Abstract
We have recently devised a culture method that generates large numbers of eosinophils at high purity from unselected BALB/c mouse bone marrow progenitors [Dyer et al., 2008. J. Immunol. 181: 4004–9]. Here we present the extended scope of this approach, as we have used this method successfully to generate eosinophil cultures of virtually 100% purity from bone marrow from C57BL/6 mice, and from TLR2, TLR3, TLR7 and TLR9-gene-deleted mouse strains on the C57BL/6 background. Both wild-type and TLR3 gene-deleted bone marrow eosinophils (bmEos) are functional, releasing peroxidase in response to the secretogogue, platelet activating factor. We have also used this method to re-evaluate production of eosinophils in bone marrow cultures from ΔdblGATA mice, a strain that is eosinophil-deficient in vivo. Interestingly, bmEos can be detected in the ΔdblGATA cultures (5% of total cells at day 10), although ~80-fold fewer bmEos are detected in ΔdblGATA than in parallel wild-type (BALB/c) bone marrow cultures. Overall, we find that generation of large numbers of eosinophils at high purity from unselected bone marrow progenitors proceeds efficiently in a variety of wild-type and gene-deleted strains, and as such this approach shows promise as a universal method for the study of eosinophil structure and function.
Keywords: Cytokines, interleukin-5, toll-like receptors, mouse, hematopoiesis
Introduction
Eosinophils are granulocytic leukocytes that develop from pluripotent bone marrow progenitors in response to Th2 cytokine stimulation, most notably in response to elevated levels of interleukin-5 (reviewed in [1, 2]). Although eosinophils are readily recognized by their distinct morphology, the biological role and functions of these cells remain uncertain and controversial. For example, although eosinophilia is a prominent component of the host response to helminthic parasite infection, the presumed role of eosinophils in anti-parasite host defense is not at all clear [3]; in fact, Fabre and colleagues [4] recently presented data suggesting that eosinophils serve to promote parasite, rather than host survival in a chronic infection model. Likewise, consensus opinion holds that eosinophils play a prominent role in pathogenesis of allergic asthma, yet large-scale clinical studies lead to conflicting conclusions [5 – 7].
In order to explore eosinophil development, and ultimately the function of individual genes and gene-products within eosinophils, we developed a culture system which permits us to generate large numbers of eosinophils at high purity (> 95%) from unselected BALB/c bone marrow progenitors [8]. We found that BALB/c bone marrow-derived eosinophils (bmEos) expressed relevant transcripts and lineage-specific cell surface proteins and underwent chemotaxis in response to eotaxin-1. As only wild-type BALB/c mice were evaluated in this first study, it was important to determine whether our findings were unique to this one strain of mice, or whether this method might be applied more universally.
Here, we explore the potential of this method as a means to generate functional bmEos from the C57BL/6 mice, as well as from bone marrow cells from specific gene-deleted mice on the C57BL/6 background. We also use this improved culture system to revisit our earlier findings [9], comparing the production of bmEos ex vivo from wild-type and eosinophil-deficient ΔdblGATA mice.
Materials and Methodology
Mice
Six to eight-week old wild-type BALB/c and C57BL/6 mice were purchased from Taconic Farms (Rockville, MD). Eosinophil-deficient ΔdblGATA mice on the BALB/c background [10] were bred and maintained on site. TLR2, TLR7, and TLR9 gene-deleted mice (C57BL/6 background) [11 – 13] are used with written permission of Dr. Shizuo Akira and were graciously provided by Dr. Rachel Caspi (NEI/NIH) and Dr. Jennifer Wang (U. Mass. Medical). TLR3 gene-deleted mice [14] were graciously provided by Dr. Ivett Jelinek (NCI/NIH). All methods herein were reviewed and approved via NIAID Animal Study Proposal LAD 7E.
