Abstract
Apolipoprotein A-I (apoA-I) is the major protein component of HDL, where it plays an important role in cholesterol transport. The deposition of apoA-I derived amyloid is associated with various hereditary systemic amyloidoses and atherosclerosis; however, very little is known about the mechanism of apoA-I amyloid formation. Methionine residues in apoA-I are oxidized via several mechanisms in vivo to form methionine sulfoxide (MetO), and significant levels of methionine oxidized apoA-I (MetO-apoA-I) are present in normal human serum. We investigated the effect of methionine oxidation on the structure, stability, and aggregation of full-length, lipid-free apoA-I. Circular dichrosim spectroscopy showed that oxidation of all three methionine residues in apoA-I caused partial unfolding of the protein and decreased its thermal stability, reducing the melting temperature (Tm) from 58.7 °C for native apoA-I to 48.2 °C for MetO-apoA-I. Analytical ultracentrifugation revealed that methionine oxidation inhibited the native self association of apoA-I to form dimers and tetramers. Incubation of MetO-apoA-I for extended periods resulted in aggregation of the protein, and these aggregates bound Thioflavin T and Congo Red. Inspection of the aggregates by electron microscopy revealed fibrillar structures with a ribbon-like morphology, widths of approximately 11 nm, and lengths of up to several microns. X-ray fibre diffraction studies of the fibrils revealed a diffraction pattern with orthogonal peaks at spacings of 4.64 Å and 9.92 Å, indicating a cross-β amyloid structure. This systematic study of fibril formation by full-length apoA-I represents the first demonstration that methionine oxidation can induce amyloid fibril formation.
Keywords: aggregation, amyloidosis, atherosclerosis, protein misfolding
ApoA-I is the major structural protein component of HDL. Much of the current interest in apoA-I stems from its central role in protection against atherosclerosis, thought to be mediated by promotion of reverse cholesterol transport, combined with antiinflammatory and antithrombotic effects (1). Both lipid-bound and lipid-free apoA-I play critical roles in reverse cholesterol transport where lipid-free apoA-I, that accounts for approximately 5% of circulating apoA-I (2), is considered the primary acceptor of cholesterol from peripheral tissues (3).
Specific mutant forms of apoA-I result in familial systemic apoA-I amyloidosis that, generally, manifests as asymptomatic cholestatic hepatopathy and nephropathy (4, 5). More commonly, apoA-I amyloid deposits are observed in the arterial intima and atherosclerotic plaque tissue. These deposits arise from wild-type apoA-I, reflecting the ability of the normal protein to form amyloid fibrils in vivo (5, 6). It is not known why apoA-I is deposited as amyloid specifically in atherosclerotic plaques (7); however, these amyloid deposits are believed to be pathogenically linked to atherosclerosis progression (5–10). The pathological importance of amyloid deposits in atherosclerosis is highlighted by the findings that fibrils composed of the β-amyloid peptide or apolipoprotein C-II (apoC-II) activate macrophages, a key feature of vascular inflammation leading to lesion development (10–12).
Fibrils isolated from patients with both hereditary and nonhereditary apoA-I amyloidosis are, generally, composed of the N-terminal 80–100 residues of the protein (5, 13), and a peptide consisting of the first 93 residues of apoA-I has been shown to aggregate in vitro to form fibrils at acidic pH (14). This has lead to speculation that proteolytic cleavage of full-length apoA-I and the release of this N-terminal fragment leads to amyloid fibril formation and deposition. However, attempts to detect N-terminal fragments of apoA-I in plasma have been unsuccessful, despite its high concentrations in amyloid deposits (5). Furthermore, the nature and anatomical site of the cleavage event is not known, and no definitive study of the pathological mechanism of apoA-I amyloid formation has been reported (13). Thus, it remains possible that the cleavage event occurs after fibril formation. This mechanism has previously been proposed for a range of hereditary apoA-I amyloidoses (15–17) and is believed to occur in other amyloid pathologies (13).
ApoA-I is subject to oxidative modification in vivo and significant levels of MetO-apoA-I exist in normal plasma (18). Analysis of serum suggests that as much as 25% of circulating apoA-I contains at least one of its three methionine residues oxidized to its sulfoxide form (MetO) (19), and this level may be increased in type one diabetic subjects (20). In vivo, the methionine residues of apoA-I are oxidized via at least two specific mechanisms. ApoA-I is a selective target of oxidation by myeloperoxidase (21), a heme protein that produces a range of reactive oxidant species capable of specifically oxidising methionine residues (22). In addition, apoA-I plays an important role in the reduction of potentially pro-atherogenic oxidized lipids. Lipid hydroperoxides, transferred to HDL from low density lipoprotein via cholesterol ester transport protein are chemically reduced to their corresponding alcohols in a process that results in consecutive and specific oxidation of methionine residues in apoA-I to their MetO forms (23). Increasing evidence indicates that oxidized forms of apoA-I are dysfunctional and promote inflammatory effects (24). In particular, oxidation of specific methionine residues of apoA-I reduces its ability to activate lecithin:cholesterol acyltransferase, thereby, impairing reverse cholesterol transport (22).
To date there have been no systematic studies of the ability of full-length apoA-I to form amyloid fibrils in vitro. In the present study we examined the effect of methionine oxidation on the biophysical properties and aggregation of full-length apoA-I. Our studies show that native apoA-I is stable under a wide range of solution conditions. In contrast, oxidation of the methionine residues of apoA-I resulted in reduced conformational stability and oligomeric self-association, leading to misfolding of the protein and formation of fibrillar structures with all the characteristics of amyloid fibrils. These findings have important implications in both familial apoA-I amyloidosis and atherosclerosis, and may point to therapeutic strategies to reduce apoA-I amyloid deposition.
Results
Native apoA-I Does Not Form Fibrils Under a Wide Range of Solution Conditions.
