Abstract
An intriguing phenomenon in the neurobiology of language is lateralization: the dominant role of one hemisphere in a particular function. Lateralization is not exclusive to language because lateral differences are observed in other sensory modalities, behaviors, and animal species. Despite much scientific attention, the function of lateralization, its possible dependence on experience, and the functional implications of such dependence have yet to be clearly determined. We have explored the role of early experience in the development of lateralized sensory processing in the brain, using the songbird model of vocal learning. By controlling exposure to natural vocalizations (through isolation, song tutoring, and muting), we manipulated the postnatal auditory environment of developing zebra finches, and then assessed effects on hemispheric specialization for communication sounds in adulthood. Using bilateral multielectrode recordings from a forebrain auditory area known to selectively process species-specific vocalizations, we found that auditory responses to species-typical songs and long calls, in both male and female birds, were stronger in the right hemisphere than in the left, and that right-side responses adapted more rapidly to stimulus repetition. We describe specific instances, particularly in males, where these lateral differences show an influence of auditory experience with song and/or the bird’s own voice during development.
Keywords: auditory, development, electrophysiology, lateralization, songbird
One of the defining adaptations of the human species is communication through spoken language. Human languages consist of arbitrary signals acquired in a social context during development through a process of vocal imitation (1). A striking neurobiological feature of language is that speech processing is lateralized to one side of the brain: The left hemisphere is dominant for speech production and perception in the majority of people. The function of lateralization in speech is unknown, although various hypotheses about how lateralization increases sensory accuracy or efficiency are under investigation (2 –6). However, lateralization is not unique to language or even to audition; instead, the processing demands of language may exploit lateral differences rooted in fundamental organizational and ontogenetic principles of the vertebrate brain seen across modalities (7 –11). The extent to which lateralization in general, and lateralization for vocal communication signals in particular, depend on postnatal experience, and the functional implications of any such dependence, have yet to be clearly determined.
In this study, we directly tested the need for early experience in the establishment of lateralized processing for learned vocal signals. We manipulated the auditory environment of developing songbirds and then, in adulthood, recorded auditory responses in an avian cortical-like brain area (the caudal medial nidopallium; NCM) that is specialized for processing vocal communication signals. Songbirds are among the few animals that learn their complex, frequency-modulated vocalizations through a process of imitation similar to human speech learning. Normal song development requires auditory and social experience with a vocalizing tutor and an extensive period of vocal practice. Songbirds provide the best-developed model of vocal learning accessible to neurobiological study (1).
Results
We raised juvenile male and female zebra finches in individual isolation and manipulated their auditory experience during the critical period for vocal imitation. One group of intact juveniles was exposed to a conspecific song (Tutored), while another group received no song exposure (Untutored). We also raised juveniles that were surgically devocalized before the critical period for song learning and exposed one group of these birds to conspecific song (Devoc-Tutored), while another group received no song exposure (Devoc-Untutored). In adulthood, we recorded multiunit auditory responses bilaterally in forebrain NCM by using multiple microelectrodes. NCM neurons are known to respond more strongly to conspecific vocalizations than to other sounds (12, 13), and show stimulus-specific adaptation: a decrease in response amplitude with repeated playback of the same sound and a slower rate of adaptation for familiar than for novel sounds (12 –14). This adaptation lasts much longer for conspecific vocalizations than for other sounds and can be thought of as a long-term recognition memory for these communication signals. These properties of selectively responding to and remembering conspecific vocalizations made NCM an ideal candidate for studying the relationship between experience and lateralization for vocal signals.
Auditory stimuli included both natural vocalizations—the long calls (LCs) of individual males and females and the songs of individual males—and simple synthetic sounds. In this species, only males sing. Both sexes produce LCs in the same behavioral contexts, although only males have LCs with complex, learned features resembling song syllables (15, 16). We measured both the absolute response magnitudes (ARMs) and the adaptation rate of neural responses to these stimuli.
Hemispheric Differences in Response Magnitude.
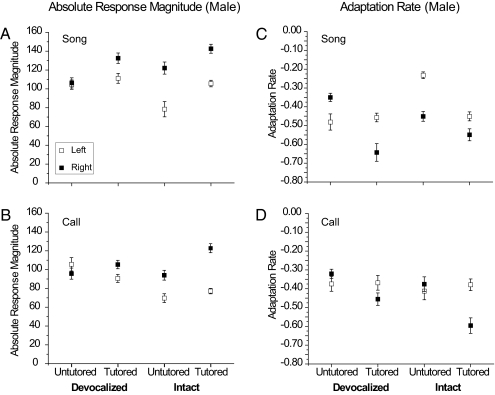
In intact males, ARMs to song stimuli were much higher in the right hemisphere than in the left (Fig. 1A; two-way ANOVA factors: left vs. right hemisphere, F = 57.40, P < 0.0001; Tutored vs. Untutored, F = 17.02, P < 0.0001). This difference was significant both in birds Tutored with song playback (see Table 1 for Bonferroni statistics on each comparison) and in Untutored birds. However, the interaction between hemisphere and tutoring experience was not significant (ANOVA; F= 0.308, P = 0.579), indicating that both Tutored and Untutored birds were lateralized.