Bone marrow isolation and culture
The method for eosinophil production from unselected bone marrow progenitors is described in detail in reference 8. Briefly, bone marrow cells were collected from the long bones by flushing with Iscove’s Modified Dulbecco’s Medium (Invitrogen), and red blood cells were lysed in dH2O followed by the addition of 10X PBS to 1X final concentration. After centrifugation, the cells were washed once in PBS containing 0.1% bovine serum albumin (BSA). The bone marrow cells were seeded at 107/mL in media containing RPMI 1640 (Invitrogen) with 20% fetal bovine (Cambrex), 100 IU/mL penicillin and 10 μg/mL streptomycin (Cellgro), 2 mM glutamine (Invitrogen), 25 mM HEPES and 1× non-essential amino acids and 1 mM sodium pyruvate (Gibco) and 50 μM β-mercaptoethanol (Sigma) and supplemented with 100 ng/mL stem-cell factor (SCF; PeproTech) and 100 ng/mL FLT3-Ligand (FLT3-L; PeproTech) from day 0 to day 4. On day 4, the media containing SCF and FLT3-L was replaced with media containing 10 ng/mL recombinant mouse interleukin-5 (rmIL-5; R&D Systems) alone. Viable (trypan blue-excluding) cells were enumerated manually via hemocytometer counts and determined on triplicate wells.
Flow cytometric detection of eosinophils
Cells were incubated with either PE-conjugated rat anti-mouse Siglec F or PE-conjugated IgG2A6 isotype control (1μg per 106 cells; BD Pharmingen) for 30 minutes at 4°C. After staining, the cells were fixed in 4% paraformaldehyde and analyzed by flow cytometry. Data were acquired with a FACSCalibur flow cytometer (BD Biosciences) and analyzed with FloJo software v 7.1 (Tree Star, Inc). Siglec F-positive cells were identified by comparison to the PE-conjugated IgG2A6 isotype control.
Degranulation assay
Detection of eosinophil peroxidase (EPO) released by platelet-activating factor (PAF)-stimulated bmEos was essentially as described [15]. Cells were collected by centrifugation and resuspended phenol-red-free Roswell Park Memorial Institute (RPMI) medium at a concentration 250,000 cells/mL and 25,000/100 uL were used per well. One uL of platelet activating factor (PAF C16) or 1 uL of vehicle, dimethyl sulfoxide (DMSO), was added to appropriate wells to achieve indicated concentration. Cells were incubated at 37°C, 5% CO2 for 30 minutes. The assay was developed using 100 uL o-phenylene-diamine (OPD) reagent (800 uL 5mM OPD in 4 mL 1M Tris (pH8.0), 5.2 mL H2O and 1.25 uL 30% H2O2) in the presence of H202. The reaction was terminated by the addition of 100 μL of 4M H2SO4 to each well. The relative production of EPO was determined by reading the plate at wavelength 492 nm. Data is reported as mean absorbance +/− standard error of the mean in relation to vehicle only control (1 % DMSO).
Results
Eosinophils (bmEos) are generated from C57BL/6 bone marrow culture
Our previous study examined production of functional eosinophils at high purity from unselected bone marrow from BALB/c mice [8]. Here we demonstrate production of bmEos from progenitors from C57BL/6 mice. Using the identical culture conditions (100 ng/mL SCF + 100 ng/mL FLT-3L for 4 days, followed by 10 ng/mL IL-5 alone thereafter), 71% of the cells in C57BL/6 bone marrow culture at day 10 were Siglec F+ bmEos, comparable to the 88% bmEos detected in the BALB/c bone marrow cultures [Figure 1]. Both BALB/c and C57BL/6 bone marrow cultures reach ~100% bmEos by day 14 [8]. BmEos can also be generated from the 129Sv mouse strain (data not shown).
Figure 1. Eosinophils generated in cultures of unselected bone marrow from both BALB/c and C57BL/6 mice.
