Abstract
The syndecans are transmembrane proteoglycans that place structurally heterogeneous heparan sulfate chains at the cell surface and a highly conserved polypeptide in the cytoplasm. Their versatile heparan sulfate moieties support various processes of molecular recognition, signaling, and trafficking. Here we report the identification of a protein that binds to the cytoplasmic domains of the syndecans in yeast two-hybrid screens, surface plasmon resonance experiments, and ligand-overlay assays. This protein, syntenin, contains a tandem repeat of PDZ domains that reacts with the FYA C-terminal amino acid sequence of the syndecans. Recombinant enhanced green fluorescent protein (eGFP)–syntenin fusion proteins decorate the plasmamembrane and intracellular vesicles, where they colocalize and cosegregate with syndecans. Cells that overexpress eGFP–syntenin show numerous cell surface extensions, suggesting effects of syntenin on cytoskeleton–membrane organization. We propose that syntenin may function as an adaptor that couples syndecans to cytoskeletal proteins or cytosolic downstream signal-effectors.
The cell surface heparan sulfate proteoglycans are at the cross-section of several different pathways (1, 2). The heparan sulfate moieties of these molecules bind various differentiation, growth, and scatter factors, facilitate the occupation and activation of the corresponding signal-transducing receptors, and are involved in the internalization and clearance of the signaling complexes from the cell surface (3). They assist receptors that are involved in cell–cell (4) and cell–matrix (5) adhesion and assist scavenging receptors that are involved in the endocytosis and transcytosis of lipoproteins and lipases (6). They also bind and activate serine proteinase inhibitors and accelerate the reactions of these inhibitors with their targets (7). Proteolysis, lipolysis, mesoderm induction, gastrulation, angiogenesis, and neuritogenesis all appear to be regulated by or to depend on heparan sulfate because this glycosaminoglycan is needed for the allosteric activation, approximation, and compartmentalization of the reactants that are engaged in these processes.
In most cells syndecans represent the major source of cell surface heparan sulfate. The four known vertebrate syndecans are small type I membrane proteins, with similar and simple domain organizations: a single ectodomain, membrane span, and cytoplasmic domain (1). The ectodomains of the different syndecans have little in common, except for the presence of three or four consensus sites for heparan sulfate attachment near the N termini of the proteins and a dibasic, presumably protease-sensitive site at the junction with the membrane spanning segment. The structures of these ectodomains have not been evolutionary conserved, except again for these shared structural elements. The membrane-spanning and the small cytoplasmic domains of the syndecans, in contrast, show extensive structural similarity (≈60% sequence identity) and have been highly conserved during evolution (1, 8). All four vertebrate syndecans and the single Drosophila syndecan share the sequence RM(K/R)KKDEGSY in the membrane-proximal segments of their cytoplasmic domains and the sequence EFYA at their C termini. This finding suggests that the extracellular heparan sulfate moieties, the cytoplasmic protein moieties, and the contiguity of these moieties are essential for syndecan function. The syndecans may provide for a transmembrane link between extracellular heparan sulfate-steered processes and intracellular structural or signaling proteins. Here we report the identification of syntenin, a protein that interacts with the cytoplasmic C-terminal FYA sequence of the syndecans.
MATERIALS AND METHODS
Yeast Two-Hybrid Screening.
Baits, consisting of the cytoplasmic domains of the syndecans fused in-frame to the DNA-binding domain of Gal4, were constructed by PCR with the BamHI/PstI restriction sites of the pAS2 vector (CLONTECH) and used to screen a human liver cDNA library (CLONTECH) cloned in pGAD10. The pAS2 plasmids and library were cotransfected in CG1945 yeast cells, and positive clones were selected on triple minus plates (Leu−, Trp−, His−) containing 5 mM 3-aminotriazol and assayed for β-galactosidase activity. Positive cDNA clones were cotransfected, by mating, with all four bait vectors and with the original pAS2 vector to confirm the interaction. Mutations in the syndecan and syntenin constructs were made by PCR. All constructs and mutants that were tested in the matings were confirmed by sequencing.
Cloning of Syntenin.
The insert from the selected clone (3p11) was subcloned in pBluescript and completely sequenced. A blast search of the National Center for Biotechnology Information databases for syntenin-derived expressed sequence tags yielded matching sequence tags in human fetal heart, liver, lung, brain, and several adult cDNA libraries, suggesting wide-spread expression of the syntenin message. Full-length cDNA for syntenin (2,193 bp) was obtained from the sequencing and consensus of the Integrated Molecular Analysis of the human genome and its Expression (I.M.A.G.E.) clones 33203 and 123057 (identified from the blast search and obtained through Research Genetics). The syntenin cDNA (clone 33203) featured an initiator ATG at position 249, a TAA stop codon at position 1,088, a polyadenylation signal (AATAAA box) at position 2,139, and a poly(A)-tail at position 2,163. The initiator ATG occurred in a Kozak sequence context (A at −3), and was preceded by two in-frame stop codons. The GenBank accession number for this syntenin cDNA sequence is AF000652.
