formation, supported by expression of BMP ligands and receptor complexes, and the presence of phosphorylated SMADs in the absence of exogenous BMP ligands. Interestingly, Komarova et al. developed a mathematical model of bone remodeling that suggested autocrine regulation of osteoclasts, although they did not identify the specific regulatory factors involved [Komarova et al., 2003]. The enhanced formation of Twsg1−/− osteoclasts in vitro in the absence of exogenous BMPs [Rodriguez et al., 2009] lends further support for a model involving autocrine BMP signaling by OCLs.
Several lines of evidence suggest that BMPs act primarily at late stages of osteoclast differentiation. Osteoclast-like cells exhibit a low level of BMP receptors and phosphorylated SMADs during the first two days of stimulation with RANKL, and we have noted that treatment with exogenous BMP2 during this time does not cause a detectible increase in p-SMAD levels (data not shown). The increased number of nuclei per cell described for Twsg1−/− osteoclasts suggest that the large osteoclast size phenotype results from excessive fusion of mononuclear progenitors [Rodriguez et al., 2009]. We found that treatment with noggin had little effect on the overall number of TRAP-positive cells, but strongly decreased the size of multinucleated osteoclast-like cells, further signifying that decreased BMP signaling impairs fusion. On the other hand, suppression of the Type II BMP receptor reduced both the number and size of TRAP-positive cells, indicative that BMP signaling plays an important role prior to fusion of TRAP-positive mononuclear precursors. The explanation for this discrepancy is not yet known. We found that BMP treatment increases expression of DC-STAMP, a transmembrane protein necessary for osteoclast fusion, as well as of TRAP, cathepsin K and ATP6v0d2, proteins involved in resorption of the bone extracellular matrix. It is not known whether increased expression of these markers is mediated directly by BMP signaling, or indirectly through increased expression of osteoclast regulatory transcription factors such as NFATc1 or through a combination of the two. Improved understanding of the precise cellular and molecular functions of BMP signaling in osteoclasts remains an important area for future investigations.
Our data from exogenous BMP2 treatment, the expression profile of BMP receptors and the progressive increase in phosphorylated SMAD levels all suggested that BMPs do not affect OCL differentiation until day 3, leading to the prediction that noggin treatment at early timepoints should not affect OCL formation. Thus, the ability of noggin treatment from day 0 until day 1 to inhibit OCL formation equally well as noggin treatment from day 0 until day 5 was unexpected. A straightforward interpretation of these data is that BMP signaling at early timepoints is indeed necessary for OCL formation. However, work by Paine-Saunders and co-workers showed that noggin binds strongly to heparan sulfate proteoglycans (HSPGs) on the cell surface and that HSPG-bound noggin retains its BMP-antagonistic activity [Irie et al., 2003; Paine-Saunders et al., 2002]. We thus speculate that when noggin is added to OCLs at early timepoints, it is retained on the cell surface and remains capable of inhibiting BMP signals at later times.
To maintain normal skeletal homeostasis, bone formation must precisely balance bone lost during resorption. Various signals from osteoclasts are thought to signal to osteoblasts to trigger the synthesis of new bone. BMPs are important positive regulators of bone formation. It is intriguing to speculate that BMPs synthesized by osteoclasts might be involved in this coupling from osteoclast activity to osteoblast activity. Indeed, a recent study found that expression of BMP6 by mature osteoclasts promotes the differentiation of mineralized osteoblasts from human mesenchymal stem cells [Pederson et al., 2008].
Collectively, our data suggest a model in which BMP2 directly enhances osteoclast-like cell formation. The important role of osteoclasts to the pathogenesis of diseases including osteoporosis, periodontal disease and cancer-associated bone disease, manifests the need for understanding the factors that regulate osteoclast formation and activity. Moreover, because BMPs are used clinically to promote localized bone growth and regeneration, further elucidation of BMP biological effects will also enable refinement of therapeutic applications.
ACKNOWLEDGEMENTS
We thank members of the Petryk laboratory for insightful comments and assistance during this project, and Chee Sohn for technical assistance. We acknowledge the use of a confocal microscope made available through National Center for Research Shared Instrumentation Grant 1 S10 RR16851.