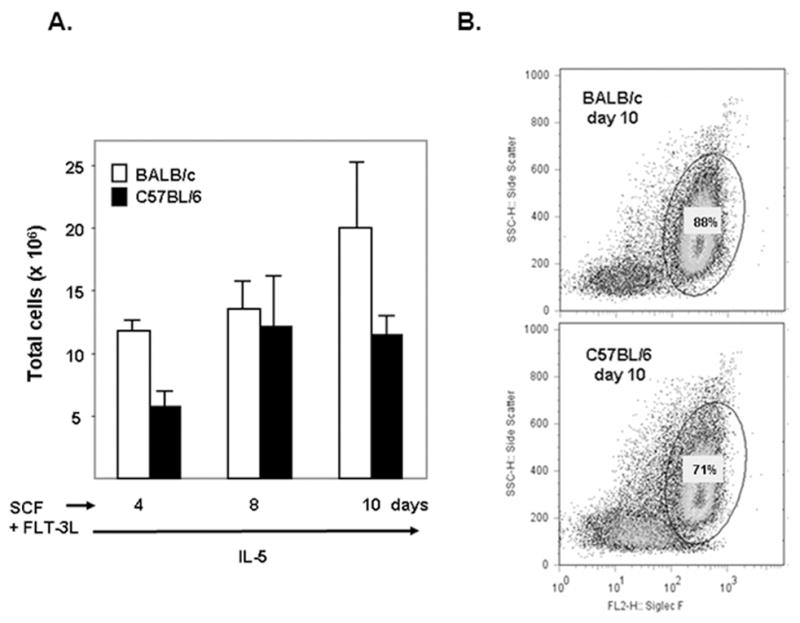
(A) Bone marrow cultures were initiated at 107 cells/mL. The number of viable cells was determined at timepoints shown in response to rmSCF (100 ng/mL) and rmFLT-3L (100 ng/mL) for 4 days after isolation, followed by rmIL-5 (10 ng/mL) alone thereafter. (B) Eosinophils were identified at day 10 in culture by side scatter (SSC, y-axis) and reactivity with anti-Siglec F+ antibodies (x-axis).
BmEos from gene-deleted progenitors
The percent bmEos were determined from cultures of C57BL/6 wild-type bone marrow and bone marrow isolated from TLR gene-deleted mice [Figure 2]. As shown, individual deletions of TLR 2, TLR3, TLR 7 and TLR9 have no impact on the rate at or extent to which bmEos are generated in culture. All cultures reached virtually 100% bmEos at days 16 – 18, including those from C57BL/6 wild-type mice.
Figure 2. Eosinophils generated from bone marrow of TLR 2, TLR 3, TLR 7, and TLR 9 gene-deleted mice.
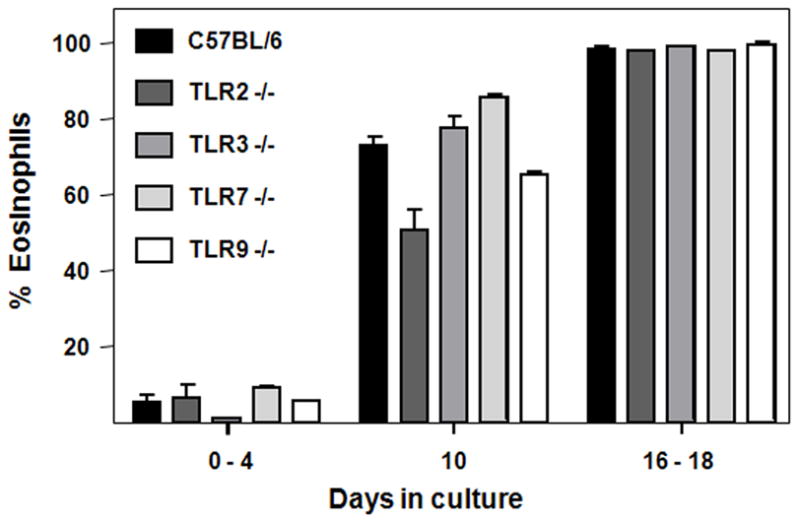
Eosinophils were identified by staining with Diff-Quik and are shown as percent of total cell number on days indicated.
BmEos degranulate
In our previous publication [8], we found that bmEos expressed typical proteins and transcripts, and underwent chemotaxis in response to gradients of mouse eotaxin-1 (CCL11). Here, we characterize another functional response, as we demonstrate that mouse bmEos degranulate in a dose-dependent fashion in response to the eosinophil-secretagogue, platelet-activating factor (Figure 3(PAF; [16, 17]).