Fig. 1.
ARM and adaptation rate of NCM neuronal responses to song and LC stimuli in males. In intact male birds exposed to playback of a male song (Tutored) or just their own isolate song (Untutored), response magnitude to both song and call stimuli was significantly higher in the right hemisphere than in the left (Left, open squares; Right, filled squares). In devocalized male birds that heard neither self-vocalizations nor a tutor song (Devoc-Untutored), no lateral difference in NCM response magnitude to either songs (A) and LCs (B) was seen. Tutored birds (Intact-Tutored) showed significant lateral differences in adaptation rates for both songs (C) and LCs (D). The left side shows lower rates (slower adaptation) than the right. The adaptation rate is the slope of the decrease in response magnitude with successive presentations, normalized by the ARM; thus, the lower symbols (more negative points) represent faster adaptation. In response to songs (C), only Devoc-Tutored males showed no hemispheric differences in adaptation rates. In contrast, in response to LCs (D), Untutored, Devoc-Untutored, and Devoc-Tutored males showed no hemispheric differences. Error bars represent means ± SEM.
Table 1.
Bonferroni post hoc probabilities for left-right differences in each of the experimental groups
| ARM | Rate | ||||
| Song | LC | Song | LC | ||
| INTACT | |||||
| Males | Tutored | <0.001 | <0.001 | 0.025 | <0.001 |
| Untutored | <0.001 | 0.031 | 0.004 | >0.99 | |
| Females | Tutored | <0.001 | <0.001 | >0.99 | 0.020 |
| Untutored | 0.827 | >0.99 | 0.825 | >0.99 | |
| DEVOCALIZED | |||||
| Males | Tutored | 0.027 | 0.258 | 0.001 | 0.413 |
| Untutored | >0.99 | >0.99 | 0.062 | >0.99 | |
| Females | Tutored | >0.99 | 0.206 | >0.99 | >0.99 |
| Untutored | 0.005 | >0.99 | >0.99 | >0.99 | |
Significant values are in bold.
The same pattern of responses was seen for the LC stimuli: ARMs were much higher in the right hemisphere than in the left (Fig. 1B; two-way ANOVA factors: left vs. right hemisphere, F = 45.72, P < 0.0001; Tutored vs. Untutored Experience, F = 12.18, P < 0.001). This difference was significant both in birds Tutored with song playback and in Untutored birds that heard only their own vocalizations. There was also a significant interaction between hemisphere and tutoring experience (ANOVA; F = 4.21 P = 0.041), indicating that the lateral difference was greater in the Tutored birds.
In devocalized males that could not produce audible vocalizations, there was a significant effect of hemisphere and of tutoring experience on ARMs for song stimuli (Fig. 1A; two-way ANOVA factors: left vs. right, F = 4.73, P = 0.030; Tutored vs. Untutored F = 9.557, P = 0.002), but not for LCs (Fig. 1B; ANOVA left vs. right F = 0.188, P = 0.66; Tutored vs. Untutored, F = 0.231, P = 0.63). However, for LCs, there was a significant interaction between hemisphere and experience (ANOVA; F = 4.84, P = 0.028), apparently because ARMs were higher in the right hemisphere in the Devoc-Tutored birds but not the Devoc-Untutored birds. Even in devocalized males, tutoring leads to some degree of lateralization. The overall result with both song and LC stimuli is that lateral differences were absent in birds raised without exposure to conspecific vocalizations; thus, the establishment of lateralized processing in males appears to require auditory experience.
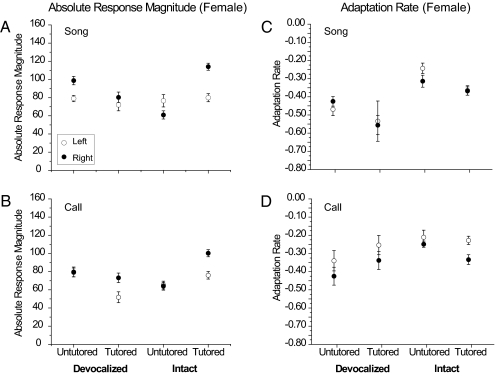
In intact females, ARMs to song stimuli were again higher in the right hemisphere than in the left in Tutored birds (Fig. 2A; two-way ANOVA factors: Tutored vs. Untutored, F = 22.48, P < 0.0001). There was also a significant interaction between hemisphere and tutoring experience (ANOVA; F = 17.47, P < 0.001), indicating that the lateral differences were greater in the Tutored birds. The lateral difference was significant in birds Tutored with song playback, but not in Untutored birds that heard only their own vocalizations (Table 1).