Native, full-length apoA-I was incubated under a range of solution conditions known to effect or induce amyloid fibril formation in other protein systems. The varied solution parameters included: protein concentration, pH, temperature, agitation, and inclusion of chaotropes. All conditions examined are detailed in Table S1. Aggregation of apoA-I in these samples was assessed by ThT fluorescence measurements, examination for light scattering, and electron microscopy of aged samples. In all cases, no fibril formation by native apoA-I was detected. In light of this apparent inability to induce in vitro fibril formation by native apoA-I we investigated the effect of methionine oxidation on the stability and aggregation of the protein.
Preparation of MetO-apoA-I.
Methionine oxidation of apoA-I by incubation with hydrogen peroxide was optimised to produce apoA-I with all three methionine residues converted to methionine sulfoxide and this material was designated MetO-apoA-I. HPLC and mass spectrometry analysis indicated that this protocol routinely yielded pure MetO-apoA-I (> 95%, Fig. S1). Alternative oxidation products were below detectable limits by tandem mass spectrometry.
MetO-apoA-I is Partially Unfolded and Has Lower Thermal Stability Than Native apoA-I.
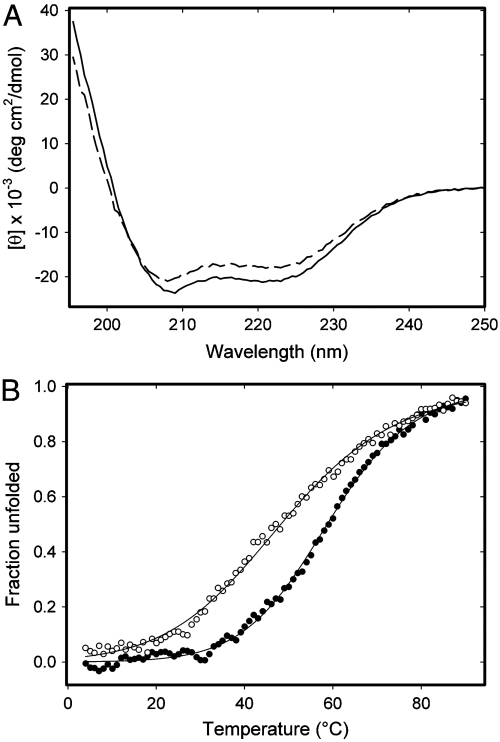
Circular dichroism (CD) spectra acquired for freshly prepared MetO-apoA-I, at both pH 7.4 and pH 6.0, showed reduced mean residue ellipticity signal intensity below 230 nm when compared with spectra obtained for native apoA-I (Fig. 1A). This suggests that methionine oxidation caused partial unfolding or destabilisation of the secondary structure of apoA-I. Accordingly, the thermal stability of MetO-apoA-I was investigated by monitoring CD ellipticity at 222 nm during thermal denaturation between 4–90 °C (Fig. 1B). Thermal denaturation curves indicated that MetO-apoA-I began to denature at much lower temperatures than native apoA-I and that both samples were completely denatured above 90 °C. Fits of these data to a two-state unfolding model yielded melting temperatures (Tm) of 58.7 ± 0.2 °C for native apoA-I, consistent with previously published data (25), and 48.2 ± 0.4 °C for MetO-apoA-I. The reduction in Tm of MetO-apoA-I by around 10.5 °C, with respect to that of native apoA-I, indicates that the structural stability of the oxidized protein is significantly impaired. The broadening of the unfolding transition of MetO-apoA-I, with respect to native apoA-I, also indicates a less cooperative unfolding and may indicate increased structural flexibility in the oxidized protein. In both samples, thermal unfolding was completely reversible.
Fig. 1.
Far UV circular dichrosim analysis of native apoA-I and MetO-apoA-I. (A) Circular dichroism spectra acquired at 0.2 mg/mL and pH 6.0 for apoA-I (Solid Line) and MetO-apoA-I (Dashed Line). (B) Thermal denaturation of apoA-I (Filled Circles) and MetO-apoA-I (Open Circles) monitored by circular dichroism ellipticity at 222 nm. Data for each sample were fitted separately to a two-state unfolding model (Solid Lines).
MetO-apoA-I is Unable to Form a Tetrameric Structure.
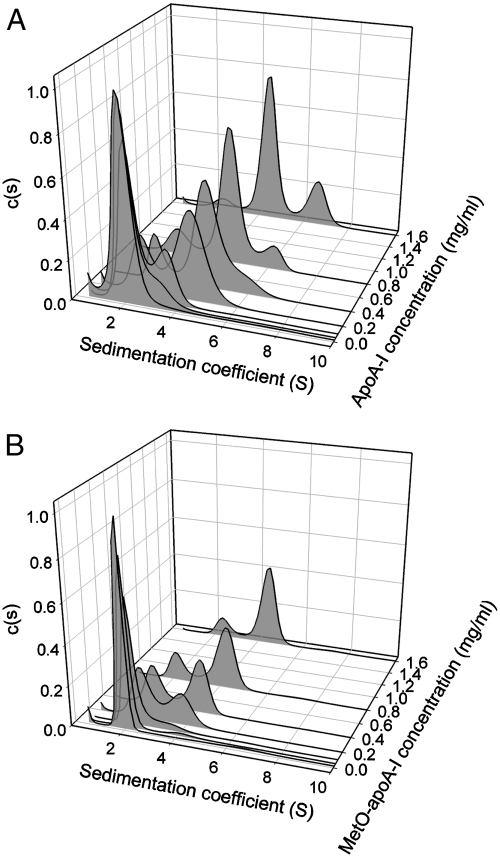
The oligomeric structures of native apoA-I and MetO-apoA-I were investigated by analytical ultracentrifugation. Sedimentation velocity experiments were carried out for both proteins at 0.02–1.6 mg/ml and velocity data were analyzed by using the program SEDFIT to produce continuous sedimentation coefficient distributions. At low concentrations (< 0.05 mg/ml) both native apoA-I and MetO-apoA-I gave sedimentation coefficient distributions consisting of a single major peak at 2.0 S (Fig. 2) indicating that both proteins were primarily monomeric at these concentrations. Increasing concentrations of native apoA-I resulted in the appearance of peaks at 4.5 S and 6.7 S, indicating the formation of two higher order oligomers. These peaks were ascribed to dimer and tetramer, respectively, consistent with early work on the self-association of apoA-I (26). In contrast, increasing concentrations of MetO-apoA-I resulted in the appearance of only one further peak, around 4.5 S. This indicates that MetO-apoA-I is unable to form the tetrameric species observed for native apoA-I. Examination of the distributions also reveals that the 4.5 S peak was proportionally smaller for MetO-apoA-I than for native apoA-I at equivalent concentrations, suggesting that dimerisation is weaker in the oxidized preparation.