Figure 3. Eosinophils generated from bone marrow of wild-type and TLR3 gene-deleted mice release eosinophil peroxidase in response to challenge with PAF.
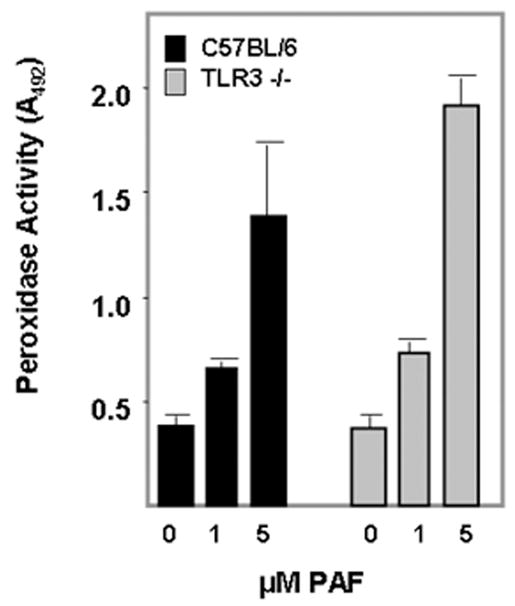
Extracellular peroxidase activity was measured as hydrolysis of o-phenylene diamine (OPD) as described [15]; peroxidase activity detected at baseline (zero concentration) represents the response to 1% DMSO vehicle control.
BmEos from eosinophil-deficient ΔdblGATA mice
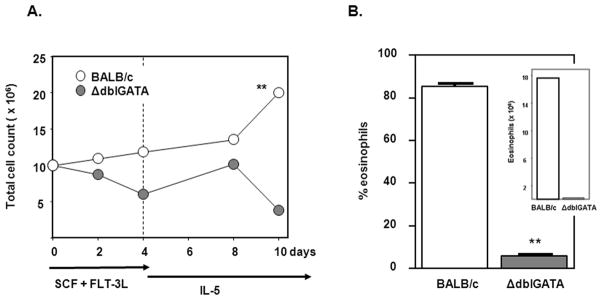
Interestingly, and in contrast to our previous study [9], with this cytokine regimen, markedly fewer bmEos were detected in bone marrow cultures from eosinophil-deficient ΔdblGATA mice when compared to those generated from BALB/c wild-type mice [Figure 4].
Figure 4. Eosinophils generated from bone marrow of ΔdblGATA eosinophil-deficient mice.
(A) Bone marrow cultures were initiated at 107 cells/mL. The number of viable cells was determined at timepoints shown in response to the cytokine regimen described in the Legend to Figure 1. Significant difference between counts detected at day 10, **p < 0.01. (B) Eosinophils identified by side scatter and Siglec F expression are shown as percent total cell number on day 10, **p < 0.01. Inset: number of eosinophils in bone marrow culture at day 10 as calculated from the data in (A) and (B).
Discussion
In this manuscript, we demonstrate the general nature of our bone marrow eosinophil culture protocol. Specifically, we document production of bmEos from C57BL/6, a genetically-distinct wild-type strain used extensively for creating gene-deleted mice. We also generated bmEos from four independent gene-deleted mouse strains on the C57BL/6 background. The ability to generate bmEos from gene-deleted strains is a critical feature of this method, as mature eosinophils isolated from mouse blood and spleen are biosynthetically inert and have relatively little mRNA; as such, introduction of RNA-silencing agents are unlikely to have a substantial impact on protein targets. Specifically, we generated substantial numbers of TLR2, TLR3, TLR7 and TLR9 gene-deleted eosinophils that can be used for functional studies. Numerous TLRs have been detected in both human and mouse eosinophils [18 – 21]; TLR2 has been implicated in interactions of eosinophils with mycobacteria [19], and TLR7 was shown to be crucial for eosinophil-mediated clearance of respiratory viruses in vivo [21]. There is also substantial evidence supporting a role for TLRs in hematopoeisis [22]; TLRs are expressed on pluripotent progenitors and they direct differentiation toward the myeloid (granulocyte, macrophage) lineages [23, 24].