Contract grant sponsor: NIH Grant number: T32-DE007288, T32-AR050938, R01-AR48147, R01-DE016601
This work was supported by grants from the National Institutes of Health Minn CResT T32-DE007288 to L.P., Musculoskeletal Research Training Grant T32-AR050938 to C.J.B, and R01-AR48147 to J.J.W, and R01-DE016601 to A.P.
The abbreviations used are
- OCL
osteoclast-like cells
- Twsg1
Twisted gastrulation 1
- P-SMAD
phosphorylated SMAD1,5,8
- RANKL
receptor activator of NF-κB ligand
- OPG
osteoprotegerin
- BMP
bone morphogenic protein
- BMPR
bone morphogenic protein receptor
- TRAP
Tartrate-resistant Acid Phosphatase
References
- Abe E, Yamamoto M, Taguchi Y, Lecka-Czernik B, O'Brien CA, Economides AN, Stahl N, Jilka RL, Manolagas SC. Essential requirement of BMPs-2/4 for both osteoblast and osteoclast formation in murine bone marrow cultures from adult mice: antagonism by noggin. J Bone Miner Res. 2000;15:663–73. doi: 10.1359/jbmr.2000.15.4.663. [DOI] [PubMed] [Google Scholar]
- Anderson HC, Hodges PT, Aguilera XM, Missana L, Moylan PE. Bone morphogenetic protein (BMP) localization in developing human and rat growth plate, metaphysis, epiphysis, and articular cartilage. J Histochem Cytochem. 2000;48:1493–502. doi: 10.1177/002215540004801106. [DOI] [PubMed] [Google Scholar]
- Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev. 2003;24:218–35. doi: 10.1210/er.2002-0023. [DOI] [PubMed] [Google Scholar]
- Cao X, Chen D. The BMP signaling and in vivo bone formation. Gene. 2005;357:1–8. doi: 10.1016/j.gene.2005.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchini MG, Hofstetter W, Halasy J, Wetterwald A, Felix R. Role of CSF-1 in bone and bone marrow development. Mol Reprod Dev. 1997;46:75–83. doi: 10.1002/(SICI)1098-2795(199701)46:1<75::AID-MRD12>3.0.CO;2-2. discussion 83–4. [DOI] [PubMed] [Google Scholar]
- Felix R, Cecchini MG, Hofstetter W, Elford PR, Stutzer A, Fleisch H. Impairment of macrophage colony-stimulating factor production and lack of resident bone marrow macrophages in the osteopetrotic op/op mouse. J Bone Miner Res. 1990;5:781–9. doi: 10.1002/jbmr.5650050716. [DOI] [PubMed] [Google Scholar]
- Giannoudis PV, Kanakaris NK, Einhorn TA. Interaction of bone morphogenetic proteins with cells of the osteoclast lineage: review of the existing evidence. Osteoporos Int. 2007;18:1565–81. doi: 10.1007/s00198-007-0441-x. [DOI] [PubMed] [Google Scholar]
- Guise TA, Mohammad KS, Clines G, Stebbins EG, Wong DH, Higgins LS, Vessella R, Corey E, Padalecki S, Suva L, Chirgwin JM. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res. 2006;12:6213s–6216s. doi: 10.1158/1078-0432.CCR-06-1007. [DOI] [PubMed] [Google Scholar]
- Henriksen K, Neutzsky-Wulff AV, Bonewald LF, Karsdal MA. Local communication on and within bone controls bone remodeling. Bone. 2009;44:1026–33. doi: 10.1016/j.bone.2009.03.671. [DOI] [PubMed] [Google Scholar]
- Irie A, Habuchi H, Kimata K, Sanai Y. Heparan Sulfate is required for bone morphogenetic protein-7 signaling. Biochemical and Biophysical Research Communications. 2003;308:858–865. doi: 10.1016/s0006-291x(03)01500-6. [DOI] [PubMed] [Google Scholar]
- Itoh K, Udagawa N, Katagiri T, Iemura S, Ueno N, Yasuda H, Higashio K, Quinn JM, Gillespie MT, Martin TJ, Suda T, Takahashi N. Bone morphogenetic protein 2 stimulates osteoclast differentiation and survival supported by receptor activator of nuclear factor-kappaB ligand. Endocrinology. 2001;142:3656–62. doi: 10.1210/endo.142.8.8300. [DOI] [PubMed] [Google Scholar]