Fig. 2.
Sedimentation velocity analysis of (A) native apoA-I and (B) MetO-apoA-I. Sedimentation data acquired at protein concentrations from 0.02 mg/mL to 1.6 mg/mL were analyzed by using a continuous sedimentation coefficient distribution, c(S), and distributions were normalized with respect to integrated signal intensity.
MetO-apoA-I Aggregates to Form Thioflavin T Reactive and Congophilic Material.
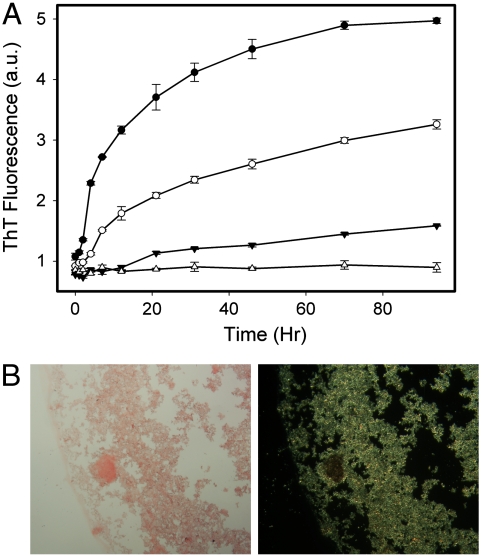
On incubation for several days at 37 °C, MetO-apoA-I formed a visible fine precipitate that was not observed on incubation of native apoA-I. The time course of aggregation of MetO-apoA-I was followed using ThT fluorescence. Incubation of MetO-apoA-I at 0.8 mg/mL at 37 °C, in the presence of ThT, resulted in a time-dependent increase in ThT fluorescence that coincided with the appearance of turbidity in the solution (Fig. 3A). This reflects the ability of the aggregates to bind ThT, which is a defining feature of amyloid fibrils. Incubation of MetO-apoA-I at 0.5 and 0.3 mg/mL, resulted in development of ThT fluorescence at a slower rate and over a longer time period. A lag phase at early timepoints, where ThT fluorescence increases little, was evident at all incubation concentrations but was more obvious at the lower concentrations. The observed decrease in lag time and accelerated rate of aggregation with increasing protein concentration is consistent with the nucleated-elongation mechanism of aggregation that is common to most amyloid fibrils.
Fig. 3.
Thioflavin T and Congo Red binding by MetO-apoA-I aggregates. (A) ThT fluorescence time course of MetO-apoA-I aggregation. MetO-apoA-I was incubated at 0.8 mg/mL (Filled Circles), 0.5 mg/mL (Open Circles), 0.3 mg/mL (Filled Triangles), and native apoA-I was incubated at 0.8 mg/mL (Open Triangles) at 37 °C and pH 6.0 in the presence of 10 μM ThT. (B) MetO-apoA-I aggregates stained with Congo Red appeared pink-red under bright field examination (Left) and exhibited strong green birefringence under cross-polarized light (Right). The width of each panel is equivalent to 620 μm (20× magnification).
Mature aggregates of MetO-apoA-I were stained with Congo Red and examined by light microscopy (Fig. 3B). Aggregates appeared pink-red under bright field examination, indicating that they were congophilic. Under cross-polarized light these aggregates displayed strong green birefringence that is characteristic and diagnostic of amyloid upon Congo Red binding.
Aggregates of MetO-apoA-I Have a Fibrillar Morphology.
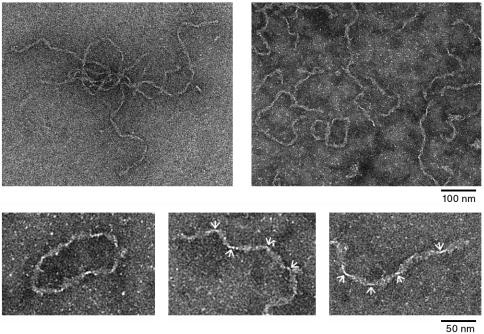
MetO-apoA-I aggregates were examined by transmission electron microscopy to determine whether these had fibrillar ultrastructural morphology. Aggregates appeared fibrillar with a high degree of fibril curling and a “ribbon” type appearance (Fig. 4). A lengthwise helical twist in the fibrils was apparent from the periodic constrictions along the fibril length. However, the periodicity of this twist was irregular, with distances between constrictions ranging 25–60 nm. At their widest projection, the fibrils were approximately 11 nm wide and examples of fibrils of several microns in length were common. The appearance of the fibrils suggests a highly flexible structure, and is similar to that of amyloid fibrils formed by another full-length apolipoprotein, apoC-II (27, 28). In a number of cases, apparently, circular MetO-apoA-I fibrils were observed, again, reminiscent of structures formed by apoC-II (28). Extensive inspection of the EM grids revealed no significant evidence of nonspecific protein aggregation, indicating that MetO-apoA-I aggregates specifically to form fibril structures.
Fig. 4.