Fig. 2.
ARM and adaptation rate of NCM neuronal responses to song and LC stimuli in females. As in males, Intact-Tutored females have higher response magnitude in the right hemisphere in response to song (A) and LC (B). In contrast to the Intact-Untutored males, Intact-Untutored females (who heard only themselves) do not show a lateral difference in the magnitude of neuronal responses (Left, open circles; Right, filled circles). Untutored males hear their own isolate song and calls (which are abnormal but contain male-typical features), whereas females hear only their own acoustically simple calls. Similar contrasts were seen in measurements of adaptation rates. Unlike Tutored males who produce both song and calls, Tutored females who were able to produce calls (Intact-Tutored) did not show significant lateral differences in adaptation rates to song (C); however, this group did show significant lateral differences in adaptation rates to LCs (D). Error bars represent means ± SEM.
A similar pattern of results were seen for the LC stimuli in Tutored females: ARMs were also higher in the right hemisphere than in the left hemisphere (Fig. 2B; two-way ANOVA factors: left vs. right, F= 6.03, P = 0.014; Tutored vs. Untutored, F = 24.96, P < 0.001). There was also a significant interaction between hemisphere and tutoring experience (ANOVA; F = 7.33 P < 0.01), indicating that the lateral difference was greater in the Tutored birds. The lateral difference was significant in Tutored birds but not in Untutored birds that heard only their own vocalizations.
Thus, in females as in males, Tutored birds showed significant lateralization. However, Untutored females (hearing only themselves) did not show a significant hemispheric difference, in contrast to the Untutored males. This contrast might be due to the different acoustic environment experienced by the males and females, rather than a sex difference per se. Although they were isolated, the intact Untutored groups experienced their own vocalizations, and the acoustic repertoires differ between the sexes. Even in the absence of a tutor song model, males still develop an “isolate song” with complex acoustic features, including frequency modulations, whereas females only produce acoustically simple calls (Materials and Methods). Thus, exposure to a more complex acoustic environment (isolate vocalizations) may be responsible for the lateralization of ARMs observed in Untutored males and not females.
In devocalized females that could not produce audible vocalizations, Tutored birds had lower ARMs to song stimuli than Untutored birds, and this difference was significant for songs (Fig. 2A; ANOVA, F = 4.61, P = 0.033), but not for LCs (Fig. 2B; ANOVA, F = 0.682, P = 0.410). In contrast to the effect seen in devocalized males, Tutoring did not produce a significant lateral difference in the ARMs for song stimuli in the Devoc-Tutored females; however, Devoc-Untutored females unexpectedly showed a significant lateral difference to song stimuli, but not for LCs (Table 1 and Fig. 2 A and B). Nevertheless, there was no significant interaction between experience and hemisphere for either songs or LCs (ANOVA for songs, F = 0.931, P = 0.336; for LCs, F = 3.73, P = 0.055), indicating that the degree of lateralization does not differ between the Tutored and Untutored groups, and making this result difficult to interpret.
To test whether lateral differences are specific to communication sounds or are seen for sounds in general, we also measured ARMs to noncommunication sounds in a subset of birds. We did not find a significant difference in the response between left and right hemispheres for pure tones (Tutored males; t = −1.846, P = 0.081; Tutored females; t = −0.593, P = 0.561) or for band-limited noise (Tutored males; t = −1.870, P = 0.078; Tutored females; t = −0.619, P = 0.544). We verified that there was no lateral difference in the sampled distribution of best frequencies that might have affected these results in any of the groups. However, the lateral differences in Tutored males approached significance, and a power analysis of the male data indicated that a larger sample would be needed to draw a firm conclusion (power ≈ 0.5). Thus, we cannot rule out some degree of experience-dependent lateralization to noncommunication sounds in males.
Hemispheric Differences in Adaptation Rate.
We further characterized the auditory responses recorded from left and right NCM by their adaptation rate, which measures the slope of the decrease in response amplitude as a function of repetition number, and can provide a measure of the familiarity of the stimuli tested (12 –14). The differences described below do not simply reflect larger initial responses because the adaptation rate measure is normalized by absolute amplitude to correct for response amplitude (Materials and Methods).