Production of Glutathione S-Transferase (GST) Fusion Proteins.
Fusion proteins between GST and syntenin or parts of syntenin (tagged with a C-terminal myc epitope, except for the M1-S226 and I103-V298 fusion proteins), and wild-type or mutant syndecan cytoplasmic domains were obtained by cloning the cDNAs into the pGEX-5X-2 vector (Pharmacia Biotech) and expression into Escherichia coli BL21 cells. Cells, induced for 3 h with 500 μM isopropyl β-d-thiogalactoside, were suspended in 100 mM NaCl, 1 mM EDTA, 50 mM Tris⋅HCl (pH 8.0) and supplemented with the proteinase inhibitors aprotinine (3 μM), benzamidine (5 mM), leupeptine (20 μM), pepstatin (3 μM), 6-aminohexanoic acid (5 mM), and phenylmethylsulfonyl fluoride (200 μM). After treatment with lysozyme for 1 h on ice, the fusion protein was recovered from the water-soluble phase and purified by affinity chromatography over glutathione-Sepharose 4B (Pharmacia Biotech). Expression of the myc tag confirmed the reading frame and expression of full-length fusion protein.
Surface Plasmon Resonance Measurements.
A total of 300 RU (resonance or reflection units) of biotinylated synthetic peptide (32 aa), corresponding to the cytoplasmic domain of syndecan-2, were immobilized on the Fc2 surface of a Streptavidin (SA)-sensor chip. Analyte was perfused (10 μl/min) over the Fc1 (control) and Fc2 (capture) surfaces in running buffer (100 mM NaCl/0.005% surfactant P20/10 mM Hepes, pH 7.4). Binding was monitored in a BIAcore 2000 instrument (Pharmacia Biosensor) and measured as the difference between Fc2 and Fc1 binding curves. The surface was regenerated through 1-min pulses of 1 M NaCl/0.05 M NaOH. The C-terminal myc epitope did not influence the binding, because GST–M92-V298 and GST–M92-V298–myc fusion proteins yielded similar profiles.
Ligand Blots.
Isopropyl β-d-thiogalactoside-induced BL21 cells expressing GST–syndecan fusion proteins were resuspended in SDS sample buffer and boiled for 5 min. The cell lysate was cleared by centrifugation and fractionated in 12% SDS/PAGE, each lane containing 0.1 μg of fusion protein. After electro transfer to Hybond-C-super membranes, the blots were blocked with 5% blocking reagent (Amersham, ECL kit) and incubated with biotinylated GST–syntenin [15 μg/ml in phosphate-buffered 75 mM NaCl (PB1/2S)]. After overnight incubation at 4°C, the blots were washed with PB1/2S and incubated for 1 h at room temperature with streptavidin-horseradish peroxidase (Amersham) diluted 1:1500 in PB1/2S. After rinsing with PB1/2S, the bound GST–syntenin was visualized with ECL Western blotting detection reagent (Amersham).
Cells, Transfections, and Fluorescence Microscopy.
Chinese hamster ovary (CHO) wild-type cells (CHO-K1: ATCC CCL61) were routinely grown in DMEM/F12 (1:1) medium supplemented with 10% fetal bovine serum and l-glutamine. Cells were prewashed with Ca/Mg-free PBS and incubated for 5 min at 4°C (3.106 cells/0.5 ml Ca/Mg-free PBS) with 30 μg of plasmid DNA. Plasmid DNA consisted of circular pEGFP-C1 (CLONTECH) or pEGFPsynt [syntenin fused to the C terminus of enhanced green fluorescent protein (eGFP)], or a mixture of 15 μg of pEGFP-C1 or pEGFPsynt and 15 μg of circular pcDNA3.1/Zeo(+) plasmid (In Vitrogen) encoding wild-type, the C9 mutant, or the C30 mutant of mouse syndecan-2. Cells were transfected by electroporation at 250 V and 960 μF with a gene pulser (Bio-Rad) and grown onto glass coverslips into 24-well plates. After 5 h of recovery 2 mM sodium butyrate was added to enhance general transcription levels. Transfectants were analyzed 36 h after transfection and fixed at room temperature with formaldehyde (3.7%) in phosphate buffer (0.1 M, pH 7.4) for 15 min. Transfectants prepared for immunocytochemistry were fixed as above, permeabilized with acetone (5 min at −20°C), and washed with PBS-glycine (pH 8.0) and 0.5% PBS-BSA. The syndecan-2-specific 10H4 antibody (9, 10) was used at a concentration of 100 μg/ml, for 1 h at room temperature. Texas X-Red-conjugated goat anti-mouse Igs (Molecular Probes), diluted 1/200, were applied for 1 h. The cells were postfixed with formaldehyde for 30 min and mounted with rubber cement (Talens, Apeldoorn, Holland). The signals were visualized by digital imaging microscopy by using a cooled charge-coupled camera (Photometrics, Tucson, AZ). Images were recorded by using the smart capture software (Vysis, Stuttgart, Germany).