Transmission electron microscope images of MetO-apoA-I fibrils indicating the gross morphology of the fibrils. Small panels show magnified views of the fibrils including an example of the, apparently, circular fibrils. Constrictions in the fibril length due to the helical twisting of the fibril ribbon are indicated with arrows.
MetO-apoA-I Fibrils Consist Solely of Full-Length Protein.
ApoA-I amyloid fibrils extracted from tissues, generally, consist of the N-terminal 80–90 residues of the protein. The nature of the proteins making up fibrils formed from MetO-apoA-I in vitro was investigated by mass spectrometry and SDS-PAGE (Fig. S2). Fibrils were prepared by repeated centrifugal pelleting and, finally, resuspended in acetonitrile to dissociate the fibrillar material. Mass spectrometry analysis of this sample revealed a pure protein sample with a molecular mass corresponding to that expected for full-length apoA-I containing three MetO residues. No fragmentation of the protein comprising the fibrils was apparent by either mass spectrometry or SDS-PAGE. This indicates that the observed fibrils consist of full-length MetO-apoA-I that has not been truncated.
X-ray Fibre Diffraction Reveals a Cross-β Amyloid Structure.
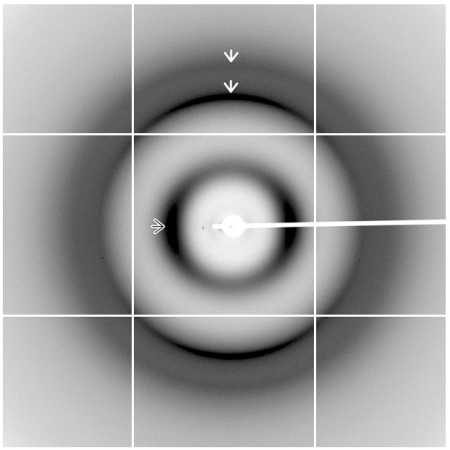
Amyloid fibrils are defined by a common core structure in which two or more β-sheets are formed from repeating β-strands oriented perpendicular to the long axis of the fibril. The spacing of these β-strands and β-sheets within the fibril produces a signature X-ray fibre diffraction pattern (29). CD spectra acquired for freshly prepared MetO-apoA-I and MetO-apoA-I fibrils (Fig. S3) indicated a significant change in secondary structure on fibril formation, consistent with a shift toward β-structure. Magnetically aligned samples of MetO-apoA-I fibrils were analyzed by X-ray fibre diffraction by using the high flux, MX2 beamline of the Australian Synchrotron. Diffraction images revealed a classical amyloid cross-β diffraction pattern with orthogonal meridional (Miller index 001) and equatorial reflections (Fig. 5). The dominant meridional reflection, a sharp peak with a spacing of 4.64 Å, is derived from the separation of hydrogen-bonded β-strands that are arranged perpendicular to the fibril axis. The more diffuse equatorial peak at 9.92 Å represents the mean spacing between β-sheets in a direction perpendicular to the fibril axis. Together, these reflections define the core cross-β structure of MetO-apoA-I fibrils. A weaker, meridional reflection with a spacing of 3.75 Å was also evident as a shoulder on the outer edge of a diffuse scattering ring. This indicates further order within the structure along the fibril axis and is consistent with diffraction patterns from a range of other amyloid fibrils (29). Partial smearing of diffraction peaks into rings indicates incomplete alignment of fibrils, possibly due to their highly flexible nature.
Fig. 5.
X-ray diffraction pattern from aligned, semi-hydrated MetO-apoA-I fibrils. Meridional (001) reflections with spacings of 4.64 and 3.75 Å are shown in the vertical axis and are indicated with White Arrows. An equatorial reflection with spacing of 9.94 Å is indicated with an Outlined Arrow. Diffraction images were acquired by using the MX2 beamline of the Australian Synchrotron operating at 12.658 keV (λ = 0.979 Å).
Discussion
ApoA-I is associated with hereditary amyloidoses and amyloid deposition in atherosclerotic tissue, where 54% of subjects have been shown to have amyloid in the intima and atherosclerotic plaques (9). Despite this, the propensity of full-length, wild-type apoA-I to form amyloid fibrils has not, previously, been systematically investigated and the mechanism of amyloid formation in vivo has not been elucidated.
We have demonstrated that oxidation of the methionine residues of apoA-I leads to aggregation of the protein into fibrils that display all the major, defining hallmarks of amyloid, including: regular fibrillar morphology, the ability to bind ThT and Congo Red, and cross-β structure. Conversely, native apoA-I is relatively stable and does not form fibrils under a range of solution conditions. This link between protein oxidation and apoA-I amyloid formation has several important implications for disease.
Substantial evidence indicates that oxidative processes and inflammation contribute to the pathogenesis of atherosclerosis (30, 31). Methionine oxidized apoA-I is a significant component of normal serum, and is a product of both general and specific oxidative processes that are increased in atherosclerosis. For this reason, MetO-apoA-I has recently gained attention as a potential marker of cardiovascular disease (32, 33). Levels of myleoperoxidase and lipid hydroperoxides, both of which cause specific oxidation of apoA-I methionines (21, 23), are greatly elevated within atherosclerotic tissue (21, 30). Thus, the proportion of MetO-apoA-I is likely to be raised at these sites, where total apoA-I concentrations are already elevated. This is supported by the demonstration that the alternative oxidation products, nitrotyrosine and chlorotyrosine, are dramatically enriched in apoA-I extracted from the atherosclerotic tissue over that in circulation (21). Furthermore, inflammatory processes within atheroma lead to elevated levels of serum amyloid A that can competitively displace apoA-I from HDL particles leading to increased local concentrations of lipid-free apoA-I (5, 8). Thus, the environment within atherosclerotic tissue would appear to favor conditions for methionine oxidation of apoA-I and fibril formation by the oxidized product. Collectively, these factors may explain the common and specific association of apoA-I amyloid deposits with atherosclerotic plaque tissue.