- Kanatani M, Sugimoto T, Kaji H, Kobayashi T, Nishiyama K, Fukase M, Kumegawa M, Chihara K. Stimulatory effect of bone morphogenetic protein-2 on osteoclast-like cell formation and bone-resorbing activity. J Bone Miner Res. 1995;10:1681–90. doi: 10.1002/jbmr.5650101110. [DOI] [PubMed] [Google Scholar]
- Kaneko H, Arakawa T, Mano H, Kaneda T, Ogasawara A, Nakagawa M, Toyama Y, Yabe Y, Kumegawa M, Hakeda Y. Direct stimulation of osteoclastic bone resorption by bone morphogenetic protein (BMP)-2 and expression of BMP receptors in mature osteoclasts. Bone. 2000;27:479–86. doi: 10.1016/s8756-3282(00)00358-6. [DOI] [PubMed] [Google Scholar]
- Kirker-Head CA, Boudrieau RJ, Kraus KH. Use of bone morphogenetic proteins for augmentation of bone regeneration. J Am Vet Med Assoc. 2007;231:1039–55. doi: 10.2460/javma.231.7.1039. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Morinaga T, Higashio K, Martin TJ, Suda T. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med. 2000;191:275–86. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide M, Murase Y, Yamato K, Noguchi T, Okahashi N, Nishihara T. Bone morphogenetic protein-2 enhances osteoclast formation mediated by interleukin-1alpha through upregulation of osteoclast differentiation factor and cyclooxygenase-2. Biochem Biophys Res Commun. 1999;259:97–102. doi: 10.1006/bbrc.1999.0715. [DOI] [PubMed] [Google Scholar]
- Komarova SV, Smith RJ, Dixon SJ, Sims SM, Wahl LM. Mathematical model predicts a critical role for osteoclast autocrine regulation in the control of bone remodeling. Bone. 2003;33:206–15. doi: 10.1016/s8756-3282(03)00157-1. [DOI] [PubMed] [Google Scholar]
- Oelgeschlager M, Reversade B, Larrain J, Little S, Mullins MC, De Robertis EM. The pro-BMP activity of Twisted gastrulation is independent of BMP binding. Development. 2003;130:4047–56. doi: 10.1242/dev.00633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Murai J, Yoshikawa H, Tsumaki N. Bone morphogenetic proteins in bone stimulate osteoclasts and osteoblasts during bone development. J Bone Miner Res. 2006;21:1022–33. doi: 10.1359/jbmr.060411. [DOI] [PubMed] [Google Scholar]
- Onishi T, Ishidou Y, Nagamine T, Yone K, Imamura T, Kato M, Sampath TK, ten Dijke P, Sakou T. Distinct and overlapping patterns of localization of bone morphogenetic protein (BMP) family members and a BMP type II receptor during fracture healing in rats. Bone. 1998;22:605–12. doi: 10.1016/s8756-3282(98)00056-8. [DOI] [PubMed] [Google Scholar]
- Otsuka E, Notoya M, Hagiwara H. Treatment of myoblastic C2C12 cells with BMP-2 stimulates vitamin D-induced formation of osteoclasts. Calcif Tissue Int. 2003;73:72–7. doi: 10.1007/s00223-002-1071-0. [DOI] [PubMed] [Google Scholar]
- Paine-Saunders S, Viviano BL, Economides AN, Saunders S. Heparan sulfate proteoglycans retain Noggin at the cell surface: a potential mechanism for shaping bone morphogenetic protein gradients. J Biol Chem. 2002;277:2089–96. doi: 10.1074/jbc.M109151200. [DOI] [PubMed] [Google Scholar]
- Paul S, Lee JC, Yeh LC. A comparative study on BMP-induced osteoclastogenesis and osteoblastogenesis in primary cultures of adult rat bone marrow cells. Growth Factors. 2009;27:121–31. doi: 10.1080/08977190802707324. [DOI] [PubMed] [Google Scholar]