RESULTS
Syntenin, a Unique PDZ Protein.
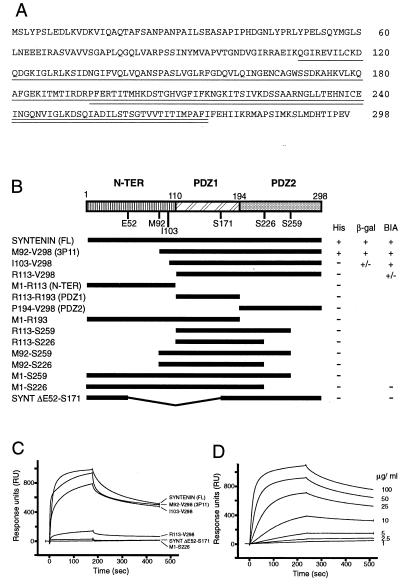
We initiated a search for tenants of the syndecan cytoplasmic domains (syntenins) by using these conserved syndecan domains as baits in yeast two-hybrid screenings (11). The insert of one clone that yielded HIS+ and LacZ+ phenotypes in combination with the four different Gal4 DNA-binding domain/syndecan fusion constructs (but not with Gal4 DNA-binding domain alone or with p53 fused to the Gal4 DNA-binding domain) was sequenced and used as a starting point to obtain a cDNA coding for the corresponding full-length protein. Full-length syntenin consists of 298 amino acids and can be divided in three parts (Fig. 1A). The first N-terminal region (aa 1–109) shows no striking homology to any known structural motif. It is relatively rich in proline and contains five tyrosines, 11 serines, and two threonines. Based on sequence alignments (see below for some examples), the second (amino acids 110–193) and third (amino acids 194–274) regions of syntenin appear to correspond to a tandem repeat of PDZ domains (postsynaptic density protein, disc-large, zonulin-1). The sequence coding for the putative second PDZ domain is extended by 24 aa (amino acids 275–298), which may still be part of the second PDZ domain or compose a fourth separate C-terminal domain. Further tests were aimed at investigating the syntenin and syndecan domains that are involved in the syndecan–syntenin interaction.
Figure 1.
Structure of syntenin and domains of syntenin necessary for the interaction with syndecans. (A) Amino acid sequence of syntenin. The two PDZ domains are indicated by the single and double underlinings. (B) Different parts of the original clone (3p11) and of the syntenin cDNA were subcloned in the pGAD10 vector or the pGEX vector to code for fusions between the activating domain of Gal4 (Gal4 AD) or GST and various full-length, truncated, and deleted versions of syntenin. Interactions between syntenin and the syndecan-2 cytoplasmic domain were scored in the yeast two-hybrid system as growth on His− plates and β-galactosidase activity. (C) Binding of GST–syntenin fusion proteins to a streptavidin-immobilized biotinylated synthetic peptide that represents the 32 cytoplasmic amino acids of syndecan-2, as detected by surface plasmon resonance. (D) Binding curves obtained at different concentrations of the GST full-length syntenin fusion protein.
Syntenin Binding Requires Both PDZ Domains.
The minimal region of syntenin necessary for the interaction with syndecans was identified in the yeast two-hybrid system and in surface plasmon resonance (BIAcore 2000) experiments (Fig. 1 B–D). Different parts of the original clone (3p11) and of the syntenin cDNA were subcloned in the pGAD10 vector to code for fusions between the activating domain of Gal4 (Gal4 AD) and various full-length, truncated, and deleted versions of syntenin. Full-length syntenin (tagged in the C terminus with myc epitope or not) and the original isolate (M92-V298, containing the two PDZ domains but missing the first 91 aa of syntenin) were able to interact with the full-length syndecan cytoplasmic domains, as revealed by growth on His− plates and β-galactosidase activity. Constructs containing only the N-terminal domain (M1-R113), the N-terminal domain and PDZ1 (M1-R193, M1-S226, M1S259), or either one of the two PDZ domains alone (e.g., M92-S259 and P194-V298) were all inactive, suggesting that the paired PDZ domains are required for the binding interaction. To confirm these data, binding was also investigated by surface plasmon resonance in a BIAcore 2000 instrument. For these experiments, we used a streptavidin-immobilized biotinylated synthetic peptide that represented the 32 cytoplasmic amino acids of syndecan-2. This peptide bound the GST–syntenin FL (full length), the GST–M92-V298, and the GST–I103-V289 proteins, but not fusion proteins composed of GST and parts of syntenin containing only one of the two PDZ domains. Binding curves obtained at different concentrations of the GST–syntenin fusion proteins were not consistent with a simple binding interaction, and suggested relatively rapid dissociation [koff, ≈1.10−4 (s−1), or a decay of ≈0.01% of the complexes per second, if one would accept an A + B ⇄ AB interaction model]. The syntenin moiety itself, isolated from the fusion protein with the help of factor Xa, also bound to the synthetic peptide, indicating that the GST moiety was not needed (not shown). No syntenin–peptide binding interaction could be observed, however, when the GST–syntenin proteins were immobilized on a biosensor chip coated with an anti-GST antibody and the syndecan peptide was perfused over the immobilized syntenin (data not shown).