We also revisited our findings on the role of the dblGATA enhancer of the mouse GATA-1 gene in promoting eosinophil hematopoiesis ex vivo. ΔdblGATA gene-deleted mice are completely devoid of mature eosinophils, both at baseline or in response to Th2 stimulation [10, 25, 26]; the molecular basis for this finding is not yet clear. In an earlier study, we found that ΔdblGATA and wild-type BALB/c mouse bone marrow cultures each yielded ~5 – 7% eosinophils in response to four days with SCF and FLT-3L, followed by various combinations of IL-5, IL-3 and GM-CSF [9]. In this work, when we restrict the secondary stimulus to IL-5 alone, wild-type cultures undergo specific expansion of the eosinophil lineage, with bmEos representing nearly 100% of the cell population by day 14. Interestingly, the ΔdblGATA bone marrow cultures can still generate eosinophils using IL-5 alone as the secondary stimulus, but the bmEo-population remains at ~5% of the total, representing ~80-fold fewer bmEos than that produced by the parallel wild-type culture. Interestingly, the original cytokine combination, which included IL-5, IL-3 and GM-CSF, skewed the distribution of GATA-1 transcripts, suggesting that the ΔdblGATA deletion might be circumvented ex vivo by use of an alternative promoter [9]; we have not evaluated transcript distribution in response to IL-5 alone. However, we have shown clearly that the ΔdblGATA bone marrow progenitors are not capable of a complete response to IL-5. As such, this system can serve as an important tool for evaluating the molecular basis of the dblGATA deletion and its direct connection to IL-5-mediated expansion of the eosinophil lineage.
Conclusion
In summary, we have shown that generation of eosinophils at high purity from unselected bone marrow progenitors proceeds effectively in a variety of wild-type and gene-deleted strains, and as such is a universal tool for the study of eosinophil structure and function.
Acknowledgments
The authors thank Dr. Rachel Caspi (NEI/NIH) for facilitating the transfer of TLR 2 −/− and TLR 9 −/− gene-deleted mice, Dr. Jennifer Wang (University of Massachusetts Medical) for the TLR7 −/− mice (strains used with permission from Dr. S. Akira), and Dr. Ivett Jelinek (NCI, NIH) for providing TLR 3 −/− mice. We also thank Dr. Alison Humbles and Dr. Craig Gerard (Harvard Medical School) for the original male mice used to set up our ΔdblGATA colony. This study was funded by NIAID Division of Intramural Research.
References
- 1.Rothenberg ME, Hogan SP. The Eosinophil. Annu Rev Immunol. 2006;24:147–74. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 2.Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, Kay AB, Rothenberg ME. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38:709–50. doi: 10.1111/j.1365-2222.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- 3.Klion AD, Nutman TB. The role of eosinophils in host defense against helminth parasites. J Allergy Clin Immunol. 2004;113:30–7. doi: 10.1016/j.jaci.2003.10.050. [DOI] [PubMed] [Google Scholar]
- 4.Fabre V, Beiting DP, Bliss SK, Gebreselassie NG, Gagliardo LF, Lee NA, Lee JJ, Appleton JA. Eosinophil deficiency compromises parasite survival in chronic nematode infection. J Immunol. 2009;182:1577–83. doi: 10.4049/jimmunol.182.3.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leckie MJ, ten Brinke A, Khan J, Diamant Z, O’Connor BJ, Walls CM, Mathur AK, Cowley HC, Chung KF, Djukanovic R, Hansel TT, Holgate ST, Sterk PJ, Barnes PJ. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356:2144–8. doi: 10.1016/s0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- 6.Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, Hargreave FE, O’Byrne PM. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–93. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 7.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, Marshall RP, Bradding P, Green RH, Wardlaw AJ, Pavord ID. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–84. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dyer KD, Moser JM, Czapiga M, Siegel SJ, Percopo CM, Rosenberg HF. Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J Immunol. 2008;181:4004–9. doi: 10.4049/jimmunol.181.6.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dyer KD, Czapiga M, Foster B, Foster PS, Kang EM, Lappas CM, Moser JM, Naumann N, Percopo CM, Siegel SJ, Swartz JM, Ting-De Ravin S, Rosenberg HF. Eosinophils from lineage-ablated Delta dblGATA bone marrow progenitors: the dblGATA enhancer in the promoter of GATA-1 is not essential for differentiation ex vivo. J Immunol. 2007;179:1693–9. doi: 10.4049/jimmunol.179.3.1693. [DOI] [PubMed] [Google Scholar]