Fibril formation may also represent a unique mechanism by which MetO-apoA-I can specifically contribute to pathology in atherosclerosis. Recent evidence has shown that amyloid fibrils initiate CD36-dependent, macrophage-inflammatory responses, including production of reactive oxygen species and secretion of inflammatory mediators (11, 12). Thus, deposition of MetO-apoA-I fibrils within atheroma can increase lesion inflammation, leading to further oxidative damage and producing a cyclical amplification of pathogenesis.
Methionine oxidation is a common modulator of fibril formation and has been shown to inhibit the aggregation of amyloid-β (34), prion protein (35), transthyretin (36), α-synuclein (37), and apoC-II (38). It has been proposed that methionine residues are involved in specific hydrophobic interactions within the fibril core structure and that these are disrupted by the more hydrophilic MetO sidechain (38), leading to the observed inhibition of fibrilisation. The induction of fibril formation by methinonine oxidation observed here may be explained by a distinct mechanism of action.
Fibril formation by full-length MetO-apoA-I appears to be induced by the disruption of the native self-assembly of the protein into dimers and tetramers (Fig. 2), coupled with impaired structural stability (Fig. 1). This mechanism is similar to that of other amyloid-forming proteins that have native oligomeric structure. Destabilisation of the oligmers of both transthyretin (39) and insulin (40) is required for the initiation of fibril formation that then proceeds via partially-folded intermediates. Thus, methionine oxidation facilitates misfolding and aggregation of apoA-I but the modified amino acids may not be directly involved in hydrophobic packing within the fibril core.
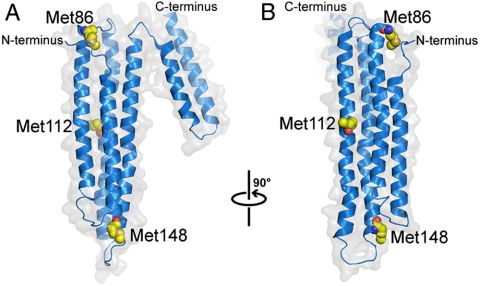
Further insight into the destabilisation of MetO-apoA-I can be gained by inspection of the crystal structure of full-length, lipid-free apoA-I (41). The three methionine residues of apoA-I, Met86, Met112, and Met148 are all contained on the four-helix bundle domain and are partially solvent accessible (Fig. 6). Whereas the oligomeric structure of apoA-I cannot be inferred from the crystal structure (41), we postulate that the four-helix bundle makes inter-subunit contacts or, at least, participates in subunit self-association in the native oligomer. Particular methionine residues may participate in binding interactions between self associating subunits, possibly forming specific hydrophobic contacts. Oxidation of the methionine side chain significantly alters its physico-chemical properties including reduction of its hydrophobic nature and gain of the ability to form hydrogen bonds. Oxidation of methionine may, therefore, directly alter or significantly disrupt intersubunit interactions resulting in reduced self-association. Alternatively methionine oxidation may have a less specific destabilising effect on the structure of the domain by weakening helix packing. This would result in a less compact structure and inhibit native self-association by altering conformation and subunit complementarity. This mechanism is consistent with the broadened melting transition exhibited by MetO-apoA-I, that suggests a more conformationally-flexible structure. It is also possible that the MetO side chain forms unique hydrogen bonds that constrain a subtly altered structure, as has been observed in fibril-forming peptides (42).
Fig. 6.
The crystal structure of lipid-free apoA-I (41, PDB code 2A01) showing the positions of methionine residues (Yellow) in the four-helix bundle. (B) depicts the structure shown in (A) rotated 90° to the right about an axis parallel to the page, as indicated.
Specific conformational perturbations within the monomer caused by the MetO side chain are likely to contribute to both the reduction in stability of the protein and to the exposure of regions critical to its aggregation and structural rearrangement into amyloid fibrils. Further work will be required to determine the specific contribution of each methionine residue to destabilisation, misfolding, and fibril formation by MetO-apoA-I.
To the authors’ knowledge, only one prior report of fibril formation by full-length apoA-I has been made (43), and this contained no characterisation of the fibrils beyond microscopic examination. The findings presented here, including our inability to replicate fibril formation with native apoA-I (Table S1), suggest that the material used in the previous study contained a significant proportion of methionine oxidized protein.
ApoA-I amyloid fibrils extracted from tissues consist, largely, of the N-terminal part of the protein (5, 13). The findings of this study establish that formation of fibrils from full-length apoA-I, followed by proteolysis, is a potential means for generation of the fragmented product, as has been proposed previously (15–17). This mechanism is also supported by the identification of individual fibril deposits comprising heterogeneous apoA-I polypeptides (15–17) that, in some cases, include a significant component of full-length apoA-I (44), and by the wide range of apoA-I fragment size observed between deposits (5, 13). Amyloid fibril formation by intact protein, followed by limited proteolysis, is thought to occur in transthyretin (45, 46) and certain light-chain amyloidoses (13), and accounts for the identification of both full-length protein and heterogeneous polypeptide fragments within the amyloid deposits.
Hereditary mutations in the apoA-I sequence that cause amyloidosis, generally, reduce the predicted α-helical propensity of the protein (5). This structural destabilisation has been proposed to result in increased proteolytic degradation or exposure of amyloidogenic regions of the protein leading to fibril formation via a mechanism similar to that observed here (15–17). In the latter case, it is likely that methionine oxidation would augment the destabilising effect of the mutation, further increasing amyloid formation. It is also important to note that any mutation causing unfolding or destabilisation of the native structure of apoA-I can also increase susceptibility of the methionines to oxidation by increasing their solvent accessibility. N-terminal fragments comprising apoA-I amyloid fibrils from patients with various hereditary apoA-I amyloidoses have been shown to contain MetO at position 86 (15–17). In these studies, methionine oxidized polypeptides were identified by mass spectrometry as the major constituent, and in one case the sole component (15), of the fibril sample. This lends further support to the involvement of methionine oxidation in apoA-I amyloid formation in disease. It is not known whether the presence of a MetO residue at position 86 alters the amyloidogenic potential of the isolated N-terminal fragment in vitro.