Syndecan Binding Requires the FYA Sequence.
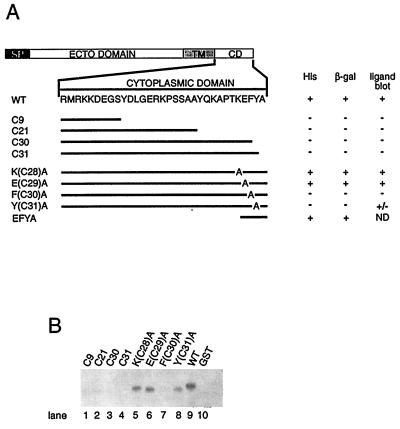
The syntenin-binding sites in the syndecan cytoplasmic domain were deduced from the testing of a series of deletion- and alanine-substitution mutants of the syndecan-2 cytoplasmic domain, both in the yeast two-hybrid system and in ligand blotting assays (Fig. 2). All the C-terminal deletions and the substitutions of alanine for the F-residue at −2 and the Y-residue at −1 abolished the binding interaction in the yeast assay. Deletion of all but the last four residues from the cytoplasmic domain, and the alanine substitution for the E residue at −3, in contrast, did not abolish the interaction in yeast. In ligand blots, GST–syntenin FL (full-length syntenin) and GST–M92-V298 (syntenin sequence starting at M92; data not shown) fusion proteins failed to bind to GST itself, to themselves (data not shown), or to fusion proteins composed of GST and the C-terminal deletions (C9, C21, C30, C31) or the F(C30)A mutant of the syndecan-2 cytoplasmic domain. The same GST–syntenin constructs, in contrast, bound to fusion proteins composed of GST and any of the other syndecan-2 mutants [including the Y(C31)A mutant, negative in the two-hybrid screening], or the four different wild-type syndecan cytoplasmic domains (only shown for syndecan-2). The results of these experiments localized the binding site to the C-terminal FYA sequence of the syndecans, consistent with the concept that PDZ proteins mediate protein–protein interactions by binding to the C termini of proteins and discriminate amongst these sequences, with a relatively low selectivity for the residue at the −1 position (12).
Figure 2.
The last three amino acids of the syndecan cytoplasmic domain are responsible for the binding to syntenin. (A) Deletion- and alanine-substitution mutants of the syndecan-2 cytoplasmic domain were cloned in pAS2, as Gal4 DNA-binding domain fusion proteins, and expressed as partners for the Gal4 activation domain full-length syntenin fusion protein. ND: not determined. (B) Ligand blot, testing for the binding of GST full-length syntenin fusion protein to GST itself and to fusion proteins composed of GST and the C-terminal deletion mutants, the alanine-substitution mutants, and the wild type (WT) form of the syndecan-2 cytoplasmic domain.
Syndecans and Syntenin Associate in Cells.
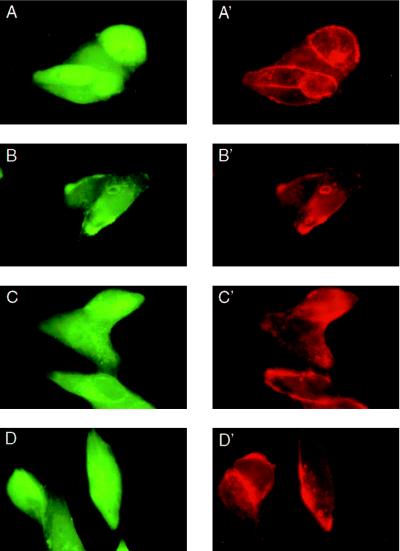
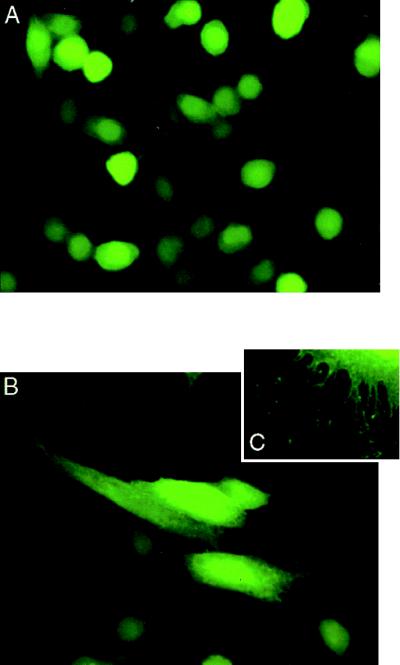
Syndecan–syntenin associations were further investigated by double fluorescence microscopy through the simultaneous overexpression of syndecans and eGFP–syntenin fusion protein in CHO cells (Fig. 3). In these experiments, the cells were cotransfected with plasmids encoding eGFP or an eGFP–syntenin fusion protein and expression plasmids encoding wild-type mouse syndecan-2, the C9-, or the C30-deletion mutant of mouse syndecan-2. In other experiments, CHO cells were also singly transfected with plasmids encoding eGFP or eGFP–syntenin (Fig. 4). In cells expressing eGFP, the green fluorescence was nearly homogeneously distributed (Figs. 3A and 4A). The plasmamembranes of these cells were barely discernable. In cells expressing eGFP–syntenin, the green fluorescence was localized to the plasmamembrane, to small intracellular vesicular structures, and to the cytoplasm (Figs. 3 B–D and 4B). Staining the double transfectants (Fig. 3) with antibody directed toward the ectodomain of syndecan-2 and Texas Red-X-labeled secondary antibody showed extensive colocalization of wild-type syndecan-2 and eGFP-syntenin (Fig. 3 B and B′), but not of wild-type syndecan-2 and eGFP (Fig. 3A and