In intact males, tested with song and LC stimuli, the results showed significant interactions of hemisphere and tutoring experience (Fig. 1C, two-way ANOVA, for songs, F = 16.466, P < 0.01; Fig. 1D; for LCs, F = 7.07, P < 0.01), but this result was not seen in females (Fig. 2C, two-way ANOVA, for songs F = 0.898, P = 0.345; Fig. 2D, for LCs; in females, F = 1.16, P = 0.283). A significantly faster (more negative slope) adaptation rate was seen in the right hemisphere than in the left hemisphere for both song and LC stimuli in Tutored males. In Tutored females, this difference was observed for LC stimuli, but not songs. Untutored males showed a faster adaptation rate on the right for songs, but Untutored females did not (Table 1).
In devocalized male birds, adaptation rates showed a significant interaction between hemisphere and Tutoring experience for both song stimuli (Fig. 2C; ANOVA, F = 19.72, P < 0.001) and LCs (Fig. 2D; ANOVA, F = 4.37, P = 0.038). For song stimuli, there were significant hemispheric differences between adaptation rates in Devoc-Tutored males, but no significant differences for LC stimuli. Devoc-Tutored female birds showed no significant lateral differences in adaptation rate for either song or LC stimuli.
Discussion
Our data show that, in Tutored birds of both sexes, ARMs are significantly greater on the right side than on the left for both conspecific songs and LCs. ARMs for simple synthetic sounds show a much smaller difference between the hemispheres, perhaps correlated with lower stimulus complexity. In addition, responses on the right side adapt more quickly than those on the left. Although the patterns of lateralization are similar for song and LC stimuli, lateral differences are most robust in response to song stimuli. The lateral asymmetries suggest that conspecific vocalizations either engage different network properties in each hemisphere or trigger a gating process that routes information differentially to each hemisphere. With respect to the latter possibility, it has been suggested that asymmetrical inhibitory influences in the brainstem auditory system may contribute to lateral differences in the mammalian cortex (17) and that sound context changes tuning functions in the brainstem auditory system of a songbird (18). However, neither the mechanism nor the functional meaning of the lateral differences in ARM’s and adaptation rates can be determined from the present experiments.
Overall, our results demonstrate lateralized acoustic processing in songbird NCM, adding to various reports of lateral differences observed in adult songbirds based on lesion and behavioral observations (19 –21), electrophysiological recording (22, 23), immediate early gene induction (24), and fMRI (25, 26). Our observation of stronger absolute responses in the right hemisphere is consistent with the finding in a recent fMRI study that BOLD responses to auditory stimuli were larger in the right hemisphere than in the left (25).
Our data further demonstrate an essential role for auditory experience in the development of lateralized responses, at least in males; however, we cannot distinguish whether our tutoring paradigm acts to induce lateralization or to rescue it. It is possible that the early brain is not lateralized and complex patterned sound somehow induces lateral differences, although this does not fit with the lateralization seen in devocalized Untutored females. It seems more likely that a bias toward lateral differences in NCM is innately specified early in development, and the failure to hear complex patterned sounds in our deprivation paradigm leads to a loss of this latent lateralization. However, this interpretation does not explain why the devocalized Untutored females show lateralized responses despite deprivation. Our data also cannot address whether lateralization originates elsewhere in the brain and we merely observe it in NCM.
Our results not only provide clear evidence of lateralized processing in an avian area specialized for discriminating species-specific communication sounds, but also demonstrate an important and, in males, apparently essential, role for auditory experience in the ontogeny of this lateralization. The songbird model may be relevant to the human case because it seems likely that both songbirds and humans have evolved the substrate for vocal learning by exploiting lateral differences in processing that are shared in the vertebrate lineage. Relevant human data are scarce, but they suggest that auditory experience could play a role in human lateralization (27 –29). In humans, there is some evidence of a critical period during which experience with language is necessary to establish neural lateralization of speech production. In one study, normally hearing adolescents and deaf adolescents who had acquired deafness after 3 years of age displayed left hemispheric dominance for productive language. However, congenitally deaf subjects and those with early acquired deafness (onset 6–36 months) showed atypical bilateral cerebral activity during productive language (27, 28). Although recent EEG and fMRI studies have suggested lateralized processing for sounds in infants, they did not find a lateral difference in phonetic processing at an early age (29), opening the possibility for an influence of experience. Again the question remains open as to whether auditory input is needed for the induction of lateral differences or only for their maintenance.
The parallel process of vocal learning humans and songbirds (1) suggests that some forms of auditory deprivation during development, due to partial or unilateral hearing loss, or inadequate stimulation with speech sounds, could produce speech pathologies because they result in the failure of normal lateralization to emerge. If these pathologies can be modeled in songbirds, then remedial paradigms could potentially be developed that use structured auditory training to rescue lateralization and improve speech processing.
Materials and Methods
Subjects and Manipulation of Postnatal Auditory Environment.