The ultrastructural morphology of amyloid fibrils formed from MetO-apoA-I is similar to those formed by apoC-II (28) but differs from the more classical fibril morphology observed for ex vivo apoA-I fibrils (44, 47). Ultrastructural remodelling of fibrils may occur in vivo during proteolysis of the fibril subunits, or due to the presence of lipid or accessory proteins such as serum amyloid P that may explain the difference in gross appearance.
X-ray fibre diffraction studies of fibrils from patients with the apoA-I Leu60Arg mutation (29) show major cross-β spacings very similar to those observed here for MetO-apoA-I fibrils. This indicates that the core structural elements within the two fibril types are similar. Diffraction patterns from ex vivo fibrils resulting from the apoA-I Leu174Ser mutation showed two closely spaced, major equatorial reflections in addition to evidence of coiled-coil structure (44); however, these features were not apparent in the diffraction patterns from MetO-apoA-I fibrils.
A diverse range of protein modifications, including other oxidation products and advanced glycation end products, have the potential to destabilise the native conformation of apoA-I, possibly leading to aggregation similar to that observed here. In addition, methionine oxidation may have a similar destabilising effect on other amyloid forming apolipoproteins that have native structure, such as apolipoprotein A-II. This is highlighted by the demonstration that methionine oxidation of apolipoprotein A-II reduces the α-helical nature of the protein (48).
Based on the observations of this study, methionine oxidation may play a crucial role in deposition of apoA-I amyloid in both inherited apoA-I amyloidoses and atherosclerosis. Thus, control or reversal of methionine oxidation in apoA-I may represent a means to decrease amyloid deposition. It is likely that levels of methionine oxidized apoA-I, particularly those in the arterial intima and atheroma, are an important consideration in atherosclerosis, and that controlling these levels can reduce this facet of cardiovascular disease pathology.
Materials and Methods
Full details in SI Text.
Materials.
Lipid-free apoA-I purified from human plasma was a kind gift from Joseph Bertolini (CSL Bioplasma, Vic, Australia). MetO-apoA-I was prepared by incubation of lipid free apoA-I with 0.3% hydrogen peroxide according to previously described methods (19, 48).
Circular Dichroism Spectroscopy.
Far UV CD spectra were recorded at a protein concentration of 0.2 mg/ml by using a Jasco J-815 CD spectrometer (Tokyo) equipped with a Peltier temperature control module. The thermal denaturation of native apoA-I and and MetO-apoA-I was studied at a protein concentration of 0.2 mg/ml by monitoring CD signal at 222 nm.
Analytical Ultracentrifugation.
Analytical ultracentrifugation experiments were performed in a Beckman XL-I analytical ultracentrifuge equipped with UV/Vis scanning optics. ApoA-I and MetO-apoA-I (0.02–1.6 mg/ml) were centrifuged at 40,000 rpm and 20 °C. Sedimentation velocity profiles were analyzed with the program SEDFIT by using the c(s) model (49).
Thioflavin T fluorescence Measurements.
ApoA-I and MetO-apoA-I were incubated at pH 6.0 and 37 °C in the presence of 10 μM ThT. Fluorescence was measured in duplicate by using a fmax fluorescence plate reader (Molecular Devices, CA).
Congo Red Staining.
Congo Red stained MetO-apoA-I fibrils were placed on poly-lysine coated slides and examined on an Olympus BX51 light microscope at 20 times magnification.
Transmission Electron Microscopy.
Negatively stained MetO-apoA-I fibrils were inspected by using a Tecnai TF30 transmission electron microscope (FEI, The Netherlands) operating at 200 kV. Images were acquired digitally by using a Gatan US1000 2k×2k CCD Camera (Pleasanton, CA).
X-ray Fibre Diffraction Studies.
MetO-apoA-I fibrils grown at 0.8 mg/ml were concentrated to approximately 15 mg/ml and aligned in the magnetic field of an unshielded 600 MHz NMR spectrometer (Fig. S4). X-ray diffraction images were acquired at the Australian Synchrotron MX2 micro-focus beamline
Supplementary Material
Acknowledgments.
We thank Dr. Joseph Bertolini for supplying apoA-I, Assoc. Prof. Paul Gooley for access to the NMR spectrometer used for alignment of fibrils, Genevieve Tan for technical assistance with light microscopy, and Kenneth Goldie and Lynne Waddington for assistance and valuable advice on electron microscopy of amyloid fibrils. Part of this research was undertaken on the MX2 beamline at the Australian Synchrotron. This work was supported by the Australian Research Council and the Melbourne Early Career Researcher Grants Scheme.
Footnotes
The authors declare no conflict of interest .
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910136107/DCSupplemental.