A′). In contrast, only occasional colocalization of green and red fluorescence was observed in cells expressing the C9- (Fig. 3 C and C′) or C30-forms (Fig. 3 D and D′) of syndecan-2 and eGFP–syntenin. Comparison of single (Fig. 4) and double transfectants (Fig. 3) suggested that the overexpression of wild-type syndecan-2 (Fig. 3B) markedly affected the distribution of eGFP–syntenin, enhancing the localization of the fusion protein to the plasmamembrane, where most of the recombinant syndecan was localized. A similar redistribution of eGFP–syntenin was not observed for the C9 and C30 syndecan-2 mutants (Fig. 3 C and D). Moreover, comparison of cells expressing eGFP, and cells expressing eGFP–syntenin revealed a striking difference in morphology. The latter cells were often large, flat, vesiculated, and decorated with numerous membrane extensions (Fig. 4 B and C). Finally, extensive colocalization of eGFP–syntenin and transfectant syndecan was also observed for syndecan-1 and syndecan-4, by using wild-type syndecan-1 and syndecan-4 transfectants and corresponding specific antibodies (data not shown).
Figure 3.
Colocaliztion of eGFP–syntenin and mouse syndecan-2 in CHO cells. Transient CHO transfectants expressing wild-type mouse syndecan-2 (A, A′, B, and B′), C9-mouse syndecan-2 (C and C′), or C30-mouse syndecan-2 (D and D′) were cotransfected with plasmids encoding eGFP (A and A′) or eGFP-syntenin fusion protein (B–D and B′–D′). Mouse syndecan-2 ectodomain was detected with mAb 10H4 (A′–D′) and Texas-X-Red-labeled second antibody.
Figure 4.
Overexpression of eGFP–syntenin in CHO cells affects membrane-cytoskeleton organization. CHO wild-type cells were transiently transfected with plasmids encoding eGFP (A) or eGFP-syntenin (B and C). Cells overexpressing eGFP–syntenin are larger, flatter, and decorated with numerous cell surface projections.
DISCUSSION
The transduction of extracellular signals, from the specialized sites that sense the signal to the intracellular effector systems that support the cellular response, calls upon a complex molecular network of interacting proteins. Recently, PDZ domains have been recognized as one of the conserved modular structures that support protein–protein interactions and networking. PDZ domains can bind to the C-terminal ends of proteins (13, 14), dimerize with other PDZ domains (15), and often occur in association with other functional modules, such as SH3 domains, protein tyrosine phosphatase domains, domains related to guanylate kinase (GUK), to band 4.1 protein, leucine zipper motifs, and additional PDZ domains (16). PDZ domains, first identified in the postsynaptic density protein PSD-95, in Dlg (the product of the Drosophila lethal(1) discs-large-1 tumor suppressor gene), and in the tight junction protein ZO-1, have now been discovered in a variety of proteins and shown to bind to membrane channels, receptors (e.g., wingless receptor and Notch), tumor suppressor proteins (adenomatous polyposis coli protein), GTPase-activating proteins, and guanine nucleotide exchange factor (17). These interactions appear to be involved in the formation of multimeric protein complexes that influence receptor positioning and clustering and the connections of receptors and receptor-associated molecules to cytoskeletal proteins and downstream signal-effectors (18). Syntenin, the molecule that we have identified here as a ligand for the C termini of the syndecans, is a member of this PDZ protein superfamily. It is an adaptor-like molecule and a candidate for linking syndecan-supported recognition processes to the cytoskeleton or cytoplasmic signal-effector systems.