Male and female zebra finches (Taeniopygia guttata) were raised in individual breeding cages, within the general aviary, with their parents and siblings until ≈10 days after hatch (phd) (14, 30). At this time, the hatchlings and the mother were placed into sound-attenuated chambers. At ≈25 phd, each bird was placed into individual isolation and its vocalizations were continuously recorded. A subset of these birds was devocalized in the subsong phase of song learning (at 25–37 phd, see ref. 31 for methods). Briefly, under anesthesia, the syrinx was exposed through an incision in the intraclavicular air sac. Openings were made in the trachea rostral to the syrinx, and in the upper portions of each bronchus below the syrinx. This procedure allowed a lower resistance path for airflow that bypassed the syrinx. After this procedure, males engaged in song-like behavior (beak movements and postures) but did not phonate, although, on rare occasions, some birds produced short breathy sounds. From 45 ± 2 phd to ≈80 phd, one group of devocalized juveniles and one group of intact sibling controls were exposed to the playback of a tutor song (Fig. 3A) in response to a key peck (sample size: male tutored intact = 11, female tutored intact = 10, male tutored devocalized = 8, female tutored devocalized = 3; Sound Analysis Live, 2.29 (30). One group of devocalized juveniles and one group of intact controls were maintained in auditory isolation (sample size: male untutored intact = 6, female untutored intact = 6, male untutored devocalized = 7, female untutored devocalized = 5).
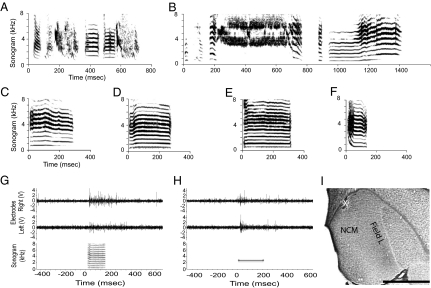
Fig. 3.
Representative vocalizations, multiunit responses, and sagittal section of NCM. (A) Sonogram of tutor song stimulus presented during development. Examples of song (B) and LC (C) produced by an Untutored male showing complex features of these abnormal “isolate” vocalizations and LC of a Tutored female (D). Representative normal LCs used as stimuli in the electrophysiology experiments (E, female call; F, male call). Representative multiunit neuronal responses from left and right NCM of a tutored male to a LC (G) and 2,750-Hz pure tone (H). (I) Representative sagittal section of NCM taken 0.75 mm from the midline. White asterisk indicates a typical lesion made at a recording site in dorsal NCM. (Scale bar: 1 mm.)
Untutored males produced an “isolate” song that is highly abnormal but includes male-typical spectral features (Fig. 3B), as do their LCs (Fig. 3C). Female zebra finches do not sing and do not copy their vocalizations from models; both Tutored and Untutored females produce typical female calls (Fig. 3D). For all groups, electrophysiological experiments were conducted in adulthood (≥110 phd) by placing multiple microelectrodes, simultaneously, in left and right NCM to record auditory responses.
It has been shown that the avian NCM, a cortical-like auditory area in the caudal telencephalon, responds selectively to conspecific songs both in adults and juveniles (12–14, 32). In addition, from experiments in adults, we know that NCM neurons encode auditory memories of the songs of individual conspecifics, which differ due to learning (14). At the end of the tutoring period, we kept all birds in isolation with no song exposure for ≥30 days before the electrophysiological testing because NCM maintains a memory for recent auditory events that can last for several days. Thus, we kept all of the cohorts of birds in the same auditory isolation so we could assess the residual effects of the developmental environment, rather than responses to songs recently experienced. During this time, for intact birds, the auditory environment consisted of birds’ own vocalizations and, for both intact and devocalized birds, subtle environmental noises (air pump hissing, cage hops, wings flapping, etc.).
Auditory Stimuli.
We tested responses to zebra finch songs and LCs (also known as distance calls). LCs are emitted in similar behavioral contexts by both males and females, e.g., when the bird can hear, but not see, a conspecific. However, there are sex differences in duration and acoustic complexity of these calls (Fig. 3 E and F; refs. 15, 16, and 33). Specifically, male LCs (Fig. 3F) are shorter and less variable in duration compared to female LCs (Fig. 3E), use higher fundamental frequencies, and incorporate fast frequency modulations. These male-typical features are learned from a tutor, and closely resemble song syllables; females do not copy their calls. It should be noted that tutoring during development was limited to a song model and did not include playback of LCs. However, in intact tutored males, the syllables in the tutor song potentially could have been used as LC models.