- Pederson L, Ruan M, Westendorf JJ, Khosla S, Oursler MJ. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc Natl Acad Sci U S A. 2008;105:20764–9. doi: 10.1073/pnas.0805133106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–14. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- Rodriguez JS, Mansky KC, Jensen ED, Carlson AE, Schwarz T, Pham L, Mackenzie B, Prasad H, Rohrer MD, Petryk A, Gopalakrishnan R. Enhanced Osteoclastogenesis Causes Osteopenia in Twisted Gastrulation-Deficient Mice through Increased BMP Signaling. J Bone Miner Res. 2009 doi: 10.1359/JBMR.090507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roodman GD. Pathogenesis of myeloma bone disease. Leukemia. 2009;23:435–41. doi: 10.1038/leu.2008.336. [DOI] [PubMed] [Google Scholar]
- Ross JJ, Shimmi O, Vilmos P, Petryk A, Kim H, Gaudenz K, Hermanson S, Ekker SC, O'Connor MB, Marsh JL. Twisted gastrulation is a conserved extracellular BMP antagonist. Nature. 2001;410:479–83. doi: 10.1038/35068578. [DOI] [PubMed] [Google Scholar]
- Sims NA, Gooi JH. Bone remodeling: Multiple cellular interactions required for coupling of bone formation and resorption. Semin Cell Dev Biol. 2008;19:444–51. doi: 10.1016/j.semcdb.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Spector JA, Luchs JS, Mehrara BJ, Greenwald JA, Smith LP, Longaker MT. Expression of bone morphogenetic proteins during membranous bone healing. Plast Reconstr Surg. 2001;107:124–34. doi: 10.1097/00006534-200101000-00018. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Nakamura K, Takahasi N, Suda T. Role of RANKL in physiological and pathological bone resorption and therapeutics targeting the RANKL-RANK signaling system. Immunol Rev. 2005;208:30–49. doi: 10.1111/j.0105-2896.2005.00327.x. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–8. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- Toth JM, Boden SD, Burkus JK, Badura JM, Peckham SM, McKay WF. Short-term osteoclastic activity induced by locally high concentrations of recombinant human bone morphogenetic protein-2 in a cancellous bone environment. Spine (Phila Pa 1976) 2009;34:539–50. doi: 10.1097/BRS.0b013e3181952695. [DOI] [PubMed] [Google Scholar]
- Vaananen HK, Laitala-Leinonen T. Osteoclast lineage and function. Arch Biochem Biophys. 2008;473:132–8. doi: 10.1016/j.abb.2008.03.037. [DOI] [PubMed] [Google Scholar]
- Wada T, Nakashima T, Hiroshi N, Penninger JM. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12:17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW, Jr., Ahmed-Ansari A, Sell KW, Pollard JW, Stanley ER. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci U S A. 1990;87:4828–32. doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildemann B, Kadow-Romacker A, Lubberstedt M, Raschke M, Haas NP, Schmidmaier G. Differences in the fusion and resorption activity of human osteoclasts after stimulation with different growth factors released from a polylactide carrier. Calcif Tissue Int. 2005;76:50–5. doi: 10.1007/s00223-004-0040-1. [DOI] [PubMed] [Google Scholar]
- Wutzl A, Brozek W, Lernbass I, Rauner M, Hofbauer G, Schopper C, Watzinger F, Peterlik M, Pietschmann P. Bone morphogenetic proteins 5 and 6 stimulate osteoclast generation. J Biomed Mater Res A. 2006;77:75–83. doi: 10.1002/jbm.a.30615. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz LD. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345:442–4. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- Zoricic S, Maric I, Bobinac D, Vukicevic S. Expression of bone morphogenetic proteins and cartilage-derived morphogenetic proteins during osteophyte formation in humans. J Anat. 2003;202:269–77. doi: 10.1046/j.1469-7580.2003.00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]