Based on the crystal structures of the PDZ-3 domain of the human homolog of Dlg (19) and of the PDZ-3 domain of PSD-95 (14), PDZ domains are essentially globular domains composed of five or six β-strands (β1/A–β6/F) and two α-helices (α1/A and α2/B) arranged into what has been described as a “β-sandwich” or “up-and-down β-barrel.” Peptide binding can be envisioned as the addition of another β-strand to the β-sheet of the PDZ domain (20). The crystal structures of hDlg and PSD-95 reveal that the C-terminal peptide ligand, which in both these cases contains the tS/TXV motif, binds within a hydrophobic pocket created by the βB-strand, the αB-helix, and the loop connecting the βA- and βB-strands. Peptide binding to this so-called carboxylate binding loop is stabilized by interactions between the backbone of the peptide and the βB-strand and by hydrogen bonding between the terminal carboxylate and residues of the βA–βB loop, including a buried arginine residue. Different PDZ domains show some variability in these regions, which probably defines their binding specificity. The two PDZ domains of syntenin are more similar to the PDZ domains of p55 and LIN-2a (both membrane-associated guanylate kinase homologs, or MAGUKs) and Tiam-1 (homologous to GEFs for Rho-like proteins), than to the PDZ domains of PSD-95, Dlg, ZO-1, and neuronal NO synthase (nNOS) (see Fig. 5). The screening of an oriented peptide library has indicated that the PDZ domains of LIN-2a, p55, and Tiam-1 select peptides with hydrophobic or aromatic side chains at the C-terminal three residues (12), whereas the PDZ domains of PSD-95, Dlg, and ZO-1 bind tS/TXV, and nNOS binds peptides that contain tDXV. This same screening suggested the existence of a least two major group of PDZ domains, and an important role for the first residue in the αB-helix (selecting for the peptide residue at position −2) in the determination of the binding specificity. The two PDZ domains of syntenin appear to belong to the LIN-2a group, lacking a basic residue at the αB1 position (S in the PDZ-1, D in the PDZ-2) and, either one or both, binding to the C-terminal FYA sequence of the syndecans.
Figure 5.
Sequence alignment of selected PDZ domains. The first (SYNT PDZ-1) and second (SYNT PDZ-2) PDZ domains of syntenin were aligned to the three PDZ domains of the rat brain postsynaptic density protein PSD-95 (M96853), PDZ-3 of the Drosophila Discs-large or Dlg protein (M73529), and its human homolog hDlg (U49089); PDZ-2 of the human tight junction protein ZO-1 (L14837); PDZ-3 of human ZO-2 (L27152), the PDZ domain of rat neuronal nitric oxide synthase or nNOS (X59949); the PDZ domain of murine Tiam-1 (U05245); the PDZ domain of the Caenorhabditis elegans protein LIN-2a (X92564); and the PDZ domain of the human p55 erythrocyte membrane protein (M64925). The numbers in parenthesis refer to the GenBank database accession numbers for the nucleotide sequences that encode these proteins. The residues forming the hydrophobic pockets are indicated by asterisks. The conserved basic residues in the carboxylate binding (CB) loop, involved in the stabilization of the terminal carboxylate group, and the αB1 residues that select for the residues at the −2 positions of the peptide are indicated in bold and with a caret. For more details we refer to the papers of Doyle et al. (14) and Morais Cabral et al. (19).
The analysis of the series of syntenin deletion mutants suggests that the two PDZ domains together are required for the interaction with the syndecans, whereas the N-terminal region is dispensable. It remains unclear at this time why the PDZ domains of syntenin do not work in isolation, but a similar requirement for paired PDZ domains has also been observed for the interactions of the GRIP adaptor protein with α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) glutamate receptors (21) and the interaction of PSD-95 with the cytoplasmic domain of the Shaker-type K+ channel (13). The failure of immobilized syntenin to bind free, presumably monomeric, syndecan cytoplasmic peptide in the biosensor experiment could be an indication that the bindings of individual domains are too weak to work in isolation. A requirement for multivalency or coupled interactions of the PDZ domains would imply that stable syntenin–syndecan interactions may require a prior dimerization or clustering of the syndecans. In the two-hybrid system the cytoplasmic domains of the syndecans are presented as dimers because they are fused to a DNA-binding domain of GAL4 which contains a dimerization domain (22). Moreover, several dimers are bound to the GAL4-responsive promoter. In situ, dimers or clusters of the cytoplasmic domains of the syndecans may form once the heparan sulfate chains of the syndecans are occupied and neutralized by ligand (23) or once the cytoplasmic domains are disconnected from the heparan sulfate moieties, e.g., by proteolysis of the ectodomain, and may be facilitated by a strong tendency for self-aggregation of the membrane-spanning domains of these molecules (24). Overexpressed syndecans may circumvent the need for induced dimerisations or amplify these processes, leading to a clear recruitment of the eGFP–syntenin fusion protein to the cell surface and intracellular membranes.
A syntenin–syndecan interaction supported by a single PDZ domain, which for some reason was not reproduced in any of the deletion mutants or conditions tested, would imply that the second PDZ domain of syntenin remains available for the C termini of other membrane receptors or cytosolic proteins and directly suggest a potential role for syntenin in cross-linking syndecans to other components. Moreover, some PDZ domains (e.g., the PDZ domain of neuronal nNOS) can bind to other PDZ domains (the PDZ-2 domains of PSD-95 and PSD-93, and the PDZ domain of α1-syntrophin) in an interaction that does not involve C-terminal regions (15). PDZ–PDZ interactions and peptide-PDZ binding of nNOS require similar tertiary structures (25). It has been suggested that PDZ–PDZ binding may represent the addition of a β-strand from one PDZ domain to a β-sheet in another domain (19, 20), but the structural basis for PDZ domain dimerizations remains at present unknown. It remains also to be investigated whether syntenin might form PDZ-supported dimers as suggested for PSD-95 (18), but such a scheme could be consistent with the binding deficiency of the deletion mutants. The N-terminal domain of syntenin, finally, contains a PXXP motif and several tyrosine residues and may therefore harbor potential recognition sequences for proteins with cognate SH3 and SH2 domains. Separate ligands for each PDZ domain, PDZ domain-supported self- and heterotypic interactions, and partners for the N-terminal region of syntenin would leave room for higher orders of syntenin and syndecan organization and networking.