References
- 1.Barter PJ, et al. Antiinflammatory properties of HDL. Circ Res. 2004;95(8):764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 2.Rye KA, Barter PJ. Formation and metabolism of prebeta-migrating, lipid-poor apolipoprotein A-I. Arterioscler Thromb Vasc Biol. 2004;24(3):421–428. doi: 10.1161/01.ATV.0000104029.74961.f5. [DOI] [PubMed] [Google Scholar]
- 3.Saito H, Lund-Katz S, Phillips MC. Contributions of domain structure and lipid interaction to the functionality of exchangeable human apolipoproteins. Prog Lipid Res. 2004;43(4):350–380. doi: 10.1016/j.plipres.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Gregorini G, et al. Renal apolipoprotein A-I amyloidosis: A rare and usually ignored cause of hereditary tubulointerstitial nephritis. J Am Soc Nephrol. 2005;16(12):3680–3686. doi: 10.1681/ASN.2005040382. [DOI] [PubMed] [Google Scholar]
- 5.Obici L, et al. Structure, function and amyloidogenic propensity of apolipoprotein A-I. Amyloid. 2006;13(4):191–205. doi: 10.1080/13506120600960288. [DOI] [PubMed] [Google Scholar]
- 6.Westermark P, Mucchiano G, Marthin T, Johnson KH, Sletten K. Apolipoprotein A1-derived amyloid in human aortic atherosclerotic plaques. Am J Pathol. 1995;147(5):1186–1192. [PMC free article] [PubMed] [Google Scholar]
- 7.Mucchiano GI, Haggqvist B, Sletten K, Westermark P. Apolipoprotein A-1-derived amyloid in atherosclerotic plaques of the human aorta. J Pathol. 2001;193(2):270–275. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH753>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 8.Mucchiano GI, Jonasson L, Haggqvist B, Einarsson E, Westermark P. Apolipoprotein A-I-derived amyloid in atherosclerosis. Its association with plasma levels of apolipoprotein A-I and cholesterol. Am J Clin Pathol. 115(2):298–303. doi: 10.1309/PJE6-X9E5-LX6K-NELY. [DOI] [PubMed] [Google Scholar]
- 9.Rocken C, et al. Prevalence and pathology of amyloid in atherosclerotic arteries. Arterioscler Thromb Vasc Biol. 2006;26(3):676–677. doi: 10.1161/01.ATV.0000201930.10103.be. [DOI] [PubMed] [Google Scholar]
- 10.Howlett GJ, Moore KJ. Untangling the role of amyloid in atherosclerosis. Curr Opin Lipidol. 2006;17(5):541–547. doi: 10.1097/01.mol.0000245260.63505.4f. [DOI] [PubMed] [Google Scholar]
- 11.Medeiros LA, et al. Fibrillar amyloid protein present in atheroma activates CD36 signal transduction. J Biol Chem. 2004;279(11):10643–10648. doi: 10.1074/jbc.M311735200. [DOI] [PubMed] [Google Scholar]
- 12.Moore KJ, et al. A CD36-initiated signaling cascade mediates inflammatory effects of beta-amyloid. J Biol Chem. 2002;277(49):47373–47379. doi: 10.1074/jbc.M208788200. [DOI] [PubMed] [Google Scholar]
- 13.Buxbaum JN. The systemic amyloidoses. Curr Opin Rheumatol. 2004;16(1):67–75. doi: 10.1097/00002281-200401000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Andreola A, et al. Conformational switching and fibrillogenesis in the amyloidogenic fragment of apolipoprotein a-I. J Biol Chem. 2003;278(4):2444–2451. doi: 10.1074/jbc.M204801200. [DOI] [PubMed] [Google Scholar]
- 15.Booth DR, et al. A new apolipoprotein Al variant, Trp50Arg, causes hereditary amyloidosis. QJM. 1995;88(10):695–702. [PubMed] [Google Scholar]
- 16.Booth DR, et al. Hereditary hepatic and systemic amyloidosis caused by a new deletion/insertion mutation in the apolipoprotein AI gene. J Clin Invest. 1996;97(12):2714–2721. doi: 10.1172/JCI118725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soutar AK, et al. ApolipoproteinAImutation Arg-60 causes autosomal dominant amyloidosis. P Natl Acad Sci USA. 1992;89(16):7389–7393. doi: 10.1073/pnas.89.16.7389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pankhurst G, et al. Characterization of specifically oxidized apolipoproteins in mildly oxidized high density lipoprotein. J Lipid Res. 2003;44(2):349–355. doi: 10.1194/jlr.M200256-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Sigalov AB, Stern LJ. Oxidation of methionine residues affects the structure and stability of apolipoprotein A-I in reconstituted high density lipoprotein particles. Chem Phys Lipids. 2001;113(1-2):133–146. doi: 10.1016/s0009-3084(01)00186-4. [DOI] [PubMed] [Google Scholar]
- 20.Brock JW, et al. Increased methionine sulfoxide content of apoA-I in type 1 diabetes. J Lipid Res. 2008;49(4):847–855. doi: 10.1194/jlr.M800015-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Zheng L, et al. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004;114(4):529–541. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shao B, Cavigiolio G, Brot N, Oda MN, Heinecke JW. Methionine oxidation impairs reverse cholesterol transport by apolipoprotein A-I. P Natl Acad Sci USA. 2008;105(34):12224–12229. doi: 10.1073/pnas.0802025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garner B, Waldeck AR, Witting PK, Rye KA, Stocker R. Oxidation of high density lipoproteins II. Evidence for direct reduction of lipid hydroperoxides by methionine residues of apolipoproteins AI and AII. J Biol Chem. 1998;273(11):6088–6095. doi: 10.1074/jbc.273.11.6088. [DOI] [PubMed] [Google Scholar]
- 24.Nicholls SJ, Zheng L, Hazen SL. Formation of dysfunctional high-density lipoprotein by myeloperoxidase. Trends Cardiovas Med. 2005;15(6):212–219. doi: 10.1016/j.tcm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Gursky O, Atkinson D. Thermal unfolding of human high-density apolipoprotein A-1: Implications for a lipid-free molten globular state. P Natl Acad Sci USA. 1996;93(7):2991–2995. doi: 10.1073/pnas.93.7.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vitello LB, Scanu AM. Studies on human serum high density lipoproteins Self-association of apolipoprotein A-I in aqueous solutions. J Biol Chem. 1976;251(4):1131–1136. [PubMed] [Google Scholar]