- 10.Yu C, Cantor AB, Yang H, Browne C, Wells RA, Fujiwara Y, Orkin SH. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J Exp Med. 2002;195:1387–95. doi: 10.1084/jem.20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–51. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 12.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 13.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 14.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–8. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 15.Adamko DJ, Wu Y, Gleich GJ, Lacy P, Moqbel R. The induction of eosinophil peroxidase release: improved methods of measurement and stimulation. J Immunol Methods. 2004;291:101–8. doi: 10.1016/j.jim.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Simon HU, Weber M, Becker E, Zilberman Y, Blaser K, Levi-Schaffer F. Eosinophils maintain their capacity to signal and release eosinophil cationic protein upon repetitive stimulation with the same agonist. J Immunol. 2000;165:4069–75. doi: 10.4049/jimmunol.165.7.4069. [DOI] [PubMed] [Google Scholar]
- 17.Bartemes KR, McKinney S, Gleich GJ, Kita H. Endogenous platelet-activating factor is critically involved in effector functions of eosinophils stimulated with IL-5 or IgG. J Immunol. 1999;162:2982–9. [PubMed] [Google Scholar]
- 18.Nagase H, Okugawa S, Ota Y, Yamaguchi M, Tomizawa H, Matsushima K, Ohta K, Yamamoto K, Hirai K. Expression and function of Toll-like receptors in eosinophils: activation by Toll-like receptor 7 ligand. J Immunol. 2003;171:3977–86. doi: 10.4049/jimmunol.171.8.3977. [DOI] [PubMed] [Google Scholar]
- 19.Driss V, Legrand F, Hermann E, Loiseau S, Gueradel Y, Kremer L, Adam E, Woerly G, Dombrowicz D, Capron M. TLR2-dependent eosinophil interactions with mycobacteria: role of alpha-defensins. Blood. 2009;113:3235–44. doi: 10.1182/blood-2008-07-166595. [DOI] [PubMed] [Google Scholar]
- 20.Mansson A, Cardell LO. Role of atopic status in TLR7 and TLR9-mediated activation of human eosinophils. J Leukoc Biol. 2009;85:719–27. doi: 10.1189/jlb.0808494. [DOI] [PubMed] [Google Scholar]
- 21.Phipps S, Lam CE, Mahalingam S, Newhouse M, Ramirez R, Rosenberg HF, Foster PS, Matthaei KI. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood. 110:1578–86. doi: 10.1182/blood-2007-01-071340. [DOI] [PubMed] [Google Scholar]
- 22.McGettrick AF, O’Neill LAJ. Toll-like receptors: key activators of leucocytes and regulator of haematopoiesis. Brit J Haematol. 2007;139:185 – 93. doi: 10.1111/j.1365-2141.2007.06802.x. [DOI] [PubMed] [Google Scholar]
- 23.Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, Kincade PW. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–12. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sioud M, Floisand Y, Forfang L, Lund-Johansen F. Signaling through toll-like receptor 7/8 induces the differentiation of human bone marrow CD34+ progenitor cells along the myeloid lineage. J Mol Biol. 2006;364:945–54. doi: 10.1016/j.jmb.2006.09.054. [DOI] [PubMed] [Google Scholar]
- 25.Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, Gerard C. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–9. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 26.Swartz JM, Dyer KD, Cheever AW, Ramalingam T, Pesnicak L, Domachowske JB, Lee JJ, Lee NA, Foster PS, Wynn TA, Rosenberg HF. Schistosoma mansoni infection in eosinophil lineage-ablated mice. Blood. 2006;108:2420–7. doi: 10.1182/blood-2006-04-015933. [DOI] [PMC free article] [PubMed] [Google Scholar]