As presented here, the experimental sessions consisted of three stimulus sets, presented in sequential order (songs, calls, and synthetic stimuli). The set of song stimuli included the Bird’s own song (BOS; in nondevocalized birds), the song model, biological father’s song, and the songs of novel conspecifics. The set of LC stimuli consisted of the bird’s own LC (in nondevocalized birds), biological father’s and mother’s LCs, and novel conspecific LCs of males and females. The set of song stimuli were presented first (25 repetitions of each song at 8 s inter-stimulus interval (ISI) in a shuffled order). Upon completion of the song stimuli, the set of LC stimuli were presented in a shuffled order (25 repetitions of each LC at 6-s ISI). In a subset of birds, after the LC stimuli, a set of synthetic stimuli to assess the tuning properties at each recording site in NCM were tested. Synthetic stimuli, consisting of pure tones and band-limited noise (500–3,000 Hz; duration = 260 ms, rise and fall cosine tapered over 5 ms; bandpass for noise stimuli ± 5% of center frequency) were presented in a shuffled order (five repetitions of each stimulus at 6-s ISI). Consistent with published reports in songbirds, this range of frequencies adequately sampled the frequency response space over the depths recorded (34 –36). All stimuli were presented at an average amplitude of 75 dBspl (“A” scale) and were presented from a speaker located at a distance of 0.45 m directly in front of the subject.
Neurophysiological Experiments and Data Analysis.
Birds were anesthetized (Nembutal 50 mg/kg; Abbott Laboratories, North Chicago, IL), placed in a stereotaxic instrument and prepared for electrophysiological testing by cementing a recording chamber and head fixation pin on the skull (Dentsply Caulk, Milford, DE). Two days later, to allow the anesthetic to be completely metabolized, awake birds were restrained by clamping the head fixation pin to the stereotaxic frame. We then placed seven electrodes bilaterally into NCM and recorded multiunit responses to auditory stimuli (for details, see ref. 14; multielectrode recording, Thomas Electronics, Ekhorn design, Germany; data collection and stimulus presentation: Spike2 and Power1401, Cambridge Electronic Design, Cambridge, UK). Multiunit activity (amplified at 19,000×, and filtered, passband: 0.5–5 kHz, to exclude field potentials) was recorded simultaneously from all seven electrodes in response to sets of auditory stimuli as described above. Examples of bilateral responses to a female call and to a tone stimulus are shown in Fig. 3 G and H (for an example of bilateral response to song stimuli, see Fig. S1). At the conclusion of each experiment, electrolytic lesions were made in several recording tracks. The birds were then anesthetized and perfused for histological preparation. Placements in NCM were verified by electrophysiological responses and then by lesion recovery and reconstruction of penetration tracks with respect to cytoarchtectonic features for NCM (Fig. 3I). Only sites that met criteria for being in NCM (histological verification and response adaptation) were included in the analyses.
We quantified the magnitude of the neuronal responses to each stimulus at each recording site by subtracting the root mean square (rms) of neural activity of the control period (0.5 s before stimulus onset) from the rms over the response period (stimulus duration plus 100 ms) for each trial. The ARMs were calculated from the average rms on the second to sixth presentations. This procedure both eliminated the effect of highly variable responses to the first presentation of each stimulus and reduced the influence of adaptation to the repeated stimuli. For the artificial stimuli, ARMs were calculated at each site by averaging responses to all five repetitions of each stimulus. Only responses to the best frequency (frequency of maximal response) were used for both tone and band-passed noise analyses. Within each bird, only responses that were significantly higher than the averaged baseline control responses of that electrode and trial stimulus were used in the calculations to determine response magnitudes. The percentage of excluded sites was <10% in the Intact experimental groups. The left and right side rejection rates were not statistically different [Mann–Whitney U; Intact-Tutored males (n = 10): Z = 0.537, P = 0.592; Intact-Tutored females (n = 9): Z = 0.512, P = 0.608].
In this study, no attempt was made to isolate single units because the constraints of the awake condition allow a very limited period for neural recordings from the brains of animals that are still naïve to song and LC stimuli in our developmental paradigm. However, to verify the lateralization measures we obtained from the rms of multiunit recordings, we used a digital spike sorting algorithm to provisionally identify the largest spike waveform on each recorded channel (for details, see Fig. S2 legend). This procedure yielded “near single-units” (NSUs). We then compared the firing rate responses of 119 NSUs identified in 11 Tutored males and 6 Devocalized-Untutored males, the groups that best exemplified complex auditory experience vs. auditory deprivation, respectively. The results showed significantly larger firing rate increases in NSUs recorded in the right hemisphere than in the left (Fig. S2). These results follow the same pattern seen for the multiunit ARM responses.
We also measured adaptation rates for songs and LC response because these measurements are stimulus-specific and can provide an index of the familiarity of the stimuli in NCM (14). Using established regression methods, we calculated the adaptation rate as the slope of the decrease in response magnitude with successive presentations over the linear region of the adaptation profile (trials 6–25) at each electrode for each stimulus (12). Examples of these adaptation profiles are shown in Fig. S3. This rate was then divided by the average ARM over the same trials (trials 6–25) to normalize the rates for differences in absolute response size between recording sites. The rate represents the percentage drop in response amplitude per stimulus repetition.