- 27.Griffin MD, et al. Phospholipid interaction induces molecular-level polymorphism in apolipoprotein C-II amyloid fibrils via alternative assembly pathways. J Mol Biol. 2008;375(1):240–256. doi: 10.1016/j.jmb.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 28.Hatters DM, MacPhee CE, Lawrence LJ, Sawyer WH, Howlett GJ. Human apolipoprotein C-II forms twisted amyloid ribbons and closed loops. Biochemistry. 2000;39(28):8276–8283. doi: 10.1021/bi000002w. [DOI] [PubMed] [Google Scholar]
- 29.Sunde M, et al. Common core structure of amyloid fibrils by synchrotron X-ray diffraction. J Mol Biol. 1997;273(3):729–739. doi: 10.1006/jmbi.1997.1348. [DOI] [PubMed] [Google Scholar]
- 30.Chisolm GM, Steinberg D. The oxidative modification hypothesis of atherogenesis: An overview. Free Radical Bio Med. 2000;28(12):1815–1826. doi: 10.1016/s0891-5849(00)00344-0. [DOI] [PubMed] [Google Scholar]
- 31.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 32.Panzenbock U, Stocker R. Formation of methionine sulfoxide-containing specific forms of oxidized high-density lipoproteins. Biochim Biophys Acta. 2005;1703(2):171–181. doi: 10.1016/j.bbapap.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Woodward M, et al. Association between both lipid and protein oxidation and the risk of fatal or non-fatal coronary heart disease in a human population. Clin Sci. 2009;116(1):53–60. doi: 10.1042/CS20070404. [DOI] [PubMed] [Google Scholar]
- 34.Hou L, Kang I, Marchant RE, Zagorski MG. Methionine 35 oxidation reduces fibril assembly of the amyloid abeta-(1-42) peptide of Alzheimer's disease. J Biol Chem. 2002;277(43):40173–40176. doi: 10.1074/jbc.C200338200. [DOI] [PubMed] [Google Scholar]
- 35.Breydo L, et al. Methionine oxidation interferes with conversion of the prion protein into the fibrillar proteinase K-resistant conformation. Biochemistry. 2005;44(47):15534–15543. doi: 10.1021/bi051369+. [DOI] [PubMed] [Google Scholar]
- 36.Maleknia SD, Reixach N, Buxbaum JN. Oxidation inhibits amyloid fibril formation of transthyretin. Febs J. 2006;273(23):5400–5406. doi: 10.1111/j.1742-4658.2006.05532.x. [DOI] [PubMed] [Google Scholar]
- 37.Uversky VN, et al. Methionine oxidation inhibits fibrillation of human alpha-synuclein in vitro. FEBS Lett. 2002;517(1-3):239–244. doi: 10.1016/s0014-5793(02)02638-8. [DOI] [PubMed] [Google Scholar]
- 38.Binger KJ, Griffin MD, Howlett GJ. Methionine oxidation inhibits assembly and promotes disassembly of apolipoprotein C-II amyloid fibrils. Biochemistry. 2008;47(38):10208–10217. doi: 10.1021/bi8009339. [DOI] [PubMed] [Google Scholar]
- 39.Kelly JW, et al. Transthyretin quaternary and tertiary structural changes facilitate misassembly into amyloid. Adv Protein Chem. 1997;50:161–181. doi: 10.1016/s0065-3233(08)60321-6. [DOI] [PubMed] [Google Scholar]
- 40.Nielsen L, et al. Effect of environmental factors on the kinetics of insulin fibril formation: Elucidation of the molecular mechanism. Biochemistry. 2001;40(20):6036–6046. doi: 10.1021/bi002555c. [DOI] [PubMed] [Google Scholar]
- 41.Ajees AA, Anantharamaiah GM, Mishra VK, Hussain MM, Murthy HM. Crystal structure of human apolipoprotein A-I: Insights into its protective effect against cardiovascular diseases. P Natl Acad Sci USA. 2006;103(7):2126–2131. doi: 10.1073/pnas.0506877103. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Hung A, Griffin MD, Howlett GJ, Yarovsky I. Effects of oxidation, pH and lipids on amyloidogenic peptide structure: Implications for fibril formation? Eur Biophys J. 38(1):99–110. doi: 10.1007/s00249-008-0363-3. [DOI] [PubMed] [Google Scholar]
- 43.Wisniewski T, Golabek AA, Kida E, Wisniewski KE, Frangione B. Conformational mimicry in Alzheimer’s disease Role of apolipoproteins in amyloidogenesis. Am J Pathol. 1995;147(2):238–244. [PMC free article] [PubMed] [Google Scholar]
- 44.Mangione P, et al. Amyloid fibrils derived from the apolipoprotein A1 Leu174Ser variant contain elements of ordered helical structure. Protein Sci. 2001;10(1):187–199. doi: 10.1110/ps.29201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Westermark P, Sletten K, Johnson KH. Ageing and amyloid fibrillogenesis: Lessons from apolipoprotein AI, transthyretin and islet amyloid polypeptide. Ciba F Symp. 1996;199:205–218. doi: 10.1002/9780470514924.ch13. discussion 218-222. [DOI] [PubMed] [Google Scholar]
- 46.Westermark P, Westermark GT. Purification of transthyretin and transthyretin fragments from amyloid-rich human tissues. Methods Mol Biol. 2005;299:255–260. doi: 10.1385/1-59259-874-9:255. [DOI] [PubMed] [Google Scholar]
- 47.Relini A, et al. Ultrastructural organization of ex vivo amyloid fibrils formed by the apolipoprotein A-I Leu174Ser variant: An atomic force microscopy study. Biochim Biophys Acta. 2004;1690(1):33–41. doi: 10.1016/j.bbadis.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 48.Anantharamaiah GM, et al. Effect of oxidation on the properties of apolipoproteins A-I and A-II. J Lipid Res. 1988;29(3):309–318. [PubMed] [Google Scholar]
- 49.Brown PH, Schuck P. Macromolecular size-and-shape distributions by sedimentation velocity analytical ultracentrifugation. Biophys J. 2006;90(12):4651–4661. doi: 10.1529/biophysj.106.081372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.