Almost all known proteins with PDZ domains are associated with the membrane, suggesting that these domains may be involved in either organizing transmembrane proteins at the plasmamembrane or recruiting proteins from the cytosol (26). Coexpression of PSD-95 with either the Shaker K+ channel or N-methyl-d-aspartate receptor in cells results in coclustering of these membrane proteins with PSD-95 on the cell surface, whereas these proteins remain diffusely distributed if expressed individually (13, 27). Similarly, overexpression of eGFP–syntenin and syndecans results in coclustering of syndecans and eGFP–syntenin, suggesting that syntenin may be involved in the organization of syndecans or vice versa. Many of the PDZ domains are found in signaling molecules, and the majority of these proteins appear to be associated with the cytoskeleton at the cell cortex. Syntenin has no obvious catalytic domain and therefore is unlikely to have a signaling function by itself, but could serve as an adaptor or scaffolding protein to attach syndecans to signaling components and the cytoskeleton. Some indirect indication for an interaction of syntenin with cytoskeletal components may be provided by the pronounced effects of the overexpression of the eGFP–syntenin fusion protein on cell shape. The numerous membrane extensions that decorate these cells suggest that syntenin has an effect on membrane dynamics and microfilament organization, implying that syntenin might be linked to pathways that are supported by the Rho, Rac, and Cdc42 families of small G proteins. The syndecans, too, have no known intrinsic signaling activity, but assist receptor tyrosine kinases of the fibroblast growth factor-receptor family (9). Although conjectural, this cofactor function of the syndecans and their binding to a PDZ protein are reminiscent of the activities of LIN-2, a MAGUK, and LIN-7, a small protein with a single C-terminal PDZ domain. Both LIN-2 and LIN-7 are required for localizing LET-23, a receptor tyrosine kinase involved in the vulval cell induction pathway in C. elegans, to cell junctions and for normal vulval development (reviewed in ref. 28). By analogy, this implies a potential role for syntenin in the positioning of syndecan-associated receptors.
Finally, the diversity of interactions mediated by PDZ domains and the apparent promiscuity of some of these domains raises the possibility that competition between different interactions may occur during the formation of macromolecular complexes by the proteins containing these domains (26). A survey of public protein sequence databases for tFXA sequences suggests several candidate ligands, in addition to syndecans, for the PDZ domains of syntenin. The strong and highly regulated fluctuations in syndecan expressions during development and wound healing (1) may therefore not only reflect modulations of extracellular heparan sulfate-catalyzed processes, but also concerted modulations of intracellular membrane–cytoplasm connections.
In conclusion, our data suggest that syntenin, a unique PDZ protein, can associate with the cytoplasmic domains of the syndecans, and may function as an adaptor molecule that links syndecan-supported recognition processes to intracellular signal-transduction, -control, and -effector systems. Further investigations should clarify the nature, significance and exclusivity of this link.
Acknowledgments
We are grateful to Dr. Paul Van Velthoven for providing a sample of the liver cDNA library, to Dr. Jackie Vandenheede for the synthesis of the syndecan-2 cytoplasmic peptide, and to Ms. Anne Mie Bruystens for culturing the cells. This work was supported by the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen, the Geconcerteerde Onderzoeksacties 1996–2000, the Interuniversity Network for Fundamental Research sponsored by the Belgian Government, the Human Capital and Mobility Programme of the European Union, and the Flanders Interuniversity Institute for Biotechnology. J.J.G. is an Aspirant and G.D. a Research Director of the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: eGFP, enhanced green fluorescent protein; FBS: fetal bovine serum; GST, glutathione S-transferase; PDZ, postsynaptic density protein, disc-large, zonulin-1; CHO, chinese hamster ovary.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF000652).