Analysis of the neural activity to song was limited to novel conspecific song stimuli. An initial statistical analysis compared responses to LC stimulus types (novel, father’s, mother’s, bird’s own call) by using a three-way ANOVA (tutoring condition by hemisphere by stimulus type). There was no significant interaction between the main effect of auditory experience on hemispheric differences (Results) for LC stimulus type (F = 0.929, P = 0.426), so responses were pooled across all stimulus types in the call set for subsequent analyses. Comparisons within each sex used two-way ANOVA: (tutoring condition by hemisphere; and Bonferroni corrected posthoc t tests between groups). The criterion for significance was set at P < 0.05 (two-tailed).
Supplementary Material
Acknowledgments
We thank Carolyn Pytte, Marc Schmidt, Blair Simpson, Karin Stromswold, Jianqiang Xiao, and Kathleen Yoder for helpful comments on the manuscript. This work was supported by National Institute of Mental Health, National Institute on Deafness and Other Communication Disorders (D.S.V. and M.L.P.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900091107/DCSupplemental.
References
- 1.Doupe AJ, Kuhl PK. Birdsong and human speech: Common themes and mechanisms. Annu Rev Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- 2.Hirnstein M, Hausmann M, Güntürkün O. The evolutionary origins of functional cerebral asymmetries in humans: does lateralization enhance parallel processing? Behav Brain Res. 2008;187:297–303. doi: 10.1016/j.bbr.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 3.Petersen MR, et al. Neural lateralization of vocalizations by Japanese macaques: communicative significance is more important than acoustic structure. Behav Neurosci. 1984;98:779–790. doi: 10.1037//0735-7044.98.5.779. [DOI] [PubMed] [Google Scholar]
- 4.Ehret G. Left hemisphere advantage in the mouse brain for recognizing ultrasonic communication calls. Nature. 1987;325:249–251. doi: 10.1038/325249a0. [DOI] [PubMed] [Google Scholar]
- 5.Hauser MD, Andersson K. Left hemisphere dominance for processing vocalizations in adult, but not infant, rhesus monkeys: Field experiments. Proc Natl Acad Sci USA. 1994;91:3946–3948. doi: 10.1073/pnas.91.9.3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halpern ME, Güntürkün O, Hopkins WD, Rogers LJ. Lateralization of the vertebrate brain: Taking the side of model systems. J Neurosci. 2005;25:10351–10357. doi: 10.1523/JNEUROSCI.3439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- 8.Zatorre RJ. Neural specializations for tonal processing. Ann N Y Acad Sci. 2001;930:193–210. doi: 10.1111/j.1749-6632.2001.tb05734.x. [DOI] [PubMed] [Google Scholar]
- 9.Rogers LJ. Factors influencing development of lateralization. Cortex. 2006;42:107–109. doi: 10.1016/s0010-9452(08)70332-0. [DOI] [PubMed] [Google Scholar]
- 10.Dorsaint-Pierre R, et al. Asymmetries of the planum temporale and Heschl’s gyrus: Relationship to language lateralization. Brain. 2006;129:1164–1176. doi: 10.1093/brain/awl055. [DOI] [PubMed] [Google Scholar]
- 11.Tommasi L. Mechanisms and functions of brain and behavioural asymmetries. Philos Trans R Soc Lond B. 2009;364:855–859. doi: 10.1098/rstb.2008.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chew SJ, Mello C, Nottebohm F, Jarvis E, Vicario DS. Decrements in auditory responses to a repeated conspecific song are long-lasting and require two periods of protein synthesis in the songbird forebrain. Proc Natl Acad Sci USA. 1995;92:3406–3410. doi: 10.1073/pnas.92.8.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chew SJ, Vicario DS, Nottebohm F. A large-capacity memory system that recognizes the calls and songs of individual birds. Proc Natl Acad Sci USA. 1996;93:1950–1955. doi: 10.1073/pnas.93.5.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phan ML, Pytte CL, Vicario DS. Early auditory experience generates long-lasting memories that may subserve vocal learning in songbirds. Proc Natl Acad Sci USA. 2006;103:1088–1093. doi: 10.1073/pnas.0510136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zann R. Structural variation in the zebra finch distance call. Z Tierpsychol. 1984;66:328–345. [Google Scholar]