References
- 1.Bernfield M, Kokenyesi R, Kato M, Hinkes M T, Spring J, Gallo R L, Lose E J. Annu Rev Cell Biol. 1992;8:365–393. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- 2.Rapraeger A C. Curr Opin Cell Biol. 1993;5:844–853. doi: 10.1016/0955-0674(93)90034-n. [DOI] [PubMed] [Google Scholar]
- 3.Schlessinger J, Lax I, Lemmon M. Cell. 1995;83:357–360. doi: 10.1016/0092-8674(95)90112-4. [DOI] [PubMed] [Google Scholar]
- 4.Leppä S, Mali M, Miettinen H M, Jalkanen M. Proc Natl Acad Sci USA. 1992;89:932–936. doi: 10.1073/pnas.89.3.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woods A, Couchman J R. Mol Biol Cell. 1994;5:183–192. doi: 10.1091/mbc.5.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chappell D A, Fry G L, Waknitz M A, Mhonen L E, Pladet M W, Iveris P-H, Strickland D K. J Biol Chem. 1993;268:14168–14175. [PubMed] [Google Scholar]
- 7.Salmivirta M, Lidholt K, Lindahl U. FASEB J. 1996;10:1270–1279. doi: 10.1096/fasebj.10.11.8836040. [DOI] [PubMed] [Google Scholar]
- 8.Spring J, Paine-Saunders S E, Hynes R O, Bernfield M. Proc Natl Acad Sci USA. 1994;91:3334–3338. doi: 10.1073/pnas.91.8.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinfeld R, Van den Berghe H, David G. J Cell Biol. 1996;133:405–416. doi: 10.1083/jcb.133.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lories V, Cassiman J J, Van den Berghe H, David G. J Biol Chem. 1992;267:1116–1122. [PubMed] [Google Scholar]
- 11.Fields S, Song O K. Nature (London) 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 12.Songyang Z, Fanning A S, Fu C, Xu J, Marfatia S M, Chisti A H, Crompton A, Chan A C, Anderson J M, Cantley L C. Science. 1997;275:73–77. doi: 10.1126/science.275.5296.73. [DOI] [PubMed] [Google Scholar]
- 13.Kim E, Niethammer M, Rothschild A, Jan N Y, Sheng M. Nature (London) 1995;278:85–88. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- 14.Doyle D A, Lee A, Lewis J, Kim E, Sheng M, MacKinnon R. Cell. 1996;85:1067–1076. doi: 10.1016/s0092-8674(00)81307-0. [DOI] [PubMed] [Google Scholar]
- 15.Brenman J E, Chao D S, Gee S H, McGee A W, Craven S E, Santillano D R, Wu Z, Huang F, Xia H, Peters M F, Froehner S C, Bredt D S. Cell. 1996;82:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- 16.Ponting C P, Philips C. Trends Biochem Sci. 1995;20:102–103. doi: 10.1016/s0968-0004(00)88973-2. [DOI] [PubMed] [Google Scholar]
- 17.Saras J, Heldin C-H. Trends Biochem Sci. 1996;21:455–458. doi: 10.1016/s0968-0004(96)30044-3. [DOI] [PubMed] [Google Scholar]
- 18.Gomperts S N. Cell. 1996;84:659–662. doi: 10.1016/s0092-8674(00)81043-0. [DOI] [PubMed] [Google Scholar]
- 19.Morais Cabral J H, Petosa C, Sutcliffe M J, Raza S, Byron O, Poy F, Marfatia S M, Chishti A H, Liddington R C. Nature (London) 1996;382:649–652. doi: 10.1038/382649a0. [DOI] [PubMed] [Google Scholar]
- 20.Harrison S C. Cell. 1996;86:341–343. doi: 10.1016/s0092-8674(00)80105-1. [DOI] [PubMed] [Google Scholar]
- 21.Dong H, O’Brien R J, Funh E T, Lanahan A A, Worley P F, Huganir R L. Nature (London) 1997;238:279–284. doi: 10.1038/386279a0. [DOI] [PubMed] [Google Scholar]
- 22.Carey M, Kakidani H, Leatherwood J, Mostashari F, Ptashne M. J Mol Biol. 1989;209:423–432. doi: 10.1016/0022-2836(89)90007-7. [DOI] [PubMed] [Google Scholar]
- 23.Martinho R G, Castel S, Ureña J, Fernández-Borja M, Makiya R, Olivecrona G, Reina M, Alonso A, Vilaró S. Mol Biol Cell. 1996;7:1771–1788. doi: 10.1091/mbc.7.11.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asundi V K, Carey D J. J Biol Chem. 1995;270:26404–26410. doi: 10.1074/jbc.270.44.26404. [DOI] [PubMed] [Google Scholar]
- 25.Stricker N L, Christopherson K S, Yi B A, Schatz P J, Raab R W, Dawes G, Bassett D E, Bredt D S, Li M. Nat Biotechnol. 1997;15:336–342. doi: 10.1038/nbt0497-336. [DOI] [PubMed] [Google Scholar]
- 26.Fanning A S, Anderson J M. Curr Biol. 1996;6:1385–1388. doi: 10.1016/s0960-9822(96)00737-3. [DOI] [PubMed] [Google Scholar]
- 27.Kim E, Cho K O, Rothschild A, Sheng M. Neuron. 1996;17:103–113. doi: 10.1016/s0896-6273(00)80284-6. [DOI] [PubMed] [Google Scholar]
- 28.Lambie E J. Curr Biol. 1996;6:1089–1091. doi: 10.1016/s0960-9822(02)70674-x. [DOI] [PubMed] [Google Scholar]