- 16.Simpson HB, Vicario DS. Brain pathways for learned and unlearned vocalizations differ in zebra finches. J Neurosci. 1990;10:1541–1556. doi: 10.1523/JNEUROSCI.10-05-01541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abrams DA, Nicol T, Zecker SG, Kraus N. Auditory brainstem timing predicts cerebral asymmetry for speech. J Neurosci. 2006;26:11131–11137. doi: 10.1523/JNEUROSCI.2744-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woolley SM, Gill PR, Theunissen FE. Stimulus-dependent auditory tuning results in synchronous population coding of vocalizations in the songbird midbrain. J Neurosci. 2006;26:2499–2512. doi: 10.1523/JNEUROSCI.3731-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams H, Crane LA, Hale TK, Esposito MA, Nottebohm F. Right-side dominance for song control in the zebra finch. J Neurobiol. 1992;23:1006–1020. doi: 10.1002/neu.480230807. [DOI] [PubMed] [Google Scholar]
- 20.Floody OR, Arnold AP. Song lateralization in the zebra finch. Horm Behav. 1997;31:25–34. doi: 10.1006/hbeh.1997.1368. [DOI] [PubMed] [Google Scholar]
- 21.Cynx J, Williams H, Nottebohm F. Hemispheric differences in avian song discrimination. Proc Natl Acad Sci USA. 1992;89:1372–1375. doi: 10.1073/pnas.89.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hauber ME, Cassey P, Woolley SM, Theunissen FE. Neurophysiological response selectivity for conspecific songs over synthetic sounds in the auditory forebrain of non-singing female songbirds. J Comp Physiol A. 2007;193:765–774. doi: 10.1007/s00359-007-0231-0. [DOI] [PubMed] [Google Scholar]
- 23.George I, Cousillas H, Richard JP, Hausberger M. New insights into the auditory processing of communicative signals in the HVC of awake songbirds. Neuroscience. 2005;136:1–14. doi: 10.1016/j.neuroscience.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Avey MT, Phillmore LS, MacDougall-Shackleton SA. Immediate early gene expression following exposure to acoustic and visual components of courtship in zebra finches. Behav Brain Res. 2005;165:247–253. doi: 10.1016/j.bbr.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Voss HU, et al. Functional MRI of the zebra finch brain during song stimulation suggests a lateralized response topography. Proc Natl Acad Sci USA. 2007;104:10667–10672. doi: 10.1073/pnas.0611515104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poirier C, Boumans T, Verhoye M, Balthazart J, Van der Linden A. Own-song recognition in the songbird auditory pathway: Selectivity and lateralization. J Neurosci. 2009;29:2252–2258. doi: 10.1523/JNEUROSCI.4650-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcotte AC, Morere DA. Speech lateralization in deaf populations: Evidence for a developmental critical period. Brain Lang. 1990;39:134–152. doi: 10.1016/0093-934x(90)90008-5. [DOI] [PubMed] [Google Scholar]
- 28.Leybaert J, D'Hondt M. Neurolinguistic development in deaf children: The effect of early language experience. Int J Audiol. 2003;42(Suppl 1):S34–S40. doi: 10.3109/14992020309074622. [DOI] [PubMed] [Google Scholar]
- 29.Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002;298:2013–2015. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- 30.Tchernichovski O, Nottebohm F, Ho CE, Pesaran B, Mitra PP. A procedure for an automated measurement of song similarity. Anim Behav. 2000;59:1167–1176. doi: 10.1006/anbe.1999.1416. [DOI] [PubMed] [Google Scholar]
- 31.Pytte CL, Suthers RA. A bird’s own song contributes to conspecific song perception. Neuroreport. 1999;10:1773–1778. doi: 10.1097/00001756-199906030-00027. [DOI] [PubMed] [Google Scholar]
- 32.Stripling R, Kruse AA, Clayton DF. Development of song responses in the zebra finch caudomedial neostriatum: role of genomic and electrophysiological activities. J Neurobiol. 2001;48:163–180. doi: 10.1002/neu.1049. [DOI] [PubMed] [Google Scholar]
- 33.Vicario DS. Using learned calls to study sensory-motor integration in songbirds. Ann N Y Acad Sci. 2004;1016:246–262. doi: 10.1196/annals.1298.040. [DOI] [PubMed] [Google Scholar]
- 34.Müller CM, Leppelsack HJ. Feature extraction and tonotopic organization in the avian auditory forebrain. Exp Brain Res. 1985;59:587–599. doi: 10.1007/BF00261351. [DOI] [PubMed] [Google Scholar]
- 35.Capsius B, Leppelsack H. Response patterns and their relationship to frequency analysis in auditory forebrain centers of a songbird. Hear Res. 1999;136:91–99. doi: 10.1016/s0378-5955(99)00112-4. [DOI] [PubMed] [Google Scholar]
- 36.Terleph TA, Mello CV, Vicario DS. Species differences in auditory processing dynamics in songbird auditory telencephalon. Dev Neurobiol. 2007;67:1498–1510. doi: 10.1002/dneu.